Abstract
Crigler–Najjar syndrome is a recessively inherited disorder characterized by severe unconjugated hyperbilirubinemia caused by a deficiency of uridine diphospho-glucuronosyl transferase 1A1. Current therapy relies on phototherapy to prevent kernicterus, but liver transplantation presently is the only permanent cure. Gene therapy is a potential alternative, and recent work has shown that helper-dependent adenoviral (HD-Ad) vectors, devoid of all viral coding sequences, induce prolonged transgene expression and exhibit significantly less chronic toxicity than early-generation Ad vectors. We used a HD-Ad vector to achieve liver-restricted expression of human uridine diphospho-glucuronosyl transferase 1A1 in the Gunn rat, a model of the human disorder. Total plasma bilirubin levels were reduced from >5.0 mg/dl to «1.4 mg/dl for >2 yr after a single i.v. administration of vector expressing the therapeutic transgene at a dose of 3 × 1012 viral particles per kg. HPLC analysis of bile from treated rats showed the presence of bilirubin glucuronides at normal WT levels >2 yr after one injection of vector, and i.v. injection of bilirubins IIIα and XIIIα in the same animals revealed excess bilirubin-conjugating capacity. There was no significant elevation of liver enzymes (alanine aminotransferase) and only transient, moderate thrombocytopenia after injection of the vector. A clinically significant reduction in serum bilirubin was observed with a dose as low as 6 × 1011 viral particles per kg. We conclude that complete, long-term correction of hyperbilirubinemia in the Gunn rat model of Crigler–Najjar syndrome can be achieved with one injection of HD-Ad vector and negligible chronic toxicity.
Keywords: adenovirus, bilirubin, gene therapy
Crigler–Najjar (CN) disease type I (OMIM 218800) is an autosomal recessively inherited disease characterized by persistent unconjugated hyperbilirubinemia (1). Normally, glucuronidation of bilirubin by uridine diphospho-glucuronosyl transferase 1A1 (UGT1A1) is required for its elimination in bile. In CN patients UGT1A1 activity is either absent (type I) or severely reduced (type II) (2). The persistently high plasma level of retained bilirubin leads to jaundice and may cause kernicterus. Current treatment consists mainly of lifelong phototherapy (10–12 h per day) (3). Although effective, phototherapy is cumbersome and inconvenient and its efficacy may diminish with age because of increased skin thickness and decreased surface/mass ratio. Liver transplantation is effective, but generally requires lifelong immunosuppression and involves substantial risks and costs. And, despite technical developments such as partial, living-related, split, in situ split, auxiliary orthotopic transplantation, or hepatocyte transplantation, donor availability is limiting in many countries (4, 5).
The full-length cDNA for human UGT1A1 has been cloned and sequenced (6), and several mutations have been discovered in CN patients that reduce or eliminate UGT1A1 activity (7). The Gunn rat (8), which is deficient in all members of the UGT1A family of conjugating isozymes (9, 10), is a useful animal model of the human disease and has been used extensively for evaluating UGT1A1 gene transfer with different vectors (11–16) or oligonucleotide-based gene therapy (17).
CN patients are potential candidates for gene therapy, because timely intervention should eliminate the risk of kernicterus. Even partial enzyme replacement should lead to significant clinical improvement because only ≈5% of normal UGT1A1 activity is adequate to significantly lower the plasma bilirubin concentration and diminish the need for phototherapy (4).
Hyperbilirubinemia in Gunn rats has been corrected for short periods of up to 4 wk by using early-generation adenoviral (Ad) vectors stripped of their E1 coding regions (13, 15). Several strategies to prolong the duration of transgene expression by reducing the immunogenicity of the vector have been explored, including induction of tolerance (12) and expression of immunomodulatory molecules (18). However, the therapeutic index of E1-deleted Ad vectors is limited because of strong immune response to viral proteins, acute toxicity, and short duration of expression. Subsequently, helper-dependent Ad (HD-Ad) vectors, also known as high-capacity or “gutless” vectors, have been developed (for review see ref. 19). These are devoid of all viral coding sequences and have negligible chronic hepatic toxicity in contrast to earlier Ad vectors (20, 21). Moreover, a single i.v. injection of HD-Ad vector can cause long-term enzyme expression (1–2.5 yr) in hepatocytes (22, 23). Why the HD-Ad genome persists for so long in vivo in an episomal state is unclear, although several models have been proposed (24).
Here, we describe the use of a HD-Ad vector expressing human UGT1A1 for the treatment of hyperbilirubinemia in the Gunn rat. Complete, stable, and clinically relevant normalization of serum bilirubin levels for >2 yr after a single HD-Ad injection was achieved. We describe the use of a HD-Ad vector for continual expression of an hepatic endoplasmic reticulum enzyme and correction of an inherited enzymatic defect. This study also is among the most complete and longest corrections so far in the Gunn rat using a viral delivery method. These encouraging results may pave the way to gene therapy of CN syndrome and similar strategies for treating other inborn errors of liver metabolism.
