Abstract
Background
Most cardiac arrest (CA) patients remain comatose post-resuscitation, prompting goals-of-care (GOC) conversations. The impact of these conversations on patient outcomes has not been well described.
Methods
Patients (n=385) treated for CA in Columbia University ICUs between 2008–2015 were retrospectively categorized into various modes of survival and death based on documented GOC discussions. Patients were deemed “medically unstable” if there was evidence of hemodynamic instability at the time of discussion. Cerebral performance category (CPC) greater than 2 was defined as poor outcome at discharge and one-year post-arrest.
Results
The survival rate was 31%(n=118); most commonly after early recovery without any discussions (57%, n=67), followed by survival due to family wishes despite physicians predicting poor neurological prognosis (20%, n=24), and then survival after physician/family agreement of favorable prognosis (17%, n=20). The survivors due to family wishes had significantly worse outcomes compared to the early recovery group (discharge:p=0.01; one-year:p=0.06) and agreement group (p<0.001; p<0.001), though 2 patients did achieve favorable recovery. Among nonsurvivors (n=267), withdrawal of life-sustaining therapy (WLST) while medically unstable was most common (31%; n=83), followed by death after care was capped (24%, n=65), then WLST while medically stable (17%, n=45). Death despite full support, brain death and WLST due to advanced directives were less common causes.
Conclusions
Most survivors due to family wishes despite poor neurological prognosis die or have poor outcomes at one-year. However, a small number achieve favorable recovery, demonstrating limitations with current prognostication methods. Among nonsurvivors, most WLST occurs while medically unstable, suggesting an overestimation of WLST due to unfavorable neurological prognosis.
Keywords: cardiac arrest, cardiopulmonary resuscitation, prognosis, goals-of-care, withdrawal of life-sustaining therapy
Background
The majority of cardiac arrest survivors remain comatose post-resuscitation,1 prompting goals-of-care (GOC) conversations with medical providers. Based on prognostication given during these conversations, families may choose to continue care, sign do-not-resuscitate (DNR) orders, or may elect for withdrawal of life-sustaining therapy (WLST). Effective GOC conversations have been shown to shorten ICU stays, reduce the time from identification of multi-organ system failure to DNR status and improve family satisfaction in medical ICU patients.1–5. The impact of GOC conversations on patient’s short- and long-term outcomes following cardiac arrest has not been well studied.
GOC conversations are particularly important when they inform decisions regarding WLST, because this accounts for over 50% of deaths following CA.6 Recent studies have demonstrated that WLST due to unfavorable neurological prognosis by a physician (WLST-N) is the most common cause of death.7,8 A thorough assessment of the GOC conversations among all patients, specifically focusing on whether consensus was reached, has not been studied. We sought to address these issues by creating a detailed categorization system for modes of survival and death based on documented GOC conversations.
Methods
Electronic medical records of 385 patients admitted for CA in Columbia University ICUs between January 2008 and March 2015 were retrospectively reviewed. Daily notes from critical care providers, events, family meetings, social worker and ethics consultation notes were utilized to collect information on GOC conversations, physician prognostication and families’ subsequent decision.
Patients were then categorized into: 1) Survival after early recovery without any GOC discussions, 2) Survival due to physician-family agreement of good prognosis, 3) Survival due to family wishes despite the physician predicting poor neurological prognosis, 4) Survival despite WLST, 5) WLST due to advanced directives, 6) WLST after physician-family agreement, 7) Death after care was capped, 8) Death despite full support before any GOC conversation, 9) Death despite full support after GOC conversation, and 10) Brain death, as shown in figure 1.
Figure 1.
Patient Categorization
Two patients were categorized as other because they did not have family or friends and thus had no opportunity for possible family discussions (one required court-appointed guardianship, and the other had care capped based on two-physician agreement with the involvement of the ethics department and patient care services).
WLST was defined as terminal extubation and then further categorized based on medical stability at the time of decision. Patients were deemed “medically unstable” if there was evidence of hemodynamic instability (refractory hypotension, need for vasopressors/inotropes, cardiovascular assist devices such as intra-aortic balloon pumps or veno-arterial ECMO), while the remaining patients were categorized as “medically stable.” “Medically stable” patients had documented poor neurological prognosis in all GOC conversations. Thus, we redefined these patients as WLST due to poor neurological prognosis (WLST-N), consistent with several recent studies.7,8
The category “death after care was capped” included patients whose families either chose no escalation of care (i.e. no further vasopressors/inotropes, antibiotics, mechanical circulatory assistance such as veno-arterial extracorporeal membrane oxygenation (ECMO), etc), or signed DNR and/or do-not-intubate (DNI) orders with subsequent respiratory or circulatory arrest.
Cerebral performance category (CPC) was calculated at hospital discharge based on neurological exam documented in discharge summaries, physical and occupational therapy notes.9 CPC was calculated at one-year post-arrest based on detailed phone interviews with patients or family members. A CPC of 1–2 was considered a good neurological recovery and z-test for proportions was used to compare various groups.
Results
Among the 385 CA patients, 72% required GOC conversations. There were 118 survivors and 267 nonsurvivors. The survivor group was younger, with lower CPC scores at baseline, less incidence of out-of-hospital cardiac arrests, shorter time to ROSC, and higher incidence of ventricular tachycardia/fibrillation on presentation (versus pulseless electrical activity or asystole), as shown in supplemental table 1.
The distribution of CPC at discharge was as follows: CPC1:6%, CPC2:8%, CPC3: 10%, CPC4:7%, CPC5:69%. At one year, the distribution was: CPC1:8%, CPC2:5%, CPC3: 4%, CPC4:2%, CPC5:79%.
Survivors
Survival after early recovery without documented discussions was the most common mode of survival (57%, n=67), followed by survival due to family wishes despite physicians predicting poor neurological prognosis (20%, n=24), then survival with physician/family agreement (17%, n=20) and finally survival despite WLST (6%, n=6).
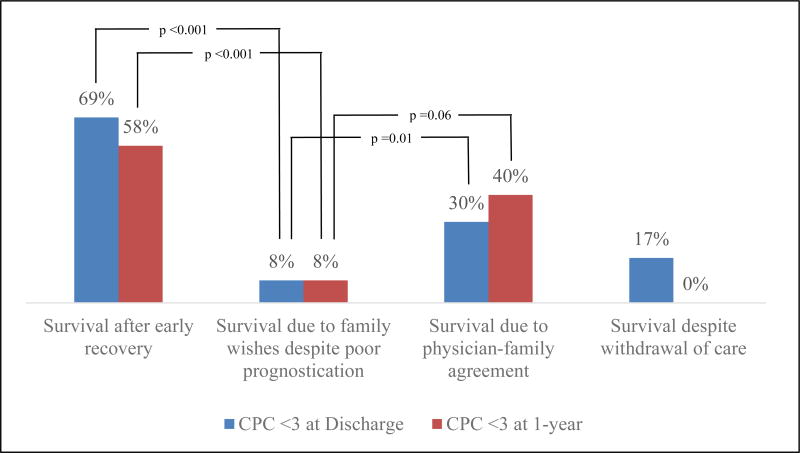
Among survivors due to family wishes despite physicians predicting poor neurological prognosis, 2 patients (8%) had favorable neurological recovery (CPC 1–2) at discharge and one-year post-arrest. This was lower than the rate of good neurological recovery among survivors due to physician-family agreement both at discharge (p=0.06), and one-year post-arrest (p=0.01). When compared to early recovery group, the survivors due to family wishes also had significantly worse outcomes at discharge (p <0.001) at one-year (p <0.001). Overall rates of good recovery are shown in figure 2. The only group to show improvement from discharge to one-year were survivors due to physician-family agreement, where the rate of good neurological recovery increased from 30% to 40%.
Figure 2.
Rate of good neurological recovery based on mode of survival
Nonsurvivors
WLST was the most common mode of death, accounting for 48% of all deaths (n=128,267); 31% (n=85/267) occurred following WLST while the patient was “medically unstable,” and only 17% (n=45/267) occurred in “medically stable” patients.
Combining the WLST while medically unstable patients with “death after care was capped” reduced the prevalence of WLST-N to 17%, making death due to hemodynamic instability the most common cause of death (55%), as shown in figure 3.
Figure 3.
Redefining Withdrawal of Life-Sustaining Therapy (WLST)
Discussion
Survival due to early recovery was the most common mode of survival among our cohort. For those who survived following a prognostication conversation, the rate of good neurological recovery at one-year post-arrest was significantly higher among those who survived due to physician-family agreement, versus those who survived due to family wishes despite poor prognostication. It is important to note that while the vast majority of patients with poor prognostication had poor neurological recovery, 2 patients in this group achieved favorable recovery both at discharge and one-year. This suggests that our current prognostication algorithms may miss some patients with potential for recovery.
Among non-survivors, the most common cause of death was WLST while medically unstable. This is in contrast to a recent study by Elmer et al, which demonstrated that even when WLST is stratified based on medical versus neurological causes, WLST-N was still the leading cause of death. One potential explanation for this is the time from arrest to withdrawal. In the Elmer et al study 57% of WLST-N occurred within the first 72 hours following arrest.8 In our cohort, only 29% of WLST-N occurred before 72 hours, and the average time to withdrawal in this group was 10 days. This delay in prognostication may result in patients who would have died due to WLST-N either recovering neurologically and therefore not undergoing WLST-N or perhaps decompensating medically, and falling under the WLST while medically unstable or death after care was capped categories. Regardless, the leading cause of death among our cohort was no longer WLST-N, and was replaced by WLST while “medically unstable.”
It is also important to note the very low rate of advanced directives among our cohort (3% of nonsurvivors). While we do not have sufficient evidence to know how a higher rate of advanced directives would have affected the outcomes, we do know that the presence of an advanced directive in our cohort took the decision-making responsibility off the family and ensured that care was in accordance with patients’ known wishes.
Conclusion
Most survivors due to family wishes despite poor prognostication die or have poor outcomes at one-year. However, a small number achieve favorable recovery at discharge and one-year, suggesting that current prognostication may miss some patients with potential for recovery. The only group of survivors to show improvement between discharge and one-year were the survivors due to physician-family agreement, suggesting that our prognostication algorithm may be more indicative of long-term outcomes rather than immediate neurological recovery. Among nonsurvivors, high rates of WLST while “medically unstable” and “death after care was capped,” suggest an overestimation of withdrawal due to poor neurological prognosis. By redefining these categories and isolating the WLST-N, we demonstrated a smaller proportion of WLST-N than previously documented.
Supplementary Material
Acknowledgments
Funding Sources
-
◦
This study was not sponsored.
Footnotes
-
◦Dr. Matthews reports no disclosures.
-
◦Dr. Magid-Bernstein reports no disclosures.
-
◦Alex Presciutti reports no disclosures.
-
◦Ashley Rodriguez reports no disclosures.
-
◦Dr. Roh reports no disclosures.
-
◦Dr. Park receives funding through NIH K01 ES026833-02
-
◦Dr. Claassen reports no disclosures.
-
◦Dr. Agarwal reports no disclosures.
References
- 1.Marion DW. Coma due to cardiac arrest: prognosis and contemporary treatment. F1000 Med Rep. 2009;1 doi: 10.3410/M1-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gay EB, Pronovost PJ, Bassett RD, Nelson JE. The intensive care unit family meeting: making it happen. J Crit Care. 2009;24(4):629.e1–12. doi: 10.1016/j.jcrc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 4.Schneiderman LJ, Gilmer T, Teetzel HD, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA. 2003;290(9):1166–1172. doi: 10.1001/jama.290.9.1166. [DOI] [PubMed] [Google Scholar]
- 5.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123(1):266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 6.Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455–1461. doi: 10.1016/j.resuscitation.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Lemiale V, Dumas F, Mongardon N, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 8.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–135. doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safar P, Grenvik A, Safar P. Brain Failure and Resuscitation. New York, Edinburgh, London, Melbourne: Churchill Livingstone; 1981. Resuscitation after brain ischemia; pp. 155–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.