Abstract
Extensive pharmacological studies have recently emerged indicating that group 2 metabotropic glutamate receptors (mGluRs) comprising mGluR2 and mGluR3 subtypes are associated with several neurological and psychiatric disorders. mGluR2 is widely distributed both presynaptically and postsynaptically in a variety of neuronal cells, but the physiological role of mGluR2 in brain function is poorly understood. This investigation involves a comprehensive behavioral analysis of mGluR2–/– knockout (KO) mice to explore the physiological role of mGluR2 in brain function. Although, under general observation, mGluR2–/– KO mice appeared to have no behavioral abnormalities, they exhibited several lines of behavioral alterations in the enforcing and defined behavioral tests. They showed a significant increase in locomotor sensitization and conditioned place preference in association with repeated cocaine administration, indicating that mGluR2 contributes to behavioral responses implicated in reinforcement and addiction of cocaine. Upon in vivo microdialysis analysis after cocaine administration, not only did extracellular levels of dopamine increase but also the response pattern of glutamate release markedly changed in the nucleus accumbens of mGluR2–/– KO mice. The mGluR2–/– KO mice also showed significant impairment in motor coordination in the accelerating rota-rod test and exhibited hyperlocomotion in novel environmental and stressful conditions, when assessed by the open-field and forced-swim tests. These results indicate that the inhibitory mGluR2 plays a pivotal role in synaptic regulation of glutamatergic transmission in the neural network.
Keywords: addiction, hyperactivity, microdialysis, glutamatergic transmission, nucleus accumbens
Glutamate receptors play central roles in neuronal excitation in the mammalian central nervous system (1). Glutamate receptors are categorized into two classes: ionotropic glutamate receptors and metabotropic glutamate receptors (mGluRs) (1, 2). mGluRs are G protein-coupled receptors and consist of eight different subtypes that are classified into three groups (1–5). mGluR2 and mGluR3 are highly homologous group 2 receptors and are both coupled to inhibitory signaling cascades (1–5). Recently, extensive pharmacological studies have emerged, indicating that group 2 mGluRs are associated with several neurological and psychiatric disorders. For example, it has been reported that agonists for group 2 mGluRs inhibit ischemia-induced hippocampal neuronal cell death (6), diminish haloperidol-induced muscle rigidity in an animal model of Parkinson's disease (7), reduce fear-potentiated startle in an animal model of anxiety (8), and attenuate behavioral abnormalities induced by phencyclidine or amphetamine (9, 10). However, the agonists or antagonists developed for group 2 mGluRs cannot distinguish the subtype specificity of group 2 mGluRs, and the specificity of group 2 mGluRs implicated in neurological and psychiatric disorders remains elusive.
mGluR2 is expressed in neuronal cells, whereas mGluR3 is distributed in both neuronal and glial cells (11–13). In neuronal cells, both mGluR2 and mGluR3 act to inhibit glutamate release as autoreceptors located on glutamatergic axon terminals, or the release of other neurotransmitters as presynaptic heteroreceptors (4, 5). mGluR2 is also concentrated at dendrites of specified interneurons, including Golgi cells in the cerebellum, cholinergic interneurons in the striatum, and starburst amacrine cells in the retina (11, 12, 14–16). Furthermore, a recent study (16) has indicated that postsynaptic mGluR2 suppresses Golgi cell excitability in an input-dependent manner and plays an important role in spatiotemporal regulation of glutamatergic transmission in the cerebellar cortical circuit. However, the physiological and behavioral role of mGluR2 in brain function is poorly understood. To explore not only the physiological role of mGluR2 in brain function but also the mGluR subtype specificity implicated in neurological and psychiatric disorders, this investigation concerns behavioral alterations of mGluR2–/– knockout (KO) mice by comprehensive behavioral analysis. Here, we report that the lack of mGluR2 alters cocaine-induced addictive responses and perturbs the dopamine (DA)–glutamate interaction in the nucleus accumbens (NAc). We also show that mGluR2 deficiency enhances stress-related hyperlocomotion and impairs movement coordination.
Materials and Methods
Animals and Behavioral Analysis. mGluR2–/– KO mice and their WT littermates were generated by mating heterozygous mGluR2+/– mice as described (17). Heterozygous mGluR2+/– mice were backcrossed to C57BL/6 background for at least 18 generations. All behavioral tests were carried out with male mice that were 10 weeks old at the start of testing. Animals were provided with food and water ad libitum. All procedures were performed according to the guidelines of Kyoto University Faculty of Medicine. The open-field test was conducted with an open-field apparatus (40 × 40 × 30 cm, Accuscan Instruments, Columbus, OH) by recording total distance traveled (cm) and time spent in the central part of the open field (sec) (18). Data were analyzed every 5 min for a 30-min period. Locomotor activity in a familiar environment was measured with an automatically monitoring system of locomotion in a home cage as described (18). Distance traveled (cm) in their home cage was calculated by using image ha software modified from nih image software (18). The forced-swim test was performed essentially according to the procedures described by Porsolt et al. (19). The apparatus consisted of four glass cylinders (30 cm in height and 13 cm in diameter) that were separated by a nontransparent shield. The cylinder was filled with water (23°C) up to a height of 15 cm. A mouse was placed into the cylinder, and its behavior was recorded by a video camera for a 10- and 6-min period for the first and second days, respectively. The immobility time was assessed by manual observation or computer-based automatic analysis (20). A mouse was judged to be immobile when it made only those movements necessary to keep its head above water. The parameter recorded was the number of seconds spent immobile. The tail-suspension test was performed for a 10-min test session according to the procedures described by Steru et al. (21). All other tests were performed as described in the behavioral test battery (18, 20).
