Background:
Capsular contracture is a common complication after breast augmentation surgery. This study pathologically evaluated the soft-tissue response to surface modifications in both smooth and textured tissue expander prostheses.
Methods:
Smooth tissue expanders and textured tissue expanders in 5 cases each were used for breast reconstruction after mastectomy. Histological samples were harvested from the capsules when the tissue expanders were replaced by silicone implants. Collagen orientation and cellular responses were assessed histologically. Capsular contracture was evaluated using the Baker classification 6 months and 2 years after the removal of the tissue expander.
Results:
The capsules surrounding the smooth tissue expanders tended to produce more contracture than those surrounding the textured tissue expanders. The collagen architecture of the capsules of the smooth tissue expanders showed random orientation with fragmentation. Conversely, the capsules of the textured tissue expanders showed parallel orientation with collagen bundles of almost normal structure. Significantly more fibrils of elastin and myofibroblasts were found in the capsules surrounding the smooth tissue than in those surrounding the textured ones.
Conclusions:
The collagen fibers surrounding the smooth tissue expanders could be cracked during expansion, which may lead to scarring and contracture. Conversely, the collagen orientation surrounding the textured tissue expanders was excellent. Moreover, the increase in elastic fibers and myofibroblasts in the capsules surrounding the smooth tissue expanders may be associated with in vivo contraction patterns. Therefore, the surface type of tissue expanders affects capsular contraction after replacement with definitive implants.
INTRODUCTION
In recent years, prostheses, such as tissue expanders and silicone implants, have been widely used in reconstructive surgery after mastectomy. Compared with reconstruction with autologous tissues, surgeries using prostheses offer the advantages of lack of damage to other sites and reduction in the operative time and length of hospital stay. However, infection and capsular contracture are unavoidable complications with prostheses. These complications are thought to be caused by low-grade bacterial infection or biofilm formation1 and collagen deposition2 around the prostheses.
Tissue expanders and silicone implants have 2 main types of surface structure: smooth or textured. Past reports on breast augmentation surgery have indicated that capsular contracture, which is a serious complication after implant insertion,3–5 occurs more commonly with smooth rather than textured surface prostheses.6 However, others have found that the incidence of capsular contracture is the same for both surface types.7
Therefore, it is possible that capsular contracture may be affected by the structure of the prosthesis surface in cases of breast augmentation. However, there have been no reports investigating the causes of contracture by comparing capsules formed by smooth and textured tissue expander prostheses in breast reconstructive surgery following mastectomy.
In this study, we histologically analyzed contracture in the capsules surrounding smooth and textured tissue expanders inserted after mastectomy.
MATERIALS AND METHODS
Participants
The study cohort comprised 10 female Japanese patients who underwent mastectomy for breast cancer. All patients were treated by immediate placement of tissue expanders, which were inserted below the pectoralis major muscle for breast reconstruction. The expanders were devised to not move by suturing neighbor pectoralis major muscles on the chest wall.
The patients were all treated between February 2011 and October 2013 at the Fujita Health University Hospital, Aichi Prefecture, Japan, or the Maehara Surgical Clinic, Aichi Prefecture, Japan. The types of tissue expander prostheses were either smooth (PMT Corporation, Minn.; 8 patients) or textured (Allergan, Calif.; 8 patients). After completion of skin expansion, the tissue expanders were subsequently replaced textured silicone implants by the same surgeon. Capsulotomy or capsulectomy was not performed at the time of the exchange to the implants. Cases who had received radiation therapy were excluded.
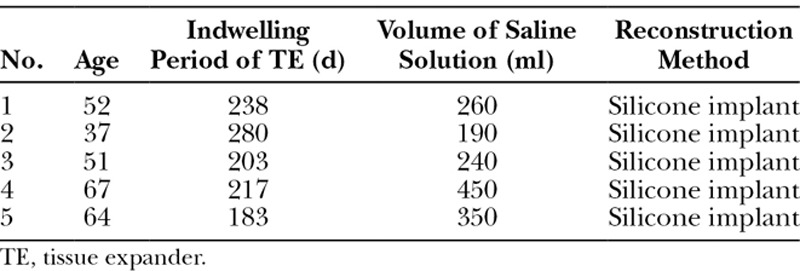
The age of the patients ranged from 43 to 60 years (51.2 ± 8.2; mean ± SD) in the smooth group and from 37 to 67 years (54.2 ± 10.6) in the textured group. The mean indwelling period of the tissue expander was 208.8 (± 16.2) days in the smooth group and 224.2 (± 33.1) days in the textured group. The mean volume of normal saline solution injected into the tissue expanders was 410 (± 108.6) mL in the smooth group and 298 (± 91.9) mL in the textured group. No significant differences were noted between the groups regarding age, the indwelling period of the tissue expander, and the amount of injected physiological saline solution (Tables 1 and 2).
