Background:
Prepectoral breast reconstruction is increasingly popular. This study compares complications between 2 subpectoral and 1 prepectoral breast reconstruction technique.
Methods:
Between 2008 and 2015, 294 two-staged expander breast reconstructions in 213 patients were performed with 1 of 3 surgical techniques: (1) Prepectoral, (2) subpectoral with acellular dermal matrix (ADM) sling (“Classic”), or (3) subpectoral/subserratus expander placement without ADM (“No ADM”). Demographics, comorbidities, radiation therapy, and chemotherapy were assessed for correlation with Clavien IIIb score outcomes. Follow-up was a minimum of 6 months.
Results:
Surgical cohorts (n = 165 Prepectoral; n = 77 Classic; n = 52 No ADM) had comparable demographics except Classic had more cardiac disease (P = 0.03), No ADM had higher body mass index (BMI) (P = 0.01), and the Prepectoral group had more nipple-sparing mastectomies (P < 0.001). Univariate analysis showed higher expander complications with BMI ≥ 40 (P = 0.05), stage 4 breast cancer (P = 0.01), and contralateral prophylactic mastectomy (P = 0.1), whereas implant complications were associated with prior history of radiation (P < 0.01). There was more skin necrosis (P = 0.05) and overall expander complications (P = 0.01) in the Classic cohort, whereas the No ADM group trended toward the lowest expander complications among the 3. Multivariate analysis showed no difference in overall expander complication rates between the 3 groups matching demographics, mastectomy surgery, risks, and surgical technique.
Conclusions:
Prepectoral and subpectoral Classic and No ADM breast reconstructions demonstrated comparable grade IIIb Clavien score complications. BMI > 40, stage 4 cancer, and contralateral prophylactic mastectomy were associated with adverse expander outcomes and a prior history of radiation therapy adversely impacted implant outcomes. Ninety-day follow-up for expander and implant complications may be a better National Surgical Quality Improvement Program measure.
INTRODUCTION
Breast reconstruction is an integral component of breast cancer and prophylactic mastectomy management.1,2 In the 1980s, prepectoral subcutaneous breast reconstruction was plagued with complications, particularly skin flap necrosis that lead to expander exposure and loss.3–5 Radovan’s invention of the tissue expander and reposition of the expander to the subpectoral plane revolutionized breast reconstruction.6–8 The subpectoral expander method has been the most conventional breast reconstruction technique for the past 25 years.9
To our knowledge, there has been no expander-based breast reconstruction study comparing a new prepectoral technique using stacked acellular dermal matrix (ADM) to traditional subpectoral/ADM sling and subpectoral/seratus/no ADM surgical techniques. This study evaluated the outcomes of both stages of reconstruction between these 3 surgical techniques.
PATIENTS AND METHODS
A retrospective review of consecutive breast reconstructions was performed using EPIC electronic medical records (Epic Systems Corp., Wis.) at a single institution from June 2008 to July 2015. The study was restricted to breasts that underwent mastectomy for breast cancer or cancer prophylaxis. Excluded breasts were those that underwent immediate single-stage implant reconstruction, reconstructions with mesh other than LifeCell Alloderm ADM (Branchburg, N.J.), or revisions. Each breast was counted once and entered in the study at the time of expander placement. The specific surgical technique used was left to the discretion of the surgeon. Each surgeon performed all 3 techniques during this period. Surgical products were consistent, given the managed care setting. The type of mastectomy (skin-sparing, nipple-sparing, or modified radical) and the final stage of reconstruction (implant placement or conversion to free flap) were recorded. Patients who underwent initial “spacer” expander placement for planned adjuvant radiation therapy with subsequent autologous reconstruction were included in the expander outcomes. The minimum follow-up for both events ranged from 6 months to 6 years. The Kaiser Foundation Research Institute Institutional Review Board approved the study.
The following patient demographics were recorded: patient age, body mass index (BMI) at the time of the initial expander placement (categorized into 5 groups according to World Health Organization classification), presence of diabetes, cardiac disease (requiring medication for hypertension, coronary heart disease, or arrhythmia), smoking history (current, former), initial cancer stage, previous breast surgeries (prior lumpectomy, reduction mammoplasty, mastopexy, augmentation mammoplasty), radiation therapy (prior history, adjuvant therapy), chemotherapy (prior history, neoadjuvant or adjuvant therapy; Table 1).10,11 Complications were limited to a grade IIIb Clavien score (i.e., “requiring surgical, endoscopic, or radiological intervention under general anesthesia”) for seroma, infection, hematoma, skin and nipple necrosis, expander deflation, and expander or implant loss (Table 2).12 It may be difficult to identify true surgical-site infections with ADM so patients given oral or intravenous antibiotics alone (Clavien type II) were not included.13
Table 1.
