Background:
Age-associated skin laxity contributes to worsening of cellulite appearance. This study evaluated the effects of microfocused ultrasound with visualization (MFU-V; Ultherapy) in combination with diluted calcium hydroxylapatite (CaHA; Radiesse) on cellulite appearance and on neocollagenesis.
Methods:
Twenty women (18–55 years old) with skin laxity and moderate-to-severe cellulite on the buttocks and thighs were retrospectively enrolled. MFU-V was applied using 4 and 7 MHz transducers (25 lines/transducer/site) and immediately followed by subdermal CaHA injection (1 ml/buttock or thigh). Photographs at baseline and 90 days were assessed by 2 independent, blinded evaluators using a 5-item cellulite severity scale. One subject scheduled for thighplasty received treatment with 6 different CaHA dilutions (0.3 ml/5 cm2) followed by MFU-V. Tissue specimens from each dilution site were examined under polarized light microscopy to assess neocollagenesis.
Results:
Both evaluators reported statistically significant improvements compared with baseline for each item on the cellulite severity scale (P < 0.001) with a 4.5-point improvement in mean overall score (P < 0.001) after a single MFU-V/CaHA treatment. At 90 days, histologic analysis showed peak neocollagenesis in samples treated with the 1:1 dilution, whether with CaHA alone or in combination with MFU-V. The highest conversion of collagen type III into collagen type I at month 3 occurred in samples injected with 1:1 and 1:0.6 CaHA dilutions without subsequent MFU-V treatment. Both procedures were well tolerated, and subject satisfaction was high.
Conclusions:
Combination treatment with MFU-V and diluted CaHA is effective for improving skin laxity and the appearance of cellulite on the buttocks and upper thighs.
INTRODUCTION
Cellulite is estimated to affect 80% to 90% of women at some point after the age of puberty.1 It occurs mainly on the buttocks and upper posterior thighs and is often described as having an orange peel, cottage cheese, or mattress-like appearance.2 Changes in the fibrous structure of the skin and increased skin laxity contribute to the worsening of cellulite with age,2 particularly in those over 35 years old.
The cutaneous changes associated with cellulite have multiple causes, but certain connective tissue anatomical characteristics found in women are thought to predispose them to this condition. Fibrous septa cross the subcutaneous fatty layer, connecting the reticular dermis to the deep fascia. In women, these are usually orientated perpendicularly to the skin surface, creating large, rectangular fat lobules.3,4 Shortening of the septa due to fibrosis leads to retraction at their cutaneous insertion points, pulling the skin down and creating the typical depressions or dimples. The raised areas result from projection of underlying fat lobules into the dermis.5 In men, the fibrous septa are organized in a crisscross pattern, which creates smaller subcutaneous fat lobules, an organization that does not favor the development of cellulite dimples.6
Cellulite involves a transformation and alteration of subcutaneous tissues and is not merely an accumulation of fat. A magnetic resonance imaging study of 60 women compared raised cellulite dimples with nondimpled control skin and found that the anatomy of subcutaneous fat was similar in both the dimple and control areas for shape, size, and thickness.7 Other studies have shown that the presence of cellulite corresponds to a thinning of the dermal layer, increase in skin laxity, and change in skin biomechanical parameters.2,8,9 Individuals with a higher body mass index (BMI) may have a weaker, less dense connective tissue structure, leading to increased extrusion of subcutaneous fat lobules into the dermis.10,11
The appearance of skin laxity and cellulite can negatively affect an individual’s quality of life. Numerous therapeutic approaches with varying degrees of effectiveness have been described, including topical products,12,13 radiofrequency,14–16 laser therapy,17–19 and carboxytherapy.20
Increasing strength and elasticity of the dermis and superficial fascia are important aims when treating skin laxity to improve the appearance of cellulite;21 such approaches require stimulation of new collagen formation and subsequent dermal remodeling. Two procedures that have unequivocally demonstrated collagen-stimulating properties and improvements in skin laxity in a variety of aesthetic indications are microfocused ultrasound with visualization (MFU-V, Ultherapy; Ulthera, Inc., Mesa, Ariz.),22–31 and injection with calcium hydroxylapatite (CaHA, Radiesse; Merz North America, Inc., Raleigh, N.C.).32–35
The aim of the current study was to evaluate whether treatment of skin laxity with a combination of MFU-V and CaHA would improve the appearance of cellulite on the buttocks and thighs. The study also sought to determine whether MFU-V would enhance the collagen-stimulating properties of CaHA.
METHODS
This was a retrospective study of women who had undergone MFU-V and CaHA treatment for skin laxity on the buttocks and thighs between December 2014 and October 2016. The study included healthy women aged 18–55 years with a BMI less than 25 kg/m2 and moderate-to-severe cellulite on the buttocks and thighs as assessed using the Hexsel, Dal’Forno and Hexsel Cellulite Severity Scale (CSS).36 Women were also required to have mild-to-severe skin laxity on the thighs and a desire for improvement in the appearance of their cellulite. Subjects were not eligible for treatment if they had undergone any treatment for skin laxity or cellulite in the last 3 months, had received liposuction in the treatment area, or had experienced an increase or decrease in body weight of more than 10% in the past 6 months. Pregnant or lactating women or those planning a pregnancy were also excluded.