Cocaine Experiments. Mice received i.p. saline or cocaine (5, 10, and 20 mg/kg). Locomotor activity was assessed in an open-field apparatus (20 × 20 × 30 cm) for a 10-min period immediately after administration of saline or cocaine as described (22). The conditioned place preference (CPP) test was performed as described (23).
In Vivo Microdialysis. In vivo microdialysis was performed with male mice at the age of 10–14 weeks. Mice were anesthetized with sodium pentobarbital, and a guide cannula (AG-5, Eicom, Kyoto) was implanted into the NAc: anterior, 1.1 mm and lateral, 1.0 mm from the bregma, and dorsal, –3.3 mm from the surface of the brain according to Franklin and Paxinos (24). Three days after surgery and 1 day before microdialysis, a dialysis probe (1 mm membrane in length, AI-5-1, Eicom) was inserted through a guide cannula. Artificial cerebrospinal fluid (aCSF) was perfused at a flow rate of 1.0 μl/min, and fractions of the outflow were collected every 20 min. After collection of three baseline fractions, cocaine (10 mg/kg) was i.p.-injected. The composition of aCSF for DA measurement was 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, 2 mM Na2HPO4 and 0.15 mM NaH2PO4 and composition of aCSF for glutamate measurement was 147 mM NaCl, 3 mM KCl, 1 mM MgCl2 and 1.3 mM CaCl2. Dialysates were assayed by HPLC with electrochemical detection (HTEC-500, Eicom) under the following conditions. For DA assay, an Eicompak PP-ODS column and a graphite electrode set at 400 mV against an Ag/AgCl reference electrode were used. For glutamate assay, an Eicompak GU-GEL column, a glutamate oxidase-immobilized column (E-ENZYMPAK), and a platinum electrode set at 450 mV against an Ag/AgCl reference electrode were used. The mobile phase for DA assay contained 100 mM sodium phosphate buffer (pH 6.0), 500 mg/liter sodium-1-decanesulfonic acid (Tokyokasei, Tokyo), 50 mg/liter EDTA (Dojindo, Tokyo), and 1.5% (vol/vol) methanol. The mobile phase for glutamate assay contained 50 mM NH4Cl and 250 mg/liter hexadecyltrimethylammonium bromide, adjusted to pH 7.4 by ammonium solution. Tissue contents of monoamines and their metabolites were measured as described (25).
Statistical Analysis. Statistical analysis was conducted by using statview (SAS institute, Cray, NC) or prism (GraphPad, San Diego). Data were analyzed by two-tailed t test, two-way ANOVA, or repeated-measure ANOVA and are presented as mean ± SEM.
Results
General Characteristics of mGluR2–/– KO Mice. Previous immunohistochemical studies (11, 12) using a monoclonal mGluR2 antibody indicate that the mGluR2 immunoreactivity is widely distributed in various brain regions, including the cerebral cortex, caudate-putamen, NAc, and cerebellum. To address the behavioral effects of mGluR2 deficiency, we subjected mGluR2–/– KO mice and their WT littermates to a comprehensive behavioral test battery (18). mGluR2–/– KO mice were healthy and showed no obvious alteration in physical characteristics (body weight, appearance of fur and whiskers, and rectal temperature) (Table 1, which is published as supporting information on the PNAS web site). There was no difference in the ability to conduct various behavioral tests between mGluR2–/– KO and WT mice, including wire hang, grip strength, sensory-motor reflexes (eye blink, ear twitch, whisker twitch, righting reflex, and acoustic startle response), and sensory-motor gating (prepulse inhibition) (Table 1).
Hyperlocomotion Under Novel Environmental and Stressful Conditions. When the locomotor activity of mGluR2–/– KO and WT mice was tested under conditions where the animals become hyperlocomotive or more mobile, hyperlocomotion phenotypes were consistently observed in mGluR2–/– KO mice. Total distance traveled by mGluR2–/– KO mice was significantly greater than that of WT mice when animals were placed in an open field (Fig. 1A). On the contrary, once animals were habituated to an environment for 3 days, total distance traveled was no longer different between mGluR2–/– KO and WT mice (data not shown). There was also no difference in a daily rhythmic locomotor activity between these two genotypes after habituation to an environment for 3 days (Fig. 1C). The hyperlocomotive tendency of mGluR2–/– KO mice in the novel environment was also noted in the light/dark transition test, the elevated-plus maze test, and the social interaction test (Fig. 1B), although the observed hyperlocomotion was mild in these tests and was not found to be statistically significant between mGluR2–/– KO and WT mice. Interestingly, the time spent in the central part of the open field, the time spent in the light box of the light/dark transition test, the time spent on an open arm in the elevated-plus maze test, and the duration of contacts in the social interaction test were all comparable between mGluR2–/– KO and WT mice (Table 1). These indices showing no statistical differences between the two genotypes are considered to be related to anxiety. It is thus more likely that the observed increase in locomotion of mGluR2–/– KO mice in the different tests is primarily caused by their hyperactivity rather than an increase in anxiety.