Patient/Breast Characteristics and Risk Factors
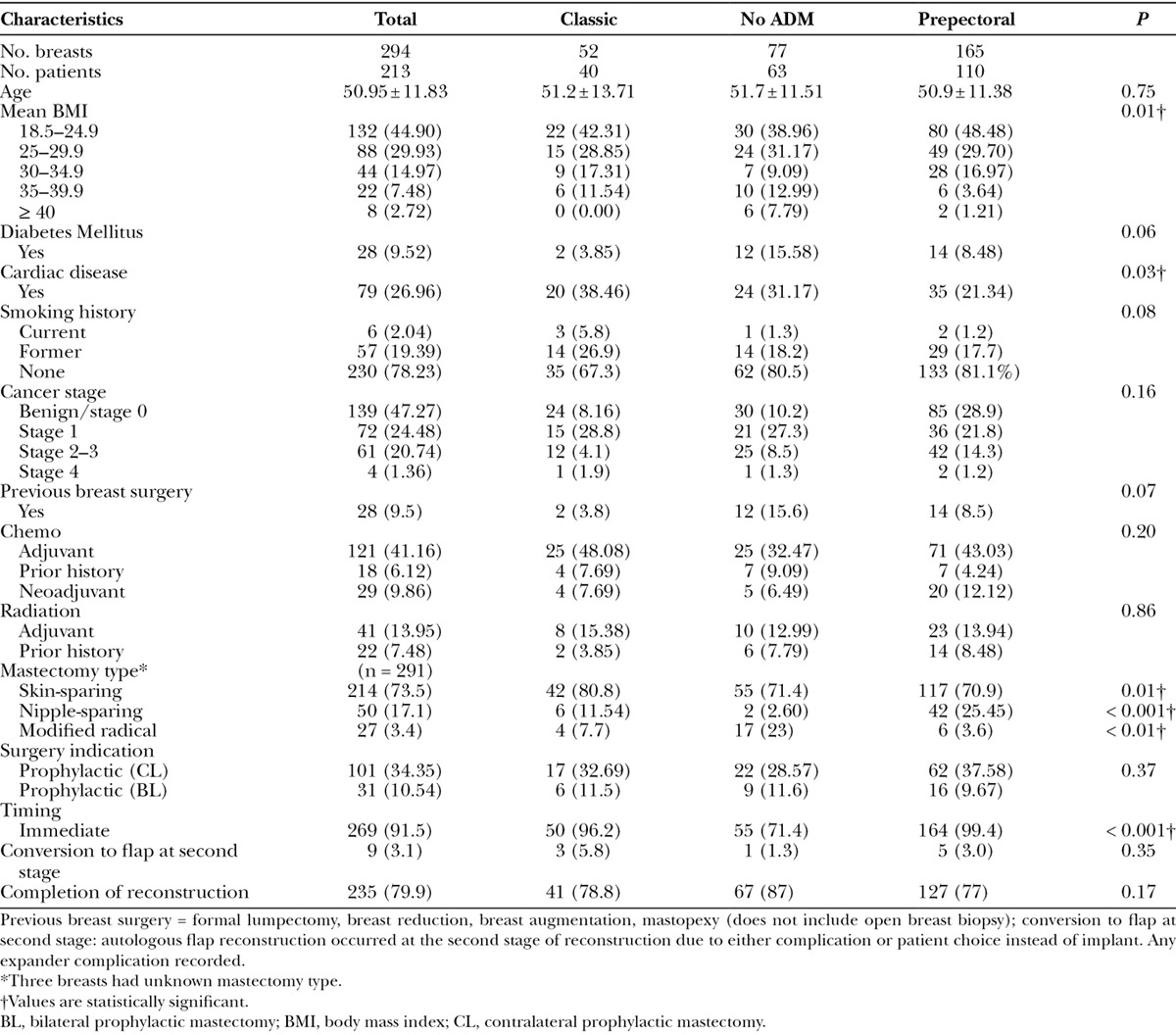
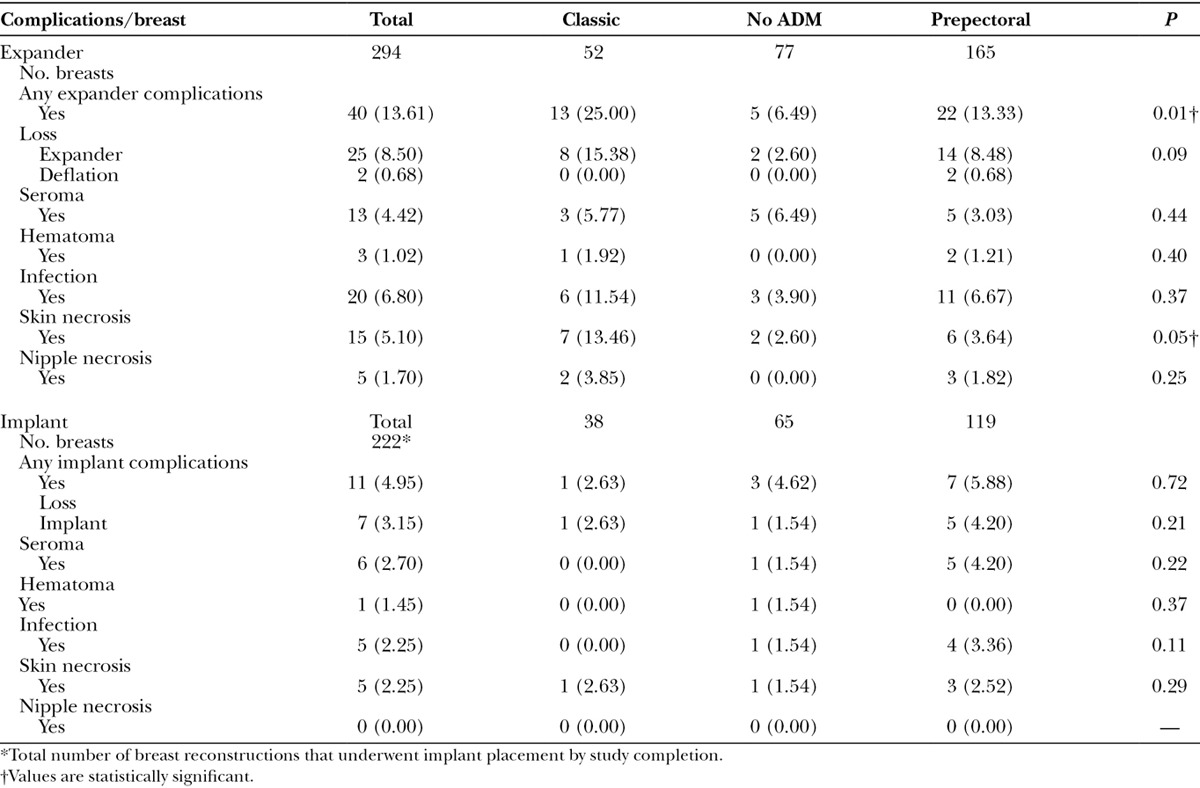
Table 2.
