Abstract
Introduction
Human sweat is a complex biofluid of interest to diverse scientific fields. Metabolomics analysis of sweat promises to improve screening, diagnosis and self-monitoring of numerous conditions through new applications and greater personalisation of medical interventions. Before these applications can be fully developed, existing methods for the collection, handling, processing and storage of human sweat need to be revised. This review presents a cross-disciplinary overview of the origins, composition, physical characteristics and functional roles of human sweat, and explores the factors involved in standardising sweat collection for metabolomics analysis.
Methods
A literature review of human sweat analysis over the past 10 years (2006–2016) was performed to identify studies with metabolomics or similarly applicable ‘omics’ analysis. These studies were reviewed with attention to sweat induction and sampling techniques, timing of sweat collection, sweat storage conditions, laboratory derivation, processing and analytical platforms.
Results
Comparative analysis of 20 studies revealed numerous factors that can significantly impact the validity, reliability and reproducibility of sweat analysis including: anatomical site of sweat sampling, skin integrity and preparation; temperature and humidity at the sweat collection sites; timing and nature of sweat collection; metabolic quenching; transport and storage; qualitative and quantitative measurements of the skin microbiota at sweat collection sites; and individual variables such as diet, emotional state, metabolic conditions, pharmaceutical, recreational drug and supplement use.
Conclusion
Further development of standard operating protocols for human sweat collection can open the way for sweat metabolomics to significantly add to our understanding of human physiology in health and disease.
Introduction
Human sweat is a biological fluid (biofluid) that is generating increasing interest across a diverse set of fields including dermatology, paediatrics, toxicology, analytical chemistry, forensic pathology, psychiatry, illicit drug testing and infectious diseases. Currently sweat is primarily used in clinical medicine for chloride sweat testing which is used in the diagnosis of cystic fibrosis (CF). Additionally, some centres around the world use a sweat patch for monitoring drugs of abuse, while others have developed an indicator test (Neuropad) to detect peripheral neuropathy in the foot sweat of diabetics.1–3 Aside from these applications, the use of sweat in medical practice is limited in part due to challenges involved with sweat collection and the range and reproducibility of testing. This is likely to change as advances in analytical technology methods within metabolomics and other related ‘omics fields allow more complex physiological information to be derived from smaller amounts of sweat with less arduous processing. This is leading to a greater understanding of the physiology of human sweating and the skin’s excretory pathways in relation to metabolites, pathogens, and xenobiotics.4 Incorporation of Bluetooth capabilities with some of the newer wearable sweat electrolyte and metabolite detecting systems reflects even wider trends in applications to enhance personalised analysis.5–7
Each type of human biofluid or tissue sample has its own signature metabolome, but most of what is known about the human metabolome is based upon findings in the ‘serum/blood metabolome’ and the ‘urine metabolome’. Further study and standardised procedures are now required to characterise the ‘sweat metabolome’ and how it fits into the bigger picture of the human metabolome, and whether the case exists for wider application of sweat metabolomic testing.
When applying a metabolomics approach to analysing human sweat, a number of variables need to be examined within the context of the origins, composition, physical characteristics and functional roles of sweat. These variables include: sweat induction and sampling techniques, timing of sweat collections, sweat storage conditions, and laboratory aspects such as metabolic quenching, extraction, concentration, fractionation, separation and other processing methods applicable to sweat. Exploring these variables within the framework of newer laboratory analytical platforms that optimise qualitative and quantitative detection of sweat metabolites will pave the way forward to make more rigorous and meaningful comparisons of sweat metabolomics studies.8 Standardising the collection, handling, processing and storage of sweat for further metabolomics analysis is vital to this endeavour and working out the further steps necessary to achieve this standardisation is the focus of this review.
Background – Metabolomics
Metabolomics is the multidisciplinary science involving the measurement and analysis of low molecular weight metabolites such as electrolytes, sugars, lipids and other compounds that exist in a selected biofluid, cell, tissue or organism under a given set of physiological conditions. It has its roots in the works of many biochemists who pioneered the discovery and detection of various vitamins in the 1940s and progressed the concepts of ‘metabolic variance’ and ‘biochemical individuality’.9–12
The exact number of unique metabolites in the human metabolome has yet to be firmly established, but it is generally thought that there is a lower number of metabolites in the human metabolome (>3,500) compared with the total number of genes (>30,000), RNAs/transcription factors (>30,000) and proteins (>100,000).13 Small changes in the transcriptome may translate into more amplified changes in metabolites.14 With presumed fewer total metabolites to analyse and a potentially more amplified signal to be detected, the power and potential of metabolomics to pick up minute but significant health-related changes holds promise.
As with all newly emerging fields, within metabolomics there is multiplicity and various expansions of terminology. Although metabolomics and metabonomics are often used interchangeably in the literature, metabonomics technically refers to the study of the interactions of metabolites over a timeframe in a complex system.15 Fluxomics refers to an extension of metabolomics, in which metabolomics is applied at various experimental time points generating kinetic data which can then be used to study metabolic pathway fluxes.16 Exposomics, another extension of metabolomics, refers to identifying metabolites linked to environmental risk factors for disease.17 Metabolites can be classified into two categories: endogenous metabolites (synthesised and utilised within a biological system) and exogenous metabolites (imported from outside the biological system into the cell, such as drugs, xenobiotics and nutrients).13,16 The Human Metabolome Project (HMP) led by Dr David Wishart of the University of Alberta in Canada published a first draft of the human metabolome in 2007 which consisted of 2180 metabolites, 1200 drugs and 3500 food components.18 A growing list of findings additional to the HMP is being compiled and verified on the Human Metabolome Database – a freely accessible and continually updated web resource (http://www.hmdb.ca/).11,13,19 Not all known human metabolites can be found in any given biofluid because different biofluids serve different functions and play different metabolic roles. As of November 2016, the HMP had identified and/or quantified over 3848 metabolites: 440 metabolites in cerebrospinal fluid, 1233 metabolites in saliva, 2287 metabolites in blood, 1746 metabolites in urine, 695 metabolites is faeces and over 172 metabolites in other tissues and biofluids including sweat.19
The methodology of metabolomics can be divided into different conceptual approaches such as targeted analysis, global metabolite profiling, metabolomics and metabolic fingerprinting/metabolic footprinting.20 A targeted metabolomics approach involves a targeted search and quantitative analysis of a set number of known metabolites or substances that play a particular role, much like a typical clinical laboratory test. Global metabolite profiling is untargeted and comprises an analysis of all measured metabolites or substances, including those known and unknown, which make up a metabolic profile of the total complement of metabolites in a particular sample.13,20,21 Metabolomics utilises complementary analytical methodologies such as liquid chromatography-dual mass spectrometry (LC-MS/MS), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR) spectroscopy in a coordinated attempt at global metabolite profiling.20 Metabolic fingerprinting refers to the metabolic ‘signature’ or mass profile of the biofluid or tissue sample of interest which is then compared in a large sample population to screen for differences between samples. When signals or significant differences can be detected, the metabolites are then identified and the biological relevance of these particular metabolites can be more easily elucidated. Metabolic footprinting is analogous to metabolic fingerprinting except the differences detected involve focus on extracellular metabolites.15
Sweat Origins, Components and Functions
Sweat Definitions
Whole body sweat is a complex mixture of cumulative secretions from millions (1.6–5 million) of eccrine, apocrine, apoeccrine and sebaceous glands as well as bacteria, yeast, fungi, other microbiota and cellular debris that reside in and on the largest organ of the human body – the skin.22 These microscopic glands dwell largely in the dermis and hypodermis layers with secretory canals through which sweat flows onto the skin surface and into hair follicles. Defining sweat precisely is complicated by confusing nomenclature across different disciplines in the scientific literature and a lack of a biological systems-based approach to studying sweat. Sweat collected from the skin surface in experimental studies, especially older studies, is often referred to as ‘eccrine’ sweat because eccrine sweat glands are the most numerous and ubiquitous glands in the skin, however many sweat samples also contain potentially trace amounts of apocrine, apoeccrine and sebaceous gland secretions (called sebum), depending upon the body site of sampling. This imprecision of nomenclature is the case in some toxicology literature dealing with sweat patch testing of illicit drugs and physiology literature studying electrolyte changes in exercise. However, in the dermatology and cosmetic literature, ‘sebum’ can figure in addition to ‘sweat’ with more emphasis on the underlying structures within the skin. The converse can also be true with studies focused on collection of sebum. The term ‘residual skin surface components’ (RSSC) is another synonym of ‘sweat’ as it comprises potential sweat glandular secretions and cellular debris (from stratum corneum – outermost epidermal skin layer).23
Mindful of the semantics surrounding sweat, it is useful to revisit the anatomy, histology and secretions of the four known gland types that can contribute to sweat. This sets the stage for better understanding and targeting of future studies to fully characterise the sweat metabolome.
Eccrine Sweat Glands and Secretions
Eccrine sweat glands exist at birth and can be located all over the body’s skin except on lips, on the nail bed and on some fields of the genitalia (e.g. glans penis). They can average 100–200/cm2 body surface area, with higher densities (600– 700/cm2) on palms and soles, and at luminal diameters of 20–60 μm at skin openings.24,25 Eccrine glands consist of single tubules ranging 4–8 mm in length that are generally divided into 3 parts: (i) deep, coiled secretory portion in deep dermis layers; (ii) upper dermal portion with straight and coiled parts; and (iii) intra-epidermal part often referred to as the acrosyringium. The dermal portion, or dermal duct, has epithelial cells connected at numerous sites by desmosomes and intercellular junctions that are believed to constitute a barrier between the luminal and extracellular compartments. The inner luminal cells contain various tonofilaments while the outer basal cells are surrounded by collagenous and fibrocyte-rich sheathes.22,26
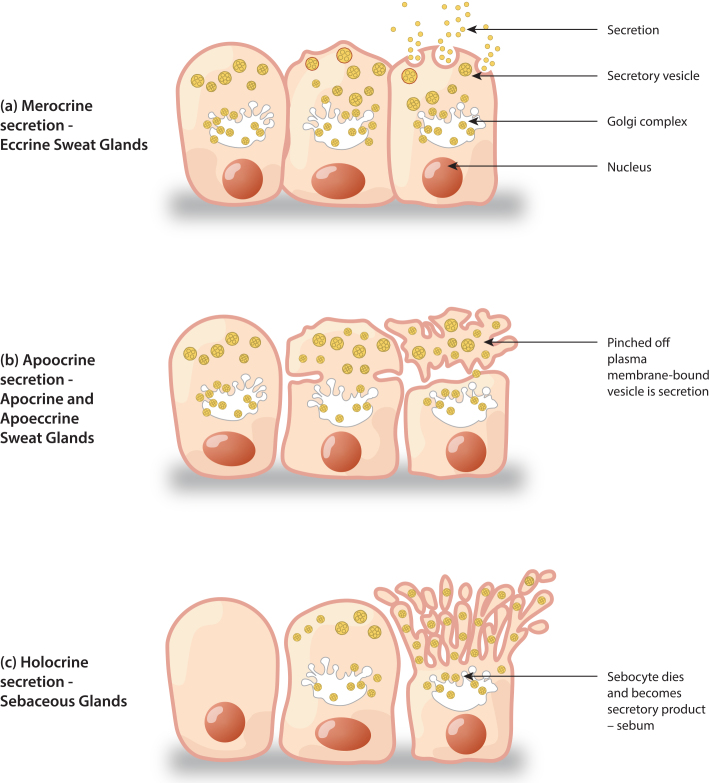
Eccrine sweat glands are classed as merocrine glands (Figure 1). Eccrine sweat gland secretions are released from cells as an aqueous fluid, without disintegration of cells, containing various electrolytes, elements, ions, amino acids, proteins and other known and unknown small molecules as outlined in Figure 2.27,4 Composition varies with many factors: rate of sweat production, transit time through the excretory duct, aldosterone activity, physical training, psychological states and acclimatisation to environmental temperatures.26 These give hints to underlying functions that have not yet been fully determined. There also exists debate whether secretory eccrine sweat is perhaps an isotonic ultrafiltrate of plasma since sweat contains many of the same solutes found in plasma, but at much lower concentrations.28 However, based on a recent proteomics study of pooled sweat samples collected from schizophrenic and control subjects, only 6 of 185 unique proteins identified in sweat were reported in serum. The authors therefore argue that sweat is not merely a plasma transudate and future metabolomics studies are required to shed more light on this topic.29
Figure 1.
