Abstract
Background
As the magnitude of sporadic colorectal cancer (CRC) in India is low, magnitude of CRC in ulcerative colitis (UC) is also considered low. As a result, screening for CRC in UC although advocated may not be followed everywhere. We report our data of UC-related CRC from a low-incidence area of sporadic CRC.
Methods
A total of 1012 patients with left-sided colitis/pancolitis having more than one full-length colonoscopy performed at least a year after the onset of symptoms were included in retrospective analysis of prospectively maintained case records. In addition, 136 patients with duration of disease >10 years underwent surveillance white-light colonoscopy prospectively during the study period.
Results
A total of 1012 individuals were finally included (6542 person-years of follow-up, 68.5% males, disease duration: 6.4 ± 6.8 years). Twenty (1.97%) patients developed CRC. Two (10%) patients developed CRC during the first decade, 10/20 (50%) during the second and 8/20 (40%) after the second decade of disease. The cumulative risk of developing CRC was 1.5%, 7.2% and 23.6% in the first, second and third decade, respectively. Of 136 high-risk UC cases, five (3.6%) had CRC on screening colonoscopy. Disease duration and increasing age of onset were associated with higher risk of CRC.
Conclusions
Cumulative risk of CRC in Indian UC patients is as high as 23.6% at 30 years. The risk of CRC increases with increasing age of onset and increasing duration of disease. A low risk of sporadic CRC does not confer a low risk of UC-related CRC, and regular screening is warranted.
Keywords: Ulcerative colitis, colorectal carcinoma, risk factors
Introduction
The burden of inflammatory bowel disease (IBD) in India and other countries in the Asia-Pacific region is low when compared to the Western population, but is steadily increasing.1,2 With an increasing disease prevalence, long-term complications of IBD are increasingly being seen by physicians treating these diseases. Colorectal carcinoma (CRC) is a deadly complication of long-standing ulcerative colitis (UC) and early recognition is the key to treating it. CRC associated with UC was first described by Crohn and Rosenberg as a case report in 1925.3 In the ensuing decades, several case reports and studies confirmed the association between CRC and UC.4,5 In some earlier reports, the risk of CRC was reported to be as high as 60%,6 and many surgeons at that time advocated prophylactic colectomy as a precaution in extensive colitis greater than 10 years’ duration. Chronic mucosal inflammation forms the background on which CRC develops in UC, and CRC associated with UC constitutes 1% of all CRC.7
The reported risk of CRC in UC varies considerably in literature. Eaden and colleagues8 pooled the results of 116 studies. The overall prevalence of CRC in UC was estimated to be 3.7%, with an overall annual incidence of 0.3%. The cumulative risk of CRC was 8.3% at 20 years and 18.4% at 30 years. In contrast to reports from Western countries, the incidence of CRC in UC has been thought to be low in the Indian subcontinent. In a study from Venkataraman9 which included 536 patients, five patients were found to have CRC. After 20 years the incidence was 2.9/1000 patient-years for colitis as a whole and 4.9/1000 patient-years for pancolitis. These older studies had a small number of patients with a short duration of follow-up and there is a lacunae in literature about the true burden of UC-related CRC. A recent study from Mumbai10 included 430 patients, of whom 12 developed CRC amounting for a prevalence rate of 2.8%. Though this study reported a burden similar to that in the Western population, the study population was small and only 67.5% of the population had left-sided colitis or pancolitis, which constitutes the risk population for CRC in UC.
Screening colonoscopy is advocated by various guidelines but it is not routinely practiced as the incidence of UC-related CRC is low and thought to be similar to that of sporadic CRC. The main reason for this neglect of screening colonoscopy is the lack of adequate data on the risk of CRC in UC patients. We undertook this study to assess the risk of CRC in Indian patients with UC, and to study the risk factors associated with UC-associated CRC. We present the largest number of data to date on UC-related CRC from the Indian subcontinent.
Material and methods
Study population
All patients of UC attending the IBD clinic at All India Institute of Medical Sciences (AIIMS), New Delhi, India, from July 2004 till July 2015 were included in the study.