Complications after Expander and Implant Surgery
The cumulative incidence of postoperative complications was computed at 30, 90, and 180 days for expander and implant placement. Complications for the expander and implant were measured separately, each with a separate postoperative 6-month follow-up period. Although no time stipulation was made for the implant placement, it typically occurred 3 months after completion of chemotherapy and 6 months after adjuvant radiation therapy.
Surgical Techniques
The 3 surgical procedures were (1) prepectoral tissue expander placement over the pectoralis muscle with ADM coverage (Prepectoral), (2) subpectoral expander placement with ADM sling as an extension of the pectoralis muscle (Classic), (3) subpectoral/subserratus placement without ADM (No ADM). Although Sterile Alloderm became available during the study, the same antibiotics were used on all breasts. We did not compare outcomes among the different generations of Alloderm.
Prepectoral
After mastectomy, an 8 × 16 cm “Thick” ADM (LifeCell Alloderm) is sutured in the subcutaneous pocket to the medial/parasternal border of the chest wall and along the inframammary fold. The lateral most 3–4 cm of the 8 × 16 cm ADM sheet is removed and sutured to the superior aspect of the ADM, covering the superior aspect of the Natrelle Style 133MV-T tabbed tissue expander (Allergan, Santa Barbara, Calif.) (Fig. 1). Bilateral reconstruction uses 16 × 20 cm, divided in half for each breast, to save costs. The partially filled expander is placed under the Alloderm. Expander tabs are then sutured to the pectoralis muscle and chest wall to maintain the expander position. The ADM is sutured laterally to create a tight pocket to prevent expander migration into the axilla. The subcutaneous layer is closed by catching the deep dermis and underlying ADM with each throw, preventing motion between the mastectomy skin flap and ADM with the intent to diminish seroma formation. A channel drain is placed over the ADM.
Fig. 1.
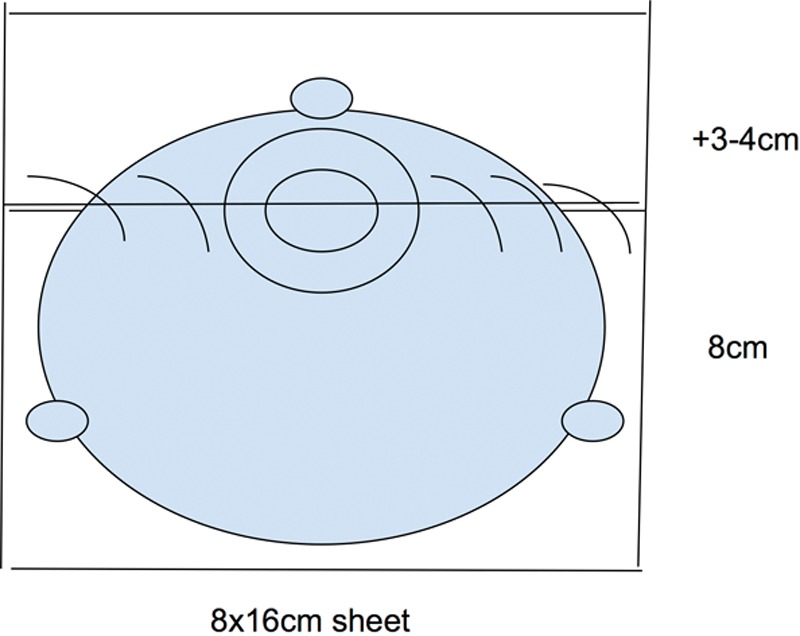
Prepectoral reconstruction technique: Stacked ADM
Classic
The leading edge of the pectoral muscle is elevated and a subpectoral pocket is fashioned. The lower medial origin muscle fibers are released for anatomical expander seating. An Alloderm sling is sutured from the leading edge of the pectoralis muscle to the inframammary fold (over the partially filled expander). The ADM is sutured laterally to close the expander pocket.
No ADM
A submuscular pocket is fashioned by elevating the pectoralis muscle and a lateral slip of the serratus anterior muscle and fascia. A partially filled expander is placed in the submuscular pocket. The serratus slip is sutured over the expander to the lateral pectoral muscle edge. ADM is not used.
Antibiotics were given uniformly throughout the study based on the current standard of care at the time of surgery. The types of expanders, dressings, postoperative drain management, and postoperative expansion schedules were similar for both expanders and implants among surgeons, given our group setting. The incisions were dressed with a gauze/transparent occlusive dressing. The drain was covered with a chlorhexidine Biopatch (Ethicon, Johnson & Johnson, New Brunswick, N.J.). Drain bulbs were infused daily with 5 cc of 0.125% Dakin’s hypochlorite solution via needless syringe into the bulb port.14 Drains were removed when output was < 30 cc/24 hours × 3 days. Implant choice was based on surgeon preference. Fat grafting was performed at implant placement to address any contour deficiencies.
STATISTICAL ANALYSIS
First, descriptive statistics were performed to compare the demographic characteristics of the 3 surgical cohorts Chi-square and t tests were used to evaluate the statistical significance of differences within categorical and continuous variables, respectively, among the 3 surgical cohorts (Table 1). Second, breast complications from either expander or implant placement were evaluated across surgical approaches and differences between the 3 approaches were compared using the chi-square test (Table 2). Third, we performed a univariate logistic regression analysis examining the likelihood of developing complications during the expander and implant procedure across a number of patient-level and surgical variables (Table 3). Lastly, a multivariate analysis was performed to specifically examine the effect of surgical technique as an independent predictor of developing a surgical complication, while controlling for a number of patient characteristics, surgical approaches, and variations in surgeon-specific techniques. Multivariate analysis of implant complications was not performed due to low numbers (Table 4).
Table 3.
Univariate Logistic Regression Analysis Predicting Development of Expander and Implant Complications
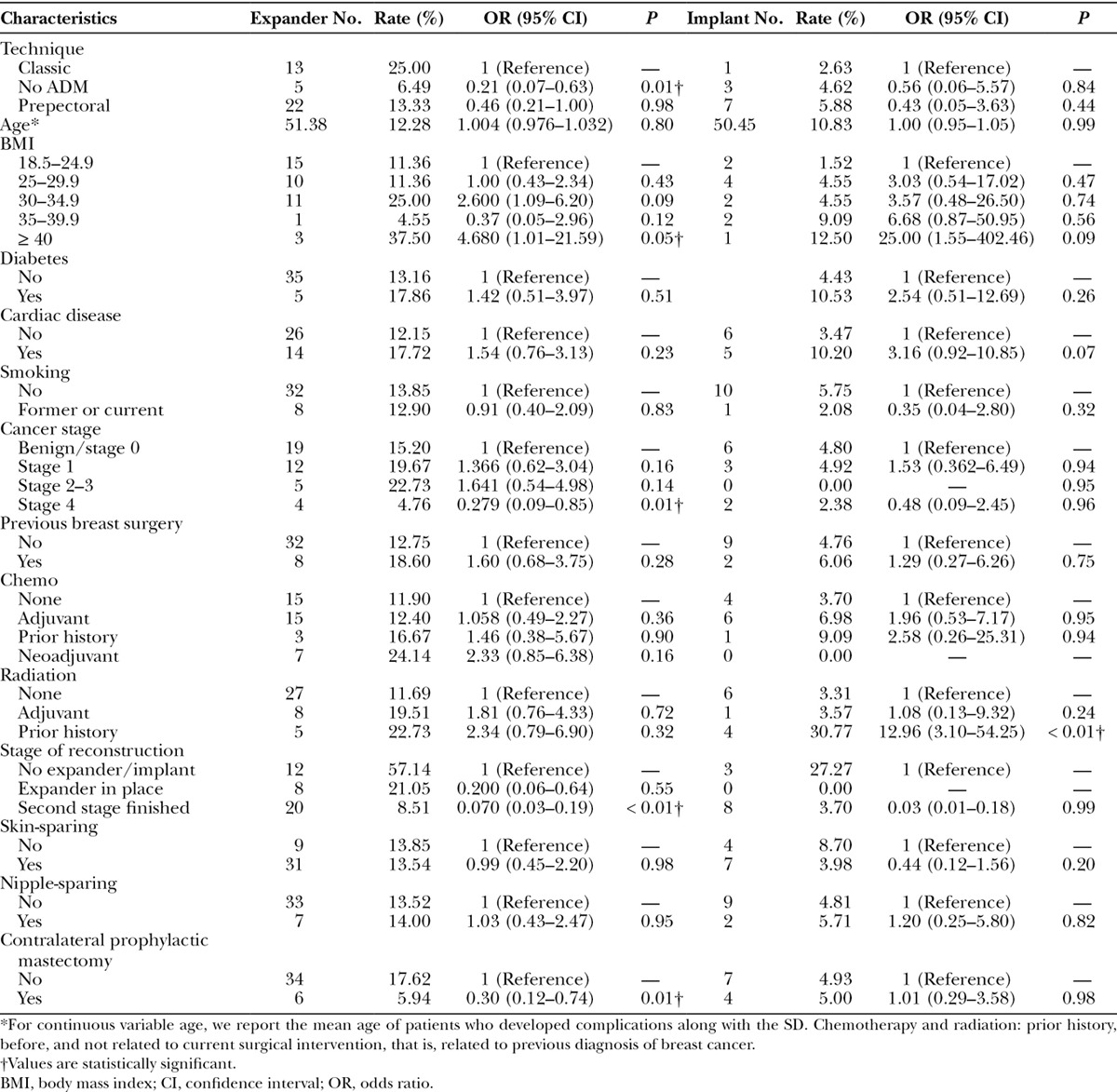
Table 4.
Association between Surgical Technique and Expander Complications
Statistical analysis occurred at the level of the breast. All P values were 2-sided, with a P < 0.05 considered statistically significant. All statistical analyses were performed with SAS Studio 3.6 (SAS Institute, Cary, N.C.).
RESULTS
Three hundred thirty-one consecutive breast reconstructions were performed between June 2008 and July 2015. Two hundred ninety-four breasts met the inclusion criteria, which consisted of 165 Prepectoral, 77 Classic, and 52 No ADM reconstructions. The mean age for all groups was 50.95 (Table 1). Demographics were similar except the Classic cohort had higher rates of cardiac disease (P = 0.03) and a trend toward higher smoking rates (current and former; P = 0.08). BMI ≥ 35 was higher in the Classic group and the No ADM group (P = 0.01). A trend toward increased rates of diabetes in the No ADM group was also noted (P = 0.06).
Among mastectomy techniques, there were more nipple-sparing mastectomies in the Prepectoral group (P < 0.001) and more skin-sparing mastectomies in the Classic and No ADM groups (P = 0.01). Unfortunately, we were not able to identify information for mastectomy type for 3 patients, and these were excluded from analysis. There were similar rates of prophylactic mastectomy in the 3 groups. No difference was noted between the 3 groups in the final stage of reconstruction (i.e., successful completion) or second-stage conversion rate to autologous flap reconstruction (Table 1).
Multiple complication variables were often seen in the same patient, causing higher complication numbers. For instance, an infected breast with necrotic skin flaps might be associated with loss of expander, recording 3 complications in a single breast. All complications were recorded in a “genomic-type format” to show this interrelationship. No pattern emerged with any of the surgical cohorts.
We looked at complications per patient as well as per breast. However, we found that complications were better examined per breast as each breast had its own individual risk factors. For example, a reconstructed breast may undergo radiation (higher risk), whereas the other breast does not but still undergoes prophylactic mastectomy (lower risk).
Expander Complications
The overall infection rate was 6.80% for expander surgery. The difference in infection among the 3 surgical groups was not statistically significant, and the incidence of seroma was similar among all 3 groups (P = 0.44).
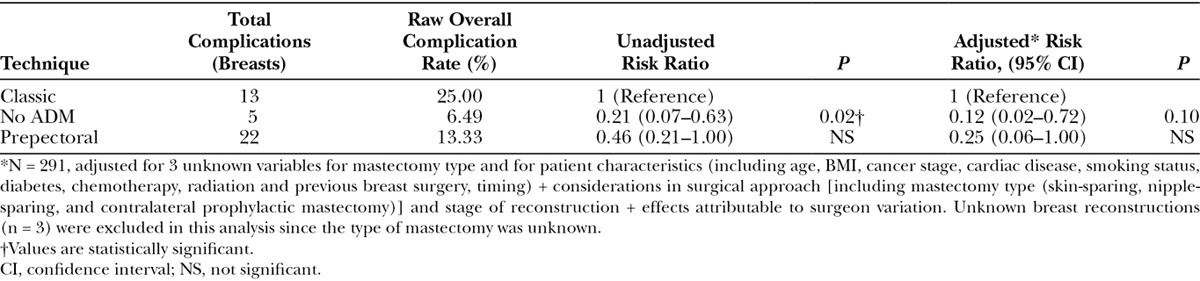
The Classic cohort had significantly more skin necrosis (P = 0.05) and overall expander complications (P = 0.01) than the other 2 surgical techniques (Fig. 2); there were more current and former smokers in the Classic group (P = 0.08). There was no statistical difference in nipple necrosis in nipple-sparing mastectomy among the surgical groups but the numbers were low (Table 2). With univariate analysis, there was a trend toward a higher loss of expander in the Classic group (P = 0.09). However, when placed in a multivariate analysis, this trend disappeared, and all 3 techniques had similar expander complication rates (Table 4).
Fig. 2.
