Abstract
To treat hypertension, combining two or more antihypertensive drugs from different classes is often necessary. β-Blockers and renin–angiotensin–aldosterone system inhibitors, when combined, have been deemed ‘less effective’ based on partially overlapping mechanisms of action and limited evidence. Recently, the single-pill combination (SPC) of nebivolol (Neb) 5 mg – a vasodilatory β1-selective antagonist/β3 agonist – and valsartan 80 mg, an angiotensin II receptor blocker, was US Food and Drug Administration-approved for hypertension. Pharmacological profiles of Neb and valsartan, alone and combined, are well characterized. In addition, a large 8-week randomized trial in stages I–II hypertensive patients (N = 4161) demonstrated greater blood pressure-reducing efficacy for Neb/valsartan SPCs than component monotherapies with comparable tolerability. In a biomarkers substudy (N = 805), Neb/valsartan SPCs prevented valsartan-induced increases in plasma renin, and a greater reduction in plasma aldosterone was observed with the highest SPC dose vs. valsartan 320 mg/day. This review summarizes preclinical and clinical evidence supporting Neb/valsartan as an efficacious and well tolerated combination treatment for hypertension.
Keywords: aldosterone, angiotensin II receptor blockers, beta-blockers, hypertension, mechanism of action, nebivolol, renin–angiotensin system inhibitors, valsartan, vasodilation
INTRODUCTION
Treatment guidelines [1] and position papers [2] have attempted to summarize available knowledge regarding the effectiveness of certain antihypertensive drug class combinations. Such efforts, although commendable, have failed to take into account heterogeneity within each drug class or the quality of available evidence. For example, combinations between β-blockers and renin–angiotensin–aldosterone system (RAAS) inhibitors have been deemed ‘less effective’ [2] based on the results of two trials [3,4] and a concern that an overlapping mechanism of action (renin suppression) would not yield a blood pressure (BP)-reducing effect that would justify treatment with two agents instead of one. A recent randomized, Phase 3 trial (NAC-MD-01; N = 4161) has provided convincing evidence that at least one β-blocker/RAAS inhibitor combination consisting of the β1-selective adrenergic blocker with β3 agonistic vasodilatory properties, Neb [5], and the angiotensin II (Angio II) receptor blocker (ARB), valsartan, is more effective in reducing BP than its component monotherapies [6]. This study, which examined a range of Neb/valsartan single-pill combination (SPC) doses (5/80, 5/160, 10/160, 10/320, or 20/320 mg/day), provided the basis for the recent US Food and Drug Administration approval of the 5/80-mg/day SPC for the treatment of hypertension. This review will examine the pharmacologic rationale for the Neb/valsartan combination and summarize the pharmacokinetic, pharmacodynamic, and clinical findings related to its use.
MECHANISMS OF ACTION AND CLINICAL PHARMACOLOGY
Nebivolol
Neb is a highly selective β1-adrenoreceptor (β1-AR) antagonist with vasodilator properties [7]. It is a racemic mixture in which the β1-selective antagonism is mediated by the d-isomer (for doses up to 20 mg/day) [8,9]. The l-isomer activates the β3-receptor, which may be the basis for the vasodilatory and endotheliotropic effects observed with Neb treatment [9–11]. The high selectivity of the d-isomer for β1-AR over beta-2 adrenoreceptor allows for limited effects of Neb on airway reactivity and insulin sensitivity, and provides a low negative inotropic effect in patients with heart failure; however, this selectivity is lessened at doses more than 10 mg and in poor metabolizers [8]. Neb has no sympathomimetic activity and inhibits peripheral α1-receptor activity only at supratherapeutic doses [9,11]. Possible mechanisms behind the antihypertensive actions of Neb include nitric oxide (NO) release [12], decreased heart rate (HR), decreased myocardial contraction, reduction in tonic sympathetic outflow from cerebral vasomotor centers to the periphery, suppression of renin activity, and vasodilation/attenuation of peripheral vascular resistance [7].
Neb is rapidly absorbed following oral administration, with plasma concentrations peaking between 1.5 and 4 h after dosing; absolute bioavailability in extensive metabolizers (most patients) is 96% [13,14]. The d-isomer has a half-life of approximately 12 h in most patients, and its levels in plasma increase in a dose-dependent manner up to 20 mg/day. Exposure to the l-isomer is higher than that of its active metabolite, with a two-fold higher area under the curve (AUC) compared with that of the d-isomer [13,14]. Less than 1% of the drug is excreted unchanged in the urine, and the primary route of metabolism is hepatic oxidation.
In the United States, Neb is available as 2.5, 5, 10, and 20 mg tablets and is approved at dosages up to 40 mg/day [7]; the range of approved doses reflects those used in the majority of Neb hypertension clinical trials in the United States (2.5–40 mg). In the European Union, Neb is available as 5-mg tablets and is approved at 5 mg/day [15]. In both the United States and the European Union, Neb is approved for use as a monotherapy or in combination with other BP-lowering drugs.
Valsartan
Angio II, an oligopeptide hormone, causes arteriolar vasoconstriction and remodeling, endothelial cell apoptosis, superoxide (O2 −) anion production, aldosterone secretion, and other processes that lead to increased peripheral vascular resistance and endothelial dysfunction; two primary angiotensin receptor types mediate these changes: type 1 (AT1) and type 2 (AT2) [16–19].
The ARB valsartan is a highly selective, ‘insurmountable’ antagonist of AT1 receptors (AT1-Rs) with a 20 000-fold greater affinity for AT1 over AT2 receptors [20]. Valsartan has one primary metabolite that has a very low affinity for the AT1-R and is essentially inactive. The main antihypertensive effect of valsartan is mediated by a reduction in Angio II activation of the AT1-R in vascular smooth muscle, resulting in decreased peripheral vascular resistance via numerous mechanisms. BP reduction is substantially present within 2 weeks, and maximal reduction is attained at approximately 4 weeks [21].
The peak plasma concentration (C max) of valsartan occurs within 2–4 h after dosing, and absolute bioavailability is roughly 25%. The AUC and C max values of valsartan increase approximately linearly with increasing doses. Orally administered valsartan is primarily excreted unchanged within feces (≈80% of dose) and urine (≈13% of dose). Approximately 20% of dose is recovered as metabolites [21].
