In the first nationwide follow-up of physicians who perform fluoroscopy-guided interventional procedures, there was no excess mortality (except leukemia, based on small numbers, in early medical school graduates), but confirmation is needed in studies with individual doses and work histories, lifestyle and other explanatory factors, and extended follow-up.
Abstract
Purpose
To compare total and cause-specific mortality rates between physicians likely to have performed fluoroscopy-guided interventional (FGI) procedures (referred to as FGI MDs) and psychiatrists to determine if any differences are consistent with known radiation risks.
Materials and Methods
Mortality risks were compared in nationwide cohorts of 45 634 FGI MDs and 64 401 psychiatrists. Cause of death was ascertained from the National Death Index. Poisson regression was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for FGI MDs versus psychiatrists, with adjustment (via stratification) for year of birth and attained age.
Results
During follow-up (1979–2008), 3506 FGI MDs (86 women) and 7814 psychiatrists (507 women) died. Compared with psychiatrists, FGI MDs had lower total (men: RR, 0.80 [95% CI: 0.77, 0.83]; women: RR, 0.80 [95% CI: 0.63, 1.00]) and cancer (men: RR, 0.92 [95% CI: 0.85, 0.99]; women: RR, 0.83 [95% CI: 0.58, 1.18]) mortality. Mortality because of specific types of cancer, total and specific types of circulatory diseases, and other causes were not elevated in FGI MDs compared with psychiatrists. On the basis of small numbers, leukemia mortality was elevated among male FGI MDs who graduated from medical school before 1940 (RR, 3.86; 95% CI: 1.21, 12.3).
Conclusion
Overall, total deaths and deaths from specific causes were not elevated in FGI MDs compared with psychiatrists. These findings require confirmation in large cohort studies with individual doses, detailed work histories, and extended follow-up of the subjects to substantially older median age at exit.
© RSNA, 2017
Introduction
The dramatic increase in use of fluoroscopy-guided interventional (FGI) procedures since the 1960s has transformed medical practice. FGI procedures involve guidance of catheters and other small devices through blood vessels or other pathways with fluoroscopic imaging to localize and treat lesions (1,2). Numerous FGI procedures replaced surgical alternatives because of clinical and economic benefits to patients (2). The occurrence of tissue reactions (deterministic effects) from radiation exposure, such as skin burns, are uncommon in patients who undergo FGI procedures (1).
Concerns have been raised about long-term radiation-related outcomes in physicians who perform FGI procedures (FGI MDs) (3–6), including dose-related posterior subcapsular lens changes (7). Despite equipment and other improvements to decrease patient and worker radiation doses, newer FGI procedures are often more complex (8), workloads have been increasing, and dose reductions in FGI procedures have been modest or not apparent (9). Estimated annual total body effective occupational radiation exposures from FGI procedures are generally within the allowable limits (50 mSv per year in the United States [10], and 20 mSv per year recommended by the International Commission on Radiological Protection [11]), but cumulative exposures may be as high as 0.5 Gy to the brain or eye lens in the absence of adequate protection (7). In several radiation-exposed populations, cumulative moderate-to-high (≥0.2 Gy) total body effective radiation exposure was linked consistently with increased cancer risk (12), circulatory system disease, and cataracts (11). Some studies suggested that cumulative protracted low-to-moderate (<0.2 Gy) total body effective radiation exposure increased risk of leukemia (13), solid tumors (14,15), and circulatory diseases (16).
Health risks among recent medical radiation workers, including those who perform FGI procedures (17), are less well studied. Excesses of certain cancers and circulatory disease were recently described in 20 982 radiologic technologists in the United States who assisted with FGI compared with 54 795 radiologic technologists who never worked with FGI procedures (18,19). Scattered radiation from the patient is the principal source of exposure to radiologic staff in the fluoroscopy suite. FGI MDs are in closer proximity to patients than technologists, and they experience higher radiation doses than most other medical radiation workers (3). We identified a nationwide cohort of physicians in the United States who are likely to have performed FGI procedures regularly on the basis of their specialties, and a comparison cohort of psychiatrists who were unlikely to have occupational radiation exposure. We compared total and cause-specific mortality rates in these physician groups over a 30-year period to determine if any differences were consistent with known radiation risks.
Materials and Methods
Because there was no direct contact with study subject physicians, the National Institutes of Health Office of Human Subjects Research Protections determined that the study was exempt from institutional human subjects board review.
Data Source and Study Population
Nationwide cohorts of FGI MDs and psychiatrists were constructed by using the American Medical Association (AMA) Physician Masterfile, which was established in 1906 and includes all U.S. physicians, regardless of AMA membership (20). The AMA obtains information about graduates of all U.S. medical schools and creates or updates the profile for each physician practicing in the United States, regardless of whether the physician graduated from a U.S. medical school, with data from accredited residency training programs, medical specialty boards, and U.S. licensing organizations.
This AMA file lists basic demographic information, up to two medical specialties per physician, vital status, and, for deceased physicians, the date (but not cause) of death. Type of practice and specialty information is updated and confirmed through self-administered surveys sent annually to one-third of physicians. All physicians are contacted every 3 years; about 40% respond. If a physician never completes a survey, specialty assignment is based on the most recent residency training obtained from the American Association of Medical Colleges through a data sharing agreement.
