Abstract
OBJECTIVE
The purpose of this study is to assess the additional value of secretin-enhanced MRCP over conventional (non–secretin-enhanced) MRCP in diagnosing disease in patients with recurrent acute pancreatitis.
MATERIALS AND METHODS
A retrospective review of a radiology database found 72 patients with recurrent acute pancreatitis who had secretin-enhanced MRCP and ERCP correlation within 3 months of each other between January 2007 and December 2011. Of these patients, 54 had no history of pancreatic tumor or surgery and underwent MRI more than 3 months after an episode of acute pancreatitis. In addition, 57 age- and sex-matched control subjects with secretin-enhanced MRCP and ERCP correlation and without a diagnosis of recurrent acute pancreatitis or chronic pancreatitis were enrolled as the control group. All studies were anonymized, and secretin-enhanced MRCP images (image set A) were separated from conventional 2D and 3D MRCP and T2-weighted images (image set B). Image sets A and B for each patient were assigned different and randomized case numbers. Two blinded reviewers independently assessed both image sets for ductal abnormalities and group A image sets for exocrine response to secretin.
RESULTS
There were statistically significantly more patients with recurrent acute pancreatitis with reduced exocrine function compared with patients in the control group (32% vs 9%; p < 0.01) on secretin-enhanced images. Patients with recurrent acute pancreatitis were more likely to have side branch dilation (p = 0.02; odds ratio, 3.6), but not divisum, compared with the control group. Secretin-enhanced images were superior to non–secretin-enhanced images for detecting ductal abnormalities in patients with recurrent acute pancreatitis, with higher sensitivity (76% vs 56%; p = 0.01) and AUC values (0.983 vs 0.760; p < 0.01).
CONCLUSION
Up to one-third of patients with recurrent acute pancreatitis showed exocrine functional abnormalities. Secretin-enhanced MRCP had a significantly higher yield for ductal abnormalities than did conventional MRI and should be part of the MRCP protocol for investigation of patients with recurrent acute pancreatitis.
Keywords: MRCP, pancreatitis, secretin
Most patients with acute pancreatitis recover completely after appropriate therapy. However, about 25–30% of patients [1, 2] have more than one such episode and are said to have recurrent acute pancreatitis. In patients with excessive alcohol consumption, this figure is higher, with about 50% having a second episode of acute pancreatitis within 10 years of follow-up [3]. The two important aims of investigating patients with recurrent acute pancreatitis are to determine whether there is a treatable risk cause for acute pancreatitis and whether there is evidence of chronic pancreatitis. In recurrent acute pancreatitis, it is assumed that the gland returns to normal between the acute episodes. In chronic pancreatitis, there is permanent damage to the gland with parenchymal fibrosis or ductal irregularities. Pancreatic ductal diseases are primarily investigated with MRCP and ERCP, with the latter usually considered to be the reference standard. Conventional MRCP is safe but has a reduced sensitivity for ductal diseases, such as divisum [4–6], and does not assess the exocrine function of the gland.
Secretin is a hormone produced by the S cells of the duodenal mucosa in response to acid in the duodenal lumen [7–9]. Its primary effect is to increase water and bicarbonate secretions by exocrine cells of the pancreas [7, 8]. Studies have shown that secretin increases the amount of fluid in the pancreatic ducts and may augment the sensitivity of MRCP in diagnosing pancreatic ductal disease, congenital anomalies, and chronic pancreatitis [9–18]. However, these studies, other than a multicenter phase 3 study [11], did not compare secretin-enhanced MRCP and conventional MRCP to determine the added benefit of secretin-enhanced MRCP. In addition, these studies usually did not correlate MRCP findings with a reference standard, such as ERCP. To our knowledge, only three prior studies have assessed the value of secretin-enhanced MRCP in recurrent acute pancreatitis [19–21].
In this study, we were interested in comparing a cohort of patients with recurrent acute pancreatitis and a control group without a history of acute or chronic pancreatitis, all of whom underwent secretin-enhanced MRCP and ERCP. The two aims of the study were to determine whether secretin-enhanced MRCP was superior to conventional MRCP in diagnosing pancreatic disease in patients with recurrent acute pancreatitis and whether there were differences in ductal anatomic and exocrine functional abnormalities between the recurrent acute pancreatitis cohort and control group on secretin-enhanced MRCP.
Materials and Methods
Patients
This retrospective HIPAA-compliant cohort study reviewed the MRI database of 2871 patients who underwent secretin-enhanced MRCP between January 2007 and December 2011. Permission for access to patient data with a waiver of consent was obtained from the institutional review board of Indiana University School of Medicine. Patients were selected for the cohort group if the secretin-enhanced MRCP was performed after a diagnosis of recurrent acute pancreatitis was made (Fig. 1). Excluding patients with pancreatic surgery or tumor resulted in a cohort of 54 patients with recurrent acute pancreatitis. In addition, a control group was selected comprising 57 patients who underwent secretin-enhanced MRCP between January 2007 to March 2007 and who had no clinical history of acute or chronic pancreatitis, pancreatic tumors, or surgery.