Study design and patient evaluation
This study was a prospective cohort study along with retrospective data analysis of a prospectively maintained database. Data regarding patients were collected from the IBD clinic records and also by interviewing them in person during their visit. The IBD clinic maintains a longitudinal database which contains all information concerning the patient’s disease, including detailed history, clinical exam, relevant test findings, and follow-up symptom assessment. The file is maintained by a team of physicians, and the parameters used for assessment were consistent among the physicians for the entire study period. To assess the prevalence of CRC and to calculate the cumulative risk of CRC in long-standing UC, patient data were collected (as per pro forma) on demographic parameters, age of onset of UC, age at which last colonoscopic assessment was performed, age at last follow-up, duration of disease, and endoscopic or histological evidence of CRC. Data regarding high risk factors for developing CRC were also collected, which included primary sclerosing cholangitis (PSC) and family history of CRC.
In addition to retrospective review of records, we also performed screening colonoscopy prospectively in consecutive patients with disease duration > 10 years who visited the IBD clinic from March 2014 till July 2015 (duration of study). Pregnant patients, patients with severe comorbidities, patients with proctitis and patients who did not give consent were excluded. Ileocolonoscopy was performed when disease activity was mild or in remission. Disease activity was assessed by using the Simple Clinical Colitis Activity Index (SCCAI). Remission was defined as SCCAI <4 and mild disease activity as a SCCAI <7.11 A polyethylene glycol electrolyte lavage solution was used in all patients for bowel preparation before ileocolonoscopy. At colonoscopy biopsies were taken from any suspicious area for malignancy. Biopsies were then reviewed by the pathologists for evidence of malignancy. The following risk factors associated with carcinoma were identified in patients found to have colitis carcinoma: (a) duration of disease, (b) extent of colonic involvement at presentation, (c) age at onset of disease, (d) presence of PSC and (e) family history of CRC. Ethical clearance for the study was obtained from the institutional review board. Informed consent was obtained from all patients who were prospectively included in the study. All patients who warranted screening consented for the same.
Outcome measures
The primary outcome measure of the study was assessment of prevalence of CRC in Indian patients of UC. Secondary outcome measures include (a) identification of risk factors for developing CRC in patients with UC and (b) studying the cumulative risk of developing CRC in patients with UC.
Statistical analysis
Descriptive statistics, i.e. mean ± standard deviation, median (interquartile range (IQR)) and frequency distribution were calculated for each variable in the study. Data were presented as mean ± SD or median (IQR) as appropriate. To compare the two groups, Student’s t test or Mann-Whitney U test (depending on normal or non-normal distribution) for quantitative variables and chi square test for categorical variables were applied as appropriate. Cumulative incidence risk was calculated by using Kaplan-Meier analysis by taking the starting point as time of onset of symptoms and end point as time of detection of carcinoma. Risk factors associated with carcinoma were identified and hazard ratios (HRs) calculated by using Cox proportional hazards method. Statistical analysis was performed using STATA for Windows version 12.1.
Results
From 2004 to July 2015, a total of 1693 patients with UC were registered at the IBD clinic, AIIMS. This prospectively maintained database of a cohort of patients with UC was used to determine the risk of CRC.
Of these, patients who had never undergone a full-length colonoscopy (n = 259), patients who underwent a full-length colonoscopy only once, within one year of onset of disease (n = 257), and patients with proctitis (n = 165) were excluded. After exclusion, a total of 1012 patients with UC were included in the analysis with a total of 6542 person-years of follow-up. Out of the 1012 patients, 136 consecutive patients warranted screening during the study period and formed the prospective arm of this study (Figure 1).
Figure 1.
Flowchart showing patients who were included and excluded for analysis.
Baseline demographics of the cohort
The mean age of the study population at onset of UC was 31.9 ± 11.7 years. Mean age at diagnosis was 32.1 ± 11.9 years. Of the patients, 693 (68.4%) were males. The mean age at last colonoscopy/colectomy was 38.4 ± 12.7 years with a mean duration of disease of 6.4 ± 6.8 years. The maximum extent of disease was left-sided colitis in 558 (55.14%) and pancolitis in 454 (44.86%) patients. Duration of disease up to the last colonoscopy was considered for analysis and there were 768 (75.88%) patients with duration less than 10 years, 186 (18.37%) patients with duration of disease ranging from 10 to 20 years and 58 (5.7%) patients with duration >20 years. No patients had a family history of CRC and two patients had PSC (0.1%) (Table 1).