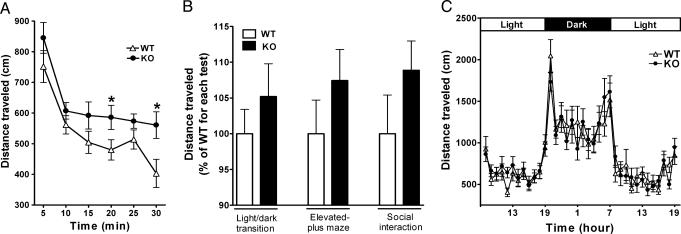
Fig. 1.
Enhanced locomotion of mGluR2–/– KO mice in novel environments. (A) Mice were placed in the open field and distance traveled was measured every 5 min for a 30-min period [(n = 19 (WT) and 17 (KO)]. There is a significant increase in locomotor activity in mGluR2–/– KO mice as compared with WT mice [genotype effect, F(1, 34) = 4.55, P = 0.040] (*, P < 0.05). (B) Distances traveled were measured for a 10-min period in the indicated tests. Data are presented as percentages of average value of WT mice. Differences in distances traveled are not statistically significant between mGluR2–/– KO and WT mice in these tests, but mGluR2–/– KO mice displayed a tendency to increase locomotor activity in all three tests; the light-dark transition test, P = 0.36, n = 21 (WT) and 19 (KO); the elevated-plus maze test, P = 0.25, n = 21 (WT) and 19 (KO); the social interaction test, P = 0.21, n = 10 pairs (WT) and 9 pairs (KO). (C) Distances traveled of mGluR2–/– KO (n = 16) and WT (n = 16) mice in their home cages exposed to a light-dark cycle of 12 h/12 h were measured at each 60-min point. There is no statistical difference between the two genotypes [genotype effect, F(1, 30) = 0.000, P = 0.999].
We also measured locomotor activity under a stressful condition by performing the forced-swim test. When mGluR2–/– KO and WT mice were placed in a water-filled cylinder, they gradually became less mobile with no statistical difference on the first day (Fig. 2A). However, mGluR2–/– KO mice were significantly more mobile on the second day as compared with WT mice (Fig. 2B). The high mobility in the forced-swim test is also related to an antidepressant activity. Tail suspension, a different depression-related behavior, was thus tested, but there was no difference in the immobility time in the tail-suspension test between WT and mGluR2–/– KO mice (WT, 269.4 ± 21.6 sec; KO, 263.7 ± 31.4 sec, P = 0.88). The mGluR2–/– KO mice thus display hyperactivity under stressful conditions.
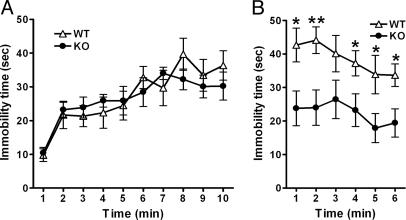
Fig. 2.
Decreased immobility of mGluR2–/– KO mice in the forced-swim test. Mice were placed in a water-filled cylinder, and their immobility time was measured every 1 min for a 10- and a 6-min period on the first (A) and second (B) days, respectively (n = 9 for both WT and KO). The immobility time is not significantly different between the two genotypes on the first day [genotype effect, F(1, 16) = 0.041, P = 0.841] but significantly decreases in mGluR2–/– KO mice as compared with WT mice on the second day [genotype effect, F(1, 16) = 9.255, P = 0.0078] (*, P < 0.05; **, P < 0.01).
Impairment of Motor Coordination in mGluR2–/– KO Mice. The cerebellum plays an important role in controlling motor coordination (26). In the cerebellum, mGluR2 is preferentially and highly expressed in Golgi cells both presynaptically and postsynaptically (11, 12), and both presynaptic and postsynaptic mGluR2 plays an important role in spatiotemporal regulation of glutamatergic transmission in the cerebellar cortical circuitry (16, 27, 28). Despite discernible response changes of mGluR2–/– KO Golgi cells in cerebellar slices (16), mGluR2–/– KO mice showed no obvious movement disorders such as cerebellar ataxia under ordinary conditions (17). We addressed whether mGluR2–/– KO mice could manage a challenging motor task that requires more demanding motor coordination. When mGluR2–/– KO and WT mice were forced to manage an accelerating rota-rod, mGluR2–/– KO mice exhibited a significant impairment in their ability to remain on a rotating rod as compared with WT mice not only at the initial trial but also throughout the course of repeated trials (Fig. 3). The performance of mGluR2–/– KO mice in remaining on a rotating rota-rod became progressively improved during repeated trials (Fig. 3). The impairment of mGluR2–/– KO mice in the rota-rod test thus most likely reflects a defect of motor coordination rather than a defect of motor learning.
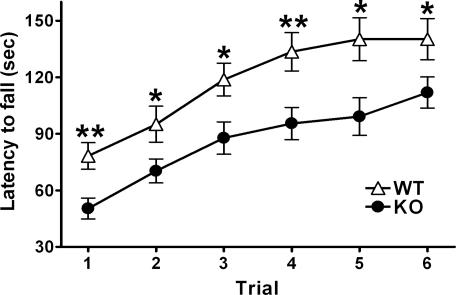
Fig. 3.