Table 1.
Background of the Patients in the Smooth TE Group
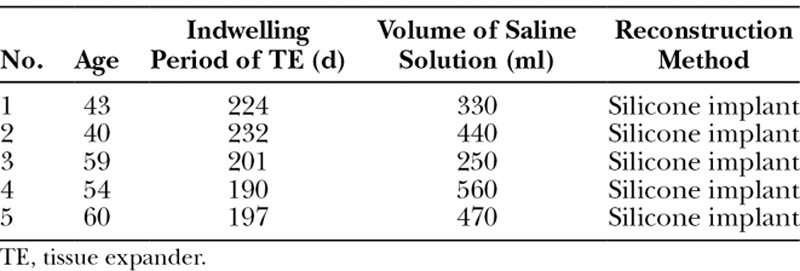
Table 2.
Background of the Patients in the Textured TE Group
Five patients belonged to the smooth group; they underwent an insertion of textured silicone implants after skin expansion with smooth surface tissue expanders. On the other hand, 5 patients belong to the textured group; they also underwent an insertion of textured silicone implants after skin expansion with textured surface tissue expanders.
At the beginning of the study, the subjects were fully informed about the study aims and the procedures involved. After receiving a description of the study, the subjects signed consent to participate. This study was approved in July 2012 by the Fujita Health University Ethics Review Committee, Japan, and was performed in accordance with the Declaration of Helsinki (1975, revised in 2008).
Clinical Diagnosis of Capsular Contracture
Contracture was assessed using the Baker classification.8 The breasts of the patients were examined 6 months and 2 years after breast reconstruction using palpation by a plastic and reconstructive surgeon who was blinded to the method of surgery and was accustomed to quantifying the symptoms of the firmness of capsules using Baker classification. In this system, class I is assigned to breasts that are soft to palpitation and easily compressible. Class II is applied with mild firmness, and class III with moderate firmness. Class IV contracture is characterized by extreme firmness.
Macroscopic Comparison of the Surface Structure of the Capsules and Expander Prostheses
The surfaces of the smooth and textured types of tissue expanders and the morphology of the surfaces of the formed capsules were observed and compared using a stereoscopic microscope at a magnification of times 50 (Power SZ−61, Olympus, Tokyo, Japan).
Sample Processing
Histological samples were harvested from the greater pectoral muscle side of the capsules when the expanders were removed and replaced by implants or autologous tissues for breast reconstruction. The samples were taken from 1 in each case, and the total was 10. The samples were cut into squares of 5 × 10 mm for examination.
The harvested tissues were fixed in 10% neutral buffered formalin solution. Briefly, 3-μm paraffin sections were prepared from the fixed tissue in the usual manner. The tissues were observed in detail using, Masson trichrome and Elastica van Gieson staining. In addition, immunostaining was performed. The sections were incubated for 1 hour at 37°C with the primary antibodies of anti-human smooth muscle actin (α-SMA) mouse monoclonal antibody (1:200, DAKO North America, Inc., Calif.) or anti-human vimentin rabbit polyclonal antibody (1:200, Bethyl Laboratories Inc., Tex.). The samples were incubated with the secondary antibody for 30 minutes at 37°C with Simple stain MAX PO MULTI (for mouse and rabbit primary antibodies; Nichirei, Tokyo, Japan). The sections were stained with the Liquid DAB + Substrate Chromogen System (DAKO). A microscope (Power BX-51, Olympus, Tokyo, Japan) was used for observation. The specimens were observed by the pathologist using anonymization in a linkable fashion.