Materials and Methods
Generation of HD-Ad Vector. A helper-dependent adenovirus is a vector with all viral coding sequences deleted and replaced by noncoding human genomic DNA. We generated a HD-Ad vector containing human UGT1A1 cDNA; the rat phosphoenol-pyruvate carboxykinase (PEPCK) promoter (25); a fragment of the human apoA-I intron 1 containing the flanking region of exons 1 and 2 (base pairs 474–698, GenBank accession no. J00098); the woodchuck hepatitis virus posttranscriptional regulatory element (26); and the bovine β-globin polyadenylation site. The expression cassette was cloned into pBGShuttle, and the corresponding Ad backbone was generated by homologous recombination in Escherichia coli as described (27). HD-Ad vector preparation followed a recently described protocol with minor modifications (28). The genomic structure of the vector was determined by HindIII digestion of viral DNA, and the parental plasmid was followed by agarose gel electrophoresis. Helper virus contamination was determined by Southern hybridization analysis of PstI-digested vector DNA with an Adinverted terminal repeat-specific probe and estimated to be <1.5% by PhosporImager analysis.
Animals. Animals were Gunn rats, HsdBlu:Gunn (Harlan Bioproducts for Science, Indianapolis), maintained on standard chow under 12-h light/dark cycles. Homozygote (j/j), 8-week-old female rats (135–155 g) were used for vector administration. WT rats with the same genetic background [HsdBlu:Gunn (+/+)] were used as controls for HPLC studies. Animal experiments were performed according to a protocol approved by the Baylor College of Medicine in conformity with National Institutes of Health guidelines.
Administration of HD-Ad Vector. The Ad vector preparation, diluted into 1 ml of PBS, was injected via the tail vein. Six animals were injected with 6 × 1011 viral particles (vp)/kg HD-Ad PEPCK-UGT1A1 (low-dose group), six received 3 × 1012 vp/kg (intermediate-dose group), and two animals received a 1 × 1013 vp/kg dose (high-dose group). Another six rats received an equal volume of PBS (control group).
Effect of Phenobarbital. Four rats were injected i.v. (tail vein) with 6 × 1011 vp/kg HD-Ad PEPCK-UGT1A1 and then treated i.p. (60 mg/kg per day) with phenobarbital (Sigma) for 8 consecutive days. Four control rats received vector followed by vehicle instead of phenobarbital. Total serum bilirubin levels in these two groups were then compared. For comparison, four saline-treated rats that had received no vector were administered phenobarbital as above, and their serum bilirubin levels were compared with those from a group of four saline-treated rats that had received only vehicle solution.
Blood Sample Analysis. Blood samples were collected from the lateral saphenous vein or tail vein. Alanine aminotransferase (ALT) levels were measured with a GP transaminase kit (Sigma), and total and direct-reacting serum bilirubin levels were determined by a diazo reagent-based method with a clinical chemistry kit (Sigma), according to the manufacturer's instructions. Serum-unconjugated bilirubin was also measured by HPLC (29). Glucuronides in bile were identified by their absorbance spectra and comparing their retention times with authentic standards. Complete blood counts were performed at the Clinical Pathology Laboratory in the Center for Comparative Medicine at Baylor College of Medicine.
Analysis of Bilirubin Glucuronides in Bile. Animals were fitted with a short (7.5 cm, PE 50) biliary cannula under ketamine anesthesia and placed under an IR heating lamp in a darkened room. After ≈30 min, a solution containing ≈0.25 mg bilirubin-IIIα and ≈0.25 mg bilirubin-XIIIα dissolved in 1 ml of WT rat serum with the aid of 0.1 ml of 0.1 M argon-degassed NaOH or DMSO was injected as a bolus into the tail vein. Bile was collected in 20-μl aliquots just before isomer injection and at frequent intervals thereafter for the next 60 min. Bile samples were flash-frozen immediately in dry ice and kept under –60°C until analysis by HPLC.
RNA Analysis. Total RNA was extracted from liver homogenates by using the RNeasy Protect kit from Qiagen (Valencia, CA). RT-PCR used specific primers to amplify a 321-bp segment of the unique 5′ domain of the human UGT1A1 mRNA as described (11). Primers and conditions for normalizing RT-PCR by rat β-actin amplification have been described (30).
Protein Analysis. Liver homogenates were prepared in a solution of 0.25 M sucrose, 10 mM Tris·HCl, pH 7.4. Microsomes were obtained by centrifugation of liver homogenates (8,000 × g for 20 min and 105,000 × g for 90 min at 4°C). Protein content was determined by using the Micro BCA protein assay reagent kit (Pierce) with BSA as standard. Aliquots of 100 μg of total homogenates or 20 μg of microsomal proteins were separated on a 7.5% SDS/PAGE gel. Western blot analysis was performed as described (11) with specific human UGT1A1 mAbs (31).