Fig. 1.
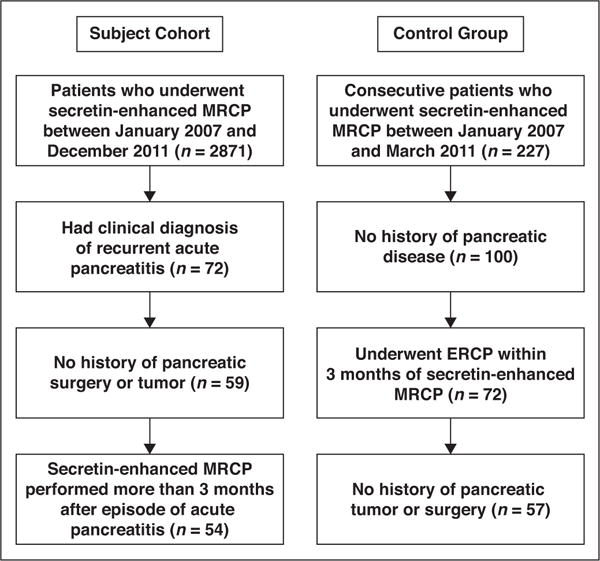
Selection flowchart for patients and control subjects in this study.
MRCP Technique
The MRI examinations were performed on a 1.5- or 3-T MRI scanner (Magnetom Avanto, Harmony, or Verio, all from Siemens Healthcare). The techniques used for T2-weighted, 2D, and 3D MRCP and secretin-enhanced MRCP sequences have been previously published [22]. The patients were advised not to eat or drink for 6 hours before the scan. Patients drank oral contrast agent (300 mL of ferumoxsil; GastroMark, Covidien Pharmaceuticals) 30 minutes before the scan to reduce the signal from luminal content of the stomach, duodenum, and proximal jejunum. Secretin-enhanced MRCP images were obtained after the injection of 16 μg of IV synthetic human secretin (ChiRhoStim, ChiRhoClin). Coronal 4-cm slab HASTE images were obtained through the upper abdomen to include the pancreas and bile ducts every 30 seconds for 10 minutes [17, 22].
Image Analysis
All images were downloaded to a computer (iMac, running Mac OS X, Apple). The secretin-enhanced MRCP images were separated from the rest of the image series and saved as the secretin-enhanced image set (set A). The T2-weighted axial and coronal images and 2D and 3D MRCP images were grouped together and saved as the non–secretin-enhanced image set (set B). Gadolinium-enhanced images were not included in this set. All images were anonymized using a DICOM viewer (OsiriX3.91, Pixmeo). The two image sets per patient were labeled with different random case numbers.
The image sets were reviewed by an abdominal radiology fellow and an abdominal radiologist with 8 years of experience who were blinded to the clinical history and ERCP results. They assessed for the presence or absence of pancreas divisum, main duct dilation, ductal stricture, dilation of three or more side branch ducts, and acinarization [23] using a 5-point scale: 1 denotes definitely present, 2 denotes probably present, 3 denotes indeterminate, 4 denotes probably absent, and 5 denotes definitely absent. Duct dilation was considered if the pancreatic duct measured greater than 5 mm in caliber. A stricture was seen when the pancreatic duct abruptly changed caliber by more than 1 mm. Any visible side branches were considered dilated. In addition, the exocrine response to secretin (assessed only on the secretin-enhanced image sets) was graded using previously published criteria, where 0 denotes no visible exocrine output, 1 denotes that output only fills the duodenal bulb, 2 denotes that output fills the duodenal bulb and the descending duodenum, and 3 denotes that output fills the entire duodenum [24]. If the two blinded reviewers diverged in their findings, a consensus agreement was made between the reviewers and a third author with 12 years’ experience in reading secretin-enhanced MRCP studies; all reviewers were blinded to clinical outcome.
Statistical Analysis
The reports of the endoscopists performing the ERCP, based on real-time evaluation of endoscopic and fluoroscopic images, were taken as the reference standard. Interobserver agreement on the initial reads before consensus review was determined using the intraclass correlation coefficient. Differences in pancreatic ductal findings (divisum, main and side branch dilation, and ductal stricture) between the recurrent acute pancreatitis cohort and the control group were determined with the Fisher exact test and odds ratios. The comparison between secretin-enhanced and non–secretin-enhanced MRCP images was performed using ROC curves. Statistical analysis was performed using MedCalc (version 12.3.0, MedCalc Software).
