Abstract
Engineered T-cell therapy using a CD19-specific chimeric antigen receptor (CD19-CAR) is a promising strategy for the treatment of advanced B-cell malignancies. Gene transfer of CARs to T-cells has widely relied on retroviral vectors, but transposon-based gene transfer has recently emerged as a suitable nonviral method to mediate stable transgene expression. The advantages of transposon vectors compared with viral vectors include their simplicity and cost-effectiveness. We used the Tol2 transposon system to stably transfer CD19-CAR into human T-cells. Normal human peripheral blood lymphocytes were co-nucleofected with the Tol2 transposon donor plasmid carrying CD19-CAR and the transposase expression plasmid and were selectively propagated on NIH3T3 cells expressing human CD19. Expanded CD3+ T-cells with stable and high-level transgene expression (~95%) produced interferon-γ upon stimulation with CD19 and specifically lysed Raji cells, a CD19+ human B-cell lymphoma cell line. Adoptive transfer of these T-cells suppressed tumor progression in Raji tumor-bearing Rag2−/−γc−/− immunodeficient mice compared with control mice. These results demonstrate that the Tol2 transposon system could be used to express CD19-CAR in genetically engineered T-cells for the treatment of refractory B-cell malignancies.
INTRODUCTION
Adoptive immunogene therapy with T-cells expressing chimeric antigen receptors (CARs) is a promising approach for the treatment of advanced malignancies. CARs are composed of an extracellular single chain fragment of variable region fused to one of the two intracellular lymphocyte signaling domains, CD28 or 4-1BB (CD137), coupled with CD3ζ to mediate T-cell activation.1 T-cells transduced with CAR-expressing vectors can recognize and kill tumor cells that express tumor-associated antigens such as CD19 in a human leukocyte antigen-independent manner. In early-phase clinical trials, the adoptive transfer of CD19-specific CAR (CD19-CAR)-transduced T-cells was found to cause anti-tumor effects in patients with chemorefractory CD19+ B-cell malignancies.2
The gene transfer of CARs into T-cells has mainly been achieved using retroviral vectors. However, DNA transposon-based gene transfer has emerged as an appealing alternative, because transposon vectors are easier and less expensive to manufacture than retroviral vectors.3 Transposon vectors work via a cut-and-paste mechanism called transposition, whereby transposon DNA containing the gene of interest is integrated into chromosomal DNA by a transposase.
Tol2 is an active transposon derived from the medaka fish (Oryzias latipes).3,4 It is active in a variety of vertebrate species and has been used for gene transfer in mammalian cells.4,5 Tol2 has a fairly large cargo capacity; it can carry a total of around 200 kb and ~ 10 kb without reducing its transpositional activity.6,7 Recently, the piggyBac (PB) transposon was shown to have a cargo capacity of 150 kb.8 Transposase itself can act as a transposition inhibitor when it exceeds a threshold concentration, enabling it to limit transposon activity in a phenomenon called overproduction inhibition (OPI). The Sleeping Beauty (SB) transposon undergoes OPI, whereas Tol2 and PB transposons exhibit limited OPI.9 Unlike SB and PB transposons that specifically integrate at TA or TTAA sequences, respectively, Tol2 does not appear to have a specific preferential target sequence.3
In the present study, we investigated whether the Tol2 transposon system could mediate the stable transfer of CD19-CAR to primary human T-cells. We show that Tol2-engineered T-cells efficiently and stably expressed CD19-CAR and exhibited CD19-dependent anti-tumor effects in vitro and in a mouse xenograft model. Our results demonstrate for the first time that the Tol2 transposon system can be used to stably express CD19-CAR in engineered T-cells for the treatment of B-cell malignancies.
RESULTS AND DISCUSSION
Transposons are promising nonviral vectors for human gene therapy. They have significantly higher integration efficiencies than electro-transferred naked DNA plasmids. Moreover, compared with retroviral vectors, transposons offer several advantages, such as low immunogenicity, simplicity of use and low manufacturing costs. The SB and PB transposon systems have also been used to stably introduce CD19-CARs into human T-cells,10,11 while the SB system recently formed part of a human clinical trial involving CAR-based T-cell therapy for B-cell malignancies.12
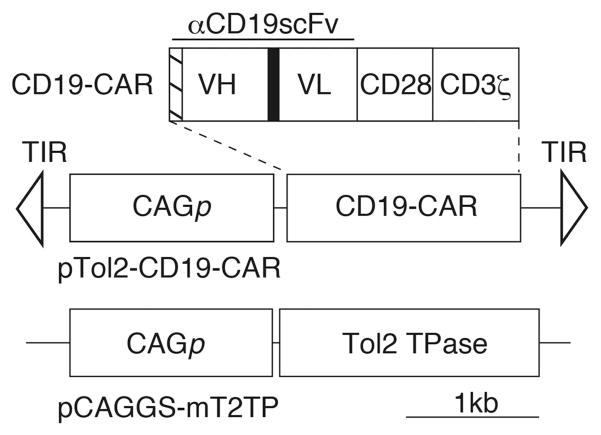
In the present study, we generated a Tol2 transposon construct carrying the CD19-CAR gene (pTol2-CD19-CAR) (Figure 1). To evaluate whether the Tol2 transposon system could be used for CD19-CAR transfer, human peripheral blood lymphocytes (PBLs) were transfected with pTol2-CD19-CAR in the presence or absence of the Tol2 transposase expression plasmid (pCAGGS-mT2TP) (Figure 1). Transfected T-cells were propagated on NIH3T3 cells expressing CD19 (3T3/CD19).
Figure 1.
CD19-CAR and the Tol2 transposon system used in this study. VH, variable heavy chain; VL, variable light chain; hatched box, CD8α signal peptide; black box, (GGGGS)3 linker; pTol2-CD19-CAR, Tol2 transposon plasmid carrying CD19-CAR; CAGp, hybrid cyto-megalovirus enhancer/chicken β-actin promoter; TIR, terminal inverted repeat; pCAGGS-mT2TP, Tol2 transposase (TPase) expression plasmid.
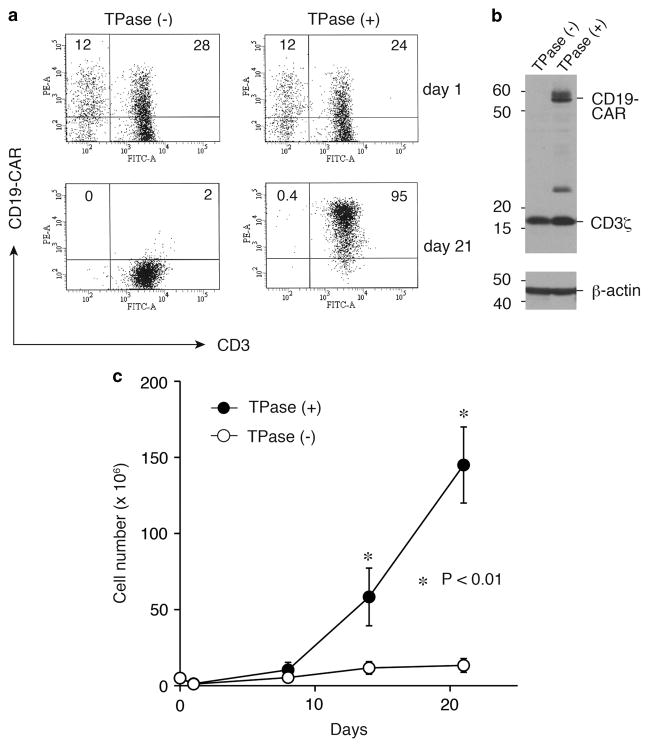
We analyzed the surface expression of CD19-CAR in transfected T-cells by flow cytometry. On day 21 of the culture, CD19-CAR+ CD3+ T-cells constituted approximately 95% of cultures transfected with both Tol2 transposon and transposase plasmids, whereas CD19-CAR expression was very low (2%) in T-cells transfected with the transposon alone (Figure 2a). We also confirmed CD19-CAR expression in T-cells co-transfected with Tol2 transposase by western blotting (Figure 2b). Co-transfected T-cells showed an approximately 29-fold expansion within 3 weeks, while T-cells transfected with transposon alone did not grow (Figure 2c). We next examined the immunophenotypes of expanded T-cells by flow cytometry. On day 21, these T-cells were positive for CD3 (99%), CD4 (61%) and CD8 (34%) after ex vivo expansion with antigen stimulation (Supplementary Figure S1). A CD4-dominant population was also previously observed in the results of SB- and PB-based T-cell gene transfer with CD19-CAR,10,13 while Saito et al.14 recently showed a CD8-dominant culture method after PB-based T-cell gene transfer with CD19-CAR. When specific T-cell subsets of engineered T-cells were analyzed in the present study, both CD45RO+CD62L+ central memory and CD45RO+CD62L− effector memory phenotypes were present (Supplementary Figure S1). These results show that large numbers of T-cells with stable and high-level transgene expression were generated by Tol2-mediated gene transfer and ex vivo expansion.
Figure 2.
CD19-CAR expression and ex vivo expansion of Tol2-modified T-cells. (a) Surface expression of CD19-CAR on T-cells after nucleofection with pTol2-CD19-CAR with or without pCAGGS-mT2TP (TPase) plasmids was examined by flow cytometry. Values represent the percentages of CD3+ CD19-CAR+ and CD3− CD19-CAR+ cells. Data are representative of one of the three independent experiments using different donors. (b) CD19-CAR expression of Tol2-tranduced T-cells in the presence or absence of TPase as detected by western blotting with an anti-CD3ζ antibody. β-Actin was used as a loading control. (c) Growth rates of transduced T-cells with or without TPase. 3T3/CD19 cells were added weekly. Viable cells were enumerated by trypan blue exclusion. Data represent mean ± s.d. from three different donors.
To examine whether stable CD19-CAR expression in Tol2-engineered T-cells was caused by genomic integration of the transposon, we measured the transgene copy number relative to the copy number of the interferon (IFN)-γ gene as a reference using real-time quantitative PCR. At 21 and 29 days posttransduction, the calculated transgene copies per T-cell were 1.75 ± 0.1 and 1.53 ± 0.6 (mean ± s.d., n = 3), respectively, for the Tol2-mediated gene transfer samples. We also mapped the transposon integration sites in these T-cells (day 29) by inverse PCR, as well as the integration sites of CD19-CAR T-cells generated by the retrovirus system in our previous study15 as a control (Table 1). These results indicate that the Tol2 transposon integrated into the human genome and stably expressed CD19-CAR in primary human T-cells.
Table 1.
Integration sites in Tol2- and retrovirus-modified T-cells
| Vector | Clone | RefSeq gene | RefSeq no. | Location | Chromosome |
|---|---|---|---|---|---|
| Tol2 | No. 1 | FAM46A | NM_017633.2 | Intron 1 | 6 |
| No. 2 | MFI2 | NM_005929.5 | Intron 1 | 3 | |
| No. 3 | VGLL1 | NM_016267.3 | 20.6 kb 3′ | X | |
| No. 4 | ZMPSTE24 | NM_005857.4 | 0.6 kb 5′ | 1 | |
| No. 5 | CDH23 | NM_001171930.1 | Intron 25 | 10 | |
| No. 6 | TMEM200A | NM_001258276.1 | Exon 1 | 6 | |
| Retrovirus | No. 7 | FYB | NM_001243093.1 | Intron 1 | 5 |
| No. 8 | BRE | NM_001261840.1 | Intron 10 | 2 | |
| No. 9 | ZNF706 | NM_001042510.1 | 40.6 kb 3′ | 8 | |
| No. 10 | SLFN5 | XM_005257934.1 | 12.8 kb 3′ | 17 | |
| No. 11 | NF1 | NM_000267.3 | Intron 36 | 17 |
Distance from the transcription start site or end sites of RefSeq genes is indicated.
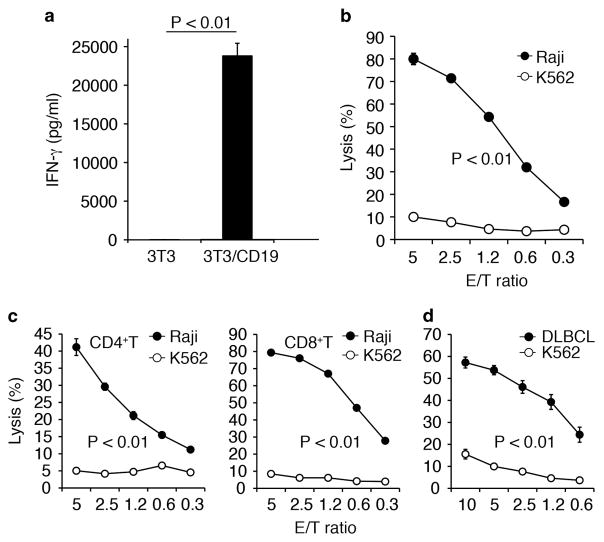
We next assessed antigen-dependent IFN-γ production of Tol2-engineered T-cells by enzyme-linked immunosorbent assay and found that they selectively produced IFN-γ only in response to CD19+ target cells (Figure 3a). CD19-CAR T-cells showed cell lytic activity against Raji cells but not against control CD19-negative K562 cells (Figure 3b). We then examined which subpopulations of T-cells were involved in targeted cell killing by cell fractionation. Both CD4+- and CD8+-sorted T-cells specifically lysed CD19+ target cells. However, CD4+ T-cell-mediated cytotoxicity was about twofold less than that observed for CD8+ T-cells (Figure 3c). Moreover, CD4+ and CD8+ T-cells also killed primary CD19+ B-cell lymphoma cells isolated from a patient with a diffuse large B-cell lymphoma (Figure 3d). Taken together, these results demonstrate that expanded CD19-CAR T-cells exhibited CD19-dependent effector functions in vitro.
Figure 3.
Antigen-specific effector functions of CD19-CAR T-cells in vitro. (a) IFN-γ secretion by CD19-CAR T-cells after co-culture with 3T3/CD19 or parental 3T3 cells. (b) Cytotoxicity of CD19-CAR T-cells against Raji and K562 cells in Calcein-AM release assays. K562 cells served as CD19− targets. (c) Cytotoxic activities in CD4- and CD8-sorted T-cells. (d) Cytotoxicity against primary CD19+ diffuse large B-cell lymphoma (DLBCL) cells. Values show the mean ±s.d. of triplicate wells.
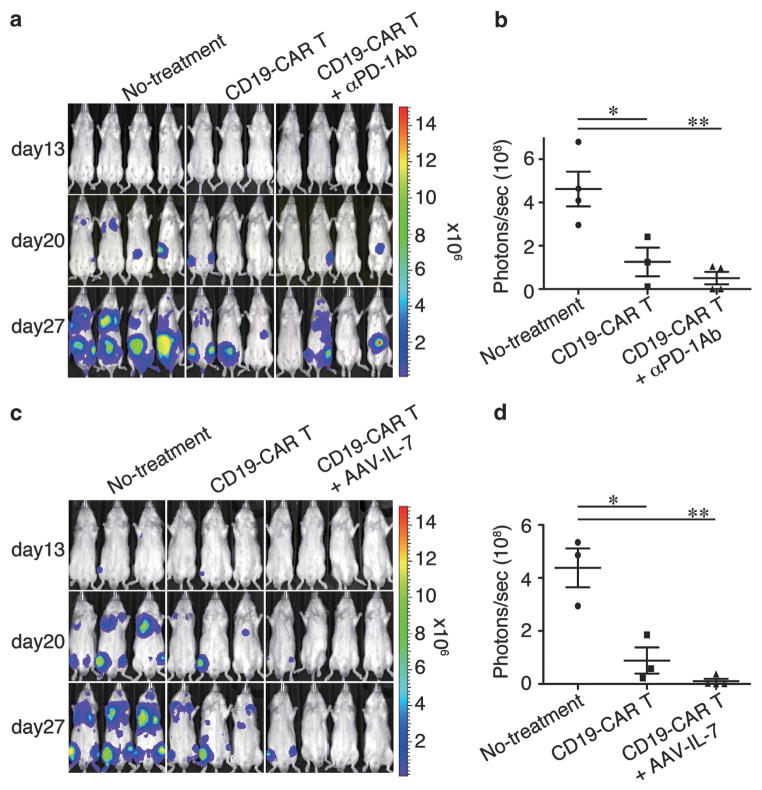
We next wanted to evaluate the in vivo anti-tumor effects of engineered T-cells in a mouse xenograft model that we previously established.15 Rag2−/−γc−/− immunodeficient mice were intravenously injected with luciferase-expressing Raji cells. Three days later, the mice were intravenously injected with CD19-CAR T-cells. Bioluminescent imaging revealed that CD19-CAR T-cells successfully suppressed Raji tumor progression, while Raji tumors grew systemically in the untreated group (Figure 4a). To augment these in vivo findings, we evaluated treatment with an antibody against the programmed death (PD)-1 protein, because our expanded T-cells partially expressed PD-1 (23 ± 6.2%; mean ± s.d., n = 3) and a PD-1 blockade by the antibody previously enhanced the anti-tumor activity of PD-1+ tumor- or virus-specific T-cells in vivo.16 We found that use of the anti-PD-1 antibody did not significantly reduce the number of tumors at the day 27 end point compared with the T-cell group alone (P = 0.30) (Figures 4a and b). However, two of the four animals treated with the anti-PD-1 antibody were tumor free, while all three mice in the T-cell group carried tumors. These observations do not support those of John et al.,17 who previously demonstrated that treatment with an anti-PD-1 antibody augmented the anti-tumor activity of PD-1+ Her-2-specific CAR T-cells cultured with antigen stimulation in their mouse model of Her-2+ breast cancer. It is possible that the types of tumors, levels of PD-1 and use of immunocompetent or immunocompromised animals in these different studies may account for these observed differences. Neither nontransduced T-cells nor the PD-1 antibody injection alone suppressed tumor progression in our present study (data not shown).
Figure 4.
(a) Bioluminescent imaging of systemic Raji tumor progression in Rag2−/−γc−/− mice following T-cell infusion. Mice inoculated intravenously with Raji/Luc cells on day 0 were infused with CD19-CAR T-cells alone on day 3 (T-cell treatment group, n =3) or in combination with three intraperitoneal injections of an anti-PD-1 antibody (n =4), whereas mice in the control group did not receive T-cells (n =4). (b) Quantitative bioluminescence imaging on day 27 as shown in panel (a). (c) Raji tumor-bearing mice were infused with CD19-CAR T-cells alone (n =3) on day 3 or in combination with a single intramuscular injection of AAV-IL-7 (n =4). (d) Quantitative bioluminescence image on day 27 as shown in panel (c). Bioluminescent imaging was performed on days 13, 20 and 27. *P<0.05, **P<0.01.
We next examined whether interleukin (IL)-7 could improve the in vivo efficacy of these T-cells in our mouse model, because IL-7 has been used to augment the anti-tumor effects of antigen-specific T-cells after adoptive cell therapy in mouse models.18 We used the adeno-associated virus (AAV) vector system to achieve systemic and long-term IL-7 expression in mice.19 The IL-7+ T-cell group showed enhanced tumor suppression via imaging analysis compared with that of the control group (P = 0.001) and approached statistical significance compared with that of the T-cell transfer group alone (P = 0.12, Figures 4c and d). AAV-mediated IL-7 administration alone did not suppress tumor growth (data not shown). These results show that Tol2-engineered T-cells function as anti-tumor effector cells in our mouse model.
CAR-based T-cell therapy is dependent on efficient gene transfer and an optimal expansion regimen to achieve both the cell number and potency required for use in the clinical setting. One day after nucleofection, expression of CD19-CAR was ~ 24% in CD3+ Tol2-engineered T-cells (Figure 2a, upper), which is similar to the transgene expression previously observed in CD3+ SB-engineered T-cells (19–65%; day 1).13,20 After 3 weeks of expansion, stable and robust CD19-CAR expression was detected in engineered T-cells (~95%; Figure 2a, lower), comparable to that observed in SB- or PB-engineered T-cells with CD19-CAR (51–98%) after 3 weeks of expansion with antigen stimulation.10,13,20 Improvements in vector design and delivery should yield even better results. Peng et al.21 showed that a green fluorescent protein (GFP)-encoded SB transposon exhibited >50% transduction without antigen stimulation in primary T-cells in the presence of an mRNA-encoded transposase. Additionally, SB100X, which encodes a hyperactive SB transposase, exhibited improved transposition activity and enhanced T-cell gene transfer efficiency.22 Thus future development of the hyperactive Tol2 transposase and its RNA delivery may improve the generation of CD19-CAR T-cells.
Some retroviral vectors integrate near transcription start sites and so have the potential to cause insertional mutagenesis.23 However, as yet there have been no reports that retroviral vectors have caused adverse events in T-cells.24 Tol2 transposons preferentially integrate around transcription start sites, CpG islands and DNaseI hypersensitive sites.25 We mapped the integration sites of Tol2- and retrovirus vector-engineered T-cells with CD19-CAR and showed that their target sequences did not overlap (Table 1). However, our sample size is too small to compare the exact integration profile of each vector, so further large-scale comparative analysis of integration sites will provide the necessary important information about the integration preference and safety of the vectors. Regardless of the vector system used, the development of safer transgene delivery systems has become an important area of study. To this end, AAV-based site-directed integration systems may be useful, because Rep proteins from AAV facilitate the integration of the viral genome into a specific locus (termed AAVS1) in chromosome 19.26 This locus is one of the safe locations in the human genome for efficient and safe transgene expression.27 Ammar et al.28 demonstrated that fusion constructs consisting of Rep and Tol2 transposase integrated marker genes from Tol2 transposon donor plasmids near the AAVS1 locus, which may offer a safer transposon-based gene delivery system for human gene and cell therapy.
In the present study, we have demonstrated that the adoptive transfer of Tol2-engineered T-cells with CD19-CAR suppressed tumor growth in tumor-bearing mice and have shown enhanced tumor suppression of Tol2-engineered T-cells in combination with systemic IL-7 delivery. IL-7 has previously been used to enhance the anti-tumor effect of tumor specific-T-cells in preclinical and clinical studies and has acceptable toxicity profiles.18 Furthermore, IL-7 preferentially maintains memory CD4+ and CD8+ T-cells over CD4+CD25+Foxp3+ regulatory T-cells in clinical trials.18 Therefore, IL-7 appears to be a potential adjuvant for engineered T-cell therapy.
The Tol2 transposon system is a promising technology for gene transfer in mammalian cells, and its efficacy is comparable with other transposon systems, such as SB and PB transposons. Tol2 also offers some advantages over SB, including a high cargo capacity and limited OPI, which are important characteristics for transposon-based gene therapy. With respect to cargo capacity and OPI, PB is similar to Tol2. Therefore, the simultaneous comparison of Tol2 and PB, with or without various augmenting reagents (for example, PD-1 antibody or IL-7) in a characterized mouse model as described in this work, would help identify an optimal transposon system for immunogene therapy.