All subjects had provided signed, informed consent to the procedures. All subjects had also given consent to the subsequent use of their photographs being rated, analyzed, and published for scientific purposes.
Treatment Procedure
The areas to be treated were marked with a pen with the subject standing upright, and topical lidocaine (Pliaglis) was applied. MFU-V was directed to the posterior buttocks, lateral buttocks, and posterior thighs using the 4 MHz transducer at a focal depth of 4.5 mm and the 7 MHz transducer at a depth of 3.0 mm, using 75 lines per transducer for each side (25 lines per buttock or thigh treatment site). Immediately after MFU-V, subjects received treatment with 1.5 ml CaHA diluted 1:1 with 1.5 ml of 2% lidocaine solution. Using a 25G, 48-mm-long cannula, the 3.0 ml of diluted CaHA was injected in the subdermis using a microdroplet fanning technique (1 ml per treatment site with 10 lines of 0.1 ml per line) to cover the same area as the MFU-V. Vigorous massage of the treatment area was performed to ensure even dispersion, and subjects were instructed to refrain from exercising the treatment area for 24 hours. Photographs were taken before treatment and at 90 days.
Assessment of Cellulite Severity
The primary objective was to evaluate the degree of skin laxity and cellulite improvement after treatment with MFU-V and CaHA. Two independent, blinded evaluators assessed cellulite severity from photographs taken at baseline and 90 days after treatment using the CSS.36 The scale identifies 5 key clinical morphologic features of cellulite: (1) number of evident depressions, (2) depth of depressions, (3) morphologic appearance of skin surface alterations, (4) grade of laxity, flaccidity or sagging skin, and (5) the classification scale originally described by Nürnberger and Müller.3 The severity of each item was graded from 0 to 3, where 0 represented the absence of cellulite and 3 represented the most severe cellulite changes. This created a final sum of scores ranging numerically from 0 to 15. Based on the final numeric score, cellulite was further classified as mild, moderate, or severe.
All subjects completed a patient satisfaction questionnaire 90 days after the procedures. Questions included does your skin have no change, is your skin smoother, is your skin rougher, are your cellulite dimples less visible, are your dimples unchanged, and are your dimples more visible? The subjects were required to respond using a 5-point scale from 1 = very unsatisfied to 5 = very satisfied.
Histologic Analysis
A secondary objective was to determine whether neocollagenesis was influenced by different CaHA dilutions compared with untreated skin and whether MFU-V enhanced the CaHA-induced neocollagenesis. One subject had consented to donate skin for a histologic study before thighplasty. She received treatment on the inner thigh with 6 different dilutions of CaHA diluted with 2% lidocaine: 1:0.16, 1:0.3, 1:0.6, 1:1, 1:2, and 1:6.5. Each inner thigh was injected with 0.3 ml of each dilution over an area of 5 cm2. The left thigh only received CaHA treatment. On the right thigh, each CaHA-treated square and 1 non–CaHA-treated area also received treatment with the MFU-V 4 MHz transducer at a depth of 4.5 mm and 7 MHz transducer at a depth of 3.0 mm at 5 lines per square for each transducer. The subject underwent a thighplasty 90 days later.
Tissue specimens from each inner thigh CaHA-injection site and 1 non–CaHA-treated site were obtained from excised skin at the time of thighplasty and fixed by direct immersion in a 10% formaldehyde solution before processing for light microscopy by dehydration, embedding in paraffin, and sectioning. The 6-μm thick sections were stained with hematoxylin and eosin to visualize the different skin layers and with picrosirius red solution 0.1% (Sirius red F3B, Sigma-Aldrich, St Louis, Mo.) for the densitometric study of collagen.
Histologic sections were analyzed by light microscopy at a magnification of 100× with a polarized light source using a Zeiss Axio 5 microscope (Carl Zeiss, Oberkochen, Germany). Under polarized microscopy, the observed color of collagen fibers stained with picrosirius red depends on fiber thickness with thin, newly formed collagen type III appearing green, and thick, mature collagen type I appearing red.37 The images were captured by a camera (Axio Cam), transmitted to a Pentium microcomputer and analyzed using the Digimizer 4.5 application (MedCalc Software, Acacialaan 22, 8400 Ostend, Belgium). This program converts the individual red and green digital images into binary images, permitting a comparative and quantitative evaluation of both mature and newly formed collagen fibers in terms of percentage areas occupied by red and green in the same reference frame of square pixels. This allows the amounts of collagen type I, type III, and total collagen (represented by the sum of collagen types I and III) to be estimated. All slides were analyzed under the same conditions and by the same pathologist.
Statistical Methodology
Statistical analyses were primarily descriptive. Quantitative variables were described using the mean, SD, and range. Rating scale scores at each visit were statistically compared with baseline scores using the Wilcoxon test for related samples. Changes from baseline were considered significant at the P < 0.05 level. Analyses were conducted using SAS System for Windows (Statistical Analysis System), version 9.2 (SAS Institute Inc, 2002–2008, Cary, N.C.).