Results
Patients
The mean age of patients in the recurrent acute pancreatitis cohort was 43.6 years (range, 14–76 years), with 17 male and 37 female patients. The mean age of the control group was 43.0 years (range, 14–74 years), with 16 male and 41 female subjects. There were no statistically significant differences in sex (p = 0.69) or age (p = 0.84) between the patients with recurrent acute pancreatitis and the control subjects. The indication for MRI in the recurrent acute pancreatitis group was to assess for chronic pancreatitis (n = 24), divisum (n = 21), sphincter of Oddi dysfunction (n = 7), and miscellaneous (n = 2). The indication for MRI in the control group was unexplained abdominal pain (n = 42), suspected sphincter of Oddi dysfunction (n = 12), and miscellaneous (n = 3). The mean time between MRCP and ERCP for all patients was 24 days (range, 0–88 days). The order in which ERCP and MRCP were performed varied.
Interobserver Agreement
The intraclass correlation coefficients for the scores given for image set A were as follows: divisum, 0.78; pancreatic duct dilation, 0.83; pancreatic duct stricture, 0.64; and side branch dilation, 0.68. Similarly for image set B, the correlation coefficients were as follows: divisum, 0.94; pancreatic dilation, 0.79; pancreatic duct stricture, 0.72; and side branch dilation, 0.65. There was good or excellent agreement between the two reviewers. Nine cases (five cases with recurrent acute pancreatitis and four control cases) needed consensus reads with the third reviewer.
Recurrent Acute Pancreatitis Versus Control Group
Table 1 gives the results of the analysis of the recurrent acute pancreatitis and control groups for pancreatic ductal and exocrine output findings. There was no statistically significant difference (p = 0.35) in the frequency of divisum between the groups. Side branch dilation (p = 0.02) was more common in the recurrent acute pancreatitis group, and patients with recurrent acute pancreatitis were more likely to show at least one ductal abnormality (p = 0.01).
TABLE 1.
Ductal Findings in Patients With Recurrent Acute Pancreatitis and in Control Subjects
| Finding at MRCP | Patients With Recurrent Acute Pancreatitis (n = 54) | Control Subjects (n = 57) | Odds Ratio (95% CI) | pa |
|---|---|---|---|---|
|
| ||||
| Divisum | 7 | 4 | 2.0 (0.5–7.2) | 0.35 |
| Pancreatic duct dilation | 13 | 5 | 3.3 (1.1–10.0) | 0.12 |
| Pancreatic duct stricture | 4 | 0 | 10.3 (0.5–195.0) | 0.05 |
| Side branch dilation | 14 | 5 | 3.6 (1.2–10.9) | 0.02 |
| All ductal abnormalitiesb | 26 | 11 | 3.8 (1.7–9.1) | 0.01 |
| Impaired exocrine output | 17 | 5 | 4.8 (1.6–14.1) | < 0.01 |
Note—Data are number of findings.
Fisher exact test.
Presence of divisum, main or side branch dilation, or ductal stricture.
Figure 2 gives the exocrine output in the recurrent acute pancreatitis and control groups. Poor exocrine output (score 1) was seen in six of 54 patients (11%) with recurrent acute pancreatitis and no control patients. The proportion of patients with impaired exocrine functions (scores 1 and 2) was statistically significantly higher in the recurrent acute pancreatitis cohort compared with the control group (31.5% vs 8.8%; p < 0.01).
Fig. 2.
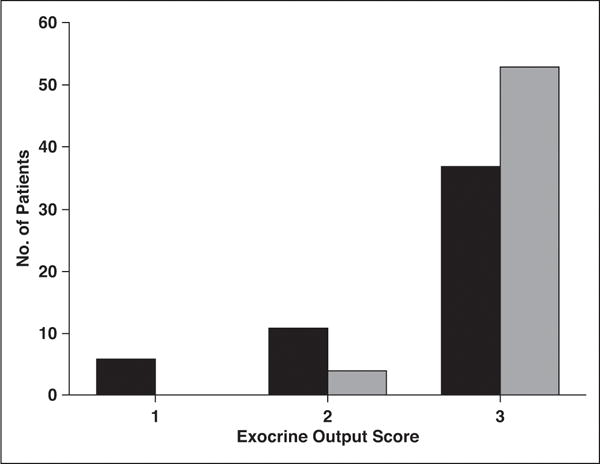
Chart of semiquantitative determination of exocrine output in response to secretin. See text for description of scoring system. Black bars denote patients with recurrent acute pancreatitis, and gray bars denote control subjects.
Usefulness of Secretin-Enhanced MRCP in Recurrent Acute Pancreatitis
Table 2 shows the sensitivity and specificity of secretin-enhanced (image set A) and non–secretin-enhanced (image set B) MRCP images in detecting abnormalities in patients with recurrent acute pancreatitis. ERCP findings were used as reference standard. Table 3 shows the AUC values for the same findings.
TABLE 2.
