Abstract
Background
Micro-inflammation and changes in gut microbiota may play a role in the pathogenesis of diverticular disease (DD).
Objective
The objective of this article is to evaluate the expression of nitric oxide (NO)-related mediators and S100B in colonic mucosa of patients with DD in an ex vivo model of bacterial infection.
Methods
Intestinal biopsies obtained from patients with diverticulosis, symptomatic uncomplicated diverticular disease (SUDD) and SUDD with previous acute diverticulitis (SUDD+AD) were stimulated with the probiotic L. casei DG® (LCDG) and/or the pathogen enteroinvasive Escherichia coli (EIEC). S100B, NO release and iNOS expression were then evaluated.
Results
Basal iNOS expression was significantly increased in SUDD and SUDD+AD patients. Basal NO expression was significantly increased in SUDD+AD. No differences in S100B release were found. In all groups, iNOS expression was significantly increased by EIEC and reduced by LCDG. In all groups, except for SUDD+AD, EIEC significantly increased NO release, whereas no increase was observed when LCDG was added to biopsies. EIEC did not induce significant changes in S100B release.
Conclusions
Colonic mucosa of patients with DD is characterized by a different reactivity toward pathogenic stimuli. LCDG plays a role in counteracting the pro-inflammatory effects exerted by EIEC, suggesting a beneficial role of this probiotic in DD.
Keywords: Diverticular disease, probiotic, nitric oxide, human colonic mucosa, enteroinvasive Escherichia coli
Introduction
Diverticular disease (DD) of the colon is a common clinical condition in the Western world. Its prevalence in the general population is estimated to range between 20% and 60%, and this condition is one of the most common gastrointestinal indications for hospital and medical visits.1,2
Diverticula of the large bowel are out-pouchings of the colon at weak points in the circular muscle where blood vessels (vasa recta) penetrate to supply the mucosa.3 The mere presence of diverticula is defined as diverticulosis. In about 20% of patients, the presence of diverticula give rise to illness, ranging from a syndrome characterized by recurrent abdominal symptoms – pain, bloating and changes in bowel habits in the absence of macroscopically evident signs of acute inflammation – defined as symptomatic uncomplicated diverticular disease (SUDD), to an acute inflammation of colonic diverticula, defined as acute diverticulitis (AD) with its typical complications (abscesses, stenoses, fistulas, perforations).4
Despite the significant burden of the pathology, the pathogenesis is poorly understood and several etiological factors may play a role in the onset of this disease.5–7
Recently, changes in the intestinal microbiota have been involved in the pathogenesis of symptoms and related acute inflammation.8 Moreover, various studies pointed out the role of the enteric nervous system (ENS) in the pathophysiology of DD.7
In a recent study by our group, in a context of intestinal inflammation, together with an increased inducible nitric oxide (NO)-synthase (iNOS) expression and NO release, we observed an increased release of S100B, a protein specifically expressed and released by enteric glial cells (EGCs).9 Previously, we demonstrated that the intestinal mucosa of patients with celiac disease and ulcerative colitis was more “sensitive” to pro-inflammatory and infectious stimuli than unaffected mucosa, and that EGCs participated in the modulation of mucosal NO production via S100B over-expression and release.10,11 Similarly, alteration in the ECGs-mediated inflammatory pathway may be involved in the pathogenesis of DD.
EGCs are able to interact with bacteria and discriminate between pathogens and probiotics via different Toll-like receptors expression and NO production.12 This different response of ECGs and the beneficial role of probiotics against the gut inflammation sustain the hypothesis of a role of probiotics in the modulation of inflammatory responses in DD induced by pathogen bacteria.
Lactobacillus casei DG® (Lactobacillus paracasei CNCM I-1572; LCDG), a probiotic strain widely used in humans, is able to modulate colonic microbiota in intestinal chronic inflammation and it also significantly modifies Toll-like receptor expression.13,14
The different clinical expressions of DD outcome (asymptomatic diverticulosis, SUDD, and AD) may be related to a different sensitivity of the colonic mucosa toward pro-inflammatory stimuli. Starting from these hypothesis, we aimed to evaluate the expression of NO-related mediators in colonic mucosa of patients with diverticulosis, SUDD and SUDD with previous episodes of AD (SUDD+AD), after bacterial infection. Moreover, we evaluated potential differences in terms of basal release of inflammatory mediators and whether LCDG is able to modulate the inflammatory response.
