Abstract
Despite recent advances in radiotherapy, a majority of patients diagnosed with pancreatic cancer (PC) do not achieve objective responses due to the existence of intrinsic and acquired radioresistance. Identification of molecular mechanisms that compromise the efficacy of radiation therapy and targeting these pathways is paramount for improving radiation response in PC patients. In this review, we have summarized molecular mechanisms associated with the radio-resistant phenotype of PC. Briefly, we discuss the reversible and irreversible biological consequences of radiotherapy, such as DNA damage and DNA repair, mechanisms of cancer cell survival and radiation-induced apoptosis following radiotherapy. We further describe various small molecule inhibitors and molecular targeting agents currently being tested in preclinical and clinical studies as potential radiosensitizers for PC. Notably, we draw attention towards the confounding effects of cancer stem cells, immune system, and the tumor microenvironment in the context of PC radioresistance and radiosensitization. Finally, we discuss the need for examining selective radioprotectors in light of the emerging evidence on radiation toxicity to non-target tissue associated with PC radiotherapy.
1. Introduction
Pancreatic cancer (PC) is predicted to affect approximately 53,670 new individuals resulting in nearly 43,090 deaths in the year 2017, making it the third leading cause of cancer-related mortality in the U.S. [1]. The 5-year survival rate for PC is ~8%, with a median survival from the time of diagnosis ranging between 3 and 6 months, neither of which have shown much improvement over the last decades [1,2]. It was noted that the incidence and death rate of PC have increased by 1.2% and 0.4%, respectively, per year since 2000 [2]. Resection, the only curative treatment for PC, is limited to only 15–20% of cases and has little success, with only 20% of resected patients living more than five years [3]. Post-resection death is often the result of recurrences occurring both locally (33%–86%) and distantly (23%–92%) [4–7]. In an attempt to improve survival, both chemotherapy (CT) and radiotherapy (RT) are used either in conjunction with resection or as the sole treatments for the majority of 80–85% of PC patients who present with unresectable tumors [8].
According to the National Comprehensive Cancer Network (NCCN) guidelines version 2.2016, resectable PC is defined as disease with no evidence of distant disease, no tumor contact with celiac axis (CA), superior mesenteric artery (SMA) or common hepatic artery and/or no tumor contact with the superior mesenteric vein (SMV) or portal vein (PV) or ≤ 180 degrees contact without vein contour irregularity. Unresectable PC is defined as distant metastatic disease or head/uncinated tumor contact with SMA/CA > 180 degrees or first jejunal SMA branch and body/tail tumor contact of >180 degrees with the SMA or CA or unreconstructable SMV/PV involvement. A borderline resectable disease is defined as those with disease status between resectable and unresectable status. In these potentially resectable patients, clinical studies have shown that a combination of CT and RT can convert the tumor to a resectable state in 8%–30% of cases [9–13]. Unfortunately, RT does not conclusively play a beneficial role in the treatment of PC, often being only mildly successful in a minority of cases for both resectable and unresectable disease. Tables 1 and 2 summarize the clinical trials that have investigated the efficacy of RT in the treatment of resectable and unresectable PCs. However, interpretability of many of these studies is limited due to lack of information in regard to radiation technique. It is important to note that the success of treatment can be greatly affected by the technique used as well as the treatment’s timing (i.e., pre-operative vs. post-operative). Further, many studies reported to be unsuccessful were based on the patient overall survival analysis only. However, as PC is very rarely curable, it may be more useful to consider the quality of life when evaluating the success of new treatments [14]. Ultimately, it appears that while some PC patients may respond to RT, the majority of PC patients are RT resistant. The main reason attributed to the ineffectiveness of RT in PC patients is the existence of intrinsic and acquired radioresistance. Several mechanisms associated with the disease have been proposed to contribute to this radioresistance of PC, including alterations in the DNA damage response, DNA repair machinery, and cell cycle checkpoint controls, as well as the hypoxic environment within the tumor and the activation of stellate cells leading to fibrosis.
Table 1.
Currently available clinical trials to base decisions of radiation therapy in resectable pancreatic cancer.
| Study/Trial | Treatment | N | OS | MS | Special considerations | Overall Conclusion | References |
|---|---|---|---|---|---|---|---|
| GITSG (1987) | RTa + CT (Bolus 5-FU during RT and maintenance for 2 yrs) | 21 | 19% f | 21 mo | Negative surgical margins only; Excluded periampullary tumors, study closed early due to poor accrual and interim analysis detection of survival benefit | Survival benefit | [351] |
| Observation | 22 | 5%f | 10.9 mo | ||||
| EORTC 40891 Updated (2007) | RTa + CT (CI 5-FU during RT, no maintenance ) | 110 | 18%g | 1.8 yr | Periampullary and pancreatic head adenocarcino mas included, positive margins allowed | No survival benefit | [352] |
| Observation | 108 | 17% g | 1.6 yr | ||||
| ESPAC-1 (2004) | RTa + CT (bolus 5-FU during RT) | 73 | 7%f | 13.9 mo | Included ductal adenocarcinoma ofthe pancreas, positive margins allowed, physician choice of randomization arms, lack of quality assurance | CT-survival benefit RT-survival detriment | [353] |
| RTa + CT (bolus 5-FU during RT and 5-FU + folinic acid maintenance for 6 mo) | 72 | 13% f | 19.9 mo | ||||
| CT (5-FU + folinic acid maintenance for 6 mo) | 75 | 29% f | 21.6 mo | ||||
| Observation | 69 | 11% f | 16.9 mo | ||||
| RTOG 9704 (2011) | GEM then 5-FU/EBRT (50.4 Gy) then GEM | 221(187 )h | 22% | 20.5 mo | The overall and median survival are for head lesions only | On multivariate analysis, GEM arm trend toward improved survival (P = 0.08) | [354] |
| 5-FU then 5- FU/EBRT (50.4 Gy) then 5-FU | 230 (201)h | 18% | 17.1 mo | ||||
| Medicare- SEER (2003) | Resection alone | 241 | 29% e | 11.5 mo | Retrospective- Data from NCI’s Surveillance, Epidemiology, and End Results (SEER) program | Adjuvant chemoradiother apy offers survival benefit | [355] |
| Resection + adjuvant chemoradiati on therapy (not further defined) | 155 | 41% e | 25.1 mo | ||||
| Johns Hopkins Hospital- | Resection alone | 509 | 16% f | 15.5 mo | Retrospective | Adjuvant chemoradiother apy offers survival benefit | [356] |
| Mayo Clinic collaborati ve study (2010) | Resection + 5-FU based CRT(median dose 50.4 Gy) | 583 | 22% f | 21.1 mo | |||
| Johns Hopkins (1997) | Standard therapy b | 99 | 80% d | 17.5 mo | Patients choose which therapy they wanted to receive. | EBRT offers a survival benefit; intensive therapy offered no survival benefit. | [357] |
| Intensive therapy c | 21 | 70% d | 21 mo | ||||
| No therapy | 53 | 54% d | 13.5 mo |
Abbreviation: GITSG = Gastrointestinal Tumor Study Group; CI=continuous infusion; CT=chemotherapy; RT=radiotherapy; CRT= Chemoradiotherapy; EBRT=external beam radiation therapy; 5-Fu= 5 fluorouracil; mo=months N=number of patients; yrs=years; OS= Overall survival; MS= Median survival.
RT dose of 40 Gy delivered by split course with a 2-week break after 20 Gy. It is important to note that the use of split course therapy allows for accelerated repopulation of malignant cells and has been associated with decreased effectiveness in anal cancer, cervical cancer and head and neck cancer [207,358–360]. Also for many cancers a minimum dose of 50 Gy is required, and therefore the 40 Gy may be inadequate [361,362].
EBRT to pancreatic bed at 4000–4500cGy in combination with two 3-day courses of 5-FU followed by weekly bolus (500mg/m2 per day) for four months.
EBRT to pancreatic bed at 5040–5760cGy as well as prophylactic hepatic irradiation at 2340–2700cGy in combination with 5-FU infusion (200mg/m2 per day) and leucovorin (5mg/m2 per day) for 5 of 7 days for 4 months.
1 year survival rate.
3 year survival rate.
5 year survival rate.
10 year survival rate.
The number inside () is for pancreatic head.
Table 2.
Currently available clinical trials to base decisions of radiation therapy in non-resectable pancreatic cancer.
| Study/Trial | Treatment | N | OS | MS | Special considerations | Overall Conclusion | References |
|---|---|---|---|---|---|---|---|
| GISTG (1981) | RTa + CT (bolus 5- FU+maintenance for 2 yrs) | 83 | 40% b | 10 mo | Other significant prognostic variables included pretreatment performance status and pretreatment CEA level | Survival benefit | [363] |
| RTc + CT (bolus 5-FU +maintenance for 2 yrs) | 86 | 40% b | 10 mo | ||||
| RTc | 25 | 10%b | 6 mo | ||||
| ECOG (1985) | RTa + CT (bolus 5-FU + maintenance) | 47 | 28%b | 8.3 mo | No survival benefit, Substantial ly greater toxicity RT +CT | [364] | |
| CT (5-FU) | 44 | 28%b | 8.2 mo | ||||
| GISTG (1988) | RT + CT (bolus 5-FU during RT; SMF maintenance for 2 yrs) | 22 | 41%b | 42 wk | Survival benefit | [365] | |
| CT (SMF for two yrs) | 21 | 19%b | 32 wk | ||||
| GERCOR(2007) | CT (GEM based combination) followed by RTd + CT (5-FU during RT) | 72 | 65.3 %b | 15 mo | Initial CT for three months, then decision to administer CRT or continue CT in non-progressive patients was the investigator’s choice. | Survival benefit | [366] |
| CT | 56 | 47.5 %b | 11.7 mo | ||||
| FFCD/SFRO (2008) | RTe+ CT (CI 5-FU+cisplatin during RT; maintenance GEMf) | 59 | 32% b | 8.6 mo | No distant metastasis, study closed early due do interim analysis showing survival detriment. | No survival benefit; Substantial ly greater toxicity RT+CT | [367] |
| GEM | 60 | 53% b | 13 mo | ||||
| ECOG (2011) | RTg +CT (GEM concurrent and maintenance) | 34 | 50%b | 11.1 mo | No distant metastasis, study closed early due to low patient accrual | Survival benefit; Substantial ly greater toxicity RT+CT | [319] |
| GEM | 37 | 32%b | 9.2 mo | ||||
| RTOG 0020 (2012) | RTg + CT (GEM and paclitaxel) | 91 | 46.2 %b | 11.5 mo | No distant metastasis | Maintenan ce R11577 is not effective and associated with broad range of toxicities | [249] |
| RTg + CT (GEM and paclitaxel) + maintenance R115777(tipifarn ib) | 94 | 34.0 %b | 8.9 mo | ||||
| LAP07 (2016) | CT (GEM with/without Erlotinib) | 136 | 24% b | 15.2 mo | CRT was associated with increased risk for grade 3 and 4 toxicity. | CRT is not superior to CT in patients with locally advanced pancreatic cancer who did not progress after 4 months of CT. | [237] |
| CT(GEM with/without Erlotinib) + CRT (50.4Gy with capecitabine) | 133 | 27% b | 16.5 mo |
Abbreviation: CI=continuous infusion; CT=chemotherapy; 5-Fu= 5 fluorouracil; GEM=gemcitabine; mo=months; n=number of patients; RT=radiotherapy; SMF= streptozocin, mitomycin, 5-FU; yrs=years.
RT dose of 40 Gy delivered by split course with a 2-week break after 20 Gy. It is important to note that the use of split course therapy allows for accelerated repopulation of malignant cells and has been associated with decreased effectiveness in anal cancer, cervical cancer and head and neck cancer [207,358–360]. Also for many cancers a minimum dose of 50 Gy is required, and therefore the 40 Gy may be inadequate [361,362].
1 year survival rate.
RT dose of 60 Gy delivered by split course with a 2-week break after 20Gy.
2 year survival rate.
RT dose 60 Gy given in 2 fractions.
RT dose of 50.4 Gy in 28 fractions.
In this review, we aim to discuss and update current knowledge and understanding of the following topics: (i) various biological processes and changes that occur during and after PC radiotherapy, (ii) possible mechanisms associated with the emergence of PC radioresistance, and (iii) currently available radiosensitizers and radioprotectors for PC therapy. The radioprotectors are discussed not only as protectors of normal cells against radiation but also as enhancers enabling cancer cell killing (differential effect) when treated with conventional or Stereotactic Body Radiation Therapy (SBRT), as an additional benefit.
2. Molecular and cellular pathways associated with radiotherapy
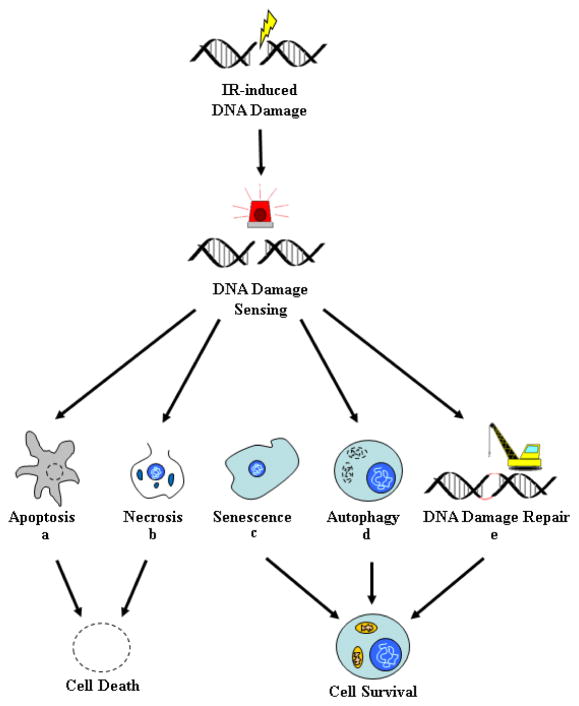
When a cell is subjected to ionizing radiation (IR), DNA damage occurs as high energy photons interact with electrons resulting in the electron gaining part of their energy, according to the Compton effect [15]. These secondary electrons can then induce DNA damage, either directly by ionizing atoms within the DNA helix, or indirectly by first ionizing oxygen-containing molecules surrounding the DNA (e.g. water), to produce highly reactive free radical species, which in turn interact with the DNA resulting in damage. This indirect mechanism has been suggested to contribute to the majority of the DNA damage [16]. Ionizing radiation can damage the DNA strands and create a single strand break (SSB) or double strand break (DSB). The SSBs are rapidly repaired by members of the poly (ADP-ribose) polymerases (PARP) family in conjunction with base excision repair (BER). A DSB probably form when two SSB are in close proximity, and these DSBs are far more difficult to repair and are therefore more toxic and lethal to cancer cells. In individual cells, exposure to radiation can elicit several possible responses: 1) transient response with survival, 2) senescence, 3) apoptosis, 4) necrosis, and 5) autophagy (Figure 1). In cells that survive irradiation, exposure to the insult induces a transient cell cycle arrest followed by DNA repair and survival. If the damage is too extensive, senescence, apoptosis, or necrosis will instead be induced to eliminate the damaged cells or at least limit their proliferative potential. Senescence is a viable state of permanent cell cycle arrest associated with the induction of markers of cellular aging. Apoptosis is a form of programmed cell death involving caspase activation followed by DNA fragmentation. Cells undergoing necrosis exhibit changes consistent with the loss of plasma membrane integrity (rupture of the plasma membrane, mitochondrial swelling, disintegrated organelles, and atypical nucleus). Apoptosis and necrosis are irreversible processes that result in cell death and their ultimate removal by macrophages. Autophagy is a process by which cells consume their own components to either self-destruct or survive conditions of limiting nutrients. In cancer cells exposed to ionizing radiation, autophagy can lead to either cell death or survival, depending on the conditions.
Figure 1. Cellular Response to Ionizing Radiation.