Immunohistochemical Analysis. Liver biopsies were fixed in Formalde-Fresh solution (Fisher Scientific) and embedded in paraffin. Sections were deparaffinized with xylene and rehydrated by incubating successively with 100%, 95%, 70%, and 50% ethanol. Endogenous peroxidase was blocked by incubation for 15 min in peroxidase quenching solution (30% H2O2/methanol, 1:9). A blocking kit (Vector Laboratories) was used to eliminate nonspecific binding of biotin and avidin. A specific anti-human UGT1A1 (dilution 1:800) was used as primary antibody. After overnight incubation with primary antibody, slides were washed in PBS and treated with a biotinylated secondary antibody (Zymed). Sections were washed with PBS, overlaid with a streptavidin-peroxidase complex for ≈5 min, and then treated with diaminobenzidine tetrahydrochloride substrate. The slides were washed, counterstained with hematoxylin, and mounted for light microscopy (32).
Statistical Analysis. Analysis of data and comparisons between control and treated groups were done with instat (GraphPad, San Diego). The significance of differences was assessed with a two-tailed Student t test for unpaired data; P < 0.05 was considered statistically significant. Results are expressed as means ± SEM.
Results
HD-Ad Administration to Gunn Rats: Effect on Serum Bilirubin. Complete normalization of total serum bilirubin concentration was achieved within ≈1 wk of a single i.v. administration of 3 × 1012 vp/kg HD-Ad PEPCK UGT1A1. Phenotypic correction, manifested as reduction in jaundice in the treated animals, was confirmed by analysis of serum (Fig. 1a) and direct, indirect (data not shown) and total bilirubin assays (Fig. 1b). Whereas pretreatment and saline-treated values of total bilirubin ranged from 5.8 to 10.5 mg/dl, animals treated with the intermediate and high doses of HD-Ad had values ranging from 0.4 to 1.4 mg/dl. The difference between total bilirubin values in the treated group was statistically significant compared with the control group throughout the experiment at all analyzed time points. Taking the 1-wk postinjection value as reference, no statistically significant difference was subsequently observed in total bilirubin level for the HD-Ad intermediate-dose and high-dose animals at any time point analyzed, indicating that the correction was stable for 2 yr. In addition, a 70% drop in total bilirubin concentration was observed after injecting a lower dose (6 × 1011 vp/kg) of vector, and the bilirubin concentration remained <3.5 mg/dl for 110 wk. These prolonged effects on serum bilirubin were confirmed by HPLC analysis of serum from high-, intermediate-, and low-dose animals 115, 102, and 107 wk, respectively, after vector injection (Fig. 1c).
Fig. 1.
HD-Ad administration into Gunn rats: effect on serum bilirubin levels. Eight-week-old female Gunn rats received saline solution or HD-Ad PEPCK UGT1A1 by tail vein injection. The dose of HD-Ad vector is expressed as vp per kg. (a) Photographs of 10-μl samples of rat serum from a representative animal from each group. (b) Serum total bilirubin levels by diazo determination. Data are indicated as mean ± SEM. The difference between the HD-Ad-treated and the saline control groups was statistically significant for all points analyzed. (c) HPLC analysis of serum from a WT rat, a high-dose rat (115 wk after treatment), an intermediate-dose rat (102 wk after treatment), and a low-dose rat (107 wk after treatment). Unconjugated bilirubin (red peak) concentrations based on HPLC analyses were 0.1, 0.1, 0.7, and 1.9 mg/dl, respectively. The top chromatogram shows, for comparison, a typical chromatogram of serum from a healthy human adult (0.4 mg/dl). Blue peaks are the injection front.
The viral preparation was administered to 8-week-old rats whose body weight (≈143 g) increased during the course of the study to ≈240 g after 2 yr. Interestingly, despite this increase, total bilirubin levels after HD-Ad injection remained stable for up to 2 yr after the first week.
Phenobarbital has been reported to induce bilirubin glucuronidation and lower plasma bilirubin levels in Gunn rats (33). To investigate whether it is possible to combine low-dose HD-Ad gene therapy with drug treatment to achieve complete correction of hyperbilirubinemia, a separate low-dose-treated group of rats was given phenobarbital (60 mg/kg per day for 8 consecutive days) as described (33) (Supporting Materials and Methods and Fig. 6, which are published as supporting information on the PNAS web site). Total bilirubin levels were then compared with a group receiving the same dose of vector but not treated with phenobarbital. No statistically significant difference was detected between the two groups, indicating that bilirubin clearance mediated by subphysiological UGT1A1 activity derived from HD-Ad-mediated expression under the rat PEPCK promoter cannot be increased by phenobarbital treatment. Nor, in contrast to ref. 33, did we observe a significant effect of phenobarbital in the Gunn rat controls.
HD-Ad Administration to Gunn Rats: In Vivo Conjugation of Bilirubin. To confirm that the persistent decline in plasma bilirubin in the treated animals was caused by elimination of bilirubin as glucuronides, we analyzed bile from one animal from each dosage group by HPLC >2 yr after HD-Ad injection and compared these chromatograms with those from a saline-treated homozygous Gunn rat control and normal nonjaundiced WT controls (Fig. 2). As expected, bilirubin glucuronides were not present in bile from the saline-treated homozygous control, but copious quantities of bilirubin monoglucuronides and diglucuronides were present in bile from the high- and intermediate-dose animals in quantities commensurate with those in WT controls. Bilirubin glucuronides were clearly present even in the low-dose rat some 107 wk after virus injection. However, the amounts were small, relative to those in WT animals and animals treated with higher doses of vector, and they contained a higher proportion of monoglucuronides.