Evaluation with Masson Trichrome Staining
The capsular tissue, which adhered to the tissue expander, was examined histologically, and the appearance of the collagen fibers was assessed after Masson trichrome staining. To compare the orientation of the collagen fibers in the smooth and textured groups, 1 field of 35,612.60 μm2 in area was observed in each specimen. For each of the 4 fields, the scores for 3 collagen fiber characteristics were determined, namely, collagen fiber orientation, density, and maturity (Table 3). The factors were arranged by the method proposed by Beausang et al..9 The mean scores for each field were calculated and compared between the smooth and textured groups. Higher scores were set to indicate nearly normal collagen fiber shape.
Table 3.
Histological Assessment Scale of Collagen Fibers

Evaluation with Elastica van Gieson Staining
The elastic fibers of the capsular tissue were examined after Elastica van Gieson staining. Red, green, blue synthesis and binary processing were conducted, and the proportion of elastic fibers was measured for 1 field (35,612.60 μm2; Fig. 1).9 Four fields in each specimen were measured by image analysis using computer software (Win ROOF v6.4, Tokyo, Japan), and the mean values were compared between the smooth and textured groups.
Fig. 1.
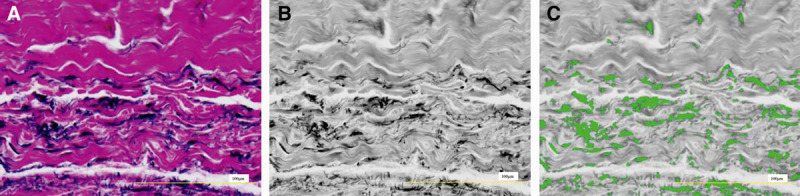
A, Image of a field stained with Elastica van Gieson (35,612.6076 μm2).
B, Blue image extracted by red, green, blue synthesis. C, Image with the range of elastic fibers selected after binary processing (green area is elastic fibers). The area ratio of elastic fibers within 1 visual field was measured. Scale bar = 100 μm.
Evaluation with Immunostaining
Anti-vimentin antibody staining was performed to evaluate fibroblasts, and anti-αSMA antibody staining was performed to evaluate myofibroblasts. Four fields in each specimen (1 field = 14,400 μm2) were observed, and the numbers of fibroblasts and myofibroblasts were counted. The mean value for each specimen of the measured cell counts in the 4 fields were calculated and compared between the smooth and textured groups.
Statistical Analysis
We used the Mann–Whitney U test to compare the score values for collagen fiber orientation, elastic fiber area, fibroblast count, and myofibroblast count between the groups. All statistical analyses were performed using Graph Pad Prism 5 (GraphPad Software, La Jolla, Calif.) SPSS 20.0 (IBM Corp, N.Y.). Data are expressed as mean ± SD. For all cases, a P value of < 0.05 (1-sided) was considered to be statistically significant.
RESULTS
Macroscopic Appearance of the Surface of the Capsules and Tissue Expanders
Observation under the stereoscopic microscope indicated that the capsular surfaces surrounding the smooth tissue expanders were smooth and that the capsules did not strongly adhere to the tissue expander surface. Meanwhile, the capsular surfaces surrounding the textured tissue expanders were rough, and the capsules strongly adhered to the tissue expander. Therefore, the capsular surfaces were concordant with the surface property of the tissue expander.
Evaluation of Capsular Contracture
Breast reconstruction was performed by replacement with textured silicone implants. The Baker classification was applied 6 months after breast reconstructive surgery to evaluate the degree of breast contracture. In the smooth group, 1 case was categorized as class I, 2 cases as class II, and 2 cases as class III of the 5 patients 0.5 year after reconstruction with textured silicone implants. There were 3 cases as class II and 2 cases as class III 2 years after operation. In the textured group, all 5 patients who had reconstruction with textured silicone implants after 0.5 year were categorized as class I. Four cases were classified as class I and 1 case as class II 2 years postoperatively. The capsules surrounding the smooth tissue expanders tended to produce more contracture than the capsules surrounding the textured tissue expanders for 2 years.
Microscopic Evaluation with Masson Trichrome Staining
The capsular surfaces were smooth in the smooth group and rough in the textured group, similar to the macroscopic appearance. Multiple collagen fibers were observed in each of the samples. The orientation of the collagen fibers of each capsule was scored and compared according to the scale in Table 3. The results indicated that the scores were significantly higher in the textured group (8.8 ± 2.0) compared with the smooth group (4.0 ± 2.0; Figs. 2, 4). The scores demonstrated that the structure of the collagen fibers in the textured group were similar to normal collagen fibers.