Nebivolol/valsartan combination
A single-center, randomized, open-label, three-way crossover trial in healthy volunteers (N = 30) found that the steady-state AUC from time 0 to the dosing interval (AUC0–τ,ss), C max at steady state (C max,ss), and time to maximum plasma concentration (T max) for Neb and its metabolites (d-nebivolol, l-nebivolol, d/l-Neb, and the Neb glucuronides) were significantly lower following treatment with a combination of Neb (20 mg) and valsartan (320 mg) vs. Neb alone [22]. For valsartan, a significantly lower AUC0–τ at steady state (AUC0–τ,ss) was observed with the Neb/valsartan combination compared with valsartan alone, but C max,ss and T max were similar. Steady-state interactions between valsartan and all Neb entities examined were observed; however, the extent of these interactions suggests that they are not clinically meaningful. A sharp increase in mean plasma renin activity (PRA) and plasma Angio II levels that occurred in participants who were receiving valsartan for 7 days was significantly attenuated with concomitant Neb administration. Compared with either monotherapy, mean 24-h urine aldosterone at Day 7 was substantially decreased after combined treatment [22].
ENDOTHELIAL EFFECTS
The endothelium plays a critical role in vascular homeostasis through fluid filtration, hormonal trafficking, modulation of tone, structure and function of blood vessels [23], and large arterial stiffness [24]. Endothelial dysfunction, an early feature in the progression of vascular damage, can lead to cardiovascular disease and chronic kidney disease [25]. NO, a metastable free radical, is a key mediator of endothelial vasodilation, renal fluid retention, and platelet aggregation/adhesion [26]. NO production is a calcium-dependent process catalyzed by the dimerized (coupled) enzyme, endothelial NO synthase (eNOS) (Fig. 1) [27]. Conditions that cause uncoupling of eNOS lead to a reduction in endothelial NO levels and production of O2 −, a free radical and proatherogenic mediator that can react with any available NO to form peroxynitrite, a proapoptotic, proinflammatory oxidant that has been associated with pathological conditions such as neurodegeneration and vascular disease [28]. Conversely, upregulation of eNOS coupling increases NO production and inhibits free-radical reactions, resulting in reduced endothelial dysfunction and hypertension.
FIGURE 1.
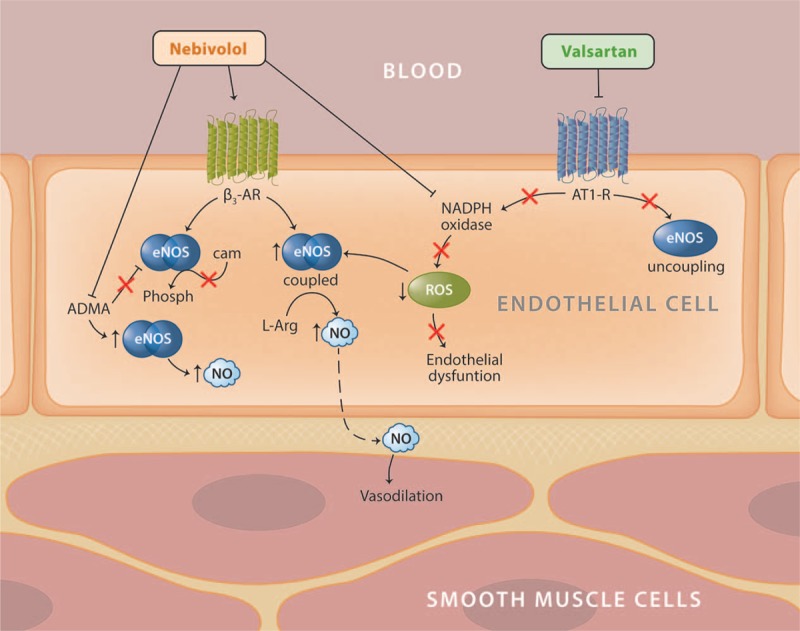
Endothelial effects of nebivolol and valsartan. β2-AR, beta-2 adrenoreceptor; β3-AR, beta-3 adrenoreceptor; ADMA, asymmetric dimethylarginine; AT1-R, angiotensin I receptor; cAMP, cyclic adenosine monophosphate; eNOS, endothelial nitric oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; ROS, reactive oxygen species.
Nebivolol
The stimulation of β3-adrenoreceptors via Neb has been shown to activate eNOS and stimulate NO production (Fig. 1) [29], which in turn activates an endothelial-derived NO/cyclic GMP (cGMP) signaling pathway [30]. Neb can stimulate NO production indirectly by lowering the levels of asymmetrical dimethylarginine (ADMA), an endogenous eNOS inhibitor and a product of methylation of arginine residues in proteins by arginine methyltransferase [5]. High ADMA levels in the plasma are associated with cardiovascular morbidity and mortality related to endothelial dysfunction [31]. In individuals with hypertension, Neb treatment results in a decrease in ADMA levels, which is closely related to a decrease in systolic blood pressure (SBP) [32,33]. Moreover, Neb can reduce endothelial dysfunction through direct interaction with free radicals in which reactive oxygen species (ROS) are scavenged in a receptor-independent manner [34]. An additional hypothesis of the vasodilatory effects of Neb posits that Neb reduces O2 − production through inhibition of eNOS uncoupling and/or stimulation of a calcium-dependent increase in eNOS within renal glomeruli [35–37].
A study in dogs found that Neb-induced vasodilation was dose-dependent and abolished upon removal of the endothelium or through eNOS inhibition, indicating a pathway that includes stimulation of eNOS [38]. These results were also supported by another study of Neb using a rat model of Angio II-induced hypertension with severe endothelial dysfunction and a marked impairment of NO/cGMP signaling [39]. In this study, Neb (10 mg/kg per day), but not metoprolol (10 mg/kg per day), normalized endothelial function and increased plasma NO bioavailability, as demonstrated by the increases in plasma nitrite and whole blood hemoglobin–NO levels. In addition, Neb, but not metoprolol, inhibited upregulation of the activity and expression of the vascular NADPH oxidase and prevented eNOS uncoupling, demonstrated by reduced vascular O2 − formation [39]. Collectively, these studies demonstrate that the effects of Neb on endothelial function are independent of β1-adrenergic receptor blockade.
