Abstract
Prior research has identified age-by-valence interactions in both behavior and neural recruitment; age has been associated with increased retrieval of positive relative to negative information as well as an increased tendency to recruit prefrontal regions during negative event retrieval and for this recruitment to correspond to decreased hippocampal connectivity. To date, the explicit relation between prefrontal recruitment and memory phenomenology has not been examined. The current study examined the link between these two measures by examining age-by-valence interactions in the relation between prefrontal recruitment and subjective ratings of memory vividness. Participants (ages 18–85) encoded visual images paired with verbal titles. During a scanned retrieval session, they were presented with titles and asked whether each had been seen with an image during encoding. Participants provided vividness ratings following retrieval of each image. Age was associated with greater prefrontally-mediated alterations in negative event phenomenology, with age-related increases in the relation between ventral prefrontal regions and negative event vividness and age-related decreases in the relation between dorsal prefrontal regions and negative event vividness. This analysis confirmed a critical role of PFC regions in age-by-valence interactions, where age reversed the relation between PFC recruitment and the subjective richness of retrieved memory representation. These findings are consistent with studies that reveal age-related enhancements in emotion regulation, and suggest that older adults may be engaging in these processes during retrieval of negative events.
Keywords: Memory, Emotion, Aging, fMRI, Vividness, Prefrontal Cortex
1. Introduction
Healthy older adults are often less able to recall specific episodic details relative to their younger counterparts (Salthouse, 2001). Despite this overall decline, research suggests that pockets of preservation may exist in which aging is not associated with such substantial deficits. Specifically, it has been suggested that memory impairments in older adults can be mitigated by the presence of emotional arousal (e.g., Kensinger, 2009), particularly when the information is of positive valence (Reed et al., 2014). This relative enhancement has been of great interest in the cognitive aging literature, because it represents a circumstance in which the ability for older adults to have access to a detailed or vivid recollection is altered by the emotional content of the memory.
In an effort to better understand this interactive influence of age and emotion on memory retrieval, recent research has examined the neural mechanisms related to this shift. These studies have highlighted age-related increases in prefrontal cortex (PFC) activity during the first several seconds of emotional memory retrieval (Murty et al., 2009; Ford et al., 2014a; Ford & Kensinger, 2014) and have further suggested that this PFC enhancement tracks with age in a relatively linear fashion: across the ages of 18 to 83, the older the individual, the greater the PFC activity during emotional memory retrieval (Ford et al., 2014a). In addition to this linear effect of age, it has also been demonstrated that older adults can rely more heavily on PFC regions during negative relative to positive event retrieval (Ford et al., 2014a; Ford & Kensinger, 2014). This valence difference, however, is significant in only the “oldest-old” participants (i.e., 70 and older), with the “young-old” (i.e., 55–69) indistinguishable from middle-aged adults (Ford & Kensinger, 2014). The emergence of a distinct valence pattern after age 70 suggests a mechanism that is separate from the more continuous effects of age that occur across all emotional memory. The nature of this valence pattern is also broadly consistent with a meta-analysis of behavioral age-by-valence interactions, revealing that such interactions were more likely to occur when the ages being compared differed more dramatically from one another – such as when the “oldest-old” were compared to young adults rather than when the “young-old” were compared to young adults (Reed, Chan, & Mikels, 2014).
The research described above implicates a memory retrieval mechanism that is prefrontally-mediated, negative valence-specific, and strongest in the oldest-old; however, the cognitive correlates of this mechanism are still unclear. One possibility—given prior research suggesting enhanced encoding of positive relative to negative information by older adults (reviewed by Kensinger & Leclerc, 2009)—is that older adults may over-recruit PFC regions to enhance vivid retrieval of negative events that were more poorly encoded initially. This possibility would be consistent with the role of the PFC in the control of retrieval and in the enhancement of memory detail (e.g., selection, maintenance, reorganization; see Badre & Wagner, 2007; Simons & Spiers, 2003). By this account, PFC activity should aide in the retrieval of vivid negative memories and thus increased PFC activity during retrieval of a particular item should be associated with increased vividness of the memory for that event. Alternatively, healthy aging has been associated with an increased motivation to regulate emotion to optimize mood during cognitive tasks (Carstensen, 1995). It is possible, then, that the PFC is being preferentially recruited by older adults during negative event retrieval to reduce the vividness of re-experienced negative content (e.g., Phillips et al., 2008; Ochsner & Gross, 2005). By this account, PFC activity should serve to down-regulate the vividness of a memory, such that increased PFC activity during retrieval of a particular event would be associated with decreased vividness for the memory of that event.
There is some support for this latter explanation. One line of support comes from analyses examining age-by-valence changes in functional connectivity between the hippocampus and the PFC during retrieval of positive and negative events (Ford et al., 2014a; Ford & Kensinger, 2014). These analyses reveal negative correlations between the left hippocampus and dorsomedial prefrontal cortex (peak voxel: −8, 30, 44) among older but not younger adults and during negative, but not positive, event retrieval (Ford et al., 2014a). This age-related pattern is consistent with an account whereby older adults’ engagement of PFC mechanisms reduces hippocampal processes, and perhaps memory vividness, during retrieval of negative events. An analysis of white matter structural integrity revealed that these changes were particularly prominent in older adults with greater estimates of integrity, suggesting that they may not be driven by age-related impairments but rather by shifts in retrieval strategy (Ford & Kensinger, 2014). In other words, older adults may be engaging PFC processes during negative event retrieval in order to dampen the vividness of those memories. These findings highlight age-related trial-by-trial shifts in neural recruitment that may support greater vividness for positive relative to negative event retrieval. However, a more direct test of this link would be to examine directly how age and valence affect the link between PFC engagement and memory vividness.
The current study examines age-by-valence interactions on the relation between PFC recruitment and ratings of memory vividness provided during retrieval. Based on the prior connectivity findings from this same dataset (Ford et al., 2014a), we hypothesize that older adults will show such an inverse relation during negative event retrieval, further supporting a regulation function of their increased prefrontal recruitment. In particular, the current study focused on the participant’s subjective experience of how vividly they recall each event rather than objective measurement of a particular event detail. A strong subjective sense of vividness may be supported by retrieval of any additional contextual information, including details about thoughts and feelings that the participant had at the time of encoding (here, called “internal details”) or details about the event itself (here, called “external details”). There is evidence to suggest that retrieval of these two types of details may be supported by distinct mechanisms. Research has shown that task instructions may be utilized to separately manipulate different types of memory details (e.g., Suengas & Johnson, 1988), and a recent analysis of the dataset utilized in the current study lab revealed a divergence of the neural networks supporting internal and external vividness ratings as early as the first two seconds of neutral memory retrieval (Ford & Kensinger, 2016). Although it is unknown how these networks may differ as a function of age, there is reason to believe that age may interact with vividness-type. Behaviorally, older adults are markedly less impaired when asked to recall internal relative to external details (see Kensinger, 2008), and during encoding they over-recruit regions associated with internal, evaluative processing (Maillet & Rajah, 2016), suggesting that they may depend on distinct cognitive and neural mechanisms.
Successful retrieval of an event includes two distinct phases: an initial search phase in which information is accessed and a subsequent elaboration phase in which additional event details are retrieved. A recent analysis from our lab (Ford, Morris, & Kensinger, 2014b) revealed significant distinctions in the neural processes supporting the search and elaboration phases, with search being associated with widespread bilateral activations across the entire cortex and elaboration primarily being associated with increased activity in the medial prefrontal cortex. This study also demonstrated a phase-by-valence interaction, with positive emotion playing a larger role during search and negative emotion playing a larger role during elaboration. These results suggest that valence-related effects may also differ across the retrieval trial in the current study. A second analysis has demonstrated unique effects of age on neural recruitment during search and elaboration, with some regions exhibiting a complete reversal in the relation between age and activity during the two phases of successful memory retrieval (Ford and Kensinger, in press). Therefore, the current analysis takes advantage of the extended retrieval period to compare critical age-by-valence-by-vividness effects in the search and elaboration phases of episodic memory retrieval. That is, we examine whether age-by-valence effects exist across both phases, or exist disproportionately in one phase compared to the other.
2. Methods
2.1 Participants
Data from fifty-nine healthy adults (mean age= 48.17, sd= 20.34, ages 19–85; mean education= 16.60, sd= 2.41; 27 females) are reported. Other findings from these participants have been reported previously, as reviewed in the introduction (Ford et al., 2014a, 2014b; Ford & Kensinger, 2014; Ford & Kensinger, 2016). Age and education did not differ across genders (p>.2 for both contrasts) and age was not significantly correlated with education (p= .64). Two additional participants were recruited but not scanned due to contraindications for MRI (ages 50 and 75; both male). Another fourteen participants were scanned, but were excluded from the current analysis due to equipment malfunction (n=1; age=49, edu=16, male), an abnormal structural scan (n=1, age= 49, edu=17, female), excessive motion in the scanner resulting in termination of MR session (n=1, age=56, edu=16, male), voluntary early termination of the MR session (n=1, age=49, edu=14, female), low behavioral performance (i.e., hit rate below 0.50 or false alarm rate above 0.50; n=6, mean age= 55.64, sd= 18.12, ages 30–83; mean education= 16.12, sd= 3.49; 2 female), or lack of variability in their vividness ratings (i.e., only providing a single value for all vividness ratings; n=4, mean age= 44.25, sd= 9.00, ages 36–53; mean education= 16.50, sd= 1.00; 2 female). Participants were right-handed native English speakers without psychiatric illness or neurological disorder and were recruited from the greater Boston area. All participants were paid for their participation and gave written informed consent in accordance with the requirements of the Institutional Review Board at Boston College.