Sensitivity and Specificity of MRCP, With or Without Secretin Enhancement, for Detecting Ductal Abnormalities in Patients With Recurrent Acute Pancreatitis
| Finding, Type of MRCP | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
|---|---|---|---|---|
|
| ||||
| Divisum | ||||
| Non–secretin enhanced | 57.1 (18.4–90.1) | 95.7 (85.5–99.5) | ||
| Secretin enhanced | 85.7 (42.1–99.6) | 91.5 (79.6–97.6) | ||
| Main pancreatic duct dilation | ||||
| Non–secretin enhanced | 46.2 (19.2–74.9) | 100 (91.4–100.0) | ||
| Secretin enhanced | 85.7 (57.2–98.2) | 97.5 (86.5–99.5) | ||
| Main pancreatic duct stricture | ||||
| Non–secretin enhanced | 66.7 (9.4–99.2) | 96.1 (86.5–99.5) | ||
| Secretin enhanced | 66.7 (9.4–99.2) | 94.1 (83.8–98.8) | ||
| Side branch duct dilation | ||||
| Non–secretin enhanced | 21.4 (4.7–50.8) | 95.0 (83.1–99.4) | ||
| Secretin enhanced | 71.4 (41.9–91.6) | 90.0 (76.3–97.2) | ||
| Any positive finding | ||||
| Non–secretin enhanced | 56.0 (34.9–75.6) | 90.0 (72.6–97.8) | ||
| Secretin enhanced | 76.0 (54.9–90.6) | 88.1 (68.3–93.8) | ||
TABLE 3.
ROC Curve Analysis of Secretin-Enhanced and Non–Secretin- Enhanced MRCP Images in Patients With Recurrent Acute Pancreatitis
| Ductal Finding | Secretin-Enhanced MRCP (Image Set A) | Non–Secretin-Enhanced MRCP (Image Set B) | p | |
|---|---|---|---|---|
|
| ||||
| Divisum | 0.918 (0.811–0.975) | 0.786 (0.653–0.886) | 0.15 | |
| Main pancreatic duct dilation | 0.921 (0.884–0.964) | 0.731 (0.593–0.842) | < 0.01 | |
| Main pancreatic duct stricture | 0.941 (0.876–0.983) | 0.824 (0.696–0.914) | 0.32 | |
| Side branch duct dilation | 0.904 (0.792–0.967) | 0.679 (0.538–0.799) | 0.01 | |
| Any positive finding | 0.983 (0.903–1.000) | 0.760 (0.624–0.866) | < 0.01 | |
Note—Data are AUC (95% CI).
In general, the secretin-enhanced images had a higher sensitivity than did the non–se-cretin-enhanced images, with a minimal reduction in specificity. This was particularly true for the detection of divisum (sensitivity, 57.1% vs 85.7%) (Fig. 3) and side branch dilation (sensitivity, 21.4% vs 71.4%) (Fig. 4). The AUC shows that the secretin-enhanced image set was substantially better at showing a ductal abnormality than the non–secretin-enhanced image set (0.983 vs 0.760; p < 0.01).
Fig. 3.
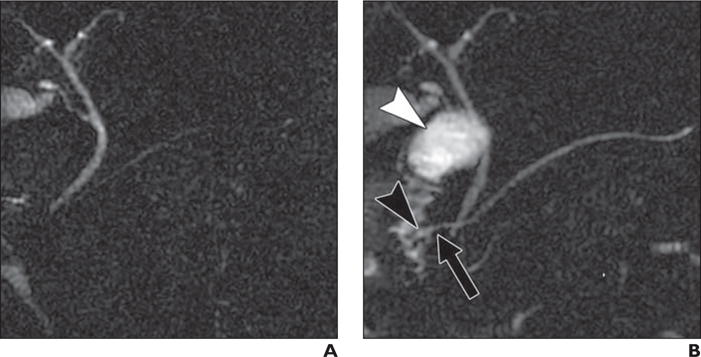
26-year-old woman with recurrent acute pancreatitis.
A, Non–secretin-enhanced MRCP is nondiagnostic for divisum because duct in pancreas head is not clearly visualized.
B, Secretin-enhanced MRCP clearly shows continuation of main duct by dorsal duct (arrow), in keeping with divisum. Dilated end of dorsal duct (Santorinicele, black arrowhead) may suggest increased pancreatic ductal pressure. Note that exocrine output predominantly fills duodenal bulb (white arrowhead) and some of descending duodenum, but not entire duodenum, indicating suboptimal exocrine response to secretin.
Fig. 4.
