Abstract
Background
Capecitabine with oxaliplatin (CAPOX) has previously demonstrated clinical activity in patients with small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC). We conducted a phase II trial to evaluate the benefit of adding bevacizumab to CAPOX.
Patients and Methods
In this phase II, single-arm, single-center, open-label study, we recruited patients aged 18 years and older with untreated advanced SBA or AAC. Patients received capecitabine 750 mg/m2 orally twice daily on days 1–14, oxaliplatin 130mg/m2 intravenously on day 1 and bevacizumab 7.5mg/kg intravenously on day 1 on a 21-day cycle. The primary endpoint was progression-free survival (PFS) at 6 months. Secondary objectives included response rate (RR), overall PFS, overall survival (OS) and toxicity.
Results
Between August 2011 and November 2014, 30 patients were enrolled into the study (M/F 13/17, median age: 63 years [range: 33–78], ECOG PS 0/1/2: 7/20/3). 23 patients (77%) had SBA (18 duodenal, 5 jejunal/ileal) and 7 patients had AAC (5 pancreaticobiliary subtype, 1 mixed subtype, 1 intestinal subtype). The most common grade 3 toxicities observed were fatigue and hypertension (7 [23%] patients each), neutropenia (6 [20%] patients), and diarrhea (3 [10%] patients). The probability of PFS at 6 months was 68% (95% confidence interval [CI] 52% to 88%). The RR was 48.3% with 1 complete response and 13 partial responses; 10 patients had stable disease. At a median follow-up of 25.9 months, median PFS was 8.7 (95% CI 4.9–10.5) months and median OS was 12.9 (95% CI 9.2–19.7) months.
Conclusions
Our results indicate that CAPOX with bevacizumab is an active and well-tolerated regimen for patients with SBA and AAC. These findings support the need for further investigation into the clinical benefit of targeting angiogenesis in SBA and AAC.
Keywords: Small bowel adenocarcinoma, Ampullary adenocarcinoma, CAPOX, Bevacizumab, Small bowel cancer
INTRODUCTION
Small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC) are rare tumors. The estimated incidence of small bowel cancer in the United States for 2016 is 10,090 patients, of which approximately 40% will be adenocarcinomas [1, 2]. The vast majority of patients present with late stage disease, which in part relates to frequent delays in diagnosis [2]. Although there are no randomized clinical trials comparing the efficacy of various chemotherapy regimens in patients with SBA, there have been four prospective studies, three of which used fluoropyrimidine and oxaliplatin as the backbone chemotherapy [3, 4, 5, 6]. We have previously demonstrated that capecitabine with oxaliplatin (CAPOX) is a safe and effective regimen for treatment of both advanced SBA and AAC [4].
Vascular endothelial growth factor (VEGF) plays a key role in tumor-associated neoangiogenesis, which helps provide a tumor with oxygen, nutrition and supports the development of metastasis [7]. We have previously conducted immunohistochemical staining for VEGF-A on 54 SBA tumor samples and found that VEGF-A is expressed in 96% of patients [8]. Another study noted universal expression of VEGF-A mRNA in 56 SBA samples at significantly higher levels compared with levels in adjacent normal intestinal mucosa [9]. The role of VEGF targeted agents has not been prospectively studied in the treatment of SBA and AAC.
Bevacizumab is a Food and Drug Administration (FDA) approved recombinant humanized monoclonal IgG antibody that binds to VEGF-A and prevents its binding to receptors on endothelial and cancer cells [10]. The combination of CAPOX and bevacizumab has been extensively studied in colorectal cancer (CRC) and has been FDA approved for first-line treatment of metastatic CRC. A phase III study comparing bevacizumab plus FOLFOX/CAPOX against placebo plus FOLFOX/CAPOX demonstrated similar rates of grade 3–4 toxicity, however, the addition of bevacizumab improved outcomes with median progression-free survival (PFS) of 9.4 months with bevacizumab compared to 8 months with placebo [11]. Given the high VEGF-A expression in metastatic SBA and AAC taken together with the benefit noted with addition of bevacizumab to chemotherapy in metastatic CRC, we hypothesized that the addition of bevacizumab to CAPOX chemotherapy would improve anti-cancer activity and improve outcome for patients with these malignancies.