Sweat gland secretion patterns.
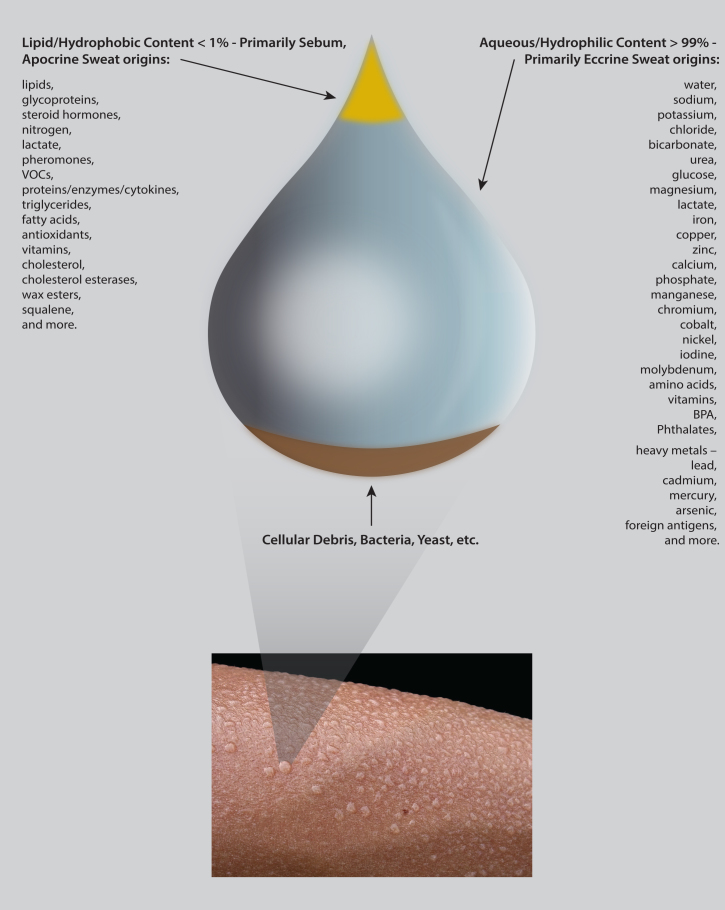
Figure 2.
Metabolomic sweat content.
Apocrine and Apoeccrine Sweat Glands and Secretions
Apocrine sweat glands also exist at birth but do not become active until the androgenic stimulation of puberty.26 They are confined to hairy body areas (i.e. axilla, mammary areola, peri-umbilicus, perineal and genital areas) since they open and secrete into adjacent hair ducts (e.g. apopilosebaceous ducts) before secretions reach the skin surface. They are generally larger than eccrine sweat glands with apocrine coil diameters of ∼800 µm compared to eccrine coil diameters of ∼500 µm, both located in the dermis and hypodermis.22 Apocrine ducts are relatively short and found in close proximity to hair follicles. The density of apocrine glands is highly variable with reports of 8–43/cm2 body surface area in one study of the axilla.30 Two different types of cells are visualised in apocrine glands: columnar secretory cells and myoepithelial cells. The secretory cells are generally noted to be full of mitochondria and different granules with convoluted cell membranes and microvilli presenting towards the lumen.22
Apoeccrine sweat glands are a mixed type gland as the name suggests and were first described in 1987 by Sato et al.30 They are also presumed to develop during puberty and be restricted to hairy body areas. As many as 50% of all axillary sweat glands are thought to be apoeccrine. Component cells of apoeccrine glands include eccrine secretory cells, apocrine secretory cells and myoepithelial cells.30 Identification of these morphologically distinct glands can be made with specific protein markers (i.e. phalloidin, S-100, CD15).22
Apocrine and apoeccrine glands are both classed as apocrine glands. With apocrine glands, secretion occurs via pinching-off of the cell’s plasma membrane producing membrane-bound vesicles, which helps to account for comparatively more viscous secretions.22,26 Sato et al. determined that Na+ and K+ concentrations obtained from the isolated ducts of the apoeccrine glands are curiously more similar to that of eccrine sweat compared to apocrine sweat.30 While the composition of apoeccrine sweat has not yet been fully elucidated, apocrine sweat has been demonstrated to contain carrier proteins for volatile odour molecules like volatile organic compounds (VOCs) and pheromones with amino acid conjugates produced by bacterial enzymes.22 Apocrine bromhidrosis (more commonly known as BO or body odour) is thought to be linked to large amounts (i.e. over 106 bacteria/cm2) of resident microflora such as aerobic cocci, diphtheroid species, Corynebacterium species and Staphylococcus epidermidis.26,31
Sebaceous Glands and Sebum
Sebaceous glands are located in the skin of all surface areas except for the palms of hands and soles of feet. They are particularly numerous on the forehead, scalp, midline back, chest, perineum and surrounding the orifices of the human body. Densities of up to 400–900 glands/ cm2 occur on the face, especially in the T-zone area of the face which starts from the midpoint and sides of the forehead, and extends downward toward the middle of the nose, including the sides of the nose and the midline part of chin.32 Sebaceous gland density decreases towards the extremities of the body. Sebaceous glands can be divided into two types: pilosebaceous glands, when associated with hair follicles, and free sebaceous glands seen mostly at transitional zones between skin and mucous membranes. A well-known example of a free sebaceous gland is the Meibomian gland of the eyelid. All sebaceous glands consist of single or multiple lobules, or acini, with ducts emptying into a main sebaceous duct. Secretory lobules contain sebaceous gland cells, or sebocytes, that are excreted in their entirety as part of the holocrine gland status. Maturing sebocytes have been visualised to accumulate high lipid content as they migrate from periphery to gland duct.33
Like apocrine sweat, sebum is thought to play a role in the generation of pheromones and body odour in its interactions with skin-residing bacteria and yeast of the microbiome.34 Sebum, however, is even more lipid-based, containing triglycerides and fatty acids (together 57% of contents) as well as cholesterol, wax esters, squalene, keratin, cellular debris, anti-microbial lipids, antioxidants, coenzyme Q10, vitamin E and other various metabolites of fat-producing cells.34,35 Interestingly, the lipids in sebum would seem to originate from both sebocytes and keratinocytes, with studies identifying differences based upon cholesterol and squalene conversion enzymes.34
Collected Sweat
Setting aside the above distinctions in gland origins, the vast majority of sweat studies in the literature have analysed a collective form of sweat with eccrine gland secretions predominating. Depending upon location of sweat sampling and various cleaning and collection strategies used during sampling or processing, trace amounts of sebum and/or apocrine and apoeccrine gland secretions, cellular matter from the epidermis and associated ∼1012 skin microbes, as well as other metabolites like xenobiotics may feature in sweat samples.36 The systems-based approach of analysing sweat with metabolomics offers the prospect of uniting all these different subcomponents of sweat. With such a metabolomics approach, studies of ‘normal’ sweat obtained from ‘healthy’ people have detected highly variable metabolite compositions with large numbers of different small molecules, of both microbial and human origin, in a primarily water-based (∼99%), relatively acidic (mean pH 6.3) solution (Figure 2).37–40 This rich complexity of sweat content hints at its functions both at the level of the skin and at the level of the organism as a whole.
Functions of Sweat
Temperature and Fluid Homeostasis
Sweat is integral to the regulation of body core temperature by water evaporative heat dissipation. Blood flow regulation and vasodilation of superficial blood vessels largely contribute to this homeothermic control and the finding that eccrine sweat production is under the control of cholinergic and, to a lesser extent, adrenergic innervation is consistent with this hypothesis.41 Various stimuli of this system include temperature, emotions, intellectual stimulation and gustatory stimulation.42 Sweat volumes vary widely as a result. Global insensible fluid losses can be approximately 1000 ml daily for the whole human body, including more than half of fluid losses through the skin via perspiration with the remaining losses being through the lungs.4,40 However, there are reports of individuals perspiring up to several litres per hour, 12 L per day under certain extreme physiological conditions.4,35,43
Eccrine sweat activity appears intermittent over a large portion of the body: cycles of periodic discharges alternating with pauses occurring from <1 to 12 geyser-like emissions per hour with single sweat gland emissions recorded every 3.3 min in one recent study.41 This activity differs among individuals, environmental circumstances and body sites with approximately 50% of total body volume of sweat being thought to be produced by the trunk, 25% by the legs and 25% by the head and upper extremities.1,48 Even in cases of profuse sweating, it is thought that only approximately 50% of sweat pores release sweat at any given time, except for the palmoplantar regions where the sweat gland activity is largely synchronised.44 How these findings fit within sweat’s overall functions in the human body is still unclear.