RESULTS
Demographic Data
A total of 20 women took part in the study and completed all study visits. Mean age (± SD) was 40.0 ± 8.2 years (range, 24–52 years) and mean BMI 21.5 ± 1.5 kg/m2 (range, 19–24 kg/m2). Mean weight was 55.7 ± 4.1 kg (range, 50–65 kg) at baseline and 56.1 ± 4.6 kg (range, 50–68 kg) at 90 days.
Cellulite Severity Scale
Two independent, blinded evaluators assessed cellulite severity using the CSS. At baseline, both evaluators rated 75% of the women as having moderate-to-severe skin depressions. Depression depth was rated as superficial in 40% of cases and medium-depth to deep in 60%. Appearance was described as “orange peel” in 8 women, “cottage cheese” in 5 women, and “mattress-like” in 5 women. Evaluators graded 50% of the women as having slight laxity, flaccidity, or sagging, and 50% as having moderate-to-severe laxity. Evaluators were also in agreement when grading women with the Nürnberger and Müller classification, with 90% of the women rated as grade 2 or 3 (Table 1).
Table 1.
Number of Patients with CSS Item Grade at Baseline and 90 Days According to the Blinded Evaluators
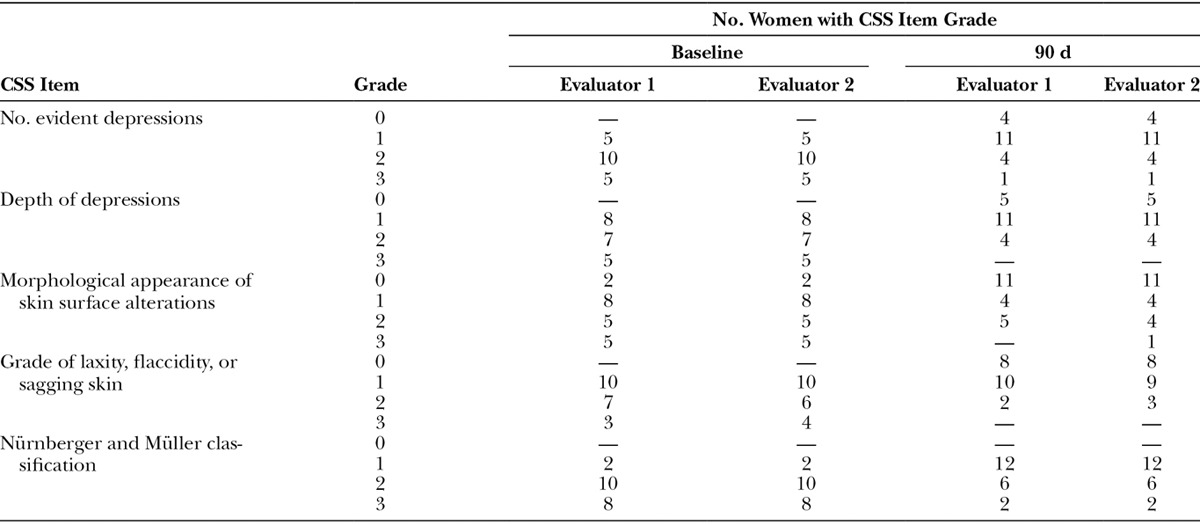
At day 90, 4 women had no evident depressions, and 11 had only a small number of depressions compared with 0 and 5 women, respectively, at baseline. The depth of depressions was also decreased, with 80% of the women having no depressions or depressions of superficial depth. Skin surface appearance was improved, with 11 women having no raised areas compared with 2 at baseline. An “orange peel” appearance was recorded for 8 women at baseline but only 4 at day 90. A “mattress-like” appearance was reported in 5 women at baseline. At day 90, only 1 evaluator used this grade for 1 subject (Table 1). Overall, 8 women showed no laxity, flaccidity, or sagging skin at day 90 compared with 0 at baseline. A further 9 or 10 women were rated as having slight skin laxity by evaluator 1 and evaluator 2, respectively. Evaluators agreed on improvements in the Nürnberger and Müller classification at day 90, with 12 subjects having a grade 1 classification compared with only 2 at baseline. Images illustrating the improvement in cellulite severity 90 days after treatment compared with baseline are shown in Figure 1 and Supplemental Figures 1 and 2 [see pdf, Supplemental Digital Content 1, which displays photographs before (A1, B1) and after (A2, B2) treatment in relaxed position (A1, A2) and at maximal squeezing (B1, B2), http://links.lww.com/PRSGO/A473; see pdf, Supplemental Digital Content 2, which displays photographs before (A1) and after (A2) treatment showing relaxed (upper photograph) and clenched (lower photograph) position views and relaxed 45-degree angle view, http://links.lww.com/PRSGO/A474].
Fig. 1.
