Abstract
We have summarized the latest findings on markers for progression of immunoglobulin (Ig)A nephropathy (IgAN), the most common primary glomerulonephritis with a high prevalence among end-stage renal disease (ESRD) patients. The clinical predictors of renal outcome in IgAN nephropathy, such as proteinuria, hypertension, and decreased estimated glomerular filtration rate (eGFR) at the time of the diagnosis, are well known. The Oxford classification of IgA nephropathy N identified four types of histological lesions (known as the MEST score) associated with the development of end-stage renal diseases ESRD and/or a 50 % reduction in eGFR. In addition, the role of genetic risk factors associated with IgAN nephropathy is being elucidated by genome-wide association studies, with 18 identified risk alleles described. Recently, biomarkers in serum (galactose-deficient IgA1, IgA/IgG autoantibodies against galactose-deficient IgA1, and soluble CD 89-IgA complexes) and urine (soluble transferrin receptor, interleukin-6/epidermal growth factor, fractalkine, laminin G-like 3 peptide, κ light chains, and mannan-binding lectin) have been identified. Some of these biomarkers may represent candidates for the development of noninvasive diagnostic tests, that would be useful for detection of subclinical disease activity, monitoring disease progression, assessment of treatment, and at the same time circumventing the complications associated with renal biopsies. These advances, along with future disease-specific therapy, will be helpful in improving the treatment effectiveness, prognosis, and the quality of life in connection with IgAN.
Keywords: IgA nephropathy, biomarkers, renal biopsy, end-stage renal disease
INTRODUCTION AND DIAGNOSIS AND PATHOGENESIS OF IgAN
Immunoglobulin (Ig)A nephropathy (IgAN) is the most common primary glomerulonephritis, leading to end-stage renal disease (ESRD) in about 30–50% % of patients, necessitating renal replacement therapy, i.e., dialysis or transplantation [1, 2]. Diagnosis of IgAN requires evaluation of renal biopsy specimens and detection of IgA dominant or co-dominant deposits, frequently concomitant with mesangial/endocapillary hypercellularity and fibrosis, as outlined in the Oxford classification [3, 4]. Renal biopsy is an invasive procedure and till now has served as a key instrument for diagnosis of IgAN and other glomerulonephritides. However, noninvasive markers of IgAN with diagnostic and prognostic significance would be valuable to complement the currently used clinical and laboratory approaches. In this review, we briefly summarize the current state of the art in this field.
Diagnosis of IgAN depends on the demonstration of mesangial IgA1-dominant or co-dominant immunodeposits (by immunofluorescence or by immunohistochemistry). IgG and/or IgM co-deposits are also detected in about 50% % of cases. Complement C3 is detected in more than 90% % of cases of primary IgAN, whereas C1q is absent. Clinically, microscopic or macroscopic hematuria with or without proteinuria (usually < 2 g/24 h) [5] is frequently observed in patients with IgAN at the time of diagnosis. The clinical presentation of macroscopic hematuria is commonly associated with an upper-respiratory-tract infection. Some patients already have renal impairment and hypertension at initial presentation.