Fig. 2.
HD-Ad-treated Gunn rats: bilirubin glucuronides in bile. HPLC chromatograms of bile from a WT control rat (a), a high-dose rat (115 wk posttreatment) (b), an intermediate-dose rat (102 wk posttreatment) (c), and a low-dose rat (107 wk posttreatment) (d). The lower chromatograms are of bile from a saline-treated homozygous Gunn rat control. BDG, bilirubin diglucuronide; BMG, bilirubin monoglucuronide; UCB, unconjugated bilirubin. (Note that the vertical scale in d is greatly expanded compared with a–c.)
Rats and humans normally have an excess capacity for bilirubin conjugation and only a small fraction of the normal complement of UGT1A1 is required to completely conjugate all bilirubin produced by heme catabolism (4, 34). To test the excess conjugating capacity of the treated Gunn rats, one animal from each treatment group was injected i.v. >2 yr after the original viral injection with an equimolar mixture of two synthetic bilirubin isomers, bilirubin-IIIα and bilirubin-XIIIα (35), and bile was collected in small (20-μl) aliquots immediately before the injection and at frequent intervals for the next hour for HPLC analysis. Bilirubins IIIα and XIIIα differ from natural bilirubin (IXα) only in the positions of the methyl and vinyl groups on their lactam rings, and they are metabolized identically to the natural isomer, requiring UGT1A1 for conjugation and biliary excretion. However, being symmetrically substituted, they each form only one monoglucuronide in addition to a diglucuronide, and these metabolites can be readily distinguished from the glucuronides of endogenous bilirubin by HPLC. The total amount of isomers injected (0.5 mg; ≈2mg/kg) is ≈35% of the daily production of bilirubin in the rat (36). Normal WT rats and a saline-treated Gunn rat control were also treated in the same way for comparison. In the saline-treated Gunn rat lacking endogenous UGT1A1 no biliary excretion of bilirubin glucuronides was observed, as expected. In the low-dose-treated rat there was no detectable excretion of bilirubin-IIIα or XIIIα glucuronides. However, in the WT controls and the intermediate-dose and high-dose rats, rapid excretion of the IIIα and XIIIα monoglucuronides and diglucuronides was observed without any notable diminution of the endogenous IXα glucuronide excretion (Fig. 3). Thus, the high-dose and intermediate-dose animals still had excess bilirubin-conjugating capacity, like the normal rat, 2 yr after injection of the vector, whereas the low-dose animal did not. Comparison of the complete bilirubin isomer glucuronide excretion profiles for these rats (data not shown) indicated that the excess conjugating capacity decreased in the order WT > high dose > intermediate dose, as might be expected.
Fig. 3.
HD-Ad-treated Gunn rats: bilirubin isomer challenge. A mixture of bilirubins IIIα (≈0.25 mg) and XIIIα (≈0.25 mg) in 1 ml of rat serum was injected i.v. into a WT control, a high-dose rat (115 wk after vector administration), and an intermediate-dose rat (102 wk after vector administration). Bile was collected just before (t = 0) and 6–15 min after isomer injection and analyzed by HPLC. (a) Representative mixture of isomers injected. (b–d) HPLC of bile from the control, high-dose, and intermediate-dose rats, respectively. BR, bilirubin; DG, diglucuronide; MG, monoglucuronide.
mRNA and Protein Analysis. Restricted hepatic expression of human UGT1A1 mRNA 20 and 52 wk after treatment in intermediate-dose HD-Ad-treated animals was confirmed by RT-PCR using primers specific for a segment of the human UGT1A1 exon 1 (Fig. 4a). No human UGT1A1 mRNA was detected by RT-PCR in stomach, intestine, spleen, kidney, heart, ovary, or lungs, both in saline-treated and HD-Ad-treated animals (data not shown).
Fig. 4.
HD-Ad administration into Gunn rats: mRNA and protein analysis. (a) RT-PCR amplification from total liver RNA extracted from Gunn rats 20 and 52 wk after administration of saline solution or 3 × 1012 vp/kg HD-Ad. Ethidium bromide-stained agarose gel shows human UGT1A1 and rat β-globin amplified fragments. Lane 1, 1 kb of Plus DNA ladder (Invitrogen); lane 2, PCR with no DNA template control; lane 3, PCR using plasmid with human UGT1A1 cDNA as template; lane 4, PCR using HD-Ad PEPCK UGT1A1 DNA as template; lane 5, no reverse transcriptase control for lane 6; lane 6, RT-PCR using liver RNA from saline-treated Gunn rat 20 wk postinjection as template; lane 7, RT-PCR using liver RNA from HD-Ad-treated Gunn rat 20 wk postinjection as template; lane 8, no reverse transcriptase control for lane 7; lane 9, RT-PCR using liver RNA from saline-treated Gunn rat 52 wk postinjection as template; and lane 10, RT-PCR using liver RNA from HD-Ad-treated Gunn rat 52 wk postinjection as template. (b) Western blot analysis of human UGT1A1 expression in liver homogenates. Protein homogenates from liver biopsy of Gunn rats 20 weeks after administration of saline solution or 3 × 1012 vp/kg HD-Ad PEPCK UGT1A1 were resolved by SDS/PAGE (7.5%). Immunoblot analysis was performed by using anti-human UGT1A1 mAbs. Lane 1, PBS-treated rat, 100 μg total protein; lane 2, HD-Ad-treated rat, 100 μg total protein; lane 3, PBS-treated rat, 25 μg microsomal protein; and lane 4, HD-Ad-treated rat, 25 μg microsomal protein. (c and d) Immunohistochemical analysis of human UGT1A1 expression in liver sections. Sections of liver biopsy of Gunn rats 20 wk after administration of saline solution (c) or 3 × 1012 vp/kg HD-Ad (d) were immunostained by using specific anti-human UGT1A1 antibodies as primary antibody. Brown staining in d indicates UGT1A1.