Impairment of mGluR2–/– KO mice in the rota-rod test. The latency to fall from an accelerating rota-rod (from 4 to 40 rpm) was measured by three trials per day [n = 21 (WT) and 19 (KO)]. mGluR2–/– KO mice spend significantly less time on the rod than did WT mice [genotype effect, F(1, 38) = 12.53, P = 0.0011] (*, P < 0.05; **, P < 0.01), although their performance improves after repeated trials [trial effect, F(5, 90) = 11.35, P < 0.0001].
Increase of Addictive Responses to Cocaine in mGluR2–/– KO Mice. The caudate-putamen and NAc play a crucial role in controlling locomotor activity (29–31). The NAc also serves as a neural substrate that is implicated in reinforcement and addiction of drugs of abuse (32–34). It has recently been reported that group 2 mGluR-selective agonists and antagonists influence hyperlocomotor activity in rodents, after single or repeated administration of drugs of abuse. However, studies concerning their effects on drug-induced behavioral changes have been contradictory (9, 10, 35–37). We addressed whether mGluR2 deficiency affects behavioral changes induced by repeated cocaine administration. Daily administration of cocaine at doses of 10 mg and 20 mg/kg progressively increased locomotor activity, called locomotor sensitization, in both mGluR2–/– KO and WT mice (Fig. 4 B and C). No such increase in locomotor activity was observed in the two genotypes after daily administration of saline (Fig. 4A). Importantly, mGluR2–/– KO mice exhibited a significant increase in locomotor sensitization at both doses of cocaine (Fig. 4 B and C), indicating that mGluR2 deficiency enhances the responses to repeated cocaine exposure.
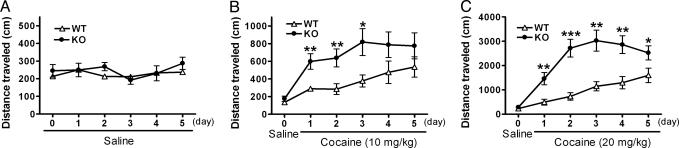
Fig. 4.
Enhanced locomotor sensitization to cocaine in mGluR2–/– KO mice. After i.p. saline injection on day 0, saline (A) and 10 mg/kg (B) and 20 mg/kg (C) cocaine were i.p.-injected once a day from day 1 to day 5; n = 8 (WT) and 11 (KO) in A; n = 10 (WT) and 9 (KO) in B; n = 9 (WT) and 13 (KO) in C. Immediately after injection of saline or cocaine, locomotor activity was counted for a 10-min period. In A, there is no difference in locomotor activity between the two genotypes [genotype effect, F(1, 17) = 0.172, P = 0.682]. Daily administration of cocaine at doses of 10 (B) and 20 (C) mg/kg induces locomotor sensitization in both mGluR2–/– KO and WT mice [10 mg in WT, F(5, 45) = 6.501, P < 0.0001; 10 mg in KO, F(5, 40) = 9.559, P < 0.0001; 20 mg in WT, F(5, 40) = 11.61, P < 0.0001; 20 mg in KO, F(5, 60) = 31.86, P < 0.0001]. In B, there is a significant increase in locomotor activity in mGluR2–/– KO mice as compared with WT mice by repeated cocaine administration [genotype effect, F(1, 17) = 6.397, P = 0.022] (*, P < 0.05; **, P < 0.01). In C, there is a significant interaction between genotype and time by repeated cocaine administration [F(4, 80) = 4.712, P = 0.0018], and a significant increase in locomotor activity is noted in mGluR2–/– KO mice as compared with WT mice at all time points after cocaine treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
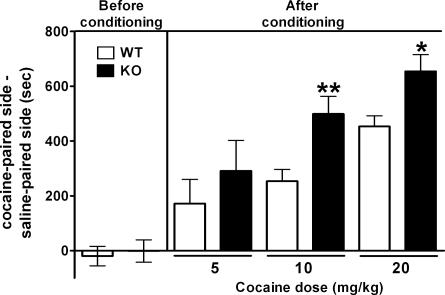
Cocaine can also establish preference for an environment associated with repeated cocaine exposure, called CPP (38, 39). This CPP paradigm serves as a model of reinforcement and addiction of cocaine administration (38, 39). We addressed whether mGluR2 deficiency results in enhancement of CPP induced by chronic cocaine exposure. Animals were conditioned with repeated cocaine administration at doses of 5, 10, and 20 mg/kg in one of two chambers that differed visually and texturally. Before conditioning, both mGluR2–/– KO and WT mice visited the two chambers with no preference (Fig. 5). After conditioning with cocaine for 3 days, both groups exhibited CPP. However, mGluR2–/– KO mice showed a significant enhancement in CPP after cocaine administration at doses of 10 and 20 mg/kg (Fig. 5). A low dose of cocaine (5 mg/kg) also tended to increase CPP in mGluR2–/– KO mice, although this difference was not statistically significant between the two genotypes (Fig. 5). The two different paradigms of cocaine-induced behavioral responses have thus provided compelling evidence that mGluR2 contributes to behavioral responses associated with reinforcement and addiction of cocaine.
Fig. 5.
Enhanced CPP to cocaine in mGluR2–/– KO mice. Shown are results of before and after conditioning with indicated doses of i.p. cocaine injection for 3 days, and the time difference was calculated by subtracting time mice spent in the saline-paired side from the time they spent in the cocaine-paired side (for each group, n > 8). mGluR2–/– KO mice spend significantly more time as compared with WT mice in the cocaine-paired side after conditioning with 10 and 20 mg/kg cocaine [genotype effect, F(1, 44) = 9.465, P = 0.0036] (*, P = 0.014; **, P = 0.006).