In contrast to eccrine glands, apocrine gland activity is reported to be more continuous in its fluid secretions, while still receiving predominantly cholinergic and some adrenergic innervation.22,42 Apoeccrine gland stimulation by physiological and pharmacological stimuli appears to be distinct from those controlling eccrine or apocrine glands. Apoeccrine glands respond quickly to psychological stress and are thought to more significantly contribute to the abundant sweat produced in the axilla.25,30
Sweat Electrolyte Regulation
Another important component of sweat that impacts human fluid balances is sodium. The concentrations of Na+ in sweat can be highly variable, ∼20–100 mmol/L, and some individuals can lose an estimated 4–6 g of Na+ per day, equivalent to 12–15 g of NaCl daily through sweating, especially if working in moderately hot conditions.43 Eccrine gland duct cells reabsorb several ions, including Na+ and Cl−, via a number of known anion exchangers such as Na+/K+-ATPases (on basolateral membranes), cystic fibrosis transmembrane conductance regulators (CFTRs, mutations of which provide the basis for Cystic Fibrosis Sweat Chloride testing), carbonic anhydrases II, and vacuolar proton pumps (V-H+-ATPase).22 Sweat Na+ and Cl− concentrations have been documented to increase with age to 12–19 years then stabilise thereafter.45,46 Sweat Na+, Cl−, and K+ concentrations also have reported body regional variations.47
Acid-base homeostatic mechanisms are presumed to be involved in sweating since sweat is more acidic than plasma, with pH ranges of 4–6.8 reported in various studies.1,4 It is noted that with increased flow rates following exercise or at temperatures above 31 °C, sweat pH increases to upper limits of approximately 6.8, which is still more acidic than plasma.47 Some non-ionised basic drugs diffuse into sweat and become ionised as a result of the lower pH of sweat, although the exact mechanisms have not been fully elucidated. This has led to projections of these non-ionised basic drugs displaying free-drug (or molecule) sweat-to-plasma (S/P) ratios of >1, as in the case of ammonia with reported S/P ratios of 20–50.48,49
Skin Protection
Sweat also provides lubricating, water-proofing, antimicrobial and skin barrier-promoting properties that support skin in the first line of defence against many environmental insults. In extreme hot conditions, the lipid-rich secretions of apocrine and sebaceous glands can emulsify sweat produced by eccrine glands to create a hydrolipid film that is not as readily evaporated. This is thought to be of importance in delaying dehydration. In colder conditions, the lipid nature of sweat becomes more solid and, in coating the hair and skin, sources of unwanted moisture like rain or snow can theoretically be more effectively repelled.50 Palmar hydration, which is directly linked to eccrine sweat production, increases the skin friction coefficient which therefore improves the adherence of hands to objects and contributes to a heightened sense of touch.41 Sweat also contains antimicrobial peptides (AMPs) like dermicidin, lactoferrin, and LL-37, an AMP of the cathelicidin family, which serve to control certain pathogenic bacterial counts on the skin surface.4,41 However, the precise qualitative and quantitative content of skin microbiota and associated microbe-microbe and microbe-host dynamics via sweat are areas of active research with early findings hinting at rich metabolic inter-relationships with impacts on skin integrity, especially in skin inflammatory states.36
The free amino acid composition of sweat is curiously different from other biofluids. Data from a recent study suggest the amino acid content of sweat is remarkably similar to the amino acid content of an epidermal protein, profilaggrin. Since profilaggrin is thought to be the key contributor of free amino acids making up the natural moisturising factor within the stratum corneum, it is postulated that sweat plays a role via interactions with profilaggrin in maintaining the barrier integrity of human skin.51
Immune System
Sweat has links to many immune-mediated mechanisms. Skin epithelial cells interact with various external stimuli to produce cytokines, and sweat directly activates epidermal keratinocytes to produce various cytokines using in vitro models with cultured human keratinocytes from surgically discarded neonatal skin samples.39 It is postulated that sweat may play both beneficial and pathological roles in immune-mediated communications. For example, sweat is well-recognised in exacerbating atopic dermatitis (AD) lesions and is associated with increased itching (pruritus) which has associations with enhanced expression of IL-31 (newer member of IL-6 family of cytokines) in tissue samples of exacerbated AD lesions.52 Sweat also contains cystatin A, a proteinase inhibitor of bacterial cysteine proteases. Given these exogenous proteases are known to break down the epidermal barrier, cystatin A in sweat may serve both immune and skin protective roles.53 Quantitative levels of IL-1α, IL-1β, IL-6, TNF-α, IL-8 and TGF-β have been measured in human sweat although the precise cellular origin of these cytokines is still unknown and could be derived from sweat, blood or epidermal cells.54
Excretion Functions and Drug Delivery Mechanisms
While the excretory function of sweat has previously been considered negligible compared to the kidney, recent studies challenge this notion. There is evidence that several toxic elements and xenobiotics may be preferentially excreted through human sweat.55–58 Some studies report arsenic, cadmium, lead and mercury being excreted in appreciable quantities via sweat, with the rates of excretion matching or exceeding urinary excretion.58 Furthermore, while excess dietary nicotinamide cannot be eliminated through urine because of its reabsorption by the renal tubules, it can be effectively excreted by sweat glands.35 Many pharmaceutical drugs are also excreted via sweat and the role of sweat patch technology in monitoring illicit drug use is based on dozens of studies examining the pharmacodynamics and pharmacokinetics of amphetamines, cocaine, cannabis, opiates and associated metabolites excreted in sweat.1,48 Drug binding to various skin fractions and reabsorption of drugs from pooled sweat on skin has also been observed. The relative concentration of unmetabolised drugs is reported to be occasionally higher in sweat than in blood, urine or saliva.59,60
The above findings suggest that molecules of drugs/metabolites/xenobiotics may reach the skin surface from blood by various proposed routes: via sweat or sebum by active or passive inter- and/or transcellular mechanisms; and transdermal migration through lipid bilayers of stratum corneum.40 The second mechanism could be linked to the concentration gradient in which only the free fraction of drug/metabolite/xenobiotic, unbound to proteins, diffuses through lipid membranes from plasma to sweat. Thus, it seems that the physical nature (i.e. molecular mass, protein-binding, pKa and lipophilicity) of each particular drug/metabolite/xenobiotic plays a role in how much ends up in sweat. In fact, dermatologists routinely take advantage of this scenario when treating cutaneous fungal infections with oral antifungal medications such as ketoconazole, terbinafine or fluconazole. It is often recommended to exercise to induce sweating while taking these oral antifungals since the drugs are transported to the skin surface by eccrine sweat and/or sebum and then often reabsorbed, thus optimising drug delivery to site of infection.61–63
Metabolic and Infectious Diseases
The alteration of sweat with different pathological conditions makes sweating a useful clinical indicator for various conditions. Over-sweating (hyperhidrosis) and under-sweating (hypohidrosis), whether regional or systemic, may represent warning signs for systemic conditions or diseases. Decreased sweat production involving the feet is the basis for the Neuropad indicator test for diabetic peripheral neuropathy.3 Impaired overall sweating is associated with preeclampsia and thought to be related to decreased clearance of plasma vasoactive amines.64 Female menopause is commonly associated with ‘hot flushes’ linked to increased sweat production.65
Night sweats can indicate serious systemic infections (e.g. tuberculosis) and malignancy, while local hyperhidrosis around a bite site can indicate toxic envenomations such as occurs with Australian redback spider bites.66 Hypoglycaemia, hyperthyroidism, hypercapnia and vagus nerve stimulation can all lead to stimulation of eccrine sweat production and alterations in local sweating may arise directly from certain skin conditions.26 For example, some hyperkeratotic disorders such as pityriasis versicolor and psoriasis interfere with the excretion of sweat and are associated with decreased sweat output as visualised with skin capacitance imaging of lesions.44 Abnormalities in the transport of sweat onto the skin’s surface may also cause a severe prickly sensation and skin inflammation resulting in the intra-epidermal retention of sweat, such as occurs with miliaria rubra which has been linked to elevated levels of IL-1 and IL-31detected in sweat.39
Therapeutic and Wellness Functions of Sweat
It is hypothesised that sweat produced by different activities may differ in composition. For example, IL-1 concentrations are increased in sweat induced by both exercise and sauna bathing,39 yet exercise is linked to increases in the generation of several end-metabolites like reactive oxygenated species that are in turn linked to oxidative stresses. This is thought not to be the case with sauna-induced sweat although this remains to be validated by further studies.35
Lipid Homeostasis
Sebum production changes have been linked to diet. Caloric deprivation in the setting of obesity decreases sebum production while a high fat diet in the setting of psoriasis increases it.67,68 Increases in energy intake have been associated with increased excretion of triglycerides, cholesterol and associated esters in sebum.35 As newer studies in sweat and skin surface lipidomics are being done, more definitive information regarding these links and potential mechanisms of action are likely to emerge.69
Methods
Pubmed, Medline, Google Scholar, Embase, Science Direct, Scopus, Ovid, Web of Science, Proquest, Toxline and UpToDate databases were initially searched with keywords ‘sweat’ and ‘metabolomics’ with restrictions of English language and of dates 2006–2016. These records were then supplemented with searches for other research by key authors, searches of citations and reference lists of key papers, and additional searches with expanded keywords relating to sweat including perspiration, sauna, exercise, secretion and/or excretion from human skin and residual skin surface components as well as expanded keywords relating to metabolomics including exposomics, xenometabolomics, toxicometabolomics and fluxomics. Older studies of sweat (before 2006) have been used in compiling background information, but not for the detailed analysis of sweat collection methods.
Of the 1320 records identified for review as of 1 June 2016, all 17 studies presenting quantitative human data utilising a sweat metabolomics methodology of analysis between the dates 2006–2016 were included, regardless of quality of experimental design. An additional three sweat proteomics-based studies were identified that utilised similar laboratory platforms relevant to metabolomics and were also included in the comparison analysis.
Results
The Table presents a summary of the pertinent information regarding sweat induction and collection methods extracted from the 20 identified studies for comparison.
Table.