Materials and methods
Patients
Biopsies were collected during endoscopy in 40 consecutive individuals, divided as follow: 10 patients with diverticulosis (three women; mean age 70 years, range 59–77), 10 patients with SUDD (three women, mean age 63, range 44–92), 10 patients with SUDD + AD (two women, mean age 60, range 31–77) and 10 people without gastrointestinal diseases (six women; mean age 53; range 33–75), who served as controls (CTRLs). Indications for endoscopy were: colorectal cancer screening, adenoma surveillance, abdominal pain, change of bowel habit, rectorragy. CTRLs were selected only among individuals undergoing screening for colorectal cancer.
For each patient we collected six random biopsies taken from the sigma region; in patients with diverticula biopsies were collected close to diverticula.
We obtained informed consent from all the participants and approval from the ethics committee of the Federico II University of Naples.
Organ culture of mucosal biopsy specimens
Whole biopsy specimens were placed on a sterilized metal grid with the mucosal side facing downward and the serosal side facing upward, to allow a polarization of the biopsies. The metal grid was put in the middle of a center-well organ culture dish (BD Europe, Le Pont De Claix, France) for 24 hours and cultured in Dulbecco’s modified Eagle medium (DMEM)-F12, (Sigma-Aldrich, MO, USA) and kept in an incubator at 37℃, continuously gassed with 95% oxygen-5% carbon dioxide. Biopsies were then stimulated with pathogen and/or a probiotic bacteria, with the stimulus always added from the serosal side of the biopsy.
Bacterial strains
Enteroinvasive Escherichia coli (EIEC; ATCC, Rockville, MD, USA) was chosen for its deleterious effects on the gastrointestinal tract,15 while L. casei DG® (LCDG; Lactobacillus paracasei CNCM I-1572, Enterolactis®, Sofar S.p.A., Trezzano Rosa, Milan, Italy, deposited by Sofar at Institute Pasteur of Paris with number I1572) was chosen for its probiotic effects.14 Bacteria were cultured and stocked as previously reported.12
Outline of the experiments
Biopsies were treated as shown in Table 1 and supernatants were collected and biopsies were put in a radioimmunoprecipitation assay (RIPA) buffer (Lonza); both were stored at –80℃ for subsequent determinations.
Table 1.
Outlines of experiments.
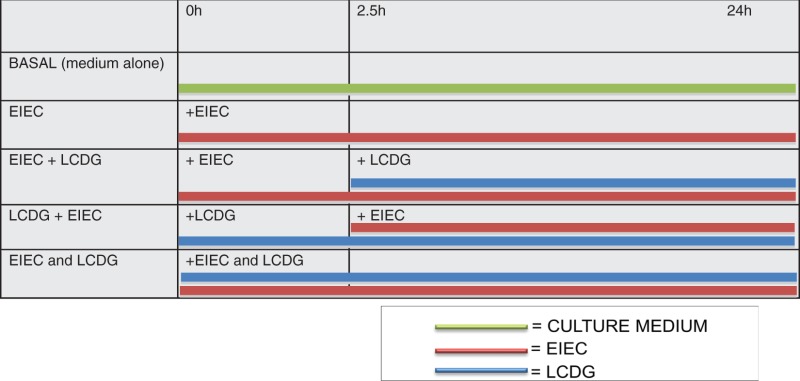
|
h: hours; EIEC: Enteroinvasive Escherichia coli; LCDG: Lactobacillus casei DG®.
Protein extraction and Western immunoblot analysis
Western blots were performed and analyzed as previously described.12 Antibodies used were: rabbit anti-iNOS (1:500 vol/vol dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-α actin (1:1000 v/v, Santa Cruz).
NO quantification
NO was measured as previously described.12
Enzyme-linked immunosorbent assay (ELISA) for S100B
ELISA for S100B (Biovendor R&D, Brno, Czech Republic) was carried out on biopsies supernatants according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed with analysis of variance (ANOVA) and multiple comparisons with Bonferroni’s test. Data presented are mean ± SD of n experiments. The level of statistical significance was set at p < 0.05.