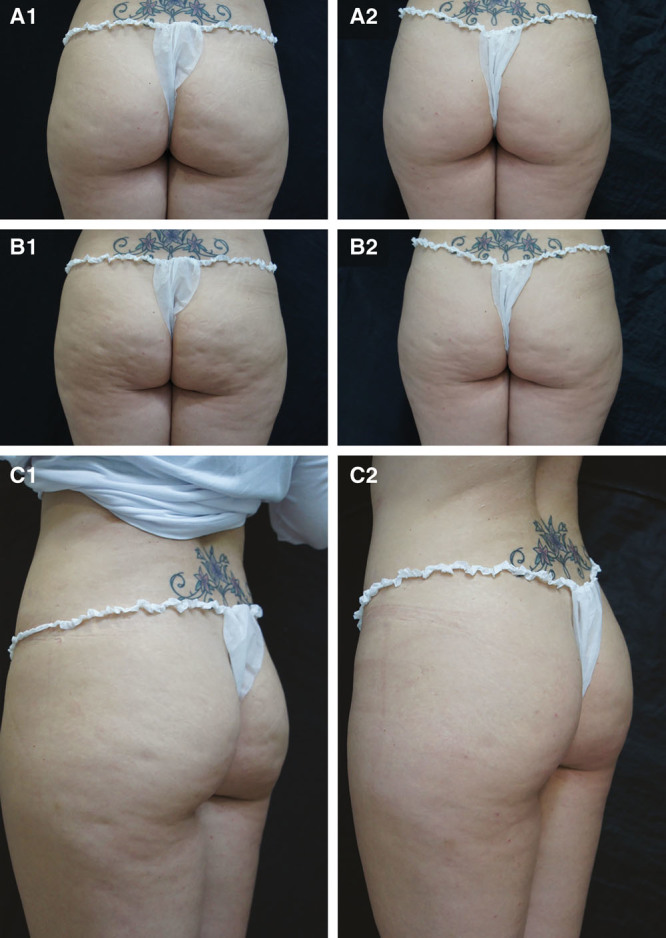
Photographs before (A1, B1, C1) and after (A2, B2, C2) treatment in relaxed position (A1, A2), at maximal clenching (B1, B2), and at 45-degree angle view (C1, C2).
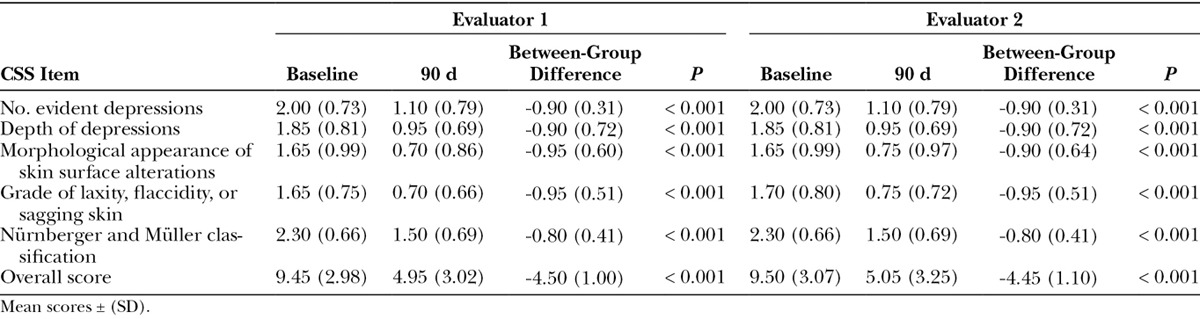
The mean evaluator score for each item and the total score is displayed in Table 2. When assessing cellulite severity 90 days after treatment, both evaluators reported statistically significant improvements for each CSS item compared with baseline. At 90 days, there was a mean improvement in the CSS overall score of 4.5 compared with baseline (P < 0.001). The slight difference in weight recorded at baseline and at 90 days (55.7 versus 56.1 kg) was not statistically significant (P = 0.5).
Table 2.
Assessment of Cellulite Severity by 2 Independent Evaluators Using the Hexsel, Dal’Forno and Hexsel CSS
Patient Satisfaction Score
Subjects were questioned about the perceived effects of treatment on their skin. All women reported that they could see a change in their skin and that it was smoother. Of the 20 women, 19 reported that their dimples were less visible and only 1 that their dimples were unchanged. When asked to rate their overall satisfaction with the treatment, 10 women (50%) reported they were very satisfied, and 9 women (45%) were satisfied.
Histologic Findings
At 90 days, polarized microscope examination of picrosirius red stained tissue sections from skin treated with a range of CaHA dilutions (Fig. 2, bars A1–A6 for CaHA alone and B1–B6 for CaHA followed by MFU-V) showed that peak total collagen gain occurred with the 1:1 dilution in both CaHA-treated skin alone and CaHA-treated skin followed by MFU-V (Fig. 2, bars A4 and B4, respectively).
Fig. 2.