Sweat induction and collection methods for metabolomics.*
| Study | Aims | n | Sweat Induction Mode | Methods | Timing | Amount | Storage | Sweat Preparation Protocols | Analytical Chemistry Platforms |
|---|---|---|---|---|---|---|---|---|---|
| Adewole et al., 201672 | Identify diagnostic biomarkers of active tuberculosis in eccrine sweat | 83 | Webster Sweat Inducer – pilocarpine iontophoresis × 5 min | Macroduct® Sweat Collector – part of Macroduct® Sweat Analysis System – covers volar forearm × 15–35 min; sweat transferred to micro-centrifuge tube | 5 min induction + 15–35 min collection | ∼10–30 µL | Samples placed on dry ice immediately, stored at −70°C until analysed | Solubilised, reduced, alkylated, and digested with protease; then dried, desalted, dried again, then resuspended in ACN, formic acid | LC – MS/MS, untargeted proteomics; FT mode for MS detection, ion trap mode for MS/MS detection |
| Jia et al., 201694 | Assess feasibility of using HPLC-MS/MS for accurate quantifying of cortisol in human eccrine sweat | 4 | Hot room set at 41°C and ∼55% humidity | Leg skin cleansed with alcohol pads, followed by dH2O and drying; sweat collected off skin into Eppendorf LoBind micro-centrifuge tubes | 25–30 min collection times | >200 µL | Samples placed on dry ice immediately, stored at −80°C until analysed | Sample mixed with ACN/ammonium acetate; addition of internal standard (in ACN); ethyl acetate extraction repeated twice, evaporated to dryness, re-constituted in ACN | HPLC-MS/MS, SRM mode, targeted |
| Sheng et al., 201693 | Monitor elimination of bioaccumulated heavy metals in humans with exercise | 17 | Exercise; no specific instruction as to type or location | Direct collection of sweat from any part of the body into glass bottle with cover; then transferred into 50 mL glass vials with lid. Referenced methods from Genuis et al. 2011 utilised | Same day as urine sample collection | >20 mL | Stored at −20°C until analysis | Samples dried in oven for standardised weight, ashed in furnace, cooled in dryer; residue reconstituted in HNO3 with heat | Flame atomic absorption spectrophotometry, targeted |
| Tang et al., 201695 | Compare levels of 5 heavy metals (Cr, Cu, Zn, Cd, Pb) in human sweat and urine after physical exercise | 9 | Exercise; playing badminton × 2 h | Upper bodies cleansed with ultrapure H2O before exercise; sweat scraped into polyethylene sample bottles. Samples allowed to stand for 30 min, then filtered using 9-mm filter paper into test tube | ∼2 h | >20 mL | Stored at 4°C until testing | 3 methods:
|
ICP-MS, targeted |
| Delgado-Povedano, Calderon-Santiago et al., 201671 | Develop and validate a method for metabolomic analysis of human sweat using GC-TOF/MS | 6 | Webster Sweat Inducer – Pilogel® Iontophoretic discs; 1.5 mA electric current × 5 min | Macroduct® Sweat Collector – part of Macroduct® Sweat-Analysis System – covers forearm skin × 15 min; sample transferred into micro-Eppendorf tube | 5 min induction + 15 min collection | >70 µL each participant – pooled into one sample | Frozen at −80°C | Pooled sweat into each of 3 protocols:
Each followed with methoxymation + silylation |
GC-TOF/MS, full scan mode, untargeted |
| Calderon-Santiago et al., 201538 | Identify metabolic markers of lung cancer in sweat to develop screening tool for diagnosis of lung cancer | 96 | Webster Sweat Inducer – Pilogel® Iontophoretic discs; 1.5 mA electric current × 5 min | Macroduct® Sweat Collector – part of Macroduct® Sweat Analysis System – covers forearm skin × 15 min; sample transferred into micro-Eppendorf tube | 5 min induction + 15 min collection | >10 µL | Frozen at −80°C until analysed | Diluted with formic acid and vortexed | LC-QTOF MS/MS, untargeted |
| Porucznik et al., 201589 | Targeted detection of BPA in sweat in comparison to urine for biomonitoring | 50 | Passive sampling – no artificial modes of sweat induction | Sweat patches (PharmChek®) applied after skin cleansed with alcohol wipes, to either upper-outer arm or front/back midriff | 7 days | Not specified | Sweat patches stored and transported in sterile, BPA- free 4-oz polypropylene sample cups; no temperature specified | Sweat patches extracted with methanol; evaporated in Turbovap®; reconstituted with ammonium bicarbonate: ACN (mobile phase) | UHPLC-MS-MS, targeted; using methods initially designed for urine samples |
| Dutkiewicz et al., 201459 | Untargeted metabolomics profiling of human sweat to evaluate hydrogel micropatch collection linked with direct mass spectrometry | 9 | Passive sampling – in room temperature, ∼25°C, 45% relative humidity | Skin pre-wiped with cellulose tissue soaked with isopropanol: H2O; fabricated agarose hydrogel micropatch embedded with PTFE probe attached to forearm area with adhesive bandage tape | 1 min–1 h | ‘single droplet’ – unable to estimate volume of sweat sample accurately | Hydrogel micropatch probe covered with glass slide, stored at 4°C | Direct coupling of hydrogel micropatch probe to nanospray desorption electrospray ionisation mass spectrometer | ESI + IT + FT-ICR-MS |
| Calderon-Santiago et al., 201437 | Untargeted global metabolomics profiling of human sweat to optimise laboratory methods and chemometrics | 96 | Webster Sweat Inducer – Pilogel® Iontophoretic discs; 1.5mA electric current × 5 min | Macroduct® Sweat Collector – part of Macroduct® Sweat Analysis System – covers forearm skin × 15 min; sample transferred into micro-Eppendorf tube | 5 min induction + 15 min collection | >5μL | Frozen at −80°C | Pooled samples diluted with formic acid:H2O with additional protocols:
|
LC-QTOF MS/MS, untargeted |
| Shetage et al., 201423 | Identify collection methods for RSSC and evaluate effects of ethnicity, gender and age on amount and composition | 315 | Passive sampling at room temperature: 18–25°C, 50– 60% relative humidity | Forehead pre-wiped with cotton soaked in diethyl ether, allowed to dry. Cigarette paper applied, held in place with elastic headband, in duplicate × 1 h, fresh cigarette paper replaced every hour for total 3 h | 3 h | Totals not specified: peak amounts 0.11–0.12 +/− 0.06–0.07 mg/ cm2 of RSSC collected in first hour | None specified | Cigarette papers dehydrated × 2 h; extracted with hexane; extract filtered through 0.2 micron PTFE membrane, concentrated by purging nitrogen | GC/MS, untargeted |
| Mark et al., 201351 | Detailed amino acid analysis of sweat to better understand key biological mechanisms governing its composition | 12 | Hot room; 40°C, 60% relative humidity × 15–40 min | Sweat droplets removed from axilla with positive displacement pipette using polypropylene tips; sample transferred directly into ‘low binding’ Eppendorf tube kept at 4°C | ∼20 min | >500 μL | Frozen at −70°C | Two methods:
|
Targeted amino acid analysis; automated amino acid analyser + GC-TOF/MS, targeted |
| Raiszadeh et al., 201229 | Untargeted and targeted analysis of healthy control and schizophrenic patient sweat, to identify candidate biomarkers of disease | 78 | Webster Sweat Inducer – pilocarpine iontophoresis applied to volar forearm | Macroduct™ Sweat Collector – Macroduct ™ Sweat Stimulation and Sweat Collection System (Elitech/WESCOR, Inc., Logan, UT, USA); sample transferred into micro-centrifuge tubes | 30 min | 50–60 μL | Stored on dry ice | Pooled samples:
|
LC-MS/MS; LC-MS/MS + spectral counting; MRM-MS verification |
| Genuis et al., 201257 | Targeted profiling of phthalate compounds in blood, sweat and urine | 20 | Self-determined by participants – infrared sauna, steam sauna, exercise | Direct collection from any body site into 500 mL glass jar using stainless steel spatula; participant-delivered to commercial laboratory; transferred to 4 mL glass jars at laboratory | No time parameters around sweat collection except conditional within 1 week of blood collection (before/after) | 100 mL | Stored at −20°C; shipped frozen on dry ice from Canada to Sweden for analysis | Not specified | HPLC/MS, targeted; GC/MS, targeted |
| Genuis et al., 201255 | Targeted profiling of BPA in blood, sweat and urine | LC-MS-MS, targeted | |||||||
| Genuis et al., 201156 | Targeted profiling of 120 compounds (toxicants) in blood, sweat and urine | ICP-MS, targeted | |||||||
| Lee et al., 201191 | Untargeted metabolomics analysis to determine biochemical composition of exercise sweat | 48 | Exercise on ergometer for 60 min | Collection with skin patch placed on the lower back | Sweat patches removed at 3 time-points: 10–20min, 30–40min, 50–60min of exercise; placed on dry ice | Not specified | Frozen at −80°C until analysis. | Not specified | GC/MS and LC/MS/MS, untargeted |
| Kutyshenko et al., 201110 | Untargeted metabolomics analysis to determine biochemical composition of human sweat | 10 | Natural environmental heat | Direct collection from forehead, upper chest, upper/lower back, arms using glass pipette or glass roller, rolled in tray with dH2 O and/or sterile spray gun filled with D2O sprayed | 3–5 min collection + 7–10 min sample preparation | >0.56 mL | Sample storage not specified; analysis performed 10–15 min after sweat collection | Diluted with D2O, centrifuged, transferred to standard NMR tube | 1H NMR Spectroscopy – high resolution, both one dimensional and two dimensional, untargeted |
| Michael-Jubeli et al., 201169 | Develop simple analytical protocol for qualitative characterisation of individual SSLs and quantitative evaluation of lipid classes | 1 | Passive sampling | Lipid-free absorbent papers placed on 6 areas
|
30 min collection | Not specified | Storage of unprocessed samples not specified | Extracted with diethyl ether twice, concentrated with rotary evaporation, transferred into 2 mL vials, dried under nitrogen stream; dried extract stored at −20°C until analysis; extracts derivatised/trimethyl-silylated; rotary evaporated, residue dissolved in isooctane | HTGC-MS, with electron impact and chemical ionisation |
| Penn et al., 200792 | Test the validity of individual odour hypothesis by analysing VOCs in sweat, urine and saliva | 197 | Passive sampling | Axillary sweat sampled with devised twister PDMS-coated stir bars, held by special rollers, placed directly on skin; samples transferred to glass vials | Once each fortnight sampling over 10-week period; unspecified sweat collection timing | Not specified | Stored at ∼4°C; shipped in cooler each week from Austria to USA for analysis | Samples directly analysed with SBSE in connection with thermal desorption GC-MS | SBSE with thermal desorption GC-MS |
| Harker et al., 200628 | Untargeted metabolomics analysis of human eccrine sweat | 60 | Hot room at 43.3°C, 65% relative humidity × 15–40 min | Underarm area wiped, then sweat collected with plastic-tipped pipette, sample transferred into sealed glass vials | 15 min collection | >50µL | Frozen at −20°C until analysis | Samples diluted and deuterated phosphate buffer (pH 7.4, 0.1M); transferred into 5 mm OK NMR tubes | 1NMR Spectroscopy – high resolution, one dimensional, untargeted |
BPA – Bisphenol A; PTFE – polytetra-fluoro-ethylene; PDMS – polydimethyl-siloxane; RSCC – residual skin surface components; SSLs – surface skin lipids; VOCs – volatile organic compounds; ACN – acetonitrile; SBSE – stir bar sorptive extraction; LC-MS/MS – Liquid Chromatography-Tandem Mass Spectrometry; HPLC-MS/MS – High Performance Liquid Chromatography-Tandem Mass Spectrometry; SRM – selected reaction monitoring; ICP-MS – Inductively Coupled Plasma mass Spectrometry; GC-TOF/MS – Gas Chromatography-Time of Flight/ Mass Spectrometry; LC-QTOF MS/MS – Liquid Chromatography-Quadripole Time Of Flight-Tandem Mass Spectrometry; ESI – Electrospray Ionisation; IT – Ion Trap; FT-ICR-MS – Fourier Transform Ion Cyclotron Resonance mass spectrometry; MRM-MS – Multiple Reaction Monitoring-Mass Spectrometry; UHPLC-MS-MS – Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry; GC / MS – Gas Chromatography / Mass Spectrometry; HTGC-MS – High Temperature Gas Chromatography-Mass Spectrometry; 1H NMR – Proton (Hydrogen -1 nuclei) Nuclear Magnetic Resonance Spectroscopy; ICP-MS – Inductively Coupled Plasma Mass Spectrometry; PTFE – polytetrafluoroethylene; dH20 – distilled water; -D2O – deuterated, heavy water
See Appendix (online supplement) for an expanded version of this table including more detailed information of sweat preparation protocols, chemometrics, databases and key findings pertaining to studies.
