Abstract
Background
Methods to detect early cognitive decline and account for heterogeneity of deficits in Parkinson's disease (PD) are needed. Quantitative methods such as latent class analysis (LCA) offer an objective approach to delineate discrete phenotypes of impairment.
Objective
To identify discrete neurocognitive phenotypes in PD patients without dementia.
Methods
LCA was applied to a battery of 8 neuropsychological measures to identify cognitive subtypes in a cohort of 199 non-demented PD patients. Two measures were analyzed from each of four neurocognitive domains: executive functioning, memory, visuospatial abilities, and language. Additional analyses examined between-groups differences in demographic and clinical characteristics (Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory [ADCS-ADL]; UPDRS-III; PD subtype (i.e., tremor-dominant (TD) versus postural instability/gait disturbance-dominant(PIGD)); and cognitive diagnosis (i.e., intact cognition versus mild cognitive impairment; MCI).
Results
LCA identified 3 distinct groups of PD patients: an intact cognition group (n=109; 54.8%), an amnestic group (n=64[32.1%]; impaired recall and recognition on verbal memory tasks, but intact performance on other measures) and a mixed impairment group with dysexecutive, visuospatial and lexical retrieval deficits (n=26 [13.1%]; relative deficits on measures of verbal fluency, visuospatial abilities, and delayed free recall on a memory task, but intact recognition memory). The amnestic and mixed impairment groups had significantly lower ratings of IADL functioning and greater motor symptoms than the cognitively intact group. Additionally, patients with PIGD vs. TD PD subtype were more likely to be classified in either cognitively impaired group. Of those diagnosed as cognitively normal according to MDS criteria (n=151), LCA classified 35 patients as amnestic (23.2%), and 15 as mixed impairment (9.9%).
Conclusions
Non-demented PD patients exhibit distinct neuropsychological profiles. One-third of patients with LCA-determined impairment were diagnosed as cognitively intact by expert consensus, indicating that classification using a statistical algorithm may assist in detection of early and subtle cognitive decline. This study also demonstrates that memory impairment is common in non-demented PD even when cognitive impairment is not clinically apparent. This study has implications for earlier detection of cognitive difficulties in PD, predicting eventual emergence of significant cognitive decline, and treatment trials for cognitive dysfunction in PD.
Keywords: Parkinson's disease, cognition, latent class analysis, neuropsychology
Introduction
Cognitive impairment is common even in early Parkinson's disease (PD),[1] impacts daily function,[2] increases risk of developing PD dementia (PDD), increases mortality,[3] and contributes to caregiver burden.[4] Up to one-third of PD patients exhibit cognitive deficits in the early stages of the disease[5], and deficits in varied cognitive domains have been demonstrated even in drug-naïve PD patients.[6] A recent longitudinal cohort study[7] found that roughly half of a sample of 141 patients with established PD and normal cognition at baseline assessment developed cognitive impairment within 6 years, and incident cases of PD mild cognitive impairment (PD-MCI) universally converted to dementia within 5 years. Early detection of cognitive deficits in PD is necessary to optimize intervention, and due to heterogeneity of clinical presentations and underlying neural substrates, novel methods to aid early detection are needed.
Although cognitive dysfunction in non-demented PD is commonly viewed as a primarily dysexecutive syndrome, there is significant heterogeneity, with impairments in memory, visuospatial processes, and language also frequently observed.[8]–[14] Deficits in these domains have been demonstrated in PD patients deemed “cognitively normal” from a clinical diagnostic perspective as well as those who meet criteria for PD-MCI. Heterogeneity in cognitive presentations is also reflected in biomarker[15]–[21] and neuropathological investigations[22], [23] in nondemented PD, which have revealed associations between cognitive impairments and diverse clinical and neuropathological biomarkers. Notably, these findings suggest comorbid disease processes, including both Lewy body and Alzheimer's disease pathology, in subgroups of PD patients.
The heterogeneity seen in cognitive, biomarker, and neuropathological investigations suggests the presence of shared or divergent neurodegenerative processes. Extant literature suggests that PD patients with primarily executive functioning deficits may be more likely to remain stable over time, while those with isolated or accompanying memory, language, or visuospatial deficits, more indicative of posterior dysfunction, may be at greater risk for future cognitive decline and dementia.[24], [25] Further understanding of cognitive heterogeneity early in the disease process has important implications regarding our ability to predict progression and better inform potential therapeutic targets. Utilizing statistical algorithms to examine neuropsychological test performance in non-demented PD patients has the potential to identify individuals at risk for decline before overt impairments are clinically apparent.