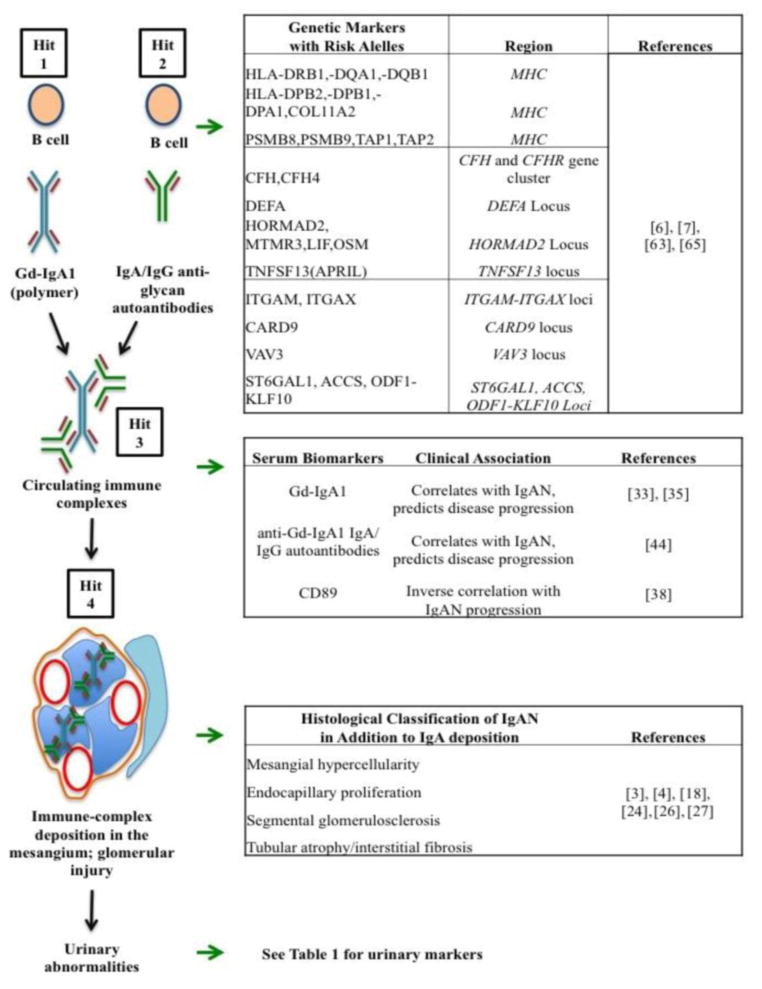
IgAN has been defined as an autoimmune disease that has a multi-hit pathobiological process with genetic and environmental contributing factors (Figure 1) [2, 6, 7]. Aberrantly glycosylated forms of IgA1 with galactose-deficient O-glycans (galactose-deficient IgA1; Gd-IgA1) play a key role in the pathogenesis of IgAN [8]. Gd-IgA1 molecules are recognized by antiglycan autoantibodies of IgG and/or IgA1 isotype, resulting in the formation of circulating immune complexes [9]. These complexes are not efficiently cleared via the normal hepatic catabolism, resulting in glomerular deposition and mesangial-cell activation, thus inciting the first step of glomerular injury [10]. The glomerular IgA1-containing immune complexes cause local activation of the complement system [11–14], proliferation of mesangial cells, production of extracellular matrix and cytokines (e.g., tumor necrosis factor-α, and transforming growth factor-β) [15], which could alter podocyte gene expression and glomerular permeability. This mesangio-podocyte injury might explain proteinuria and tubulointerstitial changes in IgAN.
Figure 1.
Summary of biomarkers related to the progression of IgAN (based on a multi-hit pathogenesis scheme of IgAN [66]). The scheme shows the hypothesis of IgAN, wherein multiple conditions (hits) are required for disease development. “Hit 1” is elevated levels of circulating Gd-IgA1, which is a necessary but not sufficient condition for disease development. The second hit, “Hit 2” is production of autoantibodies (IgG/IgA isotype) that recognize Gd-IgA1. “Hit 3” describes formation of circulating immune complexes from Gd-IgA1 and the corresponding autoantibodies. “Hit 4” is driven by the deposition of these Gd-IgA-containing pathogenic immune complexes in the mesangium and mesangial-cell activation inciting glomerular injury. Corresponding to each part of the multi-hit hypothesis is a list of IgAN-associated markers.
CLINICAL MARKERS
The following clinical predictors of renal outcome in IgAN were have been assessed in several clinical studies at the time of diagnosis: proteinuria, hypertension, decreased estimated glomerular filtration rate (eGFR) [2, 16–17] as well as histological grading [18]. Three risk factors assessed at the time of biopsy, -24-h urinary protein excretion ≥ 1.0 g, hypertension (> 140/90 mm Hg), and severe histological lesions, - were found to be significantly associated with dialysis or death [19]. The 20-year predicted survival without the need of dialysis was 96% vs. 36% between for patients with no risk factors and vs. with three risk factors was 96 % and 36 %, respectively [19]. Notably, time-averaged proteinuria was the best metric for prognosis of IgAN [20]. Moreover, control of proteinuria over time reduced the risk of future loss of kidney function [21]. These two studies showed that assessment of proteinuria during follow-up is a more powerful independent prognostic predictor than proteinuria at the onset [22]. In addition, age, gender, family historyies, hematuria, and hypoalbuminemia were also found to be associated with progression of IgAN in some studies [23].
PATHOLOGICAL MARKERS
In 2009, the Oxford classification of IgAN was proposed and it identified four types of lesions as specific pathologic features associated with the development of ESRD and/or a 50% % reduction in eGFR: mesangial hypercellularity (M0 or M1), endocapillary proliferation (E0 or E1), segmental glomerulosclerosis (S0 or S1), and tubular atrophy/interstitial fibrosis (T0 or T1 or T2) [24]. The cCombined maximal score of this MEST classification (M1+E1+S1+T2) is 5, and renal lesions with a summing score of 2 or higher were assessed as independent risk factors for progression to ESRD (dialysis or eGFR < 15 ml/min/1.73 m2). In addition, each MEST risk factor was proposed as an independent value [24]. The MEST score was independently associated with renal outcome [25]. Pathological risk factors can be associated with other clinical risk factors, such as hypertension, proteinuria and eGFR [24]. The VALIGA study (European Validation Study of the Oxford Classification of IgA Nephropathy) (VALIGA) provided a validation of the Oxford classification in a large European cohort of IgAN patients [26].
IgG co-deposits are very common and present in around 45%–60% % of IgAN biopsies. Earlier studies showed that IgAN-circulating immune complexes containing aberrantly glycosylated IgA1 bind to mesangial cells more efficiently than uncomplexed IgA. Localization of IgA with IgG in the mesangium and glomerular capillary walls correlated with higher mesangial and endocapillary cellularity scores [27]. A small-cohort study verified that mesangial IgG deposition in patients with IgAN is was associated with more severe clinical features, including more capillary-wall IgA deposits and persistent urinary abnormalities [28]. Multivariate analysis indicated that a high initial chronicity index, erythrocyturia, and mesangial IgG co-deposits were independent determinants of disease progression. These data indicate that IgG co-deposits may be involved in the development of mesangial proliferative changes. Staining for complement factors, such as C3, C4d, C5b-9, and mannan-binding lectin, may provide additional insight into the disease mechanism [28–29].
BIOMARKERS IN THE SERUM AND URINE
Recently identified serum and urine biomarkers (Figure 1, Table 1I) are related to the pathogenesis of IgAN (Gd-IgA1, anti-glycan-IgG and IgA anti-Gd-IgA1 autoantibodies [30–35], soluble CD89 [sCD89] [36–39], and urinary soluble transferrin receptor [sTfR] [40]) or to the degree of renal damage in IgAN (interleukin-6/epidermal growth factor [IL-6/EGF], or monocyte chemotactic protein-1/epidermal growth factor ratio [MCP-1/EGF], or kidney injury molecule-1 [KIM-1] [41–42]).
Table 1.I.
Biomarkers of IgAN in the urine and their clinical associations
| Biomarkers of IgAN in the urine | Clinical association | Reference |
|---|---|---|
| Soluble transferrin receptor (sTfR) | Higher in patients with IgAN or HSP, correlated with proteinuria levels | [40] |
| α1- and β2-microglobulin | Correlates with proteinuria | [42] |
| Kidney injury molecule-1 (KIM-1) | Correlates with proteinuria, combined with serum creatinine to correlates with poor renal outcome | [41] |
| Interleukin-6/epidermal growth factor (IL-6/EGF) | Marker of IgAN progression, correlates with renal outcome | [53] |
| Epidermal growth factor/monocyte chemotactic protein-1 (EGF/MCP-1) | Correlates with histologic severity and renal prognosis | [54] |
| Fractalkine | Correlates with pathogenesis of immune complex-mediated glomerulonephritis | [55] |
| Laminin G-like 3 peptide (perlecan) | Decreased levels inversely correlate with histological features | [56] |
| Free kappa light chains | Decreased levels inversely correlate with histological features | [56] |
| Uromodulin | Increased levels predict IgAN | [57] |
| α-1 antitrypsin | Increased levels in the urine associate with nephrotic syndrome | [58] |
| Podocalyxin | Associates with histologic kidney injury | [59] |
| Mannose-binding lectin | Correlates with renal function and proteinuria | [61] |
| C4a desArg peptide | Associates with severe histological changes in IgAN | [62] |
IgAN, immoglobulin A nephropathy; HSP, Henoch-Schönlein purpura.
Potential diagnostic tests for IgAN include measurement of serum levels of Gd-IgA1 and/or anti-Gd-IgA1 IgA/IgG autoantibodies [30–32]. Moldoveanu et al. [33] showed increased serum levels of Gd-IgA1 in IgAN patients compared to healthy controls of Caucasian ancestry, and other studies showed similar results for patients of Asian and African-American ancestry [34]. In children, including Caucasians and African-Americans, serum levels of Gd-IgA1 were elevated, but not associated with proteinuria.
A strong association between the serum level of Gd-IgA1 and progression of IgAN has also been shown [35]. Recent data showed [43] the potential for lectin-independent Gd-IgA1-specific assessment using a monoclonal antibody, KM55, which could facilitate the use of clinical measurement of this biomarker [43]. However, this assay is has yet to be validated in other cohorts and precise specificities of the antibody are to behave not yet been elucidated.
High levels of Gd-IgA1-specific IgG and IgA autoantibodies predict disease progression of IgAN [44]. It was suggested that biomarkers, such as serum levels of Gd-IgA1 and the corresponding autoantibodies may be useful for monitoring/predicting the progression of disease and/or evaluating responses to therapy [45–46].
The role of micro RNA (miRNA) in patients with IgAN has been investigated [47]. For example, abnormal miR-148b expression is associated with elevated production of Gd-IgA1 in peripheral blood mononuclear cells of IgAN patients [48].
Another study showed down-regulated urinary expression of miR-3613-3p in patients with IgAN [49]. Moreover, urinary levels of both miR-3613-3p and miR-4668-5p correlated with disease severity [49] and miR-29b-3p is down regulated in renal tissue of IgAN patients [50]. miR-29b-3p down-regulation causesd CDK6 overexpression, that promotes NF-κB signal by phosphorylating p65 which might enhance inflammation in IgAN [50]. Moreover, miR-223 expression in glomerular endothelial cells was associated with glomerular endothelial cell activation and was proposed as a noninvasive marker for evaluating the severity of IgAN [51]. It is not clear at this time, whether assessment of miRNA expression profile(s) may be used clinically and how predictive it may be of disease progression.
In addition, it was hypothesized that elevated soluble IgA receptor CD89 (sCD89) may be protective against progressive renal injury in IgAN [38]. However, this clinical observation is in contrast with a previously postulated pathogenic role of sCD89-IgA1 complexes [37]. Potentially, after clarification of the contrasting data, sCD89 may become a prognostic marker of the risk of ESRD or recurrent disease after transplantation, as recently published [52].
For potential urinary markers, median levels of urinary soluble transferrin receptor (sTfR) were reported to be elevated in patients with active IgAN and a related disease, Henoch-Schöoenlein purpura nephritis [40]. Urinary KIM-1 correlated with proteinuria, but not with β2-microglobulin [41–42]. Urinary excretion of KIM-1 above median was associated with poor renal outcome in patients only with serum creatinine > 135 μmol/Ll. This study showed that urinary excretion of KIM-1 was a better predictor of renal outcome than proteinuria [41]. Even for patients with clinically mild IgAN (normotension, normal renal function and proteinuria < 1.0 g/24 h), high urinary KIM-1 can be predictive of severe morphological changes found in kidney biopsies [29].
The uUrinary IL-6/EGF ratio was has been found to be a marker of the progression of IgAN [53]. Urinary IL-6/EGF ratio It was related to the severity of the disease and also predicted renal outcome. The ratio of EGF/MCP-1 in the urine was related to the severity of histologic lesions and significantly predicted renal prognosis in 132 patients with IgAN [54]. Urinary concentrations of another proinflammatory chemokines, fractalkine, and MCP-1 showed a significant inverse correlation with eGFR [55], and may be useful for predicting the activity of IgAN [55].
Decreased urinary levels of laminin G-like 3 peptide (perlecan) and free κ light chains were observed in patients with IgAN, and the levels of both inversely correlated with severity of clinical and histological features of IgAN patients [56].
Another study [57] identified a fragment of uromodulin in urine samples from patients with IgAN compared to healthy controls and patients with other glomerulonephritides [57]. Other urinary markers, such as α-1 antitrypsin and podocalyxin, were have been detected in patients with severe IgAN [58–59]. It was assumed that urinary podocalyxin was associated with severity of active glomerular injury in patients with glomerular diseases including IgAN [59].
A number of studies were have been focused on the analyses of urinary peptidome and its changes in chronic renal diseases, including IgAN [60]. Future studies will determine whether there are any urinary peptides of prognostic significance.
Complement factors and their fragments may serve as biomarkers of IgAN in serum, urine, or renal tissue [11–12]. The level of urinary mannan-binding lectin was significantly associated with renal function and proteinuria in a Chinese study of 162 patients with IgAN [61]. A low level of urinary mannan-binding lectin at the time of renal biopsy was associated with better clinical outcome and histological renal findings [61]. Another study found an association of urinary peptide fragment, complement component C4a desArg, with histological severe forms of IgAN [62].
GENETIC MARKERS
The role of genetic factors for susceptibility to IgAN has been demonstrated [63]. Genome-wide association studies in IgAN patients identified 18 risk loci associated with IgAN [64, 65]. Future assays, derived from a single-nucleotide polymorphism (SNP)-based risk score and the number of risk-associated alleles, may offer a valuable tool to predict progression of disease in addition to explaining the risk of disease and age of onset (Figure 1).
CONCLUSIONS
There are many possible candidate biomarkers of IgAN that, however, need to be validated in larger cohorts of patients and controls before they can be introduced into clinical practice. The development of noninvasive diagnostic tests would also be useful for evaluation of disease activity, monitoring disease progression and assessment of treatment effectiveness. Some IgAN biomarkers are linked to basic pathways of disease pathogenesis, as summarized in Figure 1 and Table 1I. For example, IgAN patients with high serum levels of Gd-IgA1 and/or IgG and IgA autoantibodies, have worse prognosis, and, consequently, will need close follow-up and more aggressive treatment to control clinical risk factors (proteinuria and hypertension). Moreover, some markers, such as anti-Gd-IgA1 autoantibodies, might represent a disease-specific marker and potential therapeutic target [44]. The patients with substantial disease activity (based on the clinical and histological findings) could benefit with from an early initiation of immunosuppressive treatment to reduce the incidence of ESRD. These clinical improvements will ultimately reduce the costs otherwise needed to cover renal replacement therapy (hemodialysis, peritoneal dialysis, and kidney transplantation).
Acknowledgments
The authors CR, QB, and JN have been supported in part by grants DK106341, DK079337, DK078244, DK082753, DK099228, GM098539 from the National Institutes of Health and a gift from the IGA Nephropathy Foundation of America and the authors DM and VT by grant LH15168 from the Ministry of Education, Youth and Sports of the Czech Republic.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9:e91756. doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Cattran DC, Coppo R, Cook HT, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 4.Coppo R, Troyanov S, Camilla R, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–927. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- 5.Feehally J, Floege J. IgA nephropathy and Henoch-Schönlein nephritis. In: Feehally J, Floege J, Johnson RJ, editors. Comprehensive clinical nephrology. 3. Mosby; 2007. pp. 253–264. [Google Scholar]
- 6.Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8:e1002765. doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 9.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak J, Raskova Kafkova L, Suzuki H, et al. IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant. 2011;26:3451–3457. doi: 10.1093/ndt/gfr448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maillard N, Wyatt RJ, Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503–1512. doi: 10.1681/ASN.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt R, Stahl AL, Olin Al, et al. The combined role of galactose-deficient IgA1 and streptococcal IgA-binding M protein in inducing IL-6 and C3 secretion from human mesangial cells: implications for IgA nephropathy. J Immunol. 2014;193:317–326. doi: 10.4049/jimmunol.1302249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daha MR, van Kooten C. Role of complement in IgA nephropathy. J Nephrol. 2016;29:1–4. doi: 10.1007/s40620-015-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H, Ohsawa I, Kodama F, et al. Fluctuation of serum C3 levels reflects disease activity and metabolic background in patients with IgAnephropathy. J Nephrol. 2013;26:708–15. doi: 10.5301/jn.5000278. [DOI] [PubMed] [Google Scholar]
- 15.Lai KN, Leung JC, Chan LY, et al. Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol. 2008;294:F945–955. doi: 10.1152/ajprenal.00423.2007. [DOI] [PubMed] [Google Scholar]
- 16.Maixnerova D, Bauerova L, Skibova J, et al. The retrospective analysis of 343 Czech patients with IgA nephropathy–one centre experience. Nephrol Dial Transplant. 2012;27:1492–1498. doi: 10.1093/ndt/gfr482. [DOI] [PubMed] [Google Scholar]
- 17.Reich HN, Troyanov S, Scholey JW, et al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 18.Alamartine E, Sauron C, Laurent B, et al. The use of Oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol. 2011;6:2384–2388. doi: 10.2215/CJN.01170211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–61. doi: 10.1681/ASN.2010040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbour SJ, Cattran DC, Espino-Hernandez G, et al. Identifying the ideal metric of proteinuria as a predictor of renal outcome in idiopathic glomerulonephritis. Kidney Int. 2015;88:1392–1401. doi: 10.1038/ki.2015.241. [DOI] [PubMed] [Google Scholar]
- 21.Barbour SJ, Reich HN. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis. 2012;59:865–73. doi: 10.1053/j.ajkd.2012.02.326. [DOI] [PubMed] [Google Scholar]
- 22.Coppo R, D’Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18:503–512. [PubMed] [Google Scholar]
- 23.Kaartinen K, Syrjanen J, Porsti I, et al. Inflammatory markers and the progression of IgA glomerulonephritis. Nephrol Dial Transplant. 2008;23:1285–1290. doi: 10.1093/ndt/gfm782. [DOI] [PubMed] [Google Scholar]
- 24.Roberts IS, Cook HT, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 25.Barbour SJ, Espino-Hernandez G, Reich HN, et al. Oxford Derivation, North American Validation and VALIGA Consortia. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int. 2016;89:1671–75. doi: 10.1038/ki.2015.322. [DOI] [PubMed] [Google Scholar]
- 26.Coppo R, Troyanov S, Bellur S, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellur SS, Troyanov S, Cook HT, et al. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- 28.Wada Y, Ogata H, Takeshige Y, et al. Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin Exp Nephrol. 2013;17:73–82. doi: 10.1007/s10157-012-0660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu PC, Wei L, Shang WY, et al. Urinary kidney injury molecule-1 is related to pathologic involvement in IgA nephropathy with normotension, normal renal function and mild proteinuria. BMC Nephrol. 2014;7:107. doi: 10.1186/1471-2369-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanagawa H, Suzuki H, Suzuki Y, et al. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 2014;9:e98081. doi: 10.1371/journal.pone.0098081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakata J, Suzuki Y, Suzuki H, et al. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;9:e89707. doi: 10.1371/journal.pone.0089707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Raska M, Yamada K, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289:5330–5339. doi: 10.1074/jbc.M113.512277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moldoveanu Z, Wyatt RJ, Lee J, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 34.Hastings MC, Moldoveanu Z, Julian BA, et al. Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability. Clin J Am Soc Nephrol. 2010;5:2069–2074. doi: 10.2215/CJN.03270410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao N, Hou P, Lv J, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790–796. doi: 10.1038/ki.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Launay P, Grossetête B, Arcos-Fajardo M, et al. Fcα receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-IgA complexes in patients and CD89 transgenic mice. J Exp Med. 2000;191:1999–2009. doi: 10.1084/jem.191.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793–806. doi: 10.1084/jem.20112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vuong MT, Hahn-Zoric M, Lundberg S, et al. Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int. 2010;78:1281–1287. doi: 10.1038/ki.2010.314. [DOI] [PubMed] [Google Scholar]
- 39.Lechner SM, Papista C, Chemouny JM, et al. Role of IgA receptors in the pathogenesis of IgA nephropathy. J Nephrol. 2016;29:5–11. doi: 10.1007/s40620-015-0246-5. [DOI] [PubMed] [Google Scholar]
- 40.Delanghe SE, Speeckaert MM, Segers H, et al. Soluble transferrin receptor in urine, a new biomarker for IgA nephropathy and Henoch-Schönlein purpura nephritis. Clin Biochem. 2013;46:591–597. doi: 10.1016/j.clinbiochem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Peters HP, Waanders F, Meijer E, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:3581–3588. doi: 10.1093/ndt/gfr135. [DOI] [PubMed] [Google Scholar]
- 42.Peters HP, van den Brand JA, Wetzels JF. Urinary excretion of low-molecular-weight proteins as prognostic markers in IgA nephropathy. Neth J Med. 2009;67:54–61. [PubMed] [Google Scholar]