In response to ionization radiation, a normal or cancer cell undergoes DNA damage in the form of either single strand breaks (SSB) or double strand breaks (DSB). DNA damage is sensed by the cell, resulting in progression down to any one of five biological outcomes: Apoptosis (a), Necrosis (b), Senescence (c), Autophagy (d), and/or DNA repair and survival (e). Genetic and epigenetic alterations in pathways regulating these responses can lead to radiation resistance. Following irradiation, the DNA breaks are recognized by ATM and ATR kinases, which in turn can either engage the apoptotic machinery or else protect the cell by activation of compensatory mechanisms (e.g. cell arrest and DNA repair). (a) Apoptosis is a form of programmed cell death initiated by caspases and regulated by members of the Bcl-2 family of proteins (intrinsic pathway) or members of the death receptors (extrinsic pathway). Many cancer cells have lost their ability to undergo apoptosis, thereby making them less susceptible to IR-induced apoptosis. (b) Necrosis is a form of cell death characterized by disruptions of the plasma membrane, autolysis, and breakdown of cellular organelles. The cell’s fate to undergo necrosis versus apoptosis depends on the dose of radiation, with the higher doses inducing necrosis. (c) IR-induced DNA damage can also lead to the induction of senescence. Senescence is a viable state of permanent cell cycle arrest characterized by markers of cellular aging (e.g. SA-β-galactosidase expression, increased production of matrix metalloproteinase (MMP), and decreased synthesis of extracellular matrix proteins). (d) Autophagy is a stress response characterized by the intracellular degradation of cytoplasmic constituents by the lysosomes. Autophagy can contribute to survival by the replacement of structures damaged by ROS, but the process can also contribute to cell death if autophagy is left uncontrolled. When a cell is irradiated, autophagosomes are formed that fuse with the lysosome to form autophagolysosomes. The autophagolysosomes auto-digest the damaged cell organelles and protein content for recycling. Autophagy may also be linked to radioresistance as this mechanism can be both pro-survival and pro-death depending on the conditions. (e) Activation of the ATM and ATR kinases can lead to activation of cell cycle checkpoints, formation of DNA damage foci, and recruitment of DNA repair machinery, which then act conjointly to protect the cells from the effects of radiation.
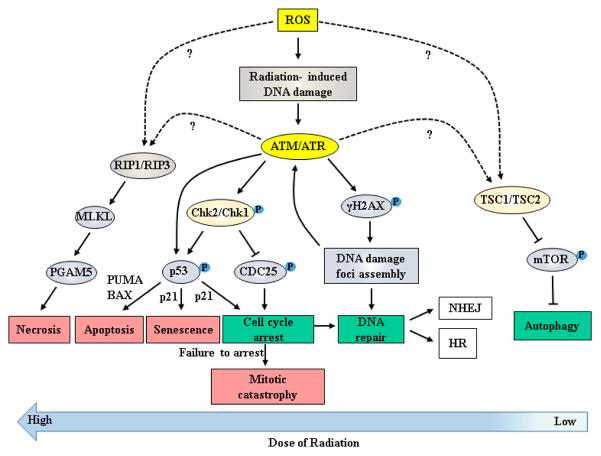
Cells exploit various responses to DNA damage and the associated biochemical pathways to protect themselves from the effects of radiation. Thus, alterations in components of these biological pathways can lead to the generation of radioresistance. Other biomolecular changes that can contribute to radioresistance include changes in tumor microenvironment leading to increased hypoxia at the tumor’s center. Autophagy may also be linked to radioresistance as this mechanism can be both pro-survival and pro-death depending on the system. Based on the current understanding, a model depicting the interplay of various biological processes involved during post-radiation response is presented in Figure 2.
Figure 2. Biological consequences and its associated signaling mechanism(s) in response to the high and low levels of ionizing radiation.
Ionizing radiation leads to the production of reactive oxygen species (ROS), which then react with cellular constituents to damage membranes, proteins, and genetic material. At the DNA level, the ROS create single- and double-stranded breaks in the genome (SSB, DSB). Detection of these breaks by surveillance systems results in the activation of the ATM and ATR kinases, which serve as central coordinators of the cellular response to IR. Activation of these kinases after IR and activation of their downstream effector kinases, the Chk2 and Chk1 kinases respectively, contributes to the induction of apoptosis (via activation of p53 and induction of pro-apoptotic Bcl-2 family members PUMA and BAX), induction of senescence (at least in part through the induction of p21 by p53, along with delayed induction of p16), or else transient cell cycle arrest (via p53-mediated induction of p21 and phosphorylation/inactivation of the CDC25 phosphatases). The induction of senescence and cell cycle arrest protect from mitotic catastrophe by blocking entry of cells with DSB in either the S and M phases of the cell cycle. Activation of the ATM and ATR kinases also causes phosphorylation of the H2AX histone variant, which then acts as a seed for the formation of DNA damage foci. These foci serve to amplify and coordinate the DNA damage response in addition to facilitating the recruitment of the DNA repair machinery. Repair of DSB can then proceed through use of homologous recombination (HR) or non-homologous end joining (NHEJ), depending on the phase of the cell cycle. Finally, through mechanisms that have not yet been completely elucidated, IR-induced ROS formation can also lead to necrosis via activation of the RIP1/RIP3 complex or else activation of autophagy via inhibition of the mTOR complex. This activation of autophagy can either promote or inhibit survival, depending on the conditions. Genetic and epigenetic alterations in the pathways that control these different responses can contribute to the development of radiation resistance.
While negligible information is known about the molecular mechanisms of radiation resistance in PC, radiation resistance has been the primary focus of clinical and radiobiological research in many other malignancies. Based on what is known about radiation resistance in other malignancies combined with the identification of mutated pathways in PC, herein we review some possible predictive pathways that can contribute to PC radioresistance.
3. DNA damage sensing and repair
The first step in a cell’s response to radiation is sensing the damage caused by that radiation and using these signals to activate the signaling cascades that will determine the nature of the cell’s response-whether it will be a transient cell cycle arrest, senescence, apoptosis, or necrosis, with or without activation of autophagy. Various proteins have been identified for their role in DNA damage sensing. SSBs created by ionizing radiation are immediately recognized by members of the PARP family, which PARsylate (addition of poly ADP-ribose) DNA-associated proteins in the vicinity of the break. These chains of poly (ADP-ribose) then serve to recruit components of the BER machinery which then can repair the break. Recognition of DSBs involves activation of the ATM (ataxia-telangiectasia mutated) and ATR (ATM and Rad3 related proteins) kinases, both of which are members of the phosphoinositide 3-kinase-like kinase (PIKK) family [17–22]. By mechanisms that remain unclear, ATM is immediately recruited to and activated by a DSB. The DSB is eventually trimmed by exonucleases to produce a single-stranded 3′-overhang that can lead to the recruitment of the Rad17-replication factor C (Rad17-RFC)/9-1-1 complex and subsequent activation of ATR. Together, the two kinases initiate and coordinate the cellular response to DNA breaks [23–26] and activate pathways that protect the cells from the effects of IR. Some of these pathways block the cell cycle to allow DNA repair to take place while others stimulate the activity of the DNA repair machinery. Several pathways activated by ATM that are discussed in this review are illustrated in Figure 2. Germline mutations in the ATM gene, in a disorder such as ataxia-telangiectasia, have been associated with the hypersensitization of cells to radiation. Thus, patients harboring mutations in the ATM gene are predicted to be at higher risk of cancer incidence [27]. Similarly, pharmacological inhibition of ATM in cancer cells using AZD8055 can sensitize these cells to the effects of radiation, and this inhibitor is now being pursued for radiosensitization of solid tumors. ATM and ATR modulate pathways that control cell cycle checkpoints, DNA repair machinery, senescence, autophagy, and apoptosis [27–34].
The success of radiation treatment is often determined by the balance between DNA damage and DNA repair [35–37]. If a cell is damaged beyond what can reasonably be repaired, it will most likely undergo programmed cell death. Besides these biological functions, if the extent of the damage is within the scope of what can be repaired, the cell will more often survive. Radiation induced DSBs are repaired by one of two mechanisms: homologous recombination (HR), and/or nonhomologous end-joining (NHEJ) [20–22,38–41], with NHEJ playing a dominant role while HR plays a supportive role [21,24,42–45]. However, HR has been more closely associated with radiosensitivity than NHEJ [46–48]. Iliakis et al., proposed that repair may in fact occur in a two-step process with NHEJ occurring first in order to restore continuity to the genome and HR occurring second at which point the actual sequence around the break is restored [49]. In addition, inhibition of NHEJ by DNA-dependent protein kinase (DNA-PK) inhibitor NU7026 or by RNAi was shown to radiosensitize 8988T and Panc1 PC cells [50], suggesting that both DNA repair mechanisms deserve further exploration as pathways for radiosensitization. ATM has also been linked to DNA repair through a variety of molecules including Nijmegen breakage syndrome1 (NBS1), BRCA1, and CHK2 [51]. Interestingly, cells deficient of BRCA1 has been shown to be associated with a hypersensitivity to irradiation and defects in homologous recombination (HR) [51,52]. Likewise, loss of NBS1 in cells has been linked to radiosensitivity and defects not only in HR but also in nonhomologous end-joining (NHEJ) [51,53,54]. Also, F-Box and WD Repeat Domain Containing 7 (FBXW7) was shown to facilitate NHEJ repair by binding with XRCC4. Subsequently, polyubiquitination of XRCC4 via lysine 63 will enhance the chance of binding FBXW7-XRCC complex with the KU70/80 heterodimer scaffold to initiate NHEJ process. Inhibition of XRCC4 polyubiquitylation using pharmacological inhibitor MLN4924 also resulted in impairment of NHEJ repair. Thus, effective inhibition of NHEJ appears to be an alternative therapeutic strategy for PC patients having a defective HR repair pathway [55].
One downstream mechanism of ATM that is especially interesting in regards to radiation resistance results in the phosphorylation and activation of p53 [56–62]. ATM kinase activity results in the activation of Chk1 and Chk2 kinases, with both kinases phosphorylating p53 at Ser-20 [56,63–67]. Phosphorylated p53 disassociates from MDM [56,63,67–69], whose natural function is to target p53 for degradation, resulting in p53 accumulation [56,70,71]. Activated p53 is a DNA-binding protein that recognizes binding sites (comprising of four head-to-tail copies of the pentanucleotide PuPuPuC(A/G)) in the promoter of genes involved in cell cycle control, DNA repair, and regulation of apoptosis. Among these are genes encoding for the pro-apoptotic proteins Bax and IGF-BP3 as well as the cell cycle regulators p21 and Gadd45 [56,59,65,72–80]. p21 is a potent and general inhibitor of the cyclin-dependent kinases (CDKs) that control the G1/S and G2/M transitions of the cell cycle. This induction of p21 by p53 blocks cells at the G1/S and G2/M borders of the cell cycle in response to radiation. This transient cell cycle arrest allows DNA repair to take place before cells are allowed to re-enter the cell cycle to resume cell division. Thus, the p53 activation in response to IR can lead to either DNA repair and survival [81] or else apoptosis [82], depending on the type of cell or the extent of DNA damage.
4. Activation of cell cycle checkpoints
Radiotherapy impedes growth and viability, at least in part, by the generation of DSBs. If present at the time a cell is undergoing mitosis, these DSBs can wreak havoc and trigger mitotic catastrophe, a form of cell death. To block entry into mitosis and allow time for DNA repair to take place, cells with DSB activate the G1/S and G2/M cell cycle checkpoints [83]. For the purpose of radiotherapy, activation of these checkpoints is a double-edge sword. The checkpoints transiently inhibit tumor cell proliferation, but they also protect the cells from mitotic catastrophe and promote DNA repair. Invariably, a small number of tumor cells recover, deactivate the checkpoints, and give rise to clones of proliferating cells. A new concept in radiation biology is the notion that tumors can be radiosensitized by drugs that block the activation of the G2/M checkpoint. The G1 checkpoint is largely mediated through the activation of p53 by Chk1/2 followed by subsequent activation of p21 which mediates G1 arrest [25,84–86]. In many cancer cells, the G1/S checkpoint is already inactivated as a consequence of genetic alterations in components of this checkpoint, including p53 and p16.
The G2/M checkpoint is under the controlof Cdc2/cyclin B complex, whose activity is pre-requisite for the G2/M transition of the cell cycle [87]. In response to DSB, the ATM and then ATR kinases are activated. These kinases activate their respective downstream targets, the Chk2 and Chk1 kinases. These two kinases in turn phosphorylate and inactivate the Cdc25 dual-specificity phosphatases, whose activities are needed for dephosphorylation of Tyr15 of Cdc2, activation of the Cdc2/Cyclin B complex, and for the G2/M transition [88–90]. On the other hand, 14-3-3 binds to the phosphorylated forms of Cdc25, which contributes to arrest the cells at G2/M after ATM/ATR activation [91,92]. The net result is the inhibition of the Cdc2/cyclin B complex by DSB and the accumulation of the irradiated cells at G2 phase of the cell cycle. Cdc25c phosphorylation by Chk1/2 allows its binding to 14-3-3, which decreases its catalytic capabilities and results in its sequestration in the cytoplasm [93]. Importantly, 14-3-3σ is routinely found to be overexpressed in PC and has been directly associated with increased radioresistance [94,95].
Recently, we have identified the small GTPase Rac1 as one such key factor that regulates the activation of the checkpoint in response to ionizing radiation [96,97]. Rac1 is a member of the Rho family of small GTPases (guanosine triphosphatases) [98]. It is isoprenylated at the C-terminus and this isoprenyl group is thought to be responsible for recruitment of Rac1 to membranes, both plasma and internal membranes. Like other small GTPases, Rac1 exists in either as an active GTP-bound form or inactive GDP-bound state. In its active form, Rac1 associates with and activates its downstream targets, including the p21-activated kinases 1-3 (PAK1-3). In breast cancer cells, we have discovered that Rac1 is activated within 15 minutes following IR [96]. In both breast and PC cells, pre-incubation of the cells with Rac1 inhibitor NSC23766 [99] abrogated activation of the G2/M checkpoint in response to IR. Similar outcomes were observed in cancer cells introduced with Rac1 siRNAs or infected with a dominant-negative mutant of Rac1 (Rac1-T17N) [96]. Interestingly, Rac1 inhibition also prevented activation of the ATM/Chk2 and ATR/Chk1 pathways in response to IR, as indicated by IP-kinase assays. Even more significantly, NSC23766 also sensitized the breast and PC cells to the effects of radiation [96,97]. While non-toxic to the unirradiated cells, NSC23766 synergized with radiation to drastically reduce the viability and clonogenicity of the cells.
5. Apoptosis
Some of the most commonly observed genetic dysregulations seen in aggressive cancer cells occur in apoptotic signaling pathways, that limit the effects of anticancer therapies, including radiation, which often rely on these pathways, to induce cell death [16,100–103]. Enhancing apoptotic cell death to improve therapeutic efficacy, can be accomplished in two ways: increasing pro-apoptotic genes and the pathways that control them or decreasing apoptotic inhibitors and their upstream pathways.
NF-κB, whose activity is often enhanced in response to radiation, has been shown to be constitutively activated in various cancers including PC, colon cancer, breast cancer, T-cell leukemia, lymphoma, and melanoma [104–107]. NF-κB is a major promoter of cell survival through the overexpression of its downstream targets (i.e. Bcl-XL, and BCL-2) which inhibit apoptosis. NF-κB can be activated by ATM [27] as well as by the p53-inducible death domain-containing protein (PIDD) [108] in response to DNA damage. Inhibition of NF-κB, by treatment with such molecules as genistein and indole-3-carbinol among others, has been shown to improve the efficacy of radiotherapy in various cancers [105,109–120]. While the exact mechanism(s) by which the inhibition of NF-κB improves treatment-associated cell death is not known, it has been suggested that it comes as a result of a greater number of cells undergoing apoptosis.
Bcl-2 family members are the master regulators of apoptosis and include pro-apoptotic (e.g. PUMA), anti-apoptotic (e.g. Bcl-2), and pore-forming effectors (BAX and BAK) and contain a BH3 domain (Bcl2 homology -3 domain). Bcl-2 was the first member discovered and is often considered an oncogene based on its anti-apoptotic function and overexpression in cancer [121]. On the other hand, inhibitors of apoptosis proteins (IAPs) are also often overexpressed in cancer, essentially acting as an apoptotic “brake” and thus greatly reducing the efficacy of treatments such as radiation [104,113,122–126]. Numerous IAPs, such as cIAP2, XIAP, and Survivin are commonly found to be upregulated in PC [127]. Of note, based on the presence of one to three baculovirus IAP repeat (BIR) and caspase activation recruitment (CARD) domain, IAPs can be divided into three classes: Class1 (XIAP, cIAP-1, cIAP-2, ML-IAP and ILP2); Class-2 (NAIP); and Class-3 (SURVIVIN and BRUCE) [125]. The importance of IAP’s apoptotic inhibition in radiation sensitivity is highlighted by the fact that chondrosarcomas, which overexpress BCL-2, BCL-XL, and XIAP, show significant radioresistance in vitro. Additionally, expression of IAPs increases with radiation treatment, while knockdown of IAPs by siRNA greatly increases radiosensitivity in a synergistic manner with radiation [128]. It is important to remember, however, that the pathways in which these proteins are involved are greatly interwoven with one another such that changing the expression of one molecule may not result in a change in overall apoptosis, as another pathway/molecule may be able to compensate. Therefore, many proteins are involved in the ultimate decision of cell survival or cell death [129].
The effects of IAPs are countered by IAP-antagonists including the second mitochondrial-derived activator of caspases (Smac) [126,130]. These molecules are activated during TNFα-dependent or TRAIL-induced apoptosis [131–137]. Synthetic mimetics of Smac have been shown to sensitize cancer cells, including those of breast cancer, multiple myeloma, glioblastoma, and ovarian cancer, to induced apoptotic cell death [131–133,137,138]. However, simply upregulating Smac may not be enough to enhance treatment efficacy as a recent study indicated that some IAPs inhibit IAP antagonists instead of directly inhibiting caspases [139].