Fig. 2.
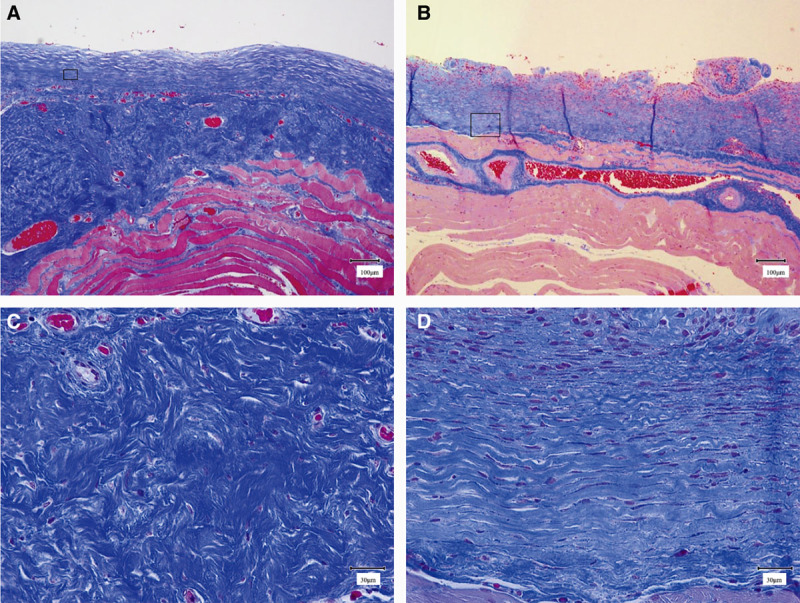
Masson trichrome staining. A, Low-power field image of a capsule formed around a smooth expander prosthesis (scale bar = 100 μm). The surface of the capsule is smooth. B, Low-power field image of a capsule formed around a textured expander prosthesis (scale bar = 100 μm). The surface of the capsule is textured. C, Enlarged field image of the square in A (scale bar = 30 μm). D, Enlarged field image of the square in B (scale bar = 30 μm).
Fig. 4.
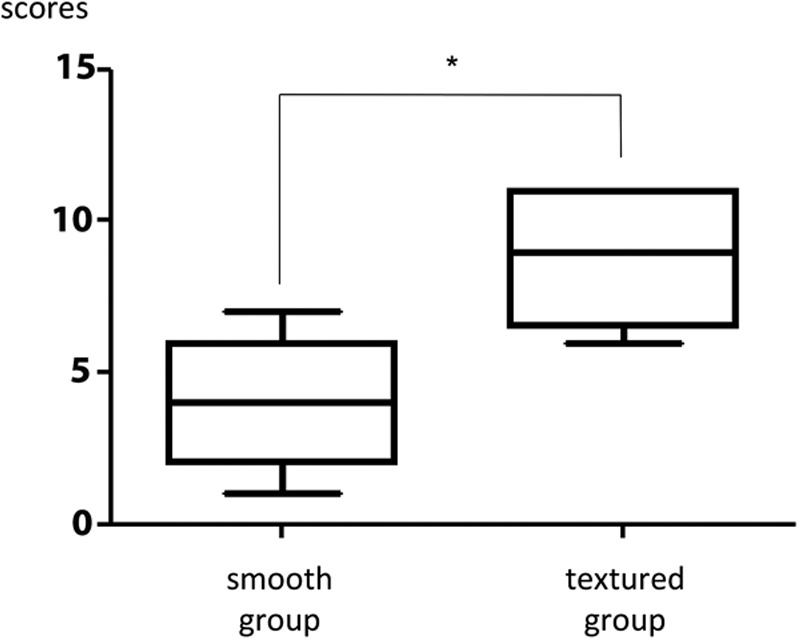
Comparison of the scores assessing the collagen fiber characteristics. Scores were significantly higher in the textured group than in the smooth group, demonstrating that the structure was closer to that of normal collagen fibers in the textured group. The results are expressed as mean ± SD (n = 5, 5); *P = 0.028 < 0.05 (Mann–Whitney U test).
