The goal of this comprehensive review is to improve understanding of testicular seminoma with a focus on radiologic-pathologic correlation, by reviewing its epidemiology and risk factors, clinical and pathologic features, common multimodality imaging findings, and important differential considerations.
Abstract
Testicular seminoma is the most common malignant tumor of the testis. It classically manifests as a painless mass. Radiologic evaluation with high-frequency ultrasonography (US) is critical for diagnosis. Seminomas are usually homogeneously hypoechoic masses at US. In challenging cases, magnetic resonance (MR) imaging may help confirm that a mass is intratesticular and provide data for local staging. Computed tomography (CT) provides valuable information for staging, including the presence and size of retroperitoneal lymph nodes. Testicular seminoma is treated with radical inguinal orchiectomy and is highly curable even at advanced stages of disease. Several neoplastic and nonneoplastic conditions may mimic testicular seminoma at imaging. Benign mimics include segmental infarction, hematoma, infection, epidermoid cyst, adrenal rests, sarcoidosis, splenogonadal fusion, and sex cord–stromal tumors. Malignant mimics include nonseminomatous germ cell tumors, lymphoma, and metastases. These conditions are individually reviewed with emphasis on features that allow differentiation from seminoma. Spermatocytic tumor, formerly known as spermatocytic seminoma, accounts for only 1% of testicular tumors. It is distinct from classic seminoma, with unique histologic, molecular, and genetic features. It affects an older patient population than classic seminoma and demonstrates indolent clinical behavior. Radiologists serve a key role in diagnosis, staging, and surveillance of patients with seminoma. A thorough knowledge of related clinical, radiologic, and pathologic findings will help the radiologist contribute to high-quality interdisciplinary care of affected patients.
See Illumination by Frazier
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the pathologic basis for the common imaging features of testicular seminoma.
■ List neoplastic and nonneoplastic conditions that may mimic testicular seminoma at imaging.
■ Describe the clinical, pathologic, and imaging features that allow distinction of spermatocytic tumor from seminoma.
Introduction
Testicular cancer is an important, but curable, medical problem for young men. Testicular seminoma is the most common malignant tumor of the testis. In this article, we discuss testicular seminoma and its mimics with a focus on radiologic-pathologic correlation. Common multimodality imaging findings and important differential considerations are included. We also update the reader on spermatocytic tumor, formerly known as spermatocytic seminoma.
Epidemiology and Risk Factors
Testicular cancer is the most common cancer in men aged 15–44 years (1). Approximately 8720 new cases of testicular cancer were diagnosed in 2016, but only 380 men will die of the disease (2). Although relatively rare, the incidence of testicular germ cell neoplasms has been rising for the past 20 years (1,3,4). In the United States, testicular seminoma is the most common subtype of testicular cancer, accounting for 55% of cases.
For seminoma, the median age at diagnosis is 35–39 years. On average, seminomas are diagnosed in patients a decade older than those with nonseminomatous germ cell tumors (1). There is significant variability in incidence rates for testicular cancer based on race. White men are at greatest risk, affected five times more often than black men and four times more often than Asian men (5).
Numerous risk factors for testicular cancer have been described in the literature (3,5). The most important of these is a personal history of a testicular germ cell tumor. Patients with a previous germ cell tumor have a 12-fold increased risk of a second testicular primary, a phenomenon that occurs in 2%–3% of patients (5,6). Untreated cryptorchidism increases a patient’s chance of developing a testicular neoplasm by eight times (Fig 1). Prepubertal orchiopexy reduces this risk to two times that of the general population.
Figure 1a.
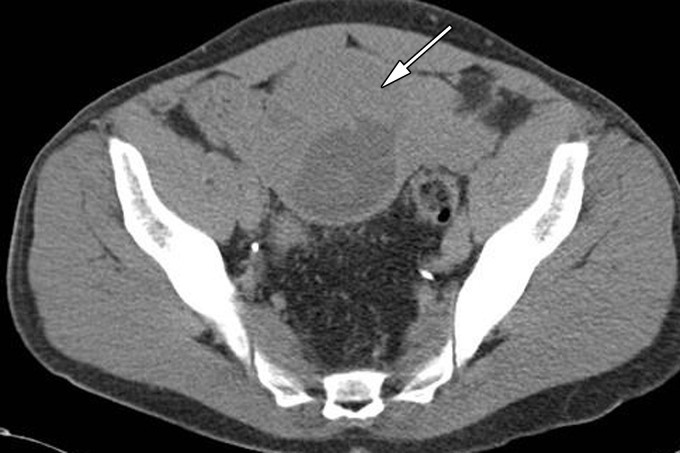
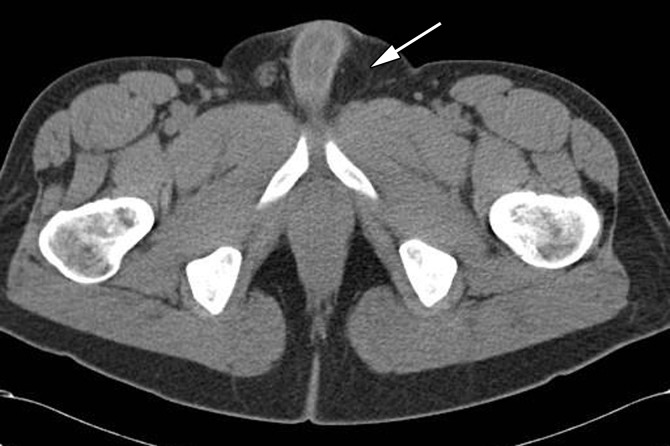
Cryptorchidism in a 39-year-old man with left flank pain. (a, b) Axial contrast-enhanced computed tomographic (CT) images show lack of a left spermatic cord (arrow in b) and an anterior pelvic mass (arrow in a), proven to be seminoma in an undescended left testis. (c) Axial contrast-enhanced CT image 6 months later after chemotherapy shows a reduction in the size of the mass (arrow).
Figure 1b.
Cryptorchidism in a 39-year-old man with left flank pain. (a, b) Axial contrast-enhanced computed tomographic (CT) images show lack of a left spermatic cord (arrow in b) and an anterior pelvic mass (arrow in a), proven to be seminoma in an undescended left testis. (c) Axial contrast-enhanced CT image 6 months later after chemotherapy shows a reduction in the size of the mass (arrow).
Family history, combined with environmental factors, increases patients’ risk up to 10-fold. Patients with male factor infertility have a threefold increased incidence of testicular cancer. Environmental exposures including organochlorines, polychlorinated biphenyls, polyvinyl chlorides, phthalates, marijuana, and tobacco have been shown to increase the incidence of testicular cancer (3,5). Testicular microlithiasis, a condition that affects approximately 2% of men, was once thought to be a risk factor for testicular cancer. Recent data have failed to show a causal link between this common condition and the much less common testicular cancer (7).
Clinical Features
Testicular cancer, including seminoma, classically manifests as a painless, palpable, solid mass. Less commonly, patients with seminoma may present with testicular swelling and mild discomfort, suggestive of epididymo-orchitis (5). After a thorough history and physical examination, most patients are evaluated with scrotal ultrasonography (US) (8). After diagnosis of a solid intratesticular mass, further clinical testing includes complete blood cell count; levels of creatinine, electrolytes, liver enzymes, and serum tumor markers; and chest radiography (3).
The primary therapy for testicular seminoma is radical inguinal orchiectomy (3,9). After surgery, patients are classified into good-, intermediate-, or poor-risk groups according to the International Germ Cell Cancer Collaborative Group classification system (3). This system uses clinically independent prognostic features such as extent of disease and levels of serum tumor markers after orchiectomy (3,10).
If the tumor histologic type is pure seminoma (Fig 2), imaging evaluation includes CT of the abdomen and pelvis to evaluate for retroperitoneal adenopathy. In the presence of retroperitoneal adenopathy or abnormal findings at chest radiography, chest CT is performed to assess for mediastinal adenopathy and detect pulmonary nodules. Magnetic resonance (MR) imaging of the brain and bone scintigraphy are indicated only if symptoms suggestive of metastases to these regions are present (3).
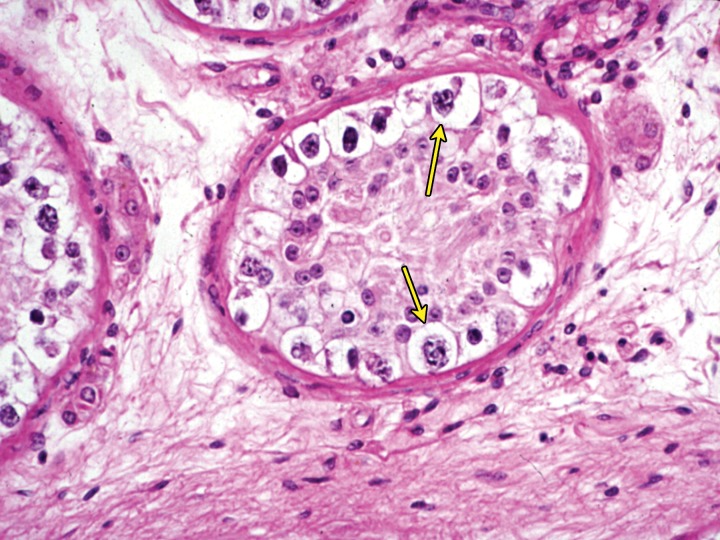
Figure 2.
Intratubular seminoma. High-power photomicrograph of a seminiferous tubule shows germ cell neoplasia in situ (GCNIS) (arrows). (Hematoxylin-eosin [H-E] stain.)
The most commonly used staging system for testicular seminoma is the American Joint Committee on Cancer staging system. Staging of testicular seminoma combines information obtained from pathologic evaluation of the radical orchiectomy specimen (T stage), results of imaging tests (N and M stages), and results of measurement of tumor marker levels (S stage). The inclusion of serum levels of tumor markers (S stage) is unique to testicular cancer. While a detailed discussion of testicular cancer staging is beyond the scope of this article, a few key points are important for radiologists to understand.
The T stage is determined pathologically. Lymph nodes are assessed with imaging and potentially with pathologic analysis. Owing to the lymphatic drainage pathways of the testes, the retroperitoneal lymph nodes are considered the regional lymph nodes. Unlike many other neoplasms, for testicular neoplasm, the greatest dimension of enlarged retroperitoneal lymph nodes is used for staging and should be reported. Key lymph node sizes that alter N staging are less than 2 cm (N1), 2–5 cm (N2), and greater than 5 cm (N3). Initial detection of metastatic disease is usually with imaging. Metastases to nonregional lymph nodes and the lungs are M1a disease, while other distant metastases are considered M1b disease (3,11).
Tumor markers used in management of testicular cancer include α-fetoprotein (AFP), β–human chorionic gonadotropin (β-hCG), and lactate dehydrogenase (LDH) (5,11). AFP levels should be normal in seminoma. When a patient with a histologically pure testicular seminoma has an elevated AFP level, it is generally assumed that an undetected focus of nonseminomatous germ cell tumor is present (3,12). Serum concentrations of β-hCG and LDH may be elevated in patients with seminoma. β-hCG elevation is more commonly seen with nonseminomatous germ cell tumors and other nonneoplastic causes including marijuana use and hypogonadism. LDH is a nonspecific marker of overall tumor burden. Tumor markers may be useful in monitoring metastatic seminomas because elevated tumor marker levels are an early sign of relapse (3).
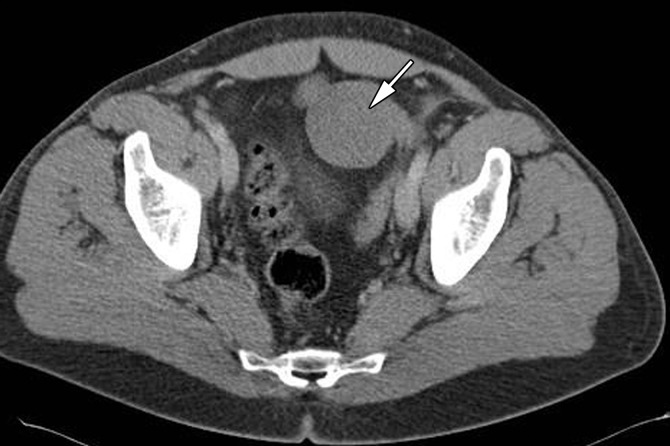
Adjuvant therapy varies by stage. Stage I pure seminoma may be managed with active surveillance, radiation therapy, or single-agent carboplatin chemotherapy. Radiation therapy is performed for low-volume stage IIA and select stage IIB seminoma. Chemotherapy regimens, including etoposide and cisplatin (EP) or bleomycin, etoposide, and cisplatin (BEP), are used for stage IIB, IIC, and III disease (3) (Fig 1c).
Figure 1c.
Cryptorchidism in a 39-year-old man with left flank pain. (a, b) Axial contrast-enhanced computed tomographic (CT) images show lack of a left spermatic cord (arrow in b) and an anterior pelvic mass (arrow in a), proven to be seminoma in an undescended left testis. (c) Axial contrast-enhanced CT image 6 months later after chemotherapy shows a reduction in the size of the mass (arrow).