Results
Basal levels of the iNOS expression and release of NO, S100B and cytokines
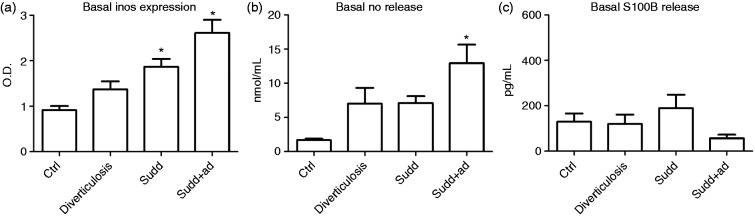
In diverticulosis patients, iNOS expression was increased compared to CTRLs, but not to a significant extent (Figure 1(a)). In SUDD and SUDD + AD patients, basal iNOS expression was significantly increased compared to controls (+2.04- and +2.86-fold increase vs CTRLs, respectively; p < 0.05) (Figure 1(a)).
Figure 1.
Basal iNOS expression and NO and S100B release in patients and healthy individuals. (a) Western blot analysis of basal iNOS expression in colonic mucosa of patients with diverticulosis, SUDD and SUDD with previous episodes of AD (SUDD + AD). (b) NO release determined by Griess assay in colonic mucosa of patients with diverticulosis, SUDD and SUDD with previous episodes of AD (SUDD + AD). (c) S100B release determined by ELISA assay in colonic mucosa of patients with diverticulosis, SUDD and SUDD with previous episodes of AD (SUDD + AD). All results are expressed as mean ± SEM of n = 10 experiments performed in triplicate. * p < 0.05 vs CTRL. iNOS: inducible nitric oxide synthase; NO: nitric oxide; SUDD: symptomatic uncomplicated diverticular disease; AD: acute diverticulitis; OD: optical density; ELISA: enzyme-linked immunosorbent assay; CTRL: control.
In diverticulosis and SUDD patients, NO release was increased compared to CTRLs, but not to a statistically significant extent (Figure 1(b)). In SUDD + AD, basal NO expression was significantly increased compared to CTRLs (+7.77-fold increase vs CTRLs; p < 0.05) (Figure 1(b)).
Because in previous works the enteroglial-derived S100B protein expression has been linked to NO release, we analyzed whether S100B was differently released in CTRLs and case individuals. As shown in Figure 1(c), CTRLs, diverticulosis, SUDD and SUDD + AD showed different S100B release but these differences were not statistically relevant.
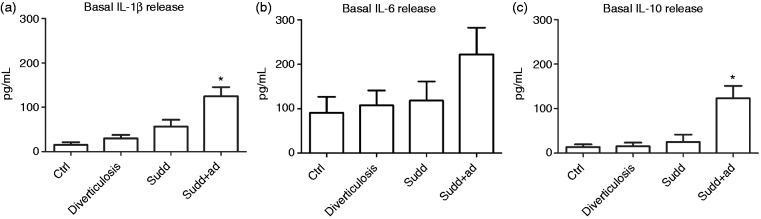
We also analyzed whether interleukin (IL)-1β, IL-6 and IL-10 were differently released among CTRLs, diverticulosis, SUDD and SUDD + AD. An increase in IL-1β release, ranging from CTRL to patients with previous AD was observed, even if only in the latter group IL-1β release was significantly increased (+8.26-fold increase vs CTRL; p < 0.05) (Figure 2(a)). Regarding IL-6, a trend in an increased release from CTRL to DD patients was also observed, even if this was not significant (Figure 2(b)). Also for IL-10, an increased release, ranging from CTRL to SUDD + AD patients, was observed and also in this case only in SUDD with previous AD patients IL-10 release was significantly greater than CTRLs (+11.25-fold increase vs CTRL; p < 0.05) (Figure 2(c)). When we analyzed the effects of bacteria stimulation on cytokines release in patients affected by every degree of the pathology, our data were characterized by a high variability and no significant results were obtained (data not shown).
Figure 2.
Basal cytokines release from colonic mucosa of patients and healthy individuals. Basal (a) IL-1β, (b) IL-6 and (c) IL-10 release determined by ELISA assay in colonic mucosa of patients with diverticulosis, SUDD and SUDD with previous episodes of AD (SUDD + AD). All results are expressed as mean ± SEM of n = 10 experiments performed in triplicate. * p < 0.05 vs CTRL. IL: interleukin; ELISA: enzyme-linked immunosorbent assay; SUDD: symptomatic uncomplicated diverticular disease; AD: acute diverticulitis; CTRL: control.
Effects of bacteria stimulation on iNOS expression
After analyzing basal levels, in order to evaluate whether bacteria stimulation differently modulated inflammatory mediators in CTRL and case individuals, we evaluated iNOS expression in mucosal biopsies.
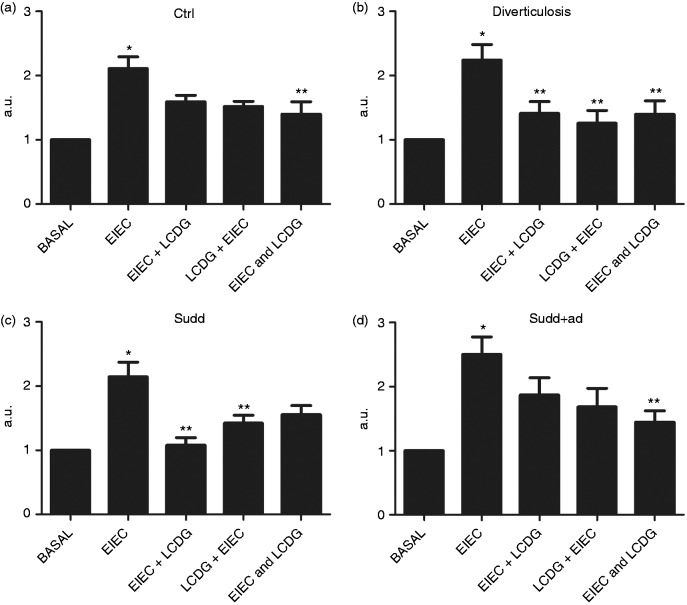
In CTRLs, EIEC stimulation significantly increased iNOS expression (+2.11-fold increase vs basal; p < 0.05, Figure 3(a)). When LCDG was added before and after EIEC, no significant changes in iNOS expression were observed (Figure 3(a)), but when LCDG was added at the same time as EIEC, we observed a significant reduction of iNOS expression compared to that induced by EIEC alone (–1.50-fold decrease vs EIEC; p < 0.05) (Figure 3(a)).
Figure 3.
iNOS expression in patients and healthy individuals after bacteria stimulation. Western blot analysis of iNOS expression after 24 hours of stimulation with enteroinvasive Escherichia coli (EIEC) and or L. casei DG® (Lactobacillus paracasei CNCM I-1572; LCDG) in colonic mucosa of (a) healthy controls, (b) patients with diverticulosis, (c) SUDD and (d) SUDD with previous episodes of AD (SUDD + AD). EIEC + LCDG: LCDG added 2.5 hours after EIEC; LCDG + EIEC: EIEC added 2.5 hours after LCDG; EIEC and LCDG: EIEC and LCDG added contemporaneously. All results are expressed as mean ± SEM of n = 10 experiments performed in triplicate. *p < 0.05 vs CTRL; ** p < 0.05 vs EIEC. iNOS: inducible nitric oxide synthase; SUDD: symptomatic uncomplicated diverticular disease; AD: acute diverticulitis; CTRL: control; a.u.: arbitrary units.
In patients with diverticulosis, EIEC significantly increased iNOS expression (+2.86-fold increase vs basal; p < 0.05) (Figure 3(b)). When LCDG was added to biopsies before, after or together with EIEC, iNOS expression results were significantly decreased compared to EIEC stimulation (–1.59-, –1.79- and +1.61-fold decrease vs EIEC, respectively; p < 0.05) (Figure 3(b)).
In SUDD patients, iNOS expression was significantly increased by EIEC stimulation (+2.14-fold increase vs basal; p < 0.05) (Figure 3(c)). LCDG addition before or after EIEC resulted in decreased iNOS expression compared to EIEC stimulation (–2.01- and –1.50-fold decrease vs EIEC, respectively; p < 0.05) (Figure 3(c)). When EIEC and LCDG were added contemporaneously, iNOS expression was decreased, even if not to a significant extent. Also in SUDD + AD, EIEC induced a significant increase in iNOS expression (+2.50-fold increase vs basal; p < 0.05) (Figure 3(d)). When LCDG was added to biopsies before or after EIEC, iNOS expression was not significantly reduced compared to EIEC (Figure 3(d)). Conversely, when EIEC and LCDG were added at the same time, iNOS expression was significantly decreased compared to EIEC stimulation (–1.73-fold decrease vs EIEC; p = NS) (Figure 3(d)).
In all participants analyzed, LCDG alone did not significantly modify iNOS expression (data not shown).