Immunoblot analyses of protein from total homogenates and microsomal preparations of liver biopsies from rats killed 20 wk after injection were performed by using a mAb specific for human UGT1A1 (31). Expression of a protein with the molecular mass of human UGT1A1 (52 kDa) was detected in HD-Ad-treated but not in saline-treated Gunn rats (Fig. 4b). This result was further confirmed by immunohistochemical analysis performed on liver sections from the same animals (Fig. 4 c and d).
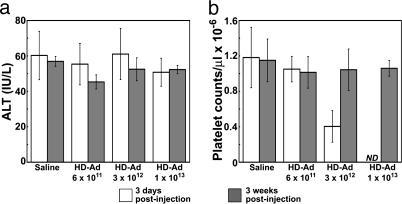
Toxicity. HD-Ad vectors are less hepatotoxic than first-generation Ad vectors. No acute liver toxicity was detected by serum alanine aminotransferase measurement after systemic injection of the HD-Ad vector in Gunn rats (Fig. 5a). Three days after injection, animals in the low-dose group (6 × 1011 vp/kg) showed no significant drop in platelet count, which is a more sensitive measure of acute toxicity. In the intermediate-dose group (3 × 1012 vp/kg) there was a 50% reduction compared with the PBS-treated controls, but the thrombocytopenia was transient and platelet counts were normal when measured 3 wk after injection (Fig. 5b).
Fig. 5.
HD-Ad administration into Gunn rats: toxicity profile. Serum alanine aminotransferase (ALT) levels (a) and circulating platelet counts (b) in Gunn rats 3 days and 3 weeks after tail vein administration of saline or HD-Ad. Doses of HD-Ad are expressed as vp per kg of body weight. Data are indicated as mean ± SEM. ND, not determined.
Discussion
CN syndrome is a rare inborn error of bilirubin metabolism caused by a deficiency of the membrane-bound phase II enzyme UGT1A1. Patients with the severe (type I) form of the disease often used to die in infancy. Since the advent of phototherapy, however, they now generally survive in otherwise good health to adulthood. But as long as their hyperbilirubinemia persists they are at constant risk of sudden bilirubin encephalopathy or severe neurologic complications such as choreoathetosis, hearing problems, or mental retardation (37). Presently the only long-lasting therapeutic alternative is liver transplantation, for which CN patients may not rank highly unless they are in acute neurological crisis.
CN syndrome has long been considered a paradigm for developing gene therapies for metabolic liver diseases. It is an attractive model for several reasons: (i) the underlying defect is well characterized at the biochemical and molecular level; (ii) the fraction of corrected hepatocytes required for clinical benefit is small, as deduced from hepatocyte transplantation studies (4); (iii) an animal model, the Gunn rat, with a similar, although not identical, genetic defect is available (9); (iv) the outcome of the experimental therapy can be easily monitored by measuring bilirubin fractions in serum and bile; (v) notwithstanding the deficiency in the UGT1A1, CN patients seem otherwise healthy if they survive infancy unscathed; and (vi) if a safe vector for enzyme correction can be developed, the risk/benefit ratio for gene therapy may be acceptable.
Several gene-transfer methods have been extensively evaluated by using the Gunn rat (38). Among nonviral approaches, gene correction via chimeraplasty has been attempted (17), but its usefulness has been questioned (39). Prolonged correction in Gunn rats has been achieved by using retroviral vectors (16, 40), but this approach requires invasive partial hepatectomy to induce hepatocyte proliferation and improve transduction. Recombinant simian virus 40 vectors effectively express human UGT1A1 for only 40 days (32). Partial correction of UGT1A1 deficiency in Gunn rats by in utero gene transfer with lentiviral vectors has also been reported (41). The therapeutic potential of Ad vectors has been demonstrated in several studies (13, 15). Their major advantage is that they preferentially localize in the liver after systemic administration and are able to mediate high-efficiency gene expression in quiescent hepatocytes without viral genome integration. Unfortunately, acute toxicity and immunogenicity of viral proteins, which cut short transgene expression, were defects of early-generation Ad vectors (42). Several strategies have been attempted to circumvent these limitations. Encouraging results were obtained by: (i) inducing tolerance in Gunn rats by administering Ad antigens (12, 43); (ii) injecting Ad vector during the neonatal period (11); (iii) including the Ad E3 region in the Ad vector (44); and (iv) coexpressing UGT1A1 and immunomodulatory molecules in the same Ad vector (18). Each strategy represented a further step toward achieving long-term expression, but toxicity remained a problem.
In comparison to the earlier-generation Ad vectors, HD-Ad vectors, which are devoid of all viral protein coding sequences, are much less toxic. Moreover, their long-term activity of up to 2 yr in liver after a single systemic administration has been achieved by using human and mouse genomic sequences as transgenes (20, 23), even though a slow loss of the Ad genome was observed (22), probably because of turnover of hepatocytes.
The current work describes the use of a HD-Ad vector for complete correction of hereditary hyperbilirubinemia in the Gunn rat, an animal model of CN syndrome type I. The clinical relevance of the study is demonstrated by the lifetime (>2 yr) normalization of hyperbilirubinemia in homozygous Gunn rats after a single injection of HD-Ad vector. The total bilirubin level was stable <1.4 mg/dl for >2 yr, which is by far the longest complete correction observed in this animal model. Sensitive HPLC analysis of serum from an intermediate-dose animal 102 wk after treatment showed a bilirubin level (0.7 mg/dl) similar to the ≈0.6 mg/dl of normal humans. An even lower concentration (0.07 mg/dl), similar to that in WT rats, was observed in a high-dose animal 115 wk after vector administration. Copious bilirubin glucuronide formation in these animals was revealed by HPLC of bile, and challenge with bilirubin isomers clearly showed excess conjugating capacity. In the low-dose group (6 × 1011 vp/kg vector) bilirubin concentrations remained <3.5 mg/dl up to 110 wk, and conjugation of bilirubin was detectable in a low-dose animal 107 wk after viral treatment. Even this reduction of hyperbilirubinemia could be considered therapeutic because phenotypically these treated Gunn rats resemble CN type II patients who have low, but not totally deficient, UGT1A1 activity. However, in CN type II patients UGT1A1 activity is derived from the endogenous UGT1A1 gene locus, whereas in the partially corrected Gunn rats, UGT1A1 expression is derived from the HD-Ad episomal genome.
In previous studies, i.v. injection of first-generation E1a-deleted and second-generation E1a/E2a-deleted Ad vectors in mice caused severe dose-related thrombocytopenia (42). This result was not caused by de novo transcription of the transgene or of viral genes because it also occurs after intravascular delivery of HD-Ad vectors lacking viral genes, and its origin remains to be determined. Possible explanations are direct damage or activation of intravascular endothelial cells with subsequent platelet sequestration by the reticuloendothelial system (45); interaction between virions and platelets leading to platelet activation; and induction of an early, innate immune response to capsid proteins with rapid release of cytokines. In the present study, toxicity associated with treatment was mild as reflected by serum transaminase levels and complete blood counts. However, the relationship between vector dose and acute toxicity may not be linear and the window between therapeutic and toxic dose might, therefore, be narrow.
The current study uses a HD-Ad vector for long-term expression of an hepatic endoplasmic reticulum protein needed for correction of an inherited cell-autonomous metabolic disorder. Most cell-autonomous diseases, such as urea cycle disorders, require correction of the phenotype in a high percentage of hepatocytes, and hence, a higher dose of vector with increasing risk of disseminated intravascular coagulation. CN syndrome, as indicated by hepatocyte transplantation studies in humans and by gene therapy research in rats, requires transfection of fewer hepatocytes to normalize or significantly reduce the plasma bilirubin concentration. In the current study, complete long-term correction was achieved in Gunn rats with a HD-Ad dose causing only mild, transient thrombocytopenia and no apparent chronic toxicity. Even a lower dose, which did not induce any measurable alteration in acute toxicity indicators, significantly reduced hyperbilirubinemia by 70% for >2 yr. A similar reduction in human patients would be adequate to greatly diminish the risk of bilirubin toxicity and eliminate the burden of daily phototherapy. However, further reduction of the acute toxicity and confirmation of the long-term safety of HD-Ad vectors may be needed before they can be used for gene therapy in humans.
Supplementary Material
Acknowledgments
We thank the Crigler-Najjar Italia-Associazione Malati Iperbilirubinemici, F. Ronchi, A. Mian, R. Cela, W. M. McCormack, and M. Ciotti. Preliminary studies by G.T. were partially supported by a postdoctoral fellowship from the American Liver Foundation. This work was supported by National Institutes of Health Grants DK56787 (to B.L.), HL51754 (to A.L.B.), and DK26307 (to A.F.M.), the Texas Gulf Coast Digestive Disease Center (through National Institutes of Health Grant DK56338), and the Baylor Mental Retardation Developmental Disabilities Research Center (through National Institutes of Health Grant HD24064).
Author contributions: G.T., V.P.M., P.N., A.F.M., A.L.B., and B.L. designed research; G.T., V.P.M., W.S.N., and A.F.M. performed research; G.T., M.J.F., A.F.M., and B.L. analyzed data; P.N. contributed new reagents/analytic tools; and G.T., A.F.M., A.L.B., and B.L. wrote the paper.