Table 1.
Demographic and clinical data of study cohort (N = 1012).
| Mean age of onset ± SD (years) | 31.9 ± 11.7 |
| Mean age of diagnosis ± SD (years) | 32.1 ± 11.9 |
| Males/Females | 693/319 |
| Mean age at last colonoscopy (years) | 38.45 ± 12.7 |
| Mean duration of disease at last colonoscopy or colectomy (years) | 6.4 ± 6.8 |
| Maximum extent of disease | |
| Left-sided colitis | 558 (55.14%) |
| Pancolitis | 454 (44.86%) |
| Duration of disease at last colonoscopy/colectomy | |
| <10 years | 768 (75.88%) |
| 10–20 years | 186 (18.37%) |
| >20 years | 58 (5.7%) |
| Family history of colorectal cancer (CRC) | 0 |
| Primary sclerosing cholangitis | 2 (0.1%) |
| Pseudopolyps | 130 (12.8%) |
Characteristics of patients with CRC (Table 2)
Table 2.
Characteristics of subgroup of patients with colorectal carcinoma (n = 20).
| Mean age of onset ± SD (years) | 34.55 ± 13.56 |
| Mean age of diagnosis ± SD (years) | 34.95 ± 13.66 |
| Males (%) | 14 (70%) |
| Mean age at last colonoscopy or colectomy (years) | 53.25 ± 12.6 |
| Mean duration of disease at last colonoscopy or colectomy (years) | 18.7 ± 11.3 |
| Maximum extent of disease | |
| Left-sided colitis | 4 (20%) |
| Pancolitis | 16 (80%) |
| Duration of disease at last colonoscopy/colectomy | |
| <10 years | 2 (10%) |
| 10–20 years | 10 (50%) |
| >20 years | 8 (40%) |
| Family history of colorectal cancer (CRC) | 0 |
| Primary sclerosing cholangitis | 0 |
| Pseudopolyps | 5/20 (25%) |
Twenty (1.97%) patients with CRC were identified in our study. All these patients had histologically proven adenocarcinoma. Of the 136 patients who underwent screening colonoscopy prospectively, five (3.5%) had CRC (Table 2). Two (10%) patients developed CRC during the first decade, 10/20 (50%) during the second and 8/20 (40%) after the second decade of disease. Four (20%) patients had left-sided colitis and 16/20 (80%) had pancolitis. The majority of CRC in our study were in the left-sided colon with most occurring in the rectum (60%) (Figure 2). All these patients were on maintenance therapy with 5-aminosalicylic acid (5 ASA). Seventeen of 20 (85%) patients received at least one course of oral steroids during their disease course. Three of 20 (15%) received azathioprine and none of them ever required biologicals. The crude prevalence rate of CRC in our cohort was 1.9%. Overall incidence density was 3.05/1000 person-years’ follow-up (95% confidence interval (CI), 1.97–4.74). With duration of disease <10 years, it was 0.7/1000 person-years, 4.25/1000 person-years between 10 and 20 years and 5.12/1000 person-years at >20 years’ duration.
Figure 2.
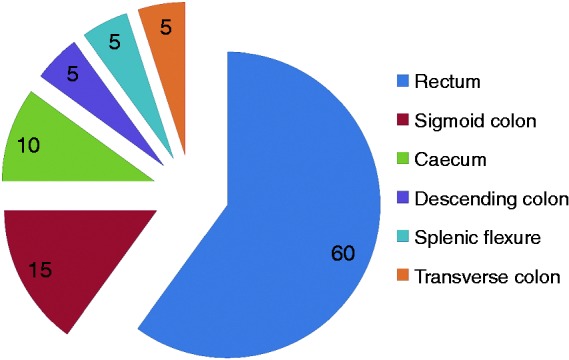
Site of location in patients with colorectal cancer (%).
Cumulative risk of developing CRC over three decades
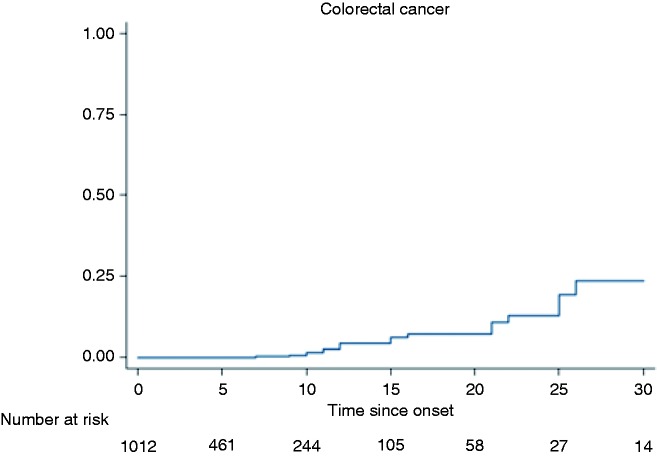
At 30 years of disease duration, 18 cases of CRC were present. Two patients developed CRC after 30 years of disease and were not included in this analysis. We analysed the cumulative risk of developing CRC till the third decade. Using the Kaplan-Meier analysis, the cumulative risk of developing CRC in patients with left-sided colitis and pancolitis was 1.5% in the first decade, 7.2% during the second decade, and 23.6% in the third decade (Figure 3).
Figure 3.
Kaplan-Meier curve showing increasing incidence of colorectal cancer with increasing duration of disease.
Risk factors for CRC
Risk factors for the development of CRC which were specific to UC were assessed. Duration of disease was found to be an independent risk factor for development of CRC (Figure 3). With an increasing extent of disease (pancolitis vs left-sided colitis) the risk of developing CRC increased, although the risk was not statistically significant (HR: 2.61 (0.85–7.9), p = 0.09). The risk of CRC also increased with increasing age of onset (HR: 1.07 (1.03–1.11), p = 0.0001). This means that there was a 7% increase in the risk of CRC for each one-year increase in the age of onset (Table 3).
Table 3.
Risk factors for colorectal carcinoma in ulcerative colitis.
| Risk factor | Hazard ratio | p value (95% CI) |
|---|---|---|
| Sex | 1.46 | 0.47 (0.52–4.10) |
| Age at onset | 1.07 | 0.001 (1.03–1.11) |
| Extent | 2.61 | 0.09 (0.85–7.9) |
CI: confidence interval.
Discussion
The present study assessed the risk of CRC in Indian patients with UC. Twenty patients (1.97%) with a mean disease duration of 18.7 ± 11.3 years developed CRC during the study period. In addition, of 136 patients screened prospectively, five (3.6%) were found to have CRC. The incidence density of CRC in our study was 3.05/1000 person-years. This would mean an annual incidence of 0.3%, which is similar to that reported in Western literature (0.3–3.3%).8,12–16 The cumulative probability of developing CRC in the present study was 1.5%, 7.2% and 23.6% in the first, second and third decade, respectively. Similar results were reported in the meta-analysis by Eaden et al.8 The meta-analysis found that the cumulative probability of developing CRC was 2%, 8% and 18% in the first, second and third decade, respectively. Therefore the risk of CRC in UC in Indian patients is similar to that in the West, where the risk of sporadic CRC is much higher than India and other Asian countries.
Asian patients and those from India have been known to have a much lower risk of sporadic CRC when compared to the Western population. Recent estimates of CRC in India show an age-adjusted incidence of 7.2/100,000 population and 5.1/100,000 population in males and females respectively and an overall incidence rate of 6.1/100,000 population.17 Thus patients with UC were thought to have a lesser risk than those that of their Western counterparts.