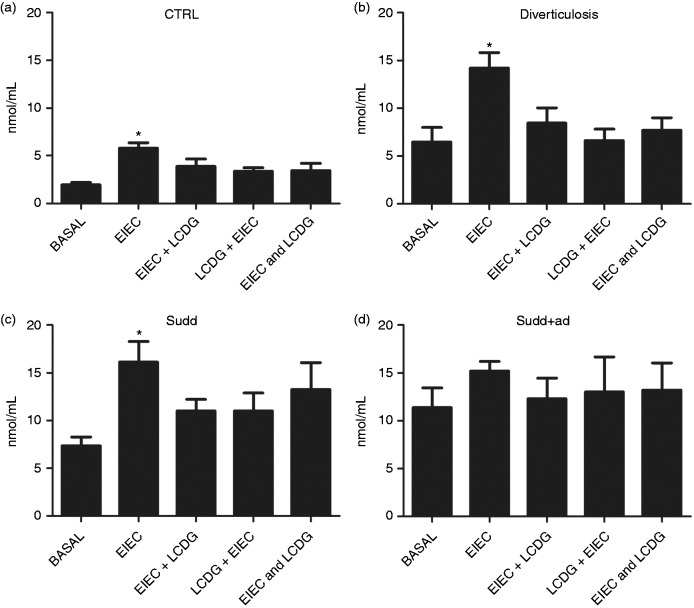
Effect of bacteria stimulation on NO release
In CTRLs EIEC stimulation significantly increased NO release (Figure 4(a)). When LCDG was added to biopsies before, after or together with EIEC, NO release was not different from basal level (Figure 4(a)). Similarly, in patients with AD and with SUDD (Figure 4(b) and 4(c)), EIEC stimulation significantly increased NO release, whereas no increase of NO release was observed when LCDG was added to biopsies before, after or together with EIEC. Rather, in patients with diverticulosis, when LCDG was added before EIEC, we observed a significant reduction in NO release (–2.35-fold decrease vs EIEC; p < 0.05) (Figure 4(b)).
Figure 4.
NO release in patients and healthy individuals after bacteria stimulation. NO release, determined by Griess assay, after 24 hours of stimulation with enteroinvasive Escherichia coli (EIEC) and/or L. casei DG® (Lactobacillus paracasei CNCM I-1572; LCDG) in colonic mucosa of (a) healthy controls, (b) patients with diverticulosis, (c) SUDD and (d) SUDD with previous episodes of AD (SUDD + AD). EIEC + LCDG: LCDG added 2.5 hours after EIEC; LCDG + EIEC: EIEC added 2.5 hours after LCDG; EIEC and LCDG: EIEC and LCDG added contemporaneously. All results are expressed as mean ± SEM of n = 10 experiments performed in triplicate. * p < 0.05 vs CTRL; ** p < 0.05 vs EIEC. NO: nitric oxide; SUDD: symptomatic uncomplicated diverticular disease; AD: acute diverticulitis; CTRL: control; a.u.: arbitrary units.
In SUDD + AD, basal NO release was higher compared to other groups (Figure 4D). In this setting, EIEC stimulation does not significantly increase NO release (Figure 4D). Similarly, no differences in terms of NO release were observed when LCDG was added to biopsies before, after or together EIEC (Figure 4D).
In all individuals analyzed, LCDG alone did not significantly modify NO release (data not shown).
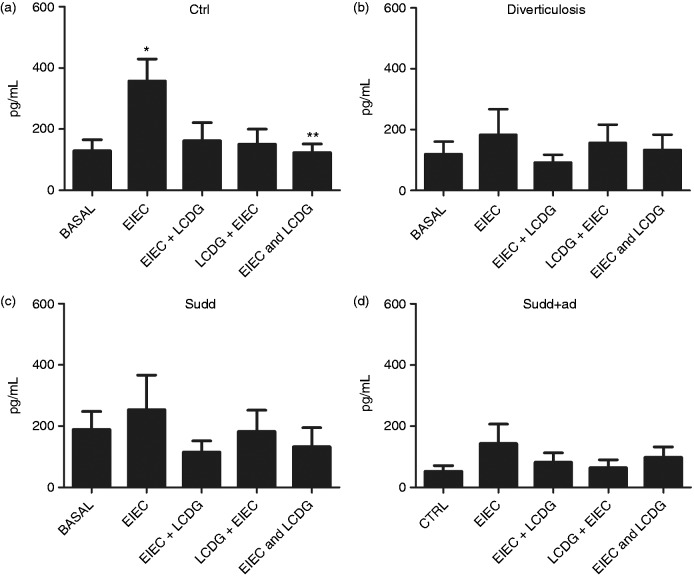
Effect of bacteria stimulation on S100B release
In CTRLs, EIEC induced a significant increase in S100B release (+2.76-fold increase vs basal; p < 0.05) (Figure 5(a)). When LCDG was added together with EIEC, S100B release results were significantly decreased compared to EIEC stimulation (–2.90-fold decrease vs EIEC; p < 0.05) (Figure 5(a)).