In conclusion, we used the Tol2 transposon system to stably transfer CD19-CAR into primary human T-cells. We found that expanded T-cells contained one or two copies of CD19-CAR leading to efficient transgene expression and further demonstrated that they exhibited CD19-dependent anti-tumor effects in vitro and in a xenograft mouse model of human B-cell lymphoma. Our results indicate that the Tol2 transposon system is a useful gene transfer platform and could be applied to CD19-CAR-based T-cell therapy for refractory B-cell malignancies.
MATERIALS AND METHODS
Plasmids
The Tol2 transposon plasmid pT2AL200R175-CAGGS-EGFP has been described previously.7 The Tol2 transposon plasmid encoding CD19-specific CAR with CD28 and CD3ζ signaling domains (CD19-CAR) from the SFG-1928z retroviral vector29 pTol2-CD19-CAR was generated by replacing the EGFP sequence of pT2AL200R175-CAGGS-EGFP with the CD19-CAR sequence. The Tol2-transposase plasmid pCAGGS-mT2TP carrying a modified transposase cDNA5 with codons optimized for mammalian use was also transfected. The human IL-7 expression vector AAV-IL-7 was generated by ligating IL-7 cDNA into the pAAV-MCS vector (Agilent Technologies Inc., Palo Alto, CA, USA).
Recombinant AAV-IL-7 production
The recombinant AAV-IL-7 vector (serotype 1) was prepared as previously described,30 and vector titers were measured using real-time PCR.30
PBLs and cell lines
PBLs from three healthy donors and a clinical sample from a patient with non-Hodgkin B-cell lymphoma were collected under a protocol approved by the Jichi Medical University Institutional Review Board, with written informed consent obtained from each donor. The mouse fibroblast NIH3T3 cell line expressing human CD19 (3T3/CD19)31 was maintained in Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA). The human Burkitt lymphoma cell line Raji (Health Science Research Resources Bank, Osaka, Japan), Raji cells expressing luciferase (Raji/Luc)15 and the human erythroleukemia cell line K562 (Riken BRC, Ibaraki, Japan) were also used. Cell lines were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum.
Transfection and expansion of T-cells
PBLs (5 × 106) were nucleofected with pTol2-CD19-CAR and pCAGGS-mT2TP (5 μg each) using the Human T-cell Nucleofector Kit and Amaxa Nucleofector II program U-014 (Lonza, Basel, Switzerland) on day 0. Transfected PBLs were transferred to X-Vivo 15 (Takara Bio, Shiga, Japan) supplemented with 5% human AB serum (NOVA Biologics, Oceanside, CA, USA) (T-cell complete medium) and cultured overnight. The next day, cells were stimulated with γ-irradiated (50 Gy) 3T3/CD19 cells at a 1:1 ratio for selective propagation and cultured in T-cell complete medium supplemented with 1 nM recombinant human IL-2 (Life Technologies) in G-Rex10 culture flasks (Wilson Wolf Manufacturing Corporation, New Brighton, MN, USA). 3T3/CD19 cells were added to the T-cell cultures on days 1 and 8.
Flow cytometry
CD19-CAR surface expression on transduced T-cells was assessed using a biotin goat anti-mouse F(ab′)-specific antibody, phycoer-ythrin–Streptavidin (Jackson Immunoresearch, West Grove, PA, USA) and fluorescein isothiocyanate anti-human CD3 (Biolegend, San Diego, CA, USA). Samples were analyzed using the BD LSR Fortessa with FACSDiva software (BD Biosciences, San Diego, CA, USA).
Western blotting
CD19-CAR protein expression was examined by western blotting using an anti-human CD3ζ antibody as described previously.15
Transgene copy number and integration sites
Transgene copy numbers were measured by real-time quantitative PCR using Thermal Cycler Dice Real Time System II (Takara Bio). Genomic DNA was isolated from CD19-CAR T-cells by a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) on days 21 and 29 after transduction. PCR was performed using a Cycleave PCR Core kit (Takara Bio) with sets of primers and probes specific for CD19-CAR and human IFN-γ. For CD19-CAR, these were the forward primer A: 5′-TGCACAGTGACTACATGAACA-3′; the reverse primer B: 5′-CGTCCTAGATTGAGCTCGTTA-3′; and probe: 5′-gctcc(A)gag-3′; for IFN-γ, these were the forward primer C: 5′-CAGGGTCACCTGACACATTCA-3′; the reverse primer D: 5′-ACTAGGCAGCCAACCTAAGC-3′; and probe: 5′-acaatgaac(A)ct-3′. Uppercase letters of probe sequences represent RNA bases. Serially diluted CD19-CAR and human IFN-γ plasmid standards (1 μl containing 6 × 101–3 × 106 copies each) were used. Each standard curve (threshold cycle values versus copy numbers) is shown in Supplementary Figure S2 (day 29). The average CD19-CAR copy number per cell was calculated by normalizing to the endogenous number of diploid human IFN-γ copies.