PATIENTS AND METHODS
Patients
All eligible patients were required to have histologically confirmed SBA or AAC; measurable metastatic disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) criteria [12]; ECOG performance status of 0 to 2; and adequate hematologic (absolute neutrophil count [ANC] ≥1,500/μl, platelets ≥100,000/μl), hepatic (total bilirubin ≤1.5 times the upper limit of normal, AST and ALT <3 times the upper limit of normal) and renal function (creatinine clearance >50mL/min). Prior adjuvant chemotherapy (including 5-fluorouracil [FU], capecitabine and oxaliplatin) was allowed if completed ≥ 52 weeks prior and previous capecitabine or 5-FU administered as a radiosensitizing agent was allowed. A minimum of 4 weeks must have elapsed from completion of any prior chemotherapy, radiotherapy or surgery. Patients with any of the following were not eligible to participate in this study: prior chemotherapy for metastatic disease, a known history of dihydropyrimidine deficiency, peripheral neuropathy of grade 3 or greater, inadequately controlled hypertension (systolic blood pressure >140 mmHg and/or diastolic blood pressure > 90 mmHg) or history of hypertensive crisis/encephalopathy, NYHA grade II or greater congestive heart failure, and myocardial infarction or unstable angina or cerebrovascular accident or transient ischemic attack or significant vascular disease within 6 months. The University of Texas MD Anderson Cancer Center (UTMDACC) Institutional Review Board approved this study protocol and all patients gave written informed consent.
Study Design
The study was an open-label, single arm, single-institution, phase II study conducted at UTMDACC. Treatment consisted of intravenous oxaliplatin (130 mg/m2) administered on day 1, intravenous bevacizumab (7.5mg/kg) administered on day 1, and oral capecitabine (1500 mg/m2) divided twice daily on days 1–14 of each treatment cycle. Treatment cycles were repeated every 21 days and imaging studies were conducted every 3 cycles. Treatment was continued until progression of disease, inter-current illness preventing further administration of treatments, severe predefined treatment related toxicities or treatment delay of more than four weeks due to toxicity.
Dose Reductions
Toxicity was graded according to the NCI CTCAE version 4.0 except for neurosensory and skin toxicity. Neurosensory toxicity was graded according to the Neurologic Toxicity Scale for Oxaliplatin Dose Adjustments. Neurosensory toxicity did not result in dose reduction for capecitabine or bevacizumab. A new cycle of chemotherapy with capecitabine, oxaliplatin, and bevacizumab was delayed until the ANC was ≥ 1000/mm3, the platelet count was ≥ 75,000/mm3 and recovery was achieved from any treatment-related non-hematological toxicity (except alopecia, and oxaliplatin-related neurosensory toxicity) to baseline or was ≤ grade 1.
Capecitabine was interrupted during a cycle for grades 3 or 4 hematologic toxicity (excluding anemia) or for ≥ grade 2 non-hematologic toxicity (excluding grade 2 nausea or vomiting). Capecitabine dosage was reduced 25% for grade 2 hand-foot syndrome and 50% for grade 3 hand-foot syndrome; 25% for grade 3 non-hematologic toxicity and 50% for grade 4 non-hematologic toxicity; and 25% for a delay in hematologic recovery of ≥ 1 week (excluding anemia). Oxaliplatin dosage was reduced by 25% for grades 3 or 4 hematologic toxicity (excluding anemia), grade 3 or 4 non-hematologic toxicity (excluding hand-foot syndrome), a delay in hematologic recovery of ≥ 1 week (excluding anemia), or paresthesias with pain or functional impairment ≥ 7 days. Oxaliplatin was discontinued if paresthesias with pain or functional impairment persisted throughout a cycle. Patients were allowed to continue on study after oxaliplatin discontinuation. Two dose reductions were allowed for capecitabine and oxaliplatin. If a third reduction was required, the patient was removed from the study. There were no dose adjustments for bevacizumab.