We defined FGI MDs as physicians in the AMA Physician Masterfile with a primary or secondary specialty of interventional cardiology, cardiac electrophysiology, or other cardiovascular specialties; vascular and interventional radiology; and endovascular surgical neuroradiology or neuroradiology (hereafter designated neuroradiology).
We selected psychiatrists as the comparison group because these physicians do not perform FGI in their practice. The stratified random sample was selected to ensure a 1:1.5 ratio (FGI MD-to-psychiatrist ratio) in each stratum defined by birth year (5-year categories) for men and a 1:4 ratio (FGI MD-to-psychiatrist ratio) for the substantially smaller subgroup of women. The source of the study population and other study methods were similar to those used in our parallel study that compared mortality in radiologists in the United States with that in psychiatrists in the United States (21).
Mortality Follow-up
If date of birth and other identifying information were missing from the AMA Physician Masterfile, we linked the physicians with commercial databases and used interactive tracing to obtain these data. We then submitted all records to the Social Security Administration to determine vital status. Records of physicians who were known or presumed to be deceased and who died between 1979 and 2008 were then sent to the National Death Index (established in 1979) to ascertain cause of death (22). If we found no record of death, the physician was presumed to be alive.
Physicians were followed from January 1, 1979, or from 1 year after medical school graduation (after 1979) until date of death, age 85 years (because cause of death is reported less reliably after that age) (23), loss to follow-up (matched with the Social Security Administration initially but not in subsequent years), or December 31, 2008, whichever occurred earliest.
Assessment of Outcomes
We assessed mortality from all causes, all cancers, the more common specific cancers, leukemia subtypes and related disorders, and several nonmalignant diseases, including those of the circulatory system. The objective was to assess risks for these causes of death, chosen because of links between these conditions and low-to-moderate radiation exposure (≤0.5 Gy) in other populations (12).
Statistical Analysis
We used year of medical school graduation plus 1 year and the cumulative number of years since medical school graduation as proxies for year first exposed and total duration of exposure, respectively.
Poisson regression was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for FGI MDs versus psychiatrists. All analyses were adjusted (via stratification) for year of birth and attained age in 5-year groups. We also evaluated risks according to year of medical school graduation (20-year groups), number of years since graduation (<20, 20–29, 30–39, and ≥40 years), sex, and specialty of the FGI MDs.
A secondary analysis compared the observed number of deaths from various causes to the expected number on the basis of rates in the general U.S. population by using standardized mortality ratios adjusted for age, sex, and year of death.
We conducted a sensitivity analysis that was restricted to physicians who were confirmed dead (through National Death Index) or confirmed alive (through the Social Security Administration) to evaluate the potential for bias related to completeness of follow-up. All analyses were conducted by using risk regression software (Epicure; Risk Sciences International, Ottawa, Ontario).
Results
We identified 45 634 physicians who performed FGI (4148 women [9.1%]). The total included 37 311 cardiologists (9933 interventional cardiologists and cardiac electrophysiologists, and 27 378 cardiovascular disease specialists), 5520 interventional radiologists, and 2803 neuroradiologists (Table 1). The comparison group included 64 401 psychiatrists (17 555 women [27.3%]). The median age at entry to follow-up was 29 years for FGI MDs and 32 years for psychiatrists. The median age at exit was 55 years for FGI MDs and 58 years for psychiatrists. Two-thirds of each group was born in the United States; country of birth was not available for 14%–15% of the physicians. During the follow-up (1979–2008), 3506 (7.7%) of FGI MDs and 7814 (12.1%) of psychiatrists died. Cause of death could not be established for 149 FGI MDs (4.2%) and 375 psychiatrists (4.8%) (data not shown).
Table 1.
Characteristics of FGI MDs versus Psychiatrists Overall and according to Sex
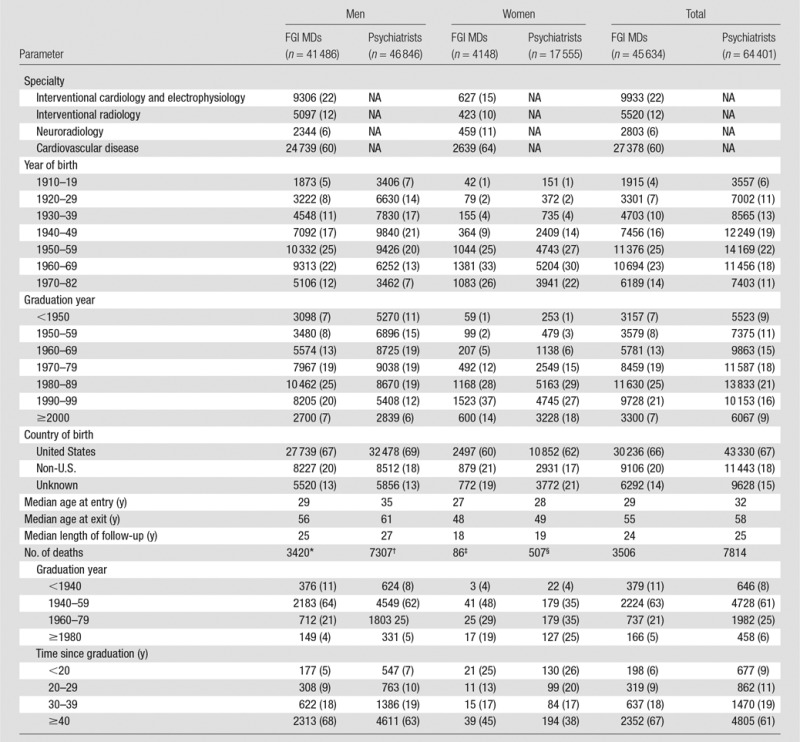
Note.—Data in parentheses are percentages. NA = not applicable.
