Abstract
Chronic wounds, in particular venous leg ulcers (VLU), represent a substantial burden for economies, healthcare systems and societies worldwide. This burden is exacerbated by the recalcitrant nature of these wounds, despite best practice, evidence-based care, which substantially reduces the quality of life of patients. Furthermore, co-morbidities such as diabetes and cardiovascular disease within ageing populations further contribute to the increasing prevalence in developed countries. This review provides an overview of the literature concerning the cellular and molecular mechanisms of wound healing and aspects where this process fails, resulting in a chronic wound. VLU may arise from chronic venous disease, which presents with many clinical manifestations and can lead to a highly complex disease state. Efforts to comprehend this state using various omics based approaches have delivered some insight into the underlying biology of chronic wounds and revealed markers of differentiation at the genomic, transcriptomic, proteomic and metabolomic levels. Furthermore, this review outlines the array of analytical tools and approaches that have been utilised for capturing multivariate data at each of these molecular levels. Future developments in spatiotemporal analysis of wounds along with the integration of multiple omics datasets may provide much needed information on the key molecules that drive wound chronicity. Such biomarkers have the potential to be developed into clinically relevant diagnostic tools to aid in personalised wound management.
Introduction
Chronic leg ulcers are a debilitating and costly affliction that impact tens of millions of individuals around the world. The etiology of these chronic wounds is attributed to a combination of a myriad of dysfunctional physiological and biochemical mechanisms, which result in a disease state that is highly complex and difficult to treat.
Patients with venous ulcers will often undergo a standard regime of compression therapy in order to restore dysfunctional physiology and repair the wound. However, in 30% of cases this intervention fails to resolve the wound and alternative therapies must be considered. It is unknown why this subset of individuals fail to respond to conventional therapy and, likewise, why the vast number of chronic wounds of varying aetiologies are recalcitrant to best-practice care.
Empirical evidence to date suggests a strong link to the underlying biochemistry of the wound as a contributing factor in non-healing ulcers. Thus, research within the last decade has focused on resolving the molecular aspects of chronic wounds, particularly in what drives the formation of these ulcers and contributes to their recalcitrant nature or ability to reepithelialise and subsequently heal. The molecular strategies employed to investigate wounds has been wide ranging and includes genomic, transcriptomic, proteomic and metabolomic methods. In addition, these methods are ideal for biomarker discovery and the subsequent development of diagnostic and prognostic tools for use in clinical practice. To date, a number of molecules have been shown to correlate with chronic wounds of varying aetiologies, however there are no diagnostic tools currently used within routine clinical care.
This review will discuss molecular aspects of chronic wounds with a focus predominantly on venous leg ulceration (VLU). The purpose of this review is to provide an overview of wound healing, aspects where this process goes wrong, particularly in the event of chronic venous disease (CVeD), and the various molecular analytical strategies that can be employed to further our understanding in this field and in the search for clinically-relevant biomarkers. An excellent review on omics investigations of venous ulcers has recently been published,1 however, we aim to build on this work and further describe various approaches for future research, additional biological considerations and the use of bioinformatics for analysis and data integration.
Chronic Wound Prevalence
The prevalence of chronic wounds is a major healthcare concern worldwide. Although the precise global burden is unknown, there is substantial data from research undertaken by developed nations that provide an implicit global perspective. These statistics have been summarised in Table 1.
Table 1.
The prevalence of chronic wounds across global regions.
| Geographical Region | Prevalence | Number of people affected | Number of inhabitants | Reference |
|---|---|---|---|---|
| Australia | 3–61 / 1000 people | 0.73–1.48 million | 24.3 million | 201 |
| Africa | 19–130 / 1000 people | 20–135 million† | 1.033 billion‡ | 202 |
| USA | 21 / 1000 people* | 6.6 million | 314 million | 4 |
| India | 4.48 / 1000 people | 5.5 million | 1.237 billion | 203 |
| Europe | 3–4 / 1000 people | 1.5–2.0 million | 500+ million | 204 |
| UK | 3.5 / 1000 people | 220,000† | 63 million‡ | 202 |
estimated from affected individuals
estimated from prevalence data
based on available historical population data
Such a pandemic-like condition also engenders serious economic ramifications. The impact of chronic wounds is observed in healthcare systems worldwide, where treatment expenses have become burdensome.2–5 Within Australia, the treatment of chronic wounds was reported to cost the annual health budget AU$400–$500 million.6 However, based on a predicted 3% expense of total healthcare expenditure as observed in other developed nations, $2.9 billion per annum has been estimated as the most current direct healthcare cost for Australia.3 This represents a substantial burden on the Australian economy and is unsustainable for future economic prosperity. Moreover, with increased life expectancy, due to advancements in medical science, there exists a greater incidence of age-related pathologies, including chronic wounds and delayed healing outcomes.7–10
This has been, in part, due to the nation’s ageing population,11,12 but is also attributed to a rise in co-morbidities that lead to non-healing wounds; including obesity,13 diabetes mellitus,14 cardiovascular disease,15 age-related degeneration of the immune system,10 and poor nutrition.16 In addition to the pressures placed on hospital systems, local communities suffer from chronic wounds as well, with patients experiencing significant losses in work productivity and increases in social disengagement. Patients will often ostracise themselves, avoiding contact with others and subsequently ensuring noncompliance with their treatment or therapy.17–21 Moreover, there is mounting evidence that suggests psychological factors, and not just the underlying wound pathophysiology, have a profound impact on healing trajectory.19,22 Patients with chronic leg ulcers will experience restricted mobility, social isolation, and an overall decrease in their quality of life.23–26 These aspects disturb a patient’s mental health and subsequently manifest with increases in sleep disturbance,27 negative emotions,28 depression and anxiety.29
From the literature, it is clear that chronic wounds have a grave impact upon the state, the economy, clinics and the hospital system, communities, and patients. This impact within Australia and societies worldwide has acted as a strong driver of research and development into chronic wounds. This is supported by recent data which suggest that, although chronic wounds represent a very significant clinical challenge, there is a higher priority to invest in research, rather than additional hospital beds, in order to meet the challenge and reduce the systemic burden of wound perpetuation.30
Wound Healing: The Acute Context
The Phases of Healing: an Insight to Where Healing Stalls
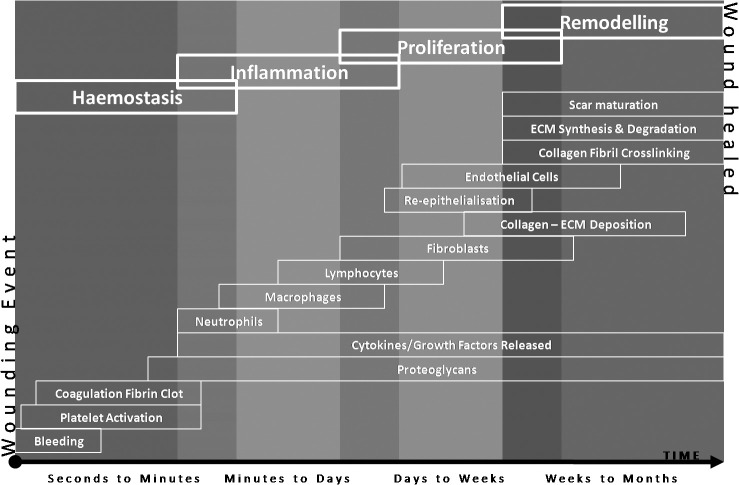
In order to comprehend the molecular problems associated with wound chronicity, the underlying processes involved in normal wound repair must be understood as failure or dysregulation at any point during the healing continuum could result in a non-healing wound. Acute wounds share a number of physiological similarities, as they do differences, with chronic wounds. Through reflection on the normal wound healing processes, one can begin to identify where, why, and how chronic wounds can form. Under normal conditions, acute wounds will heal in a time-efficient manner through an orchestrated progression of four major overlapping phases of wound healing (Figure 1), which include: haemostasis, inflammation, proliferation, and remodelling.31–34
Figure 1.
Four phases of wound healing including: (i) Haemostasis; (ii) Inflammation; (iii) Proliferation; and (iv) Remodelling. Many events occur during the various stages of the wound healing process and at any stage a wound can stall or become perturbed. Adapted from: 33, 42, 198, 199.
In stark contrast to acute wound healing, chronic wounds fail to reach the same endpoint, with prolonged inflammatory, and/or perturbed proliferative or remodelling phases, eventuating in non-healing ulceration or an undesired fibrosis of the tissue.35 The highly orchestrated process of wound healing involves multiple cell types, which include: platelets, mast cells, macrophages, neutrophils, lymphocytes, myofibroblasts, fibroblasts, epithelial cells, pericytes, endothelial cells, nerve cells, and stem cells.34,36–38 The majority of these cells will interact with each other and the extracellular matrix (ECM) environment through a series of complex signalling pathways involving a multitude of proteins, peptides, metabolites, and biomolecules, including small interfering and micro ribonucleic acids (miRNAs).39, 40 Thus, these molecules reflect the driving forces associated with repair and regeneration; with molecular milestones met during each stage of the wound healing process, and which progress the wound towards a successful healing outcome. In order to distinguish those molecular differences inherent in chronic wounds that lead to prolonged or non-healing events, it is crucial to review the process of healing in the acute wound environment.
Haemostasis
Haemostasis occurs immediately following an injury to vascularised tissues. Vasoconstriction occurs through pain receptors, injury to vascular smooth muscle, and the activation of platelets following exposure to extravascular collagen. The activated platelets also initiate the blood clotting cascade at the site of the injury. Platelets subsequently release various cytokines and growth factors, including platelet-derived growth factor (PDGF), insulin-like growth factor-I (IGF-I), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and transforming growth factor beta/alpha (TGF-β/TGF-α). In addition, adhesive glycoproteins, such as fibrinogen, fibronectin, thrombospondin, von Willebrand factor, and vitronectin, are also released and cause the platelets to become adherent and aggregate together.41–43 As platelet aggregation continues, locally expressed thrombin catalyses the conversion of fibrinogen to fibrin resulting in the formation of a fibrin clot.44 This temporary barrier not only protects against pathogenic microorganisms and continued water loss, but acts as a provisional matrix for the subsequent phases of healing.45 As the platelets continue to release growth factors and chemokines, these attractants facilitate the migration of various different cell types, including leukocytes, into the wound site to initiate the inflammatory phase of wound repair.
Inflammation and Humoral Immune Response
Inflammation occurs within minutes of wounding, wherein inflammatory cells are attracted to the wound site by complement activation, degranulation of platelets and products of bacterial degradation.46 An influx of neutrophils to the wound site marks the initial inflammatory cell response.47 These cells represent the first line of cell-mediated host defence and begin the phagocytosis of bacteria, simultaneously generating oxygen-derived free radical species, in an attempt to destroy foreign organisms. In addition, neutrophils release high levels of proteases (elastase, neutrophil collagenase, and matrix metalloproteinases (MMPs), such as MMP8 and MMP9), which actively degrade damaged cells and components of the extracellular matrix.42 Mast cells subsequently activate at the wound site and release granules containing serine proteases, histamines, and other bio-active amines that are responsible for immune defence and the physical characteristics of inflammation, including redness, heat, swelling, and pain.48 After 48–72 hours post-wounding, the neutrophils undergo apoptosis and are replaced by circulating monocytes that mature into phagocytic macrophages.49
Macrophages are essential to the regulation of wound healing and, similar to neutrophils, possess a dual role in the healing process. They phagocytose microbes and remove dead and damaged tissue through the extracellular secretion of MMPs and elastase. However, unlike neutrophils, macrophages are able to regulate the proteolytic destruction of wound tissue through the secretion of protease inhibitors.50 Secondly, macrophages mediate the transition of wound healing into the proliferative phase by the release of a number of growth factors and cytokines, including interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), epidermal growth factor (EGF), interferon-gamma (IFN-γ), PDGF, TGF-β, TGF-α, FGF, and IGF-I.42,51,52 The net result of these mediators is the stimulation of migration and proliferation of keratinocytes (epithelial cells), fibroblasts, and endothelial cells, culminating in the end of the inflammatory phase and the beginning of the proliferative phase of healing.