It is important to note that testicular seminoma is highly curable even at advanced stages. If all stages of testicular seminoma are included, the 3-, 5-, and 10-year survival rates are 95%, 86%, and 71%, respectively (13). If a patient presents with stage I disease, the most common stage at presentation, the disease-specific survival is 99% (3,14).
Pathologic Features
An updated World Health Organization (WHO) Classification of Tumours of the Urinary System and Male Genital Organs was published in 2016 (15). Before this update, the classification of testicular tumors was based on morphology alone. Currently, germ cell tumors are divided into two groups, those derived from germ cell neoplasia in situ (GCNIS) and those unrelated to GCNIS. Seminoma is a GCNIS-derived tumor. There may be a transient stage of seminoma that is intratubular (Fig 2). In more than 80% of cases, seminoma shows amplification of genetic material from the short arm of chromosome 12, often in the form of isochromosome 12p (16).
At gross pathologic analysis, classic seminoma is typically tan to pale yellow, solid, and fleshy. It is most often a well-circumscribed mass. Frequently, small foci of hemorrhage and necrosis are present (16). In intertubular seminoma, a distinct mass may not be apparent and instead sporadic irregularities in texture or color are seen (16).
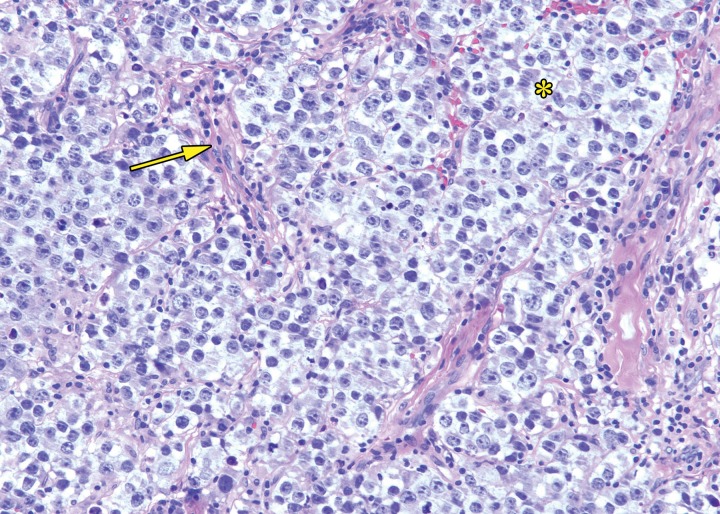
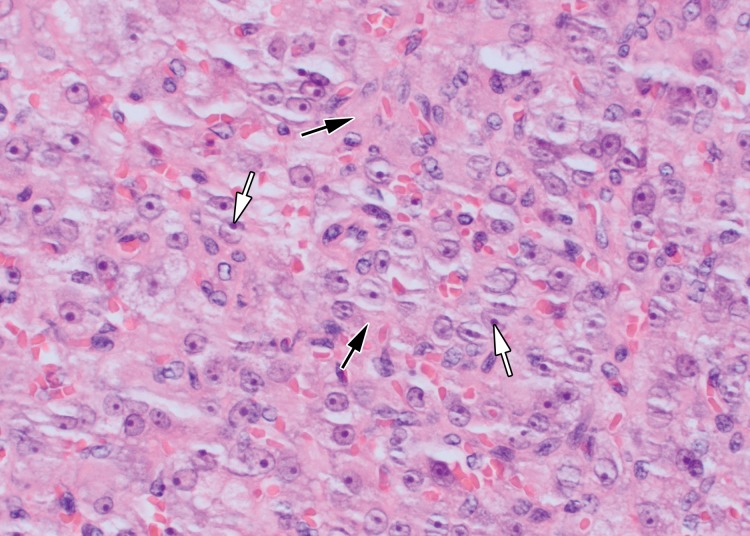
At histologic assessment, seminoma is seen as nests and sheets of cells with intervening thin fibrous septa (Fig 3). The septa separate the tumor into lobules of tumor cells (16). The septa may contain lymphocytes. Seminoma is composed of monotonous cells with clear to pale to eosinophilic cytoplasm. Nuclei are typically large and contain prominent nucleoli. Coexistent granulomatous inflammation may be seen.
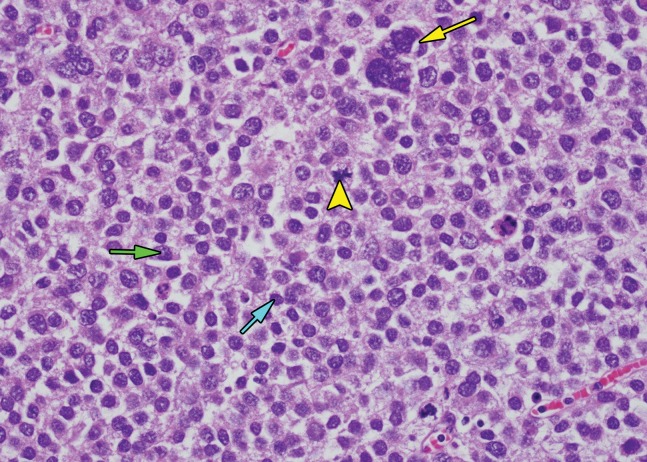
Figure 3.
Histologic features of seminoma. High-power photomicrograph of a seminoma shows sheets of monotonous cells with pale cytoplasm and large nuclei (*) with intervening thin fibrous septa (arrow). The uniform appearance of seminoma at histologic analysis helps explain the uniform appearance at imaging. (H-E stain.)
In a minority of cases, neoplastic cells may be found in the interstitium of the testis in areas distinct from the main mass, a finding described as intertubular seminoma (17). Intertubular seminoma is frequently associated with GCNIS and is associated with rete testis invasion and a worse prognosis (17). Histologic variants of seminoma include tubular, signet ring, and rhabdoid (16). Syncytiotrophoblasts may be present within the tumor and can be associated with mild elevation of serum β–human chorionic gonadotropin. Seminoma demonstrates immunoreactivity to SALL4, OCT3/4, CKIT, D2-40, and SOX17 (16).
Imaging Features
Ultrasonography
High-frequency US with a linear-array transducer is the initial imaging modality used in evaluation of a suspected testicular mass (8). US is highly accurate in localizing lesions as intratesticular or extratesticular, an important distinction since most intratesticular masses are malignant (18). US is excellent in differentiating benign cysts from more concerning solid or complex masses.
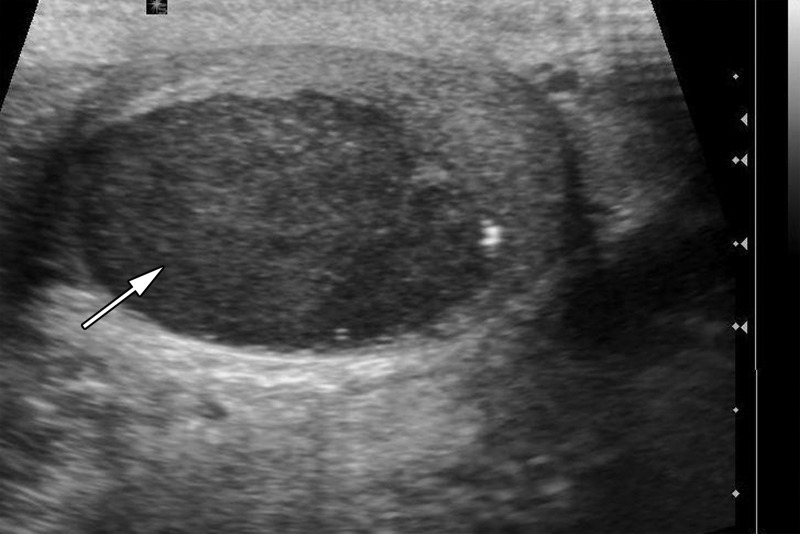
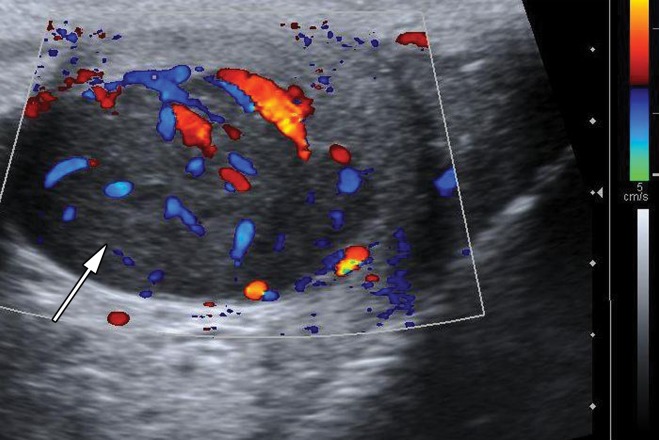
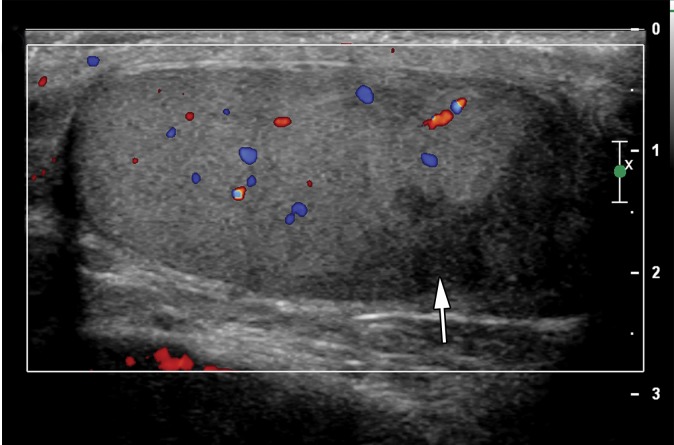
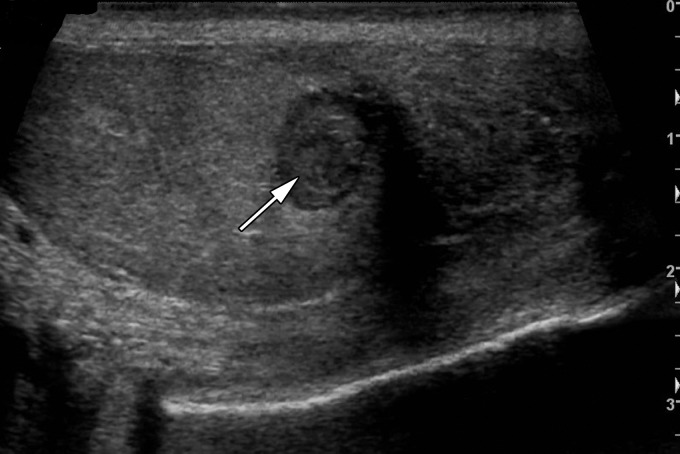
Testicular seminomas are typically unilateral (19). At gray-scale US, seminomas are hypoechoic compared with the background testis (Fig 4). They tend to be homogeneous and may be lobulated or multinodular (19) (Fig 5). Densely echogenic areas or calcifications and cystic spaces are relatively uncommon, seen in 30% and 10%, respectively (20).
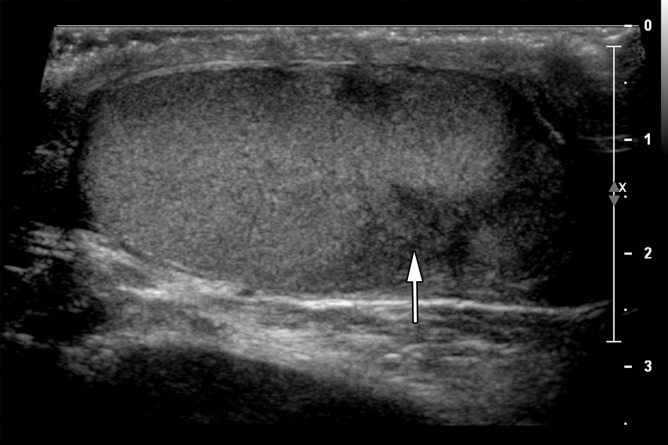
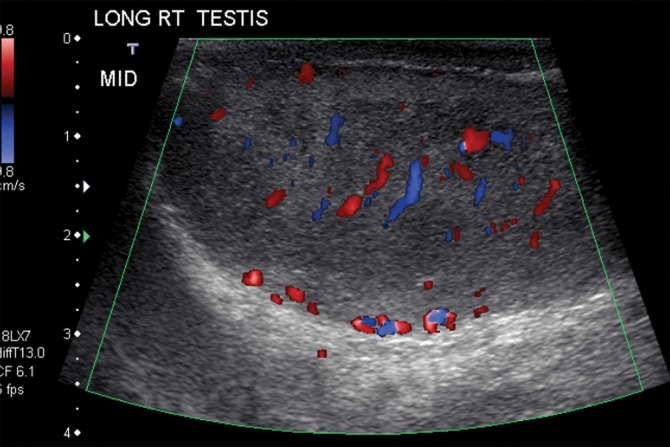
Figure 4a.