*One hundred forty unknown, missing, or invalid causes of death.
†Three hundred twenty-three unknown, missing, or invalid causes of death.
‡Nine unknown, missing, or invalid causes of death.
§Fifty-two unknown, missing, or invalid causes of death.
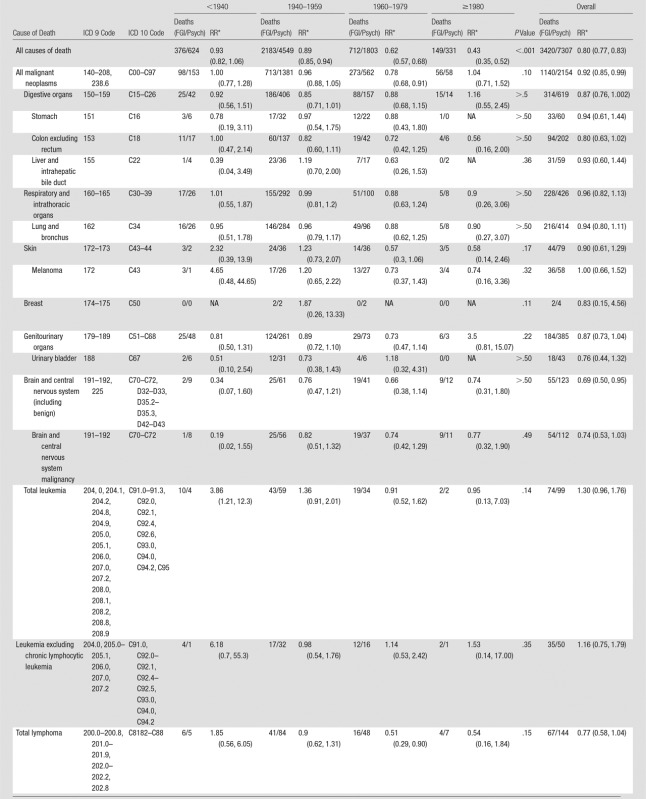
Male FGI MDs had a significantly lower risk of death overall than the male psychiatrists (RR, 0.80; 95% CI: 0.77, 0.83); this difference increased with more recent year of graduation (Table 2). All-cancer mortality was modestly reduced (RR, 0.92; 95% CI: 0.85, 0.99). An increased risk of death was observed for leukemia (RR, 3.86; 95% CI: 1.21, 12.3), but it was on the basis of small numbers and restricted to men who graduated before 1940. Risk of death was reduced for all brain tumors (RR, 0.69; 95% CI: 0.50, 0.95) and malignant brain tumors (RR, 0.74; 95% CI: 0.53, 1.03). Because of small numbers of female FGI MDs, the analyses focused primarily on men. Details on the women are presented in Tables E1–E4 (online).
Table 2.
Mortality Risks from All Causes and Specific Cancers in Male FGI MDs versus Male Psychiatrists Overall and according to Year of Medical School Graduation
Note.—Data in parentheses are 95% CIs. FGI/Psych = FGI MDs/psychiatrists, NA = not applicable.
*Stratified by year of attained age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years), year of birth (1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1969, and 1970–1982), and year of medical school graduation (<1940, 1940–1959, 1960–1979, and ≥1980).
Risks of death because of broad categories and specific types of myeloid and lymphoid malignancies and related disorders were not elevated in male FGI MDs compared with male psychiatrists (Table 3).
Table 3.
Mortality Risks from Hematopoietic and Lymphoproliferative Disorders in Male FGI MDs versus Male Psychiatrists according to Year of Medical School Graduation
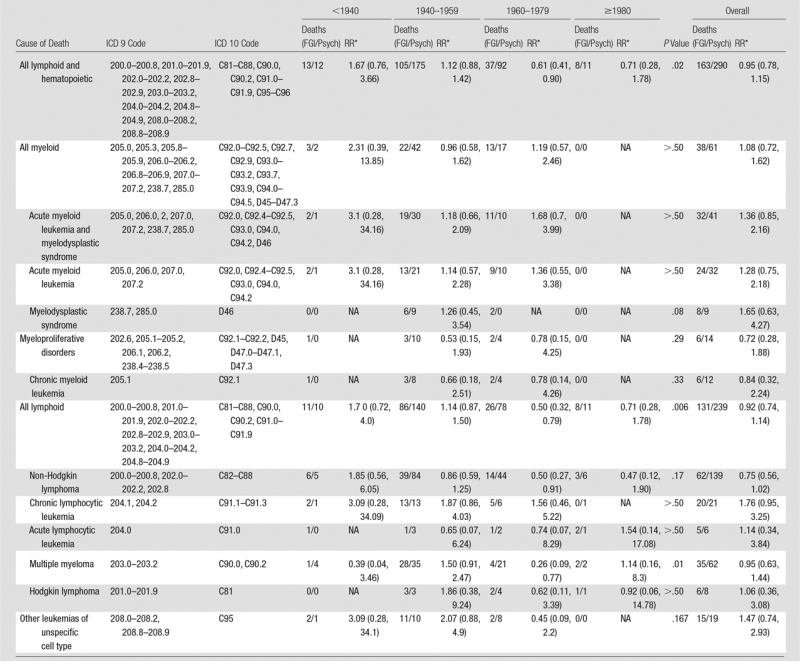
Note.—Data in parentheses are 95% CIs. FGI/Psych = FGI MDs/psychiatrists, NA = not applicable.