Migration and Proliferation
The proliferative phase of healing is associated with angiogenesis, re-epithelialisation, the formation of granulation tissue, and the formation of a new extracellular matrix through the deposition of collagen fibres, elastin, proteoglycans, and fibronectin. Fibroblasts are the main cell type present during this stage. Generally quite sparse in normal dermal tissue, these cells migrate and proliferate to great numbers within the wound area.42 They produce proteases, including MMPs, to clear the wound site, and proteoglycans, fibronectin, and collagen for construction of the provisional ECM. A number of important growth factors regulate fibroblast activity, including PDGF, TGF-β, EGF, IGF-I, and FGF.49 Macrophage-secreted FGF and PDGF stimulate fibroblast proliferation, chemotaxis, and collagenase expression. In addition, TGF-β, also secreted by macrophages, increases the overall production of mature matrix proteins and reduces matrix degradation through the stimulation of the secretion of tissue inhibitor of metalloproteinases (TIMPs), which inhibit MMPs.51 The developing granulation tissue eventually undergoes angiogenesis due to the presence of low oxygen, low pH, high lactate levels, and matrix associated mediators (such as VEGF, TGF-β, and bFGF).53, 54 The formation of new capillaries continues until the tissue oxygen and metabolic needs are met.
Re-epithelialisation subsequently takes place, wherein the epithelial cells around the wound margin migrate to cover the exposed wound surface.55 Keratinocytes begin the process; migrating, proliferating, and then differentiating into a functional epidermis. Growth factors, such as EGF, keratinocyte growth factor (KGF), and TGF-α, stimulate the mass migration of keratinocytes over the collagen fibres of the granulation tissue.42 More recently, human antigen R, a post-translational modifier of mRNAs that translate proteins involved in cell growth, differentiation, function, and death, has been observed to reduce cellular adhesion and promote keratinocyte migration.56 In addition, proteases that are produced by the leading edge of epithelial cells degrade the provisional matrix and allow the movement of these cells across the wound bed. Once the keratinocytes cover the entire surface their behaviour changes and they focus on proliferation and differentiation. As the basal keratinocytes proliferate, the number of cell layers increase and these cells undergo differentiation into the various distinct stratified layers of the new epidermis.57 This re-epithelialisation and cornification of keratinocytes hallmarks the completion of the proliferative phase of wound healing and is the clinical indicator of healing; however, this is not the final phase of healing as the underlying granulation tissue is yet to be remodelled.
Remodelling and Maturation
The remodelling phase is the final phase of wound healing where granulation tissue matures to form scar tissue and the repaired tissue tensile strength is increased. Essentially, the number of capillaries is reduced as they aggregate to form larger vessels; cell density and metabolic activity within the granulation tissue decreases; and, changes to the collagen type, abundance, and organisation occurs.42,58 Type 3 collagen, initially produced at high levels during the proliferative phase, is replaced by type 1 collagen, which becomes the dominant collagen within the skin. Tissue tensile strength is subsequently enhanced through the reorganisation of the collagen fibres, which were randomly deposited during granulation tissue formation. Over several months, key enzymes such as lysyl oxidase and the transglutaminases, present within the ECM, begin covalently cross-linking the collagen molecules and, in concert with proteolytic MMPs and collagen-stimulating TGF-β, the matrix is transformed.59 The final outcome is the formation of a scar with a maximum of 80% of the tensile strength of the original skin.42 Normally, the majority of acute wounds will heal completely; however, extrinsic and intrinsic factors can complicate the process, causing the wound to resolve into a chronic state.
Wound Healing Breakdown and the Rise of Persistent Ulceration
A number of factors, both systemic and within the local microenvironment, can alter the physiological response to wounding (Table 2). Previous reports have described considerable differences between acute and chronic wound environments; however, there are still many biochemical ‘unknowns’ related to why wounds become chronic. Moreover, there is even less information on the biochemical changes that drive a chronic wound to eventually close. It is clear, however, that the dysregulation and disruption of molecular mechanisms play a key role in the formation and persistence of chronic wounds. It is through a deeper understanding of the changes occurring at the protein level that mechanisms leading to wound resolution can be determined.
Table 2.
An inventory of some of the underlying local and systemic factors that influence the formation and persistence of chronic wounds.*
| Local Factors | Systemic Factors |
|---|---|
| Infection (e.g. pathogenic bacterial colonisation) | Chronic disease (e.g. venous disease, diabetes mellitus) |
| Tissue maceration | Malnutrition |
| Foreign bodies | Congenital healing disorders (e.g. Marfan’s syndrome) |
| Altered protein abundance | Alcoholism |
| Free radical oxidative stress | Glucocorticoid steroids |
| Ischemia-reperfusion injury | Chemotherapeutic drugs |
| Localised cancer | Advanced age |
| Venous insufficiency | Distant cancer |
| Mechanical trauma (e.g. pressure sore) | Uremia |
| Hypoxia | Anemia |
| Toxins | Obesity |
| Radiation | Smoking |
| Iatrogenic (side effects of interventions) | Systemic infection |
| Biochemical dysregulation | Genetic variants |
Aetiologies of Chronic Wounds
The aetiologies of chronic wounds are diverse, however, more than 80% of these wounds are related to diabetes mellitus, venous insufficiency, or high arterial blood pressure.60 The majority of chronic wound aetiologies encountered in clinical settings include ulcers of venous disease and insufficiency,61,62 arterial disease,63 mixed (venous and arterial disease),64 pressure,65 and diabetic neuropathy.66 Although venous disease represents a dominant ulcer aetiology, there are many different underlying conditions that can lead to the formation of a chronic wound.67–70 Less prominent aetiologies can arise from malignancies,71 rheumatoid arthritis,72 and lymphoedema.73 Despite differing aetiologies, most chronic wounds show similar behaviour and progression.74 This can be due to similarities in the basal genetic, proteomic and metabolomic response to injury and repair. As mRNA, enzymes, peptides and other metabolites play key roles during the phases of healing, any alterations to their actions can have dramatic effects on the healing outcome.42 Importantly, different aetiologies may have different effects on the wound biochemistry, however, the end result remains a disrupted healing process and the formation of a chronic wound.67–70
Often the wound aetiology becomes an important factor in the choice of treatment. For example, venous insufficiency requires compression to the afflicted limb through the use of bandaging or stockings, which improves circulation and can aid in the exchange of molecules from the wound site.75 However, compression therapy often involves uncomfortable levels of pressure that lead to low patient compliance following the closure of a chronic wound, which results in wound reoccurrence rates of up to 70% within five years.76 Moreover, it is often the underlying health of the limb that contributes to the healing of the wound or the persistence of an ulcer77 and in some cases these systemic aspects are overlooked in treating the wound. In addition, a number of wounds with varying aetiologies are encountered in clinical practice; these are compounded by other independent factors associated with wound persistence making wound management quite complex. Thus, the molecular changes that occur at the wound site that are similar and disparate between aetiologies represent an important consideration in the development of clinically relevant biomarkers.
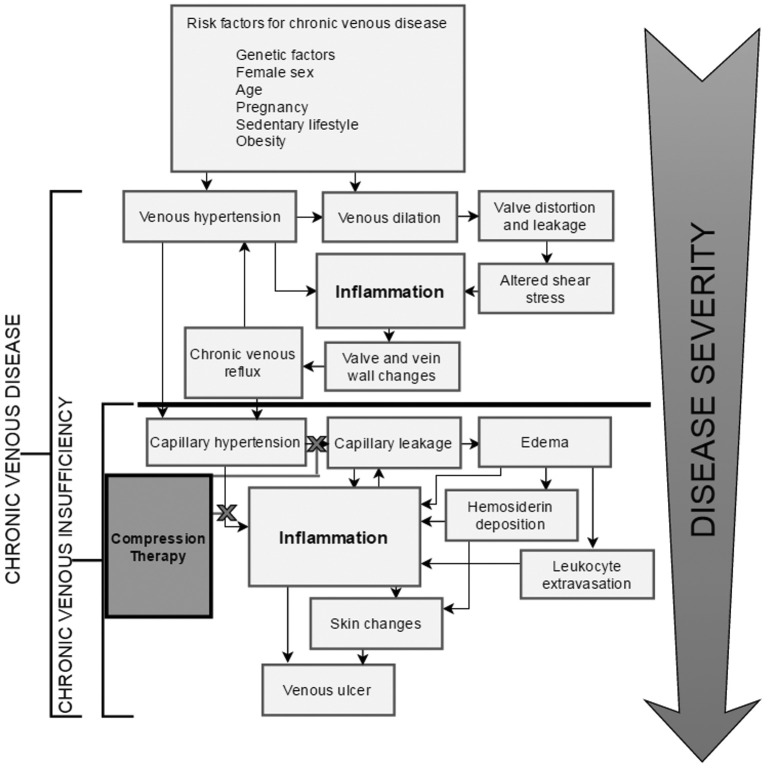
Venous ulcers represent the vast majority of ulcers, with reports in the literature of between 45% and 90% of all ulcers.78,79 Venous leg ulceration represents the most severe manifestation of CVeD. As the literature suggests, CVeD is a multifaceted disease state with an array of clinical manifestations. The onset of the early stages of CVeD are subtle and tend to proceed undetected until the disease is well underway, hence the lack of aetiological understanding. There is firm evidence that CVeD is a progressive disease and that some lifestyle and environmental factors exacerbate the condition. As outlined in Figure 2, the progression of CVeD would appear to follow a discernible trend of conditions that facilitate perpetual inflammation. What remains elusive then, is the propensity for some patients to gradually advance to more severe stages of CVeD while others stagnate.
Figure 2.