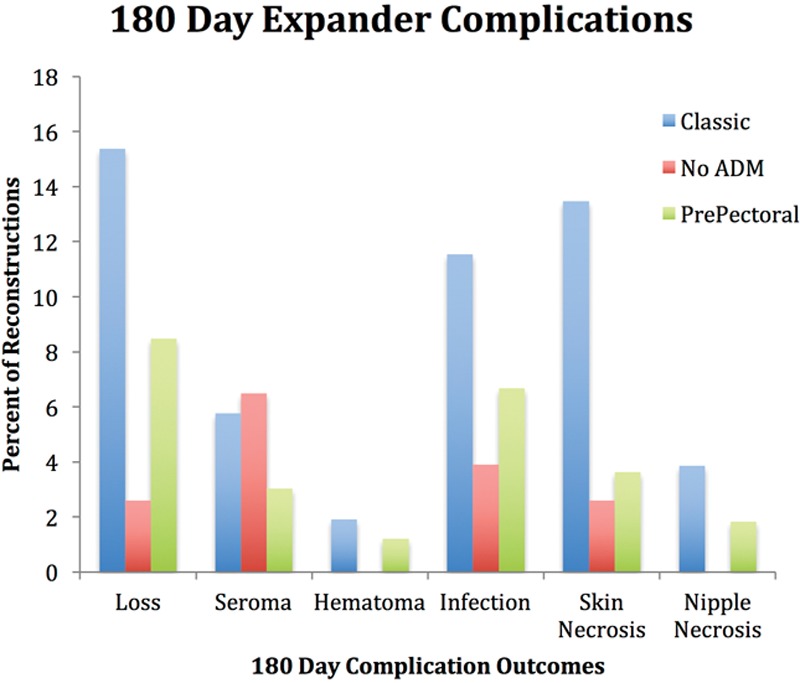
Expander complications at 180 days. Percentage of reconstruction complications.
On the other hand, the No ADM technique had the least number of expander complications among the 3 groups (P = 0.02) in a univariate analysis. Although a trend persisted when analyzed in a multivariate fashion, the statistical significance was lost (P = 0.12).
Implant Complications
The overall infection rate for implant surgery was 2.25% and the seroma formation rate was 2.70%. There was no statistically significant difference in implant complications between the Classic, Prepectoral, and No ADM surgical groups using univariate analysis (Table 2). The number of implant complications was too low to perform multivariate analysis.
Risk Factors
Of the 294 breasts entered in the study, 13.6% (n = 40) had complications related to the expander, whereas 3.7% (n = 11) were implant-related. A history of radiation before initial expander placement appeared to show a nonsignificant increase in expander complications but a striking increase (30%) in implant complications (P < 0.01; Table 3). Of the total number of implant complications, almost half (5/11 = 45%) were associated with either adjuvant or preexpander radiation. Although the numbers were low, implant loss in the Prepectoral group was seen either with radiation (3/5 = 60%) or cardiac disease (2/5 = 40%). Chemotherapy, whether neoadjuvant, adjuvant, or a prior history, did not increase the risk of complications for expander or implant surgery (Table 3).
With univariate analysis, there was a significantly higher rate of overall expander complications for patients with a BMI of 40 or greater (P = 0.05) in all surgical cohorts. There was no difference in outcome complications with both expander and implant placement among patients with diabetes (P = 0.26). Despite the higher rate of cardiac disease in the Classic group, univariate analysis did not reach significance to indicate that cardiac disease was associated with higher rates of complications; however, there was a trend toward significance that may suggest an association if the power of the test is increased (P = 0.07).
Previous breast surgery did not affect outcomes among the 3 surgical cohorts. There was no statistical difference in outcomes with respect to mastectomy type and unilateral prophylactic mastectomy between the 3 surgical cohorts (Table 3). Bilateral prophylactic mastectomy reconstructions had too few complication numbers to perform this analysis.
DISCUSSION
With increasing prophylactic breast reconstruction and nipple-sparing mastectomy for both cancer and cancer prophylaxis, women are demanding a breast comparable with their natural breast, with less discomfort, less contour deformity with minimal surgical sequela, scarring, and downtime. A renewed interest has been seen with suprapectoral reconstruction (thick mastectomy flaps without ADM) and prepectoral reconstruction techniques that now use ADM to avoid the dynamic deformity sometimes seen with subpectoral reconstruction.15–20 A “bioengineered” biodimensional subpectoral breast reconstruction including ADM both at mastectomy and implant surgery, an anatomical/form-stable implant, and fat grafting aims to recreate a better breast.21
Fewer complications have been noted in reconstructions without ADM.22,23 Adverse outcomes with ADM have been inconsistent in the literature but generally associated higher rates of seroma, infection, skin necrosis, and increased loss of expander are seen.24–32 In this study, seroma formation requiring surgical intervention was 4.42% overall for expanders and 2.70% for implants. We did not find a statistical difference in seroma or infection rates when comparing techniques that did and did not use ADM.
Increased complication rates have been noted in patients with diabetes, hypertension, BMI > 30, and contralateral prophylactic mastectomy.33–37 In this study, diabetes did not cause a higher complication rate among the 3 groups. Univariate analysis showed statistically higher expander complications with BMI ≥ 40 (P = 0.05) and stage 4 cancer but not in final implant placement. We also found reconstructed breasts with a contralateral prophylactic mastectomy had more overall expander complications (P = 0.01).
The Classic cohort had a statistically greater incidence of cardiac disease (P = 0.03), more skin necrosis (P = 0.05), and a higher overall expander complication rate (P = 0.01). More skin necrosis was found with the Classic cohort in which surgical release of the pectoralis lower medial border is usually performed for anatomical seating of the expander but not performed in Prepectoral or No ADM. Like others, we observed “window-shading” of scarred, anatomically foreshortened pectoralis muscles intraoperatively for surgical complications in the Classic cohort.16 This window-shading may contribute to poorer chest skin/pericapsular vascularity. In contrast, in No ADM, the pectoralis lateral margin is anchored inferiorly by the serratus slip, counteracting window-shading. Prepectoral reconstruction involves no disruption of the muscle origin or division of the underlying perforators.