*Stratified on year of attained age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years), year of birth (1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1969, 1970–1982), and year of medical school graduation (<1940, 1940–1959, 1960–1979, and ≥1980).
Male FGI MDs had significantly lower mortality than psychiatrists for all infectious, mental and behavioral, all and specific types of circulatory system (except cerebrovascular), respiratory, and gastrointestinal diseases, accidents, suicide, and homicide; these differences were greatest for those who graduated in more recent years (Table 4). Mortality did not differ between the groups of male physicians for all diseases of blood forming tissue combined or for aplastic anemia, specifically. Similar findings (Tables 2–4) were observed when stratified by number of years since medical school graduation (Table E1 [online]).
Table 4.
Mortality Risks from Noncancer Causes in Male FGI MDs versus Male Psychiatrists according to Year of Medical School Graduation
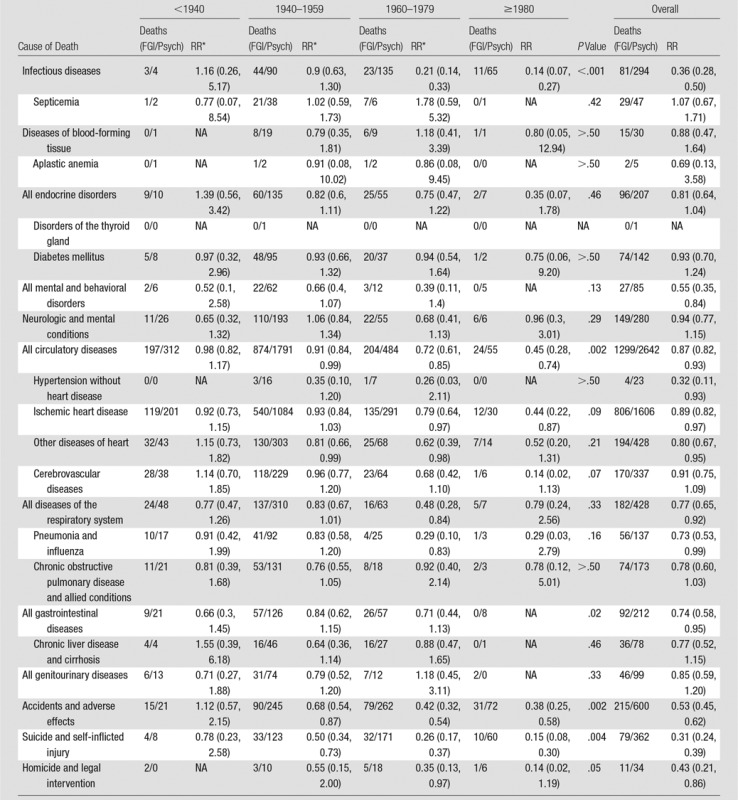
Note.—Data in parentheses are 95% CIs. FGI/Psych = FGI MDs/psychiatrists, NA = not applicable.
*Stratified by year of attained age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years), year of birth (1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1969, and 1970–1982), and year of medical school graduation (<1940, 1940–1959, 1960–1979, and ≥1980).
The small number of deaths among FGI MDs in the specific specialties limited assessment of rare outcomes (Table 5). Increased risks of death were not observed for male interventional cardiologists and electrophysiologists, interventional radiologists, or neuroradiologists compared with male psychiatrists. Male cardiovascular disease specialists had elevated total leukemia mortality (including nonsignificant increases in leukemias, excluding chronic lymphocytic leukemia and in acute myeloid leukemia) (Table 5). The elevated leukemia in the cardiovascular disease specialists derived primarily from excess risks in those who graduated before 1940 (data not shown).
Table 5.
Mortality Risks from All and Specific Causes in Male FGI MDs according to Specialty Compared with Male Psychiatrists
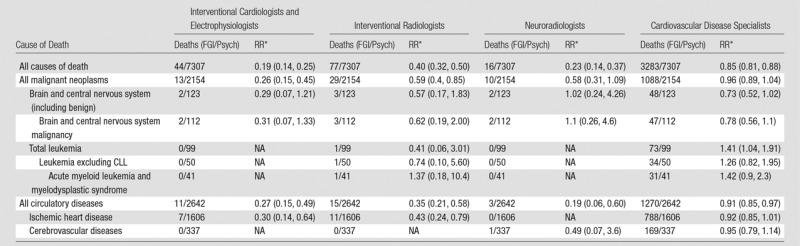
Note.—Data in parentheses are 95% confidence intervals. The specialties were as follows: 9306 interventional cardiologists and electrophysiologists, 5097 interventional radiologists, 2344 neuroradiologists, and 24 739 cardiovascular disease specialists. There were 46 846 psychiatrists. CLL = chronic lymphocytic leukemia, NA = not applicable.