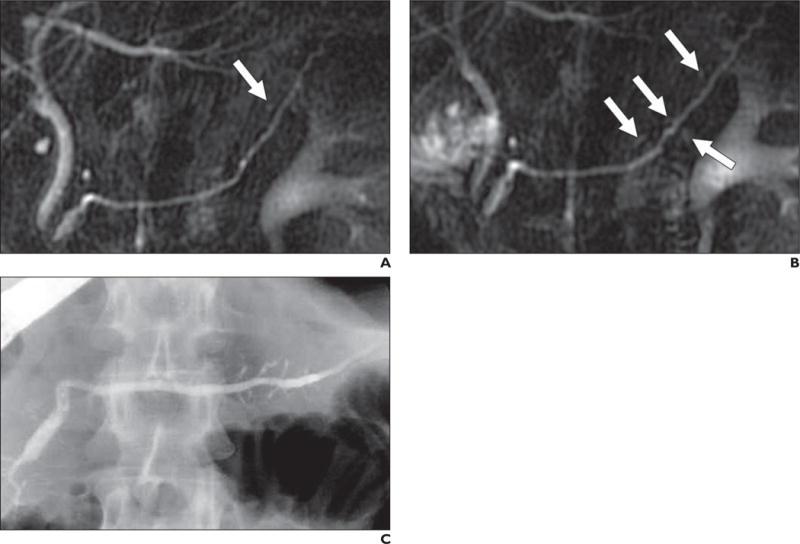
47-year-old woman with recurrent acute pancreatitis.
A, Non–secretin-enhanced MRCP shows one possible dilated side branch (arrow). This is insufficient to make diagnosis of chronic pancreatitis.
B, Secretin-enhanced MRCP shows several mildly dilated side branch ducts (arrows) consistent with mild chronic pancreatitis.
C, ERCP confirms these findings.
Discussion
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis with complete or almost complete relief of symptoms between acute episodes. Patients with recurrent acute pancreatitis do not have continuous pain requiring treatment, clinical evidence of endocrine or exocrine pancreatic insufficiency, or permanent histologic changes such as calcification or fibrosis [25]. Studies suggest that 10–30% of patients with acute pancreatitis develop recurrent acute pancreatitis [1, 26–28]. For 70– 80% of patients with recurrent acute pancreatitis, a specific cause may be identified [27], such as gall stone disease (especially biliary microlithiasis), excessive alcohol consumption, hypertriglyceridemia, medications, genetic mutations, hypercalcemia, sphincter of Oddi dysfunction, and pancreatobiliary ductal anomalies. Apart from laboratory and imaging tests to identify causes of acute pancreatitis, the investigation of recurrent acute pancreatitis requires a detailed assessment of pancreatic ductal anatomy. MRCP and ERCP are traditionally used for this purpose, though some authors have also espoused the utility of endoscopic ultrasound [2]. Increasingly, testing for mutations in cystic fibrosis transmembrane conductance regulator, serine protease inhibitor Kazal type 1, and cationic trypsinogen genes is performed in the investigation of recurrent acute pancreatitis, especially in children [27, 29]. The rate of progression of recurrent acute pancreatitis to chronic pancreatitis is unknown. It is thought that persistence of the causes of acute pancreatitis, such as genetic defects or continued alcohol abuse, increases the risk of progression of chronic pancreatitis [28, 30, 31].
Patients With Recurrent Acute Pancreatitis Versus Control Subjects
The correlation between pancreas divisum and recurrent acute pancreatitis has been debated [27, 28]. A study comparing patients with recurrent acute pancreatitis to community control subjects showed a higher frequency of divisum in patients with recurrent acute pancreatitis [32]. However, our study and others did not find this to be the case [12]. It is unclear whether divisum per se is a cause of acute pancreatitis or whether other associated factors, including genetic abnormalities, play a role [33]. Secretin-enhanced MRCP sometimes shows cystic dilatation of the end of the Santorinicele, which may suggest pancreatic ductal hypertension [34]. Reports have suggested that sphincterotomy at the minor ampulla, in cases of divisum, reduces the frequency of recurrent acute pancreatitis [35, 36]. Thus, it is clinical practice to investigate for anomalous ductal anatomy in patients with recurrent acute pancreatitis.
To our knowledge, no study has reported the ductal changes in patients with recurrent acute pancreatitis compared with a control group. Prior studies have advocated the use of secretin-enhanced MRCP in assessing patients after episodes of acute pancreatitis. A prospective study of 37 patients with idiopathic recurrent acute pancreatitis, followed over a mean period of 31 months, reported that morphologic features of ductal anatomy revealed by secretin-enhanced MRCP can obviate the need for ERCP [21]. In a study of patients with chronically elevated amylase (a group distinct from patients with recurrent acute pancreatitis), secretin-enhanced MRCP found evidence of chronic pancreatitis in one-third of cases [37]. In our study, we found evidence of multiple dilated side branches in 26% of the recurrent acute pancreatitis group compared with 9% in the control group (p = 0.02). According to the Cambridge classification of chronic pancreatitis, three or more side dilated side branches without main duct disease constitutes mild chronic pancreatitis [38]. Thus, early (subclinical) ductal changes of chronic pancreatitis were seen more commonly in patients with recurrent acute pancreatitis.