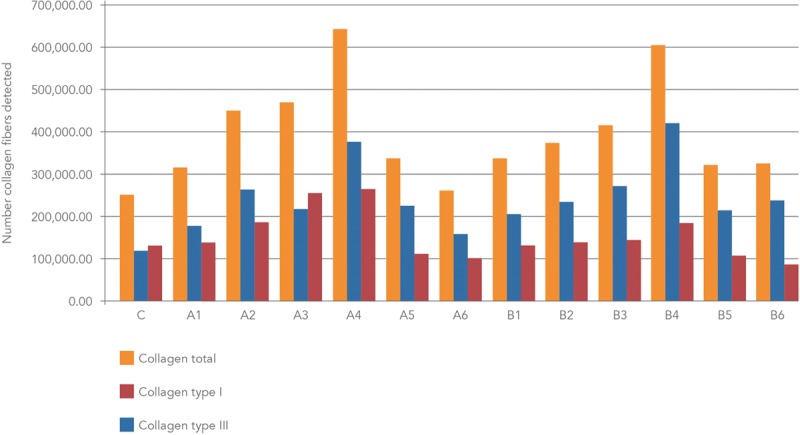
Mean numbers of collagen fibers detected after staining histologic sections with picrosirius red and observing under polarized light microscopy. C, control. A, CaHA treatment only. B, CaHA followed by microfocused ultrasound. Dilutions: A1, B1 (1:0.16), A2, B2 (1:0.3), A3, B3 (1:0.6), A4, B4 (1:1), A5, B5 (1:2), A6, B6 (1:6.5).
In CaHA-treated skin alone, the 1:1 (A4) and 1:0.6 (A3) dilutions showed the highest conversion to type I collagen at 90 days compared with untreated control tissue (C), with increases of 103% and 93%, respectively. When CaHA was combined with MFU-V, the 1:1 dilution (B4) was associated with a 251% increase in the number of collagen type III fibers compared with control, but conversion to type I collagen was only slightly evident (increase of 41%). Picrosirius-red-stained histologic sections of the dermis viewed under polarized microscopy are shown in Figure 3 for the different CaHA dilutions with or without MFU-V treatment.
Fig. 3.
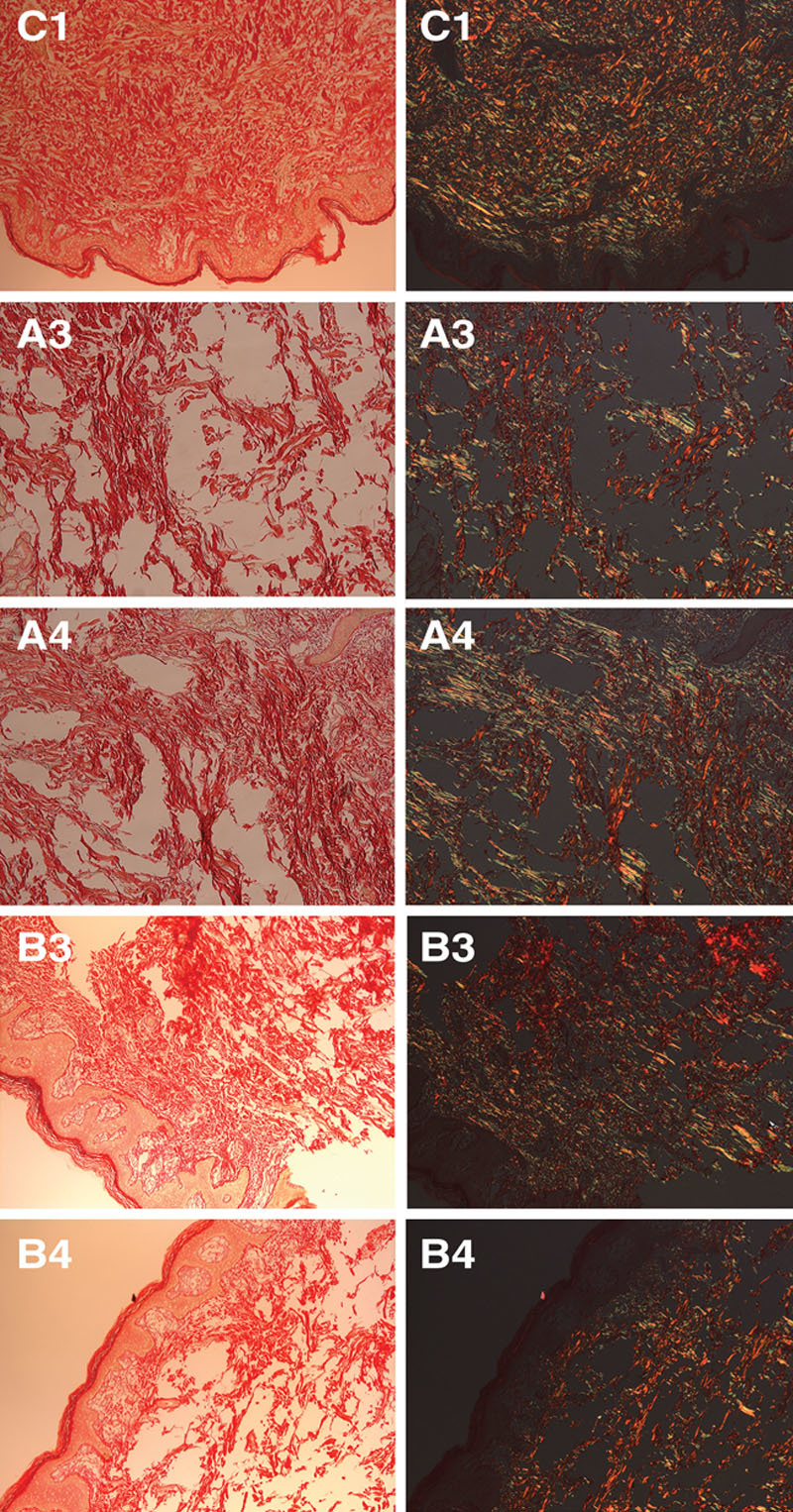
Picrosirius-red-stained histologic sections of the dermis viewed under normal microscopy (left columns) or with polarized light (right columns) are shown for control tissue (C1) and at 2 different CaHA dilutions (1:0.6, A3, B3; 1:1, A4, B4) for CaHA alone (A3, A4) or CaHA followed by MFU-V treatment (B3, B4).