Microscopic Evaluation with Elastica van Gieson
Many elastic fibers were observed in the capsular tissue in both groups. The area ratio of the visual field that was comprised of elastic fibers was significantly greater in the smooth group (2.46 ± 1.13%) than in the textured group (0.78 ± 0.39%; Figs. 3, 5).
Fig. 3.
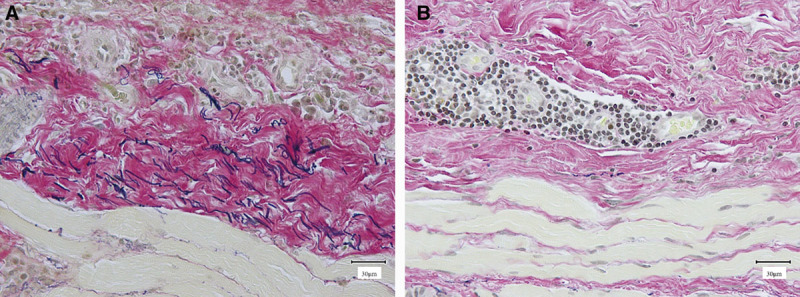
Elastica van Gieson staining. A, Image of a capsule formed around a smooth expander prosthesis (scale bar = 30 μm). B, Image of a capsule formed around a textured expander prosthesis (scale bar = 30 μm).
Fig. 5.
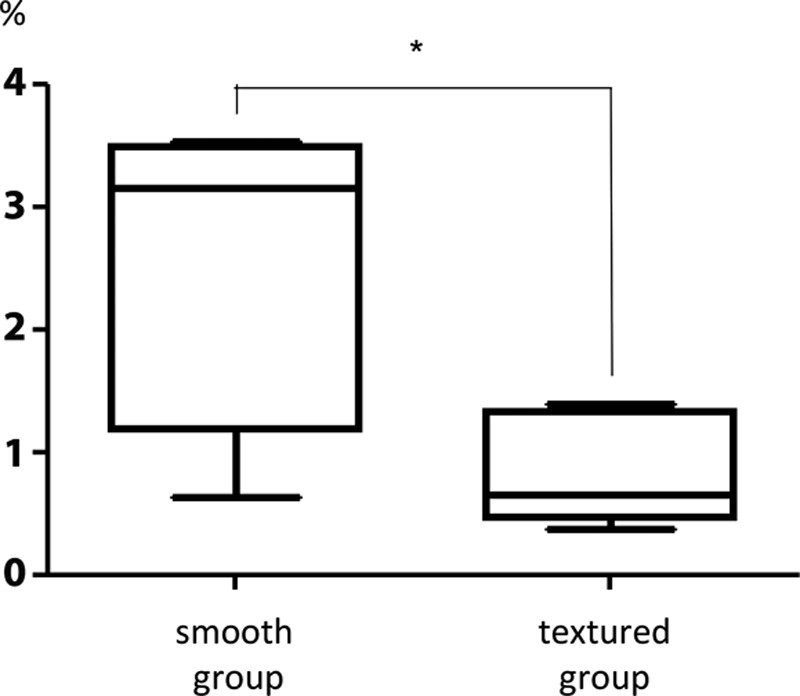
Comparison of the ratio of the area within the visual fields composed of elastic fibers. There was an increase in the number of elastic fibers in the smooth capsules versus the textured ones. The results are expressed as mean ± SD (n = 5, 5); P = 0.047 < 0.05 (Mann–Whitney U test).
Microscopic Evaluation with Immunostaining
Anti-α-SMA antibody staining indicated the presence of myofibroblasts in both the smooth and textured groups. There was a significant increase in myofibroblasts in the capsule around the smooth prostheses (9.74 ± 3.67) than in the textured prostheses (3.78 ± 1.45; Fig. 6).
Fig. 6.
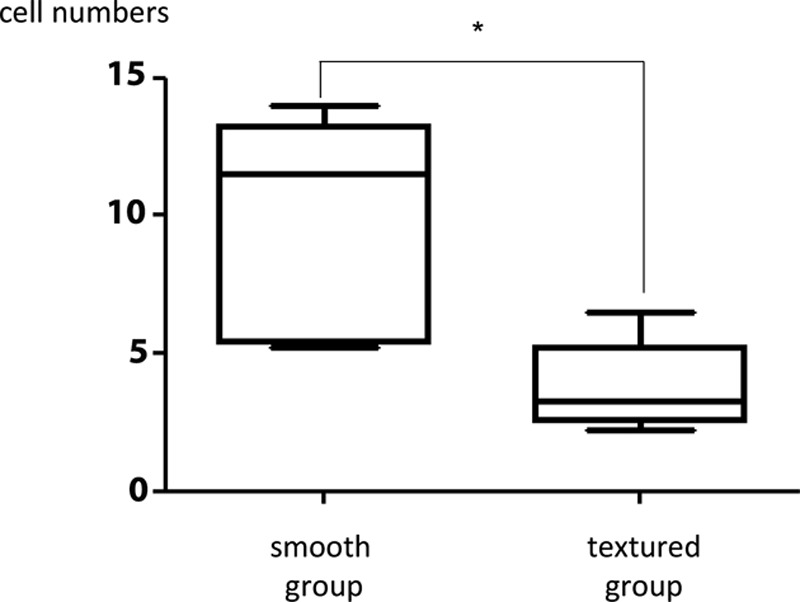
Comparison of myofibroblast counts in the visual fields of the smooth and textured groups. There was a significant increase in myofibroblasts in the capsule around the smooth prostheses than in the textured prostheses. The results are expressed as mean ± SD (n = 5, 5); *P = 0.031 < 0.05 (Mann–Whitney U test).
With anti-vimentin antibody staining, many fibroblasts were noted in both the smooth and textured groups. No significant difference was noted in fibroblast count between the smooth group (15.64 ± 7.90) and the textured group (5.82 ± 3.12; Fig. 7).
Fig. 7.
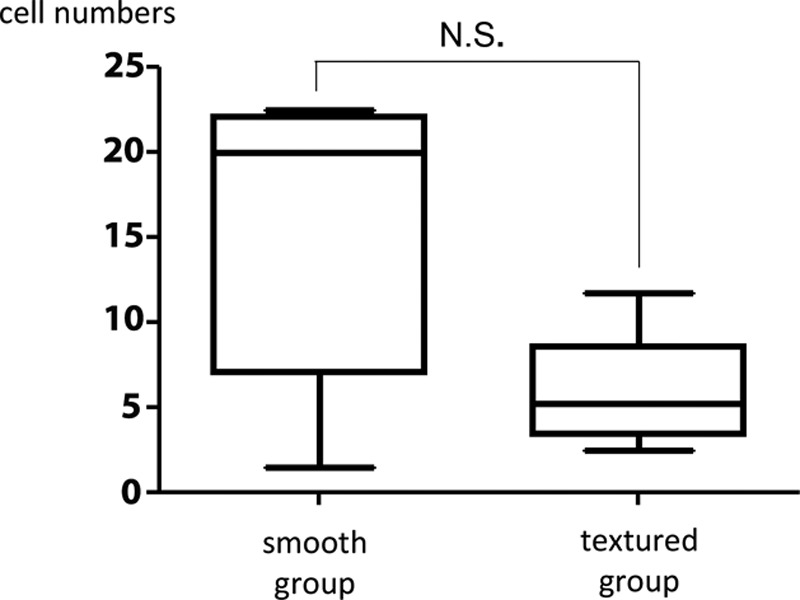
Comparison of fibroblast count in the visual fields of smooth and textured groups. No significant difference was noted in fibroblast count between the capsule around the smooth prostheses versus the textured prostheses. The results are expressed as mean ± SD (n = 5, 5); P = 0.15 > 0.05 (Mann–Whitney U test).
DISCUSSION
It is well known that capsular contracture formation is a common complication following breast augmentation surgery. Silicone implant shells have either a smooth or a textured surface; smooth surfaces demonstrate a higher incidence of contracture formation.3–7,10 No clear explanation has yet been found as to why capsular contracture is less likely to develop in textured implants; however, Valencia et al.10 suggested that rough surfaces provide a greater area of attachment compared with smooth surfaces.
In this study, we assessed capsular contracture using the Baker classification, 6 months after 2-stage breast reconstructive surgery in breast cancer patients. Ten patients eventually underwent insertion of the textured type of silicone implants, and it was revealed that the differences in the degree of contracture were produced by the surface property of the tissue expander. In this study, capsular contracture was observed in tissues that were expanded using a smooth type tissue expander. The results indicate that different types of tissue expander surfaces may lead to differences of 2 years in the incidence of subsequent contracture, even if they are later replaced with the same texture-type of silicone implant. Therefore, it is suggested that differences in the incidence of capsular contracture are due to differences in tissue reactions during tissue expansion.