Secretin-enhanced MRCP has the potential to quantify pancreatic exocrine reserve. Studies have shown that patients with suspected or established chronic pancreatitis have a reduced exocrine output in response to secretin, compared with control subjects [13, 14, 39–41]. Our study also shows a statistically significantly higher proportion of patients with recurrent acute pancreatitis with impaired exocrine function, compared with control subjects (32% vs 9%; p < 0.01). This suggests that functional deficiencies occur in about one-third of patients with recurrent acute pancreatitis, even when no anatomic ductal disease is found.
Added Value of Secretin-Enhanced MRCP in Recurrent Acute Pancreatitis
We investigated the sensitivity and specificity of secretin-enhanced MRCP and conventional MRCP for detection of four ductal abnormalities: divisum, main duct dilation, main duct stricture, and side branch dilation. Initial reports suggested a high accuracy of routine MRCP in diagnosing divisum [4]. More recent studies indicated that MRCP had a sensitivity of only 32–73% in diagnosing divisum [5, 6, 42]. Three recent studies have showed that secretin-enhanced MRCP had a higher sensitivity and specificity compared with routine MRCP for diagnosing divisum [6, 43, 44]. In one study, secretin-enhanced MRCP was shown to detect pancreas divisum with satisfactory specificity (97%) and sensitivity (73%), whereas MRCP without secretin was found to be nondiagnostic for this condition [6]. In another study [43], the AUC value for secretin-enhanced MRCP was statistically significantly higher than that for non–secretin-enhanced images for diagnosing divisum (0.86 vs 0.74; p = 0.01). Our study mirrors these findings. Secretin-enhanced MRCP had a higher sensitivity than did conventional MRCP for diagnosing divisum (86% vs 57%), with only a marginal loss of specificity (96% vs 92%). Secretin-enhanced MRCP was also superior to conventional MRCP in diagnosing main duct dilation (86% vs 42%), but not ductal strictures.
The most impressive added benefit of se-cretin-enhanced MRCP was for the detection of side branch dilation, for which the sensitivity of secretin-enhanced MRCP was 71.4%, compared with a meager 21.4% for conventional MRCP. The AUC values for secretin-enhanced MRCP and conventional MRCP were 0.904 versus 0.679 (p = 0.01). This suggests that secretin-enhanced MRCP is superior to conventional MRCP for the diagnosis of early chronic pancreatitis. Our study is the first, to our knowledge, to prove this in a blinded comparison using ERCP as a reference standard.
The strength of this study is the blinded independent evaluation of a large number of patients with recurrent acute pancreatitis and a control group. The method used for image assessment was rigorous and similar to that used in a multicenter phase 3 trial of a new secretin agent [11]. Nevertheless, we are aware of some limitations of the study. The principal of these is the retrospective nature of the study. In addition, the reviewers were blinded to clinical information, which may not reflect clinical practice. It was possible for the reviewers to determine which image sets were secretin-enhanced MRCP because of the increasing fluid content in duodenum from the exocrine output. However, the reviewers were blinded to findings on the non–secretin-enhanced images for the same patient. Secretin is an expensive drug in the United States, costing several hundred dollars per dose. Nevertheless, we think that it is useful in the MRI evaluation of selected patients with recurrent acute pancreatitis.
In conclusion, we have shown that secretin-enhanced MRCP is superior to conventional MRCP and T2-weighted sequences in detecting ductal abnormalities in patients with recurrent acute pancreatitis, particularly for findings related to early chronic pancreatitis. This may obviate the need for ERCP. In addition, secretin-enhanced MRCP showed a reduction in exocrine function in up to one-third of patients with recurrent acute pancreatitis. We believe that secretin-enhanced MRCP should be part of the MRI protocol for investigating patients with recurrent acute pancreatitis. Further studies are required to determine whether patients with recurrent acute pancreatitis with impaired exocrine function on secretin-enhanced MRCP may need to be treated differently from those with normal exocrine function.
Footnotes
Based on a presentation at the Radiological Society of North America 2012 annual meeting, Chicago, IL.
This article is available for credit.