Abbreviations: CN, Crigler–Najjar; Ad, adenoviral; HD-Ad, helper-dependent Ad; PEPCK, phosphoenolpyruvate carboxykinase; UGT1A1, uridine diphospho-glucuronosyl transferase 1A1; vp, viral particles.
References
- 1.Schmid, R. & McDonagh, A. F. (1978) in The Metabolic Basis of Inherited Diseases, eds. Stanbury, J. B., Wyngaarden, J. B. & Fredrickson, D. S. (McGraw–Hill, New York), pp. 1221–1257.
- 2.Ciotti, M., Obaray, R., Martin, M. G. & Owens, I. S. (1997) Am. J. Med. Genet. 68, 173–178. [PubMed] [Google Scholar]
- 3.van der Veere, C. N., Sinaasappel, M., McDonagh, A. F., Rosenthal, P., Labrune, P., Odievre, M., Fevery, J., Otte, J. B., McClean, P., Burk, G., et al. (1996) Hepatology 24, 311–315. [DOI] [PubMed] [Google Scholar]
- 4.Fox, I. J., Chowdhury, J. R., Kaufman, S. S., Goertzen, T. C., Chowdhury, N. R., Warkentin, P. I., Dorko, K., Sauter, B. V. & Strom, S. C. (1998) N. Engl. J. Med. 338, 1422–1426. [DOI] [PubMed] [Google Scholar]
- 5.Rela, M., Muiesan, P., Vilca-Melendez, H., Dhawan, A., Baker, A., Mieli-Vergani, G. & Heaton, N. D. (1999) Ann. Surg. 229, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson, M. R., McCarthy, L. R., Harding, D., Wilson, S., Coughtrie, M. W. & Burchell, B. (1987) Biochem. J. 242, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadakol, A., Ghosh, S. S., Sappal, B. S., Sharma, G., Chowdhury, J. R. & Chowdhury, N. R. (2000) Hum. Mutat. 16, 297–306. [DOI] [PubMed] [Google Scholar]
- 8.Schmid, R., Axelrod, J., Hammaker, L. & Swarm, R. L. (1958) J. Clin. Invest. 37, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyanagi, T., Emi, Y. & Ikushiro, S. (1998) Biochim. Biophys. Acta 1407, 173–184. [DOI] [PubMed] [Google Scholar]
- 10.Sato, H., Aono, S., Kashiwamata, S. & Koiwai, O. (1991) Biochem. Biophys. Res. Commun. 177, 1161–1164. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi, M., Ilan, Y., Chowdhury, N. R., Guida, J., Horwitz, M. & Chowdhury, J. R. (1996) J. Biol. Chem. 271, 26536–26542. [DOI] [PubMed] [Google Scholar]
- 12.Ilan, Y., Attavar, P., Takahashi, M., Davidson, A., Horwitz, M. S., Guida, J., Chowdhury, N. R. & Chowdhury, J. R. (1996) J. Clin. Invest. 98, 2640–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Askari, F. K., Hitomi, Y., Mao, M. & Wilson, J. M. (1996) Gene Ther. 3, 381–388. [PubMed] [Google Scholar]
- 14.Seppen, J., Tada, K., Ottenhoff, R., Sengupta, K., Chowdhury, N. R., Chowdhury, J. R., Bosma, P. J. & Oude Elferink, R. P. (1997) Hum. Gene Ther. 8, 27–36. [DOI] [PubMed] [Google Scholar]
- 15.Li, Q., Murphree, S. S., Willer, S. S., Bolli, R. & French, B. A. (1998) Hum. Gene Ther. 9, 497–505. [DOI] [PubMed] [Google Scholar]
- 16.Tada, K., Chowdhury, N. R., Neufeld, D., Bosma, P. J., Heard, M., Prasad, V. R. & Chowdhury, J. R. (1998) Liver Transpl. Surg. 4, 78–88. [DOI] [PubMed] [Google Scholar]
- 17.Kren, B. T., Parashar, B., Bandyopadhyay, P., Chowdhury, N. R., Chowdhury, J. R. & Steer, C. J. (1999) Proc. Natl. Acad. Sci. USA 96, 10349–10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thummala, N. R., Ghosh, S. S., Lee, S. W., Reddy, B., Davidson, A., Horwitz, M. S., Chowdhury, J. R. & Chowdhury, N. R. (2002) Gene Ther. 9, 981–990. [DOI] [PubMed] [Google Scholar]
- 19.Zhou, H., Pastore, L. & Beaudet, A. L. (2002) Methods Enzymol. 346, 177–198. [DOI] [PubMed] [Google Scholar]
- 20.Morral, N., Parks, R. J., Zhou, H., Langston, C., Schiedner, G., Quinones, J., Graham, F. L., Kochanek, S. & Beaudet, A. L. (1998) Hum. Gene Ther. 9, 2709–2716. [DOI] [PubMed] [Google Scholar]
- 21.O'Neal, W. K., Zhou, H., Morral, N., Langston, C., Parks, R. J., Graham, F. L., Kochanek, S. & Beaudet, A. L. (2000) Mol. Med. 6, 179–195. [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhardt, A. & Kay, M. A. (2002) Blood 99, 3923–3930. [DOI] [PubMed] [Google Scholar]