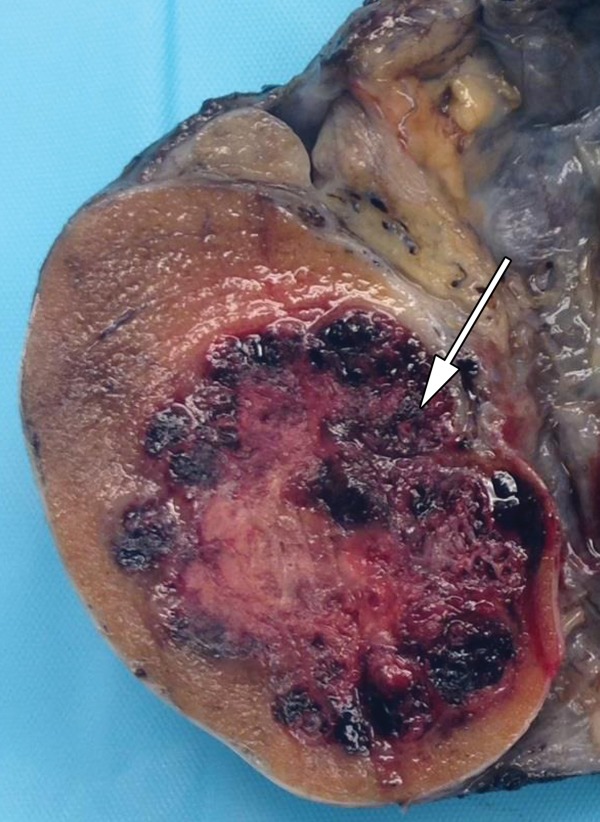
Seminoma in a 27-year-old man with a painless, palpable left testicular mass. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic mass (arrow in a) with marked internal color flow (arrow in b), characteristic of seminoma. (c) Photograph of the gross pathology specimen shows a tan, fleshy, well-circumscribed mass (*) typical of seminoma and surrounding normal testicle (arrow).
Figure 5a.
Lobulated seminoma in a 39-year-old man with a painless, palpable left testicular mass. (a) Gray-scale US image shows a well-circumscribed, hypoechoic, lobulated mass (arrow) typical of seminoma. (b) Photograph of the gross pathology specimen shows a lobulated fleshy mass (arrow) characteristic of seminoma.
Figure 4b.
Seminoma in a 27-year-old man with a painless, palpable left testicular mass. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic mass (arrow in a) with marked internal color flow (arrow in b), characteristic of seminoma. (c) Photograph of the gross pathology specimen shows a tan, fleshy, well-circumscribed mass (*) typical of seminoma and surrounding normal testicle (arrow).
Figure 4c.
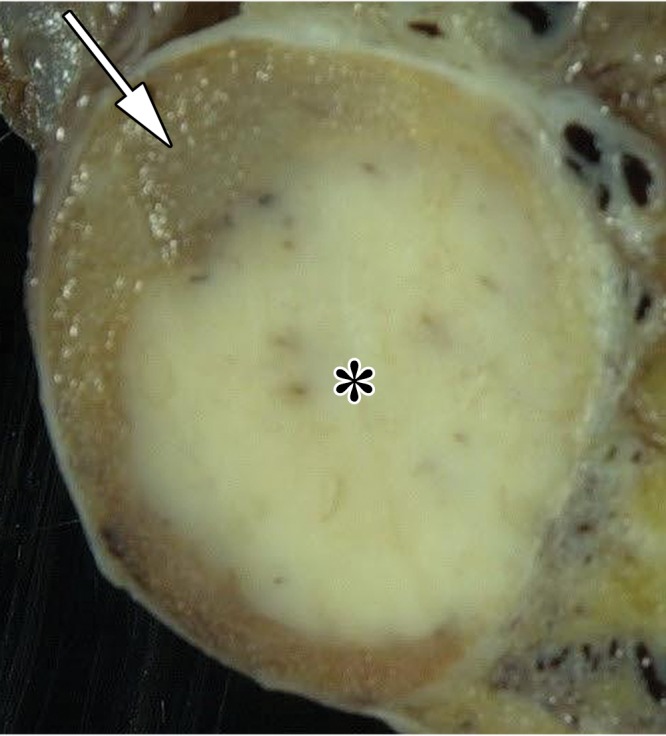
Seminoma in a 27-year-old man with a painless, palpable left testicular mass. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic mass (arrow in a) with marked internal color flow (arrow in b), characteristic of seminoma. (c) Photograph of the gross pathology specimen shows a tan, fleshy, well-circumscribed mass (*) typical of seminoma and surrounding normal testicle (arrow).
Figure 5b.
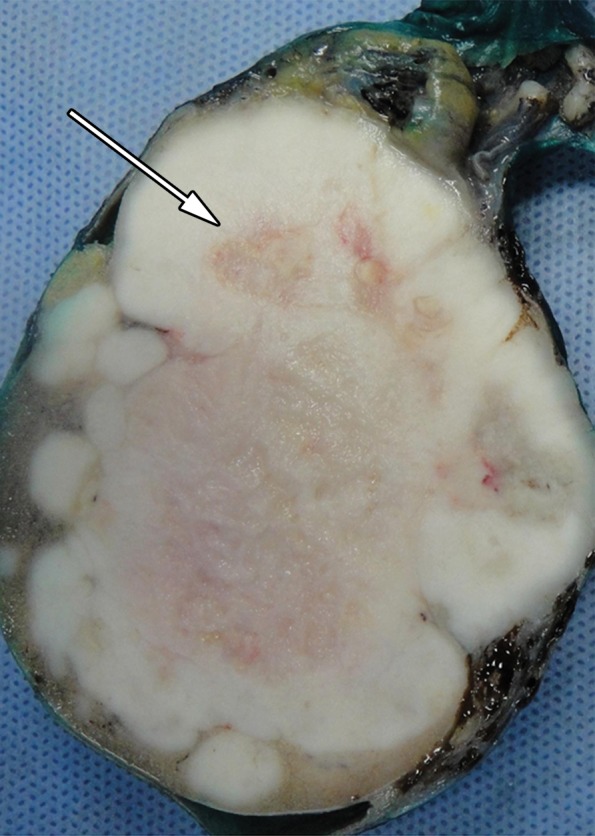
Lobulated seminoma in a 39-year-old man with a painless, palpable left testicular mass. (a) Gray-scale US image shows a well-circumscribed, hypoechoic, lobulated mass (arrow) typical of seminoma. (b) Photograph of the gross pathology specimen shows a lobulated fleshy mass (arrow) characteristic of seminoma.
At Doppler US, seminoma demonstrates increased vascularity compared with adjacent normal testis (21). Testicular seminomas are variable in size and in more than 50% of cases may be large enough to replace the entire normal testis (22–24). Sonoelastography reveals increased stiffness in the tumor compared with background normal testis due to the tumor’s cellularity and fibrous content (25).
In a small subset of patients with metastatic seminoma, US will demonstrate a normal testicular echotexture without a discrete lesion or only a small lesion or calcification within the testes (26). In the setting of a retroperitoneal mass or bulky adenopathy, the differential diagnosis includes metastatic disease from a “burned-out” testicular tumor or a rare primary retroperitoneal seminoma (23).
Computed Tomography
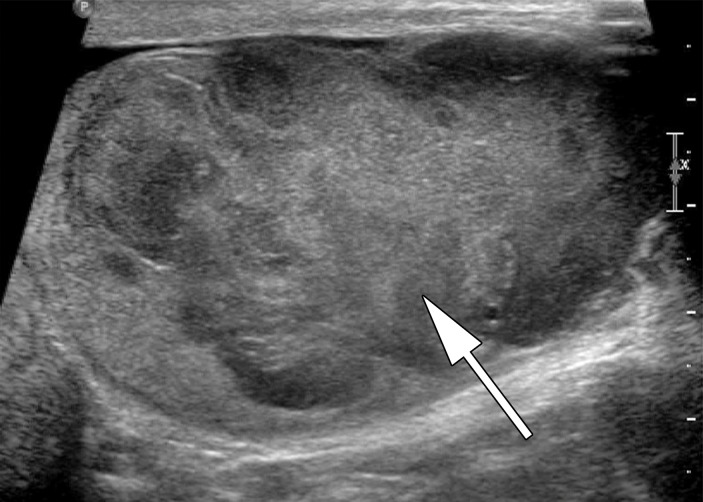
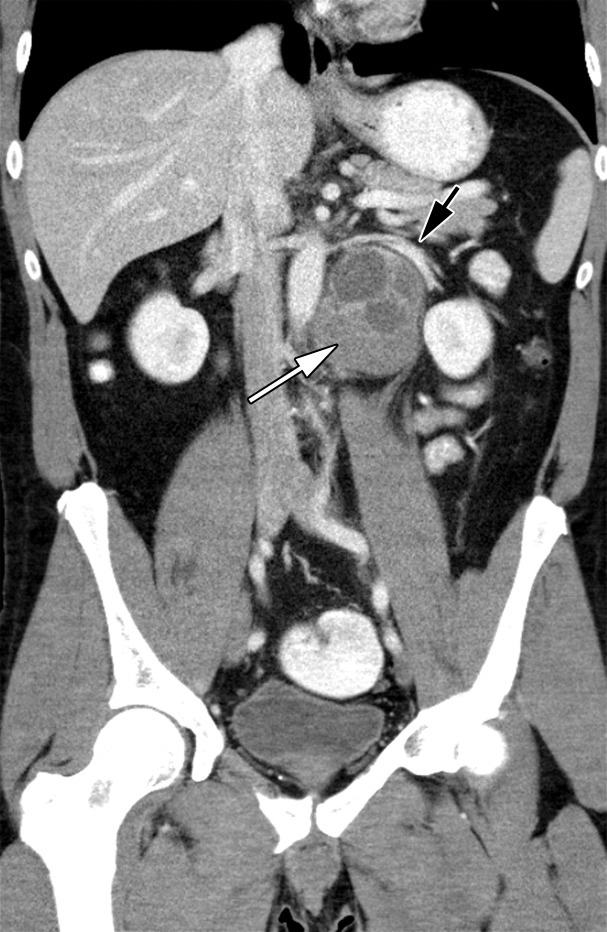
Approximately 20% of patients have metastatic disease at diagnosis, most commonly to the retroperitoneal lymph nodes. Patients with retroperitoneal adenopathy may present with abdominal or back pain or systemic symptoms (Fig 6) (11). CT may be the first imaging modality in the case of these nonspecific symptoms. Tumors of the right testis typically drain to the interaortocaval nodal group inferior to the renal hilar vessels. Left-sided tumors drain to the para-aortic and preaortic lymph nodes (11). CT is not used in evaluation of the primary testicular lesion.
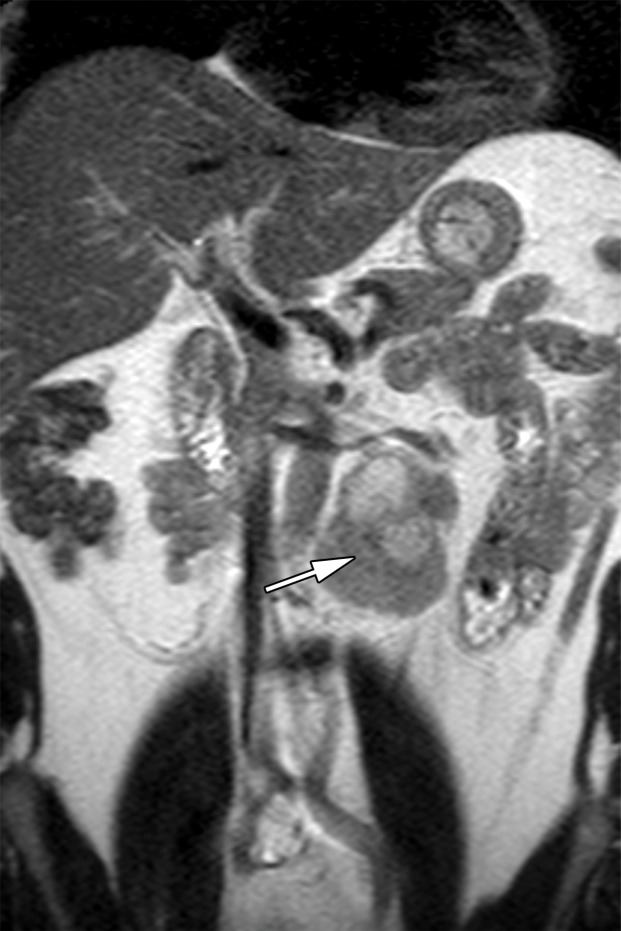
Figure 6a.
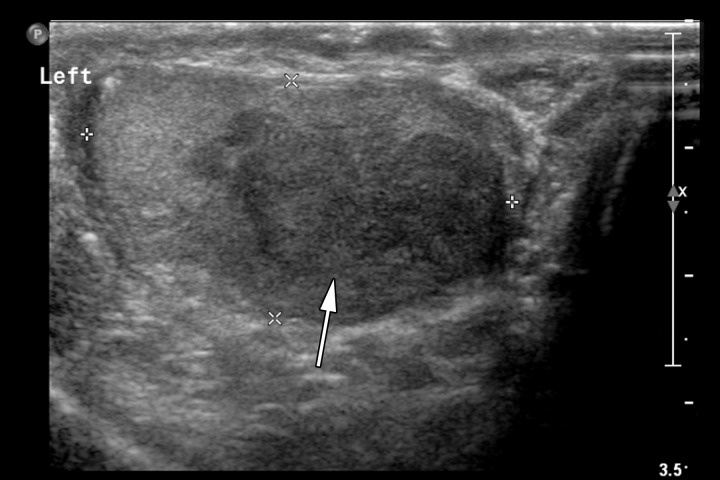
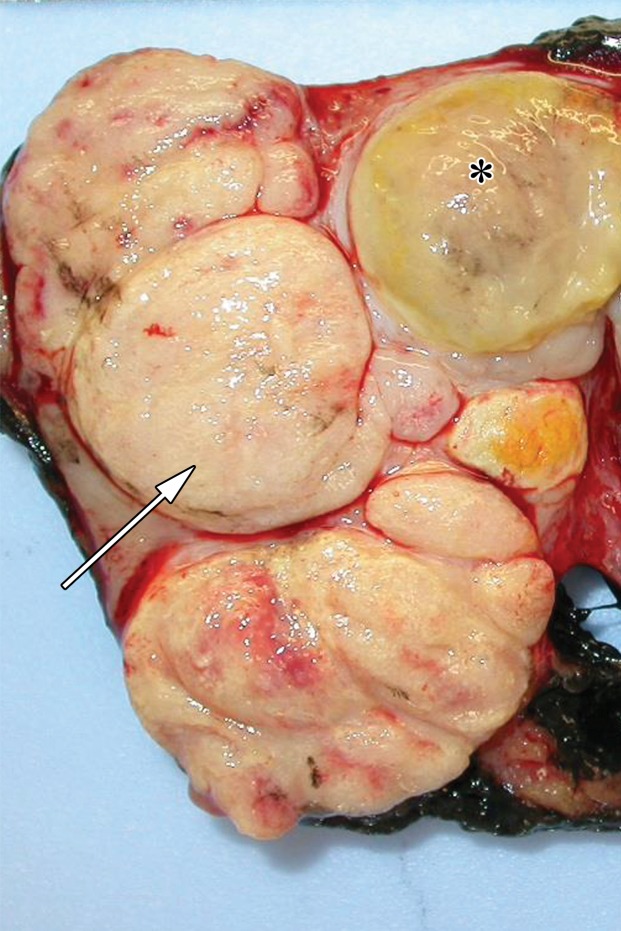
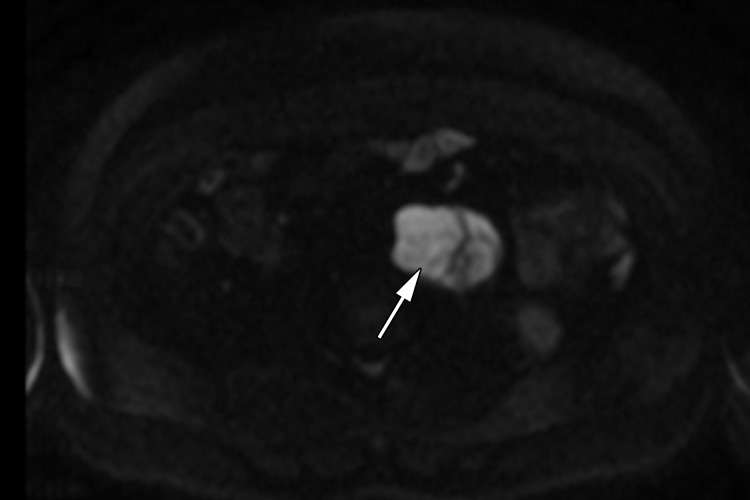
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
Figure 6c.
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
Figure 6e.
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
Figure 6f.
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
MR Imaging
Although not routinely performed as part of evaluation of an intratesticular mass, MR imaging has been shown to allow accurate distinction between seminomatous and nonseminomatous testicular neoplasms (27,28). Seminomas appear as multinodular, sharply defined, homogeneous tumors of low signal intensity on T2-weighted images (27,28). Areas of signal intensity heterogeneity related to hemorrhage or necrosis may be seen.
A key feature in seminoma is visualization of fibrovascular septa, which demonstrate low signal intensity on T2-weighted images and enhance more than the background tumor on postcontrast T1-weighted images. The fibrovascular septa may be thick or thin. A low-signal-intensity halo is variably seen, which correlates with a fibrous capsule at histologic analysis (27).
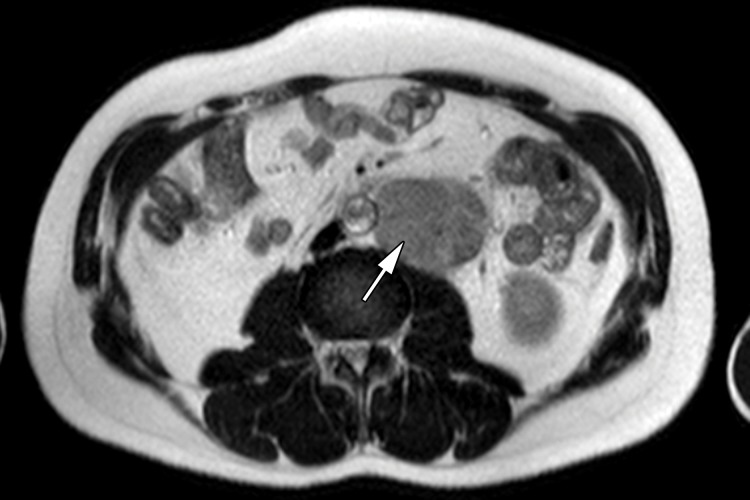
MR imaging may be used to accurately determine the local extent of disease in testicular carcinoma (29). Although CT is the current imaging test of choice, abdominopelvic MR imaging may also demonstrate retroperitoneal adenopathy and visceral metastases (Fig 6b–6d). MR imaging’s lack of ionizing radiation allows a reduction in radiation exposure compared with CT in this young patient cohort with excellent long-term survival rates.
Figure 6b.
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
Figure 6d.
Retroperitoneal adenopathy in a 45-year-old man with left back pain. (a) Coronal contrast-enhanced CT image shows left para-aortic adenopathy (white arrow) just below the level of the left renal artery (black arrow). (b–d) Coronal T2-weighted single-shot fast spin-echo (SSFSE) (b), axial T2-weighted SSFSE (c), and axial diffusion-weighted (d) MR images show the retroperitoneal adenopathy (arrow in b and c) with restricted diffusion (arrow in d). (e) Gray-scale US image obtained to look for a primary malignancy shows a well-circumscribed hypoechoic mass (arrow) representing primary testicular seminoma. (f) Photograph of the gross pathology specimen shows the retroperitoneal adenopathy with cystic space seen at imaging (*) and a solid, tan, fleshy, well-circumscribed mass (arrow) characteristic of seminoma.
Differential Diagnosis
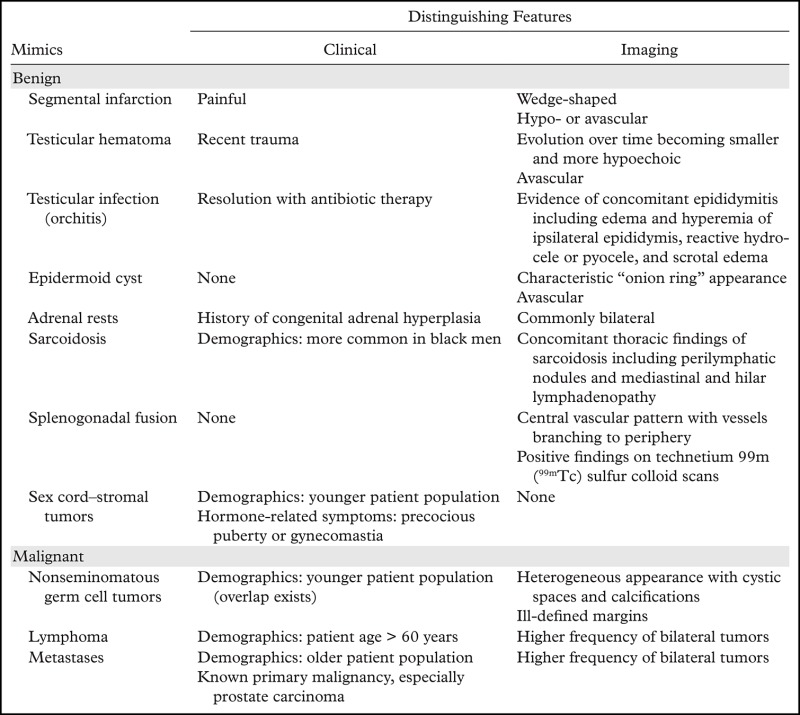
A large number of neoplastic and nonneoplastic conditions may mimic testicular seminoma at imaging. The following sections review important differential considerations and suggest distinguishing clinical and imaging features (Table).
Key Clinical and Imaging Features of Mimics of Testicular Seminoma
Benign Lesions
Segmental Infarction
Segmental infarction is an uncommon condition that may be idiopathic or represent a complication of epididymo-orchitis, prior testicular torsion, sickle cell anemia, vasculitis, or other hypercoagulable states (30,31). In cases of segmental infarction due to epididymo-orchitis, it has been suggested that venous compromise can result in a rounded lesion (30). The median age of patients with segmental infarction is 37 years, which overlaps with the age of the population that develops testicular seminoma.
At US, segmental infarction appears as a wedge-shaped or rounded area of hypoechogenicity within either testis (32) (Fig 7). When the lesion is wedge shaped, its apex is located at the mediastinum testis. When the area of infarction is rounded and homogeneous, it may mimic seminoma. A helpful US feature to distinguish segmental infarction from seminoma is markedly decreased or absent vascular flow at Doppler imaging (32). Clinically, segmental infarction typically manifests as acute pain, unlike the classic presentation of seminoma as a painless mass (30).
Figure 7a.
Testicular infarct in a 35-year-old man with a history of left epididymitis-orchitis. Gray-scale US (a) and color Doppler (b) images of the left testis show a wedge-shaped hypoechoic region (arrow in a) without internal vascular flow (arrow in b), compatible with segmental infarction.
Figure 7b.
Testicular infarct in a 35-year-old man with a history of left epididymitis-orchitis. Gray-scale US (a) and color Doppler (b) images of the left testis show a wedge-shaped hypoechoic region (arrow in a) without internal vascular flow (arrow in b), compatible with segmental infarction.
Testicular Hematoma
Testicular hematomas are a common consequence of testicular trauma. The US appearance of a testicular hematoma evolves over time (33). Acutely, hematomas are iso- to hyperechoic. Over time, hematomas decrease in size and become progressively more hypoechoic (33) (Fig 8).
Figure 8a.
Testicular hematoma in a 29-year-old man with scrotal pain after trauma. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic lesion (arrow in a) without color Doppler flow (arrow in b), compatible with testicular hematoma. (c) Color Doppler image 10 days later shows the lesion to be smaller and more hypoechoic (arrow), characteristic of testicular hematoma.
Figure 8b.
Testicular hematoma in a 29-year-old man with scrotal pain after trauma. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic lesion (arrow in a) without color Doppler flow (arrow in b), compatible with testicular hematoma. (c) Color Doppler image 10 days later shows the lesion to be smaller and more hypoechoic (arrow), characteristic of testicular hematoma.
Figure 8c.
Testicular hematoma in a 29-year-old man with scrotal pain after trauma. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a well-circumscribed hypoechoic lesion (arrow in a) without color Doppler flow (arrow in b), compatible with testicular hematoma. (c) Color Doppler image 10 days later shows the lesion to be smaller and more hypoechoic (arrow), characteristic of testicular hematoma.
Depending on the interval between the traumatic event and the imaging assessment, there may be overlap in the imaging findings of hematoma and seminoma. Fortunately, at Doppler evaluation hematomas are avascular, a helpful distinguishing feature from seminoma (34). If there is concern about an incidental testicular tumor discovered at testicular US performed for trauma, follow-up imaging to ensure resolution should be performed.
Testicular infection
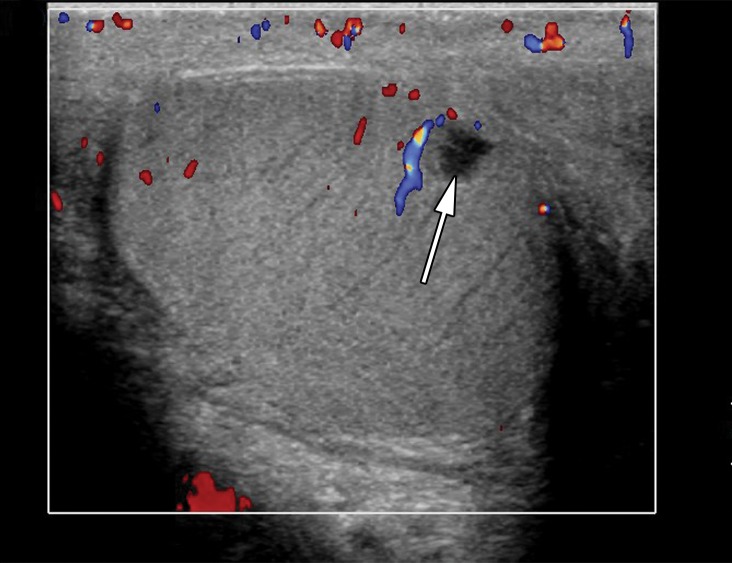
Testicular infection, including orchitis with or without abscess formation, may mimic seminoma. In the acute phase of orchitis, diffuse testicular edema results in a hypoechoic appearance of the testis (35). As the infection and inflammation evolve, the regions of hypoechogenicity become more localized (36). Since seminoma may appear as focal, multinodular, or diffuse regions of hypoechogenicity, the gray-scale US appearance may overlap. Furthermore, both orchitis and seminoma result in increased vascularity at Doppler imaging (35) (Fig 9).