All participants completed the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) to examine self-reported symptoms of anxiety, as well as the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the Geriatric Depression Scale (Sheikh & Yesavage, 1986) to evaluate symptoms of depression. In addition, participants engaged in a series of tests intended to examine general cognitive ability, vocabulary, verbal fluency, working memory, and memory (both immediate and delayed). Finally, all participants completed a battery of cognitive tests implemented in CogState, a computerized neuropsychological test battery, that was approximately 30 minutes in duration. The battery included 6 subtests that examine a range of cognitive abilities, including: Detection Task (speed of processing), Identification Task (visual attention), One Card Learning Task (visual learning and memory), One Back Task (attention/working memory), Two Back Task (attention/working memory), and Set-Shifting (executive function); these have acceptable criterion and construct validity in a neuropsychological context (see www.cogstate.com; Maruff et al., 2009). The relations of age with all cognitive variables are reported in Supplementary Table 1. In brief, healthy aging was associated with decreased anxiety, increased vocabulary, and impairments on tasks involving long term memory (e.g., delayed visual pairs) and executive control (e.g., digit/symbol task, mental arithmetic, mental control, digit span, and set shifting). In addition, older adults showed significantly greater retrieval times in four of the six CogState computer tasks.
2.2 Materials
Stimuli were 480 pictures (160 positive, 160 negative, and 160 neutral) that were each paired with a neutral title (e.g., the title “lettuce” paired with the negative image of a piece of rotting lettuce with bugs on it). These stimuli have been used in prior studies from our lab (Ford et al., 2014a, 2014b; Ford & Kensinger, 2014, Ford & Kensinger, 2016) to allow presentation of a neutral cue (the title) during retrieval to elicit an emotional memory. The use of a neutral cue avoids the potential confound of on-line emotional processing of the retrieval cue (see also Maratos et al., 2001; Sterpenich et al., 2006) and ensures that valence differences at retrieval are related to the mnemonic content retrieved. The 480 title-picture pairs were divided into 4 sets of 120 pictures each (40 positive, 40 negative, and 40 neutral) for counterbalancing purposes. One quarter of participants studied list A and were tested on lists A (“old”) and B (“new”), one quarter studied list B and were tested on lists A (“new”) and B (“old”), one quarter studied list C and were tested on lists C (“old”) and D (“new”), and one quarter studied list D and were tested on lists C (“new”) and D (“old”). The length of the study list was selected to reduce the likelihood of floor or ceiling effects across the age range.
2.3 Procedure
Following instruction and a short practice, participants encoded one set of 120 title-image pairs. In an intentional encoding task (outside of the scanner) participants were given 3 seconds to make a decision regarding the appropriateness of the word as a description of the image (1= poor description, 2= acceptable description, and 3= very good description). After a half-hour delay (M= 34.3 minutes, sd= 7.8), participants took part in a scanned retrieval task. Participants were presented with the 240 titles (120 neutral titles that were studied during the encoding phase and 120 unstudied neutral titles) randomly across 6 retrieval runs of equal length. Participants were given up to 4 seconds to decide whether the word was “old” (i.e., seen previously) or “new” (i.e., not seen previously). The screen was removed following the participant’s button press. Across participants, it was varied which items were studied and which were reserved as foils on the recognition test.
Immediately following an “old” response, 80% of the time, participants were asked to “Elaborate” on the old item (i.e., think about the image presented with the title and the experience with that title and image at encoding) for 5 seconds. To discourage participants from beginning to elaborate during the search phase, and to distinguish activity during search from activity during elaboration, 20% of trials were catch trials; instead of an elaboration phase, the next trial was presented. Following a “new” response, 80% of the time, participants moved on to the next trial. To minimize the likelihood that participants would automatically begin preparing for the next trial after a “new” response, on 20% of the trials, participants were asked to “Imagine” an image that could have accompanied the new item for 5 sec.
Following the elaboration phase, participants were asked to consider how well they were able to remember each item in two separate rating scales, with the order counterbalanced across participants. Participants were given five seconds each for two scales: 1) On a scale of 1–5, how well did they remember the details of the image associated with the cue word or phrase (“External Vividness”) and 2) On a scale of 1–5, how well did they remember their own personal thoughts and feelings from encoding the title-picture pair (“Internal Vividness”). Following each trial, participants viewed a fixation cross for 0–6 seconds to introduce jitter. A visual schematic of this procedure is presented in Figure 1.
Figure 1.
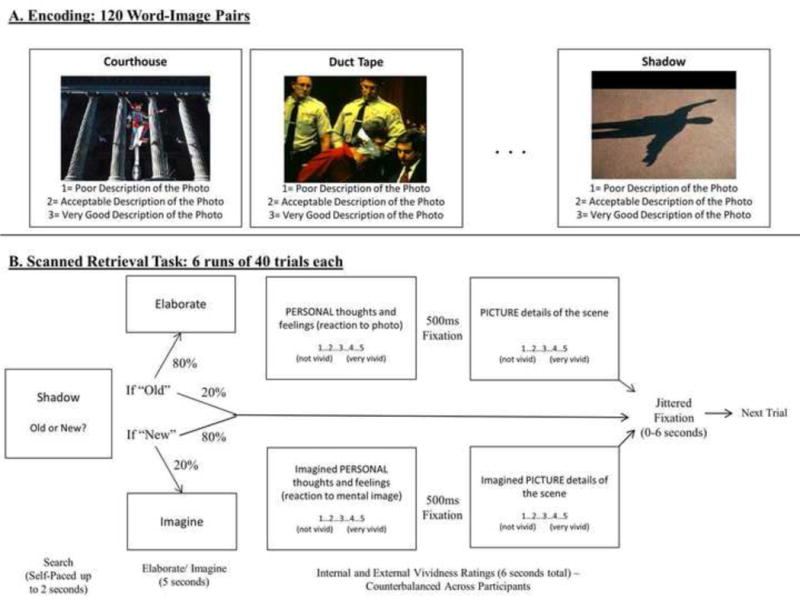
Visual schematic of behavioral methods for A) the encoding task and B) the scanned retrieval task.
After being removed from the scanner, participants were re-presented with all studied images. They rated valence and arousal on a 1–7 scale and indicated which specific emotions they experienced with each image. This portion was self-paced and participants were encouraged to respond based on their initial reaction.
2.4 Data Acquisition
Participants’ heads were stabilized in a Siemens Tim Trio 3 Tesla scanner. A localizing scan and auto-align scout were followed by a high resolution multi-echo T1 structural scan for anatomical visualization (176 1mm slices, TR=2200ms, TE1=1.64ms, TE2= 3.5ms, TE3= 5.36ms, TE4= 7.22ms). Six runs of whole brain, gradient-echo, echo planar images (31 3mm slices aligned along the line between the anterior and posterior commissures, 20% skip, TR=2s, TE=30ms, Flip angle=90) were acquired during memory retrieval using interleaved slice acquisition. A diffusion weighted scan was collected but will not be discussed. Response data were collected using a magnet-safe button response box.
2.5 Preprocessing and Data Analysis
Images were preprocessed and analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Images were co-registered, realigned, normalized (resampled at 3 mm at the segmentation stage and written at 2mm at the normalization stage) and smoothed using a Gaussian 8 mm kernel. The current analysis examined effects of vividness on recruitment during memory search, modeled as an epoch at stimulus onset, and memory elaboration, modeled as a five-second block beginning at the participant’s old/new button response.
The first level fMRI analysis examined the effect of vividness on neural activity during accurate “old” responses to studied items (i.e., “hits”). Neutral, positive, and negative hits were modeled as conditions of interest with internal and external vividness as parametric modulators of interest. Parametric effects on search and elaboration phases were modeled using separate conditions. Incorrect responses and correct “new” responses to positive, negative, and neutral items, although not relevant for the current analysis, were included in each model as separate nuisance variables. Because SPM automatically orthogonalizes parametric regressors in fixed-effects models, two separate models were generated for each subject to capture the independent effects of internal and external vividness on recruitment during retrieval. In other words, two models were generated, one with internal vividness ratings included as a parametric modulator for neutral, negative, and positive items and the other with external vividness ratings. By taking the independent correlates of internal and external vividness rather than the values orthongonalized on one another, this analysis was able to identify both the common effects of internal and external vividness as well as the type-specific effects at the second level (See supplementary materials for analysis conducted using fixed-effects models in which external and internal vividness were orthogonalized).