Likewise, the role of p53 in apoptosis has been linked to radiation resistance. While many p53 mutations have been shown to affect cell cycle check point control, others have no effect on the cell cycle but instead solely affect the cell’s ability to undergo apoptosis [140]. Importantly, the majority of p53 mutations, which occur in approximately 50% of all human cancers, are point mutations in the DNA-binding domain, resulting in the overproduction of p53 protein that is unable to activate the Bax or PUMA promoter, key apoptosis inducers [72,140–143].
Another molecule that has been implicated in apoptosis is the signal transducer and activator of transcription 1 (STAT1). STAT1 is a transcription factor downstream of the interferon signaling pathway that has been shown to be overexpressed in various other malignancies such as head and neck cancer, renal cell carcinoma, and squamous cell carcinoma [144–146]. STAT1 is activated in response to IR, and this activation likely contributes to radioresistance [147,148]. While in some instances STAT1 can increase apoptosis through the activation of pro-apoptotic genes and suppression of anti-apoptotic proteins produced by the NF-κB pathway, its constitutive overexpression has also been shown to promote cell survival likely via the modulation of pathways activated by ATM, including Chk2 and NBS1 [148–150]. This overexpression of STAT1, which is often seen in cancers, is likely linked to their observed radioresistance as knockdown of STAT1 has been shown to increase radiosensitivity in the cells of squamous cell carcinoma [148] and in renal cell carcinoma [145]. Researchers have suggested that this radiosensitization is a result of the modulation of IL-6 and IL-8 as the expression of these cytokines greatly diminishes following the downregulation of STAT1 [144]. It is believed that these cytokines may act as prosurvival signals essentially saving the tumor cells from apoptosis. Additionally, IL-1, TNFα, VEGF, HGF, and IGF-1 have been found to be radioprotective in a variety of cancers, mostly thought to be due to their activations of the NF-κB or PI3K pathways [72].
Insulin-like growth factor 1 receptor (IGF1R) signaling, which promotes ATM stability, has been shown to be involved in cellular transformation, growth, motility, and apoptotic protection. IGF1R is overexpressed in radiation resistant melanoma and breast cancer cells and blocking its expression results in decreased cell proliferation and increased radiosensitivity. It is theorized that this radiomodulating effect is primarily due to the IGF1R’s anti-apoptotic activities [151]. Genetic blockade of IGF1R by expression of a truncated, dominant negative-IGF1R increased radiation-induced apoptosis in BxPC3 PC cells [152].
Cell stress, often seen in cancer due to hypoxia, acidic microenvironments, or suboptimal protein turnover, can cause activation of the unfolded protein response (UPR) which, without cellular adaptation, can lead to apoptosis. However, adaptation can, and often does, occur; resulting in up or down-regulation of specific UPR pathways, ultimately leading to an upregulation of GRP78/BiP chaperones, which inhibit apoptosis and cause overall cytotoxic resistance [153]. Interestingly, UPR also leads to a significant increase in NF-κB activity, which further inhibits apoptosis [154].
In PC specifically, it has also been shown that regenerating gene 4 (REG4 ) acts as anti-apoptotic factor via the AKT pathway and its expression results in decreased radiosensitivity. Briefly, REG4 is a C-type lectin involved in the regulation of proliferation and differentiation of liver, pancreas, gastric and intestinal cells, discussed in detail in a recent review article [155]. The effect of REG4 on gemcitabine resistance, however, is modest, indicating that chemo-and radio-sensitivity may not fully coincide phenotypically or mechanistically. Importantly, REG4 is particularly promising as a predictor of PC radiosensitivity as it is found to be in higher levels in the sera of PC patients, with increased levels correlating with increased incidence of local recurrence after radiotherapy [156].
Of great importance in the context of the role of apoptosis dysregulation in radioresistance, however, is the understanding that radiosensitivity cannot be predicted solely depending on apoptosis or the intactness of apoptotic pathways. Studies that have used apoptosis as their sole benchmark for radiosensitivity have shown high rates of both false positive and false negative results. Additionally, as seen across multiple cancers, screening for apoptotic pathway mutations and apoptotic induction post-RT has shown limited predictive capabilities with regard to patient radioresponse and treatment outcome [72].
6. Autophagy
Autophagy, literally meaning ‘to eat oneself’, is an evolutionarily conserved mechanism that results in the large-scale lysosomal degradation of cellular contents including proteins, macromolecules, ribosomes and organelles. The role of autophagy in cancer cells can be both pro-survival and pro-death depending on the circumstances. In some instances, defective autophagy has been associated with malignant transformation of a normal cell and oncogenesis, therefore appearing to play a tumor suppressive role. In addition, in some cancer cells, as well as most normal cells, autophagy protects cells against cellular stress, thereby giving cells a chance to repair themselves instead of being automatically destined for apoptotic death upon damage [157–159]. Currently, the role of autophagy in radioresistance is both complicated and unclear, though many studies are underway to investigate its role in several types of cancer.
Autophagy, like apoptosis can also be a type of programmed cell death. In cells in which apoptosis is defective or repressed, often resulting in radiation resistance, autophagy provides another mechanism by which cell death can occur. Unfortunately, key autophagic proteins are also often underexpressed in cancers. Beclin1 (BECN1), an autophagy-related protein, is often deleted or underexpressed in cancers including prostate, breast, ovarian, colon, brain tumors, hepatocellular carcinomas and cervical cancers [160–168], suggesting that BECN1 along with its corresponding autophagic role may be repressed in some cancer cells [157]. Targeting of proteins involved in autophagy could therefore potentially offer therapeutic benefit in radiation-resistant cancer cells.
Previously, several research groups have demonstrated that inhibition of protein kinase C delta (PKCδ), Bcl-2, or tissue transglutaminase 2 (TGM2) in PC cells triggers autophagy-mediated cell death [157,169,170]. Another study found that IGF-II stimulation in combination with ionizing radiation increases cell death in pancreatic (AsPC-1, Panc-1) and breast (MCF-7) cancer cells under hypoxic conditions, most likely due to autophagy [171]. This effect, however, was not observed when cells were exposed to ionizing radiation alone. Importantly, IGF-II stimulation enhanced cell death only under hypoxic conditions, while under normoxic conditions promoted cell survival, underscoring the complexity of autophagy as both a pro-survival and pro-death mechanism, depending on both the type of cell and microenvironment. This is especially intriguing, as PC cells are radioresistant possibly due to inhibition of apoptosis. Activation of the pro-death function of autophagy could potentially be exploited in PC as an alternative means of inducing cell death in irradiated PC cells that may have lost the ability to undergo apoptosis.
However, autophagy can also act as a prosurvival mechanism in cancer cells, helping them withstand their highly hypoxic and acidic tumor microenvironments, ER stress, and therapy-induced cell stress and damage, including those caused by radiation. All these mechanisms and biological process associated with autophagy were discussed in detail in recent reviews articles [157,172–174]. Resistance to radiation has been hypothesized to be a result of the induction of autophagy leading to the sequestration of mitochondria damaged by the radiation; that protects the cells against apoptotic cell death. For example, in one study overexpression of FKBP51, a regulator of NF-κB, was associated with lower levels of apoptosis and higher levels of autophagy in melanoma cells after radiation treatment [175]. Further, it has been shown that incubation of PC cell lines with chloroquine leads to a post-irradiation cell death, which may at least in part be due to the inhibition of autophagy by chloroquine [176]. These studies suggest that, in at least some cases, autophagy acts as a prosurvival mechanism in response to radiation [175].
7. Genetic alterations affecting radioresponse of pancreatic cancer
In total, an average of 63 mutations in a core set of 12 biochemical pathways have been detected in high-throughput sequencing of PC specimens [177]. These mutations are in systems that control the response of the cells to the microenvironment, extracellular stimuli, intracellular signals, and cellular stresses. These mutations significantly alter the expression and activation of downstream molecules in their respective biochemical pathways, creating a cascading effect that serves to inhibit cell death and promote survival, repair, and proliferation via multiple mechanisms.
The earliest and most common mutations detected in PC occur at the 12th codon of the Kras gene, at which the naturally occurring glycine is commonly replaced with either an aspartic acid or valine. This mutation results in constitutive activation of Kras and continuous activation of its downstream effectors, most notably the PI3K/AKT pathway, Raf/MAPK/ERK pathway, and Rac1 GTPase [178,179]. Kras has been shown to contribute to chemotherapy resistance and radioresistance of PC. Treatment with Kras-targeted siRNA resulted in radiation sensitization in vitro and in xenografts of PC cell lines with oncogenic Kras mutations (Panc-1, ASPC-1, Capan-2, MiaPaCa-2, and PSN-1) [180]. ERK activation leads to increased proliferation and survival, as well as increased transcription of SHH and anti-apoptotic RSK (RPS6KA) protein [178]. AKT activation leads to increased VEGF, increased angiogenesis via NOS3, and activation of the NF-κB pathway, as well as inhibition of p53-mediated transcription, apoptosis, and autophagy.
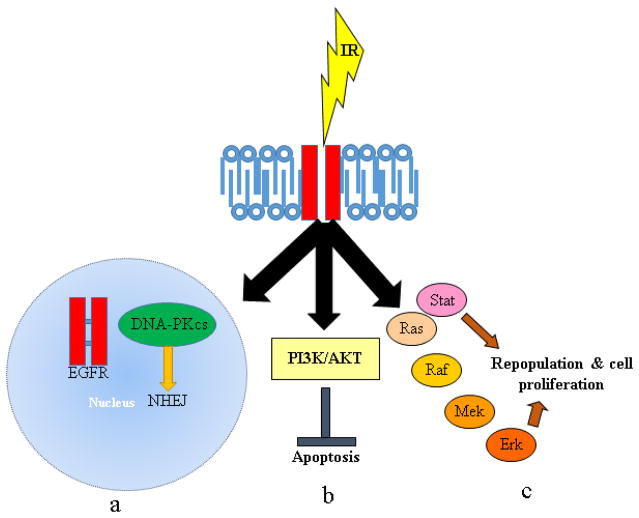
Among the many genetic alterations detected in PC, many contribute to inactivation of the G1/S cell cycle checkpoint. Among these genetic alterations are the loss of p53 and of p16, found in approximately 50% and 98% of PCs respectively [178,179,181]. Kras activation further contributes to the inactivation of this checkpoint by promoting the expression of D-type cyclins. The net effects are the absence of a G1/S cell cycle arrest in response to IR in PC cells. This lack of a G1/S response makes these cells more susceptible to abrogators of the G2/M checkpoint, as they are now more reliant on the activity of this checkpoint to promote cell cycle arrest and prevent mitotic catastrophe. But the loss of p53 in PC cells is a double-edge sword, as it can also contribute to decreased susceptibility of PC cells to apoptosis. The role of p53 in cell cycle arrest and DNA repair, likely through its induction of p21 and GADD45, has been linked to radiation sensitivity and resistance in various cancers. For example, overexpression of Bcr-Abl, the oncogenic form of c-Abl’s nuclear tyrosine kinase gene product implicated in the activation of p53, has been linked to radioresistance in growth factor-dependent lymphoma cells, likely a result of a prolonged G2/M cell cycle block that provides extra time for cells to repair DNA after radiation [72,182,183]. In a similar manner, loss of the G2/M checkpoint, which is partially controlled by p53, resulted in radiosensitization of lymphoma, carcinoma, and breast cancer cells. This observation is thought to occur because cells with DNA damage enter mitosis before they had a chance to repair the damage, thereby resulting in chromosome loss, mitotic catastrophe, and cell death [72,184,185]. Another pathway that shows frequent alterations in PC is the overexpression of the epidermal growth factor receptor (EGFR). This molecule is often overexpressed in carcinomas, including those of the lungs, colon, and breasts [186–188]. Studies have indicated that, in response to certain stimuli like radiation, EGFR translocates to the nucleus where it interacts with DNA-dependent protein kinase catalytic subunit (DNA-PKcs), a kinase implicated in the repair of DSBs through NHEJ [20,189–201]. This mobilization of EGFR can also inhibit apoptosis through activation of the PI3K/AKT pathway as well as provide a survival advantage through repopulation and tumor progression as a result of the activation of the Ras/Raf/Mek/ERK and STAT signaling pathways [20,202–208]. Multiple pathways and mechanisms through which EGFR activation imparts radioresistance in cancer cells is depicted in Figure 3.
Figure 3. EGFR-mediated responses to radiation.
Three distinct mechanisms contribute to the EGFR-mediated radioresistance in cancer cells (a) In response to irradiation, EGFR activity is induced in a ligand-independent manner which in turn leads to activation of downstream signaling which is primarily involved in the DNA repair mechanism. The putative nuclear localization sequence (NLS) present in the EGFR can be recognized by karyopherin further leading to nuclear import. In the nucleus, EGFR physically interacts with the catalytic subunit of DNA-dependent protein kinase (DNA-PKCs) to regulate the non-homologous end joining DNA repair pathway. (b) As a consequence of IR, EGFR autophosphorylates and activates its corresponding downstream signaling cascades, such as PI3K/AKT and RAS/RAF/ERK pathways to regulate survival signaling (indirectly inhibiting apoptosis) and directly promoting proliferation of irradiated cells. (c) The phosphorylated tyrosine residues in EGFR can also function as an adaptor molecule for the JAK/STAT pathway.
A mutation in the tyrosine kinase domain of EGFR in non-small cell lung carcinoma patients resulted in the failure of EGFR to enter into the nucleus and bind to DNA-PKcs, resulting in a decreased capacity to repair DSBs that strongly correlated with enhanced radiosensitivity [209,210]. Furthermore, studies have shown that cells treated with cetuximab, an anti-EGFR monoclonal antibody, have greatly diminished DNA repair and survival, likely as a result of the sequestration of EGFR in the cytoplasm and its inability to interact with DNA-PKcs [200]. Cetuximab has been shown to effectively sensitize cells to the effects of IR in several cancers, including head and neck squamous cell carcinoma, colorectal carcinoma, and non-small cell lung cancers [211–217], further suggesting the potential role of EGFR in the radiosensitization of cancer cells.
A third pathway often mutated in PC that has significant potential to enhance PC radioresistance is the Sonic hedgehog/Indian hedgehog (SHH/IHH) pathway. It is estimated that the rate of mutations in this pathway approaches 100% in PC, with 70% of PC tumors expressing 35-fold higher levels of SHH and IHH on an average [179,181]. Additionally, smoothened (SMO) is also found to be frequently overexpressed in PC [181]. Further, the SHH pathway results in activation of GLI and BMP2 along with increased IGF2 expression. Activated GLI serves to increased expression of VEGF, which promotes angiogenesis as well as increased activation of the AKT and NF-κB pathways. BMP2 activation increases PI3K activity and transcription of IGF-1. Increased levels of IGF-1 and IGF-2 interact with and activate IGFRs which, along with EGFRs, are overexpressed in 20–64% of PC tumors [178,179]. Activation of these receptors increases the activity of the PI3K, NF-κB, AKT, and JNK pathways along with stimulation of angiogenesis, generation of reactive oxygen species, and enhanced NO production via NOS2 and NOS3 activation. Increased NF-κB activity leads to increased apoptosis inhibition via increased transcription of XIAP, cIAP2, TNFAIP3 Interacting Protein 2 (TNIP2), and Bcl-2 [218]. Conversely, activation of the JNK pathway is pro-apoptotic, as it can lead to Bcl-2 and 14–3–3 inhibition, though these functions are likely appreciably tempered by the anti-apoptotic effects of the AKT pathway.
Upregulation of the cholecystokinin (CCK) B and gastrin receptor (CCKBR) is also commonly found in PC along with an increase in overall gastrin expression, resulting in increased activation of the CCKBR pathway [179]. This activation serves primarily to activate the PI3K pathway, further solidifying PI3K and its downstream pathways as a predominant mechanism for PC survival to cytotoxic stress. As multiple upstream pathways activate PI3K, inhibition of individual upstream molecules, such as EGFR, IGFR, and even Kras, may not deactivate PI3K due to compensatory effects.
Aberrant activation of the Wnt/β-catenin pathway is also found in approximately 65% of PC tumors [179,181] further contributing to redundancy in the anti-apoptotic mechanisms present in PC. TGFβ signaling is also affected by mutations commonly found in PC, namely by mutations in TGFBR1, TGFBR2, and SMAD4 occurring in 4%, 1%, and 50% of PCs respectively [181]. While these mutations result in decreased TGFβ-mediated growth inhibition, they also have the potential to exert pro-apoptotic effects by causing decreased Bcl-2 levels. These pro-death effects, however, are likely compensated for directly by NF-κB mediated upregulation of Bcl-2 and indirectly by AKT mediated activation of 14-3-3 and inhibition of BAD. To obtain a global perspective of the genes modulated in response to radiation across the cancers we have listed a few metabolic and non-metabolic genes differentially expressed and associated with radioresistance of pancreatic and other cancers in Table 3.