Figure 5.
S100B release in patients and healthy individuals after bacteria stimulation. S100B release, determined by ELISA assay, after 24 hours of stimulation with enteroinvasive Escherichia coli (EIEC) and/or L. casei DG® (Lactobacillus paracasei CNCM I-1572; LCDG) in colonic mucosa of (a) healthy controls, (b) patients with diverticulosis, (c) SUDD and (d) SUDD with previous episodes of AD (SUDD + AD). EIEC + LCDG: LCDG added 2.5 hours after EIEC; LCDG + EIEC: EIEC added 2.5 hours after LCDG; EIEC and LCDG: EIEC and LCDG added contemporaneously. All results are expressed as mean ± SEM of n = 10 experiments performed in triplicate. * p < 0.05 vs CTRL; ** p < 0.05 vs EIEC. ELISA: enzyme-linked immunosorbent assay; SUDD: symptomatic uncomplicated diverticular disease; AD: acute diverticulitis; CTRL: control; a.u.: arbitrary units.
Regarding diverticulosis, SUDD and SUDD + AD patients, bacteria stimulation did not induce significant changes in S100B release (Figure 5(b), 5(c) and 5(d)).
Moreover, when LCDG alone was added to biopsies, nonsignificant changes in S100B release were observed (data not shown).
Discussion
Our results showed an activation of NO-dependent inflammation related to iNOS expression and NO release that appeared progressively increased from diverticulosis to SUDD with previous diverticulitis. In addition, we showed a different response of the colonic mucosa of these patients to infectious stimuli and a role of the probiotic LCDG in preventing these effects.
NO is an important mediator of direct and indirect smooth muscle relaxation of the wall bowel.17 NO is also involved in gut inflammation and in the antibacterial response.18 Previous studies have demonstrated an increase in endogenously NO-mediated responses in patients with diverticulosis19 and abnormal nitrergic activities and an alteration in NOS activity in the gut of patients with SUDD.20,21 Longitudinal muscle shows abnormal relaxation responses to NO and contains altered levels of iNOS in uncomplicated DD.21
While previous studies usually focused their attention only on a single set of patients—those with diverticulosis rather than patients with SUDD or previous AD—in our first set of experiments, we sought to determine whether an increase in the severity of the disease matched an increase in the iNOS expression and NO release from the colonic mucosa of patients with a different grade of DD. Our data confirmed this hypothesis, as in colonic mucosal biopsies iNOS expression was about 1.5-fold higher in patients with diverticulosis, two-fold higher in patients with SUDD and about three-fold higher in patients with previous AD, compared to CTRLs. These data indicate the presence of an “inflammatory nitrergic gradient,” going from CTRLs to patients with SUDD and previous AD. In fact, it is known that while NO is physiologically released on demand for short periods of time following activation of constitutively expressed endothelial NO synthase (eNOS) or neuronal NO synthase (nNOS), iNOS expression is induced only after cell activation and produces NO for long periods of time, in a pathophysiological, inflammatory, context.22 Our data match previous reports that show that the immune cell infiltrates in colonic mucosa are increased according to disease severity.23
The presence of this inflammatory gradient appears further confirmed by the analysis of basal cytokine release from the colonic mucosa of our patients. Indeed, our data show an overall increase in the release of IL-1β, IL-6 and IL-10 that goes in parallel with the increase in the grade of disease severity.
We also evaluated basal NO release from colonic biopsies and we found that it was about four-fold higher in patients with diverticulosis and SUDD and eight-fold higher in patients with previous AD than in CTRLs. The differences in iNOS expression and NO release between diverticulosis and SUDD patients are unclear, but, as proposed by other authors, it may be due to a different and altered nitrergic neuro-muscular transmission in these two subsets of patients,19,21 where other than iNOS, nNOS also has a major role in producing NO.