Statistical Analysis
The primary end point was PFS at 6 months. PFS was defined as the interval between start of treatment to the date of first documentation of progression or symptomatic deterioration or death due to any cause. A previous study with 25 patients treated with CAPOX alone showed that PFS at six months is 52% [4]. The accrual goal was 30 patients. This sample size ensured that, if the trial is not terminated early, a posterior 90% confidence interval (CI) of PFS at 6 months would be (0.52–0.79), assuming that PFS at 6 months would be 0.67 (20/30) with the new treatment.
Secondary end points included response rate (RR), overall PFS, overall survival (OS) and toxicity. Responses were determined according to RECIST criteria in all evaluable patients. Toxicity data were analyzed in all patients who received at least one dose of study medication. For the unplanned exploratory analysis, we analyzed historical data from the metastatic cohort (n=25) from our previous CAPOX study [4]. Both sets of patients, current and historical, had metastatic disease.
OS was defined as the time from first study treatment to date of death or last follow-up. Comparisons were conducted using Wilcoxon rank sum test, Fisher exact test or Log rank test. Kaplan-Meier curves were used to estimate unadjusted OS and PFS time distributions. All computations were carried out in SAS version 9.4 and TIBCO Spotfire S+ version 8.2.
RESULTS
Between August 2011 and November 2014, 30 patients with advanced SBA or AAC were enrolled. The baseline characteristics of the study population are listed in Table 1. The median age of the study population was 63 years. Twenty three patients (77%) had SBA (18 of duodenal origin and 5 of jejunal/ileal origin) while seven patients (23%) had AAC (5 pancreatobiliary subtype, 1 intestinal subtype, 1 mixed subtype). Poorly differentiated histology was present in 60% of patients and mucinous histology was present in 16.7%. Inflammatory bowel disease was present in one patient, while none of the patients had a known history of Lynch syndrome.
Table 1.
Baseline patient characteristics (n = 30).
| Variable | N (%) | |
|---|---|---|
| Median age (years) | 63 (33, 78) | |
| ECOG Performance Status | 0 | 7 (23.3) |
| 1 | 20 (66.7) | |
| 2 | 3 (10.0) | |
| Grade | Moderate | 12 (40.0) |
| Poor | 18 (60.0) | |
| Mucinous features | None | 25 (83.3) |
| Present | 5 (16.7) | |
| History of inflammatory bowel disease | No | 29 (96.7) |
| Yes | 1 (3.3) | |
| History of Lynch Syndrome | No | 19 (63.3) |
| Unknown | 11 (36.7) | |
| Race | African American | 3 (10.0) |
| Asian | 1 (3.3) | |
| Hispanic | 4 (13.3) | |
| White | 22 (73.3) | |
| Gender | Female | 17 (56.7) |
| Male | 13 (43.3) | |
| Location | Ampulla | 7 (23.3) |
| Small bowel | 23 (76.7) | |
| Liver metastases | No | 13 (43.3) |
| Yes | 17 (56.7) | |
| Peritoneal metastases | No | 22 (73.3) |
| Yes | 8 (26.7) | |
Outcomes related to the efficacy of this regimen are listed in Table 2. The primary end point for this study was PFS at 6 months. The probability of PFS at 6 months with CAPOX-bevacizumab was 68% (95% CI 52%–88%; 90% CI: 54%–84%). Secondary end-points included ORR, overall PFS and OS. The ORR was 48.3% with 1 complete response (CR) and 13 partial responses (PR). Ten patients had stable disease (SD). The one inevaluable patient received one cycle of study treatment for metastatic duodenal adenocarcinoma, but due to the development of duodenal obstruction and biliary obstruction with subsequent decline in performance status, the patient was deemed not a candidate for additional chemotherapy. Figure 1 depicts a waterfall plot of best tumor response per RECIST criteria. The patient with CR was an AAC patient with pancreatobiliary subtype who developed a CR in multiple liver metastases following 12 cycles of CAPOX and bevacizumab and remained in CR for an additional 10 months. A second patient with duodenal adenocarcinoma developed radiographic resolution of extensive mesenteric and retroperitoneal lymphadenopathy but due to an elevated tumor marker is categorized as PR and currently remains on study at 15 months from study start. The RR was similar between SBA (50%) and AAC (43%). Of the five patients with pancreatobiliary subtype AAC, one patient had CR as noted above, another patient had PR while the remaining three patients had SD as their best response to treatment with CAPOX and bevacizumab. Meanwhile, best response achieved by the patient with intestinal subtype AAC was PR and best response achieved by the patient with mixed subtype AAC was SD.