*Stratified by year of attained age (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years), year of birth (1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1969, and 1970–1982), and year of medical school graduation (<1940, 1940–1959, 1960–1979, and ≥1980).
On the basis of small numbers, and mortality overall and from specific causes, all cancer, including breast cancer, and all circulatory system diseases were nonsignificantly reduced in female FGI MDs compared with female psychiatrists (Table E2 [online]).
After restricting the population to physicians confirmed to be alive or deceased, results were similar to those of the entire population of FGI MDs (Table E3 [online]).
Compared with the general population, both FGI MDs and psychiatrists had substantially lower mortality because of all causes, all cancers, and almost all specific cancers, including total leukemia (Table E4 [online]). Standardized mortality ratios were also lower in both physician groups than in the general population for total circulatory diseases and most other nonmalignant conditions.
Discussion
We found no evidence of increased risk of death from all causes, all cancers combined, specific cancers, or nonmalignant diseases for FGI MDs compared with psychiatrists. Elevated leukemia risk was restricted to physicians who graduated before 1940; this finding was driven by results for the cardiovascular disease specialists and not the other specialty groups. The number of deaths in the other specialty groups was small because of the young ages and more recent years of graduation than the cardiovascular disease specialists. The small numbers of deaths and the rarity of hematopoietic and lymphoproliferative disorders may have limited statistical power to detect increased risks.
Excess leukemia has been observed in populations with moderate-to-high radiation exposure and is associated more strongly than most solid cancers. Dose-response relationships for leukemia, excluding chronic lymphocytic leukemia, have been found in many studies of radiation-exposed populations with a higher relative risk compared with risks for most other cancers (24–27). Elevated risks were reported in nuclear workers with low-to-moderate protracted radiation exposures (13). The elevated leukemia risk in FGI MDs (mostly cardiovascular disease specialists) who graduated before 1940 may reflect higher occupational radiation doses in earlier years and is consistent with findings in early medical radiation workers from the United Kingdom, the United States, and China (17). Cardiac procedures were among the earliest and most rapidly increasing FGI procedures. The annual occupational radiation dose limit in the United States was reduced from 300 mSv in 1934 to 150 mSv in 1949 and further decreased to 50 mSv in 1958 (28).
Moderate-to-high-dose exposures have shown radiation dose-response relationships for schwannoma in atomic bomb survivors (29) and for glioma and other brain tumors in patients irradiated in childhood for benign or malignant conditions (30). Brain tumor risks associated with low-dose radiation are less clear (30), but increased risks were reported in children who underwent computed tomographic (CT) scans (31) and adults who underwent three or more CT scans and had a family history of cancer (32). Other epidemiologic studies of medical radiation workers did not observe excess risks (17,33). In FGI MDs, the brain is potentially vulnerable because leaded eyewear and thyroid shields do not protect it from occupational radiation exposures, and ceiling-suspended leaded shields, which afford good protection, are often not used (3). The reduced risk of brain tumors, primarily malignant brain neoplasms, in FGI MDs compared with psychiatrists contrasts with previous small case series (5) and elevated risks in U.S. radiologic technologists who performed FGI procedures (19). The difference in the brain tumor findings between the U.S. radiologic technologists who performed FGI procedures and the current study may be because of the older median age at exit of the technologists compared with the FGI MDs, differences in work exposures to other potential carcinogens (eg, darkroom chemicals) between the two groups of medical radiation workers, or that the finding in the technologists was due to chance.
Despite similarities in the source of the study populations, the comparison with psychiatrists, the methods used, and the findings of lower overall mortality among FGI MDs and our previously reported study of radiologists (21), important differences in age and periods of exposure complicate direct comparisons. A higher proportion of male FGI MDs graduated from medical school in 1970 or later (70.7%; 29 334 of 41 486) compared with male radiologists (60.0%; 20 947 of 34 912). The median age at cohort entry was 29 years for male FGI MDs versus 30 years for male radiologists. More importantly, the radiologists included physicians who had practiced throughout the last century in the United States, whereas FGI procedures were not generally used by cardiologists, radiologists, or interventional neuroradiologists until the 1970s, 1980s, and subsequently. Notable declines in occupational radiation exposures to medical radiation workers have been documented since the 1930s and this continued for many years after 1958, when the last reduction in recommended occupational radiation doses occurred in the United States (17). Given the differences in age, length of follow-up, and practice, it is not surprising that the elevated mortality risks of acute myeloid leukemia and possibly other cancers and cerebrovascular disease observed in radiologists who graduated before 1940 are not apparent in the FGI MDs, with the possible exception of acute myeloid leukemia among the subset of cardiovascular disease specialists. The more modest decrease in overall mortality for the radiologists versus psychiatrists (RR, 0.94; 95% CI: 0.90, 0.97) than for the FGI MDs versus psychiatrists (RR, 0.80; 95% CI: 0.77, 0.83) may also reflect greater radiation exposure among the early radiologists than the FGI MDs, although the increased mortality risks from acute myeloid leukemia, melanoma, non-Hodgkin lymphoma, and cerebrovascular disease observed in early radiologists, but not FGI MDs, could also be because of differences in statistical power (eg, insufficient length of follow-up of the FGI MDs to capture relatively rare outcomes, particularly in women), lifestyle factors, or chance.
In the extended follow-up of U.S. radiologic technologists who assisted with FGI procedures (66.4% women; 13 925 of 20 982), modest elevations of breast cancer and melanoma incidence were identified (19). Excess mortality risks from skin cancer were described in early medical radiation workers from the United Kingdom, the United States, and China (17), but other malignancies were not consistently increased. Work history information could not be evaluated in most radiation workers, including the current medical radiation or nuclear worker studies (12,14,15), nor could potential confounders such as smoking. Breast cancer was increased in the large cohort of U.S. radiologic technologists who first worked before 1940 (34), and most cancers (except for prostate cancer, chronic lymphocytic leukemia, and a few others) were associated with radiation exposure in the atomic bomb survivors (29). The overall reduced risks of total and some radiogenic cancers in the FGI MDs compared with psychiatrists may have reflected a young age at exit, healthier lifestyles and the nonsedentary nature of the work in the former, or inadequate power because of the small numbers of outcomes, particularly in women.
We found reduced risks of all and certain types of circulatory diseases. Convincing evidence linked radiation-related heart disease with high doses of radiation therapy to the chest. Associations of circulatory disease with low-to-moderate radiation exposure were described in a meta-analysis (16) in U.S. radiologists, in U.S. radiologic technologists (17), in U.S. radiologic technologists who worked with FGI procedures (18), and in atomic bomb survivors (29). The absence of an increase in mortality from circulatory disease in the FGI MDs may be because of the comparison groups used (eg, psychiatrists may have a higher risk of circulatory disease than FGI MDs because of a higher risk factor profile, such as greater smoking, more sedentary work, and other circulatory disease risk factors). Alternatively, the FGI MDs are younger than other radiation-exposed populations reported to have increased risks (17,29) and may not have yet reached the age when these outcomes were observed.
Associations of radiation dose with certain gastrointestinal and respiratory diseases reported in atomic bomb survivors (29) were not observed in the FGI MDs.
Data on annual or cumulative estimated occupational radiation exposures of FGI MDs are limited, particularly for earlier periods, and generally based on small numbers. To our knowledge, there are no national surveys of dose estimates for FGI MDs. Very few countries distinguish between medical radiation workers who perform conventional radiologic procedures versus those who perform FGI procedures (26). The United Nations Scientific Committee on the Effects of Atomic Radiation Global Survey of Occupational Radiation Exposures for all medical radiation workers found an average annual effective dose of about 0.5 mSv (range, 0.02–1.24 mSv) to workers who performed conventional radiologic procedures versus 1.6 mSv (range, 0.4–29.5 mSv) to workers who performed FGI procedures (26). Studies of interventional cardiologists suggest an annual average effective dose of 2–4 mSv per year (3), but this does not account for scatter radiation to unprotected sites (eg, hands, other parts of the extremities, brain, or, if unprotected, eyes and thyroid). A comprehensive literature review of dose per procedure and trends in doses to physicians who performed FGI cardiac procedures does not support a notable decline in doses over time (9), perhaps because of greater complexity of newer FGI procedures (8). Thus, the observed decline in relative risks with more recent year of medical school graduation is not likely to be explained by decreased occupational radiation doses.
The strengths of our study are the size and nationwide population-based nature of the cohort, and the relatively long follow-up. We also had nearly complete and systematic follow-up through a large number of tracing sources. Use of the National Death Index was another strength because of the high level of ascertainment of U.S. deaths.
The AMA Physician Masterfile does not include detailed information on work history or procedures performed, occupational radiation doses, or known risk factors for cancer or circulatory diseases. The lower risks of death from several causes in the FGI MDs compared with psychiatrists may be because of confounding exposures if psychiatrists differ from FGI MDs in smoking habits (although risks of lung cancer and chronic obstructive pulmonary disease, both smoking related, did not differ between FGI MDs and psychiatrists), sedentary working conditions, and other characteristics. We lacked data on these potential confounding exposures. Earlier studies (21) reported modestly higher risks of death in psychiatrists compared with other physicians for all causes combined, ischemic heart disease, injury and poisoning, and suicide. We had considered selecting comparison physicians from surgical specialties for comparable lifestyle characteristics with FGI MDs, but concluded that it would be difficult to identify surgical specialists unexposed to radiation because of the dramatic increase in fluoroscopy in many operating suites. Although psychiatrists are not an ideal physician comparison group, our parallel study (21) that compared an overlapping population of psychiatrists with noninterventional radiologists found mortality risks consistent with those observed in other long-term follow-up studies of radiologists.
We evaluated risks separately for the different FGI MD specialties, but the numbers of key outcomes in each group were relatively small. The lack of individual work history information and potential misclassification of medical specialty would probably bias our results toward the null. FGI MDs within each major specialty category may have experienced substantial variation in occupational radiation exposures because of differences in the types of procedures performed within and across time periods (eg, endovascular surgical neuroradiologists vs neuroradiologists). Lifetime individual occupational radiation doses were not sought. Reliable dose data for FGI MDs are difficult to determine retrospectively because many of these individuals do not wear their radiation monitors regularly (35).