Considering that colonic mucosa of CTRLs and patients showed basal differences in expression and release of NO-related mediators and that changes in gastrointestinal microflora are hypothesized to influence the etiology of DD,24,25 we asked whether the colonic mucosa of these patients also had a different “sensitivity” toward pathogenic and non-pathogenic bacteria stimuli. We found that, after a challenge with the pathogenic bacteria EIEC, iNOS expression was significantly higher than baseline, both in CTRLs and patients. Conversely, when the biopsies were challenged with the probiotic LCDG no significant increase compared to baseline was observed. To determine a possible preventive or healing effect of the probiotic, it was added to biopsies before, after or together with EIEC. Even with some differences among the different sets of patients, the presence of LCDG counteracted the increase in iNOS expression induced by EIEC. We also analyzed NO release and we obtained similar results as iNOS, with the difference that in patients with previous AD, EIEC-induced NO increase did not reach a statistical significant extent. This may probably be due to an already high baseline NO level, which does not allow for the appreciation of any changes induced by further bacterial stimulation. Taken together these data point out a different reactivity of the mucosa of patients affected by every degree of the pathology toward pathogenic stimulation and a role of probiotics in counteracting these effects. Although the exact mechanisms of action exerted by the probiotic are unclear, the observation that LCDG appears effective in decreasing EIEC-induced iNOS expression and NO release further support previous reports indicating promising effects of probiotics’ use in DD.13
In recent years, various studies found an association between distinct abnormalities of the ENS and EGC and the disturbed motility patterns underlying DD. Patients with diverticulitis showed decreased EGC and interstitial cells of Cajal density and S100B immunoreactivity in myenteric ganglia.26 Although the overall glial cell density was reduced in DD, a study in 2010 showed that a subgroup of myenteric ganglia displayed bulbous protrusions almost exclusively composed of glial cells,27 but this reported EGC activation phenomenon has not yet been confirmed by other investigations. We previously reported that the EGCs-derived S100B protein—a pivotal signaling molecule that participates in the onset and progression of the inflammatory status in the gut—regulates NO production via iNOS interaction.11 For this reason we also evaluated the involvement of S100B in DD. Analyzing basal S100B expression, no statistically significant differences between CTRLs and patients were found, even if a trend toward reduced S100B expression was evident in patients with previous diverticulitis. In CTRLs, stimulating biopsies with EIEC, but not with LCDG, significantly increased S100B expression. LCDG also exerted a preventive and healing effect because when biopsies were co-stimulated with EIEC and LCDG, the EIEC-induced S100B increase was not evident. Instead, considering biopsies of patients affected by various grade of diverticulitis, bacteria stimulation exerted no significant effects on S100B expression. Considering our data, S100B seems not to be involved in NO production. Nevertheless, in view of previous studies,28 involvement of EGC in this pathology can neither be confirmed nor excluded.
In conclusion, we showed that the colonic mucosa of patients affected by diverticulosis, SUDD and previous AD is basally characterized by dissimilar levels of iNOS expression and NO release and that these levels increase with the degree of disease severity. We also showed that colonic mucosa of patients with different grades of DD has a different reactivity toward pathogenic stimuli and that the LCDG may be effective in counteracting the inflammatory effects exerted by EIEC. Changes in gut microbiota have been reported to occur in patients with symptomatic DD;29 for this reason, adjusting the gut microbiota by the use of probiotics could influence the natural history of the disease. These data further support the beneficial role of LCDG in an inflammatory context, as previously reported.14
Our preliminary data may lay the basis for further studies addressing the concept of a different reactivity of the mucosa of patients with various-grade DD toward luminal stimuli and for addressing, in a large-scale cohort study, the real effectiveness of probiotic therapy in prevention of diverticulitis. Studies further addressing the relation between EGCs and DD are warranted to better understand the pathophysiology of this disease and to eventually lead innovative therapeutic approaches to DD.
Acknowledgments
Author contributions: F.T. was involved in study concept, experimental work, data acquisition, data analysis, data interpretation, drafting and revision of manuscript; P.A. was involved in data acquisition, data analysis, data interpretation, drafting and revision of manuscript; I.P. was involved in experimental work, data acquisition, data analysis and data interpretation; F.P.Z. was involved in data acquisition and data analysis; M.C. was involved in data acquisition and data analysis; W.F. was involved in study concept and revision of manuscript; N.G. was involved in collecting specimens; G.D.P. was involved in collecting specimens; G.S. was involved in revision of manuscript; and R.C. was involved in study concept, data acquisition, data analysis, data interpretation, drafting and revision of manuscript.
Declaration of conflicting interests
W.F. is an employee of Sofar S.p.A. The other authors have nothing to declare.