Table 2.
Efficacy analysis.
| Outcome measure | All Patients (n = 30) | ||
|---|---|---|---|
| Number | % | 95% CI | |
| Response | |||
| Yes | 14 | 48.3 | |
| No | 15 | 51.7 | |
| Type of response | |||
| Complete response | 1 | 3.4 | |
| Partial response | 13 | 44.8 | |
| Stable disease | 10 | 34.4 | |
| Progressive disease | 5 | 17.2 | |
| Inevaluable | 1 | 3.4 | |
| Probability of progression free survival at 6 months | 68 | 52 – 88 | |
| Median progression free survival, months | 8.7 | 4.9 – 10.5 | |
| Median overall survival, months | 12.9 | 9.2 – 19.7 | |
Figure 1.
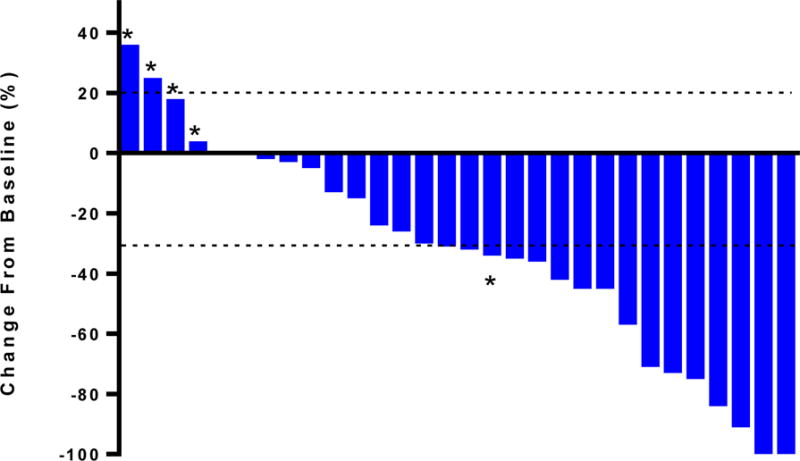
Waterfall plot with best tumor response per RECIST. * refers to patients with progressive disease per RECIST.
At a median follow-up of 25.9 months, the median PFS was 8.7 months (95% CI: 4.9–10.5 months; 90% CI: 6.5–10.3 months) and the median OS was 12.9 months (95% CI: 9.2–19.7 months; 90% CI 10.5–17.2 months) (Figure 2; Table 2). Three patients currently remain on study.
Figure 2.
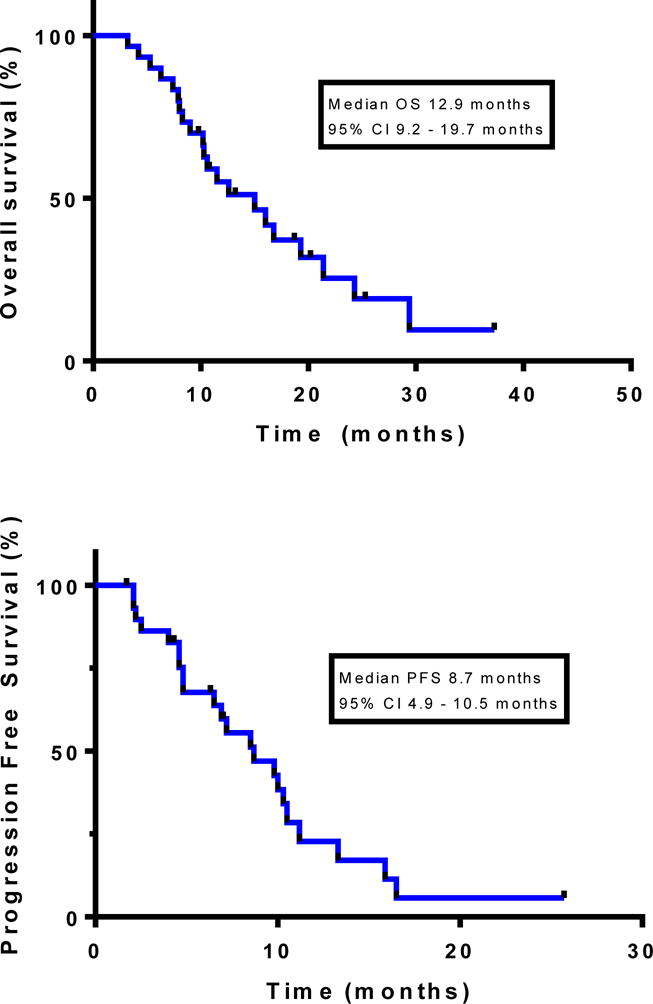
Kaplan-Meier estimates of overall survival (top) and progression-free survival (bottom).
The most common treatment-related grade 1–4 adverse events are listed in Table 3. Treatment was well tolerated, with the most common grade 3 toxicities of fatigue (7, 23%), hypertension (7, 23%), neutropenia (6, 20%), and diarrhea (3, 10%). Common grade 2 toxicities were anorexia (15, 50%), fatigue (14, 47%), and nausea (11, 37%). Only 1 grade 4 toxicity occurred; this patient developed grade 4 neutropenia after 8 cycles of treatment. There were no treatment related deaths.
Table 3.
Number of patients who experienced toxicities (n = 30).
| Toxicity type | Toxicity grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hematologic | ||||
| Anemia | 12 | 5 | 0 | 0 |
| Thrombocytopenia | 11 | 3 | 0 | 0 |
| Neutropenia | 1 | 3 | 6 | 1 |
| Non-hematologic | ||||
| Abdominal pain | 1 | 4 | 3 | 0 |
| ALT elevation | 13 | 2 | 1 | 0 |
| Alkaline phosphatase elevation | 5 | 1 | 1 | 0 |
| Anorexia | 6 | 15 | 2 | 0 |
| Ascites | 0 | 0 | 1 | 0 |
| AST elevation | 18 | 1 | 2 | 0 |
| Total bilirubin elevation | 3 | 2 | 1 | 0 |
| Colitis | 0 | 0 | 1 | 0 |
| Creatinine elevation | 2 | 0 | 0 | 0 |
| Dehydration | 3 | 8 | 1 | 0 |
| Diarrhea | 17 | 3 | 3 | 0 |
| Dysesthesia | 20 | 4 | 2 | 0 |
| Fatigue | 3 | 14 | 7 | 0 |
| Headache | 1 | 1 | 1 | 0 |
| Hypertension | 0 | 4 | 7 | 0 |
| Hyponatremia | 1 | 0 | 2 | 0 |
| Nausea | 10 | 11 | 2 | 0 |
| Noncardiac chest pain | 0 | 0 | 1 | 0 |
| Pancreatitis | 0 | 0 | 1 | 0 |
| Peripheral neuropathy | 18 | 3 | 2 | 0 |
| Pneumonitis | 0 | 0 | 1 | 0 |
| Portal Hypertension | 0 | 0 | 1 | 0 |
| Proteinuria | 11 | 1 | 0 | 0 |
| Syncope | 0 | 0 | 1 | 0 |
| Thromboembolic event | 0 | 0 | 1 | 0 |
| Vomiting | 13 | 3 | 0 | 0 |
| Weight loss | 6 | 1 | 0 | 0 |
An exploratory analysis comparing the current study to the 25 patient metastatic cohort from our prior phase II CAPOX study was performed. As shown in Supplemental Table 1, there were no significant differences in the baseline characteristics of the study populations involved in both studies. Statistical analysis demonstrated no significant difference in RR (48.3% vs. 52%, p=0.79) or PFS (8.7 months vs. 6.6 months; p=0.73; hazard ratio 1.125, 95% CI 0.585–2.163) between CAPOX with bevacizumab vs. CAPOX alone (Supplemental Figure 1).
DISCUSSION
In this study, we demonstrated that the combination of CAPOX with bevacizumab is an active regimen with an ORR of 48.3%, median PFS of 8.7 months and median OS of 12.9 months. Treatment was well tolerated with the most common grade 3 toxicities of fatigue (23%), hypertension (23%), neutropenia (20%) and diarrhea (10%). Our findings demonstrate that CAPOX with bevacizumab is a safe and efficacious combination for patients with advanced SBA or AAC. This is the first prospective clinical trial evaluating the use of targeted therapies in SBA and AAC.
Based on phase II clinical trials and retrospective data, the standard first-line treatment regimen for SBA is a combination of a fluoropyrimidine and oxaliplatin [3, 4, 5, 6]. The results from phase II studies of CAPOX and FOLFOX appear similar with response rates of 39 to 52% and a median PFS of 7.8 to 11.3 months [4, 6]. Recently, the addition of irinotecan to CAPOX was explored, resulting in a median PFS of 8.7 months and median OS of 12.7 months [5]. The results from our study appear similar to these prior studies. In addition, toxicity was similar to prior studies with this treatment combination and despite the presence of intact small bowel primaries in 60% of study patients, there were no episodes of bowel perforation.
Given its rarity and proximity to the large bowel, SBA has often been treated in a similar manner to CRC [2]. However, there are a number of epidemiological and molecular differences between SBA and CRC. According to Surveillance, Epidemiology and End Results program data, stage IV disease is present in 32% of SBA cases in contrast to 20% of CRC cases [13]. Furthermore, poor differentiation is present in 33% of SBA cases in contrast to 21% of CRC cases [13]. The incidence of these malignancies is diverging, with the incidence of SBA increasing and that of CRC declining in the United States [2]; it should be noted, however, that there is no routine screening for SBA and AAC while there are strict screening guidelines for CRC. Additionally, recent work has demonstrated that the stage-stratified cancer-specific survival rate was worse for patients with SBA than for those with CRC [13]. Although less is known about the molecular basis of SBA, the rate of APC mutations is markedly less, ranging from 7–13%, in contrast to 60–68% in CRC [2]. SMAD4 mutations are more common in SBA (30%) compared to CRC (5–16%) [2]. BRAF V600E mutations are very rare in SBA with one study finding no BRAF mutations in 99 cases [14]. The rate of KRAS mutations (codons 12 and 13) in SBA (40–60%) is comparable to CRC [2]. Taken together, such fundamental differences support continued efforts to better understand this malignancy and determine the optimal treatment approach.
An exploratory analysis comparing the current study with a preceding phase II clinical trial of CAPOX alone in SBA and AAC conducted at the same institution demonstrated no significant difference in RR or PFS. However, it is important to note that both studies enrolled a limited number of patients and were not designed or powered to detect differences between the two regimens. The benefit of addition of bevacizumab to chemotherapy in CRC has been demonstrated in several large randomized trials. The most recent NO16966 phase III clinical trial comparing bevacizumab or placebo combined with FOLFOX/CAPOX in the first-line setting demonstrated that the addition of bevacizumab improved outcome with median PFS of 9.4 months with bevacizumab compared with 8 months with placebo (HR 0.83, p=0.0023) [11]. Median OS was 21.3 months in the bevacizumab group and 19.9 months in the placebo group (HR 0.89, p=0.077). Response rates were similar in both arms, 47% in the bevacizumab group and 49% in the placebo group (p=0.31). Interestingly, phase III clinical trials in gastric cancer have not demonstrated a benefit with the addition of bevacizumab. The AVAGAST phase III clinical trial comparing bevacizumab or placebo combined with cisplatin/capectitabine in the first-line setting failed to meet its primary end-point, with a median OS of 12.1 months with bevacizumab compared with 10.1 months with placebo (HR 0.87, p=0.1002) [15]. Median PFS was significantly improved from 5.3 months with placebo to 6.7 months with bevacizumab (HR 0.80, p=0.0037). The response rate was also significantly improved from 37.4% with placebo to 46% with bevacizumab (p=0.0315). The results from this study taken together with data from other alimentary tract adenocarcinomas suggest the rationale for further evaluation of VEGF targeting in SBA and AAC. However, further investigation will require large multicenter efforts in order to provide the needed comparative data within this disease type.
This trial demonstrates the feasibility of completing prospective clinical trials of novel targeted agents in orphan tumor types. Although our findings suggest a novel combination therapy for this rare malignancy, they also raise an important question regarding the utility of costly targeted therapies in a population of patients for whom there are no randomized clinical trials. Recent successful efforts to target rare subsets of common tumors support the potential of improved clinical trial enrollment for patients with rare cancers. However, at present, oncologists and the patients they treat must rely upon phase II clinical trials to guide treatment decisions.
In conclusion, the combination of CAPOX and bevacizumab is an efficacious and well tolerated regimen in SBA and AAC. Toxicities were limited and there were no treatment related deaths. Further exploration of anti-angiogenic approaches in SBA and AAC is warranted.
Supplementary Material
Supplemental Table 1. Patient characteristics comparison between CAPOX+Bevacizumab (current study) and CAPOX (prior study Overman et al. [4])
Supplemental Figure 1. Kaplan Meier Estimates of overall survival (top) and progression-free survival (bottom) for CAPOX+Bevacizumab (current study) and CAPOX (prior study Overman et al. [4]).
Condensed Abstract.
We demonstrate that the combination of Capecitabine and Oxaliplatin with Bevacizumab is a safe and effective regimen for treatment of patients with advanced small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC) with an overall response rate of 48.3%, median progression-free survival of 8.7 months and median overall survival of 12.9 months. This is the first prospective clinical trial evaluating the use of targeted therapies in SBA and AAC.
Acknowledgments
We thank the patients who participated in this study as well as their family members for their help in advancing our knowledge about this rare malignancy. The authors thank Tamara Locke for editorial suggestions.
FUNDING
Financial support provided by Roche/Genentech and the Kavanagh Family Foundation.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Raghav K, Overman MJ. Small bowel adenocarcinomas—existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10(9):534–44. doi: 10.1038/nrclinonc.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 4.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 5.McWilliams RR, Mahoney MR, Marchello BT, et al. Pharmacogenetic dosing by UGT1A1 genotype as first-line therapy for advanced small-bowel adenocarcinoma: A North Central Cancer Treatment Group (NCCTG) trial. J Clin Oncol. 2012;30(Suppl 4):a314. [Google Scholar]
- 6.Xiang XJ, Liu YW, Zhang L, et al. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23:561–566. doi: 10.1097/CAD.0b013e328350dd0d. [DOI] [PubMed] [Google Scholar]
- 7.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Review Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 8.Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterization of adenocarcinoma of the small intestine. Br J Cancer. 2010;102(1):144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Rahden BH, Brucher BLDM, Langner C, et al. Expression of cyclo-oxygenase 1 and 2, prostaglandin E synthase and transforming growth factor beta1, and their relationship with vascular endothelial growth factors A and C, in primary adenocarcinoma of the small intestine. Br J Surg. 2006;93(11):1424–32. doi: 10.1002/bjs.5426. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Reviews Drug Discovery. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guidelines (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Overman MJ, Hu CY, Kopetz S, et al. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol. 2012;19:1439–1445. doi: 10.1245/s10434-011-2173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu T, Pappou EP, Guzzetta AA, et al. CpG Island Methylator Phenotype—Positive Tumors in the Absence of MLH1 Methylation Constitute a Distinct Subset of Duodenal Adenocarcinomas and Are Associated with Poor Prognosis. Clinical Cancer Research. 2012;18(17):4743–4752. doi: 10.1158/1078-0432.CCR-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Patient characteristics comparison between CAPOX+Bevacizumab (current study) and CAPOX (prior study Overman et al. [4])
Supplemental Figure 1. Kaplan Meier Estimates of overall survival (top) and progression-free survival (bottom) for CAPOX+Bevacizumab (current study) and CAPOX (prior study Overman et al. [4]).