Discussion
Sweat Induction Protocols
Induction of perspiration represents a phenomenon involving a complex chain of metabolic reactions, with many possible triggers, as already discussed. Exercise, temperature, stress, psychological state, relative humidity, hormonal and sympathetic/parasympathetic nervous system parameters, diet, skin colonisation factors, xenobiotics exposure – both purposeful and non-purposeful – can influence sweat volumes and content.40 Refer to the fourth column in the Table describing sweat induction modes utilised in the reviewed studies. A number of important factors are apparent when obtaining sweat for metabolomics analysis: (i) ensuring adequate amounts of sweat are available to complete the analysis, including enough volume for controls and potential further analysis; (ii) ensuring the mode of sweat induction does not interfere with the utility of the results; and (iii) ensuring that sweat induction and sweat collection happen in a timely manner that optimises metabolic quenching and metabolite stability.8,70
Pilocarpine Iontophoresis
Several active research groups rely on a chemical pilocarpine iontophoresis method of inducing sweat.29,37,38,71,72 This method takes advantage of the bioelectric properties of skin which allow the application of low intensity electrical current (i.e. 1.5 mA) for 5 min. The resulting opposition offered by skin to this electrical current, called bio-impedance, is present in intra-and extra-cellular fluids and the capacitive reactance of cell membranes. For a topically applied chemical such as pilocarpine (0.5% pilocarpine nitrate solution), a drug with cholinergic parasympathomimetic activity which aims to stimulate primarily the muscarinic receptors of eccrine sweat glands, to be absorbed through human skin, the electrical current must overcome the bio-impedance imposed on its flow to reach the target tissue of sweat glands with sufficient intensity. This bio-impedance can be influenced by a range of factors, some of which are electricity source-dependent such as the distance between electrodes positioning, pulsed direct current vs constant direct current source, and size and content of iontophoresis electrodes (typically containing 70% copper, 30% zinc with diameter of 30 mm).27,73
Some of the important host-dependent factors involved with this mode of sweat induction include the amounts of keratin and the variable thickness of stratum corneum (SC) at different body sites, fluctuating amounts of fluid in skin layers with overall hydration status, ambient temperature increasing or decreasing hydration of keratin, adipose tissue thickness (especially with some sweat glands residing in deep dermis/ subcutaneous fat) and individual pain/tolerance to the electric current. All of these factors can alter biological responses, thereby potentially confounding metabolic results. Therefore, the argument can be made that using pilocarpine with iontophoresis induces production of a particular type of primarily eccrine sweat but whether the detailed metabolomic contents of this type of sweat are the same as physiologic sweat and/or thermally-induced sweat and/or exercise-induced sweat remains unknown.
After all, the original method of cholinergic stimulation with pilocarpine iontophoresis on the skin to facilitate sweat production dates back to the 1959 Gibson and Cooke publication describing implementation and standardisation of the ‘classic sweat test’ targeting sweat chloride levels for the purposes of diagnosing cystic fibrosis (CF).74 The Webster Sweat Inducer system coupled with a patented Macroduct Sweat Collector used in more recent sweat metabolomics studies originates from a further enhancement of the pilocarpine method, again designed to specifically improve the classic sweat test for CF.75 The quantitative pilocarpine iontophoresis test (QPIT) remains the gold standard for sweat induction in terms of CF-related testing and now has over 50 years of progressive standardisation.76 Despite better uniformity in collecting sweat samples and improved reference intervals based on age, defined rates of sweating and the volume of sweat to be collected at standardised sites as well as newer confirmatory CFTR-based testing, there are still complicating factors.77–79 Documented reports of false positive and false negative sweat chloride tests are in the literature, hypothesised to be due to such wide ranging factors as contaminating topical gels, interfering dermatological lesions (i.e. atopic dermatitis), autonomic nervous system dysfunction, prostaglandin use and other medication uses (e.g. topiramate), arsenic toxicity, malnutrition states, immunoglobulin deficiencies, autoimmune disorders such as systemic lupus erythematosus, and various abnormal endocrine states such as untreated hypothyroidism and Addison’s Disease.27, 80, 81
Exercise/Sauna/Hot Rooms
Other forms of sweat induction used in the studies presented in the Table include exercise and sauna activity or exposure to elevated temperatures with varying humidity levels. Older non-metabolomics studies have suggested distinct differences in metabolic content when sweat is obtained from exercise or sauna activity, especially in Ca2+ and Mg2+ concentrations.82 As this is an active area of ongoing research, it cannot be assumed that metabolomics studies using exercise and/or sauna-produced sweat have interchangeable results. A further potential confounder for sweat studies is the humidity level of sauna or hot rooms as this may contaminate sweat samples with condensation of airborne water droplets potentially containing bacteria, viruses, fungi, and/or xenobiotics. This is especially the case if direct analysis of sweat without detailed cleaning and extraction methods is employed. There is also a documented progressive decline in sweat rates when the skin is thoroughly wetted and/or with higher humidity conditions, referred to as hidromeiosis. This can be followed by an aftereffect of increased sweating when the skin is outside the exposed high humidity environment. The timing and rate of sweating therefore depends upon ambient temperature and humidity.83
Passive Sampling/Physiological Sweating
Some of the studies in the Table used only passive sampling of sweat without any imposed modes of sweat induction. Most of the time, longer periods of sweat collection were deemed necessary to obtain the same or smaller amounts of sweat or residual skin surface components (RSCCs). This may compromise the results, especially in terms of metabolic quenching of enzymatic reactions and metabolite stability. With untargeted metabolomics analysis, this is highly relevant due to unknown metabolites and thus unknown metabolite stability profiles. With more targeted metabolomics, as in the case of sweat testing for various drugs of abuse, this would depend on desired levels of quantification and the relative stability of the target molecule in certain specified conditions, necessitating controlled stability studies to be done, as discussed more extensively in a recent review by de Giovanni and Fucci.1 With newer technological trends of sweat analysis requiring smaller amounts of sweat, the need for complicated sweat induction methods will likely be reduced or redundant.6,59,84,85
Sweat Collection Protocols
A brief overview of the sweat collection methods in the Table reveals a diverse range of techniques employed to collect sweat for metabolomics studies. These techniques vary from simple, direct collection of sweat off skin into microcentrifuge tubes or glass jars, to elaborate specifically-designed implements (i.e. glass pipettes and rollers, hydrogel micropatches) to more commercially-available products like the Macroduct® Sweat Collector or PharmChek® sweat patches. Large variations between individuals in the amounts and location of sweat produced create major difficulties for those attempting to design a universal sweat collection device. Skin irritation, alterations of skin pH, disruptions to skin barrier properties and interactions with differing individuals’ skin microbiota are just some of the difficulties to be encountered in designing an ideal sweat collecting apparatus.1
Commercial Sweat Collection Devices
The Macroduct (ELITECH Wescor® Inc., Logan, UT, USA) is a popular commercially-available sweat collector that employs a plastic capillary-coil device of 29 mm diameter to wick the sweat off the skin surface, usually of the forearms.86 Since its introduction in 1986, it has been used in several sweat studies, including several addressing sweat metabolome optimisation.37,71 The Macroduct® is a component of the Macroduct® Sweat Analysis system, involving a commercial apparatus covering the skin after iontophoresis stimulation using pilocarpine29,37,38,71,72 that was developed to reduce the problems encountered with older filter pad- and tissue paper-based sweat collections of sweat chloride testing for CF.86 Macroduct® helps to overcome issues of background contamination, encapsulation (which increases local skin temperature and sweat gland secretion) and hidromeiosis (the progressive decline in sweat rates that occurs when skin is thoroughly wetted and/or with higher humidity) encountered with the older methods.86 The Macroduct® has a capacity of ∼0.1 mL of sweat collection per device session. Some researchers have considered placing more than one Macroduct® simultaneously to collect larger amounts of sweat that are then pooled for analysis, but differing sweat rates at different collection sites, amplification of inconsistent dilutional effects and difficulty in attaching the Macroduct® to other body sites produces confounding results.29
The same corporation (ELITECH Wescor®, Inc., Logan, UT, USA) developed a larger version of the Macroduct® called the Megaduct®. This is a round, plastic concave-based device with a larger collection area of 22.1 cm2 and a central aperture through which sweat collects into coiled capillary tubing. While the Megaduct® has an increased sweat volume capacity of ∼0.5 mL,40,87 its utility is limited by the duration of heat and/or exercise necessary to sweat long enough to fill the Megaduct® reservoir. For example, in one study, it required 65–75 min to collect the full 0.5 mL reservoir of sweat in 10 healthy men, with varying exercise intensities (VO2 = 0.5–2.0 L/min), temperatures (20–40 °C) in a controlled 50% humidity environment.87 Increasing sweat collection times to this range (>60 min) can potentially impact the power of metabolic findings, especially with the issues of metabolic quenching and time course of metabolic changes.87 For example, it is known that concentrations of sweat electrolytes and minerals such as zinc and iron change in relatively short periods of time (<30 min).87,88 Furthermore, both the Macroduct® and Megaduct® are designed primarily for forearm placement. As discussed already, the human body does not have a uniform sweat rate or composition over all skin locations. In fact, results of one study suggest forearm sweat rate is 30–60% less than that of the chest or back.87
Another popular commercial sweat collection device is the PharmCheck® or PharmChek® (PharmChem Inc., Fort Worth, TX, USA) sweat patch which has been available since 1990 (refer to the sweat collection method used by Porucznik et al., 2015 in the Table).89 This device is a nonocclusive patch consisting of a medical-grade cellulose paper absorption pad covered by a thin layer of polyurethane and acrylate adhesive. To use it, the skin site must first be cleaned with a tolerable solvent (e.g. isopropyl alcohol swabs) and thoroughly dried before application. The adhesive film of the patch is a semipermeable barrier that allows oxygen, carbon dioxide and water (vaporised by body heat) to diffuse freely. Larger non-volatile molecules (such as drugs, metabolites, metals and other xenobiotics) are retained on the inert cellulose absorption pad of the patch. Contaminants from the environment cannot penetrate the adhesive barrier from the outside once it is in place, enabling the patch to be worn during normal activities, including bathing, swimming and other exercise. It has a release liner that allows removal of the collection pad only once from the adhesive layer after use thereby preventing removal, reapplication or tampering with the patch. Underneath the polyurethane layer is a unique 9-digit number printed on the patch that is visible through a purpose-made window for legal (or research) applications. These features make this device useful in sweat testing for illicit drugs.1
Some of the disadvantages of PharmChek® include high inter-subject variability (potentially due to variable body site placement), high cost, possibility of environmental contamination either before patch application or after patch removal, risk of accidental removal before desired monitoring period and differing rates of drug/metabolite/xenobiotic penetration through the membrane, depending upon charged or uncharged state. Molecules in an uncharged state have been recorded to migrate more rapidly than charged species in studies of PharmChek®.1,90
Non-Commercial Sweat Collection Techniques
A newer form of sweat patch with commercial potential described by Dutkiewicz et al. is a specifically-designed agarose hydrogel micropatch with polytetrafluoroethylene (PTFE) support that has been developed for simplified collection of very small amounts of sweat that can be analysed directly within minutes using various MS platforms.59 This new method of sweat collection shows promise, but still requires further validation and optimisation of signal sensitivity and performance at higher temperatures and at increased sweat rates.59
Other noncommercial techniques of sweat collection for metabolomics studies are also documented in the Table. Lee et al. describe a ‘sweat collection patch’ placed on the lower back with sweat collected at three time points (10–20 min, 30–40 min, 50–60 min) while participants exercised on a cycling ergometer.91 Sweat was frozen on dry ice, and then stored at −80 °C until prepared and analysed. Unfortunately, there is limited mention of skin preparation, the type of sweat collection patch used, how the sweat is frozen, either intact in patch or transferred to another collection tube, or how sweat is prepared for untargeted metabolomics analysis.91 Occlusive skin patches consisting of 2–3 layers of filter paper or gauze have been used in other sweat collection studies but limitations of excessive pH variations and skin irritation with some degree of presumed skin disruption have been significant detractors.40
Shetage et al. and Michael-Jubeli et al. both use passive sampling with ‘cigarette paper’ and ‘lipid-free absorbent paper’ to collect the desired RSCCs or surface skin lipids (SSLs), respectively. These collection methods have advantages of economics and simplicity but still have the disadvantages of encapsulation and hidromeiosis already discussed as well as long collection times of 3 h and 30 min respectively.23,69
Kutyshenko et al. describe specially-designed glass rollers and glass pipettes for sweat collection. The rollers were used on lower sweat-producing regions (e.g. arms) moisturised with a sterile distilled water spray gun beforehand whilst the glass pipettes were used on heavier sweat-producing areas, namely forehead, chest and back.10 Although the use of glass is compelling with its relative inertness and is certainly of benefit when metabolomically targeting plastics-related xenobiotics, the confounders of varied locations of sweat harvesting, dilutional effects of adding sprayed distilled water and a lack of standardisation of temperature and humidity are likely to complicate the untargeted findings of this study.