Barone et al.11 conducted an experiment in which tissue expanders were inserted under the muscle layer in rabbits, and the capsules that formed around the expanders were then analyzed. The results showed that the capsules that formed around the textured tissue expanders exhibited marked pores on the surface with a small amount of dense connective tissue compared with the capsules surrounding the smooth tissue expanders. Therefore, they proposed that expansion with textured expander prostheses resulted in expansion of pores and rupture of collagen fibers, thereby preventing contracture.11 However, rupture of collagen fibers should increase myofibroblasts during healing and cause scarring, and therefore, this mechanism contradicts the prevention of contracture.
We used the scale for the assessment of collagen fiber organization (Table 3) for the evaluation of capsular tissue surrounding the tissue expanders. Beausang et al.12 reported the histologic assessment scales of skin-scar scale focuses on collagen fibers, and they found a strong correlation between the macroscopic and microscopic of scars. In this study, we scored 3 elements of collagen fiber organization, that is, orientation, density, and maturity. The results of these scores indicated that in the capsules surrounding the smooth tissue expander prostheses, the collagen fiber orientation was clearly irregular and that there was marked rupturing and uneven thickness. In contrast, for the capsules surrounding the textured tissue expander prostheses, the thickness of the collagen fibers was even, the arrangement was regular, and there was hardly any collagen fiber rupture.
Based on these results, we hypothesize that the differences in growth patterns of collagen fibers between capsules surrounding smooth and textured tissue expanders are related to contracture. First, cracking of the collagen fibers in the capsules surrounding the smooth tissue expanders may have been a result of a “slipping” effect between the expander and the capsule due to the smoothness of the capsular surface. It may have cracked at the weak point of the surrounding tissue. This would cause scarring of the smooth capsular tissue and appear as a large amount of marked irregularity in collagen fiber thickness and direction on microscopy.
Meanwhile, uniform thickness and organized collagen fibers were observed in the capsules surrounding the textured tissue expanders. This may have been due to the absence of the above-mentioned “slipping” effect between the capsule and the expander prosthesis. The surface protrusion of the textured prosthesis lodges in the soft tissue around the prosthesis and may result in equal and slow expansion around the entire prosthesis. As a consequence, the collagen orientation is excellent in the capsule surrounding the textured type of tissue expander.
In addition, a significant increase in elastic fibers was observed in the capsule surrounding the smooth tissue expanders compared with the textured tissue expanders. Elastic fibers are abundant in tissues, for example, arteries, lungs, and skin, and they are periodically exposed to contraction. The cross-linked structure of elastin, which is the principle component of elastic fibers, causes the elastic fibers to return to their original length after expansion.13 This means that the presence of a large amount of elastic fibers should strongly ensure contraction.
Moreover, we detected a significant increase in myofibroblasts in the capsule around the smooth tissue expanders than in the textured tissue expanders. Increase in myofibroblasts can be along with the mechanism by rupture of collagen fibers. It is generally accepted that myofibroblasts represent key players in the physiological reconstruction of connective tissue after injury and in the generation of the pathological tissue deformations that characterize fibrosis.14 Brazin et al.15 examined capsules surrounding silicone implants and histologically compared the number of fibroblasts according with the Baker classification. They reported that Baker grade IV capsules had an increased number of fibroblasts compared with Baker grade I capsules.15 Prantl et al.16 also reported that capsules with Baker class III or IV contracture were clearly thicker than those with Baker class II contracture after breast augmentation with smooth silicone implants. These results suggest that a thick capsule is caused by the existence of many fibroblasts, meaning that the contracture is strong. But, in this study, there were no significant differences between the smooth and textured groups regarding fibroblast counts. This results show that the influence of fibroblast is small as a cause of contracture.
Ibrahim et al.17 indicated that prolonged inflammation is associated with fibrosis and hypertrophic scar contracture. Frequent cracking of the collagen fibers will cause persistent inflammation and may lead to contracture as well. Therefore, we are considering the examination of the function of macrophages and mast cells and deposition of transforming growth factor-β to prove it.