The individual results from these fixed-effects analyses were used in a full-factorial random-effects model with twelve conditions of interest. These twelve conditions reflected the parametric relation of activity with item-level ratings of:
Internal vividness on search for neutral events
Internal vividness on search of positive events
Internal vividness on search of negative events
External vividness on search of neutral events
External vividness on search of positive events
External vividness on search of negative events
Internal vividness on elaboration of neutral events
Internal vividness on elaboration of positive events
Internal vividness on elaboration of negative events
External vividness on elaboration of neutral events
External vividness on elaboration of positive events
External vividness on elaboration of negative events
These conditions were organized into three factors of vividness type (internal v. external), memory phase (search v. elaboration), and valence (positive v. negative). Age was included as a covariate of interest so that the model could identify regions in which age was associated with increased or decreased coupling with vividness ratings. The current analysis examined the relation between age and main effects of increased and decreased vividness (across memory phase and vividness type) as well as interactions with emotional valence, phase, and vividness type. Although the current analysis focused on these valence differences within emotional memory retrieval, comparisons between neutral and emotional event retrieval can be found in Supplementary Table 2. Main effects of phase, vividness type, and emotion (controlling for age) can be found in Supplementary Table 3 (see Ford et al., 2014b for reporting of effects of emotion on activity during search and elaboration phases).
The significance threshold for all analyses was set at p < .005 (uncorrected). Monte Carlo simulations (Slotnick et al., 2003), incorporating the smoothness of the data and run with the normalized voxel size of 2×2×2, determined that a 29-voxel extent corrected results to p < .05. Therefore, we discuss all clusters that reach this threshold. Clusters reaching significance were overlaid on anatomical images from MRIcron. For all analyses, reported coordinates reflect the peak activity within active regions in MNI space. These coordinates were converted from MNI coordinates to Talairach space, localized using the Talairach Client, and confirmed with the Talairach and Tournoux atlas (Talairach & Tournoux, 1988). For visualization purposes and to help clarify the directionality of interaction effects, activity within a 10mm sphere around peak voxels was extracted from regions of interest using the REX toolbox (downloaded from http://web.mit.edu/swg/software.htm).
3. Results
3.1 Behavioral Results
Vividness ratings were examined using an ANOVA with vividness type (internal v. external vividness) and valence (negative v. positive) as within-subject factors, and age as a continuous covariate of interest. Ratings for external vividness (M= 3.64, SE=.08) were significantly greater than those for internal vividness (M=3.21, SE= .09; F(1,57)= 8.08, p=.006, partial η2=.12). Positive and negative events did not differ in ratings of vividness, although there was a trend toward higher ratings for positive (M=3.49, SE= .07) relative to negative events (M=3.36, SE= .08; F(1,57)= 3.81, p=.06, partial η2=.06). The emotion-by-vividness type interaction was insignificant (p=.28), as was the effect of age (p=.76), the age-by-vividness type interaction (p=.37) and the three-way interaction (p=.33). The age-by-emotion interaction was significant (F(1,57)= 11.71, p<.001, partial η2=.17), with age being associated with greater decreases in vividness ratings for negative relative to positive events (see Table 1 for behavioral results).
Table 1.
Average vividness ratings as a function of rating type and memory valence
| Mean | Standard Error | Relation with Age | |
|---|---|---|---|
| External vividness ratings | |||
| Negative Events | 3.58 | 0.09 | −0.20 |
| Positive Events | 3.69 | 0.08 | 0.04 |
| Internal vividness ratings | |||
| Negative Events | 3.14 | 0.09 | −0.06 |
| Positive Events | 3.28 | 0.09 | 0.11 |
Note. No relations with age are significant at p<.05
Correlations between external and internal vividness ratings, across trials, were calculated for each subject and for neutral, positive, and negative events separately. These correlation values were examined using an ANOVA with valence (negative v. positive) as a within-subject factor and age as a continuous covariate of interest. On average, there was a strong, but not perfect, correlation between external and internal vividness ratings (Mcorr= .57, SE= .02; F(1,57)= 78.10, p< .000, partial η2=.58). This correlation was not related to age (F(1,57)= 1.34, p=.25, partial η2=.02) or valence (F(1,57)= 1.34, p=.25, partial η2=.02) or the age-by-valence interaction (F(1,57)= .24, p=.63, partial η2=.00).
3.2 Imaging Results
The following contrasts all represent interactions with age. Therefore, for the peak voxels reported in each contrast, the relevant simple effects of age were further interrogated at p<.05 to clarify directionality of the interaction. In cases where no effects were significant at p<.05, the peaks were examined at p<.1. All results are presented in Table 2. For clusters larger than 500 voxels, two additional sub-peaks are included in the table in addition to the cluster peak.
Table 2.
Regions in which the relation between activity and vividness was subject to significant age-by-memory phase, age-by-vividness type, and age-by valence interactions.
| MNI Coordinates
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region of Interest | Hemisphere | BA | x | y | z | t-value | k | ||
| Interactive effects of age and memory phase on the relation between activity and vividness ratings, regardless of vividness type and valence | |||||||||
| Search | Elaboration | ||||||||
| Frontal Lobe | |||||||||
| Premotor Cortex | L | 6 | −6 | 10 | 62 | 3.8 | 104 | # | |
| L | 6 | −42 | 2 | 48 | 3.39 | 77 | # | ||
| R | 6 | 14 | 6 | 64 | 3.76 | 100 | # | ||
| R | 6 | 42 | 2 | 48 | 3.33 | 47 | # | * | |
| R | 6 | 20 | −10 | 54 | 3.09 | 34 | # | ||
| Dorsolateral Prefrontal Cortex | R | 9 | 50 | 18 | 28 | 3.6 | 113 | # | * |
| Temporal Lobe | |||||||||
| Inferior Temporal Gyrus | L | 20 | −40 | −12 | −22 | 3.27 | 57 | * | # |
| Superior Temporal Gyrus | L | 38 | −34 | 4 | −38 | 2.96 | 41 | * | |
| L | 38 | −36 | 12 | −20 | 2.99 | 38 | * | # | |
| Parietal Lobe | |||||||||
| Inferior Parietal Lobule | R | 40 | 58 | −24 | 24 | 3.59 | 77 | # | |
| L | 40 | −54 | −30 | 38 | 3.12 | 53 | # | * | |
| Superior Parietal Lobule | R | 7 | 38 | −44 | 56 | 3.54 | 122 | # | * |
| R | 7 | 26 | −62 | 48 | 3.41 | 118 | # | * | |
| Precuneus | R | 7 | 8 | −52 | 50 | 3.35 | 76 | # | |
| Limbic Lobe | |||||||||
| Anterior Cingulate | L | 24 | 0 | 4 | 26 | 3.22 | 44 | # | * |
| Parahippocampal Gyrus | L | 34 | −14 | −4 | −20 | 3.21 | 60 | * | # |
| Cerebellum | |||||||||
| L | na | −12 | −44 | −12 | 3.19 | 33 | * | ||
| Interactive effects of age and vividness type on the relation between activity and vividness ratings, regardless of memory phase and valence | |||||||||
| External Vividness | Internal Vividness | ||||||||
| Frontal Lobe | |||||||||
| Anterior Medial Prefrontal Cortex | R | 9 | 12 | 60 | 12 | 3.64 | 99 | # | |
| L | 10 | −16 | 52 | 0 | 3.36 | 94 | # | ||
| Lateral Prefrontal Cortex | L | 10 | −38 | 42 | 14 | 3.45 | 93 | * | |