References
- 1.Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol. 2002;97:1959–1962. doi: 10.1111/j.1572-0241.2002.05907.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Haddad M, Wallace MB. Diagnostic approach to patients with acute idiopathic and recurrent pancreatitis, what should be done? World J Gastroenterol. 2008;14:1007–1010. doi: 10.3748/wjg.14.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelli H, Lappalainen-Lehto R, Piironen A, Sand J, Nordback I. Risk factors for recurrent acute alcohol-associated pancreatitis: a prospective analysis. Scand J Gastroenterol. 2008;43:614–621. doi: 10.1080/00365520701843027. [DOI] [PubMed] [Google Scholar]
- 4.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 5.Carnes ML, Romagnuolo J, Cotton PB. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas. 2008;37:151–153. doi: 10.1097/MPA.0b013e318164cbaf. [DOI] [PubMed] [Google Scholar]
- 6.Mosler P, Akisik F, Sandrasegaran K, et al. Accuracy of magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum. Dig Dis Sci. 2012;57:170–174. doi: 10.1007/s10620-011-1823-7. [DOI] [PubMed] [Google Scholar]
- 7.Chey WY, Chang TM. Secretin: historical perspective and current status. Pancreas. 2014;43:162–182. doi: 10.1097/01.mpa.0000437325.29728.d6. [DOI] [PubMed] [Google Scholar]
- 8.Tirkes T, Sandrasegaran K, Sanyal R, et al. Secretin-enhanced MR cholangiopancreatography: spectrum of findings. RadioGraphics. 2013;33:1889–1906. doi: 10.1148/rg.337125014. [DOI] [PubMed] [Google Scholar]
- 9.Sandrasegaran K, Lin C, Akisik FM, Tann M. State-of-the-art pancreatic MRI. AJR. 2010;195:42–53. doi: 10.2214/ajr.10.4421. [DOI] [PubMed] [Google Scholar]
- 10.Matos C, Metens T, Deviere J, et al. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435–441. doi: 10.1148/radiology.203.2.9114101. [DOI] [PubMed] [Google Scholar]
- 11.Sherman S, Freeman ML, Tarnasky PR, et al. Administration of secretin (RG1068) increases the sensitivity of detection of duct abnormalities by magnetic resonance cholangiopancreatography in patients with pancreatitis. Gastroenterology. 2014;147:646.e2–654.e2. doi: 10.1053/j.gastro.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Matos C, Metens T, Deviere J, Delhaye M, Le Moine O, Cremer M. Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointest Endosc. 2001;53:728–733. doi: 10.1067/mge.2001.114784. [DOI] [PubMed] [Google Scholar]
- 13.Balci NC, Alkaade S, Magas L, Momtahen AJ, Burton FR. Suspected chronic pancreatitis with normal MRCP: findings on MRI in correlation with secretin MRCP. J Magn Reson Imaging. 2008;27:125–131. doi: 10.1002/jmri.21241. [DOI] [PubMed] [Google Scholar]
- 14.Czakó L. Diagnosis of early-stage chronic pancreatitis by secretin-enhanced magnetic resonance cholangiopancreatography. J Gastroenterol. 2007;42(suppl 17):113–117. doi: 10.1007/s00535-006-1919-6. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal R, Stevens T, Novak E, Veniero JC. Secretin-enhanced MRCP: review of technique and application with proposal for quantification of exocrine function. AJR. 2012;198:124–132. doi: 10.2214/AJR.10.5713. [DOI] [PubMed] [Google Scholar]
- 16.Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR. 2006;186:499–506. doi: 10.2214/AJR.04.1775. [DOI] [PubMed] [Google Scholar]
- 17.Akisik MF, Sandrasegaran K, Aisen AA, Maglinte DD, Sherman S, Lehman GA. Dynamic secretin-enhanced MR cholangiopancreatography. RadioGraphics. 2006;26:665–677. doi: 10.1148/rg.263055077. [DOI] [PubMed] [Google Scholar]
- 18.Mensel B, Messner P, Mayerle J, et al. Secretin-stimulated MRCP in volunteers: assessment of safety, duct visualization, and pancreatic exocrine function. AJR. 2014;202:102–108. doi: 10.2214/AJR.12.10271. [DOI] [PubMed] [Google Scholar]
- 19.Khalid A, Peterson M, Slivka A. Secretin-stimulated magnetic resonance pancreaticogram to assess pancreatic duct outflow obstruction in evaluation of idiopathic acute recurrent pancreatitis: a pilot study. Dig Dis Sci. 2003;48:1475–1481. doi: 10.1023/a:1024747319606. [DOI] [PubMed] [Google Scholar]
- 20.Mariani A, Arcidiacono PG, Curioni S, Giussani A, Testoni PA. Diagnostic yield of ERCP and secretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Dig Liver Dis. 2009;41:753–758. doi: 10.1016/j.dld.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Testoni PA, Mariani A, Curioni S, Zanello A, Masci E. MRCP-secretin test-guided management of idiopathic recurrent pancreatitis: long-term outcomes. Gastrointest Endosc. 2008;67:1028–1034. doi: 10.1016/j.gie.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Tirkes T, Akisik F, Tann M, Balci NC. Imaging of the pancreas with secretin enhancement. Top Magn Reson Imaging. 2009;20:19–24. doi: 10.1097/RMR.0b013e3181b483f0. [DOI] [PubMed] [Google Scholar]