An increase in nipple necrosis has been seen with neoadjuvant and adjuvant chemotherapy with suprapectoral reconstruction and nipple-sparing mastectomy, but we did not see this in our study (Table 3).38 Timing of radiation is known to influence both expander and implant outcomes.39,40 We found no statistical increase in complications with adjuvant radiation therapy alone, but there was a 10-fold increase in implant complication rate with prior history of radiation (i.e., before expander).
We find that expander/implant complications are not always captured by the 30-day National Surgical Quality Improvement Program (NSQIP) benchmark.41,42 Periprosthetic joint literature divides complications into “early” at 90 days and “late” at 6 months or later.43,44 We tracked our complications at 30, 90, and 180 days postoperatively. All our complications occurred within 90 days for both expander and implant placement. Our study outcomes would lead us to conclude that 90 days “early” follow-up is more accurate than existing 30-day NSQIP measures and a “late” follow-up of 180 days is appropriate.
The results of our study seem to indicate that for those breasts with increased risk, subpectoral/serratus reconstruction results in fewer complications. Subpectoral/ADM sling reconstruction should be more closely studied for the vascular impact of window-shading particularly in the setting of cardiac disease and a history of smoking. Prepectoral breast reconstruction appears to be impacted by radiation before implant surgery, similar to subpectoral techniques. Contralateral prophylactic mastectomy reconstruction carries greater overall complications and should be shared with patients in their decision making.
Strengths and Limitations
Study strengths include a comparative study designed to evaluate major complication outcomes requiring surgical intervention with the prepectoral breast reconstruction technique compared with traditional methods of subpectoral breast reconstruction with 30, 90, and 180-day follow-up of both expander and implant outcomes.
Study limitations include small cohorts in a retrospective review at a single institution. Randomization to a surgical technique would not be ethical. We did not evaluate “phase 2” efficacy for capsular contracture nor contour deformity. The Pusic’s Breast Q score questionnaire could be valuable for patient satisfaction.45 Cost analysis would be important as prepectoral reconstruction uses the largest ADM sheet, whereas No ADM uses none. We did not perform a power analysis, given a potential risk of type II error. The results of this study should be interpreted in the context of relatively low numbers. Further studies with larger numbers are warranted.
CONCLUSIONS
All 3 surgical cohorts had comparable Clavien IIIb score outcomes with the No ADM trending to the lowest complication rate. BMI ≥ 40, stage 4 cancer, and contralateral prophylactic mastectomy were associated with adverse expander outcomes. Prior radiation therapy caused an overall 10-fold increase in implant loss among the 3 surgical groups. Diabetes, prior breast surgery, type of mastectomy, and chemotherapy played no role in complications in our study. We did not see a higher rate of seroma in the cohorts with ADM techniques. A higher rate of skin necrosis in the Classic group may have been related to higher incidence of cardiac disease or the impact of window-shading of the pectoralis muscle. NSQIP may consider tracking 90-day outcomes for expander/implant surgery, rather than 30 days. Larger, prospective studies including aesthetic outcomes would be beneficial.
ACKNOWLEDGMENTS
We thank John Tamaresis, PhD, MS, Stanford Department of Biomedical Data Science, Stanford University School of Medicine, for statistical support. We wish to thank Ashley N. Reese for creation of our Data Tabulation Model and her valuable manuscript editing.
Footnotes
Supported by a National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Award (KL2 TR000083 and UL1 TR001085). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. CTSA Grant # UL1 TR001085.
Disclosure: None of the authors has a financial interest in any of the products or devices mentioned in this article. Dr. Daniel Jacobs is a co-founder for AirXpanders, Inc., not involved in this study. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Platt J, Baxter NN, McLaughlin J, et al. Does breast reconstruction after mastectomy for breast cancer affect overall survival? Long-term follow-up of a retrospective population-based cohort. Plast Reconstr Surg. 2015;135:468e–476e.. [DOI] [PubMed] [Google Scholar]
- 2.Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008;359:1590–1601.. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg. 2015;42:399–404.. [DOI] [PubMed] [Google Scholar]
- 4.Slade CL. Subcutaneous mastectomy: acute complications and long-term follow-up. Plast Reconstr Surg. 1984;73:84–90.. [PubMed] [Google Scholar]
- 5.Apfelberg DB, Laub DR, Maser MR, et al. Submuscular breast reconstruction—indications and techniques. Ann Plast Surg. 1981;7:213–221.. [PubMed] [Google Scholar]
- 6.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208.. [DOI] [PubMed] [Google Scholar]
- 7.Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482–492.. [DOI] [PubMed] [Google Scholar]
- 8.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317.. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro PG, Jazayeri L. Two-stage implant-based breast reconstruction: an evolution of the conceptual and technical approach over a two-decade period. Plast Reconstr Surg. 2016;138:1–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. BMI classification. Available at https://en.wikipedia.org/wiki/Body_mass_index. Accessed April 17, 2017.