A recent study demonstrated that Neb induces endothelium-dependent and NO-dependent relaxation of the pulmonary arteries in rats with pulmonary arterial hypertension (PAH) [40]. This effect was abolished when the endothelium was removed or when NOS was inhibited. In addition, Neb corrected PAH-related endothelial dysfunction and the proinflammatory phenotype of the PAH-related phenotype of endothelial cells taken from PAH patients and decreased vascular remodeling in experimental pulmonary hypertension. Although clinical studies are necessary to confirm the effects of Neb on PAH [41], the various mechanisms by which Neb can potentially decrease and reverse cardiovascular dysfunction are of great interest.
Valsartan
Activation of the AT1-R limits bioavailability of NO by reducing NO release and increasing NO inactivation, whereas blocking the AT1-R using ARBs has been shown to significantly increase endothelium-dependent vasodilation in individuals with hypertension [42–44]. Valsartan significantly improves endothelial function and reduces oxidative stress in patients with essential hypertension [45]. This reduction in oxidative stress has been associated with an increase in NO production [43,45,46], but valsartan also increases NO levels through inhibition of eNOS uncoupling and activation of coupled eNOS [47,48]. In addition, valsartan reduces endothelial dysfunction via a cyclooxygenase-mediated mechanism, which is thought to increase the levels of NO precursors, eNOS, cofactors (e.g. arginine and tetrahydrobiopterin), and reduce the levels of endogenous eNOS inhibitors [45,49–51].
EFFECTS ON THE RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM AND THE KIDNEY
Stimulation of the RAAS through low BP causes the kidneys to release the enzyme renin, which triggers a signal transduction cascade resulting in production of Angio II. Angio II can stimulate secretion of vasopressin and aldosterone (thus increasing fluid retention), trigger release of adrenaline and noradrenaline (thereby increasing vasoconstriction), and induce changes in cardiac contractility and HR [52]. Of note, Angio II also affects vascular structure through autocrine/paracrine secretion of inflammatory cytokines and growth factors [52]. It should also be mentioned that aldosterone can be produced in extra-adrenal tissues (notably, the blood vessels, the heart, and the brain) [53] and that its receptor is expressed in numerous tissues as well [54], allowing for multiple pathways in which this hormone can affect BP regulation and cardiovascular risk.
Nebivolol
Reduced PRA has been observed following treatment with Neb [51,55] and other β-blockers [56,57] through a mechanism that is thought to act via β1-dependent inhibition of the sympathetic innervation of the juxtaglomerular apparatus (i.e., through β1-mediated inhibition of renin release) (Fig. 2). Neb also has been shown to reduce the concentration of plasma aldosterone (resulting in less fluid retention and decreased blood volume) [58] as well as to reduce proteinuria (through an increase in bioavailable NO) and to lower the activity of NADPH oxidase, thereby lowering the concentration of ROS, which are implicated in the pathogenesis of hypertension [39,59,60]. Neb has also been shown to induce relaxation of glomerular microvasculature through adenosine triphosphate (ATP) efflux, which stimulates NO release from glomerular endothelial cells via stimulation of P2Y purinergic receptors [37].
FIGURE 2.
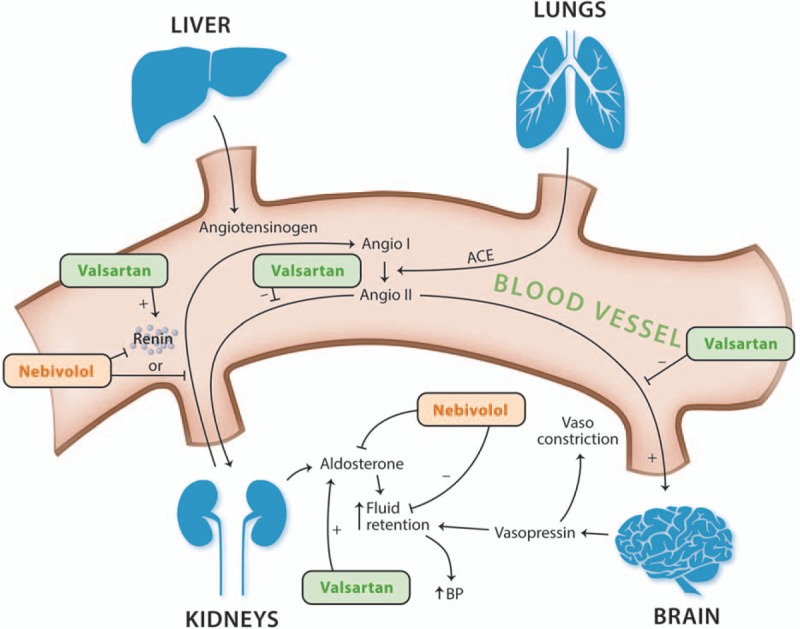
Effects of nebivolol and valsartan on the renin–angiotensin–aldosterone system. ACE, acetylcholinesterase; Angio I, angiotensin I; Angio II, angiotensin II; BP, blood pressure; RAAS, renin–angiotensin–aldosterone system.
Valsartan
Valsartan achieves its antihypertensive effects through inhibition of Angio II to the AT1-R [61], which also results in a reactive increase in PRA [52] and, in some patients, an increase in aldosterone concentration back to baseline levels (so-called aldosterone escape) [62]. In addition, valsartan has been shown to reduce microalbuminuria in patients with type 2 diabetes in a BP-independent manner [63], a phenomenon observed with other ARBs as well [64,65]. Finally, valsartan reduces the levels of urinary angiotensinogen (a marker of intrarenal RAAS activity), renal tissue gene expression of angiotensinogen, and Angio II immunoreactivity in the kidneys of patients with immunoglobulin A (IgA) nephropathy [66].
EFFECTS ON CENTRAL BLOOD PRESSURE
Central BP may be a better predictor of certain aspects of cardiovascular morbidity and mortality than peripheral (brachial) BP [67–69], and 24-h central BP has been shown to be better than 24-h peripheral BP in predicting left ventricular (LV) structure and function [70,71]. Compared with other treatments, both valsartan and Neb have been shown to be efficacious in decreasing central BP. For example, the combination of valsartan–amlodipine was shown to reduce central systolic pressure, pulse pressure (PP), and the augmentation index (AIx) more than the atenolol–amlodipine combination, despite a similar effect of the two combinations on brachial BP [72]. Similarly, Neb was found to reduce the parameters of central hemodynamics to a greater extent than other β-blockers (metoprolol, carvedilol, and atenolol), despite similar reductions in brachial BP [73–76]. Furthermore, the reductions in brachial PP and central BP observed with Neb, but not metoprolol, were shown to correlate with a reduction in LV wall thickness [74].