The progression of chronic venous disease (CVeD) into venous ulceration. CVeD progresses from mild symptoms (seen in CEAP classes 1–3) to severe pathology (seen in CEAP classes 4–6). There is a clear relationship between sustained venous hypertension and the development of microangiopathy and chronic venous insufficiency, from which more severe disease states such as lipodermatosclerosis and venous ulceration can arise. The fundamental aspect in the progression of venous pathology is the perpetuation of inflammatory processes. The current standard of treatment for patients who experience severe symptoms of CVeD is compression therapy, designed to apply continual pressure on the microvasculature of the lower limb and reduce peripheral hypertension and its implications in inflammation. Although many patients experience relief from such treatment, a large proportion continue to endure severe symptoms of CVeD despite compliance with best practice care.80, 200
Chronic Venous Disease of the Lower Limb
Chronic venous disease is an extremely common, wide-reaching disease; the prevalence of which increases significantly with age.80 CVeD can manifest itself with a range of clinical symptoms, the most distinctive being oedema, varicose veins, pigmentation and sclerosis of the skin, and venous ulceration. Although a patient may be asymptomatic or present only with an isolated symptom of CVeD, most patients suffer from several symptoms in combination. This is due to the complexity of the pathophysiological mechanisms that underpin these symptoms, some of which are associated with more detrimental effects on patient health than others. The term ‘Chronic Venous Disease’ therefore encompasses a wide range of disease states that appear in varying degrees of severity among patients. The CEAP (Clinical, Etiological, Anatomical and Pathophysiological) Classification system, outlined in Table 3, was developed to allow clinicians to categorise the severity of a patient’s CVeD. Higher CEAP values correspond to worse clinical pathologies, where the highest value of six indicates an active ulcer. Moreover, a clinical cohort study has shown that patients classified at a higher CEAP classification reported a reduced quality of life.81 Longitudinal studies have also shown that over six years (between 2000 and 2006), up to 32% of patients advance from C2 to higher CEAP classes,82 showing that CVeD is a progressive disease. This presents an implicit problem for developed countries with an increasingly ageing population.
Table 3.
Categories of CEAP (Clinical, Etiological, Anatomical and Pathophysiological). Categories are based on the presentation of symptoms increasing in severity. Note that the C4 category can describe a number of different changes to the skin. A classification of C4a describes the presentation of pigmentation and venous eczema. A classification of C4b describes the presentation of lipodermatosclerosis plus atrophie blanche.
| CEAP Classification | Pathophysiology | Corresponding Symptom |
|---|---|---|
| C0 | Asymptomatic | No clinical symptom present |
| C1 | Mild venous reflux | Telangiectasia/reticular veins |
| C2 | Aberrant ECM remodelling | Varicose veins |
| C3 | Venous hypertension, vasodilation, and fibrin cuff formation | Oedema |
| C4a|C4b | Leukocyte extravasation / Iron deposition and sclerosis | Pigmentation + eczema Lipodermatosclerosis + atrophie blanche |
| C5 | Venous insufficiency with adequate healing | Healed venous ulcer |
| C6 | Inflammatory dysregulation | Active venous ulcer |
Varicose Veins
Varicose veins can be described as convoluted and dilated veins, typically affecting the superficial veins of the lower leg. Varicose veins affect approximately one third of the adult population.83 Some prevalence studies, however, report the incidence of varicose veins to be as high as 56% and 60% in men and women, respectively.84 These patients often experience discomfort, oedema, and changes in appearance to the skin85 as a result of the condition, in addition to higher rates of depression and anxiety than the general population.86 The tortuous appearance of varicose veins can be attributed to structural and biochemical changes that occur within the vein wall,83 as well as the mechanical stresses resulting from venous reflux and turbulence.87 It has been suggested that the mechanical stresses sustained by the vein wall and valves are the predominant cause of these structural changes.83 However, this causal relationship has not been conclusively validated, and there is evidence to suggest that, not only the structural, but the biochemical changes within the vein may precede venous reflux and incompetence.88 Thus, the nature of biochemical changes within the lower limb are just as important in developing a comprehensive understanding of venous health.
While the thickness and distribution of cell types and ECM components are typically uniform throughout vein walls, this is not observed in varicose veins. Histologically, varicose veins have a highly heterogeneous distribution of tissue layers. The walls of varicose veins are sporadically arranged with sections of hypertrophy and atrophy, giving rise to their highly convoluted appearance. Compared to healthy veins, ECM components were found to be overabundant in varicose veins.89 This is likely due to the observed increase in growth factors such as TGFβ1 and bFGF which post-translationally modify TIMP and MMP expression90 in favour of ECM overproduction.89 In addition, ECM alteration could, at least in part, be explained by the differentiation of smooth muscle cells from a contractile to a proliferative phenotype,87 which secrete a different variety of proteins. Smooth muscle cells have been shown to produce excess levels of type I collagen and deficient levels of type III collagen.91 This disparity in typical collagen production may explain the loss of elasticity observed in varicose veins.
Oedema
Oedema is the localised pooling of bodily fluid within the interstitium of tissues, and is often driven by venous hypertension. Venous hypertension is caused by sustained pressure in the vasculature of the leg, arising from valvular incompetence, failure of the calf muscle pump or blockage within the vein. Persistent pressure in the macroscopic vascular system is transferred to the capillary system, resulting in increased capillary permeability and other manifestations of microangiopathy. Fluid and cellular components of blood are less restricted to entering the interstitial regions of the blood vessel walls and the dermis, resulting in swelling of the lower limb. Oedema has been reported to be the most commonly presented symptom among patients with CVeD,92 and its presence indicates a level 3 CEAP classification.
Lipodermatosclerosis
As increased permeability of capillaries also facilitates other pathology, oedema is rarely observed in isolation. Extravasation of leukocytes is another consequence of increased vessel permeability and is associated with the deposition of extraneous materials such as iron and fibrin in the pericapillary space and dermis. Browse et al. hypothesised as early as 1982 that the accumulation of fibrin in the dermis of the lower limb played a crucial role in venous ulceration, resulting from the inability of nutrients to efficiently diffuse into dermal tissues.93 Falanga et al. also noted decreased fibrinolytic activity in patients with lipodermatosclerosis and venous ulceration.94 Presence of these ‘fibrin cuffs’ in the pericapillary region of the lower limb has been shown to be heavily associated with the presence of lipodermatosclerosis and venous ulceration in patients.95–97
Lipodermatosclerosis is a term that describes changes to the dermis of the lower limb. It is characterised by inflammatory lesions and hyperpigmentation of the dermis, finally progressing to fibrotic hardening of the skin and in an ‘inverted bottle’ appearance of the leg. Patients suffering from lipodermatosclerosis experience pain and tenderness in the area; the site is often warm, scaly and rigid to the touch.98 The condition is usually confined to the medial aspect of the lower limb. The presence of lipodermatosclerosis in patients is frequently associated with venous ulceration.99 Nemeth et al. reported that the extent of lipodermatosclerotic symptoms in patients is strongly associated with venous ulcer recalcitrance.100 Histological examination of subcutaneous lipodermatosclerotic tissue reveals dilated veins, haemosiderin and fibrin deposition, sclerosis and haemorrhage.101 Acute phase lipodermatosclerosis also shows lymphocytic and inflammatory cell infiltrate, which diminishes as the disease progresses and the tissue becomes sclerotic.101
Although the exact aetiology of lipodermatosclerosis has not been established, there is evidence to suggest that sustained venous hypertension leads to increased attachment of leukocytes to the endothelium wall resulting in their activation, analogous to an inflammatory response.102 Macrophages and T lymphocytes were found to be the most commonly extravasated cells in lipodermatosclerotic skin biopsies, coinciding with increased ICAM-1 expression. Biopsies that represented persistent lipodermatosclerosis demonstrated increased quantities of the proinflammatory cytokines IL-1α and IL-1β103 that were not observed in acute cases. Pericapillary fibrin was also present in these specimens, in accordance with previous observations. Despite the strong correlation between pericapillary fibrin deposition and the presentation of severe dermal pathology, a number of studies have refuted the causal relationship between pericapillary fibrin and venous ulceration.99,104–106 There is evidence to suggest, however, that the presence of fibrin within the dermis recruits additional leukocytes to the area,107,108 perpetuating the inflammatory response.
Iron Overload
Another key symptom of CVeD is iron overload. Excess iron is sequestered by the protein complex, haemosiderin, which is located in the intracellular region of cells that predominantly populate the dermis of the skin. Excessive levels of haemosiderin within the dermis have been shown to induce hyperpigmentation of the dermis,109 exacerbate the inflammatory response and delay wound healing in cases of venous ulceration. This appears to be due to the direct roles of haemosiderin in ECM degradation and inhibition of tissue repair mechanisms.110 Moreover, iron overload within hepatocytes has been directly correlated with liver fibrosis in patients with iron storage disorders such as haemochromatosis,111 and it may be plausible that this also occurs in other tissues such as the dermis. Leonarduzzi et al. has demonstrated that oxidative stress resulting from iron overload stimulates the expression of macrophage-associated-cytokines in vitro, which can lead to the aberrant deposition of fibrotic tissue.112
Collectively, these studies suggest that excessive iron has direct implications in chronic inflammation and the consequential development of sclerotic tissue. This contention is supported by a study conducted by Caggiati et al., to investigate the extent of haemosiderin deposition within the dermis using Perl’s Prussian Blue (PPB) stain.113 Dermal biopsies of several specimens of differing stages of chronic venous disease were examined. Haemosiderin deposition was absent in normal and mildly pigmented skin samples, whereas increased staining was observed in samples taken from severely pigmented skin and lipodermatosclerotic skin. Importantly, PPB stain revealed that haemosiderin was also present in samples from the wound edge and bed of ulcerated specimens.113 Since specimens’ that exhibited regression of ulceration, yielded comparatively lower levels of staining, it is hypothesised that haemosiderin deposition diminishes with tissue healing. However, as specimens from fully healed ulcers could not be obtained due to ethical considerations, this hypothesis has not yet been substantiated.
Genomics and Transcriptomics
Genetic association studies are integral to understand the epidemiology of complex diseases; the ultimate goal being to identify genetic variants that contribute to pathology and their role in disease aetiology. This process may not only provide further information about the pathophysiology of a disease, but also reveal novel and potentially targetable biomarkers for disease onset and progression. There are a variety of methods to perform genetic association studies, including genome-wide approaches and candidate-gene approaches.
Genetic linkage and association studies have largely been successful in identifying genetic variants that contribute to Mendelian inherited diseases such as Huntington’s disease,114,115 cystic fibrosis116 and haemochromatosis117 leading to effective screening tools for early diagnosis. These diseases, however, are generally the result of a small number of rare mutations that confer a high risk of disease onset and progression. More recently, genome-wide association (GWA) studies have been used to identify genetic variants that confer a greater risk of developing complex diseases, which potentially involve multiple genetic variants.118 For this reason, it has been difficult for GWA studies to comprehensively identify all genetic variants within a complex disease. This is because there can exist a large number of common, moderate risk genetic variants that may, collectively or in various combinations, predispose an individual to a disease. This is further confounded by environmental and lifestyle factors that may accumulate into a larger conferred risk of disease. It is therefore difficult to identify significant variants associated with complex disease traits, particularly when affected patients may display a range of disease phenotypes, such is the case for chronic venous disease. Successful GWA studies, therefore, require large numbers of cases and controls (typically in the thousands) and strict phenotyping criteria to identify variants that may only contribute a small amount to disease pathogenesis and/or progression.
To date, no GWA study has been performed to identify variant alleles that contribute to CVeD or VLU. This is most likely due to considerations in the financial cost of such a study, the immense sample size required to achieve statistical power,119 considerations in the demographic of the sample population, as well as the substantial chance of a high false discovery rate. An alternative to this method is the candidate-gene approach, where-by the presence of genetic variants associated with a pathway within a multifactorial disease are compared between a patient and control population, and the relative risk of the variant to disease characteristics is calculated, usually as an odds ratio.120 This method has been performed with respect to CVeD and VLU with some success, and has shown several genetic polymorphisms that are associated with disease characteristics of VLU.120–125 These include variants in genes that function in vascular development, iron homeostasis, haemostasis, extracellular matrix homeostasis and inflammation. The obvious shortcoming with the candidate gene approach, however, is that potentially significant genetic variants may be neglected. Due to the complexity of the intricate molecular mechanisms that underpin wound healing, particularly within the context of CVeD, there may be countless biochemical pathways and genetic variants therein that could possibly be overlooked. Despite this, the candidate gene approach has so far revealed valuable information, as the genetic variants identified to date may potentially act as valuable prognostic markers for wound healing, or perhaps even as therapeutic targets.