Penn et al. describe another unique, specially-designed method of collecting sweat with a polydimethylsiloxane-coated stir bar that is rolled directly onto skin. The fact that sweat samples can then directly go through the necessary extraction step with a thermal desorption GC-MS setup is attractive. However, the fact that samples had to be shipped at 4 °C overseas to a special laboratory is a limitation and raises the issues of sample contamination and metabolite degradation during transportation.92
Unsupervised Sweat Collection Techniques
In studies by Genuis et al. and Sheng et al., participants were instructed to collect perspiration from any site on their body directly into a laboratory-provided, pre-cleaned, acid- and water-rinsed 500 mL glass jar or by using a stainless steel spatula against their skin to transfer perspiration directly into the same laboratory glass jar.55–57,93 Sweat was collected within one week before or after specified blood collection and participants delivered the collected sweat sample themselves to a laboratory without any specified storage or transport timeframes. The choice of glass for storage container concurs with the previous study discussed above. Including the option of stainless steel spatulas for sweat collection is intriguing. A grade of stainless steel was reportedly chosen to match the composition of laboratory needles used in standard blood collections since sweat was being directly compared to similarly-targeted detections of compounds in blood and urine in these studies. Recognising that stainless steel contains varying amounts of primarily iron, nickel and chromium that can also be found in trace amounts of physiological sweat, this is indeed important to factor in with future sweat metabolomics studies.56 However, the relative absence of controlled timing, temperature, humidity and storage conditions of sweat samples and the presumed delay of a metabolic quenching step make the sweat collection protocol of these series of studies less than ideal for metabolomics studies.
Direct Sweat Sampling Techniques
In a yet different approach, Harker et al. detailed subjects rinsing and drying their axillae with water just before entering a hot room (set at 43.3 °C and 65% relative humidity) for 15–40 min.28 Subsequently, the underarms were ‘wiped’ in an unspecified way and sweat was collected with a plastic-tipped pipette and transferred directly into glass vials with a collection period of approximately 15 min. The glass vials were then immediately sealed and stored frozen at −20 °C until analysis. This method included several commendable presampling controls by limiting use of pharmaceutical medications and topical applications of antiperspirant and soap products, detailing dietary limitations and specifying shaving of the axillary hair. However, the wiping of underarms immediately before sweat collection introduces potential issues of altered skin integrity on a molecular level which may impact the content of sweat analysis with 1H NMR spectroscopy. As mentioned in the section discussing sweat functions, the skin integrity is thought to influence pathways of water and other molecules/metabolites either via transmembrane proteins or lipid membranes or sweat glands during fluid transport from plasma to skin surface. Not specifying the exact material the underarms were wiped with and mixing plastic with glass in the sweat collection and storage before analysis create additional uncertainty.28
Some of the researchers associated with the Harker et al. study went on to publish another study of axillary sweat with similar direct sweat collection techniques, but optimised and targeted for amino acid analysis with a different metabolomics platform of GC-TOF (Time of Flight)/MS instead of 1H NMR spectroscopy.51 While direct sampling with minimal handling time and prompt metabolic quenching are advantages of this method, wiping the armpit before sampling and the use of a positive displacement pipette that might disrupt the skin surface along with the requirement for trained personnel to perform the sampling task remain as drawbacks.51 The choice of harvesting sweat from the axillae, rich in apocrine and apoeccrine sweat as well as eccrine sweat, in both of these studies complicates the comparisons to be made with other metabolomics studies harvesting sweat from other specific areas of the body with minimal apocrine or apoeccrine contributions.
Some similar advantages and disadvantages are appreciated with the sweat collection methods of Jia et al.94 Leg skin cleansing with alcohol pads followed by distilled water rinsing and drying precede the direct collection of sweat into microcentrifuge tubes which were placed immediately on dry ice to effect metabolic quenching.94 The cleansing and rinsing beforehand, as well as the physical, direct contact with the microcentrifuge tube may however disrupt the skin surface and again potentially alter skin integrity with its possible effects on fluid migrations from plasma to skin surface.
Summary
A diverse range of sweat induction modes and sweat collection methods are presented in the Table, all with their own advantages and disadvantages. Issues of variable location, timing and amounts of sweat induction and sampling as well as inconsistent sample processing steps and storage conditions confound most comparisons between methods. Optimising these parameters and exploring newer identified concepts surrounding sweat collection based upon updated information about sweat glands and the collective contents of their secretions will generate more meaningful results to build and improve our knowledge of the sweat metabolome. Standard operating protocols (SOPs) for collecting human biofluids like urine, blood and sweat for metabolomics studies are crucial to help control for the wide variety of factors that can influence metabolite concentrations. The SOPs for human sweat collection require updating beyond cystic fibrosis and illicit drug testing models to optimise metabolomics results. The following considerations need attention in future studies:
Specifying body sites of human sweat collection is of utmost importance in future comparisons of both targeted and untargeted metabolomics studies. Not all sweat collected anywhere on the body can be assumed homogenous in metabolic content.
Until further comparative studies are done, consideration should be given to subclassifying sweat based upon induction approaches – i.e. pilocarpine-induced sweat vs physiological sweat vs thermally-induced sweat vs exercise-induced sweat.
Measures to ensure adequate skin integrity other than mere visual inspection at sweat collection sites need further development and study.
Examining the molecular content of sweat induction, collection and storage devices for potential adsorption and metabolic reactivity requires further attention and attempts at standardisation.
Minimising the timing of sweat collection, transport and storage as well as ensuring a timely and adequate metabolic quenching step is important for comparing future sweat metabolome studies.
Environmental factors of temperature and humidity significantly impact the metabolic parameters of sweat and need to be specified and ideally standardised. Furthermore, the potential inter-relationship between overall core body temperature and local skin temperature at sweat collection sites may impact sweat metabolomics results.
Attention to controlling individual variables such as diet content, fasting vs postprandial state, exercise state, emotional state, pharmaceutical and/or recreational drug/ supplement use and underlying medical conditions that could impact the pH or overall metabolic state is important when interpreting any metabolomics results. This applies to controls instituted both preceding and during collection of sweat.
Defining the minimum amounts of sweat necessary to overcome intra-individual and inter-individual global metabolomic differences, stretching beyond guidelines based upon CF-specific testing of pilocarpine-induced sweat, is still a work in progress. Clarifications between physiological sweating and exercise- or thermally-induced sweating within this context are also necessary.
Age-specific influences on sweat metabolomics results will require further investigation.
Attempts at simultaneously characterising the individual skin microbiota (colonising bacteria, viruses, fungi, etc.) both quantitatively and qualitatively at sites of sweat collection might further elucidate suspected important metabolic relationships.
Conclusion
Better standardising of human sweat induction and collection methods to address the important challenges identified in this review is a key step to furthering sweat metabolomics. If this can be achieved, it is anticipated that sweat may become a more utilised biofluid capable of delivering easily accessible, individualised and instantaneously useful metabolic information that significantly enhances our knowledge of human health and disease.
Acknowledgments
This manuscript was developed as part of study conducted by Dr Joy Hussain during her PhD candidature. We wish to thank the Judy Jacka Foundation of Natural Therapies for providing an academic scholarship to support her candidature and therefore this study.
Footnotes
Competing Interests: None declared.