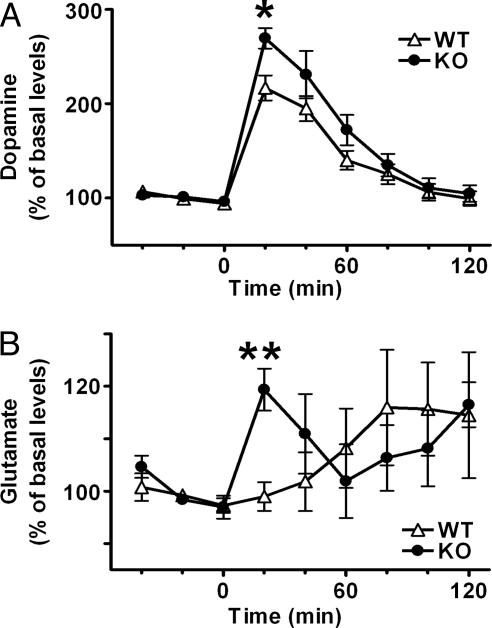
Changes in Release of DA and Glutamate in mGluR2–/– KO NAc by Cocaine Administration. The acute administration of cocaine increases extracellular levels of DA in the NAc (40). We performed in vivo microdialysis analysis to address whether the mGluR2 deficiency influences extracellular levels of DA and glutamate in the NAc after cocaine administration. Samples were collected from the NAc of freely moving mice, and extracellular levels of DA and glutamate were assessed by HPLC before and after i.p. injection of cocaine (10 mg/kg). Basal levels of DA and glutamate before cocaine administration were comparable between mGluR2–/– KO and WT mice (Fig. 6). Cocaine administration increased extracellular levels of DA in both genotypes, but the cocaine-induced increase of DA was much higher in mGluR2–/– KO mice than in WT mice, and a statistically significant increase was noted for the first 20 min after cocaine administration (Fig. 6A). As reported (41), cocaine administration had no immediate effect on glutamate release in the NAc of WT mice, and extracellular levels of glutamate gradually increased after cocaine administration (Fig. 6B). Remarkably, extracellular glutamate levels rapidly increased in the NAc of mGluR2–/– KO mice after cocaine administration (Fig. 6B), indicating that glutamate transmission markedly changes in mGluR2–/– KO mice in response to cocaine exposure. We also analyzed tissue contents of DA, serotonin, and their metabolites in the striatum of mGluR2–/– KO and WT mice and found no statistical difference between the two genotypes (n = 6 each): DA (WT, 13.64 ± 0.40; KO, 13.44 ± 0.56 ng/mg protein), 3,4-dihydroxyphenylacetic acid (WT, 786.6 ± 59.7; KO, 803.3 ± 69.1 pg/mg protein), homovanillic acid (WT, 1291.0 ± 106.0; KO, 1288.7 ± 92.0 pg/mg protein), serotonin (WT, 445.2 ± 28.5; KO, 472.4 ± 20.0 pg/mg protein), and 5-hydroxyindoleacetic acid (WT, 248.3 ± 20.4; KO, 305.0 ± 21.1 pg/mg protein). These results indicate that mGluR2 deficiency not only increases release of DA but also alters glutamatergic transmission in the cocaine-induced response within the NAc circuit.
Fig. 6.
Enhanced release of DA and glutamate in the NAc of mGluR2–/– KO mice after cocaine administration. In vivo microdialysis analysis of DA (A) and glutamate (B) were conducted for the NAc of freely moving WT and mGluR2–/– KO mice. After collection of three baseline fractions, cocaine (10 mg/kg) was i.p.-administrated and six fractions were collected every 20 min. Data are normalized to percent changes by the average value of three baseline fractions. In A, basal levels of DA in the NAc show no difference between WT and mGluR2–/– KO mice (WT, 0.60 ± 0.043 nM, n = 6; KO, 0.59 ± 0.081, n = 5). In both genotypes, cocaine significantly increases extracellular levels of DA as compared with basal levels at time points of 20, 40, and 60 min after cocaine administration (P < 0.01). There is a significant interaction between genotype and time [F(5, 45) = 2.808, P = 0.027] and a significant increase at the first fraction after cocaine administration in mGluR2–/– KO mice as compared with WT mice (*, P = 0.016). In B, basal levels of glutamate in the NAc showed no difference between WT and mGluR2–/– KO mice (WT, 82.4 ± 8.1 nM, n = 6; KO, 76.8 ± 11.6, n = 5). In mGluR2–/– KO mice, extracellular levels of glutamate significantly increase at 20 min (P < 0.01) and 120 min (P < 0.05) after cocaine administration as compared with basal levels. There are a significant interaction between genotype and time [F(5, 45) = 2.726, P = 0.031] and a significant increase at the first fraction after cocaine administration in mGluR2–/– KO mice as compared with WT mice (**, P = 0.002).