It is evident that investigating the genome may provide insights into the genetic variants that predispose an individual to VLU. In order to understand the inflammatory/healing processes at the molecular level, however, this method is not sufficient to explore how the genes are expressed within the wound environment. In particular, it is important not only to investigate the differential expression and activity of proteins between wounds at different phases of inflammation and healing, but to understand how these can affect wound healing.
Transcriptomic analysis of venous leg ulcer tissue has revealed several significant genes that are differentially expressed between healing and non-healing wounds,126 many of these with direct roles in inflammatory and apoptotic pathways. Moreover, gene expression profile analysis has revealed differentially expressed genes within other tissue types, such as varicose veins. Varicose veins have been a particular topic of interest, due to their substantial role in the progression of CVeD and initiation of inflammatory stress in the lower limb resulting from a lack of venous return. Many studies have shown several genes to be differentially expressed between varicose veins and controls,127–130 however as yet there is inconclusive evidence as to whether this dysregulation is a cause or effect of the structural changes within the tissue. Overall, these data provide an insightful ‘snapshot in time’ of the pathophysiological stages that are understood to facilitate the progression of CVeD and provides a foundation on which to expand the current understanding of its development. Although this may provide potential prognostic and therapeutic targets, there is currently limited information on why these genes are differentially expressed, their role in wound healing, and their causative relationship with disease progression. This relationship could potentially be elucidated with a temporal analysis of gene expression within varicose vein and/or chronic wound patients at various stages of CVeD and wound healing.
There is strong evidence that CVeD is a largely heritable disease, but it is also well understood that environmental and lifestyle factors can have a significant role in its onset, progression and severity. The complex interplay between each of these factors make it difficult to elucidate the causal relationship between disease mechanisms and gene expression. Wound healing, like any other complex biological process, is driven at the functional level by numerous peptides and proteins that are assembled and post-translationally modified in ways that can’t be predicted by gene expression analysis.131 It is dubious to attempt to draw accurate conclusions of the underlying molecular mechanisms of venous ulceration based on genomic and transcriptomic datasets alone, as analysis of the genome and gene expression is not sufficient to elucidate the relationship between the genome, gene expression and wound microenvironment.
Proteomics and Metabolomics
The fields of proteomics and metabolomics have undergone substantial technological advancement over the last decade, particularly in the improvement of mass spectrometry instrument sensitivity and acquisition speed. This has resulted in great leaps in our understanding of wound biochemistry at both targeted and systems levels.
Chronic Wound Protein Inventory and Markers of Differentiation: The Wound Fluid Proteome
A number of previous studies have investigated the protein constituents of chronic wounds. Those prior to 2010 have been comprehensively reviewed elsewhere.132 Subsequent studies have expanded the list of known proteins present in chronic wounds, with hundreds of proteins detected within wound fluid.133–141 Although many additional proteins have been detected in wounds, such as various cytokines and interleukins through sampling by tissue biopsy and antibody-based techniques, these are more difficult to detect using shotgun proteomics due to their relatively low abundance in wound fluid. It is likely that a single complete view of the wound proteome would require the use of multiple technologies, such as mass spectrometry with immune-enrichment/depletion strategies or sequential windows in data acquisition, to aid in the detection of low abundant molecules.
Edsberg et al. generated a large catalogue of the proteins present in wound fluid of pressure ulcers, and compared the presence or absence of these proteins in wounds that healed and those that failed to heal.141 This study demonstrated key differences in the proteomes of pressure ulcers that reflected differences between healing wounds and persistent wounds, in addition to spatial differences between the centre and periphery of the wound. The proteome of wound fluid from venous ulcers has yet to be examined to the same comprehensive extent. Moreover, the finding that spatial differences exist within a chronic wound suggests that future studies could apply higher resolution sampling in order to better understand the biochemistry of the ulcer. Eming et al.135 have compared acute wounds with venous leg ulcers and found an elevated abundance of proteins associated inflammation, in particular Annexin A1 and Protein S100-A9. This is further supported by the work by Krisp and others,133 who found elevated abundances for proteins within the annexin, S100 and MMP families within diabetic ulcers. This suggests the major inflammatory response within a chronic wound may be similar across wound aetiologies.
Protein Degradation is Increased in Chronic Wounds
Comprehensive investigations of various enzymes present in wound fluid have demonstrated that non-healing ulcers have increased protease activity, predominantly MMPs, compared to acute wounds.142–149 Moreover, the actions of human proteases are further exacerbated by the actions of bacterial proteases.150 This heightened protease activity then leads to excessive protein degradation within the wound, which impacts on normal healing processes and homeostatic responses. Critically, the consensus within the literature suggests that the vast majority of non-healing wounds will have degraded proteins, which may imply that the peptide products could be indicative of the chronic environment; potentially, these degradation products could be useful as biomarkers of wound healing or persistence. Therefore, the focus of biomarker discovery should be on the biomolecules of wound fluid as they exist within the local environment, so as not to discount the additional information that can be obtained from partially digested proteins. Moreover, the analysis of other biochemical constituents of wound fluid, such as proteins or polypeptides produced by wound microbes, which have been overlooked in previous studies of fluid, provides a unique perspective for biomarker discovery research.
In comparison to acute wounds, chronic wounds have elevated levels of protease activity at the wound site.151 In chronic wounds, increased levels of MMPs released by fibroblasts, macrophages, eosinophils, and, in particular, neutrophils, actively remove components of the ECM. This degradation becomes a problem when MMP activity is poorly regulated and the equilibrium between MMPs and their inhibitors, the TIMPs, shifts in favour of the proteases, resulting in a stagnant inflammatory phase of healing.152 It has been noted that in chronic pressure ulcers the major collagenase present is the neutrophil-derived protease, MMP8.42 This suggests that neutrophils, present in the early stages of healing, play an important role in the development of chronic wounds. In addition to increased protease activity, chronic wounds also have elevated levels of pro-inflammatory cytokines compared to acute wounds.153, 154 There are two key inflammatory cytokines present in chronic wounds, interleukin-1 (IL-1) and TNF-α, which initiate the cascade of inflammatory mediators by targeting the endothelium.155 Neutrophils attached to the endothelium subsequently infiltrate the wound site, producing reactive oxygen species and damaging host tissues. A high abundance of these cytokines signal the infiltration of more neutrophils that further exacerbate localised tissue damage.156 Interestingly, neutrophils also generate hypochlorous acid (HOCl) and N-chloramines through the myeloperoxidase-H2O2-halide system. These effectively inhibit TIMPs within the wound site and shift the protease-antiprotease equilibrium further towards degradation.151 Furthermore, excessive and prolonged inflammation is suspected to be partially attributed to the presence of sustained quantities of TNF-α within the wound area, but research in this area is still limited and further investigation is required.157
The Chronic Wound Metabolome
Compared to proteomic studies, metabolomic investigations into venous leg ulceration is still within its infancy. To date, the major metabolite targets within venous leg ulcer research have been L-arginine, oxidative free radicals, nitric oxide, and iron, with each molecule found to be significantly elevated within the chronic wound environment and strongly associated with aspects of inflammation.1,158,159 These data correlate with proteomic data on the presence of a local inflammatory phenotype within chronic wounds. Critically, within the literature there are limited examples of discovery-based or large screening metabolomic approaches for investigating venous leg ulcers. This has resulted in an extensive knowledge deficit on the dynamic functional aspects of VLU compared to the proteome. Much of the current perspective of the wound metabolome has been derived from targets assays, leaving a considerable gap within the literature that is yet to be fully explored.
Proteomics Approaches
Mass spectrometry is predominantly used over traditional chemical, gel or antibody based methods for large scale proteome analysis. This is primarily the result of research questions focussed on both the qualitative identification of proteins and the quantification of these proteins within a given system.160,161 Mass spectrometry facilitates this and can provide high mass accuracy with high confidence in protein identification and abundance.162,163 At present, there is a wide selection of mass spectrometry instruments available for use in a variety of different research investigations.
Top Down Proteomics
Top-down proteomics focusses on the native protein or polypeptide. This is ideal in proteomic investigations where accurate masses for whole proteins can reveal the presence of post-translational modifications (PTM).164 Due to the size and physiochemical properties of some native proteins, aspects of ionisation and collision induced fragmentation within the mass spectrometer do not perform well. In the majority of top-down approaches, protein sequence information is not obtained, but rather crucial data on the macro-changes to the proteome can be observed, which can include protein-protein interactions.
An approach often taken in top-down proteomics involves profiling the biomolecular constituents of a sample and then later identifying those target analytes with differential abundance. These profiling experiments are highly suited to discovery phase analyses, wherein changes within a biological system can be more easily deduced.165 These analyses will often use MS1 survey scan data to fingerprint a sample and generate a mass spectrum that reflects the underlying biochemical landscape. Such methods are key to surface enhanced or matrix assisted laser desorption/ionisation (SE/MA-LDI) time of flight (TOF) mass spectrometry (MS), where profiles of proteins/polypeptides of a sample represent the detectable biochemistry of that sample at a given point in time.
SELDI-TOF MS is a biomarker discovery phase technology. The technology utilises chromatography-based chemistries to selectively bind proteins and peptides onto the surface of a metal target. This allows for the chemical separation of a complex proteome and with subsequent mass spectrometry, generates data by analysing molecules based on differences in their mass-to-charge ratio (m/z). It has advantages in high-throughput capabilities and is well suited to early discovery phase research, but has become a defunct technology due to concerns with reproducibility.168,169 Alternatively, MALDITOF MS continues to be used to examine wound biology, particularly through bacterial profiling and biopsy tissue imaging approaches.170,171 MALDI imaging requires the use of tissue sections and thus provides protein level information in relation to the architecture of the skin. This is ideal for investigating protein abundance gradients throughout the depth of tissue and those associated with major features, such as hair follicles or the wound edge. The investigation of pressure ulcers by Taverna et al.171 using MALDI imaging MS revealed that the biochemistry differs between the wound bed and the adjacent dermis in chronic wounds, whereas in healing wounds these areas show less variation.
Bottom Up Proteomics
The identification of proteins by mass spectrometry predominantly takes a bottom-up or shotgun approach; wherein proteolytically generated peptide fragments are sequenced by the mass spectrometer and then mapped to original proteins using in silico methods. Bottom-up approaches will often separate peptides using liquid chromatography systems coupled directly to the mass spectrometer. Typical methodology requires the use of a proteolytic enzyme, commonly trypsin, which hydrolyses peptide bonds of specific amino acid residues (after lysine or arginine) and creates a pool of peptides. These peptides can then a separated by liquid chromatography and, due to the amino acid characteristics of tryptic peptides, these molecules retain a positive charge at an acidic pH, which allows easy manipulation and measurement of their gas-phase adducts in the mass spectrometer. The bottom-up proteomics approach generates data at the peptide level, which provides insight into, not only the amino acid sequence, but also the specific amino acid PTM or substitutions of a target protein.