EFFECTS ON BLOOD PRESSURE AND BIOMARKERS IN PATIENTS WITH HYPERTENSION
An 8-week, randomized, double-blind, placebo-controlled trial (NAC-MD-01; N = 4161) was conducted to assess the efficacy and safety of the Neb/valsartan SPCs in adults with Stage 1 or 2 hypertension [Joint National Committee 7 (JNC7) criteria [77]] [6]. In that trial, participants were randomized to receive Neb (5 or 20 mg/day) monotherapy, valsartan (80 and 160 mg/day) monotherapy, Neb/valsartan SPC (5/80, 5/160, or 10/160 mg/day), or placebo for 4 weeks; the dosages were doubled during Weeks 5–8 (Neb 10 or 40; valsartan 160 or 320; and SPC 10/160, 10/320, or 20/320 mg/day).
At the end of the study, the DBP reduction from baseline (the primary efficacy parameter) with the Neb/valsartan SPCs was significantly lower than with the corresponding monotherapies (Fig. 3a). In addition, significantly greater reductions in favor of the Neb/valsartan SPCs vs. monotherapy components were observed for all SBP (Fig. 3b) comparisons by study end. The results at Week 4, which include the approved SPC 5/80 mg/day dose, mirrored those of the results at study end, with significantly greater BP-lowering effects (DBP and SBP) of the majority of the Neb/valsartan SPCs compared with monotherapy components; the only exception was the 5/160 SPC vs. valsartan 160 for SBP (Fig. 3a and b). No dose response was observed for the SPCs, which likely reflects the relatively flat dose responses of the component monotherapies [78,79].
FIGURE 3.
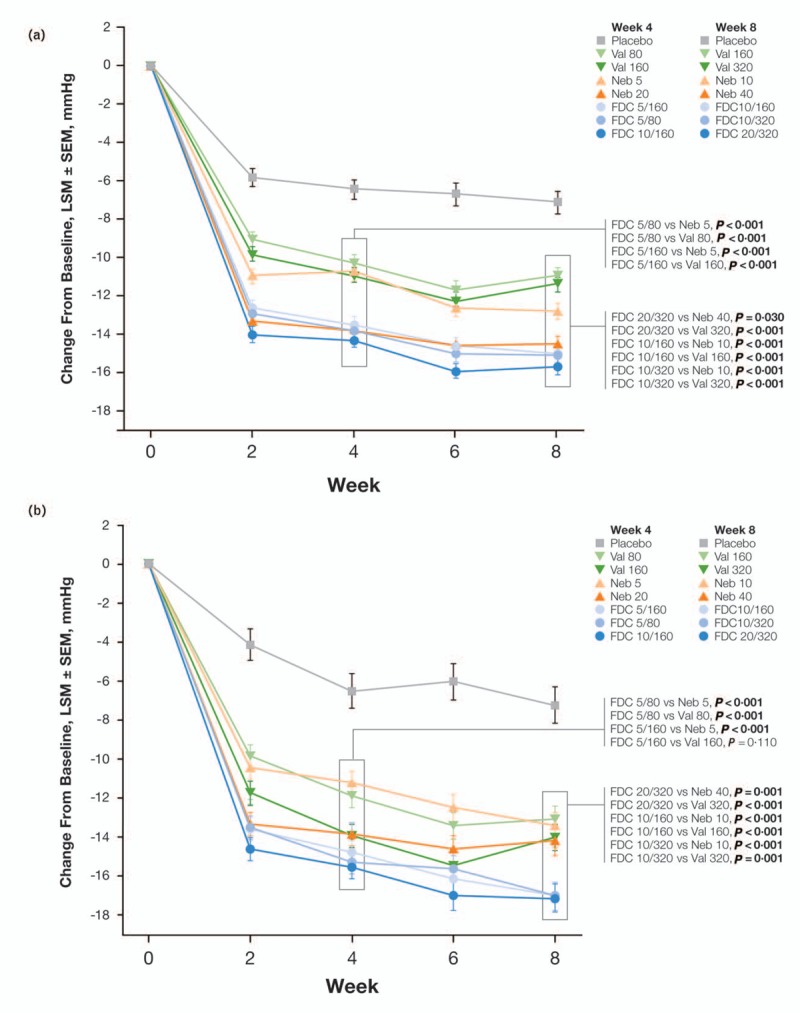
. Trial NAC-MD-01: changes from baseline in trough seated DBP (a) and SBP (b) by visit. Bold P values indicate significance. LSM, least squares mean; Neb, nebivolol; SEM, standard error of the mean; SPC, single-pill combination; Val, valsartan. Reproduced with permission from [6].
The percentage of patients who reached BP control (JNC7 criteria [77]) at endpoint was greater in the SPC groups than in the monotherapy groups. Specifically, the percentage achieving control in the SPC 10/160 group (49%) was significantly greater than in patients who were treated with Neb 10 (39%; P < 0.001) or valsartan 160 (36%; P < 0.001); similarly, a significantly greater percentage of patients achieved control in the SPC 20/320 group (52%) than in the valsartan 320 (36%; P < 0.0001) or Neb 40 groups (45%; P = 0.023). This effect was also evident at Week 4 with the SPC 5/80 (42%) vs. monotherapy components (31% Neb 5, 33% valsartan 80; P < 0.01, both) and SPC 5/160 (41%) vs. monotherapies (31% Neb 5, 32% valsartan 160; P < 0.001, both). Additional analyses revealed that the SPCs were efficacious across a wide range of phenotypes and that a reduction in PP with SPC 10/160 was significantly greater than the one observed with Neb 10 (P = 0.021), suggesting an added benefit on central hemodynamics. Finally, the adverse events and clinical laboratory parameters were similar between the SPCs and their component monotherapies.