Figure 9a.
Epididymitis-orchitis with abscess in a 40-year-old man with a 2-week history of right scrotal pain. Gray-scale US (a) and color Doppler (b) images of the right testis show an enlarged hyperemic epididymis (* in a and b) and increased testicular flow (white arrow in b). A focal area of decreased echogenicity (arrow in a) with no internal flow (black arrow in b) represents an intratesticular abscess.
Figure 9b.
Epididymitis-orchitis with abscess in a 40-year-old man with a 2-week history of right scrotal pain. Gray-scale US (a) and color Doppler (b) images of the right testis show an enlarged hyperemic epididymis (* in a and b) and increased testicular flow (white arrow in b). A focal area of decreased echogenicity (arrow in a) with no internal flow (black arrow in b) represents an intratesticular abscess.
Helpful imaging findings to suggest orchitis over seminoma include edema and hypervascularity of the ipsilateral epididymis, reactive hydrocele or pyocele, and associated scrotal edema (36). These findings are uncommon in seminoma. Clinically, seminoma may manifest with signs and symptoms that mimic orchitis. If doubt exists after the initial imaging assessment, follow-up imaging after a course of antibiotics should be performed.
Epidermoid Cyst
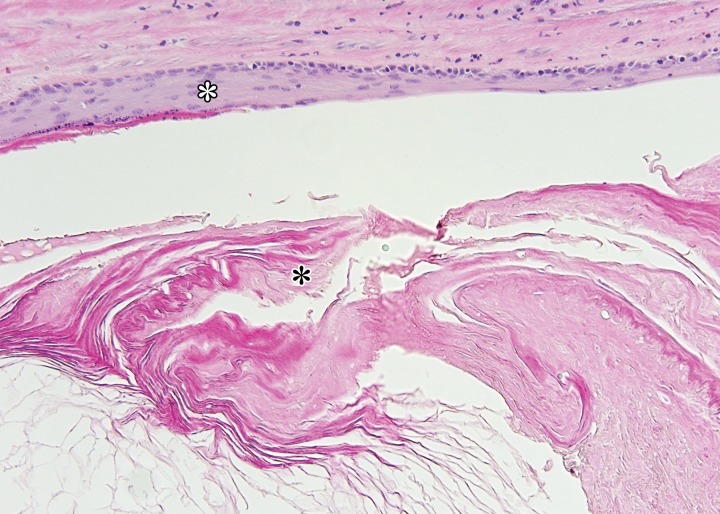
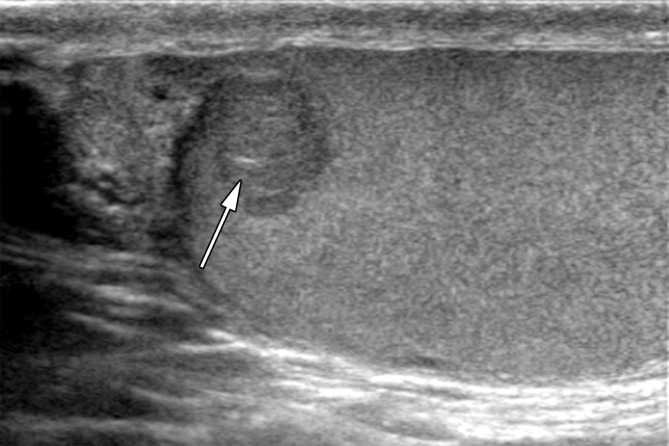
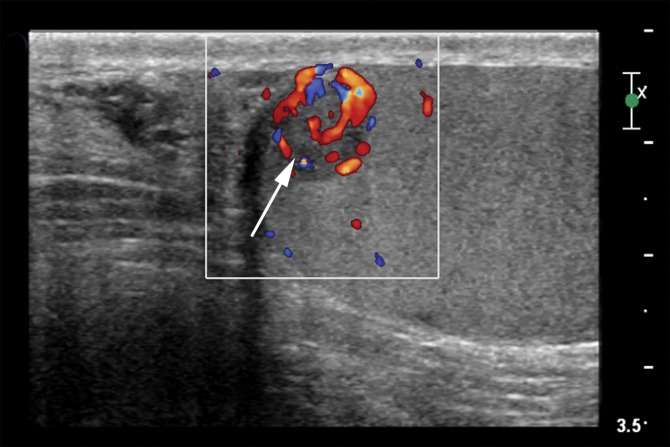
Epidermoid cysts, which account for 1% of testicular lesions, are composed of keratinizing, stratified, squamous epithelium surrounded by a fibrous wall (37). At US, epidermoid cysts are well-defined rounded lesions with a characteristic “onion ring” internal pattern of echoes (38) (Fig 10). Although these lesions are cystic, they may mimic an avascular or hypovascular solid lesion due to their internal echoes. When the characteristic imaging findings are present, this lesion should be distinguishable from the homogeneously hypoechoic appearance of seminoma (19).
Figure 10a.
Epidermoid cyst in a 45-year-old man with a painless, palpable right testicular mass. (a) Gray-scale US image of the right testis shows a well-circumscribed hypoechoic mass with an “onion skin” appearance (*), classic for epidermoid cyst. (b, c) Photograph of the gross pathology specimen (b) and low-power photomicrograph (c) show the characteristic keratinous material with a laminated appearance (* in b, black * in c). The cyst is lined by keratinizing squamous epithelium (white * in c). (H-E stain.)
Figure 10b.
Epidermoid cyst in a 45-year-old man with a painless, palpable right testicular mass. (a) Gray-scale US image of the right testis shows a well-circumscribed hypoechoic mass with an “onion skin” appearance (*), classic for epidermoid cyst. (b, c) Photograph of the gross pathology specimen (b) and low-power photomicrograph (c) show the characteristic keratinous material with a laminated appearance (* in b, black * in c). The cyst is lined by keratinizing squamous epithelium (white * in c). (H-E stain.)
Figure 10c.
Epidermoid cyst in a 45-year-old man with a painless, palpable right testicular mass. (a) Gray-scale US image of the right testis shows a well-circumscribed hypoechoic mass with an “onion skin” appearance (*), classic for epidermoid cyst. (b, c) Photograph of the gross pathology specimen (b) and low-power photomicrograph (c) show the characteristic keratinous material with a laminated appearance (* in b, black * in c). The cyst is lined by keratinizing squamous epithelium (white * in c). (H-E stain.)
Adrenal Rests
Adrenal rests commonly affect patients with congenital adrenal hyperplasia (CAH), a heterogeneous group of autosomal recessive genetic conditions (39). Patients with CAH have enzymatic defects that result in inadequate cortisol production. Owing to a lack of negative feedback, patients produce excess corticotropin, which may result in hypertrophy of ectopic adrenal cells within otherwise normal testes.
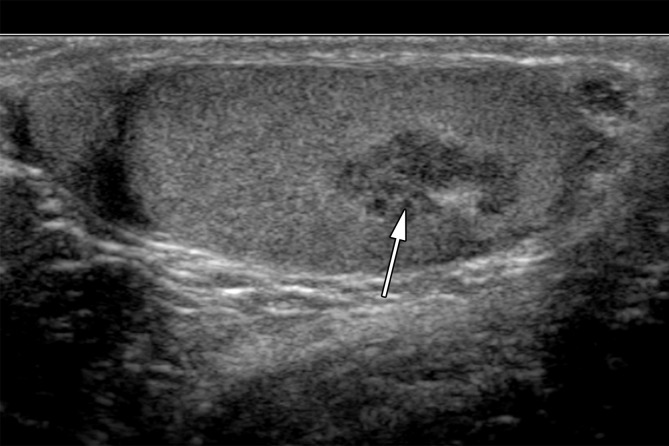
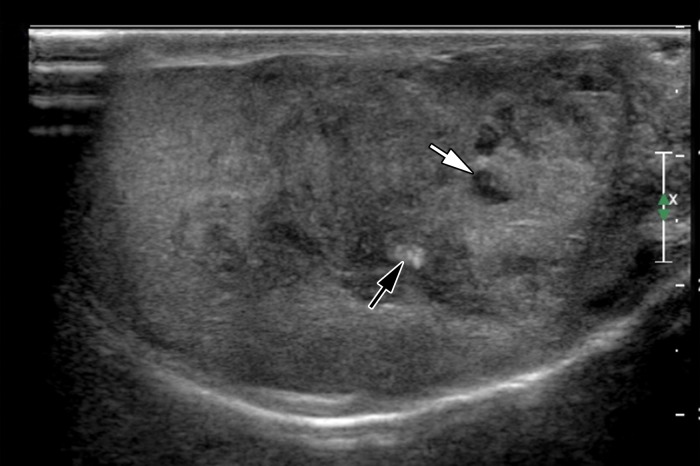
At US, adrenal rests are commonly hypoechoic masses, similar to seminoma (40). They can be variable in size, with one recent series describing a range of 4–38 mm (39). Adrenal rests are usually bilateral, an uncommon occurrence in seminoma (Fig 11). Although the imaging features of seminoma and adrenal rests may overlap, the clinical history should make differentiation possible. Additionally, alterations in treatment focused on reducing excess corticotropin may cause the lesions to regress at follow-up imaging (39).
Figure 11a.
Adrenal rests in a 25-year-old man with known congenital adrenal hyperplasia who presented with rising laboratory values despite treatment. Gray-scale US images of the right (a) and left (b) testes show bilateral hypoechoic masses (arrows) representing adrenal rests.
Figure 11b.
Adrenal rests in a 25-year-old man with known congenital adrenal hyperplasia who presented with rising laboratory values despite treatment. Gray-scale US images of the right (a) and left (b) testes show bilateral hypoechoic masses (arrows) representing adrenal rests.
Sarcoidosis
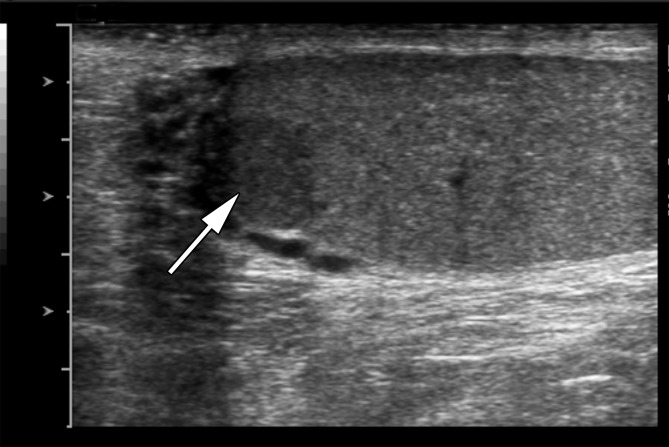
Sarcoidosis is a systemic disease characterized by formation of noncaseating granulomas. Involvement of the testis in systemic sarcoidosis is uncommon, affecting approximately 4% of patients (41). The clinical presentation is variable, ranging from an asymptomatic incidental finding to acute testicular pain. At US, testicular sarcoidosis is most commonly a focal hypoechoic mass lesion, similar to seminoma (41,42).
Clinical factors may allow differentiation of sarcoidosis from seminoma. Since isolated testicular involvement is rare, the presence of characteristic findings of pulmonary sarcoidosis may help, although mediastinal adenopathy can be seen with both conditions. Epidemiology may also be helpful, as seminoma commonly affects white men while sarcoidosis more frequently affects black men (42).
Splenogonadal Fusion
Splenogonadal fusion is a rare condition that involves congenital fusion of the testis with ectopic splenic tissue (43). Splenogonadal fusion is associated with cryptorchidism, also a risk factor for seminoma (43). Patients most commonly present with a painless mass, the most common presentation of seminoma.