References
- 1.De Giovanni N, Fucci N. The current status of sweat testing for drugs of abuse: a review. Curr Med Chem. 2013;20:545–61. doi: 10.2174/0929867311320040006. [DOI] [PubMed] [Google Scholar]
- 2.Papanas N, Papatheodorou K, Christakidis D, Papazoglou D, Giassakis G, Piperidou H, et al. Evaluation of a new indicator test for sudomotor function (Neuropad (R)) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2005;113:195–8. doi: 10.1055/s-2005-837735. [DOI] [PubMed] [Google Scholar]
- 3.Quattrini C, Jeziorska M, Tavakoli M, Begum P, Boulton A, Malik R. The Neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia. 2008;51:1046–50. doi: 10.1007/s00125-008-0987-y. [DOI] [PubMed] [Google Scholar]
- 4.Peng Y, Cui X, Liu Y, Li Y, Liu J, Cheng B. Systematic review focusing on the excretion and protection roles of sweat in the skin. Dermatology. 2014;228:115–20. doi: 10.1159/000357524. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–14. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dam VAT, Zevenbergen MAG, van Schaijk R. Toward wearable patch for sweat analysis. Sens Actuators B Chem. 2016;236:834–8. [Google Scholar]
- 7.Nayak R, Wang L, Padhye R. Electronic textiles for military personnel . Woodhead Publishing Ltd; Cambridge, UK: 2015. [Google Scholar]
- 8.Álvarez-Sánchez B, Priego-Capote F, de Castro ML. Metabolomics analysis I. Selection of biological samples and practical aspects preceding sample preparation. Trends Anal Chem. 2010;29:111–9. [Google Scholar]
- 9.Williams RJ. Biochemical Individuality: The Basis for the Genetotrophic concept: John Wiley & Sons; 1956. University of Texas Press, 1969 to 1979; Keats Publishing, 1998; 1956. [Google Scholar]
- 10.Kutyshenko VP, Molchanov M, Beskaravayny P, Uversky VN, Timchenko MA. Analyzing and mapping sweat metabolomics by high-resolution NMR spectroscopy. PLoS One. 2011;6:e28824. doi: 10.1371/journal.pone.0028824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehlaut R, Yadav S. Metabolomics a new tool to molecular imaging technology. Int J Therapeut Appl. 2012;2:33–42. [Google Scholar]
- 12.Orešič M. Metabolomics, a novel tool for studies of nutrition, metabolism and lipid dysfunction. Nutr Metab Cardiovasc Dis. 2009;19:816–24. doi: 10.1016/j.numecd.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Emwas A-HM, Salek RM, Griffin JL, Merzaban J. NMR-based metabolomics in human disease diagnosis: applications, limitations, and recommendations. Metabolomics. 2013;9:1048–72. [Google Scholar]
- 14.Kell DB, Goodacre R. Metabolomics and systems pharmacology: why and how to model the human metabolic network for drug discovery. Drug Discov Today. 2014;19:171–82. doi: 10.1016/j.drudis.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosmides AK, Kamisoglu K, Calvano SE, Corbett SA, Androulakis IP. Metabolomic fingerprinting: challenges and opportunities. Crit Rev Biomed Eng. 2013;41:205–21. doi: 10.1615/critrevbiomedeng.2013007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulimiri SV, Berger A, Sonawane B. The potential of metabolomic approaches for investigating mode (s) of action of xenobiotics: case study with carbon tetrachloride. Mutat Res. 2011;722:147–53. doi: 10.1016/j.mrgentox.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Shen H, Xu W, Peng S, Scherb H, She J, Voigt K, et al. Pooling samples for top-down molecular exposomics research: the methodology. Environ Health. 2014;13:8. doi: 10.1186/1476-069X-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35(Suppl 1):D521–6. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HMDB. The Human Metabolome Database: TMIC, The Metabolomics Innovation Centre http://www.hmdb.ca/. (Accessed 7 November 2016)
- 20.Roessner U, Bowne J. What is metabolomics all about? Biotechniques. 2009;46:363–5. doi: 10.2144/000113133. [DOI] [PubMed] [Google Scholar]
- 21.Goncharov N, Ukolov A, Orlova T, Migalovskaia E, Voitenko N. Metabolomics: on the way to an integration of biochemistry, analytical chemistry, and informatics. Biol Bull Rev. 2015;5:296–307. [Google Scholar]
- 22.Wilke K, Martin A, Terstegen L, Biel SS. A short history of sweat gland biology. Int J Cosmet Sci. 2007;29:169–79. doi: 10.1111/j.1467-2494.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 23.Shetage SS, Traynor MJ, Brown MB, Raji M, Graham-Kalio D, Chilcott RP. Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Skin Res Technol. 2014;20:97–107. doi: 10.1111/srt.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- 25.Piérard GE, Elsner P, Marks R, Masson P, Paye M, EEMCO Group EEMCO guidance for the efficacy assessment of antiperspirants and deodorants. Skin Pharmacol Appl Skin Physiol. 2003;16:324–42. doi: 10.1159/000072072. [DOI] [PubMed] [Google Scholar]
- 26.Noël F, Piérard-Franchimont C, Piérard GE, Quatresooz P. Sweaty skin, background and assessments. Int J Dermatol. 2012;51:647–55. doi: 10.1111/j.1365-4632.2011.05307.x. [DOI] [PubMed] [Google Scholar]
- 27.Beauchamp M, Lands LC. Sweat-testing: a review of current technical requirements. Pediatr Pulmonol. 2005;39:507–11. doi: 10.1002/ppul.20226. [DOI] [PubMed] [Google Scholar]
- 28.Harker M, Coulson H, Fairweather I, Taylor D, Daykin CA. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1H NMR spectroscopy. Metabolomics. 2006;2:105–12. [Google Scholar]
- 29.Raiszadeh MM, Ross MM, Russo PS, Schaepper MA, Zhou W, Deng J, et al. Proteomic analysis of eccrine sweat: implications for the discovery of schizophrenia biomarker proteins. J Proteome Res. 2012;11:2127–39. doi: 10.1021/pr2007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae. Am J Physiol. 1987;252:R166–80. doi: 10.1152/ajpregu.1987.252.1.R166. [DOI] [PubMed] [Google Scholar]
- 31.Barzantny H, Brune I, Tauch A. Molecular basis of human body odour formation: insights deduced from corynebacterial genome sequences. Int J Cosmet Sci. 2012;34:2–11. doi: 10.1111/j.1468-2494.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsatsou F, Zouboulis CC. Anatomy of the Sebaceous Gland. In: Tsatsou F, Zouboulis CC, Kligman AM, editors. Pathogenesis and Treatment of Acne and Rosacea. Springer; 2014. pp. 27–31. [Google Scholar]
- 33.Jung Y, Tam J, Jalian HR, Anderson RR, Evans CL. Longitudinal, 3D in vivo imaging of sebaceous glands by coherent anti-stokes Raman scattering microscopy: normal function and response to cryotherapy. J Invest Dermatol. 2015;135:39–44. doi: 10.1038/jid.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi VY, Leo M, Hassoun L, Chahal DS, Maibach HI, Sivamani RK. Role of sebaceous glands in inflammatory dermatoses. J Am Acad Dermatol. 2015;73:856–63. doi: 10.1016/j.jaad.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhou S-S, Li D, Zhou Y-M, Cao J-M. The skin function: a factor of anti-metabolic syndrome. Diabetol Metabc Syndr. 2012;4:4–15. doi: 10.1186/1758-5996-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fyhrquist N, Salava A, Auvinen P, Lauerma A. Skin Biomes. Curr Allergy Asthma Rep. 2016;16:40. doi: 10.1007/s11882-016-0618-5. [DOI] [PubMed] [Google Scholar]
- 37.Calderón-Santiago M, Priego-Capote F, Jurado-Gámez B, Luque de Castro MD. Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J Chromatogr A. 2014;1333:70–8. doi: 10.1016/j.chroma.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 38.Calderón-Santiago M, Priego-Capote F, Turck N, Robin X, Jurado-Gámez B, Sanchez JC, et al. Human sweat metabolomics for lung cancer screening. Anal Bioanal Chem. 2015;407:5381–92. doi: 10.1007/s00216-015-8700-8. [DOI] [PubMed] [Google Scholar]
- 39.Dai X, Okazaki H, Hanakawa Y, Murakami M, Tohyama M, Shirakata Y, et al. Eccrine sweat contains IL-1α, IL-1β and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS One. 2013;8:e67666. doi: 10.1371/journal.pone.0067666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jadoon S, Karim S, Akram MR, Kalsoom Khan A, Zia MA, Siddiqi AR, et al. Recent developments in sweat analysis and its applications. Int J Anal Chem. 2015;2015:164974. doi: 10.1155/2015/164974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Gasecka P, Formanek F, Galey JB, Rigneault H. In vivo single human sweat gland activity monitoring using coherent anti-Stokes Raman scattering and two-photon excited autofluorescence microscopy. Brit J Dermatol. 2016;174:803–12. doi: 10.1111/bjd.14292. [DOI] [PubMed] [Google Scholar]
- 42.Shields SA, MacDowell KA, Fairchild SB, Campbell ML. Is mediation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology. 1987;24:312–9. doi: 10.1111/j.1469-8986.1987.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 43.Bates GP, Miller VS. Sweat rate and sodium loss during work in the heat. J Occup Med Toxicol. 2008;3:4. doi: 10.1186/1745-6673-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xhauflaire-Uhoda E, Paquet P, Quatresooz P, Piérard GE. Characterization of the skin using capacitance imaging. Expert Rev Dermatol. 2010;5:149–58. [Google Scholar]
- 45.Kirk JM, Keston M, McIntosh I, al Essa S. Variation of sweat sodium and chloride with age in cystic fibrosis and normal populations: further investigations in equivocal cases. Ann Clin Biochem. 1992;29:145–52. doi: 10.1177/000456329202900204. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Greaves R, Massie J. The limitations of sweat electrolyte reference intervals for the diagnosis of cystic fibrosis: a systematic review. Clin Biochem Rev. 2007;28:60–76. [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor NA, Machado-Moreira CA. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem Physiol Med. 2013;2:4. doi: 10.1186/2046-7648-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cone EJ, Hillsgrove MJ, Jenkins AJ, Keenan RM, Darwin WD. Sweat testing for heroin, cocaine, and metabolites. J Anal Toxicol. 1994;18:298–305. doi: 10.1093/jat/18.6.298. [DOI] [PubMed] [Google Scholar]
- 49.Brusilow SW, Gordes EH. Ammonia secretion in sweat. Am J Physiol. 1968;214:513–7. doi: 10.1152/ajplegacy.1968.214.3.513. [DOI] [PubMed] [Google Scholar]