A substudy conducted within the NAC-MD-01 trial (N = 805) examined patients’ BP using ambulatory BP monitoring (ABPM) and their levels of PRA and plasma aldosterone [80]. Those examinations revealed that at Week 8, the SPC 10/160 was significantly more effective in lowering ABPM than the component monotherapies valsartan 160 (SBP/DBP; P < 0.001, both) and Neb 10 (DBP; P < 0.01); in addition, the SPC 20/320 reduced 24-h DBP and SBP significantly more than valsartan 320 (P < 0.01, both) but not Neb 40. From baseline to endpoint, PRA increased in valsartan-treated groups (53.8–72.8%) and decreased in Neb-treated (51.3–65.4%) and SPC-treated groups (3.2–39.0%) (Fig. 4a). At Week 8, all SPC doses were effective in reducing PRA compared with their corresponding valsartan monotherapy doses (P < 0.001, all), but not when compared with the corresponding Neb doses (Fig. 4a). Plasma aldosterone increased with placebo (17.1%) and decreased with all active treatments at endpoint [range: 11.1 (valsartan 160)–35.1% (SPC 20/320)] (Fig. 4b). The SPC 20/320 produced significantly greater decreases than valsartan 320 but not Neb 40 (P < 0.05); numerical decreases were observed in the other active treatment groups (Fig. 4b). A post-hoc analysis with pooled active treatment groups demonstrated a significant correlation between 24-h, daytime, and nighttime ABPM reduction and baseline PRA in participants treated with Neb and SPCs, but not with valsartan; baseline aldosterone levels were correlated with 24-h, daytime, and night-time ABPM reduction in those treated with the SPCs, but not with the monotherapies [80].
FIGURE 4.
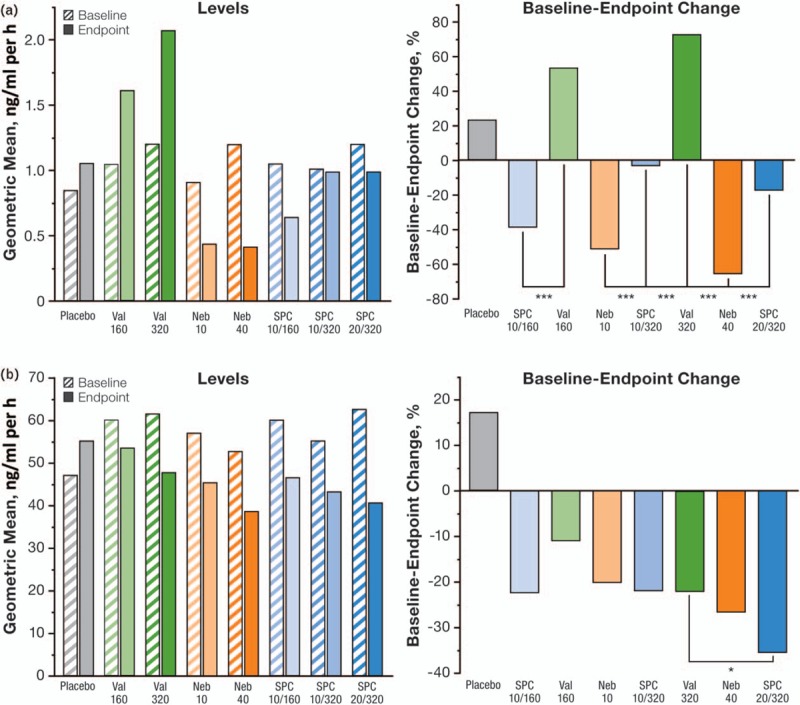
. Plasma renin activity (a) and plasma aldosterone (b) levels (b1) and baseline–endpoint change (b2) following 8 weeks of treatment with placebo, valsartan, nebivolol, or the single-pill combination. Neb, nebivolol; PRA, plasma renin activity; SPC, single-pill combination; Val, valsartan. Modified with permission from [80]. ∗ P < 0.05; ∗∗∗ P < 0.001.
Neb has previously been shown to decrease PRA in a dose-dependent manner [58,81], and the substudy data indicate that it can attenuate the reactive rise in PRA observed with valsartan treatment, suggesting that the Neb/valsartan combination can be used to attain dual RAAS blockade. Furthermore, a significantly greater reduction in aldosterone levels observed with SPC 20/320 mg/day than with valsartan 320 mg/day suggests a potential of the combination to counter the valsartan-associated ‘aldosterone escape’. The results from this substudy were in contrast to those seen when aliskiren (a direct renin inhibitor) was added to valsartan to produce a dual RAAS blockade. Following treatment with this combination, a synergistic increase in PRA occurred [82]. Moreover, no favorable clinical response was produced from the aliskiren/valsartan combination, possibly due to the excessive increase in renin and prorenin activity [83,84]. It should also be noted that increases in PRA such as these may give rise to unfavorable cardiovascular outcomes that are independent of BP reduction [85].
β-Blocker/RAAS inhibitor combinations have been considered less effective for BP reduction compared with other antihypertensive drug combinations based on a lack of additive drug effects observed in a study examining the combination of atenolol and enalapril [3] and from the primary analysis of the COSMOS study examining carvedilol and lisinopril [4]. β-Blockers, however, can vary in vasodilatory, β1-selectivity, and other properties. The mechanisms that contribute to the effectiveness of Neb (i.e. endothelium-dependent vasodilation via NO, high β1 selectivity, and/or β3 receptor agonism) [86,87] may differentiate the Neb/valsartan combination from previously studied β-blocker/RAAS inhibitors.
CONCLUSION
Neb and valsartan reduce BP via complementary mechanisms. In combination, their efficacy surpasses that of component monotherapies. Additive effects have not been observed with other β-blocker/RAAS combinations [3,4]. Hence, a Neb/valsartan combination is an efficacious and well tolerated treatment option for patients with hypertension.
ACKNOWLEDGEMENTS
The article was supported by funding from Forest Research Institute, Inc, an Allergan affiliate. The authors acknowledge the contributions of Bryn Gaertner, PhD, Vojislav Pejović, PhD, Leah Richmond, and Mark Blade of Prescott Medical Communications Group (Chicago, Illinois, USA) for their editorial suggestions, literature searches, and assistance in developing tables and figures.
Conflicts of interest
In the past 2 years, T.D.G. has received personal fees from Forest Laboratories, an Allergan affiliate. J.R.C. received honoraria for advisory board meetings and research funding from Forest. B.P. has no potential conflicts to disclose. A.J. and H.M.W. are employees, present (A.J.) or former (H.M.W.) of the article's sponsor.