- 23.Kim, I. H., Jozkowicz, A., Piedra, P. A., Oka, K. & Chan, L. (2001) Proc. Natl. Acad. Sci. USA 98, 13282–13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrhardt, A., Xu, H. & Kay, M. (2003) J. Virol. 77, 7689–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short, M. K., Clouthier, D. E., Schaefer, I. M., Hammer, R. E., Magnuson, M. A. & Beale, E. G. (1992) Mol. Cell. Biol. 12, 1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donello, J. E., Loeb, J. E. & Hope, T. J. (1998) J. Virol. 72, 5085–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toietta, G., Pastore, L., Cerullo, V., Finegold, M., Beaudet, A. L. & Lee, B. (2002) Mol. Ther. 5, 204–210. [DOI] [PubMed] [Google Scholar]
- 28.Ng, P., Parks, R. J. & Graham, F. L. (2002) Methods Mol. Med. 69, 371–388. [DOI] [PubMed] [Google Scholar]
- 29.McDonagh, A. F., Lightner, D. A., Kar, A. K., Norona, W. S., Ciotti, M., Obaray, R., Martin, M. G. & Owens, I. S. (2002) Biochem. Biophys. Res. Commun. 293, 1077–1083. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto, H., Jiang, J. Y., Mitani, D. & Sato, E. (2002) J. Exp. Zool. 293, 641–648. [DOI] [PubMed] [Google Scholar]
- 31.Peters, W. H., Allebes, W. A., Jansen, P. L., Poels, L. G. & Capel, P. J. (1987) Gastroenterology 93, 162–169. [DOI] [PubMed] [Google Scholar]
- 32.Sauter, B. V., Parashar, B., Chowdhury, N. R., Kadakol, A., Ilan, Y., Singh, H., Milano, J., Strayer, D. S. & Chowdhury, J. R. (2000) Gastroenterology 119, 1348–1357. [DOI] [PubMed] [Google Scholar]
- 33.Cohen, A. N., Kapitulnik, J., Ostrow, J. D., Zenone, E. A., Cochrane, C., Celic, L. & Cheney, H. (1985) Hepatology 5, 310–316. [DOI] [PubMed] [Google Scholar]
- 34.Asonuma, K., Gilbert, J. C., Stein, J. E., Takeda, T. & Vacanti, J. P. (1992) J. Pediatr. Surg. 27, 298–301. [DOI] [PubMed] [Google Scholar]
- 35.McDonagh, A. F. & Assisi, F. (1972) J. Chem. Soc. Chem. Commun. 3, 117–118. [Google Scholar]
- 36.Brown, S. B. & King, R. F. (1978) Biochem. J. 170, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabarki, B., Khalifa, M., Yacoub, M., Tlili, K. & Essoussi, A. S. (2002) Pediatr. Neurol. 27, 234–236. [DOI] [PubMed] [Google Scholar]
- 38.Roy-Chowdhury, N., Kadakol, A., Sappal, B. S., Thummala, N. R., Ghosh, S. S., Lee, S. W. & Roy-Chowdhury, J. (2001) J. Perinatol. 21, Suppl. 1, S114–S118; discussion S125–S127. [DOI] [PubMed] [Google Scholar]
- 39.Taubes, G. (2002) Science 298, 2116–2120. [DOI] [PubMed] [Google Scholar]
- 40.Aubert, D., Menoret, S., Chiari, E., Pichard, V., Durand, S., Tesson, L., Moullier, P., Anegon, I. & Ferry, N. (2002) Mol. Ther. 5, 388–396. [DOI] [PubMed] [Google Scholar]
- 41.Seppen, J., van der Rijt, R., Looije, N., van Til, N. P., Lamers, W. H. & Oude Elferink, R. P. (2003) Mol. Ther. 8, 593–599. [DOI] [PubMed] [Google Scholar]
- 42.O'Neal, W. K., Zhou, H., Morral, N., Aguilar-Cordova, E., Pestaner, J., Langston, C., Mull, B., Wang, Y., Beaudet, A. L. & Lee, B. (1998) Hum. Gene Ther. 9, 1587–1598. [DOI] [PubMed] [Google Scholar]
- 43.Ilan, Y., Prakash, R., Davidson, A., Jona, Droguett, G., Horwitz, M. S., Chowdhury, N. R. & Chowdhury, J. R. (1997) J. Clin. Invest. 99, 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilan, Y., Droguett, G., Chowdhury, N. R., Li, Y., Sengupta, K., Thummala, N. R., Davidson, A., Chowdhury, J. R. & Horwitz, M. S. (1997) Proc. Natl. Acad. Sci. USA 94, 2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morral, N., O'Neal, W. K., Rice, K., Leland, M. M., Piedra, P. A., Aguilar-Cordova, E., Carey, K. D., Beaudet, A. L. & Langston, C. (2002) Hum. Gene Ther. 13, 143–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.