Cluster analysis and related statistical approaches have been utilized to examine heterogeneity regarding numerous clinical variables in PD, including cognition.[26]–[29] In addition to cluster analysis structural equation modeling methods, such as latent class analysis (LCA), have proven useful in detection of underlying homogenous groups. Like cluster analysis, LCA aims to classify individuals based on shared characteristics and to identify qualitatively different subgroups. The aim of the present study was to use LCA to further understanding of neuropsychological heterogeneity in non-demented PD. This is of particular importance for clinical trials, as drug development for cognitive decline has begun to emphasize intervention in preclinical stages.[30] Further, group differences were examined among LCA-derived classes to further characterize and validate the cognitive subgroups. Finally, LCA class differences in clinical cognitive diagnosis by expert consensus (i.e., intact versus PD-MCI) were assessed.
Methods
Participants
PD patients aged 50 or older were recruited from the University of Pennsylvania Udall Center of Excellence in Parkinson's Disease Research. PD patients with a consensus diagnosis of dementia were excluded from these analyses. The University of Pennsylvania Institutional Review Board approved the study, and informed consent was obtained from all participants.
Neuropsychological Assessment
All PD participants were administered a comprehensive neuropsychological battery by trained research staff. Global cognition was assessed with the Dementia Rating Scale-2 (DRS-2)[31] and the Montreal Cognitive Assessment (MoCA).[32] Eight core parameters from the neuropsychological battery were analyzed to derive statistically-determined cognitive subgroups. LCA was performed using 2 representative measures from each of 4 cognitive domains: 1) executive functioning (Letter-Number Sequencing[33], phonemic verbal fluency); 2) language (Boston Naming Test[34], semantic verbal fluency); and 3) verbal memory (Hopkins Verbal Learning Test-Revised (HVLT-R)[35] delayed free recall and recognition discriminability scores); 4) visuospatial (Judgement of Line Orientation[36], Clock Drawing command condition[37]). All assessments were performed in the PD medication “on” state.
Motor and Psychiatric Assessments
The Unified Parkinson's Disease Rating Scale (UPDRS) Part III[38] and Hoehn and Yahr staging[39] were performed by trained research staff to assess motor impairment and disease severity. Motor assessments were performed while participants were taking their regular regimen of PD medications. Subtype of motor impairment was also coded, i.e., tremor dominant (TD) versus postural-instability gait disturbance dominant (PIGD) for a subset of patients (91%). Subtype was determined based on Jankovic et al., and 12 subjects had an indeterminate subtype according to these criteria.[40] Levodopa equivalent daily dose (LEDD) was also recorded for each participant. Depression was assessed using the Geriatric Depression Scale-15 (GDS-15).[41]
Activities of Daily Living
A subset of patients (75%) had data regarding everyday functioning assessing basic and instrumental activities of daily living (IADLS) with the Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL).[42] A knowledgeable informant (defined as a spouse, child, close relative, or friend seeing the patient a minimum of once per week) completed the ADCS-ADL for PD participants. The ADCS-ADL contains 23 items, with items 1-6 assessing basic ADLs and 7-17 assessing IADLs.
Cognitive Diagnosis
Assessment of cognitive status involved a consensus process performed by neurologists and psychiatrists with expertise in movement disorders and PD cognition at the University of Pennsylvania Udall Center, as previously described.[7] Multiple pairs of raters examined demographic and clinical data to reach a consensus cognitive diagnosis for each PD participant, based on Movement Disorders Society (MDS) level 1 criteria[43] (i.e., intact cognition versus MCI), using published demographically-corrected normative data for each neuropsychological assessment. For each neuropsychological test, standardized scores greater than or equal to 1.5 SD below the mean was deemed impaired, although discretion among raters was allowed.
Statistical Analyses
LCA
LCA was conducted in Mplus Version 7.1[44] using demographically-corrected normative scores obtained from the eight core neuropsychological variables. First, a one-class model was fit to the data. The number of classes was then increased one at a time until there was no additional model improvement.[45] The best-fitting model was selected based on a preponderance of evidence both quantitative (e.g., model fit statistics, class sizes) and qualitative (e.g., parsimoniousness, theoretical and clinical interpretability).[46]
Quantitative indicators of model fit included the following: Goodness-of-fit statistics included the Akaike Information Criterion (AIC)[47] and Bayesian Information Criterion (BIC)[48]; smaller values indicate better fit. The Vuong-Lo-Mendel-Rubin (VLMR)[49] likelihood ratio test was used to compare the model with k classes to the model with k-1 classes; a significant test indicates that the model with k classes better fits the data than the model with k-1 classes. Posterior probabilities (i.e., estimates of class probabilities for each individual) and entropy, both indices of classification quality [50], were also used to identify the best-fitting model; higher entropy values indicate better fit, with entropy values ≥.8 indicating adequate fit. Finally, class size was used as an indicator of model fit, as classes comprised of <10% of the total sample suggest possible over-fitting.