Discussion
mGluR2 is widely distributed in a variety of neuronal cells (11, 12, 42), but mGluR2–/– KO mice show no obvious behavioral abnormality under ordinary conditions (17). The present investigation has indicated that the mGluR2-null mutation causes several lines of altering responses in the more enforcing and defined behavioral tests. One clear behavioral alteration in mGluR2–/– KO mice is the significant increase of cocaine-induced behavioral responses implicated in reinforcement and addiction of this drug (32, 38, 39). The important neural network for cocaine reinforcement and addiction is the mesolimbic dopaminergic system that originates in the ventral tegmental area and that targets the NAc (32, 33). Cocaine blocks the activity of DA transporters, and the resultant increase of DA leads to cocaine-induced neural adaptation in the NAc neural circuitry (33). In addition to the dopaminergic system, the glutamatergic pathway originates in the prefrontal cortex and projects to the NAc (43). Consequently, the convergent interactions of DA and glutamate control the cellular and behavioral responses to drugs of abuse (34). Gene targeting and pharmacological studies have indicated that both ionotropic GluRs and mGluRs participate in biochemical and behavioral responses to abused drugs and psychostimulants (44). It has been reported that the group 2 mGluR-selective agonists LY379268 and LY354740 inhibit the hyperlocomotion induced by psychostimulants and psychotomimetic drugs such as amphetamine and phencyclidine (9, 10). However, it has also been reported that the group 2 mGluR-selective antagonist (2S)-α-ethylglutamic acid injected into the NAc similarly inhibit amphetamine-induced hyperlocomotion (35). In the present investigation, the suppressive effect of mGluR2 on cocaine-induced adaptation is explicitly revealed by locomotor sensitization and CPP analysis. Interestingly, mGluR5 coupling to inositol 1,4,5-trisphosphate/Ca2+ signaling has been shown to be essential for cocaine-induced adaptation, so that mGluR 5–/– KO mice displayed no cocaine-induced locomotor sensitization, nor self-administration of cocaine after repeated cocaine administration (45). The specific and different subtypes of mGluRs thus play a critical role in neural adaptation implicated in drug addiction.
The other behavioral change of mGluR2–/– KO mice is the significant increase in locomotion under novel environmental and stressful conditions. A number of pharmacological experiments by using group 2 mGluR-selective agonists and antagonists have revealed a critical role for group 2 mGluRs in locomotor function, but the results of these experiments are also contradictory. The systemic and the local NAc injection of the group 2 mGluR-selective antagonist LY341495 has been shown to induce hyperlocomotion (46, 47), but the NAc injection of group 2 mGluR-selective agonists, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) and (2S′5,2′S)-2-(carboxycyclopropyl)glycine (L-CCG-1), has also been reported to induce hyperlocomotion (36, 48). This discrepancy may result from different experimental conditions, cross-reaction with other glutamate receptors or differences in the overall and local effects of the agonists/antagonists used. The present investigation has indicated that mGluR2 is suppressive in stress-related hyperlocomotion. Interestingly, forced swimming and tail suspension are both related to animal models of depression, but the behavioral change of mGluR2–/– KO mice was observed only in the repeated forced-swim test but not in the tail suspension test. It has recently been reported that the dopaminergic system is involved in mobility in the forced-swim test but not in the tail suspension test (49). Furthermore, the close relationship between enhanced responsiveness to cocaine and reduced immobility in the forced-swim test has been reported by herpes simplex virus-mediated overexpression of the dominant-negative cAMP response element-binding protein transcription factor in the NAc (50). The altered dopaminergic system in the NAc of mGluR2–/– KO mice may thus also participate, at least partly, in their behavioral alterations under stressful conditions.
The impaired ability to conduct the accelerating rota-rod test is another characteristic of mGluR2 deficiency. Motor coordination is controlled by various brain regions, including the motor cortex, basal ganglia, and cerebellum (51). In the cerebellum, Golgi cells receive glutamatergic input from granule cells, and in turn, suppress these cells by means of the inhibitory GABA transmitter (26, 28). mGluR2 is intensely distributed both presynaptically and postsynaptically in Golgi cells, and the presynaptic and postsynaptic mGluR2 plays an important role in processing and integrating incoming input in the cerebellar cortical network (16, 28). It would thus be interesting to investigate whether, and how, the lack of inhibitory mGluR2 in Golgi cells is involved in dysfunction of motor coordination as exemplified by the rota-rod test.
Finally, in vivo microdialysis analysis has indicated that extracellular levels of DA significantly increase in the NAc of mGluR2–/– KO mice after cocaine administration. More interestingly, extracellular glutamate levels markedly increase in the NAc of mGluR2–/– KO mice immediately after cocaine administration. Cocaine activates the mesolimbic dopaminergic system at both prefrontal cortex and NAc and increases DA levels in both regions (40, 41). Furthermore, it has been reported by pharmacological studies that group 2 mGluRs inhibit pyramidal neurons postsynaptically at the prefrontal cortex and presynaptically at the NAc in a stimulus-dependent manner (52–56). It is thus conceivable that the lack of mGluR2 relieves the inhibition of pyramidal neurons at both the prefrontal cortex and NAc and leads to increase glutamate release in the NAc. Under this situation, the excess glutamate could stimulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and NMDA receptors at the presynaptic terminals of DA neurons in the NAc (57, 58), and further increase DA levels in the NAc. Thus, there is close interaction between mGluR2 and the dopaminergic system, and mGluR2 would play an important role in regulating the dopaminergic system both presynaptically and postsynaptically. The precise mechanism underlying mGluR2-mediated synaptic regulation needs further investigation, but the obvious behavioral alterations of mGluR2–/– KO mice will provide a clue to study mGluR2-mediated glutamatergic transmission in the neural network.