Data Dependent Acquisition (DDA)
The traditional mass spectrometry approach using liquid chromatography tandem mass spectrometry (LC-MS/MS) in DDA mode permits the identification of dominant peptide sequences and, subsequently, protein identification through sequence database matching. The majority of DDA experiments are performed using quadrupole-orbitrap or quadrupole-TOF based instruments due to their high resolution capabilities. Within the mass spectrometer, an initial survey scan of a complex mix of peptides will determine dominant precursor ions, which are subsequently fragmented by collision-induced-dissociation with an inert gas and the abundance of these product ions detected and displayed as mass-to-charge peaks within a spectrum. The majority of the wound fluid proteome has been identified using this approach, however, a more comprehensive interrogation of the venous ulcer wound is required.
Selected / Multiple Reaction Monitoring (SRM/MRM)
In targeted mass spectrometry, the precursor and product ion pairs, known as transitions, for a target peptide are known. Using DDA data, unique and highly detectable transitions can be targeted using a triple quadrupole mass spectrometer, where precursor masses are isolated in the first quadrupole, fragmentation within the second, and product ions isolated in the third quadrupole prior to detection. This approach is useful for multiplex experiments that measure the abundance of multiple peptide targets within a single run. Although MRM can facilitate the verification of biomarker targets and be used in the development of clinical diagnostic tests, there is limited information within the literature where this is the case.
Data Independent Acquisition (DIA)
Data independent acquisition utilises consecutive precursor isolation windows, of a fixed or custom variable size, to systematically capture the presence and quantity of peptides/molecular targets.172 The detection of all fragmentation ions via this unbiased approach effectively digitises a biological sample for interrogation against spectral libraries of known peptides. Critically, there has been wide acceptance of the technology in multiple areas of research.173–181 However, DIA has not yet been applied to the proteomic profiling of wound fluid and, thus, offers a unique opportunity to increase the current knowledgebase on venous leg ulcers. The DIA approach would be expected to produce quantitative data on hundreds, potentially thousands, of proteins within wound fluid samples and may substantially progress our understanding of chronic wound biochemistry.
Metabolomics Approaches
Metabolomics includes the identification and quantification of metabolites generated as a result of cellular physiology.182 Metabolomics captures the metabolome at a fixed point in time and thus contains a snapshot of both end-products and intermediates of cell and tissue metabolism. Metabolites include, but are not limited to, carbohydrates, lipids, vitamins, steroids, amino acids, nucleic acids, and peptides183,184 and are viewed as ideal biomolecules for diagnostic biomarkers within clinical practice.182,185 The two main approaches to metabolite discovery and measurement are nuclear magnet resonance (NMR) spectroscopy and mass spectrometry using either gas or liquid chromatography (GC-MS / LC-MS).
NMR spectroscopy is a quantitative, reproducible and nondestructive method capable of profiling liquid and tissue samples.186–188 Moreover, NMR is able to provide data on metabolites that cannot be ionised for mass spectrometry based detection, making it a valuable tool for biomarker discovery research. Mass spectrometry based detection of metabolites provides improved sensitivity and specificity over NMR spectroscopy. Moreover, this approach requires substantially less volume of sample, which is ideal for the analysis of microlitre volumes of wound fluid that are often collected. Both GC and LC utilise upstream columns (chromatography) to separate out metabolites prior to ionisation and detection within the mass spectrometer. Various column chemistries can be used to detect different classes of molecule, in addition to variable parameters (e.g. m/z range filters, selected reaction monitoring, collision energies) within the mass spectrometer.
Bioinformatic Integration of Omics Data
The generation of large multivariate datasets from multiple omics platforms can present a challenge when attempting to integrate these together to extract meaningful biological data. It is clear from the literature that any omics data integration relies on, at least, a three-step process of identifying a common variable to connect datasets (at minimum by sample name), delineation of relationship networks using ontological enrichment or clusters and modules, and application of a statistically sound model of the system that could permit simulation and prediction of system phenotypes.189–191 A number of quality reviews on the topic provide a wealth of resources to attempt omics data integration, with potential frameworks,189,190 available software and databases,85,191,192 and analytical methods189 described. The integration of multiple omics datasets could reveal new mechanism of action for the persistence or healing of chronic wounds and may enable the implementation of personalised medicine within clinical practice.190 Moreover, by integrating multi-omics datasets, the issues surrounding high false positive rates with genome wide association studies could potentially be mitigated by the incorporation and clustering of the other levels of molecular/omics data.
Consideration of the temporal component within omics data integration represents a large challenge in itself, with transcription, translation and metabolic functions occurring in dynamic flux. This can confound the process of elucidating new regulatory mechanisms within a system and may require the use of correlation analyses to determine relationships between molecules.190 This aspect will be of paramount importance for the future of omics research into venous ulcer pathophysiology. To date, the integration of more than two omics datasets from complex systems (in particular, data from transcriptomic, proteomic and metabolomic analyses) has not been widely explored.193 It can be expected that once more comprehensive integrations are achieved within model systems, new approaches may be developed to address more complex diseases and systems, such as VLU and other chronic wound aetiologies.
Clinical Challenges in Biomarker Research
The discovery and development of biomarkers from wound fluid holds promise to help inform wound management practices and reduce this impact. Indeed, it has been described in the literature that the proteomic analysis of wound fluid is crucial for the discovery and delivery of indicators of healing.197 Wound fluid characteristics can differ dramatically between patients and between ulcers, including volume, biomolecule abundance, pH, microbiome, and trace metals. With respect to the investigation of the wound fluid proteome, the concentration of protein can vary dramatically.194 Critically, the normalisation of each sample by protein concentration or total protein is crucial to the comparative analysis of healing and non-healing wounds.
In addition, as wound fluid shares similarities to serum, there is the concern regarding dynamic range effects.134 For example, proteins present in wound fluid can vary in their abundance from those that are very high, such as albumin, to those that are in very low abundance, such as growth factors or cytokines.195,196 This wide dynamic range of protein abundance complicates the detection of low abundant protein species and often requires sample fractionation or complexity reduction through other methods. Likewise, metabolites of interest can be enriched for prior to data acquisition to remove confounding background molecules.
Furthermore, patient compliance and participation can also be problematic, as patients can choose at any time point to withdraw from a study or fail to present at an expected time point. Nevertheless, despite however complex the clinical situation may be, the advantage of using clinical samples lies in the ultimate applicability of the research results to the clinical setting and research problem. This is often not the case in in vitro studies or in vivo animal models, where the samples do not directly reflect the human response, at the genetic, protein and metabolite levels, nor the uncontrolled reality of a clinical environment. The future of research into chronic wounds is to obtain a more comprehensive understanding of the biochemical changes that occur within wounds and to deduce key factors associated with healing.
Conclusion
The identification of clinically relevant molecules of chronic wound healing outcomes is an area of limited research. However, recent improvements in omics technologies provide the means to undertake more comprehensive investigations of chronic wound and aid in biomarker discovery and increasing our understanding of VLU biology. Previous omics approaches have revealed significant differences between healing and non-healing chronic wounds. However, there are currently no clinically relevant biomarkers used in standard wound management practices. Several challenges still lie ahead for the analysis of clinical samples, cataloguing of the wound fluid proteome and metabolome, and the identification and development of biomarker tools. Advancements in MS sequencing technology, such as DIA, have not yet been applied to the analysis of wound fluid. These advances permit the comprehensive exploration of the wound fluid proteome with a greater depth and scope than that of previous studies. Moreover, metabolomic methods in biomarker discovery are yet to be fully explored with respect to the analysis of wound fluid from venous ulcers.
The use of a systems approach that includes an analysis of both the human genome, based on predisposing genetic variants, and proteome and metabolome of wound fluid represents a unique strategy towards biomarker discovery. Incorporating omics data from multiple perspectives provides a more comprehensive profile of wound fluid and the underlying pathophysiology of the wound. Through such a systems approach, the likelihood of deducing molecular markers of healing outcomes would be increased. Moreover, there is potential for such markers to be developed into tools that can assist with clinical practice and ultimately make a positive impact towards addressing a global health challenge.
Acknowledgments
The authors would like to acknowledge the student support stipend awarded to ERS from the Wound Management Innovation Cooperative Research Centre.
Footnotes
Competing Interests: None declared.
References
- 1.Mannello F, Ligi D, Canale M, Raffetto JD. Omics profiles in chronic venous ulcer wound fluid: innovative applications for translational medicine. Expert Rev Mol Diagn. 2014;14:737–62. doi: 10.1586/14737159.2014.927312. [DOI] [PubMed] [Google Scholar]
- 2.Jurd A. Expenditure on Healthcare in the UK, 1997–2010. Office for National Statistics. United Kingdom Crown copyright 2012.
- 3.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times. 2008;104:44–5. [PubMed] [Google Scholar]
- 4.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. (ix) Dermatol Clin. 2012;30:107–11. ix. doi: 10.1016/j.det.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Ramstadius B. Leg ulcer management. Aust Nurs J. 1997;5:22. [PubMed] [Google Scholar]
- 7.Eaglstein WH. Wound healing and aging. Clin Geriatr Med. 1989;5:183–8. [PubMed] [Google Scholar]
- 8.Lindholm C, Bjellerup M, Christensen OB, Zederfeldt B. A demographic survey of leg and foot ulcer patients in a defined population. Acta Derm Venereol. 1992;72:227–30. [PubMed] [Google Scholar]
- 9.Labropoulos N, Wang ED, Lanier ST, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2012;129:179–86. doi: 10.1097/PRS.0b013e3182362a53. [DOI] [PubMed] [Google Scholar]
- 10.Wicke C, Bachinger A, Coerper S, Beckert S, Witte MB, Königsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized Wound Care Center. Wound Repair Regen. 2009;17:25–33. doi: 10.1111/j.1524-475X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GF, Hussey PS. Population aging: a comparison among industrialized countries. Health Aff (Millwood) 2000;19:191–203. doi: 10.1377/hlthaff.19.3.191. [DOI] [PubMed] [Google Scholar]
- 12.Australia Productivity Commission. Economic implications of an ageing Australia: draft research report. Canberra: Productivity Commission; 2004. [Google Scholar]
- 13.Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 14.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 15.Lewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcers during cardiac surgery. AORN J. 1997;65:933–42. doi: 10.1016/s0001-2092(06)62976-1. [DOI] [PubMed] [Google Scholar]
- 16.Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg. 1998;176(2A Suppl):39S–47S. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 17.Neil JA, Munjas BA. Living with a chronic wound: the voices of sufferers. Ostomy Wound Manage. 2000;46:28–34. 36–8. [PubMed] [Google Scholar]
- 18.Leighton-Bellichach A. A personal and ethical perspective on chronic wound pain. Br J Nurs. 2006;15:909–11. doi: 10.12968/bjon.2006.15.17.21902. [DOI] [PubMed] [Google Scholar]
- 19.Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63:216–20. doi: 10.1097/00006842-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67:253–71. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Keeling D, Price P, Jones E, Harding KG. Social support for elderly patients with chronic wounds. J Wound Care. 1997;6:389–91. doi: 10.12968/jowc.1997.6.8.389. [DOI] [PubMed] [Google Scholar]
- 22.Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am. 2011;31:81–93. doi: 10.1016/j.iac.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persoon A, Heinen MM, van der Vleuten CJ, de Rooij MJ, van de Kerkhof PC, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs. 2004;13:341–54. doi: 10.1046/j.1365-2702.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 24.Price P. Defining and measuring quality of life. J Wound Care. 1996;5:139–40. doi: 10.12968/jowc.1996.5.3.139. [DOI] [PubMed] [Google Scholar]
- 25.Franks PJ, Moffatt CJ. Quality of life issues in chronic wound management. Br J Community Nurs. 1999;4:283–9. [Google Scholar]
- 26.Jones J, Barr W, Robinson J, Carlisle C. Depression in patients with chronic venous ulceration. Br J Nurs. 2006;15:S17–23. doi: 10.12968/bjon.2006.15.Sup2.21237. [DOI] [PubMed] [Google Scholar]
- 27.Hareendran A, Bradbury A, Budd J, Geroulakos G, Hobbs R, Kenkre J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care. 2005;14:53–7. doi: 10.12968/jowc.2005.14.2.26732. [DOI] [PubMed] [Google Scholar]
- 28.Ebbeskog B, Ekman SL. Elderly persons experiences of living with venous leg ulcer: living in a dialectal relationship between freedom and imprisonment. Scand J Caring Sci. 2001;15:235–43. doi: 10.1046/j.1471-6712.2001.00018.x. [DOI] [PubMed] [Google Scholar]
- 29.Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31:49–53. doi: 10.1016/s0190-9622(94)70134-2. [DOI] [PubMed] [Google Scholar]
- 30.Cowman S, Gethin G, Clarke E, Moore Z, Craig G, Jordan-OBrien J, et al. An international eDelphi study identifying the research and education priorities in wound management and tissue repair. J Clin Nurs. 2012;21:344–53. doi: 10.1111/j.1365-2702.2011.03950.x. [DOI] [PubMed] [Google Scholar]
- 31.Bennett NT, Schultz GS. Growth factors and wound healing: Part II. Role in normal and chronic wound healing. Am J Surg. 1993;166:74–81. doi: 10.1016/s0002-9610(05)80589-6. [DOI] [PubMed] [Google Scholar]
- 32.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–37. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25:321–40. [PubMed] [Google Scholar]
- 34.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 35.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411–20. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 36.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 37.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–66. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 38.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 39.Perona R. Cell signalling: growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8:77–82. doi: 10.1007/s12094-006-0162-1. [DOI] [PubMed] [Google Scholar]
- 40.Kim WJ. Cellular signaling in tissue regeneration. Yonsei Med J. 2000;41:692–703. doi: 10.3349/ymj.2000.41.6.692. [DOI] [PubMed] [Google Scholar]
- 41.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin GA, Diegelmann RF, Schultz GS. Cellular and Molecular Regulation of Wound Healing. In: Falabella AF, Kirsner RS, editors. Wound Healing. Boca Raton, FL: CRC Press; 2005. p. 21. [Google Scholar]
- 43.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 44.Kerstein MD. The scientific basis of healing. Adv Wound Care. 1997;10:30–6. [PubMed] [Google Scholar]
- 45.Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–25. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 46.Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Ross R, Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J Cell Biol. 1968;39:152–68. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivas F. In this Issue: Inflammation. Cell. 2010;140:755, 757. [PubMed] [Google Scholar]
- 49.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury. 2006;37(Suppl 1):S5–12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Wilson K. Wound healing: the role of macrophages. Nurs Crit Care. 1997;2:291–6. [PubMed] [Google Scholar]
- 51.Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7:1083–103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- 52.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–28. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 53.Bhushan M, Young HS, Brenchley PE, Griffiths CE. Recent advances in cutaneous angiogenesis. Br J Dermatol. 2002;147:418–25. doi: 10.1046/j.1365-2133.2002.05003.x. [DOI] [PubMed] [Google Scholar]
- 54.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(Suppl):S62–7. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 55.Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest. 1994;70:916–24. [PubMed] [Google Scholar]
- 56.Bosanquet DC, Ye L, Harding KG, Jiang WG. Role of HuR in keratinocyte migration and wound healing. Mol Med Rep. 2012;5:529–34. doi: 10.3892/mmr.2011.675. [DOI] [PubMed] [Google Scholar]
- 57.Raja, Sivamani K, Garcia MS, Isseroff RR. Wound reepithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–68. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 58.Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35:767–79. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 59.Moali C, Hulmes DJ. Extracellular and cell surface proteases in wound healing: new players are still emerging. Eur J Dermatol. 2009;19:552–64. doi: 10.1684/ejd.2009.0770. [DOI] [PubMed] [Google Scholar]
- 60.Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2:165–70. doi: 10.1046/j.1524-475X.1994.20305.x. [DOI] [PubMed] [Google Scholar]
- 61.OBrien JF, Grace PA, Perry IJ, Burke PE. Prevalence and aetiology of leg ulcers in Ireland. Ir J Med Sci. 2000;169:110–2. doi: 10.1007/BF03166911. [DOI] [PubMed] [Google Scholar]
- 62.Forssgren A, Nelzén O. Changes in the aetiological spectrum of leg ulcers after a broad-scale intervention in a defined geographical population in Sweden. Eur J Vasc Endovasc Surg. 2012;44:498–503. doi: 10.1016/j.ejvs.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 64.Stacey M, Falanga V, Marston W, Moffat C, Phillips T, Sibbald RG, et al. Compression therapy in the treatment of venous leg ulcers. Nurs Times. 2002;98:39–43. [PubMed] [Google Scholar]
- 65.Jiricka MK, Ryan P, Carvalho MA, Bukvich J. Pressure ulcer risk factors in an ICU population. Am J Crit Care. 1995;4:361–7. [PubMed] [Google Scholar]
- 66.Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. 2009;32:2056–61. doi: 10.2337/dc08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelzén O, Bergqvist D, Lindhagen A. Leg ulcer etiologya cross sectional population study. J Vasc Surg. 1991;14:557–64. [PubMed] [Google Scholar]
- 68.Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol. 1991;25:965–87. doi: 10.1016/0190-9622(91)70295-d. [DOI] [PubMed] [Google Scholar]
- 69.Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley leg ulcer study. Br Med J (Clin Res Ed) 1987;294:929–31. doi: 10.1136/bmj.294.6577.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker SR, Stacey MC, Singh G, Hoskin SE, Thompson PJ. Aetiology of chronic leg ulcers. Eur J Vasc Surg. 1992;6:245–51. doi: 10.1016/s0950-821x(05)80313-5. [DOI] [PubMed] [Google Scholar]
- 71.Hayes S, Dodds SR. The identification and diagnosis of malignant leg ulcers. Nurs Times. 2003;99:50–2. [PubMed] [Google Scholar]
- 72.McRorie ER. The assessment and management of leg ulcers in rheumatoid arthritis. J Wound Care. 2000;9:289–92. doi: 10.12968/jowc.2000.9.6.25993. [DOI] [PubMed] [Google Scholar]
- 73.Holcomb SS. Identification and treatment of different types of lymphedema. Adv Skin Wound Care. 2006;19:103–8. doi: 10.1097/00129334-200603000-00013. quiz 108-10. [DOI] [PubMed] [Google Scholar]
- 74.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–68. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 75.Partsch H. Compression therapy. Int Angiol. 2010;29:391. [PubMed] [Google Scholar]
- 76.Ruckley CV. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology. 1997;48:67–9. doi: 10.1177/000331979704800111. [DOI] [PubMed] [Google Scholar]
- 77.Parker TJ, Broadbent JA, McGovern JA, Broszczak DA, Parker CN, Upton Z. Provisional matrix deposition in hemostasis and venous insufficiency: tissue preconditioning for nonhealing venous ulcers. Adv Wound Care (New Rochelle) 2015;4:174–91. doi: 10.1089/wound.2013.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lautenschlager S, Eichmann A. Differential diagnosis of leg ulcers. Curr Probl Dermatol. 1999;27:259–70. doi: 10.1159/000060619. [DOI] [PubMed] [Google Scholar]
- 79.Mekkes JR, Loots MA, Van Der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol. 2003;148:388–401. doi: 10.1046/j.1365-2133.2003.05222.x. [DOI] [PubMed] [Google Scholar]
- 80.Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488–98. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 81.Kahn SR, Mlan CE, Lamping DL, Kurz X, Bérard A, Abenhaim LA, VEINES Study Group Relationship between clinical classification of chronic venous disease and patient-reported quality of life: results from an international cohort study. J Vasc Surg. 2004;39:823–8. doi: 10.1016/j.jvs.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Rabe E, Pannier F, Ko A, Berboth G, Hoffmann B, Hertel S. Incidence of varicose veins, chronic venous insufficiency, and progression of the disease in the Bonn Vein Study II [Abstract] J Vasc Surg. 2010;51:791. [Google Scholar]
- 83.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96:1231–42. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 84.Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology. 2008;23:103–11. doi: 10.1258/phleb.2007.007061. [DOI] [PubMed] [Google Scholar]