| Premotor Cortex | R | 6 | 12 | −8 | 64 | 3.33 | 74 | # | |
| R | 6 | 18 | 16 | 54 | 3.2 | 32 | # | ||
| L | 6 | −10 | −2 | 58 | 3.16 | 42 | # | ||
| Precentral Gyrus | L | 4 | −10 | −22 | 68 | 3.32 | 134 | * | |
| Temporal Lobe | |||||||||
| Inferior Temporal Gyrus | R | 20 | 54 | −14 | −24 | 3.66 | 179 | * | # |
| Superior Temporal Gyrus | R | 39 | 46 | −48 | 32 | 3.47 | 329 | # | |
| R | 39 | 44 | −50 | 8 | 2.92 | 79 | # | ||
| L | 40 | −48 | −42 | 28 | 3.31 | 136 | # | ||
| L | 41 | −44 | −32 | 2 | 3.15 | 71 | T | ˆ | |
| Fusiform Gyrus | R | 37 | 56 | −54 | −6 | 3.15 | 50 | T | ˆ |
| L | 37 | −48 | −62 | 4 | 3.12 | 93 | # | ||
| Parietal Lobe | |||||||||
| Precuneus | L | 7 | −2 | −46 | 56 | 3.82 | 992 | # | |
| L | 7 | −4 | −62 | 46 | 3.78 | ˆ | |||
| R | 7 | 10 | −54 | 44 | 3.3 | # | |||
| R | 7 | 8 | −30 | 50 | 2.97 | 39 | * | ||
| Occipital Lobe | |||||||||
| Lingual Gyrus | R | 18 | 4 | −86 | −6 | 3.41 | 153 | * | |
| L | 19 | −26 | −76 | 0 | 3.22 | 160 | * | ||
| Limbic Lobe | |||||||||
| Posterior Cingulate | R | 29 | 14 | −48 | 24 | 3.61 | 87 | # | |
| R | 31 | 22 | −32 | 42 | 3.35 | 105 | T | ˆ | |
| L | 31 | −8 | −22 | 36 | 3.56 | 89 | # | ||
| Anterior Cingulate | L | 24 | −8 | −2 | 40 | 3.47 | 75 | # | |
| Parahippocampal Gyrus | L | 28 | −18 | −16 | −18 | 3.14 | 86 | * | |
| Cerebellum | |||||||||
| L | na | −16 | −62 | −34 | 3.37 | 275 | # | ||
| L | na | −36 | −60 | −20 | 3.36 | 100 | # | ||
| R | na | 42 | −54 | −24 | 3.27 | 83 | # | ||
| Other | |||||||||
| Lentiform Nucleus | R | na | 16 | −6 | −6 | 4.32 | 346 | * | # |
| L | na | −22 | −12 | −4 | 3.03 | 41 | * | ||
| Putamen | R | na | 26 | 0 | 18 | 3.46 | 44 | * | |
| L | na | −24 | 14 | 14 | 3.02 | 36 | # | ||
| Thalamus | L | na | 0 | −10 | 10 | 3 | 87 | T | ˆ |
| Interactive effects of age and valence on the relation between activity and vividness ratings, regardless of memory phase and vividness type | |||||||||
| Positive Events | Negative Events | ||||||||
| Frontal Lobe | |||||||||
| Subcallosal Gyrus | L | 25 | −24 | 8 | −18 | 4.05 | 80 | # | * |
| Premotor Cortex | R | 6 | 14 | −14 | 50 | 3.15 | 35 | # | |
| Ventromedial Prefrontal Cortex | L | 11 | −6 | 32 | −18 | 3.04 | 42 | # | * |
| Temporal Lobe | |||||||||
| Middle Temporal Gyrus | L | 21 | −52 | −8 | −14 | 3.54 | 306 | # | |
| Parietal Lobe | |||||||||
| Paracentral Lobule | R | 4 | 14 | −36 | 70 | 3.97 | 178 | # | * |
| Cerebellum | |||||||||
| L | na | −34 | −58 | −22 | 3.18 | 50 | # | ||
Clusters significant at an uncorrected threshold of p<.005, k ≥ 29 voxels
BA= approximate Brodmann Area; L=Left, R=Right
Simple effects interrogated at p< .05:
= Effect of age (i.e., Older > Young adults),
= Reverse effect of age (i.e., Young > Older adults)
Peaks in which no simple effects were significant at p<.0.5 were interrogated at p<.1: T = Effect of age (i.e., Older > Young adults),
= Reverse effect of age (i.e., Young > Older adults)
For clusters with greater than 500 voxels, two additional sub-peaks are included and examined.
3.2.1 Interactions of age with memory phase and vividness type, collapsing across valence
Interaction analyses revealed that the effect of age on coupling between vividness ratings and activity differed as a function of memory phase (i.e., age-by-phase interaction; red regions in Figure 2a) and vividness type (i.e., age-by-vividness interaction; blue regions in Figure 2a). Assessment of the age-by-phase interaction revealed that age increases in vividness-related recruitment were greater during the search relative to elaboration phase in bilateral lateral temporal lobes (BA20 and BA38), left entorhinal cortex (BA34), and the cerebellum. This interaction was greater during the elaboration relative to the search phase in frontal regions— such as bilateral premotor cortex (BA6), right dorsolateral PFC (dlPFC; BA9), and left anterior cingulate (BA24)—and bilateral inferior and superior parietal lobules (BA40 and BA7). The age-by-vividness interaction revealed that age was associated with a more positive link between activity and external relative to internal vividness ratings in a widespread network including bilateral anterior PFC (aPFC; BA9 and BA 10), lateral temporal lobe (BA20, BA39, BA40, and BA41), precuneus (BA7), lingual gyrus (BA18 and BA19), and posterior cingulate (BA 29 and BA31). This interaction was also apparent in the entorhinal region of the left parahippocampal gyrus (BA28). The reverse interaction (i.e., greater age-related effects for internal relative to external ratings) did not reveal any significant regions.
Figure 2.
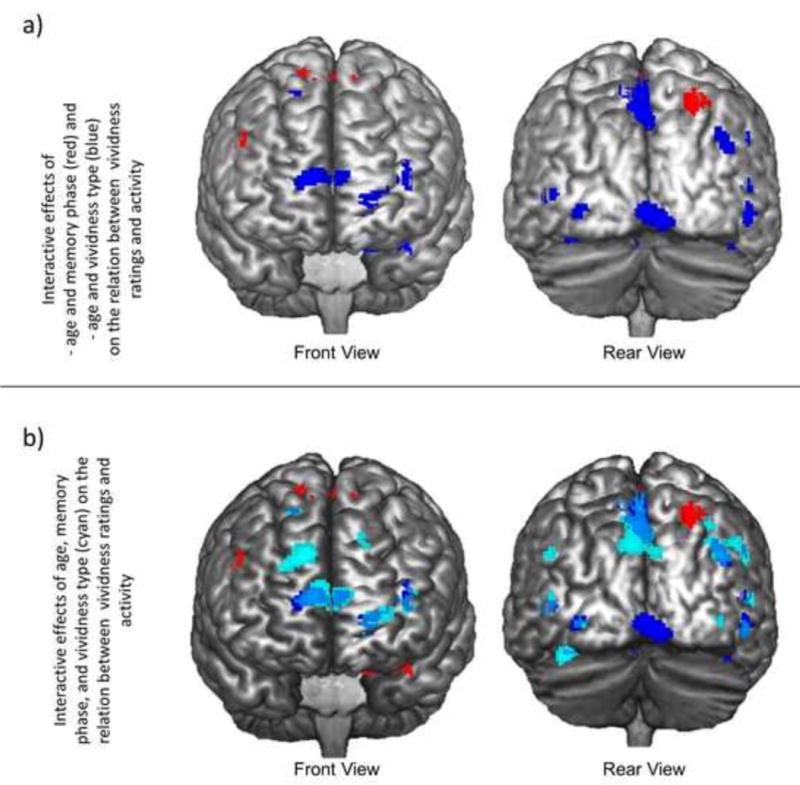
a) Regions in which the relation between neural recruitment and vividness ratings exhibited a significant age-by-memory phase interaction (red) or age-by-vividness type interaction (blue). b) Regions in which the relation between neural recruitment and vividness ratings exhibited a significant age-by-memory phase interaction (red), age-by-vividness type interaction (blue), or age-by-memory phase-by-vividness type interaction (cyan).
These interactions were further clarified by age-by-phase-by-vividness type interactions that revealed stronger age-by-vividness interactions during search relative to elaboration (Cyan regions in Figure 2b). Specifically, we see these age-by-phase-by-vividness type interactions in a widespread bilateral network including PFC (BA6, BA 10, BA8, and BA 9), lateral temporal lobes (BA20, BA37, and BA22), fusiform gyrus (BA20 and BA37), parahippocampal gyrus (BA28), and putamen. This interaction was largely driven by age-related decreases in the relation between internal vividness ratings and recruitment during memory search. In addition, age-related increases in the relation between external vividness ratings and activity during search were present in a number of regions, such as the aPFC (BA10), lateral PFC (BA10), dorsomedial PFC (dmPFC; BA8), inferior temporal gyrus (BA20), and bilateral parahippocampal gyrus (BA28).
3.2.2 Interactions of age with valence, collapsing across memory phase and vividness type
Age-by-valence interaction analyses identified a number of regions in which age was related to a stronger coupling of vividness with activity for negative relative to positive memories, including premotor cortex (BA6), paracentral lobule (BA4), middle temporal gyrus (BA21), and cerebellum. Within the prefrontal cortex, age was related to a stronger coupling of vividness with activity for negative relative to positive events in ventral PFC regions only, including the subcallosal gyrus (BA25) and left ventromedial PFC (vmPFC; BA11). In all of these regions, age was associated with a decreased relation between vividness ratings and recruitment during positive event retrieval. In a subset of these regions—specifically, the left subcallosal gyrus (BA25), left vmPFC (BA11), and right paracentral lobule (BA4)—age was also associated with an increased relation between vividness ratings and recruitment during negative event retrieval. Parameter estimates of activity extracted from a 10mm sphere around the peak voxel of the vmPFC cluster (−6, 32, −18; Figure 3) revealed that vividness ratings were associated with activity for both positive (signal change: M= .19, SE= .09) and negative (signal change: M= .16, SE= .04) events in the youngest individuals in our sample (ages 19–25), but only for negative events in the oldest individuals in our sample (ages 70–85). In this oldest sample, activity was greater for negative (signal change: M= .38, SE= .17) relative to positive events (signal change: M= −.01, SE= .15; t(11)= 2.22, p=.05).