- 23.Sandrasegaran K, Bodanapally U, Cote GA, et al. Acinarization (parenchymal blush) observed during secretin-enhanced MRCP: clinical implications. AJR. 2014;203:607–614. doi: 10.2214/AJR.13.11414. [DOI] [PubMed] [Google Scholar]
- 24.Cappeliez O, Delhaye M, Deviere J, et al. Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology. 2000;215:358–364. doi: 10.1148/radiology.215.2.r00ma10358. [DOI] [PubMed] [Google Scholar]
- 25.Deng YY, Wang R, Wu H, Tang CW, Chen XZ. Etiology, clinical features and management of acute recurrent pancreatitis. J Dig Dis. 2014;15:570–577. doi: 10.1111/1751-2980.12180. [DOI] [PubMed] [Google Scholar]
- 26.Andersson R, Andersson B, Haraldsen P, Drewsen G, Eckerwall G. Incidence, management and recurrence rate of acute pancreatitis. Scand J Gastroenterol. 2004;39:891–894. doi: 10.1080/00365520410007061. [DOI] [PubMed] [Google Scholar]
- 27.Testoni PA. Acute recurrent pancreatitis: etiopathogenesis, diagnosis and treatment. World J Gastroenterol. 2014;20:16891–16901. doi: 10.3748/wjg.v20.i45.16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khurana V, Ganguly I. Recurrent acute pancreatitis. JOP. 2014;15:413–426. doi: 10.6092/1590-8577/2417. [DOI] [PubMed] [Google Scholar]
- 29.Lucidi V, Alghisi F, Dall’Oglio L, et al. The etiology of acute recurrent pancreatitis in children: a challenge for pediatricians. Pancreas. 2011;40:517–521. doi: 10.1097/MPA.0b013e318214fe42. [DOI] [PubMed] [Google Scholar]
- 30.Gorry MC, Gabbaizedeh D, Furey W, et al. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997;113:1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- 31.Riaz C, Ochi K, Tanaka J, Harada H, Ichimura M, Miki H. Does recurrent acute pancreatitis lead to chronic pancreatitis? Sequential morphological and biochemical studies. Pancreas. 1997;14:334–341. doi: 10.1097/00006676-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Gonoi W, Akai H, Hagiwara K, et al. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. Gut. 2011;60:1103–1108. doi: 10.1136/gut.2010.230011. [DOI] [PubMed] [Google Scholar]
- 33.Bertin C, Pelletier AL, Vullierme MP, et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol. 2012;107:311–317. doi: 10.1038/ajg.2011.424. [DOI] [PubMed] [Google Scholar]
- 34.Costamagna G, Ingrosso M, Tringali A, Mutignani M, Manfredi R. Santorinicele and recurrent acute pancreatitis in pancreas divisum: diagnosis with dynamic secretin-stimulated magnetic resonance pancreatography and endoscopic treatment. Gastrointest Endosc. 2000;52:262–267. doi: 10.1067/mge.2000.107711. [DOI] [PubMed] [Google Scholar]
- 35.Kwan V, Loh SM, Walsh PR, Williams SJ, Bourke MJ. Minor papilla sphincterotomy for pancreatitis due to pancreas divisum. ANZ J Surg. 2008;78:257–261. doi: 10.1111/j.1445-2197.2008.04431.x. [DOI] [PubMed] [Google Scholar]
- 36.Liao Z, Gao R, Wang W, et al. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009;41:439–444. doi: 10.1055/s-0029-1214505. [DOI] [PubMed] [Google Scholar]
- 37.Testoni PA, Mariani A, Curioni S, Giussani A, Masci E. Pancreatic ductal abnormalities documented by secretin-enhanced MRCP in asymptomatic subjects with chronic pancreatic hyperenzymemia. Am J Gastroenterol. 2009;104:1780–1786. doi: 10.1038/ajg.2009.158. [DOI] [PubMed] [Google Scholar]
- 38.Axon AT. Endoscopic retrograde cholangiopancreatography in chronic pancreatitis: Cambridge classification. Radiol Clin North Am. 1989;27:39–50. [PubMed] [Google Scholar]
- 39.Bian Y, Wang L, Chen C, et al. Quantification of pancreatic exocrine function of chronic pancreatitis with secretin-enhanced MRCP. World J Gastroenterol. 2013;19:7177–7182. doi: 10.3748/wjg.v19.i41.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balci NC, Smith A, Momtahen AJ, et al. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT) J Magn Reson Imaging. 2010;31:601–606. doi: 10.1002/jmri.22085. [DOI] [PubMed] [Google Scholar]
- 41.Manfredi R, Perandini S, Mantovani W, Frulloni L, Faccioli N, Pozzi Mucelli R. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Radiol Med (Torino) 2012;117:282–292. doi: 10.1007/s11547-011-0774-6. [DOI] [PubMed] [Google Scholar]
- 42.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129–133. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 43.Sandrasegaran K, Cote GA, Tahir B, et al. The utility of secretin-enhanced MRCP in diagnosing congenital anomalies. Abdom Imaging. 2014;39:979–987. doi: 10.1007/s00261-014-0131-z. [DOI] [PubMed] [Google Scholar]
- 44.Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a systematic review and meta-analysis. Pancreas. 2014;43:823–828. doi: 10.1097/MPA.0000000000000143. [DOI] [PubMed] [Google Scholar]