Reviewers’ Summary Evaluations
Reviewer 1
The manuscript addresses a very interesting subject. For many years it was accepted that the combination of a β-blocker with a renin–angiotensin–aldosterone system (RAAS) inhibitor was ‘less effective’ than other combinations, due to a partially overlapping mechanism of action of these two drugs and, more important, the limited evidence. This paper shows preclinical and clinical studies on the single pill combination of nebivolol 5 mg and valsartan 80 mg, a combination recently approved by the FDA to treat hypertension, supporting that nebivolol/valsartan is an efficacious and well tolerated combination treatment for hypertension. The issue is interesting and new information is provided.
Reviewer 2
The ESH/ESC guidelines proposed the use of β-blockers as first line treatment in hypertension since they are as effective as the other major classes of antihypertensive agents in preventing cardiovascular events. Although β-blockers appear to have more side-effects (tend to increase body weight and, particularly when used in combination with diuretics, to facilitate new-onset diabetes in predisposed patients), these limitations are not shared by some of the vasodilating beta-blockers such as nebivolol. The administration of vasodilating β-blockers, and especially nebivolol, improves insulin resistance, and at the same time reduces central blood pressure. Although the combination of a renin–angiotensin system blocker with a β-blocker was not suggested by current guidelines, several studies mentioned in this manuscript suggest that nebivolol/valsartan combination is an efficacious and well tolerated treatment option for patients with arterial hypertension.
Harold M. Wright is no longer affiliated with the Forest Research Institute.
Abbreviations: β1-AR, β1-adrenoreceptor; β2-AR, beta-2 adrenoreceptor; β3-AR, beta-3 adrenoreceptor; ABPM, ambulatory blood pressure monitoring; ACE, acetylcholinesterase; ADMA, asymmetrical dimethylarginine; AIx, augmentation index; Angio I, angiotensin I; Angio II, angiotensin II; ARB, angiotensin receptor blocker; ATP, adenosine triphosphate; AT1-R, angiotensin I receptor; AT2-R, angiotensin II receptor; AUC, area under the curve; AUC0–τ,ss, steady-state AUC from time 0 to the dosing interval; BP, blood pressure; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; C max, peak plasma concentration; C max,ss, peak plasma concentration at steady state; CV, cardiovascular; eNOS, endothelial nitric oxide synthase; HR, heart rate; IgA, immunoglobulin A; JNC, Joint National Committee; LSM, least squares mean; LV, left ventricular; Neb, nebivolol; NO, nitric oxide; O2 −, superoxide; PAH, pulmonary arterial hypertension; PP, pulse pressure; PRA, plasma renin activity; RAAS, renin–angiotensin–aldosterone system; ROS, reactive oxygen species; SPC, single-pill combination; SS, steady state; T max, time to maximum plasma concentration; Val, valsartan
REFERENCES
- 1. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 2. Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. JASH 2010; 4:42–50. [DOI] [PubMed] [Google Scholar]
- 3. Wing LM, Chalmers JP, West MJ, Russell AE, Morris MJ, Cain MD, et al. Enalapril and atenolol in essential hypertension: attenuation of hypotensive effects in combination. Clin Exp Hypertens A 1988; 10:119–133. [DOI] [PubMed] [Google Scholar]
- 4. Bakris GL, Iyengar M, Lukas MA, Ordronneau P, Weber MA. Effect of combining extended-release carvedilol and lisinopril in hypertension: results of the COSMOS study. J Clin Hypertens (Greenwich) 2010; 12:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanhoutte PM, Gao Y. Beta blockers, nitric oxide, and cardiovascular disease. Curr Opin Pharmacol 2013; 13:265–273. [DOI] [PubMed] [Google Scholar]
- 6. Giles TD, Weber MA, Basile J, Gradman AH, Bharucha DB, Chen W, et al. Efficacy and safety of nebivolol and valsartan as fixed-dose combination in hypertension: a randomised, multicentre study. Lancet 2014; 383:1889–1898. [DOI] [PubMed] [Google Scholar]
- 7. Forest Pharmaceuticals Inc. Nebivolol [package insert]. St. Louis, MO: Forest Pharmaceuticals Inc; 2014. [Google Scholar]
- 8. Munzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol 2009; 54:1491–1499. [DOI] [PubMed] [Google Scholar]
- 9. Ignarro LJ. Different pharmacological properties of two enantiomers in a unique beta-blocker, nebivolol. Cardiovasc Ther 2008; 26:115–134. [DOI] [PubMed] [Google Scholar]
- 10. Maffei A, Lembo G. Nitric oxide mechanisms of nebivolol. Ther Adv Cardiovasc Dis 2009; 3:317–327. [DOI] [PubMed] [Google Scholar]
- 11. Rozec B, Quang TT, Noireaud J, Gauthier C. Mixed beta3-adrenoceptor agonist and alpha1-adrenoceptor antagonist properties of nebivolol in rat thoracic aorta. Br J Pharmacol 2006; 147:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, et al. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide-sensitive human hypertension. Hypertension 2014; 64:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Himmelmann A, Hedner T, Snoek E, Lundgren B, Hedner J. Haemodynamic effects and pharmacokinetics of oral d- and l- nebivolol in hypertensive patients. Eur J Clin Pharmacol 1996; 51:259–264. [DOI] [PubMed] [Google Scholar]
- 14. McNeely W, Goa K. Nebivolol in the management of essential hypertension: A review. Drugs 1999; 57:633–651. [DOI] [PubMed] [Google Scholar]
- 15. A. Menarini Farmaceutica Internazionale SRL. Nebivolol [patient information leaflet]. Luxembourg City, Luxembourg: A. Menarini Farmaceutica Internazionale SRL; 2014. [Google Scholar]
- 16. Dzau VJ. Local expression and pathophysiological role of renin–angiotensin in the blood vessels and heart. Basic Res Cardiol 1993; 88 (Suppl 1):1–14. [DOI] [PubMed] [Google Scholar]
- 17. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 18. Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res 1997; 81:970–976. [DOI] [PubMed] [Google Scholar]
- 19. Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res 1999; 84:1043–1049. [DOI] [PubMed] [Google Scholar]
- 20. Miura S, Karnik SS, Saku K. Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst 2011; 12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novartis Pharmaceuticals Corp. Valsartan [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2007. [Google Scholar]