Figure 3.
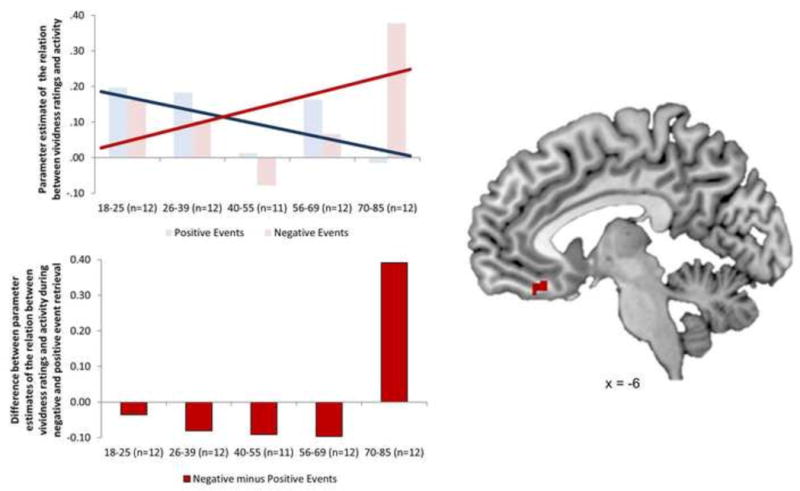
Ventromedial prefrontal cortex region in which the relation between neural recruitment and vividness ratings exhibited a significant age-by-valence interaction. In this region, age was associated with an increased relation between recruitment and vividness for negative events and a decreased relation between recruitment and vividness for positive events. Parameter estimates of this relation were extracted from a 10mm sphere around the peak voxel in this region and revealed that this interaction led to a significantly greater relation for negative relative to positive events (t(11)= 2.22, p=.05) in the oldest adults in the sample (n=12, ages 70–85).
3.2.3 Interactions of age with valence and memory phase, collapsing across vividness type
Age-by-valence interactions were further clarified by interactions with memory phase and vividness type (Figure 4a). Age-by-valence-by-phase interactions, largely driven by age-related decreases in the relation between vividness ratings and recruitment during search for positive events, were present in precentral gyrus (BA6), premotor cortex (BA6), superior temporal gyrus (BA38), middle occipital gyrus (BA37), anterior cingulate (BA33), caudate, putamen, claustrum, insula, thalamus, and into the cerebellum.
Figure 4.
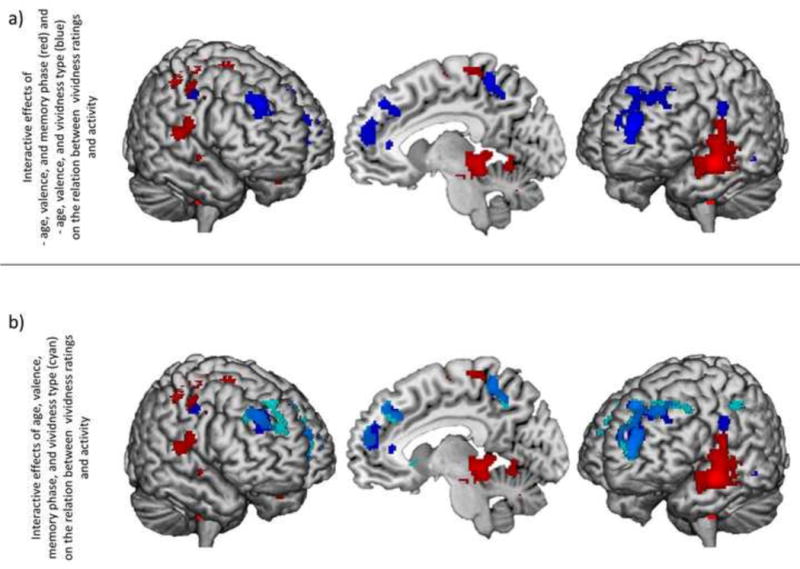
a) Regions in which the relation between neural recruitment and vividness ratings exhibited a significant age-by-valencce-by-memory phase interaction (red) or age-by-valence-by-vividness type interaction (blue). b) Regions in which the relation between neural recruitment and vividness ratings exhibited a significant age-by-valence-by-memory phase interaction (red), age-by-valence-by-vividness type interaction (blue), or age-by-valence-by-memory phase-by-vividness type interaction (cyan).
3.2.4 Interactions of age with valence and vividness type, collapsing across memory phase
Age-by-valence-by-vividness type interactions (Figure 4a, blue regions) were seen in more dorsal regions of the prefrontal cortex than were seen in the age-by-valence interactions, including aPFC (BA10) and dlPFC (BA8 and BA6). In addition, this interaction was seen in precentral gyrus (BA4), middle temporal gyrus (BA20), precuneus (BA7), postcentral gyrus (BA7 and BA2), putamen, and caudate. In a subset of these regions—aPFC (BA10), postcentral gyrus (BA4), and the caudate—this interaction was driven by age-related increases in the relation between external vividness ratings and activity during retrieval of positive events and the relation between internal vividness and activity during retrieval of negative events. In another subset—dlPFC (BA8 and BA6) and middle temporal gyrus—age was associated with a decreased relation between external vividness ratings and activity during negative event retrieval. Finally, age was associated with a decreased relation between internal vividness ratings and activity during positive event retrieval in dlPFC (BA8), precentral gyrus (BA4), precuneus (BA7), postcentral gyrus (BA7 and BA2), and putamen.
Notably, one cluster exhibiting an age-by-valence-by-vividness type interaction (peak: −24, 56, 14) overlapped with a dorsomedial prefrontal cluster exhibiting age-related decreases in hippocampal connectivity during negative relative to positive event retrieval in a previous analysis (Ford et al., 2014a). Although this prior analysis did not incorporate memory vividness, we proposed that this negative connectivity during negative event may reflect a mechanism by which older adults decrease negative event richness during retrieval by downgrading hippocampal activity. Therefore, we were particularly interested in determining whether recruitment of this region in older adults was associated with decreased negative event vividness. The cluster identified in the current study was extremely large (1379 voxels) and, as such, the peak voxels did not fall within the overlap with this prior cluster. In order to interrogate the directionality of the interaction within the region of overlap, we identified the peak voxel in a cluster identified in a conjunction analysis of each analysis (i.e., the current age-by-valence-by-vividness type interaction and the age-by-valence interaction in Ford et al., 2014a) at p<.005 and included that voxel as a sub-peak in Table 2. As predicted from our prior connectivity analysis, the age-by-valence-by-vividness type interaction at this peak was driven by an age-related increase in the relation between recruitment and external vividness ratings for positive events and, critically, an age-related decrease in the relation between recruitment and external vividness ratings for negative events.
3.2.5 Interactions of age with valence, vividness type, and memory phase
Finally, an age-by-valence-by-phase-by-vividness type analysis revealed that age-by-valence-by-vividness type interactions were stronger during search relative to elaboration (Figure 4b). Regions exhibiting this interaction include dmPFC (BA8), precentral gyrus (BA6), dlPFC (BA8), middle temporal gyrus (BA39), precuneus (BA7), postcentral gyrus (BA2), inferior parietal lobule (BA40), the caudate, and the thalamus. Although these regions all exhibited the same interaction, the patterns of activation differed across clusters. For example, in the middle temporal gyrus, the interaction was driven by an age-related increase in the relation between internal vividness ratings and activity during elaboration of positive events, an age-related decrease in the relation between external vividness ratings and activity during search of negative events, and an age-related decrease in the relation between internal vividness ratings and activity during search of positive and negative events. On the other hand, in the dlPFC cortex, the interaction was driven by age-related increases in the relation between activity during elaboration of positive events and both internal and external vividness ratings, with a stronger effect for internal vividness ratings.
Due to the size of the dmPFC cluster (777 voxels), the pattern differed across the peak voxels. The interaction in the aPFC was driven by age-related increases in the relation between external vividness ratings and activity during search for positive events, as well as age-related decreases in the relation between i) external vividness ratings and activity during search for negative events and ii) internal vividness ratings and activity during search for positive events. The age-related decreases were driven by a disappearance of the relation in older adults where there was a strong positive relation in young.
A sub-peak of this cluster, identified through a conjunction analysis with a prior analysis conducted with this dataset showing age-related decreases in hippocampal connectivity during negative relative to positive event retrieval (Ford et al., 2014a), was driven by an age-related increase in the relation between external vividness ratings and activity during search for positive events and, critically, an age-related decrease in the relation between external vividness ratings and activity during search for negative events. Parameter estimates extracted from this peak voxel revealed that this age-related decrease reflected a shift from a positive relation in the youngest adults in our sample (ages 18–25) to a negative relation in the oldest adults in our sample (ages 70–85; Figure 5). In fact, the relation between external vividness ratings and recruitment during search for negative events was significantly more negative than zero in this older adult sample (t(11)= 2.97, p=.01). A follow-up analysis of all regions exhibiting this age-by-valence-by-phase-by vividness type interaction revealed that this dorsomedial cluster was the only one that was driven by a significant negative relation in the oldest adults in our sample.
Figure 5.
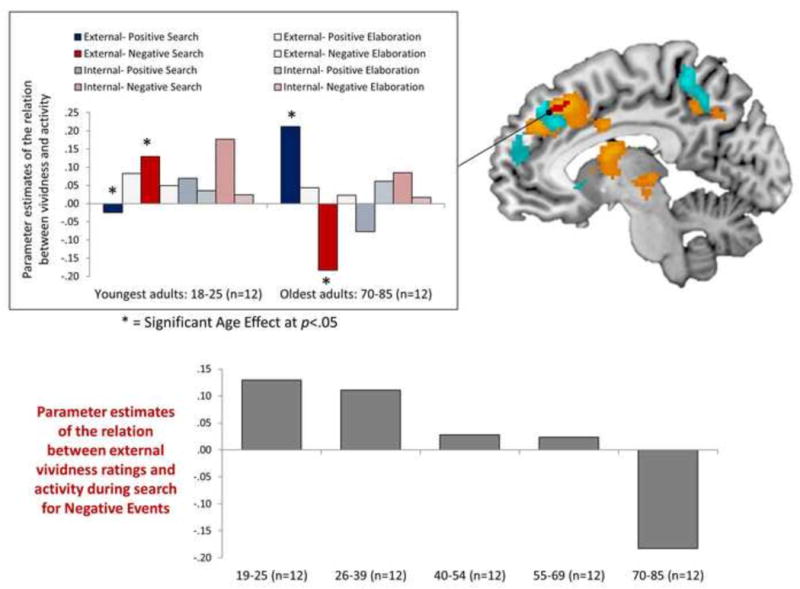
Regions exhibiting an age-by-valence-by-memory phase-by-vividness type interaction in the relation between recruitment and vividness ratings (cyan). The dorsomedial prefrontal cluster exhibiting this interaction overlapped with a dorsomedial prefrontal cluster identified in a prior analysis in which age was associated with significant decreases in hippocampal connectivity (orange regions = p< .05, red regions= p< .005; Ford et al., 2014a). To depict the relation, parameter estimates were extracted from the peak voxel from the conjunction of these two analyses. At this peak coordinate, age was associated with an increased relation between recruitment and vividness for positive events and a decreased relation between recruitment and vividness for negative events. The age-related decrease in the relation for negative events reflected a complete age reversal, where increased recruitment was associated with higher vividness ratings for negative events in the youngest adults in the sample (n=12, ages 19–25) and with lower vividness ratings for negative events in the oldest adults in the sample (n=12, ages 70–85).
4. Discussion
Aging has been associated with increases in retrieval of positive relative to negative information, leading to a behavioral positivity effect (see Reed et al., 2014). In addition, recent neuroimaging studies have revealed age-by-valence interactions, where aging is associated with increased PFC recruitment during negative relative to positive event retrieval (Ford et al., 2014a) and where this increased recruitment is associated with greater decreases in hippocampal recruitment (Ford et al., 2014a and Ford & Kensinger, 2014). Although this interaction has been described as potentially reflecting a prefrontally-mediated affective mechanism engaged by older adults to decrease the richness of negative events, the explicit relation between PFC recruitment and the phenomenology of negative events has not previously been examined. The current study was the first to directly link these two measures together by examining age-by-valence interactions in the relation between PFC recruitment and subjective ratings of memory vividness. This analysis confirmed a critical role of PFC regions in these age-by-valence interactions, where age reversed the relation between PFC recruitment and the subjective richness of the retrieved memory representation.
4.1 Age-related increases in the relation between vividness ratings and mPFC recruitment during negative relative to positive event retrieval
Age was associated with greater increases in the relation between vividness ratings and recruitment of the vmPFC during negative event retrieval relative to positive, with this interaction being driven by both an age-related increase in this relation for negative events as well as an age-related decrease in this relation for positive. Follow-up analyses revealed that young adults recruited this region during retrieval of events that were later judged as more vivid, regardless of event valence. The exaggeration of this effect for negative events in older adults, as well as the disappearance of the effect for positive, suggests that older adults are utilizing this region in a much more valence-specific way than younger adults, perhaps to enhance the overall vividness of negative events. Such a finding may be consistent with one of our competing hypotheses: that older adults over-recruit PFC regions during the retrieval of negative events in an effort to enhance vivid retrieval of poorly encoded events. In other words, older adults, if they more poorly encoded negative events than positive events initially, may require additional effort to retrieve details for these events. In support of this suggestion, prior work has linked a more subgenual region of the vmPFC to age-by-valence interactions in depth of encoding, suggesting a deeper encoding of positive than negative information in older adults (Leclerc & Kensinger, 2008).
This pattern did not differ as a function of vividness type, with age-by-valence interactions in the relation between recruitment and ratings of both external and internal vividness. If this interaction reflects an age-related reliance on prefrontal regions to enhance detail retrieval, this suggests that older adults are utilizing this vmPFC region to support retrieval of both internal and external details. However, similar age-by-valence interactions emerged for internal vividness ratings only in a slightly more dorsal region of the anterior medial PFC. The fact that age is more tightly coupled with increased internal vividness is consistent with behavioral evidence suggesting that older adults may be more likely to recall internal relative to external details (see Kensinger, 2008). Future work is needed to understand this interaction and to determine whether the same mechanism is responsible for the age-by-valence interaction in these two mPFC regions.
4.2 Age-related increases in the relation between vividness ratings and recruitment during positive relative to negative event retrieval
While vmPFC regions were characterized by greater age-related increases in the relation between vividness ratings and neural recruitment during negative relative to positive event retrieval, dorsal prefrontal regions were characterized by greater age-related increases in the relation between vividness ratings and neural recruitment during positive relative to negative event retrieval. Two patterns were identified to contribute to this interaction: a) the emergence of a significant relation between vividness and recruitment for positive events in older adults where none exists in young adults, and b) the complete age-related reversal for negative events, where young adults exhibit a positive relation between vividness and recruitment and older adults exhibit a negative relation. In other words, older adults were more likely to recruit dorsal PFC regions during retrieval of negative events that they later rated as less vivid and positive events that they later rated as more vivid.
One dmPFC cluster exhibiting this interaction overlapped with a cluster—identified in a prior analysis with the same dataset—where age was associated with significant decreases in functional connectivity with the left hippocampus. Specifically, this prior connectivity result (Ford et al., 2014a) overlaps with regions related to decreased external vividness in older adults. The overlap in these two analyses means that older adults are recruiting this dmPFC region both on trials associated with reduced hippocampal activity and on trials associated with decreased ratings of external vividness. Critically, these trial-by-trial changes may ultimately contribute to overall decreases in negative relative to positive event vividness in older adults. Further, the interaction with vividness type—driven by the selective effect in external vividness ratings— suggests that older adults are recruiting the dmPFC to reduce the impact of negative details associated with the image itself, rather than their memory for their personal reaction at encoding.
Overall, these findings are highly consistent with studies that reveal age-related enhancements in emotion regulation (e.g., Blanchard-Fields, 2007), and suggest that older adults may be engaging in these processes during retrieval of negative events. In other words, an increased motivation to regulate emotions in the oldest-old in our sample (see Mather & Carstensen, 2005) may lead older adults to preferentially recruit PFC regions in an effort to decrease the emotional impact of negative images. In support of this argument, the dmPFC has been implicated in both automatic and voluntary emotion regulation (See Phillips et al., 2008 for a review) and may be particularly involved in generating an emotional response when one anticipates an emotional event, as might be the case when trying to remember the details of a negative image associated with a neutral cue (Ochsner & Gross, 2007). Although a regulatory account is likely, the current study is unable to test this question directly. Future work is needed to examine the effect of emotion regulation on these age-by-valence interactions by determining a potential mediating role of motivation and ability to regulate negative emotions.
Notably, the age-related effects reported here were often stronger during the search phase of retrieval relative to the elaboration phase, suggesting a preferential impact of age-related changes during this phase. This pattern is consistent with recent findings demonstrating that age-related increases in prefrontal recruitment are greater during search relative to elaboration (Ford & Kensinger, in press).
4.3 Limitations and Future Directions
The current analysis examines the relation between neural recruitment and two measures of subjective vividness (i.e., internal and external). As research has demonstrated significant distinctions between the neural networks supporting subjective and objective vividness (Spaniol et al., 2009; see also Richter et al., 2016), the differences findings identified in the current study may not generalize to objective measures. Although it is plausible that participants’ vividness ratings are correlated with the amount of associative detail retrieved, it is equally possible that subjective and objective measures diverge. For instance, a person may feel that a memory is vivid even if they remember only a small subset of associative details. Importantly, low vividness in one measure in the current study (e.g., internal vividness) did not necessarily correspond to low vividness in the other (e.g., external vividness), with only 4% of all items receiving a vividness rating of “1” for both scales. In other words, low vividness ratings did not always reflect an overall memory failure as might be captured in objective memory tests. Future work is needed to confirm whether the patterns and relations reported here extend to one’s objectively-measured ability to recollect personal and event details.
