Abstract
Objective
Routine initiation β-blocker medications before vascular surgery is controversial due to conflicting data. The purpose of this analysis was to determine whether prophylactic use of β-blockers before major elective vascular surgery decreased postoperative cardiac events or mortality.
Methods
The Society for Vascular Surgery Vascular Quality Initiative (SVS-VQI) data set was used to perform a retrospective cohort analysis of infrainguinal lower extremity bypass (LEB), aortofemoral bypass (AFB), and open abdominal aortic aneurysm(AAA) repair patients. Chronic (>30 days preoperatively) β-blocker patients were excluded, and comparisons were made between preoperative (0–30 day) and no β-blocker groups. Patients were risk stratified using a novel prediction tool derived specifically from the SVS-VQI data set. Propensity-matched pairs and interprocedural specific risk stratification comparisons were performed. End points included in-hospital major adverse cardiac events (MACEs), including myocardial infarction (MI; defined as new ST or T wave electrocardiographic changes, troponin elevation, or documentation by echocardiogram or other imaging modality), dysrhythmia, and congestive heart failure, and 30-day mortality.
Results
The study analyzed 13,291 patients (LEB, 68% [n = 9047]; AFB, 11% [n = 1474]; and open AAA, 21% [n = 2770]); of these, 67.7% (n = 8999) were receiving β-blockers at time of their index procedure. Specifically, 13.2% (n = 1753) were identified to have been started on a preoperative β-blocker, 54.5% (n=7426) were on chronic β-blockers, and 32.3% (n=4286) were on no preoperative β-blockers. Among the three procedures, patients had significant demographic and comorbidity differences and thus were not combined. A 1:1 propensity-matched pairs analysis (1459 pairs) revealed higher rates of postoperative MI with preoperative β-blockers (preoperative β-blocker relative risk, 1.65; 95% confidence interval, 1.02–2.68; P = .05 vs no β-blocker), with no difference in dysrhythmia, congestive heart failure, or 30-day mortality. When stratified into low-risk, medium-risk, and high-risk groups within each procedure, all groups of preoperative β-blocker patients had no difference or higher rates of MACEs and 30-day mortality, with the exception of high-risk open AAA patients, who had a lower rate of MI (odds ratio, 0.35; 95% confidence interval, 011–0.87; P = .04).
Conclusions
Exclusive of high-risk open AAA patients, preoperative β-blockers did not decrease rates of MACEs or mortality after LEB, AFB, or open AAA. Importantly, exposure to prophylactic preoperative β-blockers increased the rates of some adverse events in several subgroups. Given these data, the SVS-VQI cannot support routine initiation of preoperative β-blockers before major elective vascular surgery in most patients.
Lower extremity bypass (LEB) or open aortic operations for peripheral arterial occlusive disease (PAOD) and abdominal aortic aneurysm (AAA) are performed in patients with a high prevalence of concomitant cardiovascular disease.1,2 These risk factors make these patients vulnerable to developing postoperative major adverse cardiac events (MACEs), including myocardial infarction (MI), arrhythmia, congestive heart failure (CHF), or sudden death.3,4 The primary factor leading to these outcomes is development of myocardial ischemia resulting from plaque rupture, prolonged subendocardial hypoperfusion, or both. β-Adrenergic blocking medications (β-blockers) theoretically offer ideal protection from MACEs because they lower myocardial oxygen demand and have plaque-stabilization properties.5 Indeed, multiple studies endorse the efficacy of preoperative (≤30-day) β-blocker initiation to reduce MACEs, and current national quality improvement programs and international consensus guidelines advocate liberal use of preoperative β-blockers.6,7
However, more recent data have failed to confirm the benefits of initiating preoperative β-blockers before noncardiac surgery.8,9 Further, prior quality initiatives within the Vascular Study Group of New England failed to document reduced preoperative heart rate or postoperative MI (POMI), despite a significant increase in preoperative β-blocker use.10 Perhaps more importantly, certain patient subgroups may be harmed by initiation of preoperative β-blockers, particularly those at a low risk of cardiac events.11 Finally, concerns regarding unsecured data in the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials, which had provided Level 1 data for the benefit of perioperative β-blockers, has now led to calls for immediate retraction of current guidelines for use of these medications.9,12
The purpose of this study was to determine whether the initiation of β-blockers before major elective vascular surgery decreased postoperative cardiac events or mortality within the Society for Vascular Surgery-Vascular Quality Initiative (SVS-VQI).
METHODS
The SVS-VQI is structured as a patient safety organization designated by the Agency for Healthcare Research and Quality. This study was approved by the SVS-VQI Research Advisory Committee and the Arterial Quality Committee and includes national data from all VQI regional quality groups. In addition, the University of Florida Institutional Review Board approved this study, and the need for patient consent was waived because no direct harm resulted from the analysis and all included data were deidentified. Details regarding this multicenter collaboration have been published and are available at www.vascularqualityinitiative.org/components/svs-pso.13,14
Study cohort
All VQI patients undergoing elective open infrainguinal LEB (any indication/conduit; n = 9594), aortofemoral bypass (AFB; aortic inflow only for any indication/conduit; n = 1599), or open AAA (OAAA; n = 3089) repair from January 2003 to June 2013 were reviewed. These procedures were selected due to the known elevated risk of postoperative MACEs for patients undergoing major open arterial reconstruction for PAOD and AAA.1,6,15,16 Patients undergoing urgent or emergency procedures were excluded. Only the first observation on a patient who received more than one operation was included. Patients were dichotomized into any preoperative β-blocker (started ≤30 days of surgery) and no preoperative β-blocker exposure groups. The analysis excluded patients on chronic β-blockers (not started prophylactically ≤30 days of surgery) because the primary goal of the analysis was to understand risk of preoperative β-blocker initiation, and patients on chronic β-blockers are known to have a higher MACE risk.10 The subgroup of patients not previously on β-blockers is most affected by current quality improvement and practice guidelines.6,7
Definitions and end points
As previously described, >100 clinical and demographic variables are collected for each patient and prospectively entered into the VQI registry. 17 Coronary artery disease (CAD) was defined as a history of MI, any coronary revascularization (coronary artery bypass grafting, percutaneous coronary intervention), or a history of angina. Additional details regarding specific definitions of various comorbidities within the VQI have been published17 and are available on-line at www.svsvqi.org. Preoperative cardiac stress testing was obtained at the clinician’s discretion and included the most recent stress electrocardiogram, stress echocardiogram, or nuclear stress test result ≤2 years.