Previous studies from India which assessed the risk of CRC in UC have included smaller study populations and have been inadequate to truly assess the risk. Kochhar et al.18 first reported the risk of UC-related CRC from India. The study consisted of a retrospective analysis of 436 patients of UC. Eight patients with UC-related CRC (1.8%) were found, which is lower than the risk in the West. Gyde et al.7 also suggested a lower incidence of UC-related CRC when compared to the Western population. Their study population consisted of 532 patients whose records were retrospectively analysed. A more recent study by Desai et al.10 though found a higher incidence, and they reported an incidence density of 3/1000 in the first 10 years, 3.3/1000 at 10 to 20 years, and 7/1000 at > 20 years, which is similar to that of our study population. They reported an overall prevalence of 2.8% in their study cohort. This study reported their data on 430 patients, of whom only 290 (67.44%) patients had left-sided colitis and pancolitis. Our data on 1012 patients are the largest number of data on UC-related CRC to date from India, where the incidence of sporadic CRC is low. All of our patients had either left-sided colitis or pancolitis, unlike the previous studies, which also included patients with proctitis, which is not a risk factor for development of CRC. This gives us a better estimate of UC-related CRC in the population at risk. Our large study showed the incidence density of CRC to be similar to that reported in studies from the Western population.
There appears to be a regional variation within Asian countries. In a study from China,19 data of 3922 patients with UC were retrospectively collected from five central teaching hospitals from 1998 to 2009. CRC was diagnosed in 34 patients, and the overall prevalence of CRC in patients with UC was 0.87%. The cumulative risk of developing CRC in the first, second and third decade was 1.15%, 3.56% and 14.36%, respectively. In a Korean multicentre study20 the cumulative risk of CRC at 30 years after UC diagnosis was found to be 5%. These estimates were lesser than that found in our study. However, both these studies also included patients with proctitis, which diluted the actual risk of CRC in these studies.
The risk of CRC in UC in our study is therefore much higher than the reported risk of sporadic CRC in India, which is about 6.1/100,000 population.17 Because of the much lower risk of sporadic CRC, screening strategies like colonoscopy have not been advocated for the general population. However, this recommendation does not seem to be valid for screening Indian UC patients for CRC. We prospectively performed screening colonoscopies in 136 patients, all with duration of disease greater than 10 years and left-sided colitis and pancolitis. Five (3.7%) patients were found to have CRC. This underscores the importance of a regular surveillance program. Therefore, screening for CRC after 10 years of disease should be routinely recommended in Indian patients with UC similar to several screening guidelines that have been endorsed by gastroenterology societies in the West. The risk of CRC appears to be low in patients with less than 10 years of disease as we found that only 2/20 (10%) of our patients with CRC had a duration of disease lesser than 10 years.
Population-based studies have always reported a lower risk of CRC when compared to hospital-based cohorts, with the annual incidence ranging from 0.06% to 0.16%.12–16 This could be attributed to the referral bias associated with hospital-based cohort studies. Patients referred to large tertiary care centres generally have a more extensive and a more severe disease course when compared to population cohorts and explains the high risk of CRC that we found in our study.
Several studies have shown that the incidence of CRC has been decreasing over time. In the recent meta-analysis by Jess et al.,21 the cumulative risk has been shown to be significantly less than that published earlier. Indian studies in the past have been scarce. Kochhar et al.18 had eight cases of colitis carcinoma (1.8%) in their study published in 1992. The study was published in 1992 and more than two decades later, our study has shown similar prevalence rates.
Risk factors for CRC in UC have been elucidated in various studies. They included duration of disease, extent of disease, coexisting PSC, family history of CRC and age of onset. In the present study, risk of CRC increased with increasing disease duration as shown by Kaplan-Meier analysis. At 30 years of disease the cumulative probability of developing CRC was 23.6%. This risk was three times greater compared to the risk at 10 years. Patients with pancolitis had a twofold increased risk of developing CRC than patients with left-sided colitis. Of the patients with CRC, 80% had pancolitis (Figure 3). Thus duration of disease and extent of disease were found to be risk factors of CRC in UC.
There is conflicting evidence with regard to whether age of onset of UC is an independent risk factor for development of UC CRC. In a British study by Rutter et al., patients who developed CRC had a median age of onset of UC greater than those without CRC.22 Another study found that patients who were diagnosed with UC after 40 years of age had a higher risk of CRC, than those diagnosed before 40 years of age.23 We also found that with increasing age of onset of UC, the risk of CRC increased significantly, suggesting a later age of disease onset to be an independent predictor for the risk of CRC.