Safety
MFU-V/CaHA combination treatment was well tolerated, with no cases of severe bruising or severe pain. All women reported mild pain the day after the procedures with a mean duration of 2 days. Mild bruising was observed in 18 women and had resolved within 1 week. Other mild events observed were erythema (2 women), edema (10 women), and injection-site induration (5 women), all of which resolved in 2–3 days.
DISCUSSION
In this retrospective study of 20 women with skin laxity and moderate-to-severe cellulite, treatment with MFU-V and diluted CaHA resulted in a significant improvement in cellulite appearance as assessed by 2 independent evaluators. Results were consistent between evaluators and showed a statistically significant improvement compared with baseline for each CSS item, including number and depth of depressions, skin surface appearance, degree of skin laxity, and grading according to the Nürnberger and Müller scale.3 The mean total CSS score improved from 9.5 ± 3 (moderate-to-severe) at baseline to 5.0 ± 3 (mild) at day 90, after only a single MFU-V/CaHA treatment session. Both procedures were well tolerated. Transient erythema, edema, and bruising were rated as mild and resolved within a few days. Pain associated with MFU-V treatment was mild. Topical lidocaine was applied, but the minimal pain might also reflect the relatively low number of lines per treatment site (25 for each transducer). Patient satisfaction with the procedure was high, with 19 of the 20 women reporting that they were satisfied or very satisfied with the treatment results.
There is considerable rationale for combining MFU-V and CaHA to treat skin laxity and thereby improve the appearance of cellulite. Both MFU-V and CaHA are skin-tightening procedures that result in remodeling of the dermis and collagenous structures in the superficial fascia with neocollagenesis and elastogenesis. MFU-V uses dual function transducers to noninvasively deliver microfocused ultrasound energy at preselected depths below the skin’s surface while simultaneously providing high-resolution ultrasound imaging of the skin layers to ensure the precision of energy delivery.38 Injection of small amounts of diluted CaHA exploits its collagen-stimulating properties without creating a volumizing effect.32,39,40 The result is targeted neocollagenesis in the area of injection.41 Current consensus is that MFU-V should be performed first but can be followed immediately by CaHA injection.42 The rationale for this is to avoid contamination of the MFU-V transducers and excess pressure on the injected CaHA.
To evaluate the collagen-stimulating properties of the 2 procedures, tissue sections from skin injected with 1 of 6 CaHA dilutions were examined using a combination of picrosirius red staining and polarized microscopy, which allows newly formed collagen type III and mature collagen type I fibers to be distinguished.43 The maximum total collagen gain was observed with the 1:1 CaHA:lidocaine dilution, whether from CaHA-treated skin alone or CaHA-treated skin followed by MFU-V. In CaHA-treated skin alone, the 1:1 and 1:0.6 dilutions showed the greatest effects on type I collagen at 3 months compared with untreated control tissue. When CaHA was combined with MFU-V, the 1:1 dilution was associated with a 251% increase in collagen type III fibers compared with control. However, conversion to collagen type I at 3 months was more evident in the tissue sections that were not subject to MFU-V, suggesting that thermal coagulation stimulation might lengthen the time required to replace collagen type III with collagen type I, probably as a result of the greater level of neocollagenesis required. These results are supported by a similarly designed study with a 6-month period between treatment and histologic analysis, which demonstrated increased dermal thickening and more dense collagen fibers in skin samples excised from CaHA/MFU-V combination sites compared with CaHA or MFU-V sites alone.44 At 3 months, the process of tissue remodelling is still ongoing. Indeed, studies of wound healing have shown that during physiological neocollagenesis, the remodeling phase in which collagen type III is gradually replaced by type I can take up to a year or more.45 A limitation of this pilot study was therefore the short follow-up. Both MFU-V and CaHA have demonstrated efficacy in their respective treatment indications for periods of 1 year or more46,47 and longer follow-up periods may have demonstrated even greater treatment effects and permitted the effects of ongoing tissue remodeling to be observed at a histologic level. Photographic assessment of cellulite appearance can be difficult as the visibility of the dimples is dependent on the orientation of the light source and the shadows it casts. Consistent light sources and camera angles are therefore required. A prospective study is now planned with a follow-up period of 1 year to address these limitations.