Our study has several limitations that merit discussion. First, we followed capsular contracture for only 2 years. It is possible that our results would have differed had we followed for longer periods of time. Second, our study is limited by its small sample size because of restricted number of breast reconstruction cases in Japan. Third, we used Baker classification for the clinical evaluation of capsular contracture, but it may not reflect a real contracture of capsule itself; therefore, other evaluation methods should be examined. Fourth, our microscopic study included only a part of the surface of the capsular tissue and was limited to harvesting of the tissue at the time of extraction of the tissue expander. For future study, we should investigate the capsular tissue in more detail by using the same technique in large animals, for example, pigs.
SUMMARY
We performed a histological investigation of the capsules surrounding smooth and textured tissue expanders inserted for breast reconstructive surgery after mastectomy. The capsules surrounding the smooth tissue expanders tended to produce more contracture than the textured types based on the Baker classification. The collagen fiber orientation in the group with smooth tissue expanders showed random orientation with fragmentation. Conversely, the textured tissue expander group showed parallel orientation with nearly normal structure of the collagen bundles. In addition, there were significantly more fibrils of elastin and myofibroblasts in the capsules surrounding the smooth tissue expanders compared with the textured tissue expanders. These results suggest that the type of surface of the tissue expander affects capsular contraction after replacement with definitive implants.
Footnotes
Drs. Kuriyama, Inoue, and Okumoto contributed equally to this work.
Presented at The 23rd Research Council Meeting of the Japan Society of Plastic and Reconstructive Surgery, Tokyo, Japan, 2014.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611.. [DOI] [PubMed] [Google Scholar]
- 2.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284.. [DOI] [PubMed] [Google Scholar]
- 3.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123.. [DOI] [PubMed] [Google Scholar]
- 4.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190.. [DOI] [PubMed] [Google Scholar]
- 5.Hakelius L, Ohlsén L. A clinical comparison of the tendency to capsular contracture between smooth and textured gel-filled silicone mammary implants. Plast Reconstr Surg. 1992;90:247–254.. [PubMed] [Google Scholar]
- 6.Spear SL, Spittler CJ. Breast reconstruction with implants and expanders. Plast Reconstr Surg. 2001;107:177–87; quiz 188.. [DOI] [PubMed] [Google Scholar]
- 7.Tarpila E, Ghassemifar R, Fagrell D, et al. Capsular contracture with textured versus smooth saline-filled implants for breast augmentation: a prospective clinical study. Plast Reconstr Surg. 1997;99:1934–1939.. [DOI] [PubMed] [Google Scholar]
- 8.Little G, Baker JL., Jr Results of closed compression capsulotomy for treatment of contracted breast implant capsules. Plast Reconstr Surg. 1980;65:30–33.. [DOI] [PubMed] [Google Scholar]
- 9.Nikitina L, Ahammer H, Blaschitz A, et al. A new method for morphometric analysis of tissue distribution of mobile cells in relation to immobile tissue structures. PLoS One. 2011;6:e15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valencia-Lazcano AA, Alonso-Rasgado T, Bayat A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J Mech Behav Biomed Mater. 2013;21:133–148.. [DOI] [PubMed] [Google Scholar]
- 11.Barone FE, Perry L, Keller T, et al. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90:77–86.. [DOI] [PubMed] [Google Scholar]
- 12.Beausang E, Floyd H, Dunn KW, et al. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102:1954–1961.. [DOI] [PubMed] [Google Scholar]
- 13.Urry DW. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J Protein Chem. 1988;7:1–34.. [DOI] [PubMed] [Google Scholar]
- 14.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503.. [DOI] [PubMed] [Google Scholar]
- 15.Brazin J, Malliaris S, Groh B, et al. Mast cells in the periprosthetic breast capsule. Aesthetic Plast Surg. 2014;38:592–601.. [DOI] [PubMed] [Google Scholar]
- 16.Prantl L, Pöppl N, Horvat N, et al. Serologic and histologic findings in patients with capsular contracture after breast augmentation with smooth silicone gel implants: is serum hyaluronan a potential predictor? Aesthetic Plast Surg. 2005;29:510–518.. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim MM, Bond J, Bergeron A, et al. A novel immune competent murine hypertrophic scar contracture model: a tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen. 2014;22:755–764.. [DOI] [PMC free article] [PubMed] [Google Scholar]