- 11.World Health Organization. BMI and risk factors. Available at http://www.who.int/gho/ncd/risk_factors/bmi_text/en/. Accessed April 17, 2017.
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass. Ann Plast Surg. 2015;74:30–32.. [DOI] [PubMed] [Google Scholar]
- 14.Degnim AC, Scow JS, Hoskin TL, et al. Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations. Ann Surg. 2013;258:240–247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39.. [DOI] [PubMed] [Google Scholar]
- 16.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139:287–294.. [DOI] [PubMed] [Google Scholar]
- 17.Gahm J, Wickman M, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer—prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast. 2010;19:462–469.. [DOI] [PubMed] [Google Scholar]
- 18.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205.. [DOI] [PubMed] [Google Scholar]
- 19.Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 2009;33:44–48.. [DOI] [PubMed] [Google Scholar]
- 20.Caputo GG, Marchetti A, Pozza ED, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705.. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell GP, Gabriel A. Bioengineered breast: concept, technique, and preliminary results. Plast Reconstr Surg. 2016;137:415–421.. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Park Y, Choi KW, et al. The effect of sterile acellular dermal matrix use on complication rates in implant-based immediate breast reconstructions. Arch Plast Surg. 2016;43:523–528.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg. 2012;130:1–9.. [DOI] [PubMed] [Google Scholar]
- 24.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678.. [DOI] [PubMed] [Google Scholar]
- 25.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178.. [DOI] [PubMed] [Google Scholar]
- 26.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356.. [DOI] [PubMed] [Google Scholar]
- 27.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614.. [DOI] [PubMed] [Google Scholar]
- 28.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436.. [DOI] [PubMed] [Google Scholar]
- 29.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e.. [DOI] [PubMed] [Google Scholar]
- 30.Xue DQ, Qian C, Yang L, et al. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375–381.. [DOI] [PubMed] [Google Scholar]
- 31.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274.. [DOI] [PubMed] [Google Scholar]
- 32.Parks JW, Hammond SE, Walsh WA, et al. Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg. 2012;130:739–746.. [DOI] [PubMed] [Google Scholar]
- 33.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436.. [DOI] [PubMed] [Google Scholar]
- 34.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058.. [DOI] [PubMed] [Google Scholar]
- 35.Voineskos SH, Frank SG, Cordeiro PG. Breast reconstruction following conservative mastectomies: predictors of complications and outcomes. Gland Surg. 2015;4:484–496.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momoh AO, Cohen WA, Kidwell KM, et al. Breast reconstruction in patients with unilateral breast cancer who choose contralateral prophylactic mastectomy—an assessment of postoperative morbidity. Plast Reconstr Surg. 2015;136:116–117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva AK, Lapin B, Yao KA, et al. The effect of contralateral prophylactic mastectomy on perioperative complications in women undergoing immediate breast reconstruction: a NSQIP analysis. Ann Surg Oncol. 2015;22:3474–3480.. [DOI] [PubMed] [Google Scholar]
- 38.Frey JD, Choi M, Karp N. The effect of neoadjuvant chemotherapy compared to adjuvant chemotherapy in healing after nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:10e. [DOI] [PubMed] [Google Scholar]
- 39.de Araujo TB, Jue Xu M, Susarla SM, et al. Impact of prior unilateral chest wall radiotherapy on outcomes in bilateral breast reconstruction. Plast Reconstr Surg. 2016;138:575e–580e.. [DOI] [PubMed] [Google Scholar]
- 40.Cordeiro PG, Albornoz CR, McCormick B, et al. What is the optimum timing of postmastectomy radiotherapy in two-stage prosthetic reconstruction: radiation to the tissue expander or permanent implant? Plast Reconstr Surg. 2015;135:1509–1517.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Koltz PF, Sbitany H. Lessons learned from the American College of Surgeons National Surgical Quality Improvement Program Database: has centralized data collection improved immediate breast reconstruction outcomes and safety? Plast Reconstr Surg. 2014;134:859–868.. [DOI] [PubMed] [Google Scholar]
- 42.Sinha I, Pusic AL, Wilkins EG, et al. Late surgical-site infection in immediate implant-based breast reconstruction. Plast Reconstr Surg. 2017;139:20–28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wingert NC, Gotoff J, Parrilla E, et al. The ACS NSQIP risk calculator is a fair predictor of acute periprosthetic joint infection. Clin Orthop Relat Res. 2016;474:1643–1648.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Quality Measures Clearinghouse (NQMC). Measure summary: total hip arthroplasty (THA) and/or total knee arthroplasty (TKA): hospital-level risk-standardized complication rate (RSCR) following elective primary THA and/or TKA. In: National Quality Measures Clearinghouse (NQMC) [Web site]. 2015. Rockville (MD): Agency for Healthcare Research and Quality (AHRQ); Available at https://www.qualitymeasures.ahrq.gov. [Google Scholar]
- 45.Pusic A, Chen C, Cano S, et al. Measuring quality of life in cosmetic and reconstructive breast surgery: systematic review of patient-reported outcomes instruments. Plast Reconstr Surg. 2007;120:823–837.. Available at https://webcore.mskcc.org/breastq/. [DOI] [PubMed] [Google Scholar]