- 22. Chen CL, Desai-Krieger D, Ortiz S, Kerolous M, Wright HM, Ghahramani P. A single-center, open-label, 3-way crossover trial to determine the pharmacokinetic and pharmacodynamic interaction between nebivolol and valsartan in healthy volunteers at steady state. Am J Ther 2015; 22:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 24. Schmitt M, Avolio A, Qasem A, McEniery CM, Butlin M, Wilkinson IB, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension 2005; 46:227–231. [DOI] [PubMed] [Google Scholar]
- 25. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000; 101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 26. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006; 113:1708–1714. [DOI] [PubMed] [Google Scholar]
- 27. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012; 33:829–837. 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 2007; 6:662–680. [DOI] [PubMed] [Google Scholar]
- 29. Figueroa X, Poblete I, Fernandez R, Pedemonte C, Cortes C, Huidobro-Toro J. NO production and eNOS phosphorylation induced by epinephrine through the activation of beta-adrenoreceptors. Am J Physiol Heart Circ Physiol 2009; 297:H134–H143. [DOI] [PubMed] [Google Scholar]
- 30. Feng MG, Prieto MC, Navar LG. Nebivolol-induced vasodilation of renal afferent arterioles involves beta3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol 2012; 303:F775–F782. [DOI] [PubMed] [Google Scholar]
- 31. Caplin B, Leiper J. Endogenous nitric oxide synthase inhibitors in the biology of disease: markers, mediators, and regulators? Arterioscler Thromb Vasc Biol 2012; 32:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kandavar R, Higashi Y, Chen W, Blackstock C, Vaughn C, Sukhanov S, et al. The effect of nebivolol versus metoprolol succinate extended release on asymmetric dimethylarginine in hypertension. J Am Soc Hypertens 2011; 5:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khan BV, Rahman ST, Haque T, Merchant N, Bhaheetharan S, Harris J, 3rd, et al. Vascular effects of nebivolol added to hydrochlorothiazide in African Americans with hypertension and echocardiographic evidence of diastolic dysfunction: the NASAA study. J Cardiovasc Pharmacol Ther 2012; 17:291–297. [DOI] [PubMed] [Google Scholar]
- 34. de Groot AA, Mathy MJ, van Zwieten PA, Peters SL. Antioxidant activity of nebivolol in the rat aorta. J Cardiovasc Pharmacol 2004; 43:148–153. [DOI] [PubMed] [Google Scholar]
- 35. Broeders MA, Doevendans PA, Bekkers BC, Bronsaer R, van Gorsel E, Heemskerk JW, et al. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation 2000; 102:677–684. [DOI] [PubMed] [Google Scholar]
- 36. Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frerart F, et al. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation 2005; 112:1198–1205. [DOI] [PubMed] [Google Scholar]
- 37. Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003; 107:2747–2752. [DOI] [PubMed] [Google Scholar]
- 38. Gao YS, Nagao T, Bond RA, Janssens WJ, Vanhoutte PM. Nebivolol induces endothelium-dependent relaxations of canine coronary arteries. J Cardiovasc Pharmacol 1991; 17:964–969. [DOI] [PubMed] [Google Scholar]
- 39. Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 2006; 48:677–684. [DOI] [PubMed] [Google Scholar]
- 40. Perros F, Ranchoux B, Izikki M, Bentebbal S, Happe C, Antigny F, et al. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol 2015; 65:668–680. [DOI] [PubMed] [Google Scholar]
- 41. Rubin LJ. The Beta-adrenergic receptor in pulmonary arterial hypertension: a novel therapeutic target? J Am Coll Cardiol 2015; 65:681–683. [DOI] [PubMed] [Google Scholar]
- 42. Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension 2000; 35 (1 Pt 2):501–506. [DOI] [PubMed] [Google Scholar]
- 43. Klingbeil AU, John S, Schneider MP, Jacobi J, Handrock R, Schmieder RE. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens 2003; 16:123–128. [DOI] [PubMed] [Google Scholar]
- 44. Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 2000; 101:1653–1659. [DOI] [PubMed] [Google Scholar]
- 45. Hirooka Y, Kimura Y, Sagara Y, Ito K, Sunagawa K. Effects of valsartan or amlodipine on endothelial function and oxidative stress after one year follow-up in patients with essential hypertension. Clin Exp Hypertens 2008; 30:267–276. [DOI] [PubMed] [Google Scholar]
- 46. Tzemos N, Lim PO, MacDonald TM. Valsartan improves endothelial dysfunction in hypertension: a randomized, double-blind study. Cardiovasc Ther 2009; 27:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su KH, Tsai JY, Kou YR, Chiang AN, Hsiao SH, Wu YL, et al. Valsartan regulates the interaction of angiotensin II type 1 receptor and endothelial nitric oxide synthase via Src/PI3K/Akt signalling. Cardiovasc Res 2009; 82:468–475. [DOI] [PubMed] [Google Scholar]
- 48. Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, et al. Beneficial effects of combination of valsartan and amlodipine on salt-induced brain injury in hypertensive rats. J Pharmacol Exp Ther 2011; 339:358–366. [DOI] [PubMed] [Google Scholar]
- 49. Aslam S, Santha T, Leone A, Wilcox C. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int 2006; 70:2109–2115. [DOI] [PubMed] [Google Scholar]
- 50. Schmidt B, Drexler H, Schieffer B. Therapeutic effects of angiotensin (AT1) receptor antagonists: potential contribution of mechanisms other than AT1 receptor blockade. Am J Cardiovasc Drugs 2004; 4:361–368. [DOI] [PubMed] [Google Scholar]
- 51. Florez A, de Haro J, Martinez E, Varela C, Bleda S, Acin F. Selective cyclooxygenase-2 inhibition reduces endothelial dysfunction and improves inflammatory status in patients with intermittent claudication. Rev Esp Cardiol 2009; 62:851–857. [DOI] [PubMed] [Google Scholar]
- 52. Atlas SA. The renin–angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 2007; 13 (8 Suppl B):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacKenzie SM, Fraser R, Connell JM, Davies E. Local renin–angiotensin systems and their interactions with extra-adrenal corticosteroid production. J Renin Angiotensin Aldosterone Syst 2002; 3:214–221. [DOI] [PubMed] [Google Scholar]