- 85.Campbell B. Varicose veins and their management. BMJ. 2006;333:287–92. doi: 10.1136/bmj.333.7562.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sritharan K, Lane TR, Davies AH. The burden of depression in patients with symptomatic varicose veins. Eur J Vasc Endovasc Surg. 2012;43:480–4. doi: 10.1016/j.ejvs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Badier-Commander C, Couvelard A, Henin D, Verbeuren T, Michel JB, Jacob MP. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193:398–407. doi: 10.1002/path.819. [DOI] [PubMed] [Google Scholar]
- 88.Naoum JJ, Hunter GC, Woodside KJ, Chen C. Current advances in the pathogenesis of varicose veins. J Surg Res. 2007;141:311–6. doi: 10.1016/j.jss.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol. 2000;192:105–12. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH670>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 90.Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064–71. [PubMed] [Google Scholar]
- 91.Sansilvestri-Morel P, Rupin A, Badier-Commander C, Kern P, Fabiani JN, Verbeuren TJ, et al. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38:560–8. doi: 10.1159/000051092. [DOI] [PubMed] [Google Scholar]
- 92.Nicolaides AN. Chronic venous disease and the leukocyte-endothelium interaction: from symptoms to ulceration. Angiology. 2005;56(Suppl 1):S11–9. doi: 10.1177/00033197050560i103. [DOI] [PubMed] [Google Scholar]
- 93.Browse NL, Burnand KG. The cause of venous ulceration. Lancet. 1982;2:243–5. doi: 10.1016/s0140-6736(82)90325-7. [DOI] [PubMed] [Google Scholar]
- 94.Falanga V, Kruskal J, Franks JJ. Fibrin- and fibrinogen-related antigens in patients with venous disease and venous ulceration. Arch Dermatol. 1991;127:75–8. [PubMed] [Google Scholar]
- 95.Hopkins NF, Spinks TJ, Rhodes CG, Ranicar AS, Jamieson CW. Positron emission tomography in venous ulceration and liposclerosis: study of regional tissue function. Br Med J (Clin Res Ed) 1983;286:333–6. doi: 10.1136/bmj.286.6362.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falanga V, Moosa HH, Nemeth AJ, Alstadt SP, Eaglstein WH. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol. 1987;123:620–3. [PubMed] [Google Scholar]
- 97.Claudy AL, Mirshahi M, Soria C, Soria J. Detection of undegraded fibrin and tumor necrosis factor-alpha in venous leg ulcers. J Am Acad Dermatol. 1991;25:623–7. doi: 10.1016/0190-9622(91)70242-t. [DOI] [PubMed] [Google Scholar]
- 98.Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol. 1993;28:623–7. doi: 10.1016/0190-9622(93)70085-8. [DOI] [PubMed] [Google Scholar]
- 99.Falanga V, Kirsner R, Katz MH, Gould E, Eaglstein WH, McFalls S. Pericapillary fibrin cuffs in venous ulceration. Persistence with treatment and during ulcer healing. J Dermatol Surg Oncol. 1992;18:409–14. doi: 10.1111/j.1524-4725.1992.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 100.Nemeth AJ, Eaglstein WH, Falanga V. Clinical parameters and transcutaneous oxygen measurements for the prognosis of venous ulcers. J Am Acad Dermatol. 1989;20:186–90. doi: 10.1016/s0190-9622(89)70019-0. [DOI] [PubMed] [Google Scholar]
- 101.Alegre VA, Winkelmann RK, Aliaga A. Lipomembranous changes in chronic panniculitis. J Am Acad Dermatol. 1988;19:39–46. doi: 10.1016/s0190-9622(88)70149-8. [DOI] [PubMed] [Google Scholar]
- 102.Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge Smith PD. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265–73. doi: 10.1016/s0741-5214(97)70188-5. [DOI] [PubMed] [Google Scholar]
- 103.Wilkinson LS, Bunker C, Edwards JC, Scurr JH, Smith PD. Leukocytes: their role in the etiopathogenesis of skin damage in venous disease. J Vasc Surg. 1993;17:669–75. [PubMed] [Google Scholar]
- 104.Sindrup JH, Avnstorp C, Steenfos HH, Kristensen JK. Transcutaneous PO2 and laser Doppler blood flow measurements in 40 patients with venous leg ulcers. Acta Derm Venereol. 1987;67:160–3. [PubMed] [Google Scholar]
- 105.Mani R, Gorman FW, White JE. Transcutaneous measurements of oxygen tension at edges of leg ulcers: preliminary communication. J R Soc Med. 1986;79:650–4. doi: 10.1177/014107688607901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheatle TR, McMullin GM, Farrah J, Coleridge Smith PD, Scurr JH. Three tests of microcirculatory function in the evaluation of treatment for chronic venous insufficiency. Phlebology. 1990;5:165–72. [Google Scholar]
- 107.Richardson DL, Pepper DS, Kay AB. Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol. 1976;32:507–13. doi: 10.1111/j.1365-2141.1976.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 108.Gross TJ, Leavell KJ, Peterson MW. CD11b/CD18 mediates the neutrophil chemotactic activity of fibrin degradation product D domain. Thromb Haemost. 1997;77:894–900. [PubMed] [Google Scholar]
- 109.Nicolaides AN. Chronic venous disease and the leukocyte-endothelium interaction: from symptoms to ulceration. Angiology. 2005;56(Suppl 1):S11–9. doi: 10.1177/00033197050560i103. [DOI] [PubMed] [Google Scholar]
- 110.Falanga V, Eaglstein WH. The trap hypothesis of venous ulceration. Lancet. 1993;341:1006–8. doi: 10.1016/0140-6736(93)91085-z. [DOI] [PubMed] [Google Scholar]
- 111.Britton RS, Ramm GA, Olynyk J, Singh R, ONeill R, Bacon BR. Pathophysiology of iron toxicity. Adv Exp Med Biol. 1994;356:239–53. doi: 10.1007/978-1-4615-2554-7_26. [DOI] [PubMed] [Google Scholar]
- 112.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, et al. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–7. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 113.Caggiati A, Rosi C, Casini A, Cirenza M, Petrozza V, Acconcia MC, et al. Skin iron deposition characterises lipodermatosclerosis and leg ulcer. Eur J Vasc Endovasc Surg. 2010;40:777–82. doi: 10.1016/j.ejvs.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 114.Gilliam TC, Tanzi RE, Haines JL, Bonner TI, Faryniarz AG, Hobbs WJ, et al. Localization of the Huntingtons disease gene to a small segment of chromosome 4 flanked by D4S10 and the telomere. Cell. 1987;50:565–71. doi: 10.1016/0092-8674(87)90029-8. [DOI] [PubMed] [Google Scholar]
- 115.Lee J-M, et al. Genetic Modifiers of Huntingtons Disease (GeM-HD) Consortium Identification of Genetic Factors that Modify Clinical Onset of Huntingtons Disease. Cell. 2015;162:516–26. doi: 10.1016/j.cell.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 117.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 118.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altmüller J, Palmer LJ, Fischer G, Scherb H, Wjst M. Genomewide scans of complex human diseases: true linkage is hard to find. Am J Hum Genet. 2001;69:936–50. doi: 10.1086/324069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gemmati D, Federici F, Catozzi L, Gianesini S, Tacconi G, Scapoli GL, et al. DNA-array of gene variants in venous leg ulcers: detection of prognostic indicators. J Vasc Surg. 2009;50:1444–51. doi: 10.1016/j.jvs.2009.07.103. [DOI] [PubMed] [Google Scholar]
- 121.Ng MY, Andrew T, Spector TD, Jeffery S, Lymphoedema Consortium Linkage to the FOXC2 region of chromosome 16 for varicose veins in otherwise healthy, unselected sibling pairs. J Med Genet. 2005;42:235–9. doi: 10.1136/jmg.2004.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ashworth JJ, Smyth JV, Pendleton N, Horan M, Payton A, Worthington J, et al. Polymorphisms spanning the 0N exon and promoter of the estrogen receptor-beta (ERbeta) gene ESR2 are associated with venous ulceration. Clin Genet. 2008;73:55–61. doi: 10.1111/j.1399-0004.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 123.Nagy N, Szolnoky G, Szabad G, Bata-Csörgo Z, Dobozy A, Kemeny L, et al. Single nucleotide polymorphisms of the fibroblast growth factor receptor 2 gene in patients with chronic venous insufficiency with leg ulcer. J Invest Dermatol. 2005;124:1085–8. doi: 10.1111/j.0022-202X.2005.23689.x. [DOI] [PubMed] [Google Scholar]
- 124.Sam RC, Burns PJ, Hobbs SD, Marshall T, Wilmink AB, Silverman SH, et al. The prevalence of hyperhomocysteinemia, methylene tetrahydrofolate reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. J Vasc Surg. 2003;38:904–8. doi: 10.1016/s0741-5214(03)00923-6. [DOI] [PubMed] [Google Scholar]
- 125.Xu HM, Zhao Y, Zhang XM, Zhu T, Fu WG. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J Surg Res. 2011;168:e143–8. doi: 10.1016/j.jss.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Charles CA, Tomic-Canic M, Vincek V, Nassiri M, Stojadinovic O, Eaglstein WH, et al. A gene signature of nonhealing venous ulcers: potential diagnostic markers. J Am Acad Dermatol. 2008;59:758–71. doi: 10.1016/j.jaad.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin H, Zhang X, Wang J, Yin W, Zhang G, Wang S, et al. Downregulation of desmuslin in primary vein incompetence. J Vasc Surg. 2006;43:372–8. doi: 10.1016/j.jvs.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 128.Lee S, Lee W, Choe Y, Kim D, Na G, Im S, et al. Gene expression profiles in varicose veins using complementary DNA microarray. Dermatol Surg. 2005;31:391–5. doi: 10.1111/j.1524-4725.2005.31103. [DOI] [PubMed] [Google Scholar]
- 129.Le Flem L, Mennen L, Aubry ML, Aiach M, Scarabin PY, Emmerich J, et al. Thrombomodulin promoter mutations, venous thrombosis, and varicose veins. Arterioscler Thromb Vasc Biol. 2001;21:445–51. doi: 10.1161/01.atv.21.3.445. [DOI] [PubMed] [Google Scholar]
- 130.Cui C, Liu G, Huang Y, Lu X, Lu M, Huang X, et al. MicroRNA profiling in great saphenous vein tissues of patients with chronic venous insufficiency. Tohoku J Exp Med. 2012;228:341–50. doi: 10.1620/tjem.228.341. [DOI] [PubMed] [Google Scholar]
- 131.Srivastava S. Informatics In Proteomics. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 132.Broadbent J, Walsh T, Upton Z. Proteomics in chronic wound research: potentials in healing and health. Proteomics Clin Appl. 2010;4:204–14. doi: 10.1002/prca.200900152. [DOI] [PubMed] [Google Scholar]
- 133.Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics. 2013;13:2670–81. doi: 10.1002/pmic.201200502. [DOI] [PubMed] [Google Scholar]
- 134.Steinsträßer L, Jacobsen F, Hirsch T, Kesting M, Chojnacki C, Krisp C, et al. Immunodepletion of high-abundant proteins from acute and chronic wound fluids to elucidate low-abundant regulators in wound healing. BMC Res Notes. 2010;3:335. doi: 10.1186/1756-0500-3-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9:4758–66. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 136.Wyffels JT, Fries KM, Randall JS, Ha DS, Lodwig CA, Brogan MS, et al. Analysis of pressure ulcer wound fluid using two-dimensional electrophoresis. Int Wound J. 2010;7:236–48. doi: 10.1111/j.1742-481X.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruzehaji N, Grose R, Krumbiegel D, Zola H, Dasari P, Wallace H, et al. Cytoskeletal protein Flightless (Flii) is elevated in chronic and acute human wounds and wound fluid: neutralizing its activity in chronic but not acute wound fluid improves cellular proliferation. Eur J Dermatol. 2012;22:740–50. doi: 10.1684/ejd.2012.1878. [DOI] [PubMed] [Google Scholar]
- 138.Shah JM, Omar E, Pai DR, Sood S. Cellular events and biomarkers of wound healing. Indian J Plast Surg. 2012;45:220–8. doi: 10.4103/0970-0358.101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganesh K, Das A, Dickerson R, Khanna S, Parinandi NL, Gordillo GM, et al. Prostaglandin E2 induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012;189:2563–73. doi: 10.4049/jimmunol.1102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grieb G, Simons D, Eckert L, Hemmrich M, Steffens G, Bernhagen J, et al. Levels of macrophage migration inhibitory factor and glucocorticoids in chronic wound patients and their potential interactions with impaired wound endothelial progenitor cell migration. Wound Repair Regen. 2012;20:707–14. doi: 10.1111/j.1524-475X.2012.00817.x. [DOI] [PubMed] [Google Scholar]