- 43.Yasutake J, Suzuki Y, Suzuki H, et al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant. 2015;30:1315–1321. doi: 10.1093/ndt/gfv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthoux F, Suzuki H, Thibaudin L, et al. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23:1579–1587. doi: 10.1681/ASN.2012010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hastings MC, Moldoveanu Z, Suzuki H, et al. Biomarkers in IgA nephropathy: relationship to pathogenetic hits. Expert Opin Med Diagn. 2013;7:615–27. doi: 10.1517/17530059.2013.856878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caliskan Y, Kiryluk K. Novel biomarkers in glomerular disease. Adv Chronic Kidney Dis. 2014;21:205–216. doi: 10.1053/j.ackd.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szeto CC, Li PK. Micro RNAs in IgA nephropathy. Nat Rev Nephrol. 2014;10:249–256. doi: 10.1038/nrneph.2014.50. [DOI] [PubMed] [Google Scholar]
- 48.Serino G, Sallustio F, Cox SN, et al. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012;23:814–824. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N, Bu R, Duan Z, et al. Profiling and initial validation of urinary microRNAs as biomarkers in IgA nephropathy. PeerJ. 2015;3:e990. doi: 10.7717/peerj.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li-Na Xing, Wang H, Yin PH, et al. Reduced mir-29b-3p expression up-regulate CDK6 and contributes to IgA nephropathy. Int J Clin Exp Med. 2014;7:5275–5281. [PMC free article] [PubMed] [Google Scholar]
- 51.Bao H, Chen H, Zhu X, et al. MiR 223 downregulation promotes glomerular endothelial cell activation by upregulating importin α4 and α5 in IgA nephropathy. Kidney Int. 2014;85:624–635. doi: 10.1038/ki.2013.469. [DOI] [PubMed] [Google Scholar]
- 52.Berthelot L, Robert T, Vuiblet V, et al. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int. 2015;88:815–822. doi: 10.1038/ki.2015.158. [DOI] [PubMed] [Google Scholar]
- 53.Ranieri E, Gesualdo L, Petrarulo F, et al. Urinary IL-6/EGF ratio: a useful prognostic marker for the progression of renal damage in IgA nephropathy. Kidney Int. 1996;50:1990–2001. doi: 10.1038/ki.1996.521. [DOI] [PubMed] [Google Scholar]
- 54.Torres DD, Rossini M, Manno C, et al. The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008;73:327–333. doi: 10.1038/sj.ki.5002621. [DOI] [PubMed] [Google Scholar]
- 55.Aizawa T, Imaizumi T, Tsuruga K, et al. Urinary fractalkine and monocyte chemoattractant protein-1 as possible predictors of disease activity of childhood glomerulonephritis. Tohoku J Exp Med. 2013;231:265–270. doi: 10.1620/tjem.231.265. [DOI] [PubMed] [Google Scholar]
- 56.Rocchetti MT, Papale M, d’Apollo AM, et al. Association of urinary laminin G-like 3 and free kappa light chains with disease activity and histological injury in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8:1115–1125. doi: 10.2215/CJN.05950612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Wang N, Wang J, et al. Identification of a uromodulin fragment for diagnosis of IgA nephropathy. Rapid Commun Mass Spectrom. 2010;24:1971–1978. doi: 10.1002/rcm.4601. [DOI] [PubMed] [Google Scholar]
- 58.Candiano G, Musante L, Bruschi M, et al. Repetitive fragmentation products of albumin and alpha1-antitrypsin in glomerular diseases associated with nephrotic syndrome. J Am Soc Nephrol. 2006;17:3139–3148. doi: 10.1681/ASN.2006050486. [DOI] [PubMed] [Google Scholar]
- 59.Asao R, Asanuma K, Kodama F, et al. Relationships between levels of urinary podocalyxin, number of urinary podocytes, and histologic injury in adult patients with IgA nephropathy. Clin J Am Soc Nephrol. 2012;7:1385–1393. doi: 10.2215/CJN.08110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Good DM, Zürbig P, Argiles A, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu LL, Jiang Y, Wang LN, et al. Urinary mannose-binding lectin is a biomarker for predicting the progression of immunoglobulin (Ig) A nephropathy. Clin Exp Immunol. 2012;169:148–155. doi: 10.1111/j.1365-2249.2012.04604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sogabe A, Uto H, Kanmura S, et al. Correlation of serum levels of complement C4a desArg with pathologically estimated severity of glomerular lesions and mesangial hypercellularity scores in patients with IgA nephropathy. Int J Mol Med. 2013;32:307–314. doi: 10.3892/ijmm.2013.1390. [DOI] [PubMed] [Google Scholar]
- 63.Gharavi AG, Kiryluk K, Choi M, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43:321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46(11):1187–96. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M, Foo JN, Wang JQ, et al. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun. 2015;6:7270. doi: 10.1038/ncomms8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]