In addition, as with all studies examining healthy aging, it is important to consider potential selection biases in our sample. The older adults in our sample were highly educated individuals who have been screened for potential health problems and dementia. In addition, they are older adults who are interested in research and motivated to come to the MR scanner for a 3-hour study session. In the current study, we must also consider the generalizability of the middle-aged adults, as it is often difficult for individuals in this age range to take the time to come into labs for study sessions. As such, it is possible that these findings do not generalize to all middle-aged and older adults, but rather reflect a particular subsample.
As mentioned above, the results of the current study suggest a potential regulatory mechanism supporting age-related changes in the relation between prefrontal recruitment and subjective memory vividness, but future work is needed to examine the effect of emotion regulation on these age-by-valence interactions. Such studies would focus on the mediating role of motivation (measured subjectively) and ability (measured objectively and subjectively) to regulate negative emotions on the effects identified in the current study. In addition, it is unclear from the current data whether similar dmPFC parametric modulation and hippocampal connectivity would be expected in an intentional memory suppression paradigm or if these patterns specifically reflect automatic regulation in memory retrieval.
4.4 Summary
The current study was the first to tie age-by-valence interactions in behavior to those in neural recruitment at a trial-by-trial (rather than between-subjects) level. Different patterns emerged in ventral and dorsal PFC regions. In ventral PFC regions, age was associated with an increased relation between recruitment and vividness ratings for negative events, a pattern consistent with an age-related increase in effort during negative event retrieval, perhaps due to reduced encoding of negative information. In dorsal regions, age was associated with a decreased relation between recruitment and vividness for negative events. Notably, these decreases overlap with a prior analysis with the same dataset showing age-related decreases in hippocampal connectivity. These findings are inconsistent with a retrieval-effort interpretation and instead suggest a controlled, prefrontally-mediated mechanism engaged by older adults during memory retrieval that reduces or dampens down the richness of negative events. While prior research has suggested that age-related shifts in emotional memory are related to downregulation of negative relative to positive events at their time of their occurrence (see Mather, 2012), these results raise the possibility that this dampening may also occur at the time of retrieval. Further, the tendency for older adults to engage this mechanism following presentation of a neutral retrieval cue—rather than an emotional cue—suggests that it is not the reprocessing of emotional content that drives this process, but rather internally-generated memories of negative events.
Supplementary Material
Table 3.
Regions in which the relation between activity and vividness was subject to age-by-memory phase-by-vividness type, age-by-valence-by-memory phase, age-by-valence-by-vividness type, and age-by-valence-by-memory phase-by-vividness type interactions.
| MNI Coordinates
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region of Interest | Hemisphere | BA | x | y | z | t-value | k | ||||||||
| Interactive effects of age, memory phase, and vividness type on the relation between activity and vividness ratings, regardless of valence | |||||||||||||||
| External Vividness | Internal Vividness | ||||||||||||||
| Search | Elaboration | Search | Elaboration | ||||||||||||
| Frontal Lobe | |||||||||||||||
| Premotor Cortex | L | 6 | −10 | −2 | 58 | 3.79 | 280 | # | |||||||
| R | 6 | 12 | −8 | 64 | 3.59 | 94 | # | ||||||||
| R | 6 | 18 | 16 | 54 | 3.24 | 72 | * | # | * | ||||||
| R | 6 | 46 | 6 | 40 | 3.11 | 86 | # | ||||||||
| Anterior Prefrontal Cortex | R | 10 | 10 | 62 | 10 | 3.66 | 203 | * | # | ||||||
| L | 10 | −20 | 54 | 2 | 3.36 | 104 | * | ||||||||
| Lateral Prefrontal Cortex | L | 10 | −38 | 42 | 12 | 3.37 | 143 | * | * | ||||||
| Dorsomedial Prefrontal Cortex | R | 8 | 14 | 50 | 32 | 3.36 | 156 | T | ˆ | ˆ | |||||
| L | 8 | −14 | 40 | 36 | 3.33 | 69 | * | # | |||||||
| Paracentral Lobule | R | 5 | 8 | −26 | 56 | 3.31 | 545 | # | |||||||
| Paracentral Lobule | L | 6 | −10 | −20 | 52 | 3.29 | # | ||||||||
| Posterior Cingulate | R | 31 | 22 | −32 | 42 | 3.24 | * | ||||||||
| Precentral Gyrus | R | 4 | 30 | −20 | 52 | 3.16 | 83 | * | # | ||||||
| Dorsolateral Prefrontal Cortex | L | 9 | −34 | 20 | 32 | 3.06 | 42 | # | |||||||
| R | 9 | 40 | 22 | 28 | 2.85 | 34 | # | * | |||||||
| Temporal Lobe | |||||||||||||||
| Inferior Temporal Gyrus | R | 20 | 54 | −14 | −24 | 4.05 | 296 | * | # | ||||||
| Middle Temporal Gyrus | L | 37 | −44 | −62 | 6 | 3.44 | 117 | # | |||||||
| Fusiform Gyrus | L | 20 | −48 | −4 | −32 | 3.34 | 49 | * | |||||||
| R | 37 | 54 | −52 | −8 | 3.07 | 43 | * | # | * | ||||||
| Superior Temporal Gyrus | R | 22 | 54 | −48 | 8 | 3.3 | 92 | # | * | ||||||
| L | 22 | −46 | −34 | −2 | 3.29 | 293 | # | # | |||||||
| Parietal Lobe | |||||||||||||||
| Precuneus | L | 7 | −2 | −44 | 58 | 3.86 | 2344 | # | |||||||
| Superior Temporal Gyrus | R | 39 | 46 | −48 | 32 | 3.83 | # | ||||||||
| Middle Temporal Gyrus | R | 39 | 38 | −60 | 34 | 3.80 | # | * | |||||||
| Inferior Parietal Lobule | L | 40 | −56 | −44 | 28 | 3.45 | 227 | # | |||||||
| Lingual Gyrus | L | 19 | −28 | −80 | −4 | 3.21 | 177 | * | |||||||
| R | 18 | 38 | −76 | −2 | 3.16 | 43 | # | ||||||||
| Limbic Lobe | |||||||||||||||
| Parahippocampal Gyrus | R | 28 | 18 | −12 | −14 | 3.9 | 219 | * | |||||||
| L | 28 | −18 | −16 | −18 | 3.09 | 36 | * | ||||||||
| Posterior Cingulate | L | 23 | −6 | −42 | 24 | 3 | 37 | # | |||||||
| Cerebellum | |||||||||||||||
| L | na | −38 | −60 | −20 | 3.81 | 186 | # | ||||||||
| L | na | −16 | −60 | −34 | 3.33 | 193 | # | ||||||||
| R | na | 42 | −54 | −24 | 2.99 | 44 | # | ||||||||
| Other | |||||||||||||||
| Putamen | R | na | 26 | 0 | 18 | 3.45 | 56 | * | * | ||||||
| L | na | −24 | 14 | 4 | 3.42 | 122 | T | # | ˆ | T | |||||
| Thalamus | L | na | −6 | −8 | 10 | 3.28 | 124 | # | |||||||
| Interactive effects of age, valence, and memory phase on the relation between activity and vividness ratings, regardless of vividness type | |||||||||||||||
| Positive Events | Negative Events | ||||||||||||||
| Search | Elaboration | Search | Elaboration | ||||||||||||
| Frontal Lobe | |||||||||||||||
| Precentral Gyrus | R | 6 | 40 | −4 | 38 | 3.56 | 656 | # | |||||||
| Postcentral Gyrus | R | 43 | 54 | −10 | 16 | 3.53 | # | * | |||||||
| Premotor Cortex | R | 6 | 30 | −6 | 40 | 3.49 | # | * | # | ||||||
| Premotor Cortex | L | 6 | −16 | −2 | 64 | 2.89 | 46 | # | * | ||||||
| Temporal Lobe | |||||||||||||||
| Superior Temporal Gyrus | L | 38 | −50 | −6 | −16 | 4.77 | 1257 | # | * | * | # | ||||
| Superior Temporal Gyrus | L | 22 | −54 | −6 | 0 | 3.83 | # | * | * | ||||||
| Postcentral Gyrus | L | 43 | −50 | −10 | 16 | 3.72 | # | * | * | ||||||
| Parietal Lobe | |||||||||||||||
| Paracentral Lobule | R | 4 | 14 | −36 | 70 | 4.15 | 351 | # | * | ||||||
| Inferior Parietal Lobule | R | 40 | 44 | 34 | 54 | 3.61 | 964 | # | * | * | |||||
| Premotor Cortex | R | 6 | 16 | −24 | 54 | 3.53 | # | * | # | ||||||
| Premotor Cortex | R | 6 | 16 | −8 | 52 | 3.51 | # | # | |||||||
| Inferior Parietal Lobule | L | 40 | −40 | −36 | 56 | 3.02 | 35 | # | |||||||
| Postcentral Gyrus | R | 3 | 48 | −14 | 46 | 3.07 | 31 | # | |||||||
| Occipital Lobe | |||||||||||||||
| Middle Occipital Gyrus | L | 37 | −46 | −72 | 6 | 3.25 | 67 | # | * | # | |||||
| Limbic Lobe | |||||||||||||||
| Anterior Cingulate | L | 33 | 0 | 18 | 20 | 3.31 | 45 | # | # | ||||||
| Cer ebellum | |||||||||||||||
| L | na | −14 | −38 | −12 | 4.25 | 1508 | * | # | |||||||
| L | 30 | −12 | −36 | −4 | 4.23 | # | * | * | # | ||||||
| L | na | −26 | −56 | −16 | 3.89 | # | * | * | |||||||