Table 3.
Radiation responsive differential gene expression profile and their associated signaling pathways in multiple cancers.
| Cancer type | Cell lines | Experimental method | Genes modulated | Associated Pathways and biological functions | References |
|---|---|---|---|---|---|
| Pancreatic cancer | Panc-1 and BxPc3 | cDNA micro array | FDPS, ACAT2, AG2, CLDN7, DHCR7, ELFN2, FASN, SC4MOL, SIX6, SLC12A2, and SQLE. | Cholesterol biosynthesis pathway | [311] |
| PK-1, PK-8, PK-9, T3M4, and MiaPaCa2 | cDNA micro array | AREG, MAPKAPK2, RGN, ANG-2, caspases-8, LRAT and CLCA-1. | Growth factor, cell cycle check point, angiogenesis, apoptosis, retinoid cycle, electron transport. | [370] | |
| Nasopharyngeal cancer | CNE2 | Proteomics | GRP78, Mn- SOD, 14-3-3σ, Maspin. | Cell survival, scavenging reactive oxygen species, Cell cycle, cell growth, angiogenesis, invasion, metastasis. | [371] |
| Esophageal Cancer | TE-2R, TE-9R, TE-13R, and KYSE-170R | cDNA micro array | ICEBERG, BIRC2, COX-2, CD73, PLAU, CYP1A1, GRP58, and ERP70, PRKCZ, ROR2,SGK, CASP6, CDH1, CDH3, PCDH9, MLL3, PRKCBP1, CDK6 and CCNA2. | Inflammation, apoptosis, DNA metabolism, cell growth, electron transport chain, apoptosis, cell adhesion, transcription and cell cycle. | [372] |
| Lung cancer | Anip973 vs Anip973R | cDNA micro array | DDB2, LOX, CDH2, CRYAB, GBP-1, CD83, TNNC1. | DNA damage repair, extracellular matrix, cell adhesion, apoptosis, angiogenesis, immune response, calcium signaling | [373] |
| A549 vs A549R | RNA Seq | SESN2, FN1, TRAF4, CDKN1A, COX-2, DDB2 and FDXR. | Epithelial to mesenchymal transition, migration and inflammatory response. | [374] | |
| Prostate cancer | PC3 vs LNCaP | RNAseq | BRCA1, RAD51, FANCG, MCM7, CDC6 and ORC1. | DNA repair and replication. | [375] |
| PC3 | cDNA micro array | CCDC88A, ROCK1, NEXN, FN1, MYH10 and MYH9 | Cell cycle and Cell motility | [376] | |
| Prostate cancer and colon cancer | PC3 vs Caco-2 | RT-PCR | CCDC88A, FN1, MYH9 and ROCK1 | Cell motility | [377] |
Note: Bold letters represents differentially upregulated genes and their associated biological pathways whereas, un-bold letters represents down regulated genes and their corresponding pathways.
8. Previously tested radiosensitizers in pancreatic cancer
With the significant complexity that the aforementioned genetic alterations provide, research toward clinically useful radiosensitizers in PC has been slow and challenging. To date, the primary focus of radiosenisitization research has been on identifyimg molecular target (s) and methodologie (s) to enhance the sensitivity of cancer cells to radiation as compared to normal cells. These methods, though, designed to prevent unacceptable increases in toxicity while improving therapeutic efficacy, significantly restrict the available avenues by which radiosensitization can be pursued. Additionally, with the dawn of stereotactic irradiation, it is arguable that such criteria may no longer be necessary as significant radiation doses can be avoided altogether for the vast majority of the healthy tissue in the tumor’s vicinity. However, despite advancements in both radiation methods and knowledge about the effects of IR on PC at both cellular and tissue levels, no specific radiosensitizers currently exist for PC.
Instead, radiosensitization of PC is currently accomplished by the use of concurrent chemotherapy, often with either 5-FU or gemcitabine. While chemoradiotherapy (CRT) was initially developed as an attempt to prevent/eliminate systemic metastatic growth during localized radiotherapy, it was found that chemotherapeutics and IR could have a synergistic effect on local tumor control [16]. This synergy, however, is inconsistent among different chemotherapeutics, even among the same class of drugs, and their underlying mechanisms are often unclear. This scenario applies in particular to the case of 5-FU and gemcitabine, in which modest and significant synergy with IR is found, respectively [16]. The reasons for this difference, despite both drugs being antimetabolites with similar mechanisms of action, remain unknown, and as such cannot be exploited for future improvement of radiosensitization efficacy. One notable exception to this is that gemcitabine has been found to inhibit ribonucleotide reductase (RNR) while 5-FU does not, the potential importance of which may be linked to the role of RNR in DNA repair [219]. Additionally, CRT with gemcitabine has shown significant increases in treatment toxicity, thus limiting its universal clinical use [16].
Several other methods for radiosensitization have been attempted for PC and other cancers. While a majority of approaches showed promise in both in vitro and in vivo preclinical studies, most ultimately failed to provide significant radiosensitization in clinical studies. However, identifying and understanding the underlying mechanisms that contributed to the failure of these methods, may provide greater insights for the development of successful radiosensitization strategies.
One of the first methods examined for potential radiosensitization was through treatment with halogenated pyrimidines. These nucleoside analogs, consisting of deoxycytidine, deoxyuridine or thymidine with a methyl group replaced by Cl, Br, or I, are incorporated into the DNA of a dividing cell, thus weakening it and increasing the amount of DNA damage occurring from a given dose of IR [16]. Through this mechanism, these halogenated pyrimidines have differential affinity for cancer cells due to their rapid proliferation rates. To significantly enhance the DNA damaging effect of radiation via these methods, however, many halogenated pyrimidines must be incorporated into each DNA strand, thus necessitating the presence of the altered pyrimidines at the tumor for multiple cell generations. Additionally, systemic treatment with halogenated pyrimidines is precluded by the ability of the liver to rapidly dehalogenate molecules, thereby necessitating delivery directly to the tumoral arterial blood supply [16]. While this is a significant hindrance to the utility of halogenated pyrimidines in the majority of cancers, it is particularly challenging in PC as the tumor is located deep in the abdomen, making access to the tumor both difficult and invasive. Further, hypovascular nature and high interstitial pressure encountered in PC make long-term intra-arterial infusions impractical.
The importance of tumor hypoxia in radioresistance has long been recognized and has provided an additional avenue in the pursuit of tumor radiosensitization. Initially, the majority of studies examining the ability of tumor oxygen status to allow for radiosensitization focused on increasing tumor oxygenation. Attempts at accomplishing this, however, either through subjecting the patient to hyperbaric oxygen or through a blood transfusion before RT, have met with limited success in most cancers and been largely unsuccessful in PC [16]. The likely cause for this failure is that low tumor oxygenation, especially that of PC, is often an artifact of inadequate and imperfect tumor vasculature, extensive desmoplasia, and rapid tumor growth, not anemia or poor blood-oxygen saturation. As such, the efficiency of such methods to practically increase tumor oxygenation is minimal.
Alternatively, methods seeking replacement of the radioenhancing effects of oxygen with other molecules that are more readily absorbed/concentrated in hypoxic tissues has also been attempted. These oxygen substitutes, primarily nitroimidazoles and related compounds, have exhibited some degree of clinical success for radiosensitization, though their actual utility in a therapeutic setting remains unclear [16,220]. These compounds enter the cytoplasm through the plasma membrane and chemically produce the same radical-stabilizing and DNA damage-fixing effects that oxygen allows. However, unlike oxygen, nitroimidazoles are metabolized slowly, allowing them to diffuse more than 200 μm away from capillaries [16,220]. As such, a deep penetration into the hypoxic portions of tumors is allowed, theoretically leading to great improvement in the ability of these oxygen substitutes to radiosensitize hypoxic tumors. One of the first oxygen substitutes to be tested in depth was misonidazole, which showed significant radiosensitizing effects in vitro where 10mM treatment allowed almost equal IR-induced cell killing in hypoxic and normoxic cells. In clinical trials, however, pre/co-treatment with misonidazole and IR failed to show any improvement in treatment response as compared to IR alone except for one trial in which a modest improvement was found in a small subset of patients with pharyngeal cancer [16]. This failure may be explained by the dose-limiting neurotoxicity (both peripheral and central) of the drug that prevented administration of doses at which optimal plasma concentration could be achieved. To address these problems of dose limitation, a similar compound, etanidazole, was also tested in clinical trials as an oxygen substitute radiosensitizer. In vitro etanidazole was found to be as potent radiosensitizer as misonidazole, but with a shorter half-life and lower partition coefficient in vivo, preventing it from crossing the blood-brain barrier and limiting its ability to penetrate peripheral nerves and thus reducing its neurotoxicity. These alterations allowed etanidazole to be administered at much greater dosage, but in clinical testing, it still failed to show improvement in RT efficacy and these aspects have been reviewed in detail elsewhere [16]. Interestingly, another nitroimidazole, nimorazole, that was shown to have decreased in vitro radiosensitizing efficiency as compared to the other tested nitroimidazoles, but also a significantly reduced toxicity profile, has provided more promising clinical results. Nimorazole significantly enhanced both local control and survival in irradiated patients with head and neck cancer, but to date has not been tested in cancers of other sites [16]. Another nitroimidazole, doranidazole (PR-350) has been tested in PC cells in vitro and in vivo [221–224], and has been evaluated in a Phase III trial (n = 46 patients) in combination with intraoperative radiotherapy for locally advanced PC in a Japanese cohort [225,226]. No difference in one-year survival was seen [226], but 3-year survival was significantly increased with the combination [225].
These failures, and limited successes, illustrate several important points with regard to radioresistance and radiosensitization in PC, as well as in cancer in general. First, and perhaps most importantly, results from the in vitro and limited in vivo testing available in radiation research are poor indicators of clinical efficacy of radiosensitizers. Additional factors beyond those of just the cancerous cells must be considered for all potential radiosensitizers, such as practicality of implementation, metabolism, the interplay of the tumor microenvironment and surrounding tissues with the sensitizer’s penetrative abilities into the tumor, and the toxicity that such a sensitizer will elicit. Unfortunately, most of these issues cannot currently be addressed using models available to bench/animal researchers and thus only come to light upon clinical testing, but can simultaneously subject the patients to harm through unforeseen toxicities, as well as cause significant expense for an ultimately doomed treatment. These difficulties highlight the need for novel research models to better evaluate potential radiosensitizers prior to implementation in clinical trials. Opportunities for such a model may exist through the use of in situ irradiation experiments using transgenic murine models, and thus development of irradiation models of this nature, though undoubtedly difficult, should be pursued as rapidly as possible.
9. Promising molecular targeting agents as radiosensitizers in pancreatic cancer therapy
Despite the lack of past success in the development of PC radiosensitizers, multiple novel add-on pharmaceuticals showing promising enhancement of PC radioresponse are currently at various stages of testing, both clinical and preclinical [227]. Unfortunately, no new preclinical techniques have been developed with which to test the efficacy of these potential radiotherapeutic modulators, resulting in reliance on evidence for warranting clinical testing that has previously been shown to poorly translate bench results to clinical worth. However, while most of these novel potential radiosensitizers will likely fail, their breadth and number provide the greatest hope to date that a clinically viable and practical PC radiosensitizer will be found.
Of the potential radiosensitizing targets currently under study, the most extensively tested is modulation of EGFR. EGFR inhibition is currently an FDA-approved treatment for PC, though it provides only negligible benefit in the form of a 2-week improvement in overall survival time [228]. Currently, focus is shifting from its use as monotherapy to an adjunct treatment to improve efficacy of other therapeutic modalities. For the purposes of PC radiosensitization, EGFR inhibition has been evaluated using both anti-EGFR monoclonal antibody cetuximab or small molecule inhibitors erlotinib and gefitinib. The results of these studies have been generally positive in the preclinical setting, though with notable exceptions. In at least one preclinical study, cetuximab was shown to elicit no beneficial additive or synergistic effects with IR [179]. Another, more recent study suggested that erlotinib only radiosensitizes PC cell lines containing wild-type Kras [229]. These studies are in concordant with an earlierstudy showing that both erlotinib and cetuximab have the capacity to increase cell death both in vitro and in vivo in irradiated BxPC3 cells, which express wild-type Kras [230]. A complication to the interpretation of this study, however, is the fact that all experiments with positive results utilized concurrent treatment with gemcitabine as well, potentiating that the increase in cytotoxicity after EGFR inhibition was in fact due to chemosensitization and not an increase in IR efficacy. The probability of this being the case is increased when it is considered that erlotinib plus gemcitabine results in increased cytotoxicity over gemcitabine alone while erlotinib plus IR lead to no enhancement in cell death [230]. Many other studies, however, have demonstrated positive radiosensitizing effects of EGFR inhibition in PC, both concurrent with and independent of treatment with chemotherapeutics, through multiple mechanisms including enhancement of the G1 checkpoint, release of apoptotic inhibition, and anti-angiogenesis [228].
Despite the conflicts in preclinical results, EGFR inhibition in the context of radiotherapy has been, and continues to be, the subject of many clinical trials, the majority of which are well summarized by Chang and Saif in their 2009 review [228]. Unsurprisingly, as with the preclinical studies, phase II trials testing EGFR inhibition as a method for radiosensitization have yielded conflicting results leading to confusion regarding the utility and future design of anti-EGFR therapies in the context of improving radiosensitization of PC. To date, some of the most encouraging outcomes for PC have been obtained from clinical trial testing EGFR inhibition in conjunction with chemoradiotherapy. The trial involved pretreatment with cetuximab (400 mg/m2 dl) prior to IR followed by concurrent cetuximab administration (250 mg/m2 dl) with chemoradiation (54Gy IR + gemcitabine (1,000 mg/m2/week for 4 weeks)) [231]. In this trial, which is also to date the largest such trial conducted, comprising 66 locally advanced PC patients, the 1-year survival was 61% and the 2-year survival was 20% with a median survival of 15 months, which is a significant improvement over the historical median survival time for locally advanced PC patients undergoing CRT of 11 months [228,231]. Another trial using erlotinib and gemcitabine concurrent with IR showed a median survival of almost 19 months in 20 locally advanced PC patients [232]. Other trials, however, have not been so favorable. Maurel et al., achieved a median survival of 7.5 months using gefitinib with gemcitabine and IR [233]. In the three treatment arms described by Demols et al., in another trial, median survival ranged from 3.7 to 10.7 months, indicating a treatment disadvantage despite 45% of the patients showing at least a partial response to various treatment regimens [234]. Although the majority of patients in these clinical trials were able to complete treatment, toxicity appeared to be a significant concern, with the majority of trials showing Grade 3–4 GI toxicity occurring in 15–60% of patients [228]. Additionally, dose-limiting toxicities were observed in 0–60% of patients [228,235,236]. Results were recently reported from the international Phase III LAP 07 study comparing capecitabine-based chemoradiation with gemcitabine-based therapy for non-progressive locally advanced PC, following four months of gemcitabine-based therapy with or without erlotinib. The results showed that standard chemoradiation improves local control but not overall survival, and there was no change in the overall survival with gemcitabine compared to gemcitabine plus erlotinib used as maintenance settings [237]. Treatment with erlotinib concurrent with RT and various chemotherapeutics is the subject of a single Phase III clinical trial (RTOG-0848) that is currently recruiting patients. This study will specifically evaluate the effect of gemcitabine +/− erlotinib followed by gemcitabine +/− erlotinib +/− IR + 5-FU/capecitabine. The results of this trial will allow the community to determine the true clinical applicability of EGFR inhibition, at least with erlotinib, in the context of CRT in PC. Interestingly, multiple studies have shown the preclinical and clinical efficacy of second generation pan-EGFR tyrosine kinase inhibitors such as afatinib and dacomitinib in breast cancer, non-small cell lung cancer, glioblastoma, head and neck cancer, and PC [238–240]. This class of TKI’s exhibit irreversible binding to the ATP binding pocket of the tyrosine kinase domain of all the EGFR family members thereby blocking the tyrosine kinase activity. In 2013, the USFDA approved afatinib as a first-line therapy for patients with metastatic NSCLC [241]. Recently, afatinib has been reported to radiosensitize PC cells by inhibiting the EGFR family member-mediated AKT phosphorylation and causing mitotic catastrophe-mediated cell death. However, unlike previous studies with erlotinib and cetuximab [230], afatinib showed similar radiosensitization potential in both Kras wild-type and Kras mutant harboring PC cells [242].
Beyond EGFR modulation, the agent that has been evaluated for PC radiosensitization is nelfinavir, a HIV protease inhibitor with potentially beneficial off-target effects. While designed for action against HIV proteases, nelfinavir, like most protease inhibitors, is promiscuous, decreasing human proteosome activities as well [243]. The resulting increase in misfolded proteins has been found to be sufficient to lead to an unfolded protein response (UPR) within the cell, causing many downstream events including phosphorylation of eIF2α. This phosphorylation acts as a signal for the cell to globally decrease protein synthesis as well as upregulate and activate GADD45 [243]. Activated GADD45 interacts with and activates the phosphatase PP1, leading to dephosphorylation of AKT and ultimately to radiosensitization [229,243,244]. In vitro, this radiosensitizing effect has been found to increase IR-induced PC cell death by approximately 40% with even greater effects seen in vivo, postulated to be due to vascular alterations within the tumor. Nelfinavir has been evaluated in several clinical trials in multiple cancer types both as a monotherapy and as an adjunct agent. Two clinical trials, one published and one completing, have attempted to analyze the additive/synergistic effects of nelfinavir with IR in PC [245]. The first trial, a Phase-I study testing a combination of nelfinavir, gemcitabine (at 2 separate dose levels with equal patient distribution in each), and IR (50.4Gy with a boost to 59.4Gy) in 12 patients with locally advanced PC, showed encouraging results. Of the 10 patients who were able to complete the therapeutic regimen, 6 were downstaged to resectability. Further, the objective response rates were found to be 50% and 70% based on CT and PET assessment, respectively [245]. Importantly, with this regimen, no dose limiting toxicities were observed. The second trial, a Phase-I currently concluding at the University of Nebraska Medical Center (data not yet published) is showing a similarly mild toxicity profile for nelfinavir added to stereotactic respiratory gated CRT, though with relatively less treatment efficacy.
Another avenue for radiosensitizing PC currently undergoing clinical testing is the inhibition of histone deacetylases (HDACs). Preclinical studies using HDAC inhibitors (HDACIs) on PC and various other cancers have demonstrated the role of HDACs in response to and repair of DNA damage [246]. By increasing the amount of radiation-induced DNA damage and slowing DNA repair, along with modulation of p53, pRB, cell cycle regulators, as well as regulators of apoptosis and cell proliferation, HDACIs radiosensitize cancer cells. One preclinical study in PC specifically demonstrated that treatment with vorinostat, a hydroxamic acid HDACI, significantly reduces post-IR colony survival in several PC cell lines, leading to 40–60% enhancement in cell death post-IR as compared to cells treated with IR alone [247]. Interestingly, IR plus vorinostat induced no greater apoptosis than vorinostat alone (p=0.69). Consistent with previous studies in other cancers, mechanistically vorinostat was found to delay DNA repair post-IR (via inhibition of both NHEJ and HR) as well as inhibit constitutive and IR-induced NF-κB and EGFR signaling pathways, [247]. Paradoxically, studies have also demonstrated the ability of HDACIs to radioprotect healthy tissues, particularly cutaneous, intestinal, and bone marrow tissues, both in vitro and in vivo [246]. With this preclinical foundation, two clinical trials, one published and one with unknown status, have tested the effects of HDACIs with CRT in PC [248]. The first trial, a Phase-I study testing a combination of vorinostat (dose escalation up to 400 mg), capecitabine, and IR (30Gy in 10 fractions) in 21 patients with non-metastatic PC, showed the combination was well tolerated, although three patients experienced dose limiting toxicities, including two gastrointestinal toxicities and one thrombocytopenia. The Intergroup definition of borderline resectable PC was used, and 6 weeks elapsed from completion of RT to surgery. At the time of surgical resection, 90% of patients exhibited stable disease while 10% had progressive disease and the median survival was 1.1 years [248]. The second trial (NCT01333631), is currently analyzing the effects of the HDACI and antiepileptic drug valproic acid, in combination with chemoradiotherapy with gemcitabine in PC patients.
An additional mechanism that has been explored for clinical testing, though currently stalled, for PC radiosensitization involves inhibition of Ras. Even with the activating mutations of Ras common to PC, the ability of Ras to function is dependent on post-translational farnesylation or geranylgeranylation (together called prenylation) via a farnesyltransferase or geranylgeranyltransferase, respectively, to allow for attachment to the inner envelope of the plasma membrane [179]. As such, farnesyltransferase inhibitors (FTI) and geranylgeranyltransferase inhibitors (GGTI) have been used in combination with radiation. A phase II randomized trial of gemcitabine/paclitaxel/RT, followed by the FTI tipifarnib (R115777) for unresectable PC demonstrated that maintenance FTI failed to improve clinical outcome and was associated with increased toxicities [249]. It should be pointed out that it is well established that Kras and N-Ras become alternatively geranylgeranylated and remain fully functional in cells treated with FTIs [250–252]. Beyond FTIs, a dual farnesyltransferase and geranylgeranyltransferase inhibitor (L-778123) has undergone preclinical and ultimately Phase-I evaluation as an IR adjunct in the hopes that Ras inhibition would lead to significant decrease in PC radioresistance [179,253]. Despite encouraging results from initial studies, including demonstration of enhanced radiosensitivity in patient-derived cell lines, L-778123 has been abandoned due to unacceptable cardiotoxicity encountered in clinical trials. Currently, focus is centered on reducing the toxicity either through cardioprotective add-on pharmaceuticals or alterations to the drug structure. Many researchers, including the National Cancer Institute sponsored “RAS Initiative” collaborative project, are engaged to find specific Ras inhibitors.
In addition to these potential methods for PC radiosensitization currently undergoing clinical testing, multiple other radiosensitizers have shown significant promise but have not yet moved beyond preclinical experiments. For example, HIF-1α inhibition as a mechanism for radiosensitization has been the subject of multiple studies as its nuclear translocation, which occurs in the context of cellular hypoxia, promotes the expression of various target genes involved in cell survival promotion, inhibition of apoptosis, and antioxidant function [254–256] Significantly, nuclear HIF-1α is found in 88% of PC tumor samples and only 16% of samples from normal pancreas, illustrating both the hypoxia present in PC as well as the likelihood that HIF-1α inhibition, if found to be a clinically viable method for PC radiosensitization, will differentially act on PC cells and not surrounding healthy pancreas [256]. Inhibition of HIF-1α with the orally bioavailable small molecule inhibitor PX-478 resulted in enhanced radiosensitivity in hypoxic PC cells in vitro, both with and without concurrent treatment with 5-FU or gemcitabine, showing an approximate 40% increase in IR-associated cell death [256,257]. Interestingly, the radiosensitizing effects of PX-478 were found to be enhanced in vivo, where a tumor growth inhibition 97% greater than that found with IR alone was observed when mice were concurrently treated with it and 5 daily 1Gy fractions of IR, highlighting the importance of tumor oxygenation on PC radiosensitivity [256].
Small molecule inhibitors of MEK (PD0325901) and Akt (API-2) were evaluated for their radiosensitizing potential alone and in combination [258]. MEK inhibition induced radiosensitization in vitro and in vivo. The inclusion of the Akt inhibitor with the MEK inhibitor in conjunction with radiation regimen resulted in greater radiosensitization. It should be noted that increased phosphorylation of ERK were seen at time points between two to 24 hours after radiation, and phosphorylated-Akt levels were elevated at two and six hours after radiation, but returned to baseline levels at 24 hours. Interestingly, increased phosphorylated-Akt levels were observed with MEK inhibition alone.
Chk1 and Chk2 have received attention as potential targets for radiosensitization based on their roles in DNA damage response, as mentioned above. The Chk1/2 inhibitor AZD7762 radiosensitized MiaPaCa-2 cells, and prolonged the tumor volume doubling time in MiaPaCa-2 and patient-derived xenografts in combination with radiation and gemcitabine [259]. AZD7762 treatment abrogated the IR-induced G2 checkpoint and inhibited HR. Further, specific Chk1 inhibition with MK8776 sensitized HR-proficient, but not HR-deficient PC cells and xenografts to gemcitabine-radiation without toxicity to small intestine [260]. Combination of AZD7762 and a PARP1 inhibitor [olaparib (AZD2281)] radiosensitized p53-mutant cells to a greater degree than the cells acquired with wild-type p53, while the PARP1 inhibitor fails to radiosensitize normal intestinal epithelial cells. This combination also caused G2 checkpoint abrogation, inhibited HR, and prolonged the DNA damage response [261]. PARP1 promotes BER and single-strand break repair (SSBR), and PARP inhibitors, by blocking BER, lead to an increase of collapsed replication forks generating DSBs [262]. Radiosensitization of MiaPaCa-2 cells with olaparib (AZD2281) alone has been reported [263]. Similarly, the same group of researchers have also shown that the combined inhibition of Wee1 tyrosine kinase using a small molecule inhibitor AZD1775 and PARP1 inhibitor olaparib radiosensitizes AsPC-1 and MiaPaCa-2 PC cells significantly. The Wee1 dependent radiosensitization of AZD1775 has been demonstrated to be via abrogation of homologous recombination (HR) repair and G2 checkpoint regulation in PC cells [264]. Recently, inhibition of ERK1/2 by MEK inhibitor GSK1120212 has been shown to impair several DNA-DSB repair pathways for radiosensitization of PC cells harboring oncogenic mutant Kras [265]. Finally, the Chk1 specific inhibitor MK8776 sensitized HR repair-proficient (AsPC-1, MiaPaCa-2, BxPC-3) but not HR repair-deficient (Capan-1) PC cells to gemcitabine-radiation combination, and sensitized MiaPaCa-2 xenografts to gemcitabine-radiation combination [260]. A scaffolding subunit of protein phosphatase 2A (PP2A), PPP2R1A, was identified in a siRNA library screen as a potential target for PC radiosensitization. Genetic and pharmacologic inhibition (with LB100) of PP2A induced radiosensitization in vitro and in vivo. Mechanistically, LB100 treatment caused accumulation of active form of CDC25C, a PP2A substrate, and inhibited HR [266].
ATR inhibition with the novel inhibitors VE-821 and VE-822 radiosensitized PC cells and also sensitized these cells to gemcitabine chemotherapy [267,268]. VE-822 prolonged growth delay of xenografts after radiation and gemcitabine-based chemoradiation, without increasing toxicity to normal cells or tissues. In these studies, ATR inhibition was found to abrogate the IR-induced G2 checkpoint, prolong DNA damage and reduce HR in IR-treated PC cells.
Neural Precursor Cell Expressed, Developmentally Down-Regulated 8 (NEDD8)-activating enzyme regulates the E3 ubiquitin ligase SCF (SKP1, Cullins, and F-box protein), which is implicated in DNA damage and DNA repair mechanisms [269,270]. The novel inhibitor of NEDD8-activating enzyme, MLN4924, was also shown to radiosensitize PC cells, but not normal cells, in vitro and in vivo [271]. Mechanistic studies on MLN4924 treatment resulted in accumulation of SCF substrates, including CDT1 (resulting in enhanced IR-induced damage and aneuploidy), WEE1 (resulting in enhanced G2/M arrest), and NOXA (resulting in enhanced apoptosis) [271].
Ribonucleotide reductase (RNR), which catalyzes the reduction of ribonucleotides to deoxyribonucleotides, has also been suggested to be a target for PC radiosensitization. RNR has been shown to be recruited to DNA damage sites and to be needed for effective DNA repair [272]. Inhibition of RNR with Triapine radiosensitized the PSN-1 PC cell line in vitro and in vivo, along with a glioma and prostate cancer line [219]. It should be noted that Triapine radiosensitized a normal fibroblast cell line when treated before irradiation, but not after. RNR is also inhibited by gemcitabine, suggesting that blocking production of deoxyribonucleotides required for DNA repair may be an added mechanism of gemcitabine radiosensitization [219].
Inhibition of the SHH pathway has also been examined for its effects on PC radiosensitivity. This inhibition, accomplished through treatment with cyclopamine, a steroidal alkaloid that directly interacts with SMO, was shown to increase post-IR PC cell death in SHH-expressing cells, though in an additive, not synergistic, manner [273]. The theoretical promise of SHH inhibition in the context of PC radiosenisitization has become more clear in recent years as further studies have indicated that SHH signaling is necessary for the development and maintenance of PC desmoplasia, a major contributing factor to poor PC tumor vascularization and the resulting tumor hypoxia [274,275]. As such, the ability of SHH inhibition to increase tumor oxygenation through stromal effects, thereby improving PC radiosensitivity, is likely; though proof of this ability requires extensive murine studies that have, as of yet, not been attempted. Recently, SHH signaling inhibition by BMS833932 was combined with radiation in an orthotopic PC mouse model [276]. HH signaling inhibition alone had no significant effect on primary tumor growth, but significantly reduced metastases to lymph nodes, and combining HH inhibition with focal irradiation (6 Gy one time around the pancreas site) gave a greater than additive effect in reducing lymph node metastases. Though SHH inhibitors still appear promising as potential radiosensitizers, expectations have been dampened with the completion of a Phase Ib/II study showing that addition of vismodegib (GDC-0449), an SHH antagonist, plus gemcitabine did not improve overall survival, overall response rate, or progression free survival compared to gemcitabine plus placebo in an unselected cohort of patients with metastatic PC [277].
Anti-apoptotic proteins have also been linked to radioresistance. Intratumoral injection of Bcl-XL antisense oligonucleotides that were attached to cell penetrating peptide (Antennapedia) resulted in decreased tumor volume and weight, and increased apoptosis, of ASPC-1 xenografts following treatment with IR [278]. Further, adenovirus-mediated siRNA knockdown of Mcl-1 resulted in radiosensitization of Panc-1 cells in vitro and in vivo [279].
Inhibitors of Hsp90, a molecular chaperone, have been shown to radiosensitize select PC cells. 17DMAG radiosensitized MiaPaCa and PSN-1 cells, but not ASPC1 cells [280]. In MiaPaCa cells, 17DMAG inhibited DNA repair, reduced DNA-PKcs phosphorylation, disrupted DNA-PKcs/EGFR interaction, abrogated the G2 checkpoint, and reduced MRN (MRE11/Rad50/NBS1) complex nuclear foci [281]. A novel Hsp90 inhibitor, NVP-HSP990, also radiosensitized MiaPaCa-2 cells at 37 degrees and at hyperthermic conditions [282].
IGF-1R inhibition has shown the ability to enhance radiation-induced cytotoxicity in PC cell lines as well [152,179]. In BxPC3 cells, dominant negative inhibition of IGF-1R by transduction with an adenovirus construct in combination with IR was found to result in a 1.74-fold increase in apoptosis (as measured by caspase-3 activity). Whereas, control virus transduced cells treated with IR alone was found to increase apoptosis by 1.53-fold relative to the untreated cells [152]. Unfortunately, no control for apoptotic induction resulting from IGF-1R inhibition alone was included in this study, though decreased cellular viability was clearly shown, making it impossible to determine if the increased apoptosis resulting from co-treatment was additive or synergistic. Additionally, to date, no in vivo studies have been conducted combining IGF-1R inhibition with IR to determine if the improvement of IR-induced cell death resulting from concurrent IGF-1R inhibition translates to more complicated and clinically relevant models. Consequently, substantial further evaluation regarding the ability of IGF-1R inhibition to radiosensitize PC cells is required before its true potential in this regard can be determined.
A number of additional molecules have been implicated as potential targets for sensitizing PC to radiation by genetic knockdown studies, but specific inhibitors were not used. For example, combined and individual siRNA knockdown of caveolin-1, β1-integrin, and focal adhesion kinase (FAK) sensitized the PC cell lines Panc-1 and MiaPaca-2 to radiation. Importantly, Caveolin-1 levels were massively upregulated in these cells, as well as the ATU8902 PC cell line, 12 hours and 24 hours after 2 Gy irradiation, along with induced formation of Caveolin-1-positive caveolae [283]. Interestingly, it was also shown that cholesterol depletion by beta-cyclodextrin radiosensitized these cells [283]. Recently, siRNA knockdown of Caveolin-1 was found to radiosensitize MiaPaCa-2 and Panc-1 cells grown in 3D cell culture [284], although Caveolin-1 expression levels were not seen to increase after radiation in 3D cultures, as opposed to 2D cell cultures mentioned above.
Inhibition of GSK3-beta by lithium chloride, shRNA knockdown, or expression of a kinase dead mutant GSK3-beta has been shown to increase radioresistance of Panc-1 and BxPC3 cells. This GSK3-beta inhibition resulted in stabilization of beta-catenin and shRNA knockdown of beta-catenin radiosensitized both of these cell lines, suggesting beta-catenin could be another potential target for radiosensitization [285].
Other drugs shown to act as PC radiosensitizers in vitro include natural products and small molecules. Benzyl isothiocyanate increased radiation-induced apoptosis of BxPC-3 cells, enhanced G2/M arrest, and inhibited NF-κB [286]. Curcumin, raspberry extract, and neem leaf extract each inhibited constitutive and IR-induced NF-κB, and further increased IR-induced apoptosis in PC cells [287]. Guggulsterone radiosensitized PC-Sh and BxPC3 cells, inhibited NF-κB activation, and reduced IGF1-R beta expression [288]. Hydroxychalcones activate heat shock factor 1 and were shown to radiosensitize Panc1 cells [289]. Gleevec (STI-571), which inhibits c-Kit and PDGFR, radiosensitized BxPC-3, Capan-1, and MiaPaCa-2 cells [290]. Lastly, L-canavanine, an analogue of L-arginine, exhibited synergy with radiation in killing Panc-1 and MiaPaCa-2 cells [291].
10. Drugs targeting metabolism and metabolic enzymes as radiosensitizers in pancreatic cancer
The idea of altered metabolic reprogramming contributing towards proliferation, growth, and survival of cancer cells came from the phenomenon known as the Warburg effect [292]. The Warburg effect highlights the conversion of glucose to lactate as observed in cancercells, even in the presence of adequate amount of oxygen. Ying et al., showed that mutant Kras directs glucose to be used for nucleotide biosynthesis and protein glycosylation (the hexosamine biosynthetic pathway) in PC mouse models [293]. The hypoxic tumor microenvironment of PC also drives glycolysis via HIF-1α [294,295]. Moreover, increased glucose uptake is the basis of imaging human tumors, including pancreatic tumors, with [18F]-deoxyglucose PET (FDG-PET) imaging. Interestingly, patients with locally advanced PC with high baseline metabolic volume assessed by FDG-PET scans had inferior survival after receiving stereotactic body radiation therapy [296]. This suggests that metabolic changes of a tumor may in fact contribute to the response of radiation.
Glycolysis can be inhibited using the glucose analog, 2-Deoxy-D-glucose (2DG), in cancer cells [297]. Mechanistically, 2DG competes with physiological glucose for cellular uptake through glucose transporters and is phosphorylated by hexokinase to form 2-DG-P. Thus it creates a chemically-induced glucose deprivation in cells, which in turn disrupts the conversion of glucose to lactate. In PC, the combination of 2-DG with radiotherapy demonstrated enhanced PC cell killing through disruption of thiol metabolism, in both cell culture as well as in a nude mouse model [298]. Previous studies related to diabetes and hyperglycemia showed the beneficial effects of metformin in promoting insulin-stimulated glucose uptake in cells and lowering plasma triglyceride levels and free fatty acids [299]. Several recent preclinical in vitro and in vivo studies have shown an additional property of metformin as a radiosensitizer in several cancers, including PC. Changes associated with the phosphorylation status of LKB-1 (T189) and AMPK activity was reported to be the central mechanism behind the radiosensitizing effect of metformin in PC [300]. Shimura et al., reported that long-term fractionated dose of 5 Gy to cancer cells conferred radioresistance by activating an AKT pathway-mediated alteration in aerobic glycolysis [301]. Blocking the AKT activity using AKT/protein kinase B signaling inhibitor-2 (API-2) drastically decreased the lactate production.
Recently, pharmacological ascorbate was shown to be effective in radiosensitizing MIA PaCa-2 and PANC-1 PC cells in both in vitro and in vivo. Ascorbate promoted H2O2 production which may diffuse through the cell membrane resulting in oxidative damage to the cellular contents such as lipids, proteins and DNA. Thus, ascorbate was shown to be an effective prodrug for radiosensitization of PC. Importantly, ascorbate did not enhance radiation induced killing of non-tumorigenic PC cells suggesting that ascorbate targets altered metabolic pathways in cancer cells to enhance radiotherapy in PC [302].
Son et al., demonstrated that Kras promotes glutamine uptake and use in a non-canonical pathway in PC [303]. In most normal cells, glutamine-derived glutamate will be transformed into α-ketoglutarate to enter into the TCA cycle, whereas, in PC cells glutamine is converted to aspartate which is transported to the cytoplasm and converted into oxaloacetate. Furthermore, oxaloacetate is transformed to malate and then pyruvate, increasing cellular NADPH/NADP+ ratios thereby leading to the maintenance of the redox state of cancer cells. Li et al., showed that use of Zaprinast, a phosphodiesterase inhibitor which also inhibits glutaminase, could effectively sensitize PC cells to radiation by induction of apoptosis and intracellular accumulation of reactive oxygen species [133]. Similarly, pharmacological inhibition of another glutamine pathway enzyme GLS-1 (mitochondrial glutaminase) using bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide (BPTES) exhibited growth inhibitory effects on cancer cells in culture with minor tumor growth suppression in xenograft models [304,305]. β-lapachone (β-lap) is a bioactivable drug whose therapeutic effect is dependent on the endogenous expression of NAD(P)H: quinone oxidoreductase (NQO1) in cancer cells [306,307]. It has been demonstrated that targeting glutamine metabolism, using BPTES sensitizes PC cells to β-lap by enhancing reactive oxygen species as well as PARP activation, leading to enhanced tumor cell specific cytotoxicity [308]. β-lapachone has been shown to be a potential radiosensitizer in a variety of cancers [309,310].
In our recent global analysis, we identified 11 candidate genes (FDPS, ACAT2, AG2, CLDN7, DHCR7, ELFN2, FASN, SC4MOL, SIX6, SLC12A2, and SQLE), a majority of which were related to the cholesterol biosynthesis (CBS) pathway, that were differentially expressed in radioresistant PC cells. We found that farnesyl diphosphate synthase (FDPS), a branch-point enzyme of CBS pathway, was significantly upregulated in radiation-resistant cells, and in PC patient samples. Further, genetic modulation using siRNA and pharmacological inhibition of FDPS using zoledronic acid resulted in radiosensitization of PC cells in vitro and in vivo [311]. Ying et al., also demonstrated that Kras controls mRNA expression of cholesterol synthesis enzymes in PC mouse models using doxycycline-inducible expression of KrasG12D [293]. Interestingly, measurement of DNA copy number using microarray-based comparative genomic hybridization on 23 human pancreatic ductal adenocarcinoma specimens and 6 cell lines revealed that SQLE is amplified and a potential oncogene in PC [312]. Beside our analysis showing upregulated FASN in radiation-resistant cells, treatment with the lipase and FASN inhibitor orlistat enhanced sensitivity to gemcitabine and overexpression of FASN in PC cells increased radiation resistance [313]. We believe that modulating metabolic pathways is an alternative route for the evolution of radioresistance in tumour cells. Targeting these metabolic pathways using currently available US FDA-approved agents can potentially radiosensitize PC cells.
Other metabolic pathways utilized in PC have yet to be tested as potential pathways for radiosensitization. Kras promotes macropinocytosis in PC, a process in which cancer cells uptake extracellular proteins with subsequent catabolic process of yielding aminoacids that can further enter into carbon metabolism and ATP production which is required for the cancer cells survival [314]. The role of cells in the microenvironment of PC tumors, such as stellate cells, have recently been shown to secrete metabolites, such as alanine, that are utilized as an alternative carbon source by PC cells [315].
Lastly, metabolite profiling of 38 PC cell lines identified three metabolic subtypes in PC, namely “slow proliferating,” “glycolytic,” and “lipogenic” [316]. The “glycolytic” subtype was enriched for cell lines will show greater response to inhibitors targeting aerobic glycolysis (oxamate and the LDHA inhibitor GNE-140) and glutaminolysis (BPTES), whereas the “lipogenic” subtype was enriched for cell lines that were sensitive to inhibitors of fatty acid synthesis, including the FASN inhibitors GSK1195010, cerulenin, and orlistat; and an inhibitor of SCD (enzyme which converts saturated fatty acids into mono-unsaturated fatty acids). The association of metabolic subtypes with the three clinical subtypes of PDAC, recently identified through molecular profiling of PDAC tumors, was also determined [317]. Incase of human PDAC specimens, the “lipogenic” subtype was associated with the epithelial (classical) subtype, whereas the “glycolytic” subtype intensely correlates with the mesenchymal (QM-PDA) subtype [316]. It is of utmost interest to determine if metabolic or molecular subtypes dictate response to radiation therapy and if specific pathways can be exploited for radiosensitization. This could be tested by measuring responses to radiation in human PC cell lines of known clinical and metabolic subtypes. We have briefly outlined some of the potential radiosensitizers and their respective targets along with their biological effects tested in in vitro and in vivo settings of pancreatic cancer as Table 4.
Table 4.
Currently available radiosensitizing agents, their respective targets and corresponding biological effects in pancreatic cancer
| S. No | Inhibitor(s) | Radiosensitization target (s) | Cellular effect/process | References |
|---|---|---|---|---|
| 1. | AZD8055 | mTOR | Reversing radioresistance | [378] |
| 2. | L-778123 | Ras | Decrease in PC radioresistance. | [253] |
| 3. | Cetuximab/erlotinib | EGFR | Chemo-radiosensitization | [231,232] |
| 4. | Nelfinavir | PI3K/AKT pathway | Radiosensitization (Increased radiation induced cell death) | [244] |
| 5. | PX-478 | HIF-1α | Enhanced radiosensitivity in hypoxic PC. | [256,257] |
| 6. | NVP-HSP990 | Hsp90 | Increased radiosensitivity and radiothermosensitivity. | [282] |
| 7. | NU7026 | DNA-dependent protein kinase | DNA repair pathway | [50] |
| 8. | Triapine | RNR | Inhibition of DNA repair. | [219] |
| 9. | GSK1120212 | ERK1/2 | Impairment of DNA-DSB repair pathways | [265] |
| 10. | Metformin | Phosphorylation status of LKB-1 (T189) and AMPK activity | Disrupts DNA damage signaling. | [300] |
| 11. | 17DMAG | Hsp90 | Inhibition of DNA repair and G2/M checkpoint abrogation. | [281] |
| 12. | MLN4924 | NEDD8 | Enhanced radiation-induced damage and aneuploidy | [271] |
| 13. | NSC23766 | Rac1 | G2/M checkpoint abrogation | [97] |
| 14. | AZD7762 | Chk1/2 | G2/M checkpoint abrogation and inhibition of Homologous recombination (HR) repair. | [259] |
| 15. | AZD7762 in combination with Olaparib (AZD2281) | Chk1/2 and PARP1 | Radiosensitize p53 mutant cells apart from its G2/M checkpoint abrogation and inhibition of HR repair. | [261] |
| 16. | AZD1775 and olaparib | Wee1 and PARP1 | G2/M checkpoint abrogation and inhibition of HR repair. | [264] |
| 17. | VE-821/VE-822 | ATR | G2/M checkpoint abrogation, prolonged DNA damage and inhibition of HR repair. | [267,268] |
| 18. | MK8776 | Chk1 | Inhibition of HR repair. | [260] |
| 19. | LB100 | PPP2R1A (Scaffold subunit of protein phosphatase 2A) | Inhibition of radiation-induced Rad51 focus formation and HR repair. | [266] |
| 20. | MLN4924 | XRCC4 polyubiquitylation | NHEJ repair | [55] |
| 21. | Benzyl isothiocyanate | NF-κB | Radiation-induced apoptosis and enhanced G2/M arrest. | [286] |
| 22. | Curcumin, raspberry extract, and neem leaf extract | NF-κB | Radiation-induced apoptosis | [287] |
| 23. | Guggulsterone | Inhibition of NF- κactivation and downregulation of IGF1-R β. | Inhibition of cell growth and radiation-induced DNA damage repair. | [288] |
| 24. | Afatinib | Phosphorylated forms of EGFR, HER2, HER3 and HER4 | Mitotic catastrophe-mediated cell death. | [230] |
| 25. | Vorinostat | Histone deacetylase | Increased apoptosis post-radiation treatment. | [247] |
| 26. | PD0325901 in combination with API-2 | MEK/AKT | Growth arrest and increased apoptosis. | [258] |
| 27. | Gleevec (STI-571) | c-Kit and PDGFR | Radiation-induced apoptotic cell death and cytotoxicity. | [290] |
| 28. | Zaprinast | Phosphodiesterase and glutaminase | Induction of apoptosis and accumulation of intracellular reactive oxygen species. | [133] |
| 29. | Pharmacological grade ascorbate | Promotes H2O2 production | Oxidative damage to DNA. | [302] |
| 30. | Orlistat | FASN | Decrease in cell survival. | [313] |
| 31. | Zoledronic acid | FDPS | Decrease in cell survival and reduction of xenograft tumor volume. | [311] |
| 32. | BMS833932 in combination with radiation | SHH | Reduction of primary tumor growth and decreased lymph node metastasis. | [276] |
11. Conclusions and future directions
While surgical resection remains the mainstay for pancreatic cancer, postoperative chemotherapy or chemoradiation therapy (CRT) is extensively used to improve outcomes. However, these benefits from CRT come at a cost of appreciable toxicity, with grade 3–4 hematologic and non-hematologic toxicities occurring in greater than 50% of cases [318]. Although adjuvant therapies have been studied in greater detail than their neoadjuvant counterparts, the latter can similarly include chemotherapy alone or combined CRT. Neoadjuvant chemotherapy/CRT is safe from a postoperative complication standpoint, without significant increases in complication rates compared with surgery alone [319,320]. However, RT concurrently with chemotherapy especially (gemcitabine) in patients with localized unresectable pancreatic cancer is associated with high grade 3 and 4 toxicities (79%) [319]. Stereotactic body radiation therapy (SBRT) allows for improved conformality and shortened treatment times as compared to conventionally-fractionated radiotherapy, and is being increasingly used at centers across the United States. Initial studies demonstrated feasibility and effectiveness of SBRT for pancreatic cancer including high local control [321,322]. However, despite superior conformality, in part due to single-fraction treatment regimens, there were substantial and serious acute and late gastroduodenal/bowel toxicities observed, secondary to the close anatomical relationship with the pancreas [323–327].
We recently performed a secondary dosimetric analysis correlating dosimetric parameters with histologic damage to the duodenum using patients who had pancreaticoduodenectomy from our institutional phase I trial of chemotherapy followed by SBRT and concurrent administration of the human immunodeficiency virus (HIV) protease inhibitor nelfinavir. Our study showed that duodenal histologic damage correlates with mean duodenal dose, V20–V35, and PTV mean/maximum doses [328]. As the use of high-dose SBRT regimens becomes widespread, it is imperative to explore modalities that can selectively radioprotect adjacent organs without altering the tumor response. As discussed earlier, the tumor cells and normal cells respond differently to radiation exposure. Consequently, several compounds have been demonstrated to exhibit selective radioprotective effects on normal cells and have been evaluated in several tumors with a goal to limit radiation toxicity to healthy organs. Recently, several adjuvant cytoprotectants have been evaluated in combination with radiation or chemotherapy [329]. In 1996, the US FDA approved an organic thiophosphate, amifostine, to reduce the nephrotoxicity caused by the chemotherapeutic agent cisplatin in non-small cell lung cancer patients [330]. In a randomized clinical trial in head and neck cancer patients, administration of amifostine before radiation significantly improved loco-regional control, disease free survival and overall survival rates compared with RT alone [331]. Amifostine is a pro-drug formed when a phosphate moiety is added to a sulfhydryl compound. This pro-drug can further dephosphorylate to an active thiol metabolite by alkaline phosphatase enzymes present in the normal cells. Further, the thiol metabolite will scavenge the reactive oxygen species (ROS) generated during RT [332,333]. On the other hand, the level of alkaline phosphatase is low in tumor cell; hence, the radioprotective effects of amifostine do not extend to tumor cells. Another approach is to inactivate the radiation-induced reactive oxygen species (ROS) by the addition of a novel Mn (III) tetrakis (N-ethylpyridinium-2-yl) porphyrin containing the active antioxidant (MnTE-2-PyP) to RT [334]. Apart from the radioprotective effect of MnTE-2-PyP it was also reported to be effective in inhibiting prostate cancer growth and metastasis by altering the p300 transcriptional complex binding to DNA and reduce histone acetylation [335]. In addition, AEOL-10150, a manganoporphyrin catalytic antioxidant was demonstrated to be effective in eliminating the peroxynitrate and carbonate (free radicals) to protect lung against radiation induced injury (single dose of 28 Gy) in an experimental rat model [336]. Specifically, the mode of action of this drug is neutralization of free radicals (both reactive oxygen and reactive nitrogen species), thereby protecting the normal counterparts cells against radiation. Currently, Aeolus Pharmaceuticals is initiating a phase-1 clinical trial to demonstrate its radioprotective effect against acute radiation damage to lung in healthy volunteers [337]. As free radicals are short lived, inhibiting the intracellular signaling cascade and DNA repair mechanism has emerged as an alternative radioprotectant strategy. Recently, Onconova Therapeutics has developed a drug known as Ex-RAD (the sodium salt of 4-carboxystyryl-4-chlorobenzylsulfone) which is claimed to protect normal hematopoietic cells and gastrointestinal tract cells [338]. In two phase 1 clinical trials, subcutaneous administration of Ex-RAD in healthy volunteers was shown be safe and well tolerated. Given their selective effects on normal cells, it will be of immediate interest to evaluate radioprotectors such as metallo prophyrins or other agents neutralizing the action of both ROS and reactive oxygen/nitrogen species in conjunction with conventional RT or SBRT. These agents can either be evaluated by systemic delivery or administered locally via endoscopic procedures in the vicinity of vulnerable sites like duodenum. Such agents need to be actively investigated in preclinical models of pancreatic cancer and radiation therapy.
Despite the failure of past attempts at developing a clinically relevant PC radiosensitizer, studies to date have been far from fruitless. Examining the knowledge gained from past study results, especially in the context of our ever-improving knowledge regarding both PC and radiation biology, it is clear that most theoretically promising avenues for PC radiosensitization remain untested. One of the most important steps towards this goal has been the general acceptance that chemoresistance and radioresistance are distinct phenomena, indicating that using pathways shown to be important for chemosensitization as a template for improvement of radiosensitivity may not be entirely logical [16]. While there are obvious targets that should allow for significant therapeutic improvement for all cytotoxic therapies, such as anti-apoptotic mechanisms, others appear to be dependent on therapeutic modality. With this in mind, it has been theorized that success of radiation treatment can be achieved by only two mechanisms: 1) increasing the amount of radiation-induced damage occurring from treatment or 2) decreasing the efficiency of post-IR DNA repair [16]. The effectiveness of IR is further qualified by the consideration that, increasing radiation-induced damage currently must necessarily come from increased radiation dose, which brings with it the significant risk of increased toxicity to healthy tissues as well. As such, some believe that a preferable method for enhancing IR efficacy is through targeting of DNA repair mechanisms, especially those differentially used by the cancer cells. While we agree that increasing DNA damage and inhibiting DNA repair is one of the more promising methods for improving the efficacy of radiotherapy [339], we caution that this viewpoint risks being both overly simplistic and limiting to future research as it ignores the reaction of the cell to DNA damage completely.
Further, the redundancy of pro-survival signaling resulting from common PC driving genetic alterations reduces the likelihood that targeted manipulation of a single pathway will provide any therapeutically significant radiosensitization. Consequently, we suggest that focus should not be placed on single pathway targets, especially those upstream of complex pathways that allow for substantial biochemical overlap with others, but rather on downstream targets closer to the desired effector molecules, such as TGM2 and AKT, or those that represent nodes of interaction between two or more significant pathways, such as JNK. Additionally, due to the uncanny ability of PC to overcome single-target manipulation, simultaneous targeting of two or more individual pathways or biochemical nodes may be necessary to elicit clinical radiosensitization. While such methods have been attempted to a limited extent in the past, it has only been through the use of chemicals with broad-based actions, such as protease inhibitors causing an unfolded protein response or HDACIs which alter genetic expression of a plethora of genes, or with small molecule inhibitors with multiple targets but of limited scope, such as EGFR and HER2 co-inhibition via lapatinib [229,244,246,247] or inhibiting the catalytic domain of all the EGFR family members through afatinib, neratinib and dacomitinib [340]. A notable exception to this theme is the above-mentioned study combining Akt and MEK inhibition via individual specific small molecule inhibitors which showed promising results when used in combination, though to our knowledge this combination has not been studied beyond preclinical experiments to date [258]. As such, we believe that more thoughtful and targeted manipulation of multiple pathways having little upstream interaction, or addition of specific pathway inhibition to a broad-acting agent that does not overlap is warranted. For example, agents such as NVP-BEZ235, which acts to inhibit both PI3K and mTOR, have yet to be tested in the context of PC IR response [341]. Further, HDACIs and inhibitors of the SHH pathway have shown the ability to act synergistically on PC cell cycle arrest and apoptosis while neither were found to induce substantial effects alone [342]. This combination has not been evaluated in the context of IR. A number of metabolic pathway inhibitors mentioned above, have yet to be examined as potential radiosensitizers, and combinations of inhibitors of multiple metabolic pathways may be important but may come with an enhanced risk of off-target toxicity.
In addition, a subpopulation of cells known as cancer stem cells (CSC) is thought to be a critical determinant for radioresistance of several cancers including PC. Hence, drugs that are being tested for radiosensitization potential should be examined for their ability to target CSC population, and alternatively, drugs which are differentially toxic to CSCs should be evaluated as potential radiosensitizers. Further, more studies are needed to prove the contribution of stem cells to radiation resistance.
Large portions of radiation-induced cellular damage and the resulting biochemical responses also remain largely unexplored in the context of PC radiosensitization. The vast majority of radiation research has focused on damage to the DNA from IR-induced free radicals and the downstream effects of such damage. However, the effects of expression levels of superoxide dismutases and inhibition of their activities on PC radioresistance remain unknown and unexplored. Additionally, it is well established that free radicals have the capacity to attack and alter many classes of cellular molecules, potentiating the existence of many other vital processes necessary for the survival of irradiated PC cells beyond the DNA damage [343–345]. While the effect of DNA DSBs in IR-induced cell death is without doubt of the utmost importance, it is likely unwise to ignore the effects of IR on other cellular complexities such as the plasma membrane and structural proteins, and thus we suggest these areas as potential fertile grounds for future IR cancer research.
The role of the immune system in PC radioresistance also remains largely unexplored as well, and treatment successes in other cancers with immune checkpoint inhibitors should bolster the rationale for such studies. Immune checkpoint inhibitors, which includes anti-PD-1, and anti-PD-L1 and anti-CTLA4, antibodies are effective as monotherapy in immune-sensitive cancers like melanoma, but have thus far lacked efficacy in immune-insensitive cancers such as PC [346–348]. A recent study showed that combination of radiation, vaccination (SIY) and checkpoint blockade with anti-PD-L1 antibody led to regression of established PC tumors (Panc02 mouse cells engineered to express the SIY antigen) [349]. Another recent study using the KC and KPC mouse models of PC, showed that treatment of pancreata with radiation (2 to 12 Gy) resulted in induction of macrophages to acquire an immune-suppressive phenotype which disabled the anti-tumor T-cell response. Treatment with a neutralizing antibody against MCSF prevented this IR-induced immune suppression, and slowed tumor growth [350].
Another area of cancer biology that remains unexplored in the context of PC radioresistance is the impact of tumor microenvironment in response to IR. While no study to date has examined the results of targeting tumor stroma on PC radiosensitivity, results from multiple studies indirectly hint at its importance. For example, the actions of nelfinavir as a radiosensitizer were found to be much greater in murine models than was predicted from in vitro results [229]. Similar results were found by Schwartz et al., for PX-478 mediated HIF-1α inhibition [257]. Additionally, the latter study found that PX-478 co-treatment resulted in significant stromal alterations in irradiated in vivo models which likely accounted for the improved radiosensitizing effects. Growing evidence suggest that PC desmoplasia is intimately implicated in tumor hypoxia, with the known negative effects of hypoxia on radiosensitivity, itself is evidence enough to pursue tumor microenvironmental effects on PC IR efficacy [274,275]. Further, the difference in tumor stroma found in human PC compared to that in subcutaneous xenograft murine models, the only mouse model currently available for PC IR research, may at least partially account for the failure of radiosensitizers clinically despite highly promising preclinical evidence. Currently, the only model available that faithfully recapitulates the stromal effects seen in human PC are the genetically engineered murine models of PC [274]. Alternatively, reasonable, though suboptimal, stromal recapitulation can be obtained through orthotopic transplant models as well [275]. Common to both of these models, however, is that in situ tumors occurring deep in the abdomen are produced, making their irradiation without significant/lethal damage to the surrounding healthy tissue near impossible with currently available research irradiators. Nevertheless, development of novel methods to overcome this complication inherent in such models is necessary to further PC IR research towards more innovative and clinically relevant preclinical studies, thus increasing the likelihood of future success.
Though triumphs in the realm of PC radiosensitization have been modest to date, it is doubtless that we are moving in the right direction. While PC remains one of the most complicated and redundantly therapeutically refractory cancers known to man, hope abounds that a clinically practical and efficacious adjunct therapy to improve the efficacy of radiotherapy in PC will soon be found. Several radiosensitizers are currently undergoing clinical trials in PC with many more in other cancers, leading to a high probability that one or more of these will become a part of standard PC radiotherapy in the near future. Further, many novel targets, both single and in combination, will be entering clinical study soon. Further improvement of current preclinical models, however, is necessary to allow for a better translation of preclinical results to clinical trials.
Highlights.
A majority of pancreatic cancer (PC) patients do not respond to radiotherapy.
Mutations and altered signaling pathways in PC cells have roles in radiation resistance by affecting DNA damage sensing, DNA repair, cell cycle checkpoints, and cell survival.
Potential radiosensitizers tested in clinical trials for PC include chemotherapy (5-FU, gemcitabine, capecitabine), oxygen substitutes (nitroimidazoles), EGFR inhibitors (cetuximab, erlotinib), nelfinavir, HDAC inhibitors, and prenyltransferase inhibitors.
A number of synthetic drugs and natural products have been tested as radiosensitizers or radioprotectants for PC in preclinical studies.
Acknowledgments
Funding
The authors on this review article, in part, are supported by the grants from National Institutes of Health (P50 CA 127297, RO1 CA195586, RO1 CA 206444, UO1 CA 200466 and EDRN UO1 CA200466).
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Surveillance Epidemiology and End Results Cancer Statistics Review 1975–2006. 2011 [Google Scholar]
- 4.Barugola G, Falconi M, Bettini R, Boninsegna L, Casarotto A, Salvia R, Bassi C, Pederzoli P. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP. 2007;8:132–140. [PubMed] [Google Scholar]
- 5.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 6.Fischer R. Early Recurrence of Pancreatic Cancer After Resection and During Adjuvant Chemotherapy. Saudi Journal of Gastroenterology. 2012;18(2):118–121. doi: 10.4103/1319-3767.93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rau BM, Moritz K, Schuschan S, Alsfasser G, Prall F, Klar E. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. 2012;152:S103–S111. doi: 10.1016/j.surg.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Andren-Sandberg A. Pancreatic cancer: chemotherapy and radiotherapy. N Am J Med Sci. 2011;3:1–12. doi: 10.4297/najms.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE, Lenzi R, Vauthey JN, Wong AB, Phan T, Nguyen Q, Janjan NA. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Ellis LM, Abbruzzese JL, Amos C, Xiong HQ, Ho L, Evans DB, Tamm EP, Ng C, Pisters PW, Charnsangavej C, Delclos ME, O’Reilly M, Lee JE, Wolff RA. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB., III Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 12.Jessup JM, Steele G, Jr, Mayer RJ, Posner M, Busse P, Cady B, Stone M, Jenkins R, Osteen R. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg. 1993;128:559–564. doi: 10.1001/archsurg.1993.01420170093014. [DOI] [PubMed] [Google Scholar]
- 13.Wilkowski R, Thoma M, Schauer R, Wagner A, Heinemann V. Effect of chemoradiotherapy with gemcitabine and cisplatin on locoregional control in patients with primary inoperable pancreatic cancer. World J Surg. 2004;28:1011–1018. doi: 10.1007/s00268-004-7338-z. [DOI] [PubMed] [Google Scholar]
- 14.Hazard L. The Role of Radiation Therapy in Pancreas Cancer. Gastrointest Cancer Res. 2009;3(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Compton AH. On the Mechanism of X-Ray Scattering. Proc Natl Acad Sci U S A. 1925;11:303–306. doi: 10.1073/pnas.11.6.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lippincott Williams & WIlkins; Philadelphia: 2006. [Google Scholar]
- 17.Zou L, Elledge SJ. Sensing and signaling DNA damage: roles of Rad17 and Rad9 complexes in the cellular response to DNA damage. Harvey Lect. 2001;97:1–15. [PubMed] [Google Scholar]
- 18.Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol. 2001;13:225–231. doi: 10.1016/s0955-0674(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 19.Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol. 2002;14:237–245. doi: 10.1016/s0955-0674(02)00312-5. [DOI] [PubMed] [Google Scholar]
- 20.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–6560. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson DO, Alt FW. DNA double strand break repair and chromosomal translocation: lessons from animal models. Oncogene. 2001;20:5572–5579. doi: 10.1038/sj.onc.1204767. [DOI] [PubMed] [Google Scholar]
- 22.Khanna KK, Lavin MF, Jackson SP, Mulhern TD. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 2001;8:1052–1065. doi: 10.1038/sj.cdd.4400874. [DOI] [PubMed] [Google Scholar]
- 23.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 24.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 25.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 26.Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed KM, Li JJ. ATM-NF-kappaB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets. 2007;7:335–342. doi: 10.2174/156800907780809769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckardt NA. ATM to the rescue: repairing DNA damage. Plant Cell. 2003;15:1–3. doi: 10.1105/tpc.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandita TK. A multifaceted role for ATM in genome maintenance. Expert Rev Mol Med. 2003;5:1–21. doi: 10.1017/S1462399403006318. [DOI] [PubMed] [Google Scholar]
- 30.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 31.Rotman G, Shiloh Y. ATM: from gene to function. Hum Mol Genet. 1998;7:1555–1563. doi: 10.1093/hmg/7.10.1555. [DOI] [PubMed] [Google Scholar]
- 32.Alexander A, Walker CL. Differential localization of ATM is correlated with activation of distinct downstream signaling pathways. Cell Cycle. 2010;9:3685–3686. doi: 10.4161/cc.9.18.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, Lobrich M, Shiloh Y, Chen DJ. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 34.Xue L, Yu D, Furusawa Y, Cao J, Okayasu R, Fan S. ATM-dependent hyper-radiosensitivity in mammalian cells irradiated by heavy ions. Int J Radiat Oncol Biol Phys. 2009;75:235–243. doi: 10.1016/j.ijrobp.2009.04.088. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz JL, Rotmensch J, Giovanazzi S, Cohen MB, Weichselbaum RR. Faster repair of DNA double-strand breaks in radioresistant human tumor cells. Int J Radiat Oncol Biol Phys. 1988;15:907–912. doi: 10.1016/0360-3016(88)90125-3. [DOI] [PubMed] [Google Scholar]
- 36.Kelland LR, Edwards SM, Steel GG. Induction and rejoining of DNA double-strand breaks in human cervix carcinoma cell lines of differing radiosensitivity. Radiat Res. 1988;116:526–538. [PubMed] [Google Scholar]
- 37.Giaccia AJ, Schwartz J, Shieh J, Brown JM. The use of asymmetric-field inversion gel electrophoresis to predict tumor cell radiosensitivity. Radiother Oncol. 1992;24:231–238. doi: 10.1016/0167-8140(92)90229-n. [DOI] [PubMed] [Google Scholar]
- 38.D’Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 39.Petrini JH. The mammalian Mre11-Rad50-nbs1 protein complex: integration of functions in the cellular DNA-damage response. Am J Hum Genet. 1999;64:1264–1269. doi: 10.1086/302391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 41.Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- 42.Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, Mizuta R, Varghese AJ, Alt FW, Jeggo PA. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 43.Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP, Jeggo PA. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci U S A. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeggo PA, Taccioli GE, Jackson SP. Menage a trois: double strand break repair, V(D)J recombination and DNA-PK. Bioessays. 1995;17:949–957. doi: 10.1002/bies.950171108. [DOI] [PubMed] [Google Scholar]
- 45.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Zeng ZC, Bui TA, DiBiase SJ, Qin W, Xia F, Powell SN, Iliakis G. Nonhomologous end-joining of ionizing radiation-induced DNA double-stranded breaks in human tumor cells deficient in BRCA1 or BRCA2. Cancer Res. 2001;61:270–277. [PubMed] [Google Scholar]
- 47.Wang H, Zeng ZC, Bui TA, Sonoda E, Takata M, Takeda S, Iliakis G. Efficient rejoining of radiation-induced DNA double-strand breaks in vertebrate cells deficient in genes of the RAD52 epistasis group. Oncogene. 2001;20:2212–2224. doi: 10.1038/sj.onc.1204350. [DOI] [PubMed] [Google Scholar]
- 48.Xia F, Taghian DG, DeFrank JS, Zeng ZC, Willers H, Iliakis G, Powell SN. Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc Natl Acad Sci U S A. 2001;98:8644–8649. doi: 10.1073/pnas.151253498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–5847. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- 50.Li YH, Wang X, Pan Y, Lee DH, Chowdhury D, Kimmelman AC. Inhibition of non-homologous end joining repair impairs pancreatic cancer growth and enhances radiation response. PLoS One. 2012;7:e39588. doi: 10.1371/journal.pone.0039588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 52.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 53.Shiloh Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu Rev Genet. 1997;31:635–662. doi: 10.1146/annurev.genet.31.1.635. [DOI] [PubMed] [Google Scholar]
- 54.Carney JP, Maser RS, Olivares H, Davis EM, Le BM, Yates JR, III, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, Karnak D, Tan M, Lawrence TS, Morgan MA, Sun Y. FBXW7 Facilitates Nonhomologous End-Joining via K63-Linked Polyubiquitylation of XRCC4. Mol Cell. 2016;61:419–433. doi: 10.1016/j.molcel.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satyamoorthy K, Chehab NH, Waterman MJ, Lien MC, El-Deiry WS, Herlyn M, Halazonetis TD. Aberrant regulation and function of wild-type p53 in radioresistant melanoma cells. Cell Growth Differ. 2000;11:467–474. [PubMed] [Google Scholar]
- 57.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 58.Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 59.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 60.Maltzman W, Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984;4:1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chernov MV, Stark GR. The p53 activation and apoptosis induced by DNA damage are reversibly inhibited by salicylate. Oncogene. 1997;14:2503–2510. doi: 10.1038/sj.onc.1201104. [DOI] [PubMed] [Google Scholar]
- 62.Haapajarvi T, Pitkanen K, Tsubari M, Laiho M. p53 transactivation and protein accumulation are independently regulated by UV light in different phases of the cell cycle. Mol Cell Biol. 1997;17:3074–3080. doi: 10.1128/mcb.17.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 65.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 66.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 67.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 68.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 69.Unger T, Juven-Gershon T, Moallem E, Berger M, Vogt SR, Lozano G, Oren M, Haupt Y. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 1999;18:1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 71.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 72.Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. 1999;17:56–72. [PubMed] [Google Scholar]
- 73.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 74.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 75.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 76.Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O’Connor PM, Fornace AJ., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 77.Zhan Q, Bae I, Kastan MB, Fornace AJ., Jr The p53-dependent gamma-ray response of GADD45. Cancer Res. 1994;54:2755–2760. [PubMed] [Google Scholar]
- 78.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 80.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 81.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 82.Ding HF, Fisher DE. Mechanisms of p53-mediated apoptosis. Crit Rev Oncog. 1998;9:83–98. doi: 10.1615/critrevoncog.v9.i1.60. [DOI] [PubMed] [Google Scholar]
- 83.Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–M56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 85.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 86.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 87.Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta. 2001;1519:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 88.Kharbanda S, Saleem A, Datta R, Yuan ZM, Weichselbaum R, Kufe D. Ionizing radiation induces rapid tyrosine phosphorylation of p34cdc2. Cancer Res. 1994;54:1412–1414. [PubMed] [Google Scholar]
- 89.O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 91.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14–3–3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 92.Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 93.Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 94.Guweidhi A, Kleeff J, Giese N, El FJ, Ketterer K, Giese T, Buchler MW, Korc M, Friess H. Enhanced expression of 14-3-3 sigma in pancreatic cancer and its role in cell cycle regulation and apoptosis. Carcinogenesis. 2004;25:1575–1585. doi: 10.1093/carcin/bgh159. [DOI] [PubMed] [Google Scholar]
- 95.Neupane D, Korc M. 14-3-3sigma Modulates pancreatic cancer cell survival and invasiveness. Clin Cancer Res. 2008;14:7614–7623. doi: 10.1158/1078-0432.CCR-08-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan Y, Greer PM, Cao PT, Kolb RH, Cowan KH. RAC1 GTPase plays an important role in gamma-irradiation induced G2/M checkpoint activation. Breast Cancer Res. 2012;14(2):R60. doi: 10.1186/bcr3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan Y, Hein AL, Etekpo A, Burchett KM, Lin C, Enke CA, Batra SK, Cowan KH, Ouellette MM. Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to gamma-irradiation. Oncotarget. 2014;5:10251–10270. doi: 10.18632/oncotarget.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 101.Kumar MV, Shirley R, Ma Y, Lewis RW. Role of genomics-based strategies in overcoming chemotherapeutic resistance. Curr Pharm Biotechnol. 2004;5:471–480. doi: 10.2174/1389201043376698. [DOI] [PubMed] [Google Scholar]
- 102.Algan O, Stobbe CC, Helt AM, Hanks GE, Chapman JD. Radiation inactivation of human prostate cancer cells: the role of apoptosis. Radiat Res. 1996;146:267–275. [PubMed] [Google Scholar]
- 103.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 104.Dai Y, Lawrence TS, Xu L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor-kappa B. Am J Transl Res. 2009;1:1–15. [PMC free article] [PubMed] [Google Scholar]
- 105.Munshi A, Kurland JF, Nishikawa T, Chiao PJ, Andreeff M, Meyn RE. Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther. 2004;3:985–992. [PubMed] [Google Scholar]
- 106.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 107.Huang S, Deguzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- 108.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 109.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 110.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 111.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 112.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 113.Dai Y. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res. 2008;14(23):7701–7710. doi: 10.1158/1078-0432.CCR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 117.Olivier S, Robe P, Bours V. Can NF-kappaB be a target for novel and efficient anti-cancer agents? Biochem Pharmacol. 2006;72:1054–1068. doi: 10.1016/j.bcp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 118.Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–2959. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 119.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 121.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 122.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 123.Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999;19:388–398. doi: 10.1023/a:1020502800208. [DOI] [PubMed] [Google Scholar]
- 124.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 125.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 126.Srinivasula SM, Ashwell JD. IAPs: what’s in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer. 2008;7:64. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim DW, Seo SW, Cho SK, Chang SS, Lee HW, Lee SE, Block JA, Hei TK, Lee FY. Targeting of cell survival genes using small interfering RNAs (siRNAs) enhances radiosensitivity of Grade II chondrosarcoma cells. J Orthop Res. 2007;25:820–828. doi: 10.1002/jor.20377. [DOI] [PubMed] [Google Scholar]
- 129.Zhou L, Yuan R, Serggio L. Molecular mechanisms of irradiation-induced apoptosis. Front Biosci. 2003;8:d9–19. doi: 10.2741/927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bucur O, Ray S, Bucur MC, Almasan A. APO2 ligand/tumor necrosis factor-related apoptosis-inducing ligand in prostate cancer therapy. Front Biosci. 2006;11:1549–1568. doi: 10.2741/1903. [DOI] [PubMed] [Google Scholar]
- 131.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes T. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 132.Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 134.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petrucci E, Pasquini L, Petronelli A, Saulle E, Mariani G, Riccioni R, Biffoni M, Ferretti G, edetti-Panici P, Cognetti F, Scambia G, Humphreys R, Peschle C, Testa U. A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells. Gynecol Oncol. 2007;105:481–492. doi: 10.1016/j.ygyno.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 137.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 138.Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Tomita Y, Krajewski K, Roller PP, Wang S. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–4150. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 139.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004;24:7003–7014. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rowan S, Ludwig RL, Haupt Y, Bates S, Lu X, Oren M, Vousden KH. Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. EMBO J. 1996;15:827–838. [PMC free article] [PubMed] [Google Scholar]
- 141.Runnebaum IB, Nagarajan M, Bowman M, Soto D, Sukumar S. Mutations in p53 as potential molecular markers for human breast cancer. Proc Natl Acad Sci U S A. 1991;88:10657–10661. doi: 10.1073/pnas.88.23.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ludwig RL, Bates S, Vousden KH. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol Cell Biol. 1996;16:4952–4960. doi: 10.1128/mcb.16.9.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bargou RC, Daniel PT, Mapara MY, Bommert K, Wagener C, Kallinich B, Royer HD, Dorken B. Expression of the bcl-2 gene family in normal and malignant breast tissue: low bax-alpha expression in tumor cells correlates with resistance towards apoptosis. Int J Cancer. 1995;60:854–859. doi: 10.1002/ijc.2910600622. [DOI] [PubMed] [Google Scholar]
- 144.Efimova EV, Liang H, Pitroda SP, Labay E, Darga TE, Levina V, Lokshin A, Roizman B, Weichselbaum RR, Khodarev NN. Radioresistance of Stat1 over-expressing tumour cells is associated with suppressed apoptotic response to cytotoxic agents and increased IL6–IL8 signalling. Int J Radiat Biol. 2009;85:421–431. doi: 10.1080/09553000902838566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hui Z, Tretiakova M, Zhang Z, Li Y, Wang X, Zhu JX, Gao Y, Mai W, Furge K, Qian CN, Amato R, Butler EB, Teh BT, Teh BS. Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:288–295. doi: 10.1016/j.ijrobp.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 146.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101:1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, Fornace AJ., Jr Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64:6368–6371. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 148.Khodarev NN, Minn AJ, Efimova EV, Darga TE, Labay E, Beckett M, Mauceri HJ, Roizman B, Weichselbaum RR. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 2007;67:9214–9220. doi: 10.1158/0008-5472.CAN-07-1019. [DOI] [PubMed] [Google Scholar]
- 149.Townsend PA, Cragg MS, Davidson SM, McCormick J, Barry S, Lawrence KM, Knight RA, Hubank M, Chen PL, Latchman DS, Stephanou A. STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage. J Cell Sci. 2005;118:1629–1639. doi: 10.1242/jcs.01728. [DOI] [PubMed] [Google Scholar]
- 150.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, Roizman B, Bergh J, Pawitan Y, d van V, Minn AJ. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Macaulay VM, Salisbury AJ, Bohula EA, Playford MP, Smorodinsky NI, Shiloh Y. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001;20:4029–4040. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- 152.Min Y, Adachi Y, Yamamoto H, Ito H, Itoh F, Lee CT, Nadaf S, Carbone DP, Imai K. Genetic blockade of the insulin-like growth factor-I receptor: a promising strategy for human pancreatic cancer. Cancer Res. 2003;63:6432–6441. [PubMed] [Google Scholar]
- 153.Wang G, Yang Z, Zhang K. Endoplasmic Reticulum Stress Response in Cancer: Molecular Mechansim and Therapeutic Potential. Am J Transl Res. 2010;2(1):65–74. [PMC free article] [PubMed] [Google Scholar]
- 154.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 155.Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9:2635–2641. doi: 10.3748/wjg.v9.i12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eguchi H, Ishikawa O, Ohigashi H, Takahashi H, Yano M, Nishiyama K, Tomita Y, Uehara R, Takehara A, Nakamura Y, Nakagawa H. Serum REG4 level is a predictive biomarker for the response to preoperative chemoradiotherapy in patients with pancreatic cancer. Pancreas. 2009;38:791–798. doi: 10.1097/MPA.0b013e3181ac5337. [DOI] [PubMed] [Google Scholar]
- 157.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 160.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 161.Daniel F, Legrand A, Pessayre D, Borrega-Pires F, Mbida L, Lardeux B, Degott C, van Nhieu JT, Bernuau D. Beclin 1 mRNA strongly correlates with Bcl-XLmRNA expression in human hepatocellular carcinoma. Cancer Invest. 2007;25:226–231. doi: 10.1080/07357900701206323. [DOI] [PubMed] [Google Scholar]
- 162.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 163.Koneri K, Goi T, Hirono Y, Katayama K, Yamaguchi A. Beclin 1 gene inhibits tumor growth in colon cancer cell lines. Anticancer Res. 2007;27:1453–1457. [PubMed] [Google Scholar]
- 164.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 165.Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30:429–436. [PubMed] [Google Scholar]
- 166.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shen Y, Li DD, Wang LL, Deng R, Zhu XF. Decreased expression of autophagy- related proteins in malignant epithelial ovarian cancer. Autophagy. 2008;4:1067–1068. doi: 10.4161/auto.6827. [DOI] [PubMed] [Google Scholar]
- 168.Wang ZH, Peng ZL, Duan ZL, Liu H. Expression and clinical significance of autophagy gene Beclin 1 in cervical squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:860–863. [PubMed] [Google Scholar]
- 169.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–249. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 170.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 171.Isohashi F, Endo H, Mukai M, Inoue T, Inoue M. Insulin-like growth factor stimulation increases radiosensitivity of a pancreatic cancer cell line through endoplasmic reticulum stress under hypoxic conditions. Cancer Sci. 2008;99:2395–2401. doi: 10.1111/j.1349-7006.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fang Y, Tan J, Zhang Q. Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol Int. 2015;39:891–898. doi: 10.1002/cbin.10463. [DOI] [PubMed] [Google Scholar]
- 174.Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012;22:43–61. doi: 10.1038/cr.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Romano S, D’Angelillo A, Pacelli R, Staibano S, De LE, Bisogni R, Eskelinen EL, Mascolo M, Cali G, Arra C, Romano MF. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010;17:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 176.Yang S, Kimmelman AC. A critical role for autophagy in pancreatic cancer. Autophagy. 2011;7:912–913. doi: 10.4161/auto.7.8.15762. [DOI] [PubMed] [Google Scholar]
- 177.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Karhu R, Mahlamaki E, Kallioniemi A. Pancreatic adenocarcinoma -- genetic portrait from chromosomes to microarrays. Genes Chromosomes Cancer. 2006;45:721–730. doi: 10.1002/gcc.20337. [DOI] [PubMed] [Google Scholar]
- 179.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6:412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brunner TB, Cengel KA, Hahn SM, Wu J, Fraker DL, McKenna WG, Bernhard EJ. Pancreatic cancer cell radiation survival and prenyltransferase inhibition: the role of K-Ras. Cancer Res. 2005;65:8433–8441. doi: 10.1158/0008-5472.CAN-05-0158. [DOI] [PubMed] [Google Scholar]
- 181.You L, Chen G, Zhao YP. Core signaling pathways and new therapeutic targets in pancreatic cancer. Chin Med J (Engl) 2010;123:1210–1215. [PubMed] [Google Scholar]
- 182.Nishii K, Kabarowski JH, Gibbons DL, Griffiths SD, Titley I, Wiedemann LM, Greaves MF. ts BCR-ABL kinase activation confers increased resistance to genotoxic damage via cell cycle block. Oncogene. 1996;13:2225–2234. [PubMed] [Google Scholar]
- 183.Yuan ZM, Huang Y, Whang Y, Sawyers C, Weichselbaum R, Kharbanda S, Kufe D. Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 184.Fan S, Smith ML, Rivet DJ, Duba D, Zhan Q, Kohn KW, Fornace AJ, Jr, O’Connor PM. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 185.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 186.Normanno N, De LA, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De FG, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 187.Normanno N, Bianco C, De LA, Maiello MR, Salomon DS. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr Relat Cancer. 2003;10:1–21. doi: 10.1677/erc.0.0100001. [DOI] [PubMed] [Google Scholar]
- 188.Abd El-Rehim DM, Pinder SE, Paish CE, Bell JA, Rampaul RS, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer. 2004;91:1532–1542. doi: 10.1038/sj.bjc.6602184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kamio T, Shigematsu K, Sou H, Kawai K, Tsuchiyama H. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum Pathol. 1990;21:277–282. doi: 10.1016/0046-8177(90)90227-v. [DOI] [PubMed] [Google Scholar]
- 190.Klein C, Gensburger C, Freyermuth S, Nair BC, Labourdette G, Malviya AN. A 120 kDa nuclear phospholipase C gamma1 protein fragment is stimulated in vivo by EGF signal phosphorylating nuclear membrane EGFR. Biochemistry. 2004;43:15873–15883. doi: 10.1021/bi048604t. [DOI] [PubMed] [Google Scholar]
- 191.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 192.Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- 193.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat. 2006;95:211–218. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 194.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marti U, Burwen SJ, Wells A, Barker ME, Huling S, Feren AM, Jones AL. Localization of epidermal growth factor receptor in hepatocyte nuclei. Hepatology. 1991;13:15–20. [PubMed] [Google Scholar]
- 196.Marti U, Ruchti C, Kampf J, Thomas GA, Williams ED, Peter HJ, Gerber H, Burgi U. Nuclear localization of epidermal growth factor and epidermal growth factor receptors in human thyroid tissues. Thyroid. 2001;11:137–145. doi: 10.1089/105072501300042785. [DOI] [PubMed] [Google Scholar]
- 197.Marti U, Wells A. The nuclear accumulation of a variant epidermal growth factor receptor (EGFR) lacking the transmembrane domain requires coexpression of a full-length EGFR. Mol Cell Biol Res Commun. 2000;3:8–14. doi: 10.1006/mcbr.2000.0177. [DOI] [PubMed] [Google Scholar]
- 198.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, Rimm D, Burtness BA. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 199.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 200.Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76:157–161. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 201.Lucero H, Gae D, Taccioli GE. Novel localization of the DNA-PK complex in lipid rafts: a putative role in the signal transduction pathway of the ionizing radiation response. J Biol Chem. 2003;278:22136–22143. doi: 10.1074/jbc.M301579200. [DOI] [PubMed] [Google Scholar]
- 202.Contessa JN, Hampton J, Lammering G, Mikkelsen RB, Dent P, Valerie K, Schmidt-Ullrich RK. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002;21:4032–4041. doi: 10.1038/sj.onc.1205500. [DOI] [PubMed] [Google Scholar]
- 203.Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 205.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 206.Toulany M, Dittmann K, Kruger M, Baumann M, Rodemann HP. Radioresistance of K-Ras mutated human tumor cells is mediated through EGFR-dependent activation of PI3K-AKT pathway. Radiother Oncol. 2005;76:143–150. doi: 10.1016/j.radonc.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 207.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 208.Zhan M, Han ZC. Phosphatidylinositide 3-kinase/AKT in radiation responses. Histol Histopathol. 2004;19:915–923. doi: