Summary
Lattice-like structures known as perineuronal nets (PNNs) are key components of the extracellular matrix (ECM). Once fully crystallized by adulthood, they are largely stable throughout life. Contrary to previous reports that PNNs inhibit processes involving plasticity, here we report that the dynamic regulation of PNN expression in the adult auditory cortex is vital for fear learning and consolidation in response to pure tones. Specifically, after first confirming the necessity of auditory cortical activity for fear learning and consolidation, we observed that mRNA levels of key proteoglycan components of PNNs were enhanced 4 hours after fear conditioning but were no longer different from the control groups 24 hours later. A similar pattern of regulation was observed in numbers of cells surrounded by PNNs and area occupied by them in the auditory cortex. Finally, the removal of auditory cortex PNNs resulted in a deficit in fear learning and consolidation.
Keywords: perineuronal nets, extracellular matrix, auditory cortex, memory, learning, Chondroitinase ABC
Introduction
Identifying cellular and extracellular mechanisms that establish and maintain auditory memories can contribute to the discovery of novel therapeutic agents for neuropsychiatric diseases that involve associative learning and sensory memories. PTSD is one such disorder characterized by avoidance, intrusive symptoms, cognitive disruptions, generalized hyperarousal and anxiety, often in response to sensory cues associated with the original trauma. The pathology of this disorder involves a dysregulation of the fear system, likely caused by an inability to extinguish or inhibit fear, increased generalization of fear cues, and enhanced consolidation of fear learning (Bowers and Ressler, 2015; Liberzon et al., 1999). Although the amygdala is a key site for establishing auditory fear associations (Andero and Ressler, 2012; Johansen et al., 2011; LeDoux, 2007; LeDoux, 2000), behavioral studies now also implicate the necessity of the auditory cortex in acquisition (Letzkus et al., 2011), storage (Boatman and Kim, 2006; Grosso et al., 2015; Herry and Johansen, 2014; Romanski and LeDoux, 1992) and extinction (Song et al., 2010) of auditory fear memories as well as for discrimination learning (Goosens and Maren, 2001). Studies have demonstrated that the auditory cortex contains a long-term trace of behaviorally relevant sounds (Ivanova et al., 2011; Weinberger, 2007), but the molecular mechanisms underlying the formation of this memory trace are not well understood. It is likely that the modification and maintenance of particular synaptic connections between subsets of neurons is important for the formation and maintenance of this memory process (Coultrap and Bayer, 2012; Mayford et al., 2012; Murakoshi and Yasuda, 2012). Synaptic plasticity is likely the substrate through which networks of neurons involved in a stimulus-behavior association form lasting connections (McKinney; Sultan and Day, 2011). However, the biological machinery underlying synaptic function undergoes considerable turnover (Day and Sweatt, 2011), raising the question of how long-term changes at a synapse are maintained.
The neural extracellular matrix (ECM) surrounding cells, synapses and processes in the central nervous system, could be one such player in the maintenance of synaptic morphology and memory traces through complex interactions between neurons and molecules (Levy et al., 2014). The ECM is thought to play particularly important roles in spine and synapse stability and plasticity as it provides a scaffold in the extracellular space (Celio and Blumcke, 1994) in addition to regulating neural plasticity through associations with signaling molecules in development and adulthood (Dyck and Karimi-Abdolrezaee, 2015; Sherman and Back, 2008). The ECM is composed of a meshwork of interconnected proteins and carbohydrates (Levy et al., 2014) including chondroitin sulphate proteoglycans (CSPGs) of the lectican family. Lecticans such as aggrecan, brevican and neurocan are widely implicated in the organization, development, normal maintenance and pathology of the CNS (Avram et al., 2014; Bandtlow and Zimmermann, 2000). CSPGs also form a condensed cartilage-like matrix called PNNs around certain neurons (Fawcett, 2009). PNNs wrap around synapses on the cell body and proximal neurites of specific neuron sub-types in a lattice-like structure. Therefore, they are strategically positioned to exert influences in the development and stabilization of synapses. PNNs emerge late in postnatal development and play a crucial role in the maturation of synapses and closure of critical periods by limiting synaptic plasticity (Dyck and Karimi-Abdolrezaee, 2015; Pizzorusso et al., 2002).
PNNs are prevalent in the adult rodent auditory system, including the auditory cortex (Sonntag et al., 2015). Given that these structures play a role in the inhibition of plasticity and closure of ocular dominance critical periods in the visual cortex, it is possible that they play a role in cementing or stabilizing the newly formed auditory memory traces in the auditory cortex, resulting in fear learning and consolidation. In the current study, we hypothesized that dynamic regulation of PNNs in the auditory cortex was an important component in fear learning and consolidation associated with auditory cues. We first demonstrated that the auditory cortex is necessary for the fear learning and consolidation of fear memories associated with auditory cues and subsequently determined that PNNs play a key role in these processes.
Results
Auditory cortical activity during fear conditioning is necessary for auditory fear learning and consolidation
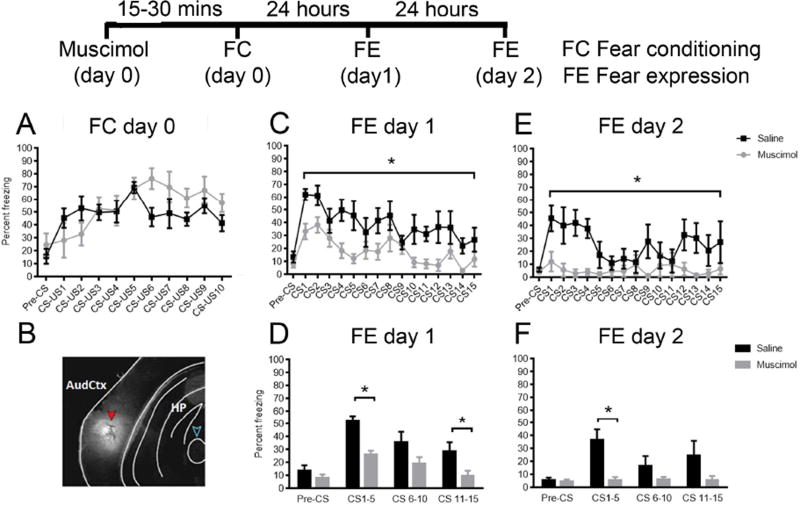
It is known that fear learning associated with complex tones requires activity in the auditory cortex (Letzkus et al., 2011) and that the auditory cortex mediates changes in sensory acuity induced by fear conditioning without affecting the specificity of learning (Aizenberg and Geffen, 2013). To determine if activity in the auditory cortex was necessary for fear learning associated with pure tones, in Experiment 1, animals were trained with 10 tone-shock pairings, 15–30 minutes after bilateral injections of muscimol (n=7) or saline (n=7) into the auditory cortex via cannulas. Muscimol is a potent GABAA receptor agonist (Johnston, 2014), resulting in an increase in inhibitory tone after administration. Following training, to test fear learning 24 hours later, mice were subjected to 15 tones (6 kHz) in the absence of any shocks in a context different from where fear conditioning occurred. Mice that have learned that the tone predicts shock will freeze when tones are presented even in the absence of a shock until they extinguish this specific memory (>30 tone presentations alone).
We observed that there were no significant differences in fear acquisition between saline and muscimol groups (F (1, 12) = 0.6217, P = 0.4457, n=7/group, Figure 1A), suggesting that neural mechanisms to hear and respond to tones were intact, despite auditory cortex inactivation. Fluorescently tagged Muscimol-BODIPY can be observed at 30 minutes after injection into the auditory cortex in Figure 1B to demonstrate the precise localization of muscimol injections and spread.
Figure 1. Muscimol mediated inactivation of auditory cortex before auditory fear conditioning decreased fear expression observed 24 and 48 hours later.
Muscimol or saline was injected into the auditory cortex of mice 15–30 minutes prior to Pavlovian auditory fear conditioning. Mice were fear-conditioned and no differences were observed in fear acquisition (A) in saline and muscimol groups. B depicts fluorescent bodipy-muscimol in the auditory cortex. Decreased fear expression was observed in muscimol injected mice 24 hours (C) after fear conditioning with significant differences between groups during CS 1–5 and CS11–15 (D) and 48 hours after fear conditioning (E) during CS1–5(F). *P < 0.05 vs. vehicle. All values are means ±SEM.
At 24 hours after fear conditioning, muscimol-treated animals exhibited lower percentages of freezing behavior in response to playback of the tone as compared to controls (main effect of treatment: F (1, 12) = 13.94, P=0.0029, n=7/group, Figure 1C). Furthermore, there were significant differences between saline and muscimol groups when the data were binned (main effect of treatment: F (1, 12) = 12.05, P = 0.0046, Figure 1D). Post hoc comparisons using Bonferroni correction showed significant differences between saline and muscimol groups during CS1–5 and CS11–15 (P<0.05, Figure 1D).
Similarly, 48 hours after fear conditioning, muscimol-treated animals exhibited lower percentages of freezing behavior in response to playback of the tone as compared to controls (main effect of treatment: F (1, 12) = 5.906, P = 0.0317, n=7/group, Figure 1E). Furthermore, there were significant differences between experimental groups, when the data were binned according to CS number (main effect of treatment: F (1, 12) = 5.900, P = 0.0318, n=7/group, Figure 1F). Post hoc comparisons using Bonferroni correction showed significant differences between saline and muscimol groups at the CS1–5 bin (P<0.05, Figure 1F). These data suggested that while muscimol did not affect auditory behavioral responses or auditory fear acquisition, that the auditory memory consolidation may be impaired with cortical inactivation.
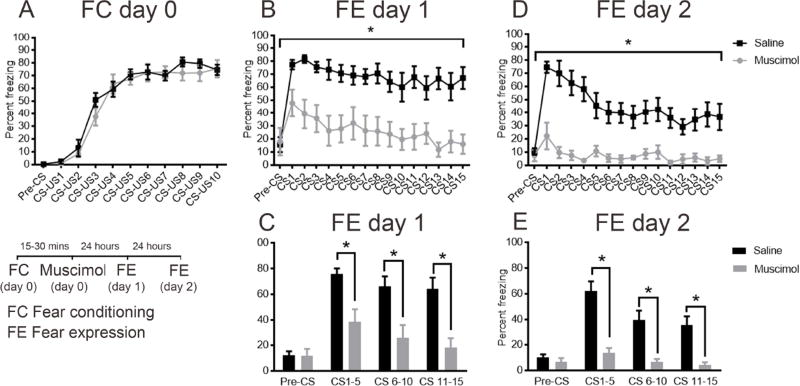
To specifically determine if the auditory cortex plays a role in fear consolidation, in Experiment 2, animals were first trained with 10 tone-shock pairings before bilateral injections of muscimol (n=7) or saline (n=7) were administered into the auditory cortex via cannulas within 30 min following training. We observed that there were no significant differences in fear acquisition between saline and muscimol groups (F (1, 17) = 0.7119, P=0.4105, n=6–12/group, Figure 2A), suggesting that fear acquisition to tones was intact before pharmacological silencing of auditory cortex.
Figure 2. Muscimol mediated inactivation of auditory cortex after auditory fear conditioning decreased fear expression observed 24 and 48 hours later.
Muscimol or saline was injected into the auditory cortex of mice within 30 minutes after Pavlovian auditory fear conditioning. No differences were observed in fear acquisition (A) in saline and muscimol groups. Decreased fear expression was observed in muscimol injected mice 24 hours (B) after fear conditioning with significant differences between groups during CS1–5, CS 6–10 and CS 11–15 (C) and 48 hours after fear conditioning (D) during CS1–5, CS 6–10 and CS 11–15 (E). *P < 0.05 vs. vehicle. All values are means ±SEM.
To test fear learning 24 hours later, mice were subjected to 15 tones (6 kHz) in the absence of any shocks in a context different from where fear conditioning occurred. Animals treated with muscimol after fear conditioning exhibited lower percentages of freezing behavior in response to playback of the tone as compared to controls (main effect of treatment: F (1, 15) = 15.2, P=0.0014, n=6–12/group, Figure 2B). Furthermore, there were significant differences between saline and muscimol groups when the data were binned (main effect of treatment: F (1, 15) = 13.41, P=0.0023, Figure 2C). Post hoc comparisons using Bonferroni correction showed significant differences between saline and muscimol groups during CS1–5, CS6–10 and CS11–15 (P<0.05, Figure 2C).
Similarly, 48 hours after fear conditioning, muscimol-treated animals exhibited lower percentages of freezing behavior in response to playback of the tone as compared to controls (main effect of treatment: F (1, 14) = 16.43, P=0.0012, n=6–12/group, Figure 2D). Furthermore, there were significant differences between experimental groups, when the data were binned according to CS number (main effect of treatment: F (1, 14) = 14.35, P=0.0020, n=6–12/group, Figure 2E). Post hoc comparisons using Bonferroni correction showed significant differences between saline and muscimol groups at the CS1–5, CS6–10 and CS11–15 bins (P<0.05, Figure 2E).
These results emphatically demonstrate that the auditory cortex is not needed for acquiring the association between a pure tone and a shock given there were no differences in fear conditioning between saline controls and muscimol subjects in Experiment 1. In contrast, the auditory cortex is required for learning and consolidation of auditory fear memories (Experiments 1 and 2). Auditory fear memory associated with a simple tone, tested 24 and 48 hours after fear conditioning, is impaired, despite cortical activity returning to baseline within a few hours after acquisition given the short half-life of muscimol (DiSorbo et al., 2009). Furthermore, specifically interfering with consolidation of fear memories by muscimol administration after fear conditioning also leads to significantly lower levels of fear expression 24 and 48 hours later (Experiment 2), suggesting that neural activity in the auditory cortex, even after the sound-shock pairing session, is necessary for fear memory consolidation.
Levels of mRNA transcripts of perineuronal net lecticans are dynamically regulated after auditory fear conditioning
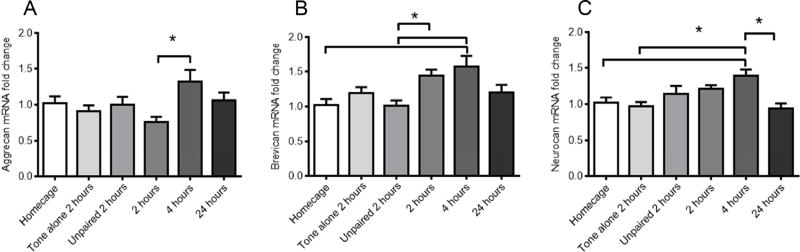
PNNs are expressed in the rodent cortex, and have previously have been demonstrated to surround Parvalbumin (Kosaka and Heizmann, 1989; Wintergerst et al., 1996) and NeuN (Galtrey et al., 2008) positive neurons (Figure S1). PNNs in the visual cortex of rodents appear over a time course that coincides with the closure of the ocular dominance critical period (Liu et al., 2013). Notably, any manipulation resulting in the disruption of PNN aggregation prevents the closure of the ocular dominance critical period (Carulli et al., 2010). Given the role of PNNs in neural plasticity associated with critical periods during development and adult paradigms of learning (Mironova and Giger, 2013; Nabel and Morishita, 2013), we hypothesized that PNNs would be regulated at the level of mRNA and protein in response to fear learning. Using real-time PCR, we measured mRNA levels of lectican components of perineuronal nets, aggrecan, brevican and neurocan (Yamaguchi, 2000), in homecage control animals as well as 2, 4 and 24 hours after fear conditioning to determine the time course over which they were regulated so as to encompass the early and late phases of fear memory consolidation in Experiment 3.
mRNA levels of aggrecan were significantly higher at 4 fours after fear conditioning as compared to levels at 2 hours after fear conditioning (main effect of experimental group: F(5, 33) = 2.839, P = 0.0301 n=5–8/group, Tukey’s post hoc comparison, P<0.05, Figure 3A). Similarly, levels of brevican mRNA were significantly higher 4 hours after fear conditioning as compared to the home cage group and 2 hour unpaired control (shock and tone were not presented in a paired manner and animals were sacrificed 2 hours after tone and shock presentation; main effect of experimental group: F (5, 39) = 4.695, P = 0.0019, n=6–8/group, Tukey’s post hoc comparison, P<0.05, Figure 3B). Note that in this experiment the brevican mRNA at 2 hours following fear condition was also significantly greater than the 2 hour unpaired control. Finally, mRNA levels of neurocan were significantly higher 4 hours after fear conditioning as compared to control home cage animals and tone-only animals (sacrificed 2 hours after tone alone presentation in the absence of shocks) as well as animals sacrificed 24 hours after fear conditioning (main effect of experimental group: F (5, 38) = 5.002, P = 0.0013, n=6–8/group, Tukey’s post hoc comparison, P<0.05, Figure 3C). Therefore, mRNA levels of three lecticans were dynamically regulated during auditory fear memory consolidation, in that they were up-regulated 4 hours after fear conditioning but were similar to baseline levels within 24 hours after fear conditioning.
Figure 3. mRNA levels of genes encoding lecticans were enhanced 4 hours after fear conditioning but returned to baseline levels 24 hours later.
mRNA levels of aggrecan were significantly higher at 4 hours after fear conditioning compared to 2 hours after fear conditioning (A). Brevican mRNA levels were highest at 4 hours after fear conditioning and significantly higher than the home cage group and unpaired group (B). mRNA levels of neurocan were significantly higher at 4 hours after fear conditioning as compared to home cage group, tone only group and 24 hours after fear conditioning (C). *P < 0.05 vs. vehicle. All values are means ±SEM.
Perineuronal nets are dynamically regulated after fear conditioning
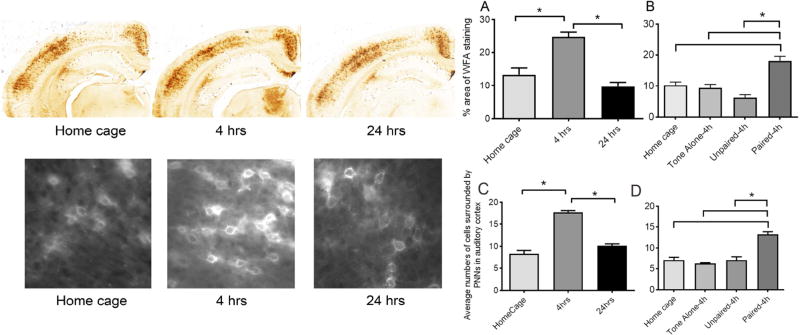
To determine whether PNN expression is also regulated after fear conditioning, in Experiment 4, we measured the percentage of area occupied by PNNs in the primary auditory cortex (Au1) and dorsal auditory cortex (AuD), as intense PNN expression was observed in these regions. Results are reported from both regions combined. In addition, we counted numbers of cells that were surrounded by PNNs in layer 2/3 of the auditory cortex.
The percentage area occupied by PNNs (Figure 4A) in the auditory cortex, as detected by wisteria floribinda agglutinin (WFA) immunohistochemistry, was higher 4 hours after fear conditioning as compared to home cage, tone alone and unpaired controls (main effect of treatment: F (2, 19) = 21.70, P < 0.0001, Tukey’s post hoc comparison, P<0.05,n=6–8/group). There were no significant differences between controls and fear conditioned subjects 24 hours after auditory fear conditioning. In a separate group of animals sacrificed at 4 hours after tone experience, area of expression of PNNs (Figure 4B) was highest in the paired group (tone paired with shock/fear-conditioned) as compared to the tone alone and unpaired (random presentation of tone and shock) groups (main effect of treatment: F (3, 16) = 19.91, P < 0.0001, Tukey’s post hoc comparison, P<0.05, n=4–6/group).
Figure 4. PNN expression was specifically enhanced 4 hours after tone-shock paired fear conditioning but returned to baseline levels 24 hours later.
Area of expression of PNNs across the auditory cortex was highest at 4 hours after fear conditioning, but not different from controls by 24 hours after fear conditioning (A). In a separate group of animals sacrificed at 4 hours after tone experience (B), area of expression of PNNs was highest for the paired group as compared to home cage controls, and groups receiving only tone presentation or unpaired stimulation. Numbers of cells surrounded by PNNs are higher at 4 hours after fear conditioning than home cage group and the group sacrificed 24 hours after fear conditioning (C). In the same group of animals as in (B), the number of cells surrounded by PNNs was highest in the paired group 4 hours after auditory fear conditioning as compared to the home cage controls, tone alone and unpaired group 4 hours after tone experience (D). *P < 0.05 vs. vehicle. All values are means ±SEM.
Furthermore, numbers of cells surrounded by PNNs (Figure 4C), as detected by WFA immunohistochemistry, were higher 4 hours after fear conditioning (averaged across 2–3 sections per subject) as compared to home cage controls. Cell numbers were no longer significantly different from home cage controls 24 hours after fear conditioning (main effect of treatment: F (2, 18) = 85.09, P < 0.0001, Tukey’s post hoc comparison, P<0.05, n=7–8/group). In the separate 4 hour group of animals, numbers of cells surrounded by PNNs (4D) were highest in the paired group as compared to the tone alone and unpaired group (main effect of treatment: F (3,15) = 22.06, P < 0.0001, Tukey’s post hoc comparison, P<0.05, n=4–6/group), confirming a transient regulation of extracellular matrix proteins in a sound learning paradigm.
Perineuronal nets in the auditory cortex are necessary for fear learning and consolidation
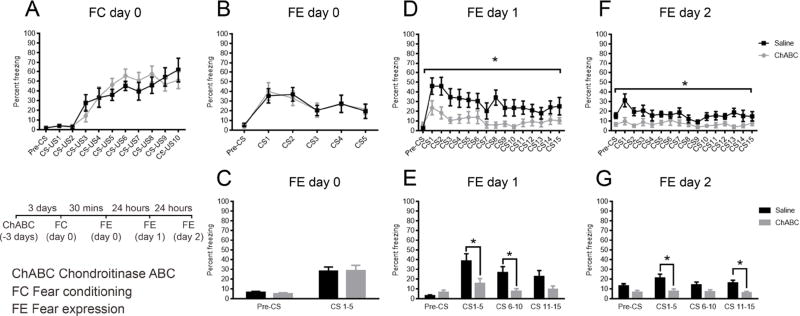
Given the observed regulation of PNNs after fear conditioning, we next asked whether PNNs are necessary for fear learning in Experiments 5, 6 and 7. In Experiment 5, the enzyme Chondroitinase ABC (ChABC) was bilaterally injected into the auditory cortex of experimental mice to specifically digest PNNs. Control mice received bilateral injections of saline. After 72 hours, ChABC animals and saline controls were both able to learn a sound-fear association (F (1, 12) = 0.1194, P = 0.7357, n=6–8/group, Figure S2A).
ChABC treatment resulted in the degradation of PNNs in the auditory cortex, confirmed with WFA staining in Figure S2B. Mice treated with ChABC had lower levels of fear expression in comparison to saline-treated controls 24 hours after fear conditioning, suggesting that the PNNs in auditory cortex are required for consolidating the acoustically-elicited fear memory (main effect of treatment: F (1, 10) = 7.533, P = 0.0207 n=6–8/group, Figure S2C). Further analysis of these data after binning CS1–5, 6–10 and 11–15 again showed a significant difference (main effect of treatment: F (1, 10) = 7.126,P = 0.0235), with post hoc differences (Bonferroni correction) between experimental groups during CS1–5 and CS6–10 (P<0.05, Figure S2D). At 48 hours after fear conditioning, there was a trending difference between experimental groups (F (1, 11) = 3.626, P = 0.0834, Figure S2E). After CS trials were binned (main effect of treatment: F (1, 11) = 4.101, P = 0.0678, n=6–8/group, Figure S2F), significantly lower levels of fear expression in the ChABC groups were observed during CS1–5 after post hoc comparison using Bonferroni correction (P<0.05, Figure S2F).
We next sought to replicate our results in Experiment 6 using a different set of mice, while also testing short-term learning at 30 minutes after fear conditioning, and repeat fear conditioning using a novel tone 3 months after ChABC administration into the auditory cortex. Since previous work demonstrated that PNNs return to control levels 2 months after ChABC treatment (Romberg et al., 2013), Experiment 6 allowed us to ascertain whether any long-term damage was incurred from the ChABC manipulation.
As observed in Experiment 5, there were no differences observed in fear acquisition between control and ChABC groups (F (1, 16) = 0.05000, P = 0.8259, n=9/group, Figure 5A). Interestingly, there were also no differences in fear expression 30 minutes after fear conditioning (F (1, 17) = 0.0006487, P = 0.9800, Fig. 5B), even after CS trials were binned from CS1–5 (F (1, 17) = 0.01434, P = 0.906, Figure 5C).
Figure 5. Removal of PNNs in the auditory cortex using ChABC before auditory fear conditioning decreased fear expression observed 24 and 48 hours later, but did not decrease fear expression 30 minutes later.
ChABC or saline was injected into the auditory cortex of mice 72 hours prior to Pavlovian auditory fear conditioning. Mice were fear-conditioned and no differences were observed in fear acquisition (A) or fear expression 30 minutes after fear conditioning (B) between saline and ChABC groups (C). Decreased fear expression was observed in ChABC injected mice in comparison to controls at 24 hours after fear conditioning (D) wherein significant differences between groups were observed during CS1–5 and CS6–10 (E) and at 48 hours after fear conditioning during CS1–5 and CS11–15(F). *P < 0.05 vs. vehicle. All values are means ±SEM
Fear expression between saline and ChABC groups was significantly different at 24 hours after fear conditioning on a trial-by-trial basis (F (1, 17) = 6.119, P = 0.0242, Figure 5D), and after data were binned according to CS1–5, CS6–10 and CS11–15 (main effect of treatment: F (1, 17) = 5.139, P = 0.0367, Figure 5E). Significantly lower levels of fear expression were observed in the ChABC group as compared to the saline group during CS1–5 and CS6–10 after post hoc comparison using Bonferroni correction (P<0.05, Figure 5E).
Similarly at 48 hours after fear conditioning, lower levels of fear expression were observed in the ChABC group compared to the saline group (main effect of treatment: F (1, 17) = 10.70, P = 0.0045, Figure 5F). This was also true after data were binned (main effect of treatment: F (1, 17) = 11.12, P = 0.0039, Figure 5G), with significant differences between groups observed during CS1–5 and CS11–15 after post hoc comparison using Bonferroni correction (P<0.05, Figure 5G).
We then tested whether the same subjects as in Experiment 6 could be fear conditioned to a novel tone of a higher frequency (11kHz) three months after our initial experiment (Figure S4), when the PNNs were expected to have re-aggregated (Romberg et al., 2013). Our prediction was that fear learning would no longer differ between ChABC and saline treated subjects given the reappearance of PNNs after ChABC treatment. As predicted, fear acquisition was similar in both saline and ChABC groups (F (1, 17) = 0.001162, P = 0.9732, n=9/group, Figure S4A). Furthermore, there were no differences in fear expression between the groups 24 hours after fear conditioning (F (1, 17) = 0.03322, P = 0.8574, n=9/group, Figure S4B). PNNs were observed to have regrown in ChABC subjects when sacrificed 3 months after ChABC administration in the auditory cortex (Figure S4C). Taken together, these data both replicated our prior results, and demonstrated the specificity of the ChABC effect to long-term fear learning and not to initial acquisition or immediate expression of fear associated with auditory cues.
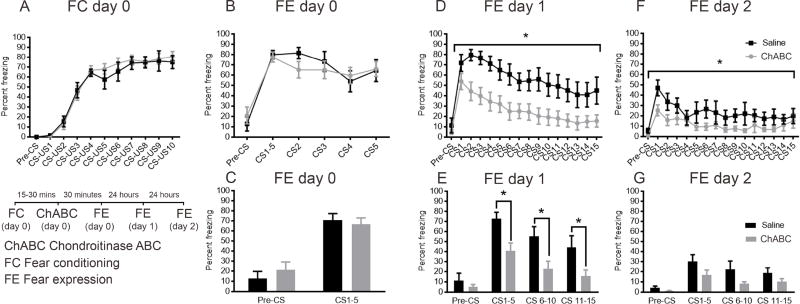
The ChABC effect on long-term rather than short-term expression of fear led us to next investigate the role of PNNs specifically in the consolidation of auditory fear memories in Experiment 7. We administered ChABC (bilateral auditory cortex stereotaxic administration) immediately after fear conditioning on day 0, and tested short-term (30 minutes) as well as long-term expression 24 and 48 hours after auditory fear conditioning. We had previously confirmed that ChABC completely dissolved PNNs in the auditory cortex within 4 hours after administration into the auditory cortex (data not shown). There were no differences in fear acquisition between control and ChABC groups (F (1, 20) = 0.1994, P=0.6600, n=8–14/group, Figure 6A). Interestingly, there were also no differences in fear expression 30 minutes after fear conditioning (F (1, 20) = 0.04082, P=0.8419, Fig. 6B), including when CS trials were binned from CS1–5 (F (1, 20) = 0.04792, P=0.8289, Figure 6C). This could be because the nets were not entirely dissolved, or as demonstrated in Experiment 6, short term consolidation of auditory cue associated fear memories was not dependent on the presence of PNNs in the auditory cortex.
Figure 6. Removal of PNNs in the auditory cortex after fear conditioning, results in decreased fear expression observed after 24 and 48 hours but not 30 minutes after fear conditioning.
ChABC or saline was injected into the auditory cortex of mice within 30 minutes after auditory fear conditioning. No differences were observed in fear acquisition (A) or fear expression 30 minutes after fear conditioning (B) between saline and ChABC groups (C). Decreased fear expression was observed in ChABC injected mice in comparison to controls at 24 hours after fear conditioning (D) wherein significant differences between groups were observed during CS1–5, CS6–10 and CS11–15 (E) and at 48 hours after fear conditioning (F). No significant differences between groups were observed after binning according to CS number (G). *P < 0.05 vs. vehicle. All values are means ±SEM
There were significant differences in fear expression between saline and ChABC groups at 24 hours after fear conditioning (F (1, 19) = 7.880, P = 0.0112, Figure 6D). After data were binned according to CS1–5, CS6–10 and CS11–15, significantly lower levels of fear expression were observed in the ChABC group as compared to the saline group for all bins (main effect of treatment: F (1, 19) = 8.186, P=0.0100, Figure 6E) after post hoc comparison using Bonferroni correction (P<0.05, Figure 6E).
Similarly, at 48 hours after fear conditioning, lower levels of fear expression were observed in the ChABC group compared to the saline group (main effect of treatment: F (1, 19) = 4.764, P = 0.0418, Figure 6F). After data were binned, a significant main effect of treatment was observed (F (1, 19) = 5.26, P=0.0334, Figure 6G), although significant differences were not observed between groups in individual CS bins after post hoc comparison using Bonferroni correction (P>0.05, Figure 6G). These data suggest that PNNs play a key role in the consolidation of fear memories associated with auditory cues.
Furthermore, no main effect of treatment was found on freezing during the inter-trial-intervals on fear expression days (Figure S3), indicating that group differences were specific to the tone playback period versus generalized contextual freezing and that auditory processes were intact in both saline and ChABC subjects. Specifically, there was no significant main effect of treatment (saline versus ChABC) on freezing either pre-CS or during the ITIs either one (F(1,19)=2.959, p>0.05, Figure S3A) or two (F(1,19)=0.037, p>0.05, Figure S3B) days after fear-conditioning. On day 1, there was a significant interaction between ITI number and treatment (F (14, 266) = 2.182, P=0.0088) driven by the first ITI, wherein saline-treated animals showed significantly greater freezing during the first between-tone period (ITI1) compared to the ChABC animals. No other significant differences were observed during any of the ITIs.
Discussion
This study demonstrates a dynamic regulation of PNNs in the adult brain in response to a paradigm of learning, Pavlovian fear conditioning. Traditionally, PNNs have been observed to be dynamically regulated during development, unlike in adulthood when they are considered to be stable structures unless perturbed by disease or injury in regions such as the spinal cord (Fawcett, 2015). Therefore, to the best of our knowledge, this is the first study to implicate a change in cortical PNN expression in response to adult learning. Furthermore, this is the first study to show that PNNs in the auditory cortex are necessary for fear learning and consolidation in response to auditory fear conditioning. Finally, although previous studies have suggested that a decrease in PNNs leads to enhanced performance in tests of memory (Happel et al., 2014; Romberg et al., 2013), this study provides evidence that transient auditory cortical PNN enhancement and the possible reduction of plasticity (Pollock et al., 2014; Valenzuela et al., 2014) 4 hours after fear conditioning is key for the formation of long-term memory traces associated with auditory cues. Here we show for the first time that removal of PNNs, possibly resulting in decreased inhibition and enhanced plasticity in the auditory cortex, prevented consolidation of fear learning. Therefore, it is possible that the brakes on plasticity dynamically asserted by PNNs after fear conditioning are an important step for auditory cortex-associated fear memory formation, potentially by preventing the interference of the fear memory by subsequent sound experience.
We first demonstrated that activity in the auditory cortex is necessary for consolidation of auditory fear memory in Pavlovian fear conditioning tasks using simple tones (Figures 1 and 2). The amygdala has long been known to play an essential part in processing fearful environmental stimuli and in fear conditioning. More recently, new circuits have been identified to mediate fear learning and memory consolidation (Herry and Johansen, 2014). One such region that has been implicated in the storage of auditory fear memories is the auditory cortex (Grosso et al., 2015). In our studies, mice that had decreased activation in the auditory cortex due to GABA receptor agonist muscimol administration prior to fear conditioning or immediately after fear conditioning (using simple tones), acquired fear similar to controls but had decreased fear expression in comparison to controls 24 and 48 hours after fear conditioning (Figure 1 and 2). These results are in agreement with a previously published study wherein the authors demonstrated that muscimol inactivation of the auditory cortex prior to fear conditioning using complex FM sweeps resulted in decreased fear expression 24 hours later (Letzkus et al., 2011). Interestingly, our results further demonstrate a requirement for neural activity in auditory cortex after the sound conditioning itself, pointing to a role for ongoing activity (perhaps in the form of activity replay (Qin et al., 1997)) in memory consolidation. As we established the importance of auditory cortical activity in fear learning involving simple tones associated with footshocks, we further explored the molecular mechanisms underlying plasticity in the auditory cortex that contributes to this fear learning.
The cortical ECM consisting of CSPGs is a key player in the inhibition of juvenile and adult plasticity. CSPG expression in primary visual cortex of rodents increases through the critical period of ocular dominance from P19–35 (Pizzorusso et al., 2002). A seminal study showed that dark rearing, which delays critical period closure, also delayed the developmental increase in CSPGs. After CSPG degradation with ChABC in adult rats, monocular deprivation caused an ocular dominance shift toward the non-deprived eye. Therefore the mature ECM of the adult visual cortex blocks experience-dependent plasticity, and removal of CSPGs reactivates plasticity (Levy et al., 2014; Pizzorusso et al., 2002). Furthermore, during early postnatal development, fear memories are easily erased via extinction paradigms as compared to adulthood (Kim and Richardson, 2007). In fact, CSPGs in the amygdala of adult rodents are a key player in the resilience and maintenance of fear memories (Gogolla et al., 2009; Pizzorusso et al., 2002; Quirk et al., 2010). Following a developmental profile similar to the visual cortex, the appearance of PNNs (formed by aggregation of CSPGs) in the amygdala coincided with the developmental switch in fear memory resilience. Enzymatic degradation of PNNs in the amygdala in adulthood led to subsequently acquired fear memories being susceptible to erasure via extinction (Gogolla et al., 2009). These studies provided the impetus to explore the contribution of PNNs in the auditory cortex to adult fear learning. We hypothesized that the PNNs would be necessary for the maintenance of fear memories, thereby contributing to fear learning.
We found that mRNA levels of lecticans were enhanced 4 hours after fear conditioning but were no different from home cage controls 24 hours after fear conditioning (Figure 3). In keeping with this pattern of change in response to fear conditioning, the number of WFA-positive cells or cells surrounded by PNNs as well as area occupied by PNNs, was significantly higher compared to home cage, tone alone and unpaired controls at 4 hours after fear conditioning, but were no different from home cage group, 24 hours after fear conditioning (Figure 4). In sum, these data suggest that the deficits in fear learning observed in ChABC treated adult mice, 24 hours after fear conditioning, are due to an absence of a dynamic upregulation in PNNs surrounding cells, observed at 4 hours after fear conditioning in control animals. Therefore, the transient enhancement of PNNs shortly after fear conditioning is likely necessary for the recently acquired fear cue to be consolidated.
To demonstrate a role for PNNs in learning and memory processes in adults, we enzymatically destroyed PNNs in the auditory cortex prior to, as well as after, fear conditioning in separate experiments. We observed that although fear acquisition was similar to controls, expression of fear was decreased in experimental groups 24 and 48 hours after fear conditioning, but not 30 minutes after fear conditioning (Figures S2, 5 and 6). Our results suggest that PNNs are necessary for the storage of long-term memories, and their absence results in decreased fear expression observed 24 and 48 hours after auditory fear conditioning. Significantly, 3 months after ChABC treatment, when PNNs had regrown in the auditory cortex (Figure S4), no differences in fear expression were observed 24 hours after fear conditioning to a different tone. This suggests that the deficits in fear learning due to PNN degradation in the auditory cortex can be reversed after PNN regrowth.
In contrast to our results, several other studies have shown that PNN removal in adulthood can enhance learning and memory. For example, Crtl1 knock-out mice, which had attenuated PNN expression in the cortex, displayed enhanced long-term object recognition memory and facilitated long-term depression in the perirhinal cortex (Romberg et al., 2013). Similar effects on memory were observed when PNNs were digested by ChABC in the perirhinal cortex, and recognition memory returned to baseline over time as the PNNs reformed after enzymatic degradation. In a different study of drug-induced conditioned place preference, extinction learning over several days was found to be improved when combined with intra-amygdala injections of ChABC, possibly by potentiating the function of plasticity related proteins there (Xue et al., 2014). Even within auditory cortex, digestion of PNNs resulted in enhanced performance after several days of retraining in a cue reversal learning task, suggestive of increased cognitive flexibility (Happel et al., 2014). In sum, degradation of adult PNNs across various brain areas has mainly, albeit not always (see, for example, Slaker et al. (2015)), been associated with improvements in learning paradigms.
That learning can be disrupted by removing auditory cortical PNNs just before or after a single session of pure tone fear conditioning may therefore seem contrary to the idea that PNNs are normally inhibitory to plasticity. However, drawing on recently revealed cortical circuit mechanisms for auditory fear learning (Letzkus et al., 2015), we speculate that the temporally delayed upregulation of PNNs helps protect recent memories that are still consolidating from interference by other experiences. A large population of PNNs have been observed surrounding inhibitory interneurons expressing the calcium binding protein parvalbumin (Figure S1) (Berretta et al., 2015). The inhibition of such parvalbumin interneurons in layer 2/3 by footshocks during auditory fear conditioning normally disinhibits layer 2/3 pyramidal cells in auditory cortex, and preventing this disinhibition pharmacologically or optogenetically results in decreased memory consolidation (Letzkus et al., 2011). PNNs around parvalbumin interneurons help maintain the tone of inhibitory neurotransmission within cortex, and their reduction is associated with weakened inhibitory activity and enhanced excitatory neuron plasticity (Deidda et al., 2015; Kinden Lensjø et al., 2016; Sale et al., 2007; Slaker et al., 2015). Hence, a transient increase in PNN expression 4 hours after sound-shock pairing may then increase the inhibitory-to-excitatory balance onto pyramidal neurons arising from inputs that are either spontaneously active or evoked by newly experienced sounds. Neurons still undergoing cellular changes during their late-phase consolidation of the fear cue memory (Izquierdo et al., 2006) may then be protected against the decay of memory traces or creation of interfering memory traces, which could impair learning (Banai et al.; Brashers-Krug et al., 1996; Seitz et al., 2005; Wright et al., 2010). In mice treated with ChABC, the changes in inhibition and excitation mediated by transient PNN upregulation would not occur after fear conditioning, possibly contributing to decreased fear consolidation. Future studies will need to tease apart the neural responses in layer 2/3 interneurons and pyramidal cells after fear conditioning in the presence and absence of PNNs.
Our interpretation of these data is focused on disrupted consolidation of fear, due to the demonstration that the initial cued freezing during all post-manipulation tests showed very low freezing. However, as discussed above, a number of prior studies of PNN disruption have suggested that this type of manipulation may lead to a rapid, erasure-like extinction of the initial memory process. We cannot completely rule out this possibility, and such an interpretation would be largely consistent with the existing literature, including facilitated reversal learning and other measures of behavioral flexibility (Gogolla et al., 2009; Happel et al., 2014; Xue et al., 2014). However, enhancement of extinction is usually demonstrated behaviorally by a more rapid within-session extinction process (with similar initial levels of fear), and/or a more robust extinction-retention test (Walker et al., 2002). Given that we observed lower levels of fear at the very first fear expression test, we believe the most parsimonious explanation is a disruption of initial fear consolidation, although this may be augmented by more rapid extinction as well.
A slew of ECM enzymes play a role in extracellular matrix stability. Tissue inhibitors of metalloproteinases (TIMPs) inhibit matrix metalloproteinases (MMPs) as well as the closely related ‘A Disintegrin and Metalloprotease’ (ADAMs) and ADAMs with thrombospondin motifs (ADAMTSs), all of which are involved in ECM proteolysis (Arpino et al., 2015; Levy et al., 2015; Pizzi and Crowe, 2007; Seals and Courtneidge, 2003; Senkov et al., 2014). Therefore, PNN stability in response to Pavlovian fear conditioning could be influenced by the activity and expression of these enzymes. It is likely that auditory fear conditioning results in the regulation of such enzymes in a time-dependent manner. Future experiments will involve assaying the expression and activity of these enzymes in the auditory cortex in response to fear conditioning, potentially leading to further targets for therapies for PTSD.
Overall our work has shown that PNNs are necessary for auditory fear learning and consolidation in adults. The uncovering of dynamic cellular pathways that are influenced by the regulation of PNNs will shed light on the storage of long-term memories and lead to potential therapeutic avenues to decrease the learning of traumatic memories.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Kerry Ressler (kressler@mclean.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All experiments were conducted with 2-month-old C57Bl/6J male mice purchased from Jackson Laboratory (Bar Harbor). After arrival at the Yerkes Vivarium, mice from the same cage were assigned randomly to control and experimental groups. Animals were housed on a 12 hr light/dark cycle in standard group cages (≤5/cage) with ad libitum access to food and water. All experiments were conducted during the light half of the cycle. All procedures were approved by Emory University’s IACUC and followed guidelines set by NIH.
METHOD DETAILS
Surgery and local drug injection
Effect of Muscimol on auditory fear conditioning (Figures 1 and 2): Mice were anaesthetized with ketamine-dormitor and fixed in a stereotaxic frame (Stoelting Instruments). Analgesia was provided by local injection of metacam s.c. and lidocaine under the scalp. Guide cannulas (26 gauge, with dummy screw caps, Plastics One) were implanted bilaterally to inject at the following coordinates: 2.46 mm posterior of bregma, ± 4.5 mm lateral of midline, 0.6 mm below cortical surface. After surgery all animals received postoperative analgesic metacam for 2 days and as needed. Mice were then given 2 weeks to recover from surgery, during which time they were handled 5–6 times to habituate them to the injection procedure. Fifteen minutes before fear conditioning (Experiment1) or within 30 minutes after fear conditioning (Experiment 2), 32-gauge stainless steel injectors attached to 10 µl Hamilton syringes were inserted into the guide cannulas and an injection volume of 0.25 µl per hemisphere was delivered within 120 s using a microinfusion pump (Stoelting). Drug animals received bilateral injections of muscimol (100 ng per hemisphere) whereas control mice were injected with saline solution only. In a subset of mice, fluorescent muscimol bodipy (625 µM with 5% DMSO) was injected after fear expression to quantify spread of the drug. After completion of the experiment, mice were transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PFA), their brains extracted and post-fixed in paraformaldehyde overnight. For histological verification of the injection site, 50-µm coronal brain sections were made on a microtome (Leica Microsystems) and imaged on a microscope.
Effect of Chondroitinase ABC (ChABC) on auditory fear conditioning (Figures 5, 6 and S2): Mice were anaesthetized with ketamine-dexdormitor and fixed in a stereotaxic frame (Stoelting Instruments). Analgesia was provided by local injection of metacam s.c. and lidocaine under the scalp. In Experiments 5 (Figure 5) and 6 (Figure S2), mice received bilateral injections of either saline or ChABC into the auditory cortex via a Hamilton syringe lowered to the following co-ordinates: 2.46 mm posterior of bregma, ± 4.5 mm lateral of midline, 0.6 mm below cortical surface. Following this procedure, mice were administered post-operative analgesia 12 hours apart. 3 days later, they underwent fear conditioning. Experiments 6 was a replication of Experiment 5 with the exception that fear expression was tested 30 minutes after fear conditioning in addition to 24 and 48 hours later.
In Experiment 7 (Figure 6), mice were anaesthetized with ketamine-dormitor and fixed in a stereotaxic frame (Stoelting Instruments). Analgesia was provided by local injection of metacam s.c. and lidocaine under the scalp. Guide cannulas (26 gauge, with dummy screw caps, Plastics One) were implanted bilaterally to inject at the following coordinates: 2.46 mm posterior of bregma, ± 4.5 mm lateral of midline, 0.6 mm below cortical surface. After 2 weeks of recovery, mice underwent fear conditioning. Within 30 minutes after fear conditioning, drug animals received bilateral injections of ChABC whereas control mice were injected with saline solution only.
After completion of the experiment, a subset of mice was transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PFA), their brains extracted and post-fixed in paraformaldehyde overnight. For histological verification of the injection site, 50-µm coronal brain sections were made on a microtome (Leica Microsystems) and imaged on a microscope.
Auditory Fear Conditioning
Mice were pre-exposed to sound attenuated conditioning chambers (San Diego Instruments) (grid floors, room light on, cleaned with Quatricide) for 3 consecutive days before training. On the day of auditory fear conditioning in context A, mice received 10 CS-US pairings (CS: 30 s, 6 kHz, 75 db tone) (US: 1s, 0.6 mA foot-shock) wherein the tone co-terminated with the mild foot-shock with a 120 second intertrial interval (ITI). Where an unpaired condition was used, the same CS and US parameters were used with no cotermination and presented in a random sequence. For the tone alone group, mice were subjected to the tones identical to the paired group with 120 second ITI but no shock was delivered at any point. The percentage of time spent freezing during fear acquisition was measured by SR-LAB software (San Diego Instruments). Fear expression was tested 30 minutes after fear conditioning (context B) and fear learning was tested 24 hours (context C) and 48 hours (context D) after fear conditioning in a novel context (modular test chambers; Med Associates Inc. with plexiglass floor, room light off/on, red chamber lights on/off, cleaned with EtOH) when mice were exposed to 15 CS tones on two consecutive days. Freezing during the tone presentations was measured with FreezeView software (Coulbourn Instruments). 3 months after the first training mice were retrained to a novel tone (context D) (10 CS-US pairings, CS: 30 s, 11 kHz, 75 db tone,US: 1s, 0.6 mA foot-shock) wherein the tone co-terminated with the mild foot-shock with a 120 second intertrial interval (ITI). Fear learning was tested (context E) 24 hours later with 10 CS presentations. For all experiments (i.e. Experiments 5, 6 and 7), freezing was analyzed by observers blind to treatment groups.
mRNA Quantification in the auditory cortex
In Experiment 3 (Figure 3), male mice were subjected to auditory fear conditioning (Paired and Unpaired groups) or subjected to tones alone in the absence of shocks (Tone only controls). Brains from these animals and Home Cage controls were collected 2, 4, and 24 hours after fear conditioning and were rapidly frozen on dry ice. After micropunching the auditory cortex, mRNA were extracted from the tissue punches using the RNeasy Kit (QIAGEN). The SABiosciences RT2 First Strand Kit was used to reverse transcribe the mRNA to cDNA. cDNA samples were coded to allow for the experimenter to blind to the treatment group in the following steps. RT-PCR was then performed using the cDNA as template in a SYBR green Universal PCR Master Mix mixture. The primers included Mouse Gapdh (GAPDH) as Endogenous Control, Mouse Brevican (Mm00435249_m1), Mouse Aggrecan(Mm00803077_m1), and Mouse Neurocan (Mm00496902_m1). The plate was run in the Applied Biosystems 7500 Fast Real-Time PCR System under the Standard 7500 run mode (one cycle 50.0°C, 2 min; one cycle 95.0°C, 10 min; 40 cycles 95.0°C, 15 s and 60°C, 1 min with fluorescence measured during 60°C step). Data were then analyzed using the 2−ΔΔCT method(Livak and Schmittgen, 2001). All collected data were normalized to the Home Cage group, and statistical analysis involved ANOVA on the fold change values with Bonferroni post hoc correction.
Immunohistochemistry for WFA
In Experiment 4 (Figure 4), subjects were anesthetized with ketamine-domitor 4hrs or 24 hours after fear conditioning and transcardially perfused with 0.1M PBS followed by 4% PFA. Brains were dissected out and stored in 4% PFA (24 hours) followed by 30% sucrose in 0.1M PBS (72 hours). Brains were sectioned on a Leica microtome at 50um thickness and sections were stored in 0.1MPBS. Every eighth section was processed for WFA immunoreactivity. After extensive washing, sections were incubated overnight at 4 °C in biotiny lated WFA (1:500; Vector labs) or anti-NeuN antibody (1:500, abcam) or anti-Parvalbumin antibody (1:500, Sigma) in PBS and 0.1% Triton X-100. After three washes in PBS, tissue sections were either visualized using VectaStain ABC kit (Vector Laboratories) and developed in DAB peroxidase substrate (Sigma) or exposed to fluorescent secondary antibodies; streptavidin, Texas Red conjugate or Alexa Fluor 488 conjugate (Life technologies). Sections were mounted on Fisherbrand electrostatic slides and coverslipped.
Quantitation of WFA positive cell numbers and area
Cells surrounded by WFA staining were counted as WFA positive. Cell numbers within a constant grid area kept constant within sections and placed in the auditory cortex, were quantified in Image J (NIH). Percentage area occupied by nets was measured in Image J within a constant grid area in the auditory cortex kept constant between sections. All cell counts and measurements were performed blind to treatment groups. Cell counts or percentage area occupied by PNNs were obtained from 2–3 sections which were then averaged to attain a single value per animal that was used in statistical analyses.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical software Graphpad Prism 6.1 was used for all statistical analyses. Data were analyzed using the one-way ANOVA (mRNA and immunohistochemistry) or two-way repeated measures ANOVA (fear conditioning and fear expression) for CS trial and treatment over time. This was followed by Tukey’s post hoc analyses or Bonferroni correction with P<0.05. Statistical assumptions of independence and equal variance between groups were met in all experiments. The numbers (n) of animals or samples in Experiments 1–7 have been listed in the Results section of the manuscript. Specifically, ‘n’ refers to the number of mice that underwent surgeries and behavioral testing in Experiments 1,2,5,6 and 7. In experiments 3 and 4, ‘n’ refers to the number of mice from which tissue punches (for RT-PCR analysis) or brain slices (for immunohistochemistry analysis) were obtained. The numbers of animals planned for each experiment was based on previously demonstrated numbers that have been sufficient to reveal group differences for the expected effect size (Gafford et al., 2012; Mahan et al., 2012). One data point from the cell count and area of stain analysis was excluded as an outlier based on the Grubbs’ test in Graphpad. No data points were excluded from any other experiments.
Supplementary Material
Highlights.
Auditory cortex activity after auditory fear conditioning is necessary for learning
Removal of PNNs from the auditory cortex of adult mice decreases fear learning
Regrowth of PNNs restores the ability to learn new memories
Temporal regulation of PNNs occurs in response to fear learning
Acknowledgments
The authors thank the staff of the Yerkes Neuroscience vivarium for animal husbandry and veterinary support. This work was supported by NIH R21MH102191 grant to KJR and RCL, NIH R01DC008343 grant to RCL, and was funded in part by ORIP/OD P51OD011132 (formerly NCRR P51RR000165). The authors would like to thank Dr. Dennis Choi and Dr. Orion Keifer who provided training in stereotaxic surgery as well as Dr. Mallory Bowers who assisted with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
SBB, VAG, JB, HSA and NKD conducted the experiments. SBB, RCL and KJR designed the experiments and wrote the paper.
Disclosures: The authors have nothing to disclose.
References
- Aizenberg M, Geffen MN. Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nat Neurosci. 2013;16:994–996. doi: 10.1038/nn.3443. [DOI] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes, Brain and Behavior. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biology. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Avram S, Shaposhnikov S, Buiu C, Mernea M. Chondroitin Sulfate Proteoglycans: Structure-Function Relationship with Implication in Neural Development and Brain Disorders. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/642798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Ortiz JA, Oppenheimer JD, Wright BA. Learning two things at once: Differential constraints on the acquisition and consolidation of perceptual learning. Neuroscience. 2010;165:436–444. doi: 10.1016/j.neuroscience.2009.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the Developing Brain: New Conceptual Insights for Old Proteins. Physiological Reviews. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: Potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophrenia Research. 2015;167:18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. European Journal of Neuroscience. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biological Psychiatry. 2015;78:E15–E27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JCF, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Celio MR, Blumcke I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain research Brain research reviews. 1994;19:128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends in Neurosciences. 2012;35:607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day Jeremy J, Sweatt JÂD. Epigenetic Mechanisms in Cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G, Bozzi Y, Caleo M, Cancedda L. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nature Neuroscience. 2015;18 doi: 10.1038/nn.3890. 87-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSorbo A, Wilson GN, Bacik S, Hoxha Z, Biada JM, Mickley GA. Time-dependent retrograde amnesic effects of muscimol on conditioned taste aversion extinction. Pharmacology, biochemistry, and behavior. 2009;92:319–326. doi: 10.1016/j.pbb.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck SM, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Experimental neurology. 2015;269:169–187. doi: 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Fawcett J. Progress in Brain Research (Elsevier) 2009. Molecular control of brain plasticity and repair; pp. 501–509. [DOI] [PubMed] [Google Scholar]
- Fawcett JW. Chapter 10 - The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. In: Numa Dancause SN, Serge R, editors. Progress in Brain Research. Elsevier; 2015. pp. 213–226. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Guo J-D, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Kwok JCF, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. European Journal of Neuroscience. 2008;27:1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal Nets Protect Fear Memories from Erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and Auditory Fear Conditioning are Mediated by the Lateral, Basal, and Central Amygdaloid Nuclei in Rats. Learning & Memory. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso A, Cambiaghi M, Concina G, Sacco T, Sacchetti B. Auditory cortex involvement in emotional learning and memory. Neuroscience. 2015;299:45–55. doi: 10.1016/j.neuroscience.2015.04.068. [DOI] [PubMed] [Google Scholar]
- Happel MFK, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proceedings of the National Academy of Sciences. 2014;111:2800–2805. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Matthews A, Gross C, Mappus RC, Gollnick C, Swanson A, Bassell GJ, Liu RC. Arc/Arg3.1 mRNA expression reveals a subcellular trace of prior sound exposure in adult primary auditory cortex. Neuroscience. 2011;181:117–126. doi: 10.1016/j.neuroscience.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LRM, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends in Neurosciences. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Johansen Joshua P, Cain Christopher K, Ostroff Linnaea E, LeDoux Joseph E. Molecular Mechanisms of Fear Learning and Memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GR. Muscimol as an Ionotropic GABA Receptor Agonist. Neurochem Res. 2014;39:1942–1947. doi: 10.1007/s11064-014-1245-y. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kinden Lensjø K, Elle Lepperød M, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. The Journal of Neuroscience. 2016 doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Heizmann CW. SELECTIVE STAINING OF A POPULATION OF PARVALBUMIN-CONTAINING GABAERGIC NEURONS IN THE RAT CEREBRAL-CORTEX BY LECTINS WITH SPECIFIC AFFINITY FOR TERMINAL N-ACETYLGALACTOSAMINE. Brain Research. 1989;483:158–163. doi: 10.1016/0006-8993(89)90048-6. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Letzkus Johannes J, Wolff Steffen BE, Lüthi A. Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Levy AD, Omar MH, Koleske AJ. Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Frontiers in Neuroanatomy. 2014;8 doi: 10.3389/fnana.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C, Brooks JM, Chen J, Su J, Fox MA. Cell-specific and developmental expression of lectican-cleaving proteases in mouse hippocampus and neocortex. Journal of Comparative Neurology. 2015;523:629–648. doi: 10.1002/cne.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: A symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu H, Yu T, Yao J, Zhao C, Yin ZQ. Expression of Perineuronal Nets, Parvalbumin and Protein Tyrosine Phosphatase σ in the Rat Visual Cortex During Development and After BFD. Current Eye Research. 2013;38:1083–1094. doi: 10.3109/02713683.2013.803287. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu J-H, Worley PF, Ressler KJ. Epigenetic Modulation of Homer1a Transcription Regulation in Amygdala and Hippocampus with Pavlovian Fear Conditioning. The Journal of Neuroscience. 2012;32:4651. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and Memory Storage. Cold Spring Harbor Perspectives in Biology. 2012;4 doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. The Journal of Physiology. 2010;588:107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YA, Giger RJ. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends in Neurosciences. 2013;36:363–373. doi: 10.1016/j.tins.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends in Neurosciences. 2012;35:135–143. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EM, Morishita H. Regulating Critical Period Plasticity: Insight from the Visual System to Fear Circuitry for Therapeutic Interventions. Frontiers in Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi MA, Crowe MJ. Matrix metalloproteinases and proteoglycans in axonal regeneration. Experimental neurology. 2007;204:496–511. doi: 10.1016/j.expneurol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of Ocular Dominance Plasticity in the Adult Visual Cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pollock E, Everest M, Brown A, Poulter MO. Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis. 2014;70:21–31. doi: 10.1016/j.nbd.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Qin Y-L, McNaughton BL, Skaggs WE, Barnes CA. Memory Reprocessing in Corticocortical and Hippocampocortical Neuronal Ensembles. Philosophical Transactions: Biological Sciences. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing Fear Memories with Extinction Training. The Journal of Neuroscience. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. The Journal of Neuroscience. 1992;12:4501–4509. doi: 10.1523/JNEUROSCI.12-11-04501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, Bussey TJ, Fawcett JW, Pizzorusso T, Saksida LM. Depletion of Perineuronal Nets Enhances Recognition Memory and Long-Term Depression in the Perirhinal Cortex. The Journal of Neuroscience. 2013;33:7057–7065. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya-Vetencourt JF, Medinin P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nature Neuroscience. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes & Development. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Yamagishi N, Werner B, Goda N, Kawato M, Watanabe T. Task-specific disruption of perceptual learning. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14895–14900. doi: 10.1073/pnas.0505765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkov O, Andjus P, Radenovic L, Soriano E, Dityatev A. Chapter 3 - Neural ECM molecules in synaptic plasticity, learning, and memory. In: Alexander Dityatev BWH, Asla P, editors. Progress in Brain Research. Elsevier; 2014. pp. 53–80. [DOI] [PubMed] [Google Scholar]
- Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends in Neurosciences. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of Perineuronal Nets in the Medial Prefrontal Cortex Impairs the Acquisition and Reconsolidation of a Cocaine-Induced Conditioned Place Preference Memory. The Journal of Neuroscience. 2015;35:4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song EY, Boatman JA, Jung MW, Kim JJ. Auditory cortex is important in the extinction of two different tone-based conditioned fear memories in rats. Frontiers in Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag M, Blosa M, Schmidt S, Rübsamen R, Morawski M. Perineuronal nets in the auditory system. Hearing Research. 2015 doi: 10.1016/j.heares.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Sultan FA, Day JJ. Epigenetic mechanisms in memory and synaptic function. Epigenomics. 2011;3:157–181. doi: 10.2217/epi.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JC, Heise C, Franken G, Singh J, Schweitzer B, Seidenbecher CI, Frischknecht R. Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philosophical Transactions of the Royal Society B-Biological Sciences. 2014:369. doi: 10.1098/rstb.2013.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of Conditioned Fear Extinction by Systemic Administration or Intra-Amygdala Infusions of d-Cycloserine as Assessed with Fear-Potentiated Startle in Rats. The Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst ES, Weisenhorn DMV, Rathjen FG, Riederer BM, Lambert S, Celio MR. Temporal and spatial appearance of the membrane cytoskeleton and perineuronal nets in the rat neocortex. Neuroscience Letters. 1996;209:173–176. doi: 10.1016/0304-3940(96)12643-4. [DOI] [PubMed] [Google Scholar]
- Wright BA, Sabin AT, Zhang Y, Marrone N, Fitzgerald MB. Enhancing Perceptual Learning by Combining Practice with Periods of Additional Sensory Stimulation. The Journal of Neuroscience. 2010;30:12868–12877. doi: 10.1523/JNEUROSCI.0487-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y-X, Xue L-F, Liu J-F, He J, Deng J-H, Sun S-C, Han H-B, Luo Y-X, Xu L-Z, Wu P, Lu L. Depletion of Perineuronal Nets in the Amygdala to Enhance the Erasure of Drug Memories. The Journal of Neuroscience. 2014;34:6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. CMLS, Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.