Supplementary Material
Acknowledgments
We thank Kouichiro Ohnuki for technical advice and assistance, Shigeyuki Chaki for advice, and James Monypenny for careful reading of the manuscript. This work was supported in part by research grants from the Ministry of Education, Science, and Culture of Japan. Y.M. is a Fellow of the Japan Society for the Promotion of Science.
Author contributions: Y.M., T.M., T.F., Y.T., H.M., and S.N. designed research; Y.M. performed research; T.M. and S.N. contributed new reagents/analytic tools; Y.M., T.M., T.F., Y.T., and S.N. analyzed data; and Y.M. and S.N. wrote the paper.
Abbreviations: mGluR, metabotropic glutamate receptor; KO, knockout; DA, dopamine; NAc, nucleus accumbens; CPP, conditioned place preference.
References
- 1.Meldrum, B. S. (2000) J. Nutr. 130, 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi, S. (1992) Science 258, 597–603. [DOI] [PubMed] [Google Scholar]
- 3.Conn, P. J. & Pin, J. P. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 205–237. [DOI] [PubMed] [Google Scholar]
- 4.Anwyl, R. (1999) Brain Res. Brain Res. Rev. 29, 83–120. [DOI] [PubMed] [Google Scholar]
- 5.Schoepp, D. D. (2001) J. Pharmacol. Exp. Ther. 299, 12–20. [PubMed] [Google Scholar]
- 6.Pizzi, M., Consolandi, O., Memo, M. & Spano, P. F. (1996) Eur. J. Neurosci. 8, 1516–1521. [DOI] [PubMed] [Google Scholar]
- 7.Rouse, S. T., Marino, M. J., Bradley, S. R., Awad, H., Wittmann, M. & Conn, P. J. (2000) Pharmacol. Ther. 88, 427–435. [DOI] [PubMed] [Google Scholar]
- 8.Helton, D. R., Tizzano, J. P., Monn, J. A., Schoepp, D. D. & Kallman, M. J. (1998) J. Pharmacol. Exp. Ther. 284, 651–660. [PubMed] [Google Scholar]
- 9.Moghaddam, B. & Adams, B. W. (1998) Science 281, 1349–1352. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. H. & Vezina, P. (2002) Pharmacol. Biochem. Behav. 73, 333–337. [DOI] [PubMed] [Google Scholar]
- 11.Neki, A., Ohishi, H., Kaneko, T., Shigemoto, R., Nakanishi, S. & Mizuno, N. (1996) Neurosci. Lett. 202, 197–200. [DOI] [PubMed] [Google Scholar]
- 12.Ohishi, H., Neki, A. & Mizuno, N. (1998) Neurosci. Res. 30, 65–82. [DOI] [PubMed] [Google Scholar]
- 13.Tamaru, Y., Nomura, S., Mizuno, N. & Shigemoto, R. (2001) Neuroscience 106, 481–503. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, S., Hikida, T., Watanabe, D., Ichinose, H., Nagatsu, T., Kreitman, R. J., Pastan, I. & Nakanishi, S. (2000) Science 289, 633–637. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida, K., Watanabe, D., Ishikane, H., Tachibana, M., Pastan, I. & Nakanishi, S. (2001) Neuron 30, 771–780. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe, D. & Nakanishi, S. (2003) Neuron 39, 821–829. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi, M., Kobayashi, K., Manabe, T., Takahashi, T., Sakaguchi, I., Katsuura, G., Shigemoto, R., Ohishi, H., Nomura, S., Nakamura, K., et al. (1996) Science 273, 645–647. [DOI] [PubMed] [Google Scholar]
- 18.Miyakawa, T., Leiter, L. M., Gerber, D. J., Gainetdinov, R. R., Sotnikova, T. D., Zeng, H., Caron, M. G. & Tonegawa, S. (2003) Proc. Natl. Acad. Sci. USA 100, 8987–8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porsolt, R. D., Le Pichon, M. & Jalfre, M. (1977) Nature 266, 730–732. [DOI] [PubMed] [Google Scholar]
- 20.Miyakawa, T., Yamada, M., Duttaroy, A. & Wess, J. (2001) J. Neurosci. 21, 5239–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steru, L., Chermat, R., Thierry, B. & Simon, P. (1985) Psychopharmacology 85, 367–370. [DOI] [PubMed] [Google Scholar]
- 22.Hikida, T., Kaneko, S., Isobe, T., Kitabatake, Y., Watanabe, D., Pastan, I. & Nakanishi, S. (2001) Proc. Natl. Acad. Sci. USA 98, 13351–13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hikida, T., Kitabatake, Y., Pastan, I. & Nakanishi, S. (2003) Proc. Natl. Acad. Sci. USA 100, 6169–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franklin, K. B. J. & Paxinos, G. (1997) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 25.Mizuma, H., Mizutani, M., Nozaki, S., Iizuka, H., Tohyama, H., Nishimura, N., Watanabe, Y. & Kohashi, R. (2003) Biochem. Biophys. Res. Commun. 302, 156–161. [DOI] [PubMed] [Google Scholar]
- 26.Ito, M. (1984) The Cerebellum and Neural Control (Raven, New York).