| R | na | 10 | −62 | −8 | 3.8 | 270 | # | * | # | ||||||
| R | na | 40 | −44 | −26 | 3.19 | 40 | # | ||||||||
| R | na | 22 | −50 | −36 | 3.06 | 50 | # | * | * | # | |||||
| R | na | 36 | −64 | −14 | 2.9 | 35 | # | # | |||||||
| Other | |||||||||||||||
| Caudate | L | na | −2 | 4 | −6 | 3.55 | 42 | # | * | # | |||||
| Putamen | L | na | −24 | 10 | −12 | 3.51 | 218 | # | * | * | # | ||||
| Claustrum | R | na | 32 | −12 | 18 | 3.43 | 177 | # | * | * | |||||
| Insula | L | 13 | −34 | −2 | 20 | 3.39 | 55 | # | |||||||
| R | 13 | 50 | −4 | −8 | 3.11 | 36 | # | * | # | ||||||
| Thalamus | L | na | −2 | −14 | 16 | 3.23 | 77 | # | # | ||||||
| Interactive effects of age, valence, and vividness type on the relation between activity and vividness ratings, regardless of memory phase | |||||||||||||||
| External Vividness | Internal Vividness | ||||||||||||||
| Positive Events | Negative Events | Positive Events | Negative Events | ||||||||||||
| Frontal Lobe | |||||||||||||||
| Anterior Prefrontal Cortex | L | 10 | −24 | 56 | 14 | 3.94 | 1379 | * | * | ||||||
| Caudate | R | na | 18 | 30 | −6 | 3.94 | * | * | |||||||
| Dorsomedial Prefrontal Cortex | L | −8 | 38 | 40 | 2.72 | * | # | ||||||||
| Dorsolateral Prefrontal Cortex | R | 8 | 28 | 40 | 34 | 3.56 | 237 | # | # | ||||||
| R | 6 | 30 | 14 | 38 | 3.09 | 29 | * | # | |||||||
| Precentral Gyrus | L | 4 | −34 | −16 | 36 | 3.22 | 114 | # | |||||||
| Temporal Lobe | |||||||||||||||
| Middle Temporal Gyrus | L | 20 | −58 | −44 | −10 | 3.12 | 63 | # | |||||||
| Parietal Lobe | |||||||||||||||
| Precuneus | L | 7 | −26 | −44 | 50 | 3.52 | 116 | # | |||||||
| Postcentral Gyrus | L | 7 | −10 | −46 | 68 | 3.44 | 242 | # | |||||||
| L | 2 | −52 | −14 | 30 | 3.12 | 77 | * | * | |||||||
| R | 2 | 40 | −20 | 36 | 3.11 | 111 | # | ||||||||
| Other | |||||||||||||||
| Putamen | L | na | −24 | 10 | −18 | 3.66 | 48 | # | * | ||||||
| Caudate | L | na | −18 | 20 | −6 | 3.47 | 73 | * | * | ||||||
| Interactive effects of age, valence, memory phase, and vividness type on the relation between activity and vividness ratings | |||||||||||||||
| External Vividness | Internal Vividness | ||||||||||||||
| Positive Events | Negative Events | Positive Events | Negative Events | ||||||||||||
| Search | Elaboration | Search | Elaboration | Search | Elaboration | Search | Elaboration | ||||||||
| Dorsomedial Prefrontal Cortex | L | 8 | −8 | 42 | 44 | 4.1 | 777 | * | # | # | |||||
| Anterior Prefrontal Cortex | L | 10 | −16 | 54 | 4 | 3.96 | * | # | # | ||||||
| Dorsomedial Prefrontal Cortex | L | −8 | 38 | 40 | 3.01 | * | # | ||||||||
| Precentral Gyrus | L | 6 | −32 | −8 | 34 | 3.76 | 231 | # | * | ||||||
| Dorsolateral Prefrontal Cortex | R | 8 | 28 | 16 | 38 | 3.34 | 92 | # | * | ||||||
| Temporal Lobe | |||||||||||||||
| Middle Temporal Gyrus | L | 39 | −48 | −62 | 26 | 3.37 | 85 | # | # | * | # | ||||
| Parietal Lobe | |||||||||||||||
| Precuneus | L | 7 | −26 | −44 | 50 | 3.72 | 437 | # | * | ||||||
| Postcentral Gyrus | R | 2 | 38 | −24 | 36 | 3.41 | 49 | # | |||||||
| Inferior Parietal Lobule | L | 40 | −50 | −32 | 46 | 3.00 | 98 | # | |||||||
| Other | |||||||||||||||
| Caudate | R | na | 18 | 30 | −6 | 3.97 | 281 | * | * | ||||||
| L | na | −18 | 22 | −6 | 3.37 | 91 | * | * | |||||||
| L | na | −20 | −28 | 16 | 3.16 | 63 | * | * | # | ||||||
| Thalamus | R | na | 14 | −18 | 18 | 3.82 | 279 | # | # | ||||||
Clusters significant at an uncorrected threshold of p<.005, k ≥ 29 voxels
BA= approximate Brodmann Area; L=Left, R=Right
Simple effects interrogated at p< .05:
= Effect of age (i.e., Older > Young adults),
= Reverse effect of age (i.e., Young > Older adults)
Peaks in which no simple effects were significant at p<.05 were interrogated at p<.1: T = Effect of age (i.e., Older > Young adults),
= Reverse effect of age (i.e., Young > Older adults)
For clusters with greater than 500 voxels, two additional sub-peaks are included and examined.
Peaks in bold were identified using a conjunction analysis with a previously reported analysis (Ford et al., 2014a)
Examined age-by-valence interactions on the link between recruitment and vividness.
Different age-related patterns emerged for ventral and dorsal prefrontal regions.
Age increases in relation between ventral activity and negative event vividness.
Age decreases in relation between dorsal activity and negative event vividness.
Vividness decreases overlap with age-related decreases in hippocampal connectivity.
Acknowledgments
The authors would like to thank Katherine Mickley Steinmetz for her assistance designing the current study, and John Morris and Halle Zucker for their assistance creating presentation scripts and running participants. Magnetic resonance data were collected at the Harvard Center for Brain Science. We thank the staff there, particularly Tammy Moran and Ross Mair, for their assistance with data collection and quality assurance. This work was supported by a Memory and Cognitive Disorders grant from the McKnight Endowment Fund for Neuroscience (EAK) and NIH grant MH080833 (EAK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors report no actual or potential conflicts of interest.
References Cited
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2882–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F. Everyday problem solving and emotion: An adult developmental perspective. Curr Dir Psychol Sci. 2007;16:26–31. [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Curr Dir Psychol Sci. 1995;4:151–156. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Kensinger EA. The relation between structural and functional connectivity depends on age and on task goals. Frontiers in Human Neuroscience. 2014;8:307. doi: 10.3389/fnhum.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Kensinger EA. Effects of internal and external vividness on hippocampal connectivity during memory retrieval. Neurobiology of Learning and Memory. 2016;134:78–90. doi: 10.1016/j.nlm.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Kensinger EA. Age-related reversals in neural recruitment across memory retrieval phases. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.0521-17.2017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Morris JA, Kensinger EA. Neural recruitment and connectivity during emotional memory retrieval across the adult life span. Neurobiology of Aging. 2014a;35(12):2770–2784. doi: 10.1016/j.neurobiolaging.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JH, Morris JA, Kensinger EA. Effects of emotion and emotional valence on the neural correlates of episodic memory search and elaboration. Journal of Cognitive Neuroscience. 2014b;26:825–839. doi: 10.1162/jocn_a_00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. Eur J Cogn Psych. 2009;21:192–215. [Google Scholar]
- Kensinger EA. How emotion affects older adults’ memories for event details. Memory. 2009;17:208–219. doi: 10.1080/09658210802221425. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive and Affective Behavioral Neuroscience. 2008;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci Biobehav Rev. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, Pietrzak RH. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Science. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cognitive Neurosci. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikels JA. Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychology and Aging. 2014;29:1–15. doi: 10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Richter FR, Cooper RA, Bays PM, Simons JS. Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. ELife. 2016;5 doi: 10.7554/eLife.18260. DOI: http://dx.doi.org/10.7554/eLife.18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrate of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. NY: The Haworth Press Inc; 1986. pp. 165–173. [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analysis using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewall G, Degueldre C, Luxen A, Collete F, Maquet P. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. Journal of Neuroscience. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