Class comparisons
Patients were assigned to groups based on their LCA-derived highest posterior probabilities. Differences among LCA-derived groups in demographics, global cognitive status, and clinical variables were assessed using ANOVA for continuous variables (age, years of education, Hoehn & Yahr, UPDRS-III, LEDD, GDS-15, DRS-2, and ADCS-ADL IADL Subscale) and Pearson's χ2 test for categorical variables (sex and PD subtype, i.e., TD versus PIGD). When omnibus findings were significant, follow-up tests were performed using Tukey's t-tests for continuous variables or partitioned χ2 test for categorical variables. Finally, LCA-derived classifications were compared with consensus cognitive diagnoses (i.e., intact cognition or MCI) based on Movement Disorders Society (MDS) criteria[43] using cross-tabulation, Pearson's χ2 test, and Cohen's kappa.
Results
Cohort Characteristics
Demographics and clinical characteristics of the full sample (n=199) are presented in Table 1.
Table 1. Full Sample Demographic and Clinical Characteristics (n=199).
| Age | 70.57 (7.47) |
| Education | 16.24 (2.34) |
| Sex (% male) | 66.8% |
| Disease Duration | 6.85 (5.21) |
| Hoehn & Yahr | 2.36 (0.65) |
| UPDRS-III | 22.95 (11.55) |
| PD subtype (% PIGD) | 56.9% |
| LEDD | 758.31 (481.25) |
| GDS-15 | 2.74 (2.78) |
| DRS-2 | 136.76 (5.57) |
| ADCS-ADL IADL Subscale | 50.71 (6.83) |
Results are presented as M (SD) except where noted (i.e., sex and PD subtype). UPDRS-III = Unified Parkinson's Disease Rating Scale Part III; PIGD = postural instability/gait disturbance-dominant; LEDD = levodopa-equivalent daily dose; GDS-15 = Geriatric Depression Scale-15; DRS-2 = Dementia Rating Scale-2; ADCS-ADL = Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory; IADL = Instrumental activities of daily living.
The sample was 66.8% male. Participants average age was 70.57 years old (SD=7.47) with a mean of 16.24 (SD=2.34 years of education, average disease duration of 6.85 years (SD=5.21), and average DRS-2 total score of 136.76 (SD=5.57).
Latent Class Analysis
The results of the LCA are found in Table 2. Several statistical fit indices supported the three-class model. The three-class model yielded the optimal values of entropy and BIC. AIC improved substantially through the three-class model but only minimally beyond the three-class model. The VLMR test indicated that the two-class model conferred a significant improvement in model fit over the one-class solution (p < .01), whereas the improvement in model fit conferred by the three-class model did not reach statistical significance (p = .13). Nonetheless, inspection of the two-class model suggested that one of the two classes exhibited a heterogeneous neuropsychological profile that was better differentiated by the three-class model. In addition, the smallest class yielded by the three-class model comprised 13% of the sample, which is within acceptable limits (i.e., ≥10% of the entire sample). Thus, we interpreted the three-class model as best.
Table 2. Fit Indices for Latent Class Analysis Models with 1 - 4 Classes.
| Number of classes | Number of free parameters | AIC | BIC | Log likelihood | VLMR p | Entropy | Smallest class size (% of sample) |
|---|---|---|---|---|---|---|---|
| 1 | 16 | 4944.642 | 4997.335 | -2456.321 | N/A | N/A | 100% |
| 2 | 25 | 4724.800 | 4807.133 | -2337.400 | .0006 | 0.82 | 45% |
| 3 | 34 | 4648.771 | 4760.744 | -2290.386 | .1255 | 0.88 | 13% |
| 4 | 43 | 4632.110 | 4773.722 | -2273.055 | .2048 | 0.81 | 11% |
AIC = Akaike information criterion; BIC = Bayesian information criterion
Demographics and clinical characteristics for each of the three classes as determined by LCA are presented in Supplementary Table 1. Means and standard deviations of the eight core neuropsychological parameters for the three classes as determined by LCA are presented in Figure 1. These data provide evidence for an amnestic class (n = 64; 32%) with impairments on both HVLT-R free recall and recognition memory, but performance within the normal range across other measures; a mixed impairment class with lexical retrieval and visuospatial deficits (n = 26; 13%), reflected by impaired verbal fluency performance (semantic fluency more impaired than phonemic fluency), diminished HVLT-R recall but intact HVLT recognition, and visuoconstruction difficulties on the Clock Drawing task; and a cognitively intact class (n = 109; 55%) with no impairments across all eight core neuropsychological measures.
Figure 1. Neuropsychological Test Performance of LCA-Derived Classes.