- 27.Mitchell, S. J. & Silver, R. A. (2000) Nature 404, 498–502. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi, S. (2005) Trends Neurosci. 28, 93–100. [DOI] [PubMed] [Google Scholar]
- 29.Freed, W. J. (1994) Neurosci. Biobehav. Rev. 18, 111–120. [DOI] [PubMed] [Google Scholar]
- 30.Di Chiara, G., Morelli, M. & Consolo, S. (1994) Trends Neurosci. 17, 228–233. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson, A., Waters, N., Holm-Waters, S., Tedroff, J., Nilsson, M. & Carlsson, M. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 237–260. [DOI] [PubMed] [Google Scholar]
- 32.Berke, J. D. & Hyman, S. E. (2000) Neuron 25, 515–532. [DOI] [PubMed] [Google Scholar]
- 33.Nestler, E. J. (2001) Nat. Rev. Neurosci. 2, 119–128. [DOI] [PubMed] [Google Scholar]
- 34.Mohn, A. R., Yao, W. D. & Caron, M. G. (2004) Neuropharmacology 47, Suppl. 1, 101–110. [DOI] [PubMed] [Google Scholar]
- 35.Kim, J. H., Beeler, J. A. & Vezina, P. (2000) Neuropharmacology 39, 1692–1699. [DOI] [PubMed] [Google Scholar]
- 36.Breysse, N., Risterucci, C. & Amalric, M. (2002) Pharmacol. Biochem. Behav. 73, 347–357. [DOI] [PubMed] [Google Scholar]
- 37.David, H. N. & Abraini, J. H. (2003) Neuropharmacology 44, 717–727. [DOI] [PubMed] [Google Scholar]
- 38.Tzschentke, T. M. (1998) Prog. Neurobiol. 56, 613–672. [DOI] [PubMed] [Google Scholar]
- 39.Bardo, M. T. & Bevins, R. A. (2000) Psychopharmacology 153, 31–43. [DOI] [PubMed] [Google Scholar]
- 40.Di Chiara, G. & Imperato, A. (1988) Proc. Natl. Acad. Sci. USA 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid, M. S., Hsu, K., Jr., & Berger, S. P. (1997) Synapse 27, 95–105. [DOI] [PubMed] [Google Scholar]
- 42.Ohishi, H., Shigemoto, R., Nakanishi, S. & Mizuno, N. (1993) Neuroscience 53, 1009–1018. [DOI] [PubMed] [Google Scholar]
- 43.Sesack, S. R., Carr, D. B., Omelchenko, N. & Pinto, A. (2003) Ann. N.Y. Acad. Sci. 1003, 36–52. [DOI] [PubMed] [Google Scholar]
- 44.Kenny, P. J. & Markou, A. (2004) Trends Pharmacol. Sci. 25, 265–272. [DOI] [PubMed] [Google Scholar]
- 45.Chiamulera, C., Epping-Jordan, M. P., Zocchi, A., Marcon, C., Cottiny, C., Tacconi, S., Corsi, M., Orzi, F. & Conquet, F. (2001) Nat. Neurosci. 4, 873–874. [DOI] [PubMed] [Google Scholar]
- 46.David, H. N. & Abraini, J. H. (2001) Neuropharmacology 41, 454–463. [DOI] [PubMed] [Google Scholar]
- 47.O'Neill, M. F., Heron-Maxwell, C., Conway, M. W., Monn, J. A. & Ornstein, P. (2003) Neuropharmacology 45, 565–574. [DOI] [PubMed] [Google Scholar]
- 48.Swanson, C. J. & Kalivas, P. W. (2000) J. Pharmacol. Exp. Ther. 292, 406–414. [PubMed] [Google Scholar]
- 49.Renard, C. E., Dailly, E., David, D. J., Hascoet, M. & Bourin, M. (2003) Fundam. Clin. Pharmacol. 17, 449–455. [DOI] [PubMed] [Google Scholar]
- 50.Pliakas, A. M., Carlson, R. R., Neve, R. L., Konradi, C., Nestler, E. J. & Carlezon, W. A., Jr. (2001) J. Neurosci. 21, 7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middleton, F. A. & Strick, P. L. (2000) Brain Res. Brain Res. Rev. 31, 236–250. [DOI] [PubMed] [Google Scholar]
- 52.Hu, G., Duffy, P., Swanson, C., Ghasemzadeh, M. B. & Kalivas, P. W. (1999) J. Pharmacol. Exp. Ther. 289, 412–416. [PubMed] [Google Scholar]
- 53.Xi, Z. X., Baker, D. A., Shen, H., Carson, D. S. & Kalivas, P. W. (2002) J. Pharmacol. Exp. Ther. 300, 162–171. [DOI] [PubMed] [Google Scholar]
- 54.Otani, S., Daniel, H., Takita, M. & Crepel, F. (2002) J. Neurosci. 22, 3434–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robbe, D., Alonso, G., Chaumont, S., Bockaert, J. & Manzoni, O. J. (2002) J. Neurosci. 22, 4346–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorrain, D. S., Baccei, C. S., Bristow, L. J., Anderson, J. J. & Varney, M. A. (2003) Neuroscience 117, 697–706. [DOI] [PubMed] [Google Scholar]
- 57.Krebs, M. O., Desce, J. M., Kemel, M. L., Gauchy, C., Godeheu, G., Cheramy, A. & Glowinski, J. (1991) J. Neurochem. 56, 81–85. [DOI] [PubMed] [Google Scholar]
- 58.Desce, J. M., Godeheu, G., Galli, T., Artaud, F., Cheramy, A. & Glowinski, J. (1992) Neuroscience 47, 333–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.