Outcomes were restricted to mortality. Radiation-related conditions with low mortality (eg, thyroid carcinoma) cannot be evaluated in the absence of a nationwide population-based U.S. cancer registry. Other conditions, such as nonmelanoma skin cancer or cataracts, which are not reported to population-based registries, could not be ascertained through data collection strategies restricted to database linkages. Although the AMA Physician Masterfile is comprehensive, an unknown fraction of FGI MDs may not have been included. The small number of deaths overall (7.7% [3506 deaths in 45 634 FGI MDs]; and 12.1% [7814 deaths in 64401 psychiatrists]) and those from rare outcomes limited our statistical power to assess these outcomes. The evaluation of risks from many different causes of death can result in the identification of statistically significant associations by chance alone, although there is a stronger a priori rationale for an increase in the leukemia risk in early medical school graduates than for other diseases and more recent graduation years. Nevertheless, the potential problem of multiple comparisons must be acknowledged. The young age at exit strongly supports the need for additional follow-up to capture more fully lifetime risks. Finally, there were too few women, and very few who worked during the early periods, to study their mortality rates in detail.
In conclusion, compared with psychiatrists, male FGI MDs had lower risks of death overall and from most cancers and other causes, except for leukemia among early medical school graduates, particularly cardiologists. Our results need to be confirmed in other studies with dose and work history information, including the types, frequency, and duration of performing FGI procedures and personal and general radiation protection measures. Extended follow-up of incidence and mortality are needed to obtain a clearer picture of lifetime risks, particularly for the specialists thought to have the highest exposures. Pooling of data from the United States with data from other countries would provide greater statistical power to assess health risks in these physicians, although the benefits (eg, greater statistical power and increased opportunity to evaluate subgroup characteristics) must be balanced against methodologic shortcomings (eg, heterogeneity of populations and exposures, and differences in information about potential confounders).
Advance in Knowledge
■ In a nationwide cohort of 45 634 physicians likely to have performed fluoroscopy-guided interventional (FGI) procedures (referred to as FGI MDs), total and cause-specific mortality was compared with that of 64 401 psychiatrists during the follow-up period (1979–2008); overall, total deaths and deaths from most specific causes, including those that are considered to be related to radiation, were not elevated in FGI MDs.
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgments
We thank the AMA for providing historic AMA Physician Masterfile information. We appreciate the support from the Society for Interventional Radiology, Society for Cardiovascular Angiography and Interventions, Heart Rhythm Society, Society of Neurointerventional Surgery, American College of Cardiology, Radiological Society of North America. American Association of Physicists in Medicine, and the American Board of Internal Medicine. We are grateful to Christopher McClure, Liliana R. Preiss, and John Heinrich of Research Triangle Institute, International for data collection and management; Nathan Appel of IMS, Inc. for computing support; and Annelie Landgren and Ka Lai Lou for assistance with manuscript preparation.
Received July 1, 2016; revision requested August 17 and received November 28; accepted December 12; final version accepted January 6, 2017.
Study supported by Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the U.S. Public Health Service of the Department of Health and Human Services, Bethesda, Maryland.
M.S.L. and C.M.K. contributed equally to this work.
Multi-Specialty Occupational Health Group: Stephen Balter, PhD (liaison to the American Association of Physicists in Medicine); Linda B. Bresolin, PhD (liaison to the Radiological Society of North America); Charles E. Chambers, MD (liaison to the Society for Cardiovascular Angiography and Interventions); James A. Goldstein, MD (liaison to the Society for Cardiovascular Angiography and Interventions); David E. Haines, MD (liaison to the Heart Rhythm Society); Marsha Holton, RN, RCIS, FSICP (liaison to the Society of Invasive Cardiovascular Professionals); Lloyd W. Klein, MD (liaison to to the Society for Cardiovascular Angiography and Interventions); Alexander M. Norbash, MD, MHCM (liaison to the Society of Neurointerventional Surgery); Eric M. Walser, MD (liaison to the Society of Interventional Radiology).
Disclosures of Conflicts of Interest: M.S.L. Activities related to the present article: funds for travel provided to the author from the Intramural Research Program and the National Institutes of Health. Activities not related to the present article: money paid to author’s institution from the Intramural Research Program and the National Institutes of Health. Other relationships: disclosed no relevant relationships. C.M.K. disclosed no relevant relationships. E.N. disclosed no relevant relationships. R.A.K. disclosed no relevant relationships. E.S.G. disclosed no relevant relationships. N.N. disclosed no relevant relationships. R.S.L. disclosed no relevant relationships. D.L.M. disclosed no relevant relationships. A.B.d.G. disclosed no relevant relationships.