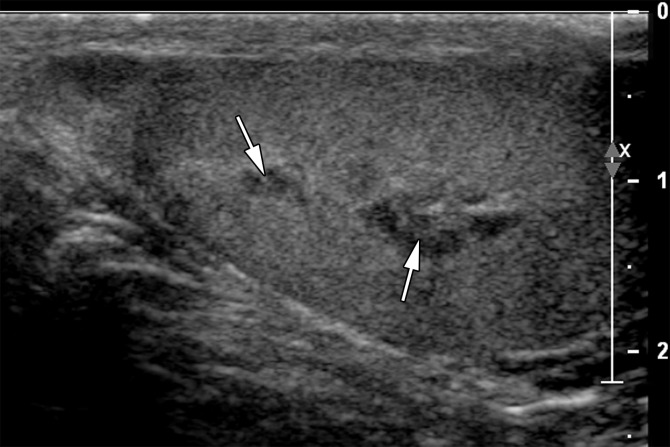
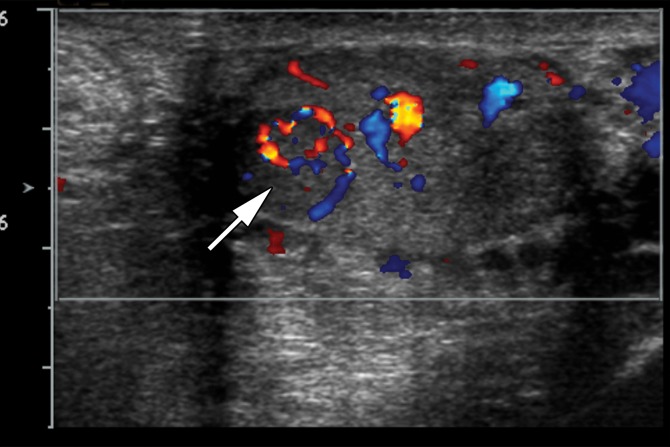
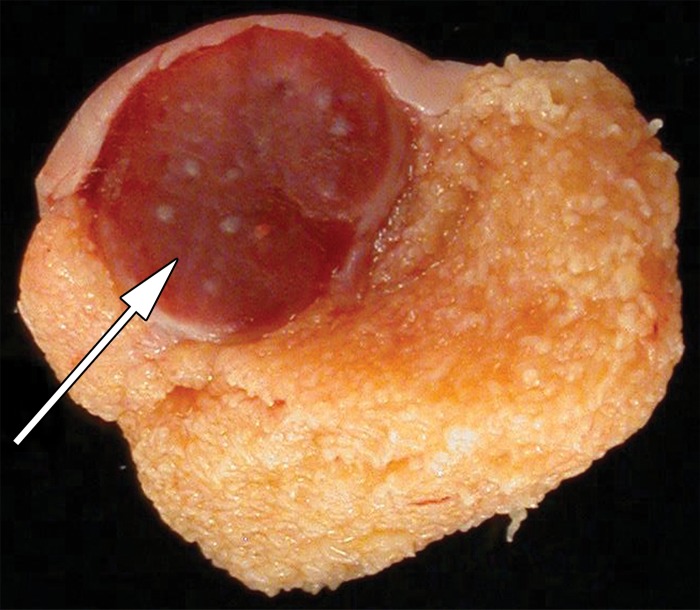
The ectopic splenic tissue is usually hypoechoic at gray-scale US and may be challenging to separate from the ipsilateral testis (41,43) (Fig 12). It has been suggested that a central vascular pattern with vessels branching toward the lesion’s periphery may be a helpful distinguishing feature (41). If splenogonadal fusion is suspected, 99mTc sulfur colloid scans can confirm activity in ectopic splenic tissue (44).
Figure 12a.
Splenogonadal fusion in a 29-year-old man with a painless, palpable left testicular mass, found during workup for infertility. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a hypoechoic mass (arrow in a) with internal flow (arrow in b). (c) Photograph of the gross pathology specimen shows a mass (arrow) representing splenic tissue.
Figure 12b.
Splenogonadal fusion in a 29-year-old man with a painless, palpable left testicular mass, found during workup for infertility. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a hypoechoic mass (arrow in a) with internal flow (arrow in b). (c) Photograph of the gross pathology specimen shows a mass (arrow) representing splenic tissue.
Figure 12c.
Splenogonadal fusion in a 29-year-old man with a painless, palpable left testicular mass, found during workup for infertility. (a, b) Gray-scale US (a) and color Doppler (b) images of the left testis show a hypoechoic mass (arrow in a) with internal flow (arrow in b). (c) Photograph of the gross pathology specimen shows a mass (arrow) representing splenic tissue.
Sex Cord–Stromal Tumors
Sex cord–stromal tumors, including Leydig cell tumor, Sertoli cell tumor, thecoma, and granulosa cell tumor, are uncommon testicular neoplasms, accounting for only 5% of testicular neoplasms (23). Sex cord–stromal tumors are typically benign, although malignant cases have been reported. At US, sex cord–stromal tumors may appear as a focal hypoechoic mass, identical to seminoma (Fig 13). Distinguishing clinical features are young age at presentation and hormone-related symptoms, such as precocious puberty or gynecomastia (24).
Figure 13a.
Leydig cell neoplasm in a 24-year-old man with a palpable right scrotal mass and new-onset gynecomastia. (a, b) Gray-scale US (a) and color Doppler (b) images of the right testis show a well-circumscribed hypoechoic mass (arrow in a) with internal color flow (arrow in b). (c, d) Photograph of the gross pathology specimen (c) and high-power photomicrograph (d) show a well-circumscribed mahogany brown mass (arrow in c) and polygonal cells with abundant eosinophilic cytoplasm (black arrows in d) and prominent nucleoli (white arrows in d), characteristic of Leydig cell tumor. (H-E stain.)
Figure 13b.
Leydig cell neoplasm in a 24-year-old man with a palpable right scrotal mass and new-onset gynecomastia. (a, b) Gray-scale US (a) and color Doppler (b) images of the right testis show a well-circumscribed hypoechoic mass (arrow in a) with internal color flow (arrow in b). (c, d) Photograph of the gross pathology specimen (c) and high-power photomicrograph (d) show a well-circumscribed mahogany brown mass (arrow in c) and polygonal cells with abundant eosinophilic cytoplasm (black arrows in d) and prominent nucleoli (white arrows in d), characteristic of Leydig cell tumor. (H-E stain.)
Figure 13c.
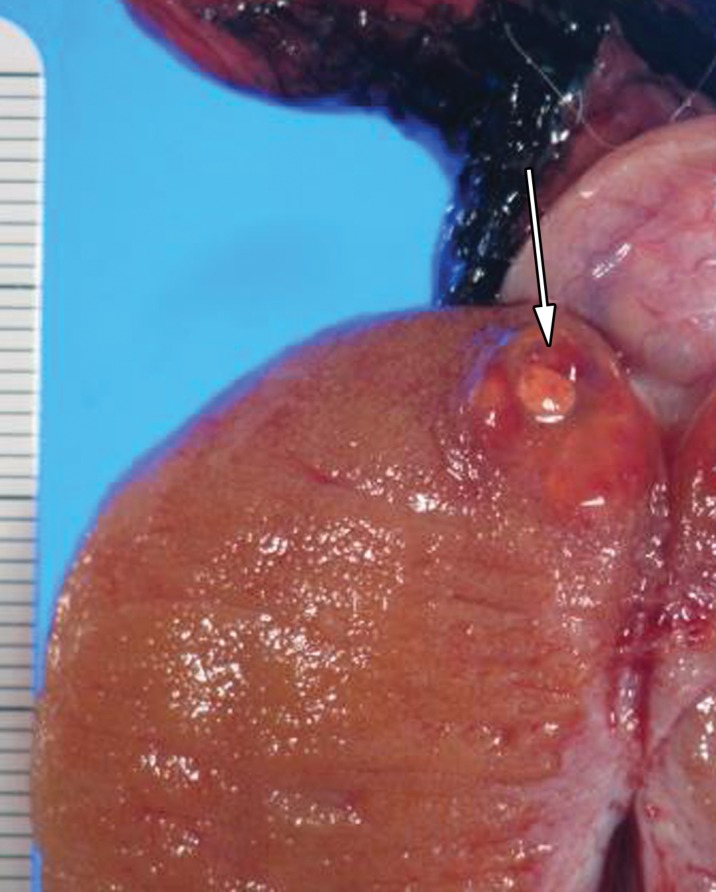
Leydig cell neoplasm in a 24-year-old man with a palpable right scrotal mass and new-onset gynecomastia. (a, b) Gray-scale US (a) and color Doppler (b) images of the right testis show a well-circumscribed hypoechoic mass (arrow in a) with internal color flow (arrow in b). (c, d) Photograph of the gross pathology specimen (c) and high-power photomicrograph (d) show a well-circumscribed mahogany brown mass (arrow in c) and polygonal cells with abundant eosinophilic cytoplasm (black arrows in d) and prominent nucleoli (white arrows in d), characteristic of Leydig cell tumor. (H-E stain.)
Figure 13d.
Leydig cell neoplasm in a 24-year-old man with a palpable right scrotal mass and new-onset gynecomastia. (a, b) Gray-scale US (a) and color Doppler (b) images of the right testis show a well-circumscribed hypoechoic mass (arrow in a) with internal color flow (arrow in b). (c, d) Photograph of the gross pathology specimen (c) and high-power photomicrograph (d) show a well-circumscribed mahogany brown mass (arrow in c) and polygonal cells with abundant eosinophilic cytoplasm (black arrows in d) and prominent nucleoli (white arrows in d), characteristic of Leydig cell tumor. (H-E stain.)
Malignant Lesions
Nonseminomatous Germ Cell Tumors
Nonseminomatous germ cell tumors include embryonal carcinoma, teratoma, yolk sac tumor, and choriocarcinoma (3). The most common nonseminomatous germ cell tumor is mixed germ cell tumor (MGCT), where multiple histologic subtypes coexist (19). MGCTs tend to be more heterogeneous in echotexture and echogenicity than seminomas.
Although seminomas, especially large seminomas, may demonstrate cystic spaces and calcifications, these findings are more commonly encountered in MGCT (Fig 14). MGCTs are more likely to have ill-defined margins than are seminomas (24). The clinical presentations of seminomatous and nonseminomatous tumors overlap, but on average men with seminoma tend to be about a decade older than patients with nonseminomatous tumors (23).
Figure 14a.
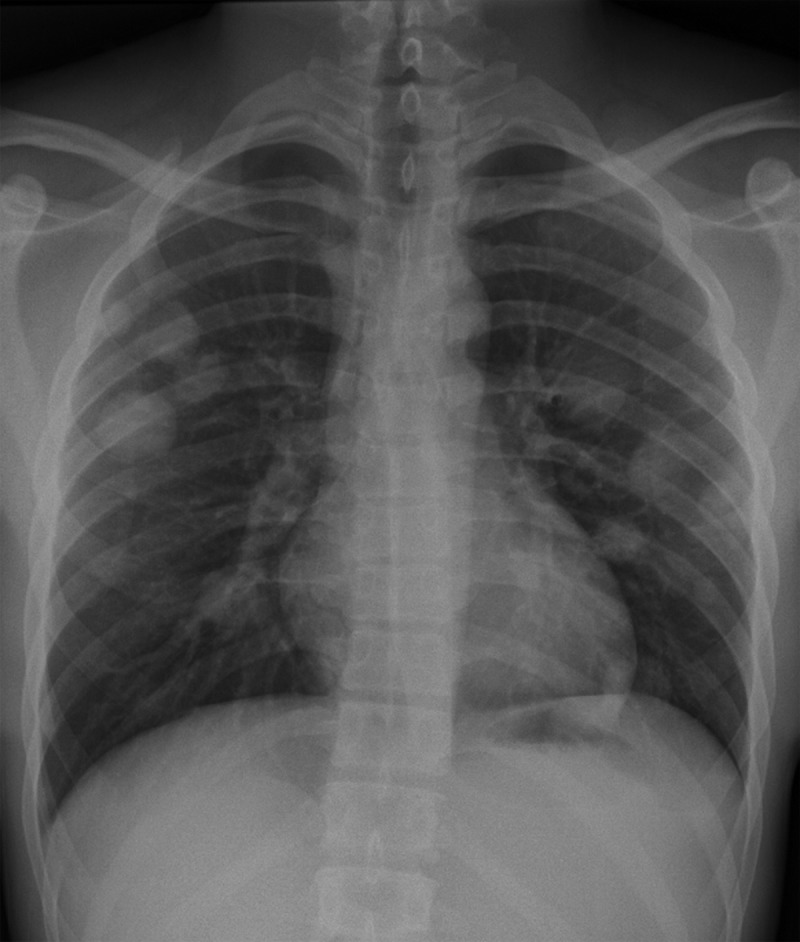
Malignant MGCT in a 20-year-old man with left chest pain. (a) Posteroanterior chest radiograph shows pulmonary metastases. Testicular US was performed to look for a primary neoplasm. (b) Gray-scale US image of the left testis shows an ill-defined heterogeneous mass with cystic spaces (white arrow) and calcifications (black arrow). (c) Photograph of the gross pathology specimen shows a variegated appearance (arrow) secondary to the heterogeneity of the tumor, which explains the heterogeneous US appearance.
Figure 14b.
Malignant MGCT in a 20-year-old man with left chest pain. (a) Posteroanterior chest radiograph shows pulmonary metastases. Testicular US was performed to look for a primary neoplasm. (b) Gray-scale US image of the left testis shows an ill-defined heterogeneous mass with cystic spaces (white arrow) and calcifications (black arrow). (c) Photograph of the gross pathology specimen shows a variegated appearance (arrow) secondary to the heterogeneity of the tumor, which explains the heterogeneous US appearance.
Figure 14c.
Malignant MGCT in a 20-year-old man with left chest pain. (a) Posteroanterior chest radiograph shows pulmonary metastases. Testicular US was performed to look for a primary neoplasm. (b) Gray-scale US image of the left testis shows an ill-defined heterogeneous mass with cystic spaces (white arrow) and calcifications (black arrow). (c) Photograph of the gross pathology specimen shows a variegated appearance (arrow) secondary to the heterogeneity of the tumor, which explains the heterogeneous US appearance.
Lymphoma
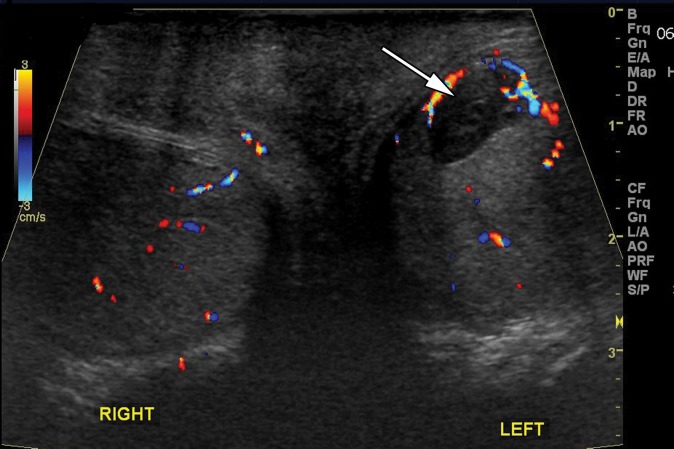
Non-Hodgkin B-cell lymphoma is the most common testicular malignancy in men older than 60 years (45). At US, testicular lymphoma is hypoechoic and hypervascular, similar to both seminoma and epididymo-orchitis (46) (Fig 15). Testicular lymphoma is typically painless, allowing clinical differentiation from epididymo-orchitis. Lymphoma is usually unilateral but can be bilateral in approximately one-third of cases, a much higher percentage than seminoma. Although the imaging appearance of lymphoma parallels that of seminoma, the affected patient population is significantly older (23).
Figure 15a.
Lymphoma in a 72-year-old man with right testicular pain and swelling. Gray-scale US (a) and color Doppler (b) images of the right testis show an ill-defined, hypoechoic, hypervascular mass replacing almost the entire testis, which was proven to be diffuse large B-cell lymphoma. Note the remaining rim of normal testicular tissue (arrow in a).
Figure 15b.
Lymphoma in a 72-year-old man with right testicular pain and swelling. Gray-scale US (a) and color Doppler (b) images of the right testis show an ill-defined, hypoechoic, hypervascular mass replacing almost the entire testis, which was proven to be diffuse large B-cell lymphoma. Note the remaining rim of normal testicular tissue (arrow in a).
Metastases
Although uncommon, a variety of primary tumors can metastasize to the testes. The most common primary malignancy that spreads to the testis is prostate adenocarcinoma, accounting for 35% of cases (24). Metastases due to tumors of the lung, skin (melanoma), colon, and kidney have also been described (24). The imaging appearance of testicular metastases is nonspecific and may overlap with that of seminoma.
Fortunately, epidemiologic factors may help. Patients affected by testicular metastases are older on average than men with seminoma. Testicular metastases usually occur in the setting of advanced metastatic disease. Additionally, most metastases are unilateral, but bilateral disease can be seen in up to 15% of patients (23).
Spermatocytic Tumor
Until the 2016 update of the World Health Organization (WHO) Classification of Tumours of the Urinary System and Male Genital Organs (15), spermatocytic tumor was known as spermatocytic seminoma, a subtype of seminoma. Currently, spermatocytic tumor is thought to be unrelated to classic seminoma (15). Specifically, spermatocytic tumor lacks an association with germ cell neoplasia in situ (GCNIS). It also lacks 12p amplification and shows a unique amplification of chromosome 9 corresponding to the DMRT1 gene (15). Spermatocytic tumor is not associated with cryptorchidism, a major risk factor for seminoma (47).
Spermatocytic tumor accounts for only 1% of testicular cancers. It affects older men than does classic seminoma, with a median age at diagnosis of 54 years (1). It is unilateral in 90% of cases, but at 10%, bilateral disease is more common than in classic seminoma (48).
Spermatocytic tumor manifests as a slowly enlarging but painless mass. The majority of tumors are larger than 5 cm at diagnosis. It demonstrates indolent behavior, rarely metastasizing in the absence of sarcomatous transformation. Coexistent sarcoma occurs in 6% of cases (47). The typical therapy for spermatocytic tumor without sarcomatous transformation is limited to curative orchiectomy.
Despite the disparate molecular features of seminoma and spermatocytic tumor, the gross features are similar. Exceptions include a more gelatinous appearance of the cut tumor surface and more cystic spaces in spermatocytic tumor than in seminoma (16).
At histologic analysis, spermatocytic tumor is composed of an admixture of small, medium, and giant tumor cells (Fig 16). The giant cells may be multinucleate. Pleomorphism, atypical mitoses, and apoptosis are common findings (16). The tumor cells lack the glycogenation seen in seminoma. At immunohistochemical staining, SALL4 shows positivity. CKIT is positive in 50% of cases. Spermatocytic tumor does not react to OCT3/4 (16).
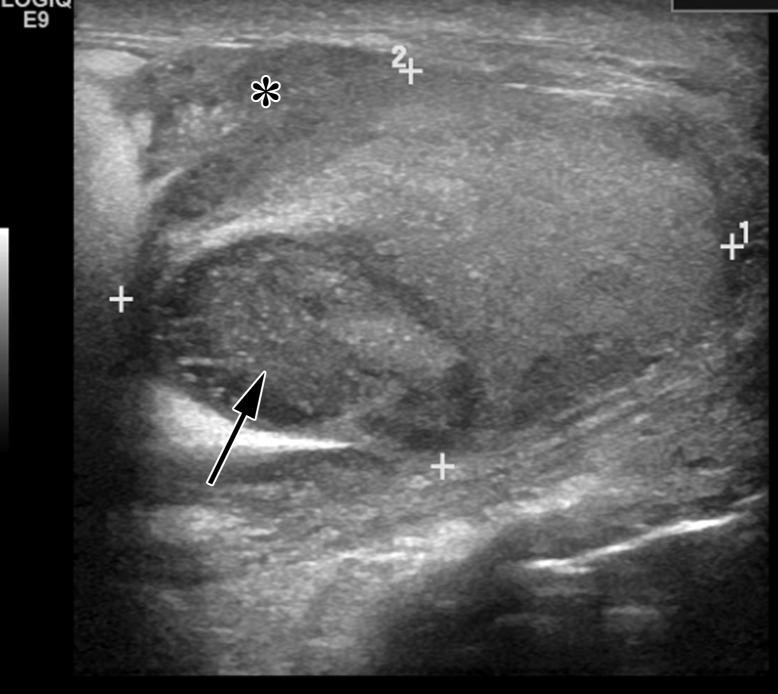
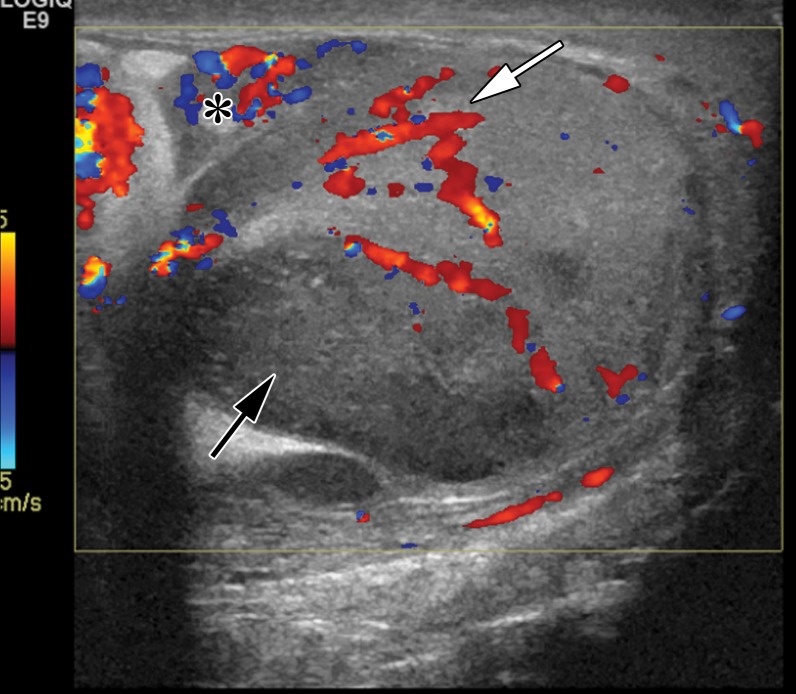
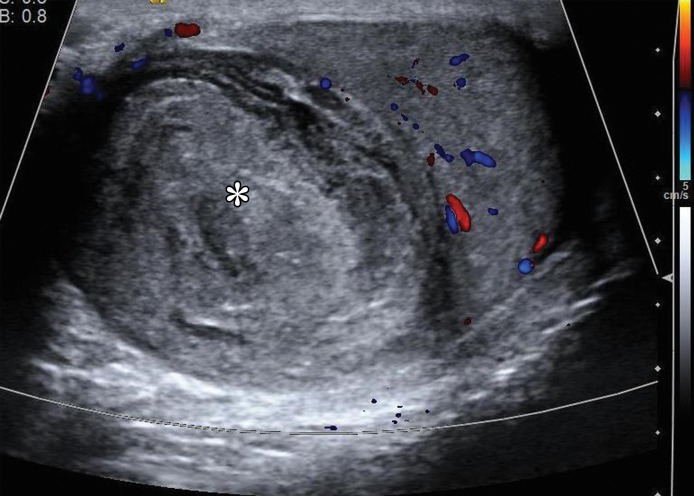
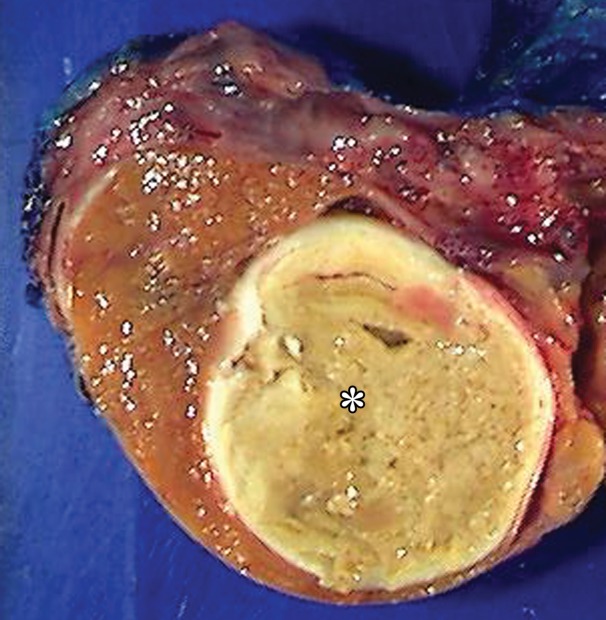
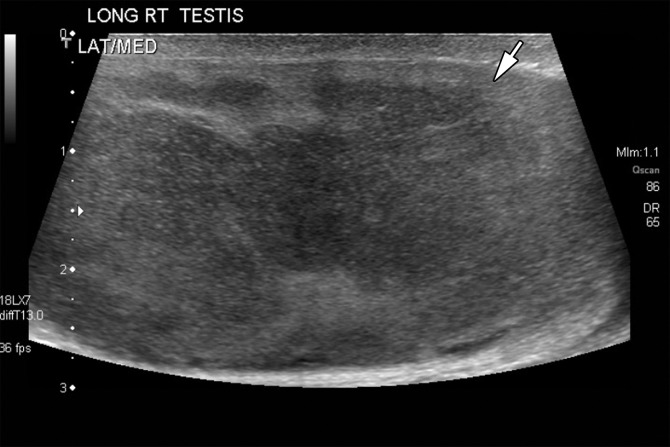
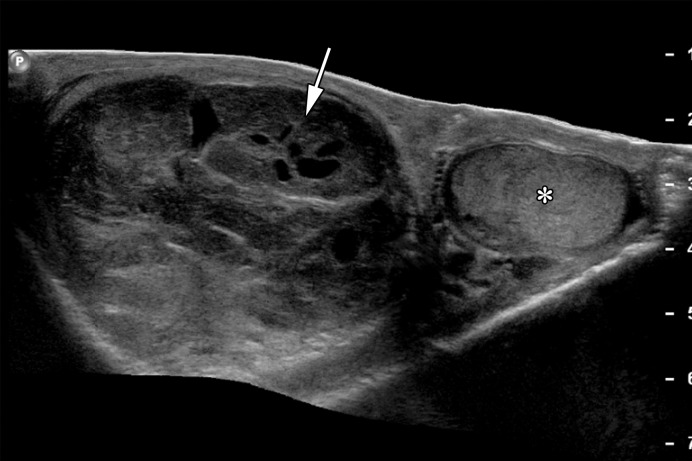
Figure 16a.
Spermatocytic tumor in an 85-year-old man with an enlarging, painless right testicular mass. (a) Panoramic gray-scale US image shows a markedly enlarged heterogeneous right testis (arrow) adjacent to the normal left testis (*). (b) Photograph of the gross pathology specimen shows the heterogeneous appearance of the tumor (arrow). (c) High-power photomicrograph shows the characteristic small cells (blue arrow), intermediate cells (green arrow), and giant cells (yellow arrow) of spermatocytic tumor. The intermediate cells show a spireme-like chromatin distribution. Mitoses (arrowhead) are also present. (H-E stain.)
Figure 16b.
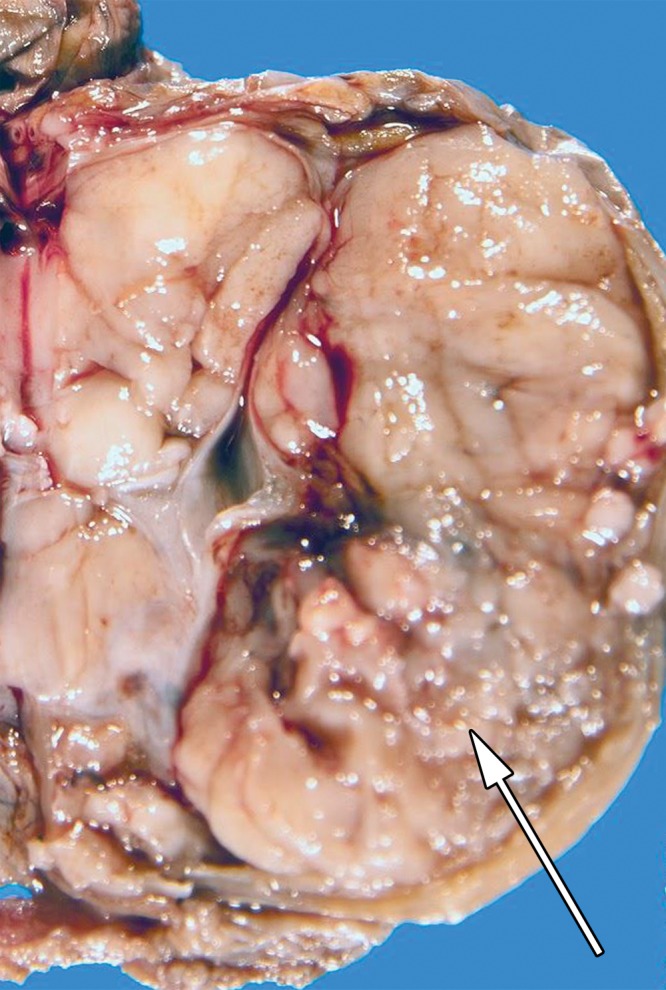
Spermatocytic tumor in an 85-year-old man with an enlarging, painless right testicular mass. (a) Panoramic gray-scale US image shows a markedly enlarged heterogeneous right testis (arrow) adjacent to the normal left testis (*). (b) Photograph of the gross pathology specimen shows the heterogeneous appearance of the tumor (arrow). (c) High-power photomicrograph shows the characteristic small cells (blue arrow), intermediate cells (green arrow), and giant cells (yellow arrow) of spermatocytic tumor. The intermediate cells show a spireme-like chromatin distribution. Mitoses (arrowhead) are also present. (H-E stain.)
Figure 16c.
Spermatocytic tumor in an 85-year-old man with an enlarging, painless right testicular mass. (a) Panoramic gray-scale US image shows a markedly enlarged heterogeneous right testis (arrow) adjacent to the normal left testis (*). (b) Photograph of the gross pathology specimen shows the heterogeneous appearance of the tumor (arrow). (c) High-power photomicrograph shows the characteristic small cells (blue arrow), intermediate cells (green arrow), and giant cells (yellow arrow) of spermatocytic tumor. The intermediate cells show a spireme-like chromatin distribution. Mitoses (arrowhead) are also present. (H-E stain.)
Only limited descriptions of the US appearance of spermatocytic tumor are present in the literature (48). These reports describe spermatocytic tumor as a well-defined but inhomogeneous mass (47). It may contain cystic spaces (48).
Conclusion
Testicular cancer, of which seminoma is the most common pure subtype, is an important disease affecting young men. Radiologists have a vital role in diagnosis, staging, and follow-up of patients affected by seminoma. A thorough understanding of the clinical, radiologic, and pathologic findings of this disease will help the radiologist contribute to high-quality interdisciplinary care of affected patients. With adherence to current diagnostic and treatment protocols, long-term survival of patients with testicular seminoma can be achieved.
Supported by the American Institute for Radiologic Pathology, the Joint Pathology Center, and Uniformed Services University of the Health Sciences. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the U.S. Government.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Abbreviation:
- H-E
- hematoxylin-eosin
References
- 1.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973-2007. Andrology 2015;3(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Network NCC. Testicular cancer (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf. Published 2016. Accessed March 17, 2016.
- 4.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 2003;170(1):5–11. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson SM, Lowrance WT. Epidemiology and diagnosis of testis cancer. Urol Clin North Am 2015;42(3):269–275. [DOI] [PubMed] [Google Scholar]
- 6.Theodore Ch, Terrier-Lacombe MJ, Laplanche A, et al. Bilateral germ-cell tumours: 22-year experience at the Institut Gustave Roussy. Br J Cancer 2004;90(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol 2012;22(11):2540–2546. [DOI] [PubMed] [Google Scholar]
- 8.Tiemstra JD, Kapoor S. Evaluation of scrotal masses. Am Fam Physician 2008;78(10):1165–1170. [PubMed] [Google Scholar]
- 9.Jones RH, Vasey PA. Part I: testicular cancer—management of early disease. Lancet Oncol 2003;4(12):730–737. [DOI] [PubMed] [Google Scholar]
- 10.International Germ Cell Cancer Collaborative Group . International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997;15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 11.Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. AJR Am J Roentgenol 2013;200(6):1215–1225. [DOI] [PubMed] [Google Scholar]
- 12.Nazeer T, Ro JY, Amato RJ, Park YW, Ordonez NG, Ayala AG. Histologically pure seminoma with elevated alpha-fetoprotein: a clinicopathologic study of ten cases. Oncol Rep 1998;5(6):1425–1429. [DOI] [PubMed] [Google Scholar]
- 13.Dong W, Gang W, Liu M, Zhang H. Analysis of the prognosis of patients with testicular seminoma. Oncol Lett 2016;11(2):1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mead GM, Fossa SD, Oliver RT, et al. Randomized trials in 2466 patients with stage I seminoma: patterns of relapse and follow-up. J Natl Cancer Inst 2011;103(3):241–249. [DOI] [PubMed] [Google Scholar]
- 15.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs: part A—renal, penile, and testicular tumours. Eur Urol 2016;70(1):93–105. [DOI] [PubMed] [Google Scholar]
- 16.Howitt BE, Berney DM. Tumors of the testis: morphologic features and molecular alterations. Surg Pathol Clin 2015;8(4):687–716. [DOI] [PubMed] [Google Scholar]
- 17.Browne TJ, Richie JP, Gilligan TD, Rubin MA. Intertubular growth in pure seminomas: associations with poor prognostic parameters. Hum Pathol 2005;36(6):640–645. [DOI] [PubMed] [Google Scholar]
- 18.Rifkin MD, Kurtz AB, Pasto ME, Goldberg BB. Diagnostic capabilities of high-resolution scrotal ultrasonography: prospective evaluation. J Ultrasound Med 1985;4(1):13–19. [DOI] [PubMed] [Google Scholar]
- 19.Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. Tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. RadioGraphics 2002;22(1):189–216. [DOI] [PubMed] [Google Scholar]
- 20.Schwerk WB, Schwerk WN, Rodeck G. Testicular tumors: prospective analysis of real-time US patterns and abdominal staging. Radiology 1987;164(2):369–374. [DOI] [PubMed] [Google Scholar]
- 21.Aganovic L, Cassidy F. Imaging of the scrotum. Radiol Clin North Am 2012;50(6):1145–1165. [DOI] [PubMed] [Google Scholar]
- 22.Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology 2003;227(1):18–36. [DOI] [PubMed] [Google Scholar]
- 23.Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics 2015;35(2):400–415. [DOI] [PubMed] [Google Scholar]
- 24.Appelbaum L, Gaitini D, Dogra VS. Scrotal ultrasound in adults. Semin Ultrasound CT MR 2013;34(3):257–273. [DOI] [PubMed] [Google Scholar]
- 25.Secil M, Altay C, Basara I. State of the art in germ cell tumor imaging. Urol Oncol 2016;34(3):156–164. [DOI] [PubMed] [Google Scholar]
- 26.Comiter CV, Renshaw AA, Benson CB, Loughlin KR. Burned-out primary testicular cancer: sonographic and pathological characteristics. J Urol 1996;156(1):85–88. [PubMed] [Google Scholar]
- 27.Tsili AC, Tsampoulas C, Giannakopoulos X, et al. MRI in the histologic characterization of testicular neoplasms. AJR Am J Roentgenol 2007;189(6):W331–W337. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JO, Mattrey RF, Phillipson J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am J Roentgenol 1990;154(3):539–543. [DOI] [PubMed] [Google Scholar]
- 29.Tsili AC, Argyropoulou MI, Giannakis D, Sofikitis N, Tsampoulas K. MRI in the characterization and local staging of testicular neoplasms. AJR Am J Roentgenol 2010;194(3):682–689. [DOI] [PubMed] [Google Scholar]
- 30.Bilagi P, Sriprasad S, Clarke JL, Sellars ME, Muir GH, Sidhu PS. Clinical and ultrasound features of segmental testicular infarction: six-year experience from a single centre. Eur Radiol 2007;17(11):2810–2818. [DOI] [PubMed] [Google Scholar]
- 31.Aquino M, Nghiem H, Jafri SZ, Schwartz J, Malhotra R, Amin M. Segmental testicular infarction: sonographic findings and pathologic correlation. J Ultrasound Med 2013;32(2):365–372. [DOI] [PubMed] [Google Scholar]
- 32.Saxon P, Badler RL, Desser TS, Tublin ME, Katz DS. Segmental testicular infarction: report of seven new cases and literature review. Emerg Radiol 2012;19(3):217–223. [DOI] [PubMed] [Google Scholar]
- 33.Deurdulian C, Mittelstaedt CA, Chong WK, Fielding JR. US of acute scrotal trauma: optimal technique, imaging findings, and management. RadioGraphics 2007;27(2):357–369. [DOI] [PubMed] [Google Scholar]
- 34.Sommers DN, Jensen J. Sonographic findings of typical and atypical scrotal trauma. Ultrasound Q 2015;31(2):99–108. [DOI] [PubMed] [Google Scholar]
- 35.Cook JL, Dewbury K. The changes seen on high-resolution ultrasound in orchitis. Clin Radiol 2000;55(1):13–18. [DOI] [PubMed] [Google Scholar]
- 36.Lentini JF, Benson CB, Richie JP. Sonographic features of focal orchitis. J Ultrasound Med 1989;8(7):361–365. [DOI] [PubMed] [Google Scholar]
- 37.Shah KH, Maxted WC, Chun B. Epidermoid cysts of the testis: a report of three cases and an analysis of 141 cases from the world literature. Cancer 1981;47(3):577–582. [DOI] [PubMed] [Google Scholar]
- 38.Atchley JT, Dewbury KC. Ultrasound appearances of testicular epidermoid cysts. Clin Radiol 2000;55(7):493–502. [DOI] [PubMed] [Google Scholar]
- 39.Delfino M, Elia J, Imbrogno N, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: prevalence and sonographic, hormonal, and seminal characteristics. J Ultrasound Med 2012;31(3):383–388. [DOI] [PubMed] [Google Scholar]
- 40.Ribagnac M, Brac De La Perrière A, Lyonnet D, Rouvière O. Testicular adrenal rests: the role of imaging [in French]. J Radiol 2007;88(5 Pt 1):631–638. [DOI] [PubMed] [Google Scholar]
- 41.Stewart VR, Sidhu PS. The testis: the unusual, the rare and the bizarre. Clin Radiol 2007;62(4):289–302. [DOI] [PubMed] [Google Scholar]
- 42.Eraso CE, Vrachliotis TG, Cunningham JJ. Sonographic findings in testicular sarcoidosis simulating malignant nodule. J Clin Ultrasound 1999;27(2):81–83. [DOI] [PubMed] [Google Scholar]
- 43.Stewart VR, Sellars ME, Somers S, Muir GH, Sidhu PS. Splenogonadal fusion: B-mode and color Doppler sonographic appearances. J Ultrasound Med 2004;23(8):1087–1090. [DOI] [PubMed] [Google Scholar]
- 44.Guarin U, Dimitrieva Z, Ashley SJ. Splenogonadal fusion: a rare congenital anomaly demonstrated by 99Tc-sulfur colloid imaging—case report. J Nucl Med 1975;16(10):922–924. [PubMed] [Google Scholar]
- 45.Leite KR, Garicochea B, Srougi M, et al. Monoclonality of asynchronous bilateral lymphoma of the testis. Eur Urol 2000;38(6):774–777. [DOI] [PubMed] [Google Scholar]
- 46.Bertolotto M, Derchi LE, Secil M, et al. Grayscale and color Doppler features of testicular lymphoma. J Ultrasound Med 2015;34(6):1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharmeen F, Rosenthal MH, Howard SA. The management of retroperitoneal lymphadenopathy in spermatocytic seminoma of the testicle. Clin Imaging 2014;38(2):202–204. [DOI] [PubMed] [Google Scholar]
- 48.Varela JR, Pombo F, Requejo I, Aguilera C, Bargiela A, Alvarez A. Spermatocytic seminoma: sonographic appearance. J Ultrasound Med 1996;15(8):607–609. [DOI] [PubMed] [Google Scholar]