Both MFU-V and CaHA are associated with little downtime, are FDA approved, and have long-established safety profiles in a wide variety of indications. In the current study, both treatments were well tolerated, and the histologic analysis did not reveal any foreign body reactions or any changes in the appearance or characteristics of CaHA. Current evidence suggests that the safety profile of MFU-V combined with other aesthetic products including CaHA is consistent with the safety profiles of the individual treatments.48
CONCLUSIONS
Combining MFU-V with diluted CaHA produced statistically significant improvements in cellulite severity after only a single procedure. By inducing neocollagenesis and improving skin laxity, MFU-V and diluted CaHA helped to improve the appearance of the skin dimples typical of cellulite. The procedures were well tolerated, and subject satisfaction was high. MFU-V and CaHA at a dilution ratio of 1:1 are effective in combination for improving skin laxity and cellulite severity in the buttocks and upper thighs.
ACKNOWLEDGMENTS
The authors thank Dr. Paula Marchese and Dr. Thais Bello for support in assessing the treatment outcomes and Jenny Grice for editorial assistance, which was funded by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by Merz Pharmaceuticals GmbH.
REFERENCES
- 1.Luebberding S, Krueger N, Sadick NS. Cellulite: an evidence-based review. Am J Clin Dermatol. 2015;16:243–256.. [DOI] [PubMed] [Google Scholar]
- 2.Lorencini M, Camozzato F, Hexsel D. Farage MA, Miller KW, Maibach HI. Skin aging and cellulite in women. In: Textbook of Aging Skin. 2016:Berlin, Germany: Springer; 1–9.. [Google Scholar]
- 3.Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221–229.. [DOI] [PubMed] [Google Scholar]
- 4.Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161–168.. [DOI] [PubMed] [Google Scholar]
- 5.Hexsel D, de Oliveira Dal’Forno T, Cignachi S. Goldman MP, Bacci PA, Leibaschoff G, Hexsel D, Angelini F. Definition, clinical aspects, associated conditions, and differential diagnosis. In: Cellulite Pathophysiology and Treatment. 2006:New York, N.Y.: Taylor and Francis Group; 7–27.. [Google Scholar]
- 6.Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118–124.. [DOI] [PubMed] [Google Scholar]
- 7.Hexsel D, Siega C, Schilling-Souza J, et al. A comparative study of the anatomy of adipose tissue in areas with and without raised lesions of cellulite using magnetic resonance imaging. Dermatol Surg. 2013;39:1877–1886.. [DOI] [PubMed] [Google Scholar]
- 8.Ortonne JP, Zartarian M, Verschoore M, et al. Cellulite and skin ageing: is there any interaction? J Eur Acad Dermatol Venereol. 2008;22:827–834.. [DOI] [PubMed] [Google Scholar]
- 9.Dobke MK, Dibernardo B, Thompson RC, et al. Assessment of biomechanical skin properties: is cellulitic skin different? Aesthet Surg J. 2002;22:260–266.. [DOI] [PubMed] [Google Scholar]
- 10.Hexsel DM, Abreu M, Rodrigues TC, et al. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35:1471–1477.. [DOI] [PubMed] [Google Scholar]
- 11.Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. Pathophysiology. J Am Acad Dermatol. 2010;62:361–370.. [DOI] [PubMed] [Google Scholar]
- 12.Hexsel D, Soirefmann M. Cosmeceuticals for cellulite. Semin Cutan Med Surg. 2011;30:167–170.. [DOI] [PubMed] [Google Scholar]
- 13.Sainio EL, Rantanen T, Kanerva L. Ingredients and safety of cellulite creams. Eur J Dermatol. 2000;10:596–603.. [PubMed] [Google Scholar]
- 14.Emilia del Pino M, Rosado RH, Azuela A, et al. Effect of controlled volumetric tissue heating with radiofrequency on cellulite and the subcutaneous tissue of the buttocks and thighs. J Drugs Dermatol. 2006;5:714–722.. [PubMed] [Google Scholar]
- 15.Mlosek RK, Woźniak W, Malinowska S, et al. The effectiveness of anticellulite treatment using tripolar radiofrequency monitored by classic and high-frequency ultrasound. J Eur Acad Dermatol Venereol. 2012;26:696–703.. [DOI] [PubMed] [Google Scholar]
- 16.De La Casa Almeida M, Suarez Serrano C, Jiménez Rejano JJ, et al. Intra- and inter-observer reliability of the application of the cellulite severity scale to a Spanish female population. J Eur Acad Dermatol Venereol. 2013;27:694–698.. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RF, Roche GC, Shanks SC. A double-blind, placebo-controlled randomized trial evaluating the ability of low-level laser therapy to improve the appearance of cellulite. Lasers Surg Med. 2013;45:141–147.. [DOI] [PubMed] [Google Scholar]
- 18.DiBernardo BE, Sasaki GH, Katz BE, et al. A multicenter study for cellulite treatment using a 1440-nm Nd:YAG wavelength laser with side-firing fiber. Aesthet Surg J. 2016;36:335–343.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petti C, Stoneburner J, McLaughlin L. Laser cellulite treatment and laser-assisted lipoplasty of the thighs and buttocks: combined modalities for single stage contouring of the lower body. Lasers Surg Med. 2016;48:14–22.. [DOI] [PubMed] [Google Scholar]