- 141.Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen. 2012;20:378–401. doi: 10.1111/j.1524-475X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 142.Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, et al. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995;104:236–40. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- 143.Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335–41. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- 144.Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31:306–10. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 145.Wagner S, Coerper S, Fricke J, Hunt TK, Hussain Z, Elmlinger MW, et al. Comparison of inflammatory and systemic sources of growth factors in acute and chronic human wounds. Wound Repair Regen. 2003;11:253–60. doi: 10.1046/j.1524-475x.2003.11404.x. [DOI] [PubMed] [Google Scholar]
- 146.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 147.Hoffman R, Starkey S, Coad J. Wound fluid from venous leg ulcers degrades plasminogen and reduces plasmin generation by keratinocytes. J Invest Dermatol. 1998;111:1140–4. doi: 10.1046/j.1523-1747.1998.00429.x. [DOI] [PubMed] [Google Scholar]
- 148.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154–65. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 149.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 150.Wysocki AB, Bhalla-Regev SK, Tierno PM, Jr, Stevens-Riley M, Wiygul RC. Proteolytic activity by multiple bacterial species isolated from chronic venous leg ulcers degrades matrix substrates. Biol Res Nurs. 2013;15:407–15. doi: 10.1177/1099800412464683. [DOI] [PubMed] [Google Scholar]
- 151.Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433–41. doi: 10.1046/j.1524-475x.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 152.Rayment EA, Upton Z. Finding the culprit: a review of the influences of proteases on the chronic wound environment. Int J Low Extrem Wounds. 2009;8:19–27. doi: 10.1177/1534734609331596. [DOI] [PubMed] [Google Scholar]
- 153.Harris IR, Yee KC, Walters CE, Cunliffe WJ, Kearney JN, Wood EJ, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol. 1995;4:342–9. doi: 10.1111/j.1600-0625.1995.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 154.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 155.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 156.Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347–52. [PubMed] [Google Scholar]
- 157.Ashcroft GS, Jeong MJ, Ashworth JJ, Hardman M, Jin W, Moutsopoulos N, et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012;20:38–49. doi: 10.1111/j.1524-475X.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kalkhof S, Förster Y, Schmidt J, Schulz MC, Baumann S, Weißflog A, et al. Proteomics and metabolomics for in situ monitoring of wound healing. Biomed Res Int. 2014;2014:934848. doi: 10.1155/2014/934848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–71. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 160.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 161.Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Han X, Aslanian A, Yates JR., 3rd Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12:483–90. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Negishi A, Ono M, Handa Y, Kato H, Yamashita K, Honda K, et al. Large-scale quantitative clinical proteomics by label-free liquid chromatography and mass spectrometry. Cancer Sci. 2009;100:514–9. doi: 10.1111/j.1349-7006.2008.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu S, Brown JN, Tolić N, Meng D, Liu X, Zhang H, et al. Quantitative analysis of human salivary gland-derived intact proteome using top-down mass spectrometry. Proteomics. 2014;14:1211–22. doi: 10.1002/pmic.201300378. [DOI] [PubMed] [Google Scholar]
- 165.Svensson AM, Whiteley GR, Callas PW, Bovill EG. SELDI-TOF plasma profiles distinguish individuals in a protein C-deficient family with thrombotic episodes occurring before age 40. Thromb Haemost. 2006;96:725–30. [PubMed] [Google Scholar]
- 166.Hutchens TW, Yip T-T. New desorption strategies for the mass spectrometric analysis of macromolecules. Rapid Commun Mass Spectrom. 1993;7:576–80. [Google Scholar]
- 167.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 168.Issaq HJ, Veenstra TD, Conrads TP, Felschow D. The SELDI-TOF MS approach to proteomics: protein profiling and biomarker identification. Biochem Biophys Res Commun. 2002;292:587–92. doi: 10.1006/bbrc.2002.6678. [DOI] [PubMed] [Google Scholar]
- 169.Bons JA, Wodzig WK, van Dieijen-Visser MP. Protein profiling as a diagnostic tool in clinical chemistry: a review. Clin Chem Lab Med. 2005;43:1281–90. doi: 10.1515/CCLM.2005.222. [DOI] [PubMed] [Google Scholar]
- 170.Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, et al. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. MBio. 2016;7:e01058–16. doi: 10.1128/mBio.01058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Taverna D, Pollins AC, Sindona G, Caprioli RM, Nanney LB. Imaging mass spectrometry for assessing cutaneous wound healing: analysis of pressure ulcers. J Proteome Res. 2015;14:986–96. doi: 10.1021/pr5010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics. 2012;11:O111.016717. doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zawadzka AM, Schilling B, Held JM, Sahu AK, Cusack MP, Drake PM, et al. Variation and quantification among a target set of phosphopeptides in human plasma by multiple reaction monitoring and SWATH-MS2 data-independent acquisition. Electrophoresis. 2014;35:3487–97. doi: 10.1002/elps.201400167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Y, Chen J, Sethi A, Li QK, Chen L, Collins B, et al. Glycoproteomic analysis of prostate cancer tissues by SWATH mass spectrometry discovers N-acylethanolamine acid amidase and protein tyrosine kinase 7 as signatures for tumor aggressiveness. Mol Cell Proteomics. 2014;13:1753–68. doi: 10.1074/mcp.M114.038273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang F, Lin H, Gu A, Li J, Liu L, Yu T, et al. SWATH™-and iTRAQ-based quantitative proteomic analyses reveal an overexpression and biological relevance of CD109 in advanced NSCLC. J Proteomics. 2014;102:125–36. doi: 10.1016/j.jprot.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 176.Haverland NA, Fox HS, Ciborowski P. Quantitative proteomics by SWATH-MS reveals altered expression of nucleic acid binding and regulatory proteins in HIV-1-infected macrophages. J Proteome Res. 2014;13:2109–19. doi: 10.1021/pr4012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Griffiths JR, Chicooree N, Connolly Y, Neffling M, Lane CS, Knapman T, et al. Mass spectral enhanced detection of Ubls using SWATH acquisition: MEDUSAsimultaneous quantification of SUMO and ubiquitin-derived isopeptides. J Am Soc Mass Spectrom. 2014;25:767–77. doi: 10.1007/s13361-014-0835-x. [DOI] [PubMed] [Google Scholar]
- 178.Zhu X, Chen Y, Subramanian R. Comparison of information-dependent acquisition, SWATH, and MS(All) techniques in metabolite identification study employing ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem. 2014;86:1202–9. doi: 10.1021/ac403385y. [DOI] [PubMed] [Google Scholar]
- 179.Collins BC, Gillet LC, Rosenberger G, Röst HL, Vichalkovski A, Gstaiger M, et al. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 1433 system. Nat Methods. 2013;10:1246–53. doi: 10.1038/nmeth.2703. [DOI] [PubMed] [Google Scholar]
- 180.Liu Y, Hüttenhain R, Surinova S, Gillet LC, Mouritsen J, Brunner R, et al. Quantitative measurements of N-linked glycoproteins in human plasma by SWATHMS. Proteomics. 2013;13:1247–56. doi: 10.1002/pmic.201200417. [DOI] [PubMed] [Google Scholar]
- 181.Vowinckel J, Capuano F, Campbell K, Deery MJ, Lilley KS, Ralser M. The beauty of being (label)-free: sample preparation methods for SWATH-MS and next-generation targeted proteomics. F1000Res. 2013;2:272. doi: 10.12688/f1000research.2-272.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Idle JR, Gonzalez FJ. Metabolomics. Cell Metab. 2007;6:348–51. doi: 10.1016/j.cmet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arakaki AK, Skolnick J, McDonald JF. Marker metabolites can be therapeutic targets as well. Nature. 2008;456:443. doi: 10.1038/456443c. [DOI] [PubMed] [Google Scholar]
- 184.Zhou B, Xiao JF, Tuli L, Ressom HW. LC-MS-based metabolomics. Mol Biosyst. 2012;8:470–81. doi: 10.1039/c1mb05350g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang A, Sun H, Wu X, Wang X. Urine metabolomics. Clin Chim Acta. 2012;414:65–9. doi: 10.1016/j.cca.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 186.Millard RW, Rosevear PR. Metabolomics: seeking a unique biomarker signature for coronary artery syndromes. J Am Coll Cardiol. 2012;59:1642–4. doi: 10.1016/j.jacc.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 187.Nicholson JK, Lindon JC, Holmes E. Metabonomics: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–9. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 188.Pan Z, Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal Bioanal Chem. 2007;387:525–7. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- 189.Bersanelli M, Mosca E, Remondini D, Giampieri E, Sala C, Castellani G, et al. Methods for the integration of multi-omics data: mathematical aspects. BMC Bioinformatics. 2016;17(Suppl 2):15. doi: 10.1186/s12859-015-0857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buescher JM, Driggers EM. Integration of omics: more than the sum of its parts. Cancer Metab. 2016;4:4. doi: 10.1186/s40170-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Joyce AR, Palsson BO. The model organism as a system: integrating omics data sets. Nat Rev Mol Cell Biol. 2006;7:198–210. doi: 10.1038/nrm1857. [DOI] [PubMed] [Google Scholar]
- 192.Masseroli M, Canakoglu A, Ceri S. Integration and querying of genomic and proteomic semantic annotations for biomedical knowledge extraction. IEEE/ACM Trans Comput Biol Bioinform. 2016;13:209–19. doi: 10.1109/TCBB.2015.2453944. [DOI] [PubMed] [Google Scholar]
- 193.Pineda S, Real FX, Kogevinas M, Carrato A, Chanock SJ, Malats N, et al. Integration Analysis of Three Omics Data Using Penalized Regression Methods: An Application to Bladder Cancer. PLoS Genet. 2015;11:e1005689. doi: 10.1371/journal.pgen.1005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schmidtchen A. Chronic ulcers: a method for sampling and analysis of wound fluid. Acta Derm Venereol. 1999;79:291–5. doi: 10.1080/000155599750010698. [DOI] [PubMed] [Google Scholar]
- 195.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996;4:234–9. doi: 10.1046/j.1524-475X.1996.40211.x. [DOI] [PubMed] [Google Scholar]
- 196.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–52. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 197.Fernandez ML, Broadbent JA, Shooter GK, Malda J, Upton Z. Development of an enhanced proteomic method to detect prognostic and diagnostic markers of healing in chronic wound fluid. Br J Dermatol. 2008;158:281–90. doi: 10.1111/j.1365-2133.2007.08362.x. [DOI] [PubMed] [Google Scholar]
- 198.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 199.Öztürk F, Ermertcan AT. Wound healing: a new approach to the topical wound care. Cutan Ocul Toxicol. 2011;30:92–9. doi: 10.3109/15569527.2010.539586. [DOI] [PubMed] [Google Scholar]
- 200.Finlayson K, Edwards H, Courtney M. Factors associated with recurrence of venous leg ulcers: a survey and retrospective chart review. Int J Nurs Stud. 2009;46:1071–8. doi: 10.1016/j.ijnurstu.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 201.Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Pract Res. 2014;22:4–12. 14–19. [Google Scholar]
- 202.Rahman GA, Adigun IA, Fadeyi A. Epidemiology, etiology, and treatment of chronic leg ulcer: experience with sixty patients. Ann Afr Med. 2010;9:1–4. doi: 10.4103/1596-3519.62615. [DOI] [PubMed] [Google Scholar]
- 203.Gupta N, Gupta SK, Shukla VK, Singh SP. An Indian community-based epidemiological study of wounds. J Wound Care. 2004;13:323–5. doi: 10.12968/jowc.2004.13.8.26657. [DOI] [PubMed] [Google Scholar]
- 204.Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care. 2009;18:154–61. doi: 10.12968/jowc.2009.18.4.41607. [DOI] [PubMed] [Google Scholar]
- 205.Hunt TK, Hopf H, Hussain Z. Physiology of wound healing. Adv Skin Wound Care. 2000;13(Suppl):6–11. [PubMed] [Google Scholar]
- 206.Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26:306–19. doi: 10.1097/01.bcr.0000169887.04973.3a. [DOI] [PubMed] [Google Scholar]