Abbreviations:
- AMA
- American Medical Association
- CI
- confidence interval
- FGI
- fluoroscopy-guided intervention
- FGI MD
- physician who performs FGI procedures
- RR
- relative risk
References
- 1.Miller DL. Overview of contemporary interventional fluoroscopy procedures. Health Phys 2008;95(5):638–644. [DOI] [PubMed] [Google Scholar]
- 2.National Council on Radiation Protection and Measurements . Radiation dose management for fluoroscopically-guided interventional medical procedures. NCRP Report No 168. Bethesda, Md: National Council on Radiation Protection and Measurements, 2010. [Google Scholar]
- 3.Cousins C, Miller DL, Bernardi G, et al. ICRP PUBLICATION 120: Radiological protection in cardiology. Ann ICRP 2013;42(1):1–125. [DOI] [PubMed] [Google Scholar]
- 4.Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory: time for a safer environment. Catheter Cardiovasc Interv 2009;73(3):432–438. [DOI] [PubMed] [Google Scholar]
- 5.Roguin A, Goldstein J, Bar O. Brain malignancies and ionising radiation: more cases reported. EuroIntervention 2012;8(1):169–170. [DOI] [PubMed] [Google Scholar]
- 6.Vaño E, Gonzalez L, Fernandez JM, Alfonso F, Macaya C. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br J Radiol 2006;79(941):383–388. [DOI] [PubMed] [Google Scholar]
- 7.Vañó E, Miller DL, Dauer L. Implications in medical imaging of the new ICRP thresholds for tissue reactions. Ann ICRP 2015;44(1 Suppl):118–128. [DOI] [PubMed] [Google Scholar]
- 8.Miller DL. Efforts to optimize radiation protection in interventional fluoroscopy. Health Phys 2013;105(5):435–444. [DOI] [PubMed] [Google Scholar]
- 9.Kim KP, Miller DL, Balter S, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys 2008;94(3):211–227. [DOI] [PubMed] [Google Scholar]
- 10.National Council on Radiation Protection and Measurements . Recommendations on limits for exposure to ionizing radiation. NCRP Report No 91. Bethesda, Md: National Council on Radiation Protection and Measurements, 1987. [Google Scholar]
- 11.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37(2-4):1–332. [DOI] [PubMed] [Google Scholar]
- 12.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Radiation. IARC Monogr Eval Carcinog Risks Hum 2012;100(Pt D):7–303. [PMC free article] [PubMed] [Google Scholar]
- 13.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2015;2(7):e276–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100(1):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. [Published correction appears in BMJ 2015;351:h6634.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little MP, Azizova TV, Bazyka D, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120(11):1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linet MS, Kim KP, Miller DL, Kleinerman RA, Simon SL, Berrington de Gonzalez A. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat Res 2010;174(6):793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajaraman P, Doody MM, Yu CL, et al. Incidence and mortality risks for circulatory diseases in US radiologic technologists who worked with fluoroscopically guided interventional procedures, 1994-2008. Occup Environ Med 2016;73(1):21–27. [DOI] [PubMed] [Google Scholar]
- 19.Rajaraman P, Doody MM, Yu CL, et al. Cancer risks in U.S. radiologic technologists working with fluoroscopically guided interventional procedures, 1994-2008. AJR Am J Roentgenol 2016;206(5):1101–1108; quiz 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Medical Association . AMA Physician Masterfile. http://www.ama-assn.org/ama/pub/about-ama/physician-data-resources/physician-masterfile.page. Accessed October 25, 2015.
- 21.Berrington de González A, Ntowe E, Kitahara CM, et al. Long-term mortality in 43 763 U.S. radiologists compared with 64 990 U.S. psychiatrists. Radiology 2016;281(3):847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . National Death Index. http://www.cdc.gov/nchs/ndi.htm. Updated March 2, 2015. Accessed July 18, 2015.
- 23.Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897-1997. Br J Radiol 2001;74(882):507–519. [DOI] [PubMed] [Google Scholar]
- 24.Hsu WL, Preston DL, Soda M, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res 2013;179(3):361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krestinina LY, Davis FG, Schonfeld S, et al. Leukaemia incidence in the Techa River Cohort: 1953-2007. Br J Cancer 2013;109(11):2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United Nations Scientific Committee on the Effects of Atomic Radiation . Sources and effects of ionizing radiation. New York, NY: United Nations Scientific Committee on the Effects of Atomic Radiation, 2008. [Google Scholar]
- 27.Zablotska LB, Bazyka D, Lubin JH, et al. Radiation and the risk of chronic lymphocytic and other leukemias among chornobyl cleanup workers. Environ Health Perspect 2013;121(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inkret WC, Meinhold CB, Taschner JC. A brief history of radiation protection standards. Los Alamos Sci 1995;23:116–123. [Google Scholar]
- 29.Mabuchi K, Fujiwara S, Preston DL, Shimizu Y, Nakamura N, Shore RE. Atomic bomb survivors: Long-term health effects of radiation. In: Shrieve DC, Loeffler JS, eds. Human radiation injury. Philadelphia, Pa: Wolters Kluwer/Lippincott Williams & Wilkins, 2011; 89–113. [Google Scholar]
- 30.Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 2012;14(11):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis F, Il’yasova D, Rankin K, McCarthy B, Bigner DD. Medical diagnostic radiation exposures and risk of gliomas. Radiat Res 2011;175(6):790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitahara CM, Linet MS, Drozdovitch V, et al. Cancer and circulatory disease risks in US radiologic technologists associated with performing procedures involving radionuclides. Occup Environ Med 2015;72(11):770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doody MM, Freedman DM, Alexander BH, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer 2006;106(12):2707–2715. [DOI] [PubMed] [Google Scholar]
- 35.Padovani R, Le Heron J, Cruz-Suarez R, et al. International project on individual monitoring and radiation exposure levels in interventional cardiology. Radiat Prot Dosimetry 2011;144(1-4):437–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.