Funding
This work was funded in part by a grant from Sofar S.p.A.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009; 136: 1134–1144. [DOI] [PubMed] [Google Scholar]
- 2.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: Evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 3.Slack WW. The anatomy, pathology, and some clinical features of divericulitis of the colon. Br J Surg 1962; 50: 185–190. [DOI] [PubMed] [Google Scholar]
- 4.Cuomo R, Barbara G, Pace F, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United European Gastroenterol J 2014; 2: 413–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich J, Möller SH, Andersen D. Colonic haustral pattern in relation to pressure activity and presence of diverticula. Scand J Gastroenterol 1977; 12: 857–864. [DOI] [PubMed] [Google Scholar]
- 6.Trotman IF, Misiewicz JJ. Sigmoid motility in diverticular disease and the irritable bowel syndrome. Gut 1988; 29: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulmer TF, Rosch R, Mossdorf A, et al. Colonic wall changes in patients with diverticular disease—is there a predisposition for a complicated course? Int J Surg 2014; 12: 426–431. [DOI] [PubMed] [Google Scholar]
- 8.Zeng MY, Inohara N and Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. Epub ahead of print 24 August 2016. DOI: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed]
- 9.Cirillo C, Sarnelli G, Turco F, et al. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil 2011; 23: e372–e382. [DOI] [PubMed] [Google Scholar]
- 10.Esposito G, Cirillo C, Sarnelli G, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 2007; 133: 918–925. [DOI] [PubMed] [Google Scholar]
- 11.Cirillo C, Sarnelli G, Esposito G, et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil 2009; 21: 1209–e112. [DOI] [PubMed] [Google Scholar]
- 12.Turco F, Sarnelli G, Cirillo C, et al. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014; 63: 105–115. [DOI] [PubMed] [Google Scholar]
- 13.Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: Mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease—a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther 2013; 38: 741–751. [DOI] [PubMed] [Google Scholar]
- 14.D’Incà R, Barollo M, Scarpa M, et al. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci 2011; 56: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 15.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998; 11: 142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Rosa M, Radomski M, Carnuccio R, et al. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun 1990; 172: 1246–1252. [DOI] [PubMed] [Google Scholar]
- 17.Burleigh DE. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology 1992; 102: 679–683. [DOI] [PubMed] [Google Scholar]
- 18.Hibbs JB, Jr, Taintor RR, Vavrin Z, et al. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun 1988; 157: 87–94. [DOI] [PubMed] [Google Scholar]
- 19.Espin F, Rofes L, Ortega O, et al. Nitrergic neuro-muscular transmission is up-regulated in patients with diverticulosis. Neurogastroenterol Motility 2014; 26: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 20.Commane DM, Arasaradnam RP, Mills S, et al. Diet, ageing and genetic factors in the pathogenesis of diverticular disease. World J Gastroenterol 2009; 15: 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golder M, Burleigh DE, Ghali L, et al. Longitudinal muscle shows abnormal relaxation responses to nitric oxide and contains altered levels of NOS1 and elastin in uncomplicated diverticular disease. Colorectal Dis 2007; 9: 218–228. [DOI] [PubMed] [Google Scholar]
- 22.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med 2000; 6: 347–373. [PMC free article] [PubMed] [Google Scholar]
- 23.Tursi A, Brandimarte G, Elisei W, et al. Assessment and grading of mucosal inflammation in colonic diverticular disease. J Clin Gastroenterol 2008; 42: 699–703. [DOI] [PubMed] [Google Scholar]
- 24.Tursi A. New physiopathological and therapeutic approaches to diverticular disease of the colon. Expert Opin Pharmacother 2007; 8: 299–307. [DOI] [PubMed] [Google Scholar]
- 25.Maconi G, Barbara G, Bosetti C, et al. Treatment of diverticular disease of the colon and prevention of acute diverticulitis: A systematic review. Dis Colon Rectum 2011; 54: 1326–1338. [DOI] [PubMed] [Google Scholar]
- 26.Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol 2005; 58: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wedel T, Büsing V, Heinrichs G, et al. Diverticular disease is associated with an enteric neuropathy as revealed by morphometric analysis. Neurogastroenterol Motility 2010; 22: 407–414, e93–e94. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa-Cortes F, Turco F, Linan-Rico A, et al. Enteric glial cells: A new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiller RC. Changing views on diverticular disease: Impact of aging, obesity, diet, and microbiota. Neurogastroenterol Motility 2015; 27: 305–312. [DOI] [PubMed] [Google Scholar]