Inverse PCR was performed as described previously7 to identify the 3′ transposon junction sequence. Genomic DNA was digested with Hpa II and self-ligated. Integration junction sequences were amplified by two rounds of PCR. The primers for first-round PCR were: F1 (5′-TGGCGGCCTCGAGTGGCTGTTA-3′) and R1 (5′-CTCTA CAAATGTGGTATGGCTG-3′); those for second-round PCR were: F2 (5′-GTGAAGGGCGTCGTAGGTGTC-3′) and R2 (5′-GTATGGCTGATTATGATCCTCTA-3′). Second-round PCR products were cloned into a pMD20-T vector (Takara Bio), which was then transformed into DH5α cells. The genomic integration sites of retrovirus vectors were determined in our previous study.32 Sequences of resulting clones were subjected to a human BLAST search at http://blast.ncbi.nlm.nih.gov/Blast.cgi and analyzed as described previously.32
Enzyme-linked immunosorbent assay
Supernatants from duplicate wells of the target 3T3/CD19 cell and effector CD19-CAR T-cell co-culture (105 cells each) were harvested after 48-h incubation. Supernatant human IFN-γ levels were measured using a human IFN-γ enzyme-linked immunosorbent assay kit (Biolegend).
Cytotoxicity assay
Cell lytic activity was examined using calcein acetoxymethyl ester (Calcein-AM) (Dojindo, Kumamoto, Japan) cytotoxicity assays.33 Calcein-AM-labeled Raji cells and K562 target cells (104 each) were co-cultured with increasing numbers of effector CD19-CAR T-cells. After 4-h incubation, supernatants were harvested and transferred into 96-well plates. Sample fluorescence was read by Fluoroskan Ascent FL (Thermo Fisher Scientific, Waltham, MA, USA). The percentage of lysed cells was calculated with the same formula used for 51Cr release assays.34
Mouse tumor model
Balb/c Rag2−/−γc−/− (10–12-weeks old) immunodeficient mice35–37 were intravenously injected with 5 × 104 Raji/Luc cells on day 0. Mice were intravenously injected with 107 CD19-CAR T-cells alone on day 3 or T-cells in combination with either intraperitoneal injection of an anti-PD-1 antibody (Clone J116; Bio-XCell, West Lebanon, NH, USA)38 at 250 μg per mouse (on days 3, 10 and 17) or intramuscular injection of AAV-IL-7 (1011 vg per body) on day 3.19 Bioluminescence imaging was performed and analyzed using an IVIS imaging system with the Living Image software (PerkinElmer, Waltham, MA, USA) as described previously.15 All mouse experiments were carried out humanely following approval from the Institutional Animal Experiment Committee of Jichi Medical University.
Statistical analysis
The Student’s t-test was used to evaluate differences in experiments. GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) was used to analyze statistical calculations. A value of P<0.05 was considered statistically significant.
Nucleotide sequence accession numbers
Reported nucleotide sequence data are available from the DDBJ/EMBL/GenBank database under accession nos. AB917147–AB917152 and LC002285–LC002289.
Acknowledgments
We thank Miyoko Mitsu and Satomi Fujiwara for the AAV preparations. This work was supported in part by a Grant-in-Aid for Scientific Research (Nos. 22700923 and 24390247), a JMU Graduate Student Start-Up Grant for Young Investigators to N Iwase and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2017. We thank Dr M Ito (Central Institute for Experimental Animals) for providing Balb/c Rag2−/−γc−/− mice. We also thank JMU Core Center of Research Apparatus for assistance with flow cytometry. This publication was subsidized by JKA through its promotion funds from KEIRIN RACE.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Davila ML, Brentjens R, Wang X, Riviere I, Sadelain M. How do CARs work?: Early insights from recent clinical studies targeting CD19. Oncoimmunology. 2012;1:1577–1583. doi: 10.4161/onci.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami K. Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 2007;8(Suppl 1):S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagita K, Yamanaka I, Emoto N, Kawakami K, Shimada S. Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy. BMC Biotechnol. 2010;10:3. doi: 10.1186/1472-6750-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami K, Noda T. Transposition of the Tol2 element, an Ac-like element from the Japanese medaka fish Oryzias latipes, in mouse embryonic stem cells. Genetics. 2004;166:895–899. doi: 10.1534/genetics.166.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suster ML, Sumiyama K, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genomics. 2009;10:477. doi: 10.1186/1471-2164-10-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rostovskaya M, Fu J, Obst M, Baer I, Weidlich S, Wang H, et al. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 2012;40:e150. doi: 10.1093/nar/gks643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabundzija I, Irgang M, Mates L, Belay E, Matrai J, Gogol-Doring A, et al. Comparative analysis of transposable element vector systems in human cells. Mol Ther. 2010;18:1200–1209. doi: 10.1038/mt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manuri PV, Wilson MH, Maiti SN, Mi T, Singh H, Olivares S, et al. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum Gene Ther. 2010;21:427–437. doi: 10.1089/hum.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebriaei P, Huls H, Jena B, Munsell M, Jackson R, Lee DA, et al. Infusing CD19-directed T cells to augment disease control in patients undergoing autologous hematopoietic stem-cell transplantation for advanced B-lymphoid malignancies. Hum Gene Ther. 2012;23:444–450. doi: 10.1089/hum.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito S, Nakazawa Y, Sueki A, Matsuda K, Tanaka M, Yanagisawa R, et al. Anti-leukemic potency of piggyBac-mediated CD19-specific T cells against refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Cytotherapy. 2014;16:1257–1269. doi: 10.1016/j.jcyt.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukahara T, Ohmine K, Yamamoto C, Uchibori R, Ido H, Teruya T, et al. CD19 target-engineered T-cells accumulate at tumor lesions in human B-cell lymphoma xenograft mouse models. Biochem Biophys Res Commun. 2013;438:84–89. doi: 10.1016/j.bbrc.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 17.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 18.Lundstrom W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24:218–224. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin KQ, Urabe M, Yang J, Nomiyama K, Mizukami H, Hamajima K, et al. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum Gene Ther. 2001;12:1047–1061. doi: 10.1089/104303401750214276. [DOI] [PubMed] [Google Scholar]
- 20.Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36:112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng PD, Cohen CJ, Yang S, Hsu C, Jones S, Zhao Y, et al. Efficient nonviral Sleeping Beauty transposon-based TCR gene transfer to peripheral blood lymphocytes confers antigen-specific antitumor reactivity. Gene Therapy. 2009;16:1042–1049. doi: 10.1038/gt.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z, Maiti S, Huls H, Singh H, Olivares S, Mates L, et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Therapy. 2011;18:849–856. doi: 10.1038/gt.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 24.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Guo H, Tammana S, Jung YC, Mellgren E, Bassi P, et al. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Mol Ther. 2010;18:1803–1813. doi: 10.1038/mt.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kogure K, Urabe M, Mizukami H, Kume A, Sato Y, Monahan J, et al. Targeted integration of foreign DNA into a defined locus on chromosome 19 in K562 cells using AAV-derived components. Int J Hematol. 2001;73:469–475. doi: 10.1007/BF02994009. [DOI] [PubMed] [Google Scholar]
- 27.Sadelain M, Papapetrou EP, Bushman FD. Safe harbours for the integration of new DNA in the human genome. Nat Rev Cancer. 2012;12:51–58. doi: 10.1038/nrc3179. [DOI] [PubMed] [Google Scholar]
- 28.Ammar I, Gogol-Doring A, Miskey C, Chen W, Cathomen T, Izsvak Z, et al. Retargeting transposon insertions by the adeno-associated virus Rep protein. Nucleic Acids Res. 2012;40:6693–6712. doi: 10.1093/nar/gks317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(Pt 1):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 30.Yagi H, Ogura T, Mizukami H, Urabe M, Hamada H, Yoshikawa H, et al. Complete restoration of phenylalanine oxidation in phenylketonuria mouse by a self-complementary adeno-associated virus vector. J Gene Med. 2011;13:114–122. doi: 10.1002/jgm.1543. [DOI] [PubMed] [Google Scholar]
- 31.Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol. 2000;18:405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- 32.Tsukahara T, Agawa H, Matsumoto S, Matsuda M, Ueno S, Yamashita Y, et al. Murine leukemia virus vector integration favors promoter regions and regional hot spots in a human T-cell line. Biochem Biophys Res Commun. 2006;345:1099–1107. doi: 10.1016/j.bbrc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannagi M, Sugamura K, Sato H, Okochi K, Uchino H, Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983;130:2942–2946. [PubMed] [Google Scholar]
- 35.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 36.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, et al. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Yi T, Qin L, Maldonado RA, von Andrian UH, Kulkarni S, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123:1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]