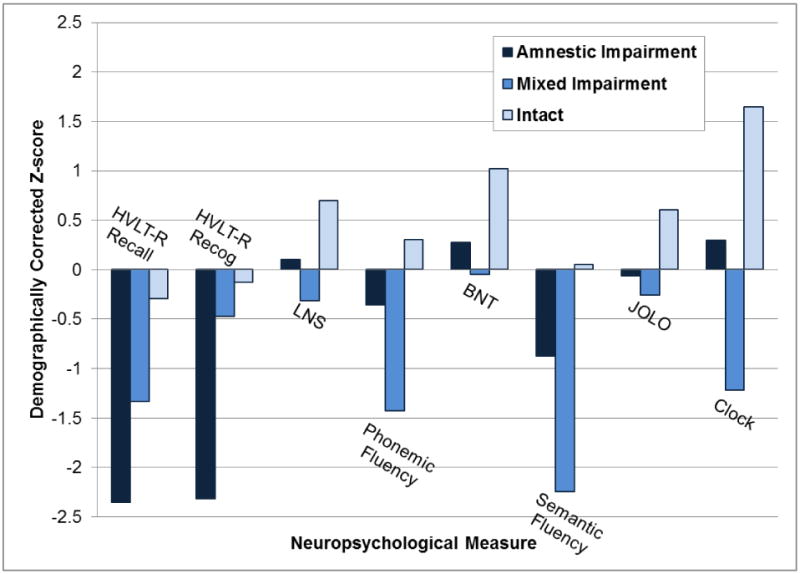
Memory: HVLT-R Recall = Hopkins Verbal Learning Test-Revised Delayed Recall, HVLT-R Recog = HVLT-R Recognition Discriminability
Executive Functioning: LNS = Letter-Number Sequencing, Phonemic Fluency = ‘FAS’ Phonemic Verbal Fluency
Language: BNT = Boston Naming Test, Semantic Fluency = Animal Verbal Fluency
Visuospatial: JOLO = Judgement of Line Orientation; Clock = Clock drawing to command
Between-Group Differences on Demographic and Clinical Variables
There were no group differences in age, education, or depression severity. There was a significant relationship between group membership and sex, χ2(2) = 6.30, p < 0.05. The overall sample was 67% male and 33% female. The greatest proportion of males was observed in the amnestic group (78%), followed by the mixed impairment (69%) and intact groups (60%). Follow-up tests revealed a significant difference in sex between the amnestic and intact groups (p = 0.01), whereas differences between the mixed impairment group and each of the other groups did not reach statistical significance.
Disease duration was significantly different among the groups, F(2,196) = 3.63, p =0.02, with the mixed group having significantly longer disease duration than the intact group (p = 0.02). Disease duration did not significantly differ between the amnestic and intact or amnestic and mixed groups. Group differences in disease severity as measured by the Hoehn & Yahr were significant F(2,192) = 7.47, p = 0.001. The amnestic group (p < 0.01) and mixed impairment group (p = 0.03) exhibited greater disease severity than the intact group. Significant group differences were also revealed with regard to UPDRS-III motor symptom severity, F(2,193) = 4.25, p = 0.02. The amnestic group exhibited significantly greater motor symptoms than the intact group (p = 0.01) and there was a trend for the mixed impairment group exhibiting greater motor symptoms (p =0.06). There were no differences between the amnestic and mixed impairment groups regarding Hoehn and Yahr or UPDRS-III scores. There were no group differences in LEDD.
Subtype of PD (i.e., TD versus PIGD) was significantly associated with group membership, χ2(2) = 7.81, p = 0.02, with the greatest proportion of PIGD observed in the mixed impairment group (88%), followed by the amnestic (66%) and intact groups (57%). Follow-up tests revealed a significant difference in subtype between the mixed impairment and intact groups (p < 0.01), and there was a trend regarding differences between the mixed impairment and amnestic groups (p = 0.06).
There were significant group differences regarding global cognition as assessed by the DRS-2 (F(2,196) = 21.43, p< 0.001). The amnestic and mixed impairment groups had lower total DRS-2 scores (p < 0.001) than the intact group. There were also significant group differences regarding the IADL subscale score of the ADCS-ADL (F(2,144) = 6.40, p = 0.002), in that the amnestic (p = 0.03) and mixed impairment groups (p = 0.03) exhibited significantly more IADL impairment than the intact group, consistent with previous research.[51]
Classification Using Latent Class Analysis Versus Clinical Diagnosis
Table 3 compares clinical diagnoses (normal/intact cognition and PD-MCI using MDS consensus criteria) versus LCA-derived statistical classifications. Consensus clinical diagnosis of PD patients with normal cognition and PD-MCI was significantly associated with group membership, χ2(2) = 37.17, p < 0.001. When the two LCA-identified impaired groups were combined into a single LCA-impaired class for the purposes of assessing diagnostic agreement, Cohen's kappa indicated fair agreement between LCA and clinical diagnosis (κ = 0.39, SE = 0.06, 95% CI = 0.27 - 0.51). Agreement was present in 71% of the sample, with 101 patients classified as intact by both methods and 40 classified as impaired by both methods. A small number of patients (n = 8, 4% of the sample) were diagnosed with PD-MCI but were classified as intact by LCA.
Table 3. Cross-Tabulation of Latent Class Analysis versus Clinical Consensus Diagnostic Classification.
| LCA classification | ||||
|---|---|---|---|---|
| Intact (n=109) | Mixed impairment (n=26) | Amnestic (n=64) | ||
| Consensus diagnostic classification | PD Patients with Normal Cognition (n=151) | 101 | 15 | 35 |
| PD-MCI (n=48) | 8 | 11 | 29 | |
LCA = latent class analysis; PD = Parkinson's disease; MCI = mild cognitive impairment.
Diagnostic disagreement was driven largely by patients who were classified as normal by consensus diagnosis but impaired by LCA. Of those PD patients diagnosed as “normal” according to consensus criteria (n = 151), 23% (n = 35) were classified as amnestic, and 10% (n = 15) were classified as mixed impairment in the present LCA. This indicates that one-third of the PD patients classified as normal/intact by clinical consensus criteria in this sample were statistically identified as having a distinct neuropsychological phenotype indicative of cognitive dysfunction using LCA.
Discussion
The present study reveals that person-centered statistical techniques such as LCA can identify distinct neuropsychological phenotypes even in clinical prediagnostic stages of cognitive decline in PD. One-third of patients in the two impaired LCA-derived groups were diagnosed as cognitively intact by expert consensus, indicating that classification using a statistical algorithm may assist in detection of early, subtle changes which may not lead to consensus diagnosis of PD-MCI. The two LCA-derived impairment groups had more severe motor symptoms and disease severity, lower ratings of IADL function, and were more likely to have PIGD- than TD-subtype PD.
Importantly, this study indicates that memory impairment is common even early in the course of PD when cognitive impairment is not clinically apparent to the treating physician. A larger proportion of the sample fell into the amnestic group (32%) than the mixed impairment group (13%). Research has suggested that executive-based deficits occur earlier in the disease course due to fronto-striatal dysfunction characteristic of PD, and memory deficits do not emerge until later in the disease course.[27] However, accumulating evidence suggests a subgroup of PD patients experience more posterior cortical dysfunction, which increases risk of dementia. In one longitudinal study examining predictors of dementia in PD, patients with deficits in measures of posterior cortical function were more predictive of dementia than measures of fronto-striatal function.[52] The presence of a primary amnestic group in the present study is in line with studies demonstrating Alzheimer's disease patterns of brain atrophy on structural imaging[19] and reduced cerebrospinal fluid levels of amyloid β 1-42 in nondemented PD patients, both of which have been found to be predictors of cognitive decline at longitudinal follow-up.[18], [21]
The present findings support the presence of heterogeneity, which has been revealed in biomarker, neuropathological, and genetic investigations of cognition in PD. Imaging studies have revealed atrophy in frontostriatal regions and cholinergic structures such as the insular cortex and caudate nucleus[15], prefrontal cortical and hippocampal atrophy and caudate dopaminergic hypofunction[16], faster rates of cortical thinning[17] and greater amyloid burden[18] in PD patients with cognitive impairment. A structural imaging study found that an Alzheimer's disease pattern of brain atrophy (i.e., hippocampal and medial temporal lobe atrophy) predicted cognitive decline over a two-year period in non-demented PD patients.[19] Neuropathological studies of individuals with PD-MCI[22], [23] have revealed the presence of neocortical or limbic Lewy bodies, Alzheimer's disease pathology, and possible cerebrovascular disease. Studies examining genetic influences on cognition in PD have indicated that risk factors associated with the etiology of Alzheimer's disease confer additional risk for cognitive impairment and decline in a subset of PD patients, indicating more rapid decline and greater cognitive impairment in PD patients with the APOE ε4 allele.[53], [54] Studies examining other genes also reflect cognitive heterogeneity; the COMT genotype has been associated with performance on measures of frontostriatal and frontoparietal functioning[53], [55], GBA mutations linked to impaired executive and visuospatial functions[56], and MAPT H1/H1 genotype associated with impaired performance on measures of temporal lobe functioning[52], [53] and parietal functions [57], [58].
Importantly, LCA offers a number of advantages over cluster analysis, as it takes into account the uncertainty in allocating cases to groups, considers class size when assigning membership, and provides fit statistics for comparison of competing models.[59] Several studies have utilized LCA and a related approach, latent profile analysis (LPA), to examine subgroups of PD patients according to motor, psychiatric, or cognitive presentations.[60]–[65] Although two studies[64], [65] included examination of neuropsychological test performance, this was not the primary outcome variable of interest. This current study is the first to utilize LCA to examine cognition in PD as the primary outcome. In other studies examining cognition in patients without PD, (i.e., cognitively normal adults and dementia) person-centered statistical techniques such as LCA have also proven useful in detection of underlying homogenous groups.[66]–[68] The current study extends this methodology to non-demented PD patients.
Limitations of the current study include our inability to examine simple attention in addition to the four domains examined, a homogenous sample (primarily white and highly educated), and the absence of longitudinal follow-up data. Due to homogeneity of the sample, the present results need to be replicated in different PD cohorts. Follow-up studies on this cohort will examine the utility of LCA-derived groups in regards to risk and rate of progression to dementia, as well as associations with biomarkers. It is important to note that the mixed impairment group had significantly longer disease duration than the intact group, and both impairment groups had greater ratings of motor impairment than the intact group, raising the possibility that the presence of a group with intact cognition may reflect differences in disease severity. Additionally, all measures were performed in the levodopa “on” state and results may differ if tested in the “off state” or in drug naïve patients. The use of these techniques requires validation in other PD cohorts as well as longitudinal studies to determine if they are useful in predicting future cognitive decline. LCA and related techniques examining longitudinal data can be implemented in other existing large cohorts of PD patients and in multi-center studies utilizing comprehensive neuropsychological test batteries. Validation in other PD cohorts using different neuropsychological assessments will also be informative, as the type of assessments used may impact results of the LCA. Finally, as we do not know the concordance between self-report of cognitive decline and cognitive assessment results, future research examining this relationship is warranted.
The use of a statistical algorithm offers a more objective approach to cognitive classification, extending our understanding of the cognitive profile beyond mean differences in performance to a more nuanced conceptualization of multiple, qualitatively distinct profiles of cognitive impairment. The presence of multiple cognitive phenotypes in early PD emphasizes the necessity of screening for impairment in a variety of cognitive domains (e.g., memory, language) in addition to those included in the conventional profile of PD-associated cognitive deficits (e.g., executive function). In addition, whereas conventional diagnostic strategies require prior assumptions about the degree of impairment, resulting in the use of potentially arbitrary cutoff scores, statistical approaches do not require such assumptions and instead may detect subtle weaknesses within an individual's neuropsychological profile. Importantly, early identification of patients at risk for cognitive decline improves ability to predict progression, better inform pharmacotherapy, and identify potential therapeutic targets for clinical trials based on these unique phenotypes of neuropsychological functioning. Accounting for heterogeneity may lead to a more personalized medicine approach to treatment of cognitive deficits in PD.
Supplementary Material
Supplementary Table 1: Demographic and Clinical Characteristics of Groups Determined with Latent Class Analysis
Acknowledgments
Study Funding: This study was funded by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488).
Kathryn M. Devlin receives research support from the National Science Foundation (DGE-1144462).
Sharon X. Xie is supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488) and by NIH grant # AG10124.
Dawn Mechanic-Hamilton receives salary support from the National Institutes of Health and The Imagination Institute and has received consulting fees from Neuronix.
Howard H. Hurtig receives salary support from NINDS P50 NS053488 and royalties from UpToDate.
Alice Chen-Plotkin is supported by the NIH-NINDS (P50 NS053488 and UO1 NS082134), the Burroughs Wellcome Fund, the Benaroya Fund, and the Pechenik Montague Award Fund.
Lama M. Chahine receives support from the NIH (P50 NS053488), Michael J Fox Foundation, and royalties from Wolters Kluwel (for book authorship).
James F. Morley receives research support from the Department of Veterans Affairs and GE Healthcare.
John E. Duda receives research support from the Department of Veterans Affairs, the National Institutes of Health, and the Michael J. Fox Foundation for Parkinson's Research.
David R. Roalf is supported by NIMH K01 MH102609.
Nabila Dahodwala receives research support from the NIH, National Parkinson Foundation, Parkinson Council, Michael J Fox Foundation, Biotie and Abbvie.
Jacqueline Rick is supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488).
John Q. Trojanowski serves as an Associate Editor of Alzheimer's & Dementia. He may accrue revenue on patents submitted by the University of Pennsylvania wherein he is inventor including and he is co-inventor on patents submitted the University of Pennsylvania wherein he is inventor that have generated income he has received from the sale of Avid to Eli Lily. Finally, he receives research support from the NIH (AG 10124, AG 17586, AG-19724AG 024904, NS053488, AG029213), Janssen, GSK, Cure PSP and the Michael J. Fox Foundation.
Paul Moberg receives support from NIH grants R21MH108895 and R01MH099156 as well as SAMHSA CFDA#93.958-CMHSBG.
Daniel Weintraub has received research support from Boehringer Ingelheim, National Institutes of Health, and Penn Center for Excellence in Research on Neurodegenerative Diseases (CERND). He has received consulting fees or honoraria from: Acadia Pharmaceuticals, Boehringer Ingelheim, General Electric, Merck Serono, Novartis Pharmaceuticals, Pfizer, Sanofi Aventis, Johnson and Johnson, and Solvay. supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488) and by NIH grant # NS065087, and by the Michael J. Fox Foundation.
Footnotes
Disclosures/Conflict of Interest: Laura Brennan has no disclosures.
Baochan Tran has no disclosures.
References
- 1.Williams-Gray C, Foltynie T, Brayne CEG, Robbins W, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 2.Cahn D, Sullivan E, Shear P, Pfefferbaum A, Heit G, Silverberg G. Differential Contributions of Cognitive and Motor Component Processes to Physical and Instrumental Activities of Daily Living in Parkinson's Disease. Arch Clin Neuropsychol. 1998 Oct;13(7):575–583. [PubMed] [Google Scholar]
- 3.Buter TC, van der Hour A, Matthews F, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease A 12-year population study. Neurology. 2008;70:1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Larsen JP, Karlsen K, Lim G, Tandberg E. Mental symptoms in Parkinson's disease and important contributors to caregiver distress. Int J Geriatr Psyciatry. 1999;14:866–874. [PubMed] [Google Scholar]
- 5.Foltynie T, Brayne CEG, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated parkinson disease: The Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 7.Pigott K, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85:1276–1282. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson G, Leverenz JB. Profile of Cognitive Impairment in Parkinson Disease. Brain Pathol. 2010;20(3):640–645. doi: 10.1111/j.1750-3639.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland D, Bronnick K, Williams-Gray C. Mild cognitive impairment in Parkinson disease A multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of Mild Cognitive Impairment in Parkinson's Disease: Progression to Dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 11.Muslimović D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson's disease. Brain. 2007;130(11):2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- 12.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 13.Yarnall AJ, et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology. 2014 Jan;82(4):308–16. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cholerton Ba, et al. Evaluation of mild cognitive impairment subtypes in Parkinson's disease. Mov Disord. 2014 May;29(6):756–64. doi: 10.1002/mds.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JE, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2014 Jan;85(1):7–16. doi: 10.1136/jnnp-2013-305062. [DOI] [PubMed] [Google Scholar]
- 16.Jokinen P, Bru A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson's disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Park Relat Disord. 2009;15:88–93. doi: 10.1016/j.parkreldis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Hanganu A, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain. 2014 Apr;137(Pt 4):1120–9. doi: 10.1093/brain/awu036. [DOI] [PubMed] [Google Scholar]
- 18.Gomperts SN, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub D, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain. 2012 Jan;135(Pt 1):170–80. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Compta Y, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24(15):2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 21.Siderowf A, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler CH, et al. Heterogeneous neuropathological findings in Parkinson's disease with mild cognitive impairment. Acta Neuropathol. 2010 Dec;120(6):827–8. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jellinger KA. Neuropathology in Parkinson's disease with mild cognitive impairment. Acta Neuropathol. 2010;120:829–830. doi: 10.1007/s00401-010-0755-1. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Garcia D, et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson's disease. Eur J Nucl Med Mol Imaging. 2012;39(11):1767–1777. doi: 10.1007/s00259-012-2198-5. [DOI] [PubMed] [Google Scholar]
- 25.Williams-Gray CH, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84:1258–64. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 26.van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, Marinus J. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord. 2010 Jun;25(8):969–78. doi: 10.1002/mds.23116. [DOI] [PubMed] [Google Scholar]
- 27.Dujardin K, et al. The spectrum of cognitive disorders in Parkinson's disease: a data-driven approach. Mov Disord. 2013 Feb;28(2):183–9. doi: 10.1002/mds.25311. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17(4):195–200. [PubMed] [Google Scholar]
- 29.Szeto JYY, et al. The relationships between mild cognitive impairment and phenotype in Parkinson's disease. npj Park Dis. 2015;1:15015. doi: 10.1038/npjparkd.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med. 2013;368(13):1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 31.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 32.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–696. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. WAIS-III, Wechsler adult intelligence scale: Administration and scoring manual. Psychological Corporation; 1997. [Google Scholar]
- 34.Kaplan E, Godglass H, Weintraub S. Boston naming test. Pro-ed; 2001. [Google Scholar]
- 35.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 36.Benton Arthur L, Sivan Abigail B, Hamsher Kerry deS, Varney Nils R, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 1994 [Google Scholar]
- 37.Hubbard EJ, et al. Clock drawing performance in cognitively normal elderly. Arch Clin Neuropsychol. 2008;23(3):295–327. doi: 10.1016/j.acn.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahn S, Elton R. Unified Parkinson's disease rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 39.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 40.Jankovic J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(1):1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink T, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 42.Galasko D, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer's Dis Assoc Disord. 1997;11(Suppl. 2):S33–S29. [PubMed] [Google Scholar]
- 43.Litvan I, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement disorder society task force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthén L, Muthén B. Mplus user's guide (version 7.0) 2007 [Google Scholar]
- 45.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct Equ Model A Multidiscip J. 2007 Oct;14(4):535–569. [Google Scholar]
- 46.Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 2005. [Google Scholar]
- 47.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 48.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 49.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 50.Muthén B. Latent variable mixture modeling. In: Marcoulides G, Schumacker R, editors. Advanced structural equation modeling: New developments and techniques. Mahwah, NJ: Erlbaum; 2000. pp. 1–33. [Google Scholar]
- 51.Rosenthal E, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010 Jul;25(9):1170–6. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams-Gray CH, et al. The distinct cognitive syndromes of Parkinson's disease : 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 53.Morley JF, et al. Genetic influences on cognitive decline in Parkinson's disease. Mov Disord. 2012;27(4):512–8. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mata IF, et al. APOE, MAPT, and SNCA genes and cognitive performnace in Parkinson disease. JAMA Neurol. 2014;71(11):1405. doi: 10.1001/jamaneurol.2014.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker Ra. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27(18):4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mata IF, et al. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord. 2016;31(1):95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nombela C, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain. 2014;(i) doi: 10.1093/brain/awu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbins TW, Cools R. Cognitive deficits in Parkinson's disease: a cognitive neuroscience perspective. Mov Disord. 2014 Apr;29(5):597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- 59.Eshghi A, Haughton D, Legrand P, Skaletsky M, Woolford S. Identifying Groups: A Comparison of Methodologies. J Data Sci. 2011;9:271–291. [Google Scholar]
- 60.Paul SS, et al. Two-Year Trajectory of Fall Risk in People With Parkinson Disease: A Latent Class Analysis. Arch Phys Med Rehabil. 2016;97(3):372–379.e1. doi: 10.1016/j.apmr.2015.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zahodne L, Marsiske M, Bowers D. A latent class analysis of psychological disturbance in Parkinson's disease. Int J Geriatr Psychiatry. 2013;28(10):1054–1060. doi: 10.1002/gps.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burn D, et al. Parkinson's disease motor subtypes and mood. Mov Disord. 2011;27(3):379–386. doi: 10.1002/mds.24041. [DOI] [PubMed] [Google Scholar]
- 63.Starkstein SE, et al. Anxiety has specific syndromal profiles in parkinson disease: A data-driven approach. Am J Geriatr Psychiatry. 2014;22(12):1410–1417. doi: 10.1016/j.jagp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Flensborg Damholdt M, Shevlin M, Borghammer P, Larsen L, Ostergaard K. Clinical heterogeneity in Parkinson's disease revisited: a latent profile analysis. Acta Neurol Scand. 2012 May;125(5):311–8. doi: 10.1111/j.1600-0404.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 65.Mavandadi S, et al. Use of latent variable modeling to delineate psychiatric and cognitive profiles in Parkinson's disease. Am J Geriatr Psychiatry. 2010;17(11):986–995. doi: 10.1097/JGP.0b013e3181b215ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Libon DJ, et al. Neuropsychological Syndromes Associated with Alzheimer's/Vascular Dementia: A Latent Class Analysis. J Alzheimers Dis. 2014 Jan;42(3):999–1014. doi: 10.3233/JAD-132147. [DOI] [PubMed] [Google Scholar]
- 67.Köhler S, et al. Progression to dementia in memory clinic patients without dementia: A latent profile analysis. Neurology. 2013;81:1342–1349. doi: 10.1212/WNL.0b013e3182a82536. [DOI] [PubMed] [Google Scholar]
- 68.Hayden KM, et al. Pre-clinical Cognitive Phenotypes for Alzheimer Disease: A Latent Profile Approach. Am J Geriatr Psychiatry. 2013 Sep;:1–11. doi: 10.1016/j.jagp.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Demographic and Clinical Characteristics of Groups Determined with Latent Class Analysis