- 20.Pianez LR, Custódio FS, Guidi RM, et al. Effectiveness of carboxytherapy in the treatment of cellulite in healthy women: a pilot study. Clin Cosmet Investig Dermatol. 2016;9:183–190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hexsel D, Hexsel C. The role of skin tightening in improving cellulite. Dermatol Surg. 2014;40:S180–S183.. [DOI] [PubMed] [Google Scholar]
- 22.Alam M, White LE, Martin N, et al. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62:262–269.. [DOI] [PubMed] [Google Scholar]
- 23.Suh DH, Shin MK, Lee SJ, et al. Intense focused ultrasound tightening in Asian skin: clinical and pathologic results. Dermatol Surg. 2011;37:1595–1602.. [DOI] [PubMed] [Google Scholar]
- 24.Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754–759.. [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Jang WS, Cha YJ, et al. Multiple pass ultrasound tightening of skin laxity of the lower face and neck. Dermatol Surg. 2012;38:20–27.. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki GH, Tevez A. Clinical efficacy and safety of focused-image ultrasonography: a 2-year experience. Aesthet Surg J. 2012;32:601–612.. [DOI] [PubMed] [Google Scholar]
- 27.Fabi SG, Massaki A, Eimpunth S, et al. Evaluation of microfocused ultrasound with visualization for lifting, tightening, and wrinkle reduction of the décolletage. J Am Acad Dermatol. 2013;69:965–971.. [DOI] [PubMed] [Google Scholar]
- 28.Gold MH, Sensing W, Biron J. Use of micro-focused ultrasound with visualization to lift and tighten lax knee skin (1.). J Cosmet Laser Ther. 2014;16:225–229.. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg DJ, Hornfeldt CS. Safety and efficacy of microfocused ultrasound to lift, tighten, and smooth the buttocks. Dermatol Surg. 2014;40:1113–1117.. [DOI] [PubMed] [Google Scholar]
- 30.Fabi SG, Goldman MP, Dayan SH, et al. A prospective multicenter pilot study of the safety and efficacy of microfocused ultrasound with visualization for improving lines and wrinkles of the décolleté. Dermatol Surg. 2015;41:327–335.. [DOI] [PubMed] [Google Scholar]
- 31.Rokhsar C, Schnebelen W, West A, et al. Safety and efficacy of microfocused ultrasound in tightening of lax elbow skin. Dermatol Surg. 2015;41:821–826.. [DOI] [PubMed] [Google Scholar]
- 32.Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13:1047–1052.. [PubMed] [Google Scholar]
- 33.Amselem M. Radiesse: a novel rejuvenation treatment for the upper arms. Clin Cosmet Investig Dermatol. 2016;9:9–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cogorno Wasylkowski V. Body vectoring technique with Radiesse for tightening of the abdomen, thighs, and brachial zone. Clin Cosmet Investig Dermatol. 2015;8:267–273.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yutskovskaya Y, Kogan E. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a pilot study. J Drugs Dermatol. 2017;16:68–74.. [PubMed] [Google Scholar]
- 36.Hexsel DM, Dal’forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23:523–528.. [DOI] [PubMed] [Google Scholar]
- 37.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455.. [DOI] [PubMed] [Google Scholar]
- 38.Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305–313.. [DOI] [PubMed] [Google Scholar]
- 39.Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6:223–226.. [DOI] [PubMed] [Google Scholar]
- 40.Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34:S64–S67.. [DOI] [PubMed] [Google Scholar]
- 41.Carruthers JD, Carruthers JA, Humphrey S. Fillers and neocollagenesis. Dermatol Surg. 2014;40:S134–S136.. [DOI] [PubMed] [Google Scholar]
- 42.Fabi SG, Burgess C, Carruthers A, et al. Consensus recommendations for combined aesthetic interventions using Botulinum toxin, fillers, and microfocused ultrasound in the neck, décolletage, hands, and other areas of the body. Dermatol Surg. 2016;42:1199–1208.. [DOI] [PubMed] [Google Scholar]
- 43.Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978;41:267–274.. [DOI] [PubMed] [Google Scholar]
- 44.Casabona G, Michalany N. Microfocused ultrasound with visualization and fillers for increased neocollagenesis: clinical and histological evaluation. Dermatol Surg. 2014;40:S194–S198.. [DOI] [PubMed] [Google Scholar]
- 45.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542.. [DOI] [PubMed] [Google Scholar]
- 46.Werschler WP, Werschler PS. Long-term efficacy of micro-focused ultrasound with visualization for lifting and tightening lax facial and neck skin using a customized vectoring treatment method. J Clin Aesthet Dermatol. 2016;9:27–33.. [PMC free article] [PubMed] [Google Scholar]
- 47.Bass LS, Smith S, Busso M, et al. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30:235–238.. [DOI] [PubMed] [Google Scholar]
- 48.Fabi SG, Goldman MP, Mills DC, et al. Combining microfocused ultrasound with botulinum toxin and temporary and semi-permanent dermal fillers: safety and current use. Dermatol Surg. 2016;42:S168–S176.. [DOI] [PubMed] [Google Scholar]