There were no patients with family history of CRC in the present study cohort as expected considering a low prevalence of CRC in the general population. We had only two patients with PSC and neither of them had CRC.
In summary, there is a definite risk of CRC in Indian patients with UC which is almost similar to the West. The risk of CRC is low in the general population but not in patients with UC. As duration of disease and extent of disease increases, the risk increases. Screening for CRC should routinely be undertaken in high-risk patients.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ahuja V, Tandon RK. Inflammatory bowel disease: The Indian augury. Indian J Gastroenterol 2012; 31: 294–296. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: A comparison with developed countries and regional differences. J Dig Dis 2010; 11: 134–147. [DOI] [PubMed] [Google Scholar]
- 3.Crohn BB, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis (nonspecific). Am J Med Sci 1925; 170: 220–227. [Google Scholar]
- 4.Bargen JA. Chronic ulcerative colitis associated with malignant disease. Arch Surg 1928; 17: 561–576. [DOI] [PubMed] [Google Scholar]
- 5.Svartz N, Ernberg T. Cancer coli in cases of colitis ulcerosa. Acta Med Scand 1949; 135: 444–447. [Google Scholar]
- 6.Devroede GJ, Taylor WF, Sauer WG, et al. Cancer risk and life expectancy of children with ulcerative colitis. N Engl J Med 1971; 285: 17–21. [DOI] [PubMed] [Google Scholar]
- 7.Gyde S, Prior P, Dew MJ, et al. Mortality in ulcerative colitis. Gastroenterology 1982; 83: 36–43. [PubMed] [Google Scholar]
- 8.Eaden JA, Abrams KR, Mayberry JF, et al. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001; 48: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman S, Mohan V, Ramakrishna B, et al. Risk of colorectal cancer in ulcerative colitis in India. J Gastroenterol Hepatol 2005; 20: 705–709. [DOI] [PubMed] [Google Scholar]
- 10.Desai D, Shah S, Deshmukh A, et al. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J Gastroenterol 2015; 21: 3644–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998; 43: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palli D, Trallori G, Bagnoli S, et al. Hodgkin’s disease risk is increased in patients with ulcerative colitis. Gastroenterology 2000; 119: 647–653. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001; 91: 854–862. [DOI] [PubMed] [Google Scholar]
- 14.Winther KV, Jess T, Langholz E, et al. Long-term risk of cancer in ulcerative colitis: A population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol 2004; 2: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 15.Lakatos L, Mester G, Erdelyi Z, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: Results of a population-based study. Inflamm Bowel Dis 2006; 12: 205–211. [DOI] [PubMed] [Google Scholar]
- 16.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: A population-based study from Olmsted County, Minnesota. Gastroenterology 2006; 130: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013, http://globocan.iarc.fr (accessed 20 September 2016).
- 18.Kochhar R, Goenka MK, Kaushik SP, et al. Colorectal carcinoma in Indian patients with idiopathic ulcerative colitis. Eur J Cancer Prev 1992; 1: 293–296. [DOI] [PubMed] [Google Scholar]
- 19.Gong W, Lv N, Wang B, et al. Risk of ulcerative colitis-associated colorectal cancer in China: A multi-center retrospective study. Dig Dis Sci 2012; 57: 503–507. [DOI] [PubMed] [Google Scholar]
- 20.Kim BJ, Yang SK, Kim JS, et al. Trends of ulcerative colitis-associated colorectal cancer in Korea: A KASID study. J Gastroenterol Hepatol 2009; 24: 667–671. [DOI] [PubMed] [Google Scholar]
- 21.Jess T, Rungoe C, Peyrin-Biroulet, et al. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population- based cohort studies. Clin Gastroenterol Hepatol 2012; 10: 639–645. [DOI] [PubMed] [Google Scholar]
- 22.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology 2006; 130: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 23.Karvellas CJ, Fedorak RN, Hanson J, et al. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can J Gastroenterol 2007; 21: 443–446. [DOI] [PMC free article] [PubMed]