The main outcome measures included development of any in-hospital, postoperative MACE (clinically significant arrhythmia, CHF or MI) and 30-day death. MI was defined as new ST or T wave, or both, electrocardiographic changes, troponin elevation, or documentation by echocardiogram or other imaging modality. Clinically significant arrhythmias included any new atrial or ventricular rhythm disturbance requiring treatment with medication or cardioversion. CHF included new pulmonary edema documented by chest radiograph and requiring treatment or monitoring in the intensive care unit. Mortality events were verified within the Social Security Death Index Master File.
Development of procedure-specific MACE prediction models
For each procedure (infrainguinal LEB, AFB, and OAAA), logistic regression was used to assign individual patient risk scores reflecting the likelihood of MACEs. The risk score was used to facilitate propensity matching between patients receiving preoperative β-blockers and those not given preoperative β-blockers and to stratify the matched cohort by MACE risk. Therefore, all possible preoperative and intraoperative predictors of MACEs were considered for the models except β-blocker status. To minimize the effect of missing variables, final risk models included only those variables whose exclusion resulted in appreciable loss of predictive power. Candidate predictors were derived from known factors associated with MACE16 as well as additional variables available in the data set that were identified by literature review15,18 (Supplementary Table I, online only). Of note, data on postoperative stroke and heart rate on arrival to the operating room before induction of anesthesia were collected and analyzed.
The R 3.0.2 statistical software (The R Foundation for Statistical Computing, http://www.r-project.org/foundation/) was used to create a logistic regression model for the composite MACE outcome that included all predictors. To reduce this model to the fewest possible predictors without diminishing predictive power, the predictive value of the full model was assessed using the area under the receiver operating characteristic curve (AUC). Next, a stepwise elimination algorithm based on the Akaike information criterion (the stepAIC function in the R package MASS) was used for model reduction. The reduced model AUC was compared with the full model AUC to ensure that loss of predictive power in the reduced model was no more than 1% lower than the full model. Risk scores (model-estimated log-odds of MACEs) were then assigned to each patient.
There were 9047 patients (94.3%) with complete information for all variables in the reduced LEB MACE model. Similarly, 1474 AFB patients (92.2%) had complete information in the reduced model. The AUC was .747 for the LEB model and .734 for the AFB model. Finally, 2770 OAAA patients (89.7%) had complete information for all variables in the reduced model (AUC = .677).
To increase the robustness of our results, two closely related but separate analyses were performed. Initially, propensity scoring was used to match each patient who received preoperative β-blockers to a patient who received no β-blockers and then we compared the results of matched pairs. This analysis attempts to mimic a clinical trial in which β-blockers are assigned randomly to participants and focuses on providing information about the overall effect of β-blocker exposure across all levels of cardiac risk. The limitations of this analysis are that it is generalizable only to that subset of preoperative β-blocker patients for whom suitable matches are found and discards information from patients not exposed to β-blockers and not used for matching.
In our second analysis, we stratified all patients by MACEs risk and analyzed the effect of β-blocker exposure separately within each risk strata. This ensured a comparison of outcomes across the complete spectrum of patients and enabled us to look at the effects of β-blocker exposure within each risk strata. This comparison was limited, however, by our ability to accurately assess cardiac risk. Although both analyses involve retrospective data and are necessarily imperfect, we believe that the similar results obtained from each approach enhance credibility of our overall conclusions.
Analysis 1: Propensity-matched pairs analysis
To understand preoperative β-blocker exposure risk and to control for treatment-selection bias, we performed a 1:1 propensity score-matched analysis (R 3.0.2). We estimated likelihood of receiving a preoperative β-blocker for each patient, based on published guidelines.6,7 Propensity scores were generated using logistic regression with β-blocker exposure (preoperative or none) as the outcome and the estimated log-odds of MACEs, procedure (infrainguinal, suprainguinal, or OAAA), statin use, CAD history, prior bypass, prior peripheral vascular intervention, prior major amputation, smoking history (current, past, or none), hypertension, diabetes mellitus (requiring insulin or medication), prior coronary artery bypass grafting, geographic region, and surgery year as predictor variables. Patient pairs who were and were not exposed to β-blockers were matched using a greedy algorithm with caliper width of 0.1 standard deviations of the estimated log-odds of receiving preoperative β-blockers. Covariate balance was assessed using the standardized difference, with values <10% indicating minimal imbalance. We used the McNemar test to compare the frequency of the primary outcomes.
Analysis 2: Procedure-specific risk stratification analysis
We used the risk scores assigned to each patient by the procedure-specific MACEs prediction models described above to classify each patient in the full data set as low-risk, medium-risk, or high-risk according to the tertiles of risk scores within each procedure. We then used χ2 or Fisher exact tests, as appropriate, to compare outcome rates between the β-blocker groups within each risk category and within each procedure.
RESULTS
Patient cohort
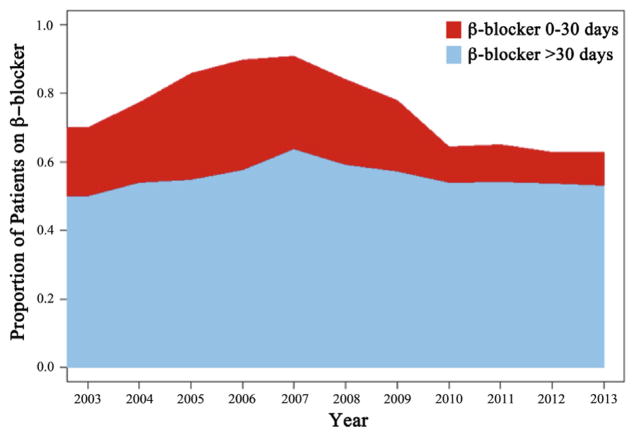
Data from 14 regions, 151 centers, and 676 surgeons were contributed to the initial VQI cohort consisting of 14,282 elective patients (LEB, 67% [n = 9594]; AFB, 11% [n = 1599]; and OAAA, 22% [n = 3089]). We excluded 991 patients (7%) due to missing data. The final analysis cohort was 13,291 patients (LEB, 68% [n = 9047]; AFB, 11% [n = 1474]; and OAAA, 21% [n = 2770]). At time of their index procedure, 67.7% (n = 8999) were on β-blockers; specifically, 13.2% (n = 1753) were identified to have been started on a preoperative (0–30 day) β-blocker, 54.5% (n = 7426) were on chronic β-blockers, and 32.3% (n = 4286) were not on preoperative β-blockers. The trend in VQI β-blocker use over time is depicted in Fig 1.
Fig 1.
This graph depicts the trends in β-blocker use within the Vascular Quality Initiative (VQI) from 2003 to 2013 for elective patients undergoing infrainguinal lower extremity bypass (LEB), aortofemoral bypass (AFB), and open abdominal aortic aneurysm (OAAA) repair. Chronic β-blocker (use for >30 days preoperatively, blue) exposure remained relatively consistent; however, a significant reduction in prophylactic preoperative β-blocker use (eg, initiation within 0–30 days of operation, red) is noted. Specifically, comparison of the peak year of use (2007) with the last complete year in the data set (2012) demonstrates a significant decline (26.9% in 2007 vs 9.0% in 2012; P < .0001) in use of this intervention.
Analysis 1: Propensity-matched pairs analysis
Overall matched-pairs outcomes
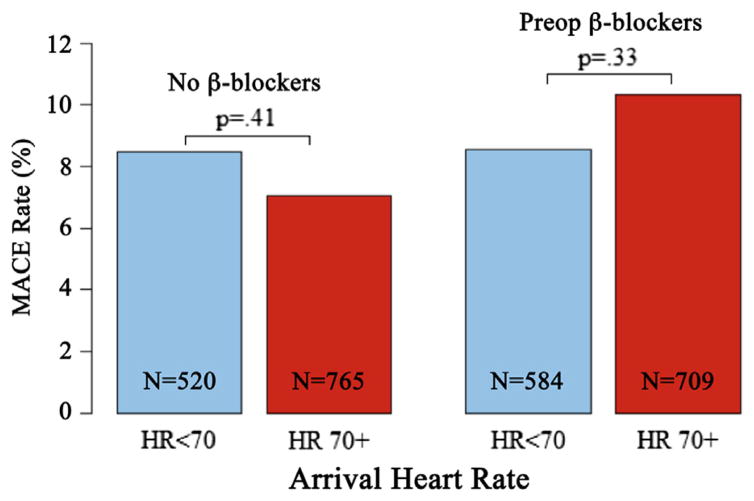
Demographic and clinical characteristics of the study cohort, before and after propensity score matching, are reported in Table I. By design, no significant differences in rates of tobacco use, hypertension, statin exposure, prior open or endovascular peripheral arterial operation history, diabetes, or pre-existing ischemic heart disease were present between the matched groups. Overall, a MACE occurred in 9.3% (n = 1236), and 30-day mortality was 1.8% (n = 235). The effect of arrival heart rate control and β-blocker exposure was analyzed among matched patients and no significant difference in MACE rate was detected (Fig 2). The postoperative stroke rate was not different between unmatched (no β-blocker, 0.6% [n = 22 of 3640] vs preoperative β-blocker, 0.9% [n = 8 of 931], P = .53) and matched (no β-blocker, 0.6% [n = 6 of 981] vs preoperative β-blocker, 0.9% [n = 8 of 924], P = .70) patients.
Table I.
Selected study cohort characteristicsa
| Variable | Full cohort, No. (%) | P valueb | Matched cohort, No. (%) | P valueb | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No β-B (n = 4249; 71%) | Pre-op β-B (n = 1733; 29%) | No β-B (n = 1459) | Pre-op β-B (n = 1459) | |||
| Risk category | ||||||
| Low | 1866 (43.9) | 622 (35.9) | 548 (37.6) | 552 (37.8) | ||
| Medium | 1357 (31.9) | 598 (34.5) | 493 (33.8) | 493 (33.8) | ||
| High | 1026 (24.1) | 513 (29.6) | <.0001 | 418 (28.6) | 414 (28.4) | .98 |
| Procedure type | ||||||
| Infrainguinal bypass | 2938 (69.1) | 1053 (60.8) | 907 (62.2) | 911 (62.4) | ||
| Suprainguinal bypass | 597 (14.1) | 206 (11.9) | 203 (13.9) | 201 (13.8) | ||
| OAAA repair | 714 (16.8) | 474 (27.4) | <.0001 | 349 (23.9) | 347 (23.8) | .99 |
| Smoking history | ||||||
| Never | 549 (12.9) | 180 (10.4) | 141 (9.7) | 160 (11.0) | ||
| Prior | 1506 (35.4) | 679 (39.2) | 565 (38.7) | 553 (37.9) | ||
| Current | 2194 (51.6) | 874 (50.4) | .003 | 753 (51.6) | 746 (51.1) | .51 |
| Hypertension | 3083 (72.6) | 1344 (77.6) | <.001 | 1149 (78.8) | 1130 (77.5) | .79 |
| Statin | 2551 (60.0) | 1146 (66.1) | <.001 | 953 (65.3) | 976 (66.9) | .39 |
| Prior vascular operationc | 1923 (45.3) | 639 (36.9) | <.001 | 537 (36.8) | 562 (38.5) | .36 |
| Prior major amputation | 112 (2.6) | 44 (2.5) | .90 | 36 (2.5) | 37 (2.5) | 1 |
| Diabetes mellitus | 1347 (31.7) | 542 (31.3) | .77 | 468 (32.1) | 449 (30.8) | .47 |
| CAD | 677 (15.9) | 366 (21.1) | <.001 | 295 (20.2) | 299 (20.5) | .89 |
| Prior PCI/CABG | 705 (16.6) | 306 (17.7) | .34 | 254 (17.4) | 261 (17.9) | .77 |
β-B, β-blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; OAAA, open abdominal aortic aneurysm; PCI, percutaneous coronary intervention.
Selected variables chosen for matching characteristics are from American College of Cardiology guidelines for use of preoperative β-blockers before noncardiac surgery.
P value is result of χ2 or Fisher exact test.
Prior vascular operation includes any history of peripheral endovascular procedure and/or open arterial reconstruction for any indication.
Fig 2.
This graph demonstrates a comparison of the overall major adverse cardiac event (MACE) rate within different groups of patients who were and were not exposed to preoperative β-blockers as a function of how well their arrival to the operating room heart rate (HR) was controlled (HR <70 beats/min [blue] vs HR >70 beats/min [red]). No significant difference in MACE rate was noted within the different HR categories irrespective of the β-blocker exposure history. When other cutoffs for HR control were analyzed, no significant differences were found in MACE rate between groups.
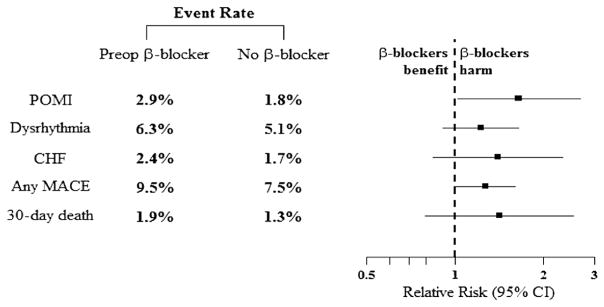
The rates of individual adverse cardiac events and 30-day mortality between the full study cohort and the matched patients are depicted in Fig 3 and reported in Table II. Among the full unmatched cohort, elevated rates of all MACEs and 30-day mortality were detected in patients receiving preoperative β-blockers. Similar trends were also observed among matched pairs. Specifically, elevated rates of dysrhythmia, CHF, and 30-day mortality were noted for patients on preoperative β-blockers, but these were not significantly different. However, matched patients receiving preoperative β-blockers were significantly more likely to develop POMI (preoperative β-blocker: relative risk, 1.65; 95% confidence interval [CI], 1.02–2.68; P = .05 vs no β-blocker).
Fig 3.
Overall major adverse cardiac event (MACE) and 30-day mortality is demonstrated for the 1459 matched pairs. Although elevated rates of multiple end points are noted among matched patients exposed to preoperative β-blockers, none reached statistical significance. The solid squares indicate the mean difference, the dashed vertical line indicates no effect, and the horizontal lines represents the 95% confidence intervals (CIs). CHF, Congestive heart failure; POMI, postoperative myocardial infarction.
Table II.
Outcomes for all Risk Groups Combined
| Variable | Full cohort, No. (%) | P valuea | Matched cohort, No. (%) | RR of pre-op β-B (95% CI) | P valueb | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| No β-B (n = 4249) | Pre-op β-B (n = 1733) | No β-B (n = 1459) | Pre-op β-B (n = 1459) | ||||
| POMI | 88 (2.1) | 49 (2.8) | .09 | 26 (1.8) | 43 (2.9) | 1.65 (1.02–2.68) | .05 |
| Dysrhythmia | 199 (4.7) | 112 (6.5) | .006 | 75 (5.1) | 92 (6.3) | 1.23 (0.91–1.65) | .21 |
| CHF | 73 (1.7) | 37 (2.1) | .33 | 25 (1.7) | 35 (2.4) | 1.4 (0.84–2.33) | .24 |
| Any MACE | 295 (6.9) | 162 (9.3) | .002 | 109 (7.5) | 138 (9.5) | 1.27 (0.99–1.61) | .06 |
| 30-day death | 50 (1.2) | 30 (1.7) | .12 | 19 (1.3) | 27 (1.9) | 1.42 (0.79–2.54) | .29 |
β-B, β-blocker; CHF, congestive heart failure; CI, confidence interval; MACE, major adverse cardiac event; POMI, postoperative myocardial infarction; RR, relative risk.
The P value is the result of the χ2 test.
The P value is result of the McNemar test for paired data.
Matched cardiac risk patient outcomes
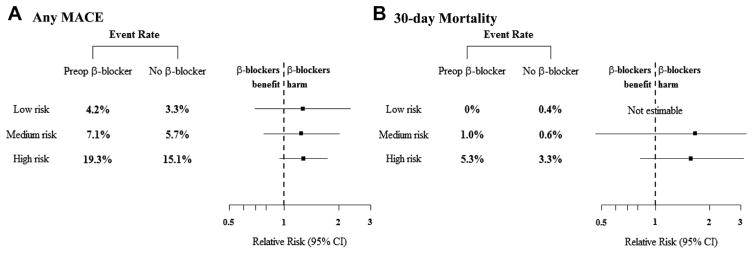
The outcomes of matched patients that were categorized by cardiac risk for development of postoperative MACEs is reported in Table III and depicted in Fig 4. When looking within the matched pairs by cardiac risk grouping, similar trends toward elevated rates in the composite MACEs end point are noted for patients who received preoperative β-blockers; however, none reached statistical significance. When examining the matched patients at high cardiac risk, the composite MACEs end point was elevated in the preoperative β-blocker patients and was primarily driven by a significantly higher rate of POMI (preoperative β-blocker: relative risk, 1.75; 95% CI, 1.01–3.03; P = .04 vs no β-blocker).
Table III.
Outcomes by cardiac risk strata among matched patients
| Risk strata | No β-B, No. (%) | Pre-op β-B, No. (%) | RR of pre-op β-B (95% CI) | P valuea |
|---|---|---|---|---|
| Low risk | 548 (49.8) | 552 (50.2) | ||
| POMI | 3 (0.5) | 3 (0.5) | 0.99 (0.20–4.90) | 1 |
| Dysrhythmia | 13 (2.4) | 20 (3.6) | 1.53 (0.77–3.04) | .29 |
| CHF | 3 (0.5) | 3 (0.5) | 0.99 (0.20–4.90) | 1 |
| Any MACE | 18 (3.3) | 23 (4.2) | 1.27 (0.69–2.32) | .53 |
| 30-day death | 2 (0.4) | 0 (0) | NA | .25 |
| Medium risk | 493 (50.0) | 493 (50.0) | ||
| POMI | 4 (0.8) | 7 (1.4) | 1.75 (0.52–5.94) | .55 |
| Dysrhythmia | 24 (4.9) | 25 (5.1) | 1.04 (0.60–1.80) | 1 |
| CHF | 1 (0.2) | 8 (1.6) | 8.0 (1.00–63.7) | .06 |
| Any MACE | 28 (5.7) | 35 (7.1) | 1.25 (0.77–2.02) | .44 |
| 30-day death | 3 (0.6) | 5 (1.0) | 1.67 (0.40–6.93) | .73 |
| High risk | 418 (50.2) | 414 (49.8) | ||
| POMI | 19 (4.5) | 33 (8.0) | 1.75 (1.01–3.03) | .04 |
| Dysrhythmia | 38 (9.1) | 47 (11.4) | 1.25 (0.83–1.87) | .30 |
| CHF | 21 (5.0) | 24 (5.8) | 1.15 (0.65–2.04) | .65 |
| Any MACE | 63 (15.1) | 80 (19.3) | 1.28 (0.95–1.73) | .12 |
| 30-day death | 14 (3.3) | 22 (5.3) | 1.59 (0.82–3.06) | .18 |
β-B, β-blocker; CHF, congestive heart failure; CI, confidence interval; MACE, major adverse cardiac event; NA, not applicable; POMI, postoperative myocardial infarction; RR, relative risk.
The P value is the result of the χ2 or Fisher exact test.
Fig 4.
The composite (A) of a major adverse cardiac event (MACE) and (B) 30-day mortality rates with the accompanying forest plot describing the 95% confidence interval (CI; horizontal line) are displayed for the different cardiac risk strata within the matched-pairs analysis. Elevated rates of (A) MACEs and (B) 30-day mortality are seen; however, these did not reach statistical significance. The solid squares indicate the mean difference, and the dashed vertical line indicates no effect.
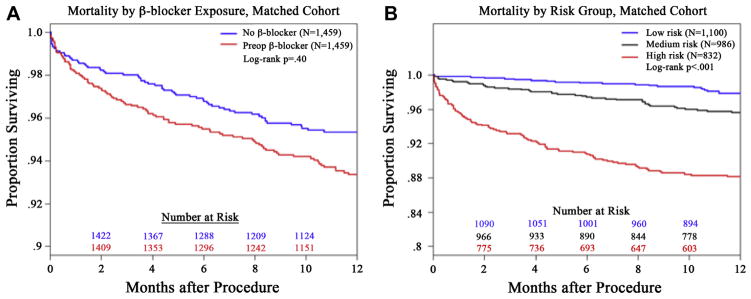
The 30-day mortality rates were also elevated for patients at medium and high cardiac risk receiving preoperative β-blockers within the matched cohorts, but these differences did not reach significance. Despite the overall trend of elevated in-hospital MACEs and 30-day mortality in patients receiving preoperative β-blockers, it did not translate into significantly different 1-year survival for the entire matched group of patients (Fig 5, A). In contradistinction, 1-year survival was significantly different among the matched patients when compared across the different cardiac risk groupings (low-risk vs high-risk, log-rank, P < .0001; Fig 5, B).
Fig 5.
The Kaplan-Meier plots demonstrate the all-cause mortality outcomes for (A) matched patients as a function of preoperative β-blocker exposure or (B) cardiac risk stratification. A, No significant survival difference was detected between matched patients exposed to preoperative β-blockers (red lines) compared with those not exposed to β-blockers (blue lines; log-rank, P = .40). B, As anticipated, differential survival is noted across the groups at low (black line), medium (blue line), and high (red line) cardiac risk. Specifically, high-cardiac-risk patients had significantly worse long-term survival (P < .0001) than the low-risk and medium-risk patients.
Analysis 2: Procedure-specific outcomes across cardiac risk strata for all patients
Infrainguinal LEB
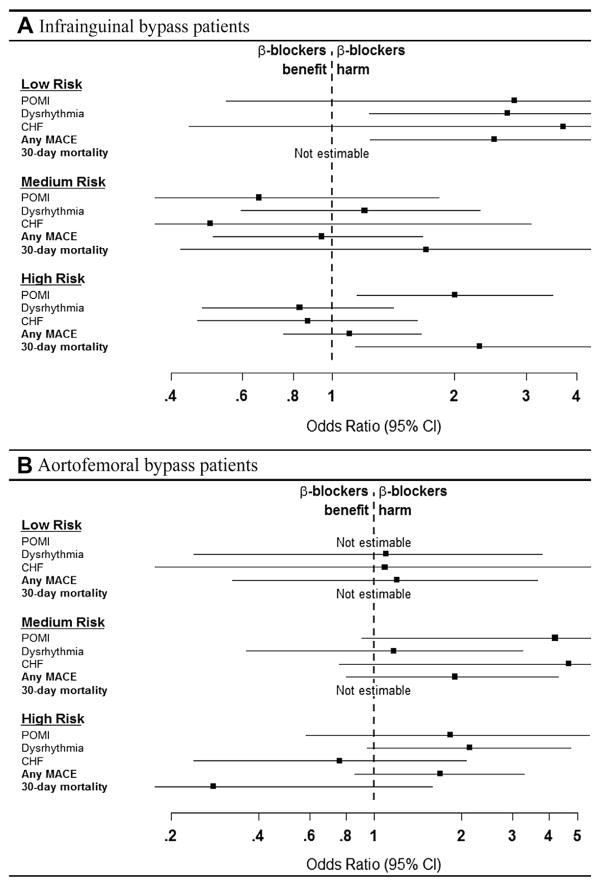
For LEB patients, when grouped into categories of low, medium, and high cardiac risk, significantly higher rates of postoperative dysrhythmia (odds ratio [OR], 2.7; 95% CI, 1.23–5.58; P = .01) and any MACE (OR, 2.5; 95% CI, 1.24–4.97; P = .008) occurred in the low-cardiac-risk patients who received preoperative β-blockers. Similarly, the high-cardiac-risk patients placed on preoperative β-blockers also had significantly higher rates of POMI (OR, 2.0; 95% CI, 1.15–3.48; P = .01) and 30-day mortality (OR, 2.3; 95% CI, 1.14–4.48; P = .02; Fig 6, A; Supplementary Table II, online only).
Fig 6.
These forest plots display the odds of an individual developing a major adverse cardiac event (MACE) or 30-day mortality, or both, among (A) elective infrainguinal lower extremity bypass (LEB) and (B) aortofemoral bypass (AFB) patients as a function of their preoperative cardiac risk stratification. A, Low-risk and high-risk infrainguinal bypass patients exposed to preoperative β-blockers had significantly higher risk of certain end points such as dysrhythmia/any MACE (low risk) or postoperative myocardial infarction (POMI)/30-day mortality (high risk). B, Although elevated rates of many end points are seen in the AFB patients exposed to β-blockers preoperatively, none reached statistical significance. The solid squares indicate the mean difference, the dashed vertical line indicates no effect, and the horizontal lines represents the 95% confidence intervals (CIs). CHF, Congestive heart failure.
Suprainguinal bypass
With respect to patients undergoing elective AFB for a PAD indication, although no significant difference in rates of MACE or 30-day death occurred between the two groups across all cardiac risk strata, there was a general trend toward elevated rates for many individual MACEs for patients receiving preoperative β-blockers. However, a differential trend was noted in the high-cardiac-risk group; specifically, elevated postoperative in-hospital MACE rates were seen in patients receiving preoperative β-blockers, which was primarily driven by increased rates of postoperative dysrhythmia, but the 30-day mortality rate was lower compared with patients not receiving a preoperative β-blocker (Fig 6, B; Supplementary Table III, online only).
OAAA repair
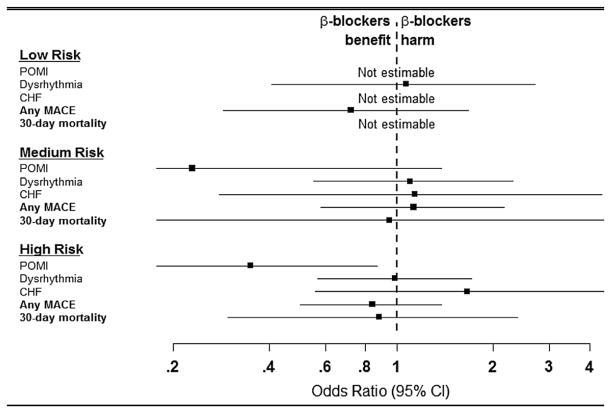
Lastly, for patients undergoing OAAA repair, no difference in MACE or 30-day mortality rates was noted in the low-risk and medium-risk patients based on preoperative β-blocker exposure; however, high-cardiac-risk patients receiving preoperative β-blockers had significantly lower rates of POMI (OR, 0.35; 95% CI, 0.11–0.87; P = .04), which identifies a patient group that may benefit from preoperative β-blockade (Fig 7; Supplementary Table IV, online only).
Fig 7.
Elective open abdominal aortic aneurysm (OAAA) patients initiated on prophylactic β-blockers between 0 and 30 days preoperatively had no benefit in the low-cardiac-risk and medium-cardiac-risk groups. However, high-cardiac-risk patients given preoperative β-blockers had a significantly lower likelihood of developing postoperative myocardial infarction (POMI) compared with patients without preoperative β-blockers. The solid squares indicate the mean difference, the dashed vertical line indicates no effect, and the horizontal lines represents the 95% confidence intervals (CIs). CHF, Congestive heart failure; MACE, major adverse cardiovascular event.
DISCUSSION
The goal of initiating preoperative β-blockers is to reduce rates of postoperative MACEs or mortality, or both; however, these effects were not observed in this analysis of VQI patients undergoing elective major vascular surgery operations. Patients were analyzed with two methods in an attempt to determine the risk of preoperative β-blocker exposure, with particular attention given to their cardiac risk status as well as to differences in primary indication and operation type. Patients started on preoperative β-blockers did not have lower rates of postoperative MACEs or 30-day mortality compared with matched patients without preoperative β-blocker therapy.
Importantly, this study identified certain subgroups that, irrespective of preoperative cardiac risk status, may be harmed by preoperative prophylactic β-blockade exposure, particularly individuals undergoing elective LEB or AFB for a PAOD indication. On the basis of these results and the increasing evidence in the reported literature, routine use of preoperative β-blockers in all vascular surgery patients within the VQI cannot be supported.
Interestingly however, we identified one subset of patients that may derive benefit from initiating preoperative β-blockers, which is high-risk patients undergoing elective OAAA repair. These data demonstrate that vascular patients are not only different from other noncardiac surgical patients with regard to β-blockers but that there are also likely significant differences within vascular disease groups, and OAAA patients may require different perioperative management. This identifies a group where a randomized trial may be warranted to further delineate the potential benefit of preoperative β-blockers for reducing postoperative MACEs.
Cardiac complications are responsible for significant morbidity and mortality after major vascular surgery. Primarily based on two randomized controlled trials (RCTs) in the 1990s,19,20 preoperative β-blockade was widely adopted as a simple intervention to reduce the risk of these events. Indeed, practice guidelines were influenced so significantly by results of various publications in the early 2000s21–23 that routine initiation of preoperative β-blockers for medium-risk and high-risk patients undergoing noncardiac surgery was linked to best practice and patient safety standards.21,22,24
However, three RCTs published in 2005 and 2006 failed to confirm the benefit of routine preoperative β-blockade.25–27 An insightful analysis by Lindenauer et al28 demonstrated that preoperative β-blockers reduced mortality only in patients with two or more revised cardiac risk index (RCRI)18 factors but increased mortality in individuals with one or no risk factors. Further concerns about the use of preoperative β-blockers in noncardiac surgery patients were raised when results from the Preoperative Ischemic Evaluation (POISE) multicenter RCT were published. 11 Patients randomized to preoperative β-blockers had lower rates of POMI but an increased risk of hypotension, stroke, and death, albeit with a higher than usual dose of perioperative β-blocker.11 As a result of these publications, best practice guidelines changed in 200629 and 2009,6 and initiation of preoperative β-blockade in vascular surgery patients with known CAD or inducible ischemia on preoperative testing was downgraded to a Class IIa recommendation.
The controversy surrounding preoperative β-blocker use is reflected in the VQI practice patterns (Fig 1). From 2003 to 2013, a significant difference in preoperative β-blocker use was noted, particularly when comparing 2007 (peak use) with 2012, most recent complete year of data for this analysis: 26.9% (2007) vs 9.0% (2012; P < .0001). The VQI preoperative β-blocker use trends were affected not only by the shifting landscape regarding use of preoperative β-blockade during this time period but also due to results of an internal regional quality initiative. A New England quality improvement effort from 2003 to 2008 significantly increased β-blocker use before vascular surgery (68% in 2003 vs 88% in 2008; P < .001) but did not decrease incidence of POMI (5.2% in 2003 vs 5.5% in 2008; P = .88).10
More recently, the scientific validity of two influential RCTs20,30 supporting use of preoperative β-blockers has been questioned.12,31 In addition, a systematic review by Bouri et al9 cited several reasons for discrediting the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE I-IV) trials. These authors demonstrated that when all other RCTs for initiation of β-blockers before noncardiac surgery are analyzed separately from the DECREASE trials, a 27% increased risk of mortality was found for patients exposed to preoperative β-blockade.9 Indeed, our matched-pairs analysis demonstrated increased rates of 30-day mortality for medium-risk and high-risk patients on preoperative β-blockers; however, the difference did not reach statistical significance (Fig 3).
Further, in the secondary analysis, low-cardiac-risk LEB patients placed on preoperative β-blockers had significantly greater risk of any MACE (OR, 2.5; 95% CI, 1.24–4.97; P = .008) that was driven primarily by postoperative dysrhythmia (OR, 2.7; 95% CI, 1.23–5.58; P = .01). Similarly, high-cardiac-risk LEB patients exposed to preoperative β-blockade were significantly more likely to have POMI (OR, 2.0; 95% CI, 1.15–3.48; P = .01) and 30-day mortality (OR, 2.3; 95% CI, 1.14–4.48; P = .02) compared with patients not on preoperative β-blockers (Fig 6, A).
To further confound the narrative about the safety and efficacy of preoperative β-blockers, a recent report from London et al32 on noncardiac surgery patients in the Veterans Health Administration demonstrated significantly lower rates of postoperative cardiac arrest, Q wave MI, and 30-day all-cause mortality for patients receiving preoperative β-blockade. Individuals with two or more RCRI predictors appeared to have the greatest benefit; however, the authors conceded they were unable to detect significant associations between β-blocker exposure and outcome in patients undergoing vascular surgery.32 This highlights the importance of registries with data regarding specific patient groups.
Because of the on-going concerns regarding preoperative β-blockers, a systematic review from the American College of Cardiology/American Heart Association was recently completed and reported that preoperative β-blockade started ≤1 day before noncardiac surgery prevents nonfatal POMI but increases the risk of stroke, death, hypotension, and bradycardia.33 Moreover, without the controversial DECREASE studies, these authors concluded that there are insufficient data on β-blockade started ≥2 days before surgery, and new multicenter RCTs are needed to address this knowledge gap.33 Notably, only 2.0% of patients in our VQI analysis were administered β-blockers ≤1 day of their operation, so a robust analysis of this subgroup was not possible to investigate this association.
This study has some important limitations. First, despite the various analytic methods, including the propensity analysis, this was not a randomized trial. Unmeasured bias and confounding certainly may have influenced these results. Despite analyzing >13,000 patients in three different high-risk vascular operations, event rates are relatively low, leading to small numbers across risk groups limiting the ability to make definitive conclusions, as evidenced by wide CIs in multiple analyses. Importantly, no dose information or postoperative data regarding reason for continuation/titration or rationale for β-blocker medication stoppage are available.
Lastly, the risk stratification models were specific to this data set and internally validated with bootstrapping. The low-risk, medium-risk, and high-risk designation was based on risk tertiles, so some may criticize this as an invalidated risk metric. However, risk models always perform better on the data from which they are developed, and our only focus was to derive the most accurate method of determining each patient’s preoperative risk of postoperative MACEs so subsequent analyses could be completed. This methodology influenced these results and makes difficult comparisons with other studies using more generalized risk prediction tools.
Notably, we did not use the RCRI18 because most patients were missing cerebrovascular disease history information. However, the RCRI is a model intended for wider clinical application and accounts for only a limited number of patient factors. We believe our risk-stratification index, which was created specifically for our data set using all variables available in the VQI, can only be more accurate in assigning risk to our patients. In fact, Bertges et al,16 whose methods we followed, demonstrated that a vascular surgery procedure-specific risk index developed from the Vascular Study Group of New England outperforms the RCRI.
CONCLUSIONS
Initiation of preoperative β-blockade did not significantly decrease MACE or 30-day mortality in most VQI patients. The rate of MACEs was increased in multiple subgroups of patients who received preoperative β-blockers. Routine initiation of β-blockers before major vascular surgery in most VQI patients is not beneficial. However, initiation of preoperative β-blockers in certain high-risk populations, such as OAAA may be protective, and a vascular surgical disease-specific RCT in this cohort may be warranted.
Supplementary Material
Acknowledgments
This work was supported in part by funding from the National Institutes of Health (NIH-NHLBI 5K23HL115673-02) and the Society for Vascular Surgery Foundation Mentored Patient-Oriented Research Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Society for Vascular Surgery Foundation.
Footnotes
Author conflict of interest: none.
Presented at the 2014 Vascular Annual Meeting of the Society for Vascular Surgery, Boston, Mass, June 5–7, 2014.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest. 0741-5214
AUTHOR CONTRIBUTIONS
Conception and design: SS, VP, AB
Analysis and interpretation: SS, VP, DN, DB, KH, JJ, JC, AB
Data collection: SS, JC
Writing the article: SS, VP, DN, AB
Critical revision of the article: SS, VP, DN, DB, KH, JJ, JC, AB
Final approval of the article: SS, VP, DN, DB, KH, JJ, JC, AB
Statistical analysis: DN
Obtained funding: SS
Overall responsibility: SS
SS and VP participated equally and share first authorship.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takigawa M, Yoshimuta T, Akutsu K, Takeshita S, Yokoyama N. Prevalence and predictors of coexistent silent atherosclerotic cardiovascular disease in patients with abdominal aortic aneurysm without previous symptomatic cardiovascular diseases. Angiology. 2012;63:380–5. doi: 10.1177/0003319711419359. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano G, Laurenzano E, Rengo C, De Rosa G, Brevetti L, Sannino A, et al. Abdominal aortic aneurysm in patients affected by intermittent claudication: prevalence and clinical predictors. BMC Surg. 2012;12(Suppl 1):S17. doi: 10.1186/1471-2482-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priebe HJ. Perioperative myocardial infarction—aetiology and prevention. Br J Anaesth. 2005;95:3–19. doi: 10.1093/bja/aei063. [DOI] [PubMed] [Google Scholar]
- 6.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task force on practice guidelines. Circulation. 2009;120:e169–276. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 7.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 8.Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962–76. doi: 10.1016/S0140-6736(08)61560-3. [DOI] [PubMed] [Google Scholar]
- 9.Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456–64. doi: 10.1136/heartjnl-2013-304262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodney PP, Eldrup-Jorgensen J, Nolan BW, Bertges DJ, Likosky DS, Cronenwett JL. A regional quality improvement effort to increase beta blocker administration before vascular surgery. J Vasc Surg. 2011;53:1316–28. e1311. doi: 10.1016/j.jvs.2010.10.131. discussion: 1327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 12.Chopra V, Eagle KA. Perioperative mischief: the price of academic misconduct. Am J Med. 2012;125:953–5. doi: 10.1016/j.amjmed.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–37. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Woo K, Eldrup-Jorgensen J, Hallett JW, Davies MG, Beck A, Upchurch GR, Jr, et al. Regional quality groups in the Society for Vascular Surgery® Vascular Quality Initiative. J Vasc Surg. 2013;57:884–90. doi: 10.1016/j.jvs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Boersma E, Poldermans D, Bax JJ, Steyerberg EW, Thomson IR, Banga JD, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001;285:1865–73. doi: 10.1001/jama.285.14.1865. [DOI] [PubMed] [Google Scholar]
- 16.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The vascular study group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the revised cardiac risk index in vascular surgery patients. J Vasc Surg. 2010;52:674–83. 683.e671–3. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 19.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–20. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 20.Poldermans D, Boersma E, Bax JJ, Thomson IR, van de Ven LL, Blankensteijn JD, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341:1789–94. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2014;135:569–75. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288:501–7. doi: 10.1001/jama.288.4.501. [DOI] [PubMed] [Google Scholar]
- 23.Shojania KG, Duncan BW, McDonald KM, Wachter RW. Making health care safer II: an updated critical analysis of the evidence for patient safety practices. Available at: http://www.ahrq.gov/research/findings/evidence-based-reports/services/quality/ptsafetysum.html. [PMC free article] [PubMed]
- 24.Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—-executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2002;105:1257–67. [PubMed] [Google Scholar]
- 25.Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg. 2005;41:602–9. doi: 10.1016/j.jvs.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Juul AB, Wetterslev J, Gluud C, Kofoed-Enevoldsen A, Jensen G, Callesen T, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ. 2006;332:1482. doi: 10.1136/bmj.332.7556.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol After Vascular Surgery (MAVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–90. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 29.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. Circulation. 2006;113:2662–74. doi: 10.1161/CIRCULATIONAHA.106.176009. [DOI] [PubMed] [Google Scholar]
- 30.Dunkelgrun M, Boersma E, Schouten O, Koopman-van Gemert AW, van Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV) Ann Surg. 2009;249:921–6. doi: 10.1097/SLA.0b013e3181a77d00. [DOI] [PubMed] [Google Scholar]
- 31.Erasmus Medical Center Follow-up Investigation Committee. Investigation into possible violation of scientific integrity: report summary. Rotterdam, The Netherlands: Eramus Medical Center; Sep 30, 2012. Available online: http://www.erasmusmc.nl/1172194/2090115/Integrity_report_2012-10.pdf. [Google Scholar]
- 32.London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309:1704–13. doi: 10.1001/jama.2013.4135. [DOI] [PubMed] [Google Scholar]
- 33.Wijeysundera DN, Duncan D, Nkonde-Price C, Virani SS, Washam JB, Fleischmann KE, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2246–64. doi: 10.1161/CIR.0000000000000104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.