- 54. Hawkins UA, Gomez-Sanchez EP, Gomez-Sanchez CM, Gomez-Sanchez CE. The ubiquitous mineralocorticoid receptor: clinical implications. Curr Hypertens Rep 2012; 14:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fortepiani LA, Ortiz MC, Atucha NM, Garcia-Estan J. Nebivolol ameliorates nitric oxide deficient hypertension. Scientific World Journal 2002; 2:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simon G, Johnson ML. Comparison of antihypertensive and beta 1-adrenoceptor antagonist effect of nebivolol and atenolol in essential hypertension. Clin Exp Hypertens 1993; 15:501–509. [DOI] [PubMed] [Google Scholar]
- 57. Ayers K, Byrne LM, DeMatteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension 2012; 59:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chan TY, Woo KS, Nicholls MG. The application of nebivolol in essential hypertension: a double-blind, randomized, placebo-controlled study. Int J Cardiol 1992; 35:387–395. [DOI] [PubMed] [Google Scholar]
- 59. Mollnau H, Schulz E, Daiber A, Baldus S, Oelze M, August M, et al. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol 2003; 23:615–621. [DOI] [PubMed] [Google Scholar]
- 60. Weber MA. The role of the new beta-blockers in treating cardiovascular disease. Am J Hypertens 2005; 18 (12 Pt 2):169s–176s. [DOI] [PubMed] [Google Scholar]
- 61. Black HR, Bailey J, Zappe D, Samuel R. Valsartan: more than a decade of experience. Drugs 2009; 69:2393–2414. [DOI] [PubMed] [Google Scholar]
- 62. Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol 2007; 3:486–492. [DOI] [PubMed] [Google Scholar]
- 63. Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106:672–678. [DOI] [PubMed] [Google Scholar]
- 64. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869. [DOI] [PubMed] [Google Scholar]
- 65. Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870–878. [DOI] [PubMed] [Google Scholar]
- 66. Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant 2011; 26:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 68. Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens 2009; 27:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17 635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Protogerou AD, Argyris AA, Papaioannou TG, Kollias GE, Konstantonis GD, Nasothimiou E, et al. Left-ventricular hypertrophy is associated better with 24-h aortic pressure than 24-h brachial pressure in hypertensive patients: the SAFAR study. J Hypertens 2014; 32:1805–1814. [DOI] [PubMed] [Google Scholar]
- 71. Zhang Y, Kollias G, Argyris AA, Papaioannou TG, Tountas C, Konstantonis GD, et al. Association of left ventricular diastolic dysfunction with 24-h aortic ambulatory blood pressure: the SAFAR study. J Hum Hypertens 2015; 29:442–448. [DOI] [PubMed] [Google Scholar]
- 72. Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine–valsartan combination decreases central systolic blood pressure more effectively than the amlodipine–atenolol combination: the EXPLOR study. Hypertension 2010; 55:1314–1322. [DOI] [PubMed] [Google Scholar]
- 73. Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens 2008; 26:351–356. [DOI] [PubMed] [Google Scholar]
- 74. Kampus P, Serg M, Kals J, Zagura M, Muda P, Karu K, et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension 2011; 57:1122–1128. [DOI] [PubMed] [Google Scholar]
- 75. Koumaras C, Tziomalos K, Stavrinou E, Katsiki N, Athyros VG, Mikhailidis DP, et al. Effects of renin–angiotensin–aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. J Am Soc Hypertens 2014; 8:74–82. [DOI] [PubMed] [Google Scholar]
- 76. Studinger P, Tabak AG, Chen CH, Salvi P, Othmane TE, Torzsa P, et al. The effect of low-dose carvedilol, nebivolol, and metoprolol on central arterial pressure and its determinants: a randomized clinical trial. J Clin Hypertens (Greenwich) 2013; 15:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 78. Makani H, Bangalore S, Supariwala A, Romero J, Argulian E, Messerli FH. Antihypertensive efficacy of angiotensin receptor blockers as monotherapy as evaluated by ambulatory blood pressure monitoring: a meta-analysis. Eur Heart J 2014; 35:1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weiss RJ, Saunders E, Greathouse M. Efficacy and tolerability of nebivolol in stage I–II hypertension: a pooled analysis of data from three randomized, placebo-controlled monotherapy trials. Clin Ther 2011; 33:1150–1161. [DOI] [PubMed] [Google Scholar]
- 80. Giles TD, Bakris GL, Oparil S, Weber MA, Li H, Bharucha DB, et al. Correlation of plasma renin activity and aldosterone concentration with ambulatory blood pressure responses to nebivolol and valsartan, alone and in combination, in hypertension. J Am Soc Hypertens 2015; 9:845–854. [DOI] [PubMed] [Google Scholar]
- 81. Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, et al. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin–angiotensin–aldosterone system in normotensive and hypertensive subjects. Am J Hypertens 1999; 12:451–459. [DOI] [PubMed] [Google Scholar]
- 82. Epstein BJ. Aliskiren and valsartan combination therapy for the management of hypertension. Vasc Health Risk Manag 2010; 6:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sealey JE, Laragh JH. Aliskiren, the first renin inhibitor for treating hypertension: reactive renin secretion may limit its effectiveness. Am J Hypertens 2007; 20:587–597. [DOI] [PubMed] [Google Scholar]
- 84. Sealey JE, Laragh JH. Aliskiren fails to lower blood pressure in patients who have either low PRA levels or whose PRA falls insufficiently or reactively rises. Am J Hypertens 2009; 22:112–121. [DOI] [PubMed] [Google Scholar]
- 85. Gonzalez MC, Cohen HW, Sealey JE, Laragh JH, Alderman MH. Enduring direct association of baseline plasma renin activity with all-cause and cardiovascular mortality in hypertensive patients. Am J Hypertens 2011; 24:1181–1186. [DOI] [PubMed] [Google Scholar]
- 86. Stoschitzky K, Stoschitzky G, Brussee H, Bonelli C, Dobnig H. Comparing beta-blocking effects of bisoprolol, carvedilol and nebivolol. Cardiology 2006; 106:199–206. [DOI] [PubMed] [Google Scholar]
- 87. Pedersen ME, Cockcroft JR. The vasodilatory beta-blockers. Curr Hypertens Rep 2007; 9:269–277. [DOI] [PubMed] [Google Scholar]