- 50.Porter AM. Why do we have apocrine and sebaceous glands? J R Soc Med. 2001;94:236–7. doi: 10.1177/014107680109400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark H, Harding CR. Amino acid composition, including key derivatives of eccrine sweat: potential biomarkers of certain atopic skin conditions. Int J Cosmet Scie. 2013;35:163–8. doi: 10.1111/ics.12019. [DOI] [PubMed] [Google Scholar]
- 52.Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012;91:552–66. doi: 10.1016/j.ejcb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Tanei R, Hasegawa Y. Atopic dermatitis in older adults: A viewpoint from geriatric dermatology. Geriatr Gerontol Int. 2016;16(Suppl 1):75–86. doi: 10.1111/ggi.12771. [DOI] [PubMed] [Google Scholar]
- 54.Marques-Deak A, Cizza G, Eskandari F, Torvik S, Christie IC, Sternberg EM, et al. Measurement of cytokines in sweat patches and plasma in healthy women: Validation in a controlled study. J Immunol Methods. 2006;315:99–109. doi: 10.1016/j.jim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 55.Genuis SJ, Beesoon S, Birkholz D, Lobo RA. Human excretion of bisphenol A: blood, urine, and sweat (BUS) study. J Environ Public Health. 2012;2012:185731. doi: 10.1155/2012/185731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genuis SJ, Birkholz D, Rodushkin I, Beesoon S. Blood, urine, and sweat (BUS) study: monitoring and elimination of bioaccumulated toxic elements. Arch Environ Contam Toxicol. 2011;61:344–57. doi: 10.1007/s00244-010-9611-5. [DOI] [PubMed] [Google Scholar]
- 57.Genuis SJ, Beesoon S, Lobo RA, Birkholz D. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Scientific World Journal. 2012;2012:615068. doi: 10.1100/2012/615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sears ME, Kerr KJ, Bray RI. Arsenic, cadmium, lead, and mercury in sweat: a systematic review. J Environ Public Health. 2012;2012:184745. doi: 10.1155/2012/184745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutkiewicz EP, Lin J-D, Tseng T-W, Wang Y-S, Urban PL. Hydrogel Micropatches for Sampling and Profiling Skin Metabolites. Anal Chem. 2014;86:2337–44. doi: 10.1021/ac4039338. [DOI] [PubMed] [Google Scholar]
- 60.Cone EJ. New developments in biological measures of drug prevalence. NIDA Res Monogr. 1997;167:108–29. [PubMed] [Google Scholar]
- 61.Harris R, Jones H, Artis W. Orally administered ketoconazole: route of delivery to the human stratum corneum. Antimicrob Agents Chemother. 1983;24:876–82. doi: 10.1128/aac.24.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lever L, Dykes P, Thomas R, Finlay A. How orally administered terbinafine reaches the stratum corneum. J Dermatolog Treat. 1990;1(Suppl 2):23–5. [Google Scholar]
- 63.Faergemann J, Laufen H. Levels of fluconazole in serum, stratum corneum, epidermis-dermis (without stratum corneum) and eccrine sweat. Clin Exp Dermatol. 1993;18:102–6. doi: 10.1111/j.1365-2230.1993.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhou S-S, Zhou Y-M, Li D, Chen N-N. Preeclampsia and future cardiovascular risk: A point of view from the clearance of plasma vasoactive amines. Hypertens Pregnancy. 2016;35:1–14. doi: 10.3109/10641955.2015.1115062. [DOI] [PubMed] [Google Scholar]
- 65.Bouloux PM. M58–Sweating and Flushing: Evaluation and Management.. ENDO2013, Meet the Professor -Clinical Case Management.; UCL: Royal Free Campus; 2013. [Google Scholar]
- 66.Nicholson GM, Graudins A, Wilson HI, Little M, Broady KW. Arachnid toxinology in Australia: from clinical toxicology to potential applications. Toxicon. 2006;48:872–98. doi: 10.1016/j.toxicon.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Pochi PE, Downing DT, Strauss JS. Sebaceous gland response in man to prolonged total caloric deprivation. J Invest Dermatol. 1970;55:303–9. doi: 10.1111/1523-1747.ep12260136. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson DI. Psoriasis and dietary fat: the fatty acid composition of surface and scale (ether-soluble) lipids. J Invest Dermatol. 1966;47:185–92. doi: 10.1038/jid.1966.129. [DOI] [PubMed] [Google Scholar]
- 69.Michael-Jubeli R, Bleton J, Baillet-Guffroy A. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J Lipid Res. 2011;52:143–51. doi: 10.1194/jlr.D008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Álvarez-Sánchez B, Priego-Capote F, de Castro ML. Metabolomics analysis II. Preparation of biological samples prior to detection. Trends Anal Chem. 2010;29:120–7. [Google Scholar]
- 71.Delgado-Povedano M, Calderón-Santiago M, Priego-Capote F, de Castro ML. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography–mass spectrometry in high resolution mode. Anal Chim Acta. 2016;905:115–25. doi: 10.1016/j.aca.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 72.Adewole OO, Erhabor GE, Adewole TO, Ojo AO, Harriet O, Wolfe LM, et al. Proteomic profiling of eccrine sweat reveals its potential as a diagnostic biofluid for active tuberculosis. Proteomics Clin Appl. 2016;10:547–53. doi: 10.1002/prca.201500071. [DOI] [PubMed] [Google Scholar]
- 73.Gomez CC, Servidoni MdeF, Marson FA, Canavezi PJ, Vinagre AM, Costa ET, et al. Pulsed direct and constant direct currents in the pilocarpine iontophoresis sweat chloride test. BMC Pulm Med. 2014;14:198. doi: 10.1186/1471-2466-14-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545–9. [PubMed] [Google Scholar]
- 75.Webster HL. Laboratory diagnosis of cystic fibrosis. Crit Rev Clin Lab Sci. 1983;18:313–38. doi: 10.3109/10408368209085074. [DOI] [PubMed] [Google Scholar]
- 76.Taylor CJ, Hardcastle J, Southern KW. Physiological measurements confirming the diagnosis of cystic fibrosis: the sweat test and measurements of transepithelial potential difference. Paediatr Respir Rev. 2009;10:220–6. doi: 10.1016/j.prrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Collie JT, Massie RJ, Jones OA, LeGrys VA, Greaves RF. Sixty-five years since the New York heat wave: advances in sweat testing for cystic fibrosis. Pediatr Pulmonol. 2014;49:106–17. doi: 10.1002/ppul.22945. [DOI] [PubMed] [Google Scholar]
- 78.Quinton PM. A synopsis of methods of sweat tests in pathology. Clin Biochem. 2014;9:757–8. doi: 10.1016/j.clinbiochem.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 79.Mishra A, Greaves R, Smith K, Carlin JB, Wootton A, Stirling R, et al. Diagnosis of cystic fibrosis by sweat testing: age-specific reference intervals. J Pediatr. 2008;153:758–63. doi: 10.1016/j.jpeds.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 80.Guglani L, Stabel D, Weiner DJ. False-positive and false-negative sweat tests: systematic review of the evidence. Pediatr Allergy Immunol Pulmonol. 2015;28:198–211. doi: 10.1089/ped.2015.0552. [DOI] [PubMed] [Google Scholar]
- 81.DeMarco ML, Dietzen DJ, Brown SM. Sweating the small stuff: adequacy and accuracy in sweat chloride determination. Clin Biochem. 2015;48:443–7. doi: 10.1016/j.clinbiochem.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Verde T, Shephard R, Corey P, Moore R. Sweat composition in exercise and in heat. J Appl Physiol. 1982;53:1540–5. doi: 10.1152/jappl.1982.53.6.1540. [DOI] [PubMed] [Google Scholar]
- 83.Iwase S, Kawahara Y, Nishimura N, Sugenoya J. Effect of micro mist sauna bathing on thermoregulatory and circulatory functions and thermal sensation in humans. Int J Biometeorol. 2016;60:699–709. doi: 10.1007/s00484-015-1064-0. [DOI] [PubMed] [Google Scholar]
- 84.Al-omari M, Liu G, Mueller A, Mock A, Ghosh RN, Smith K, et al. A portable optical human sweat sensor. J Appl Phys. 2014;116:183102. [Google Scholar]
- 85.Li Z, Tatlay J, Li L. Nanoflow LC-MS for high-performance chemical isotope labeling quantitative metabolomics. Anal Chem. 2015;87:11468–74. doi: 10.1021/acs.analchem.5b03209. [DOI] [PubMed] [Google Scholar]
- 86.Cole D, Boucher MJ. Use of a new sample-collection device (Macroduct) in anion analysis of human sweat. Clin Chem. 1986;32:1375–8. [PubMed] [Google Scholar]
- 87.Ely M, Ely B, Chinevere T, Lacher C, Lukaski H, Cheuvront S. Evaluation of the Megaduct sweat collector for mineral analysis. Physiol Meas. 2012;33:385–94. doi: 10.1088/0967-3334/33/3/385. [DOI] [PubMed] [Google Scholar]
- 88.DeRuisseau KC, Cheuvront SN, Haymes EM, Sharp RG. Sweat iron and zinc losses during prolonged exercise. Int J Sport Nutr Exerc Metab. 2002;12:428–37. doi: 10.1123/ijsnem.12.4.428. [DOI] [PubMed] [Google Scholar]
- 89.Porucznik CA, Cox KJ, Wilkins DG, Anderson DJ, Bailey NM, Szczotka KM, et al. A preliminary study of bio-monitoring for bisphenol-A in human sweat. J Anal Toxicol. 2015;39:562–6. doi: 10.1093/jat/bkv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kidwell DA, Smith FP. Susceptibility of PharmChek drugs of abuse patch to environmental contamination. Forensic Sci Int. 2001;116:89–106. doi: 10.1016/s0379-0738(00)00353-4. [DOI] [PubMed] [Google Scholar]
- 91.Lee DP, Kennedy AD, O’Neal EK, Bishop PA, Haub MD, Strecker KL, et al. Global untargeted metabolic profiling of human sweat from exercising men and women. J Int Soc Sports Nutr. 2011;8:9. [Google Scholar]
- 92.Penn DJ, Oberzaucher E, Grammer K, Fischer G, Soini HA, Wiesler D, et al. Individual and gender fingerprints in human body odour. J R Soc Interface. 2007;4:331–40. doi: 10.1098/rsif.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sheng J, Qiu W, Xu B, Xu H, Tang C. Monitoring of heavy metal levels in the major rivers and in residents’ blood in Zhenjiang City, China, and assessment of heavy metal elimination via urine and sweat in humans. Environ Sci Pollut Res. 2016;23:11034–45. doi: 10.1007/s11356-016-6287-z. [DOI] [PubMed] [Google Scholar]
- 94.Jia M, Chew W, Feinstein Y, Skeath P, Sternberg E. Quantification of cortisol in human eccrine sweat by liquid hromatography-tandem mass spectrometry. Analyst. 2016;141:2053–60. doi: 10.1039/c5an02387d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang S, Yu X, Wu C. Comparison of the levels of five heavy metals in human urine and sweat after strenuous exercise by ICP-MS. J Appl Math Phys. 2016;4:183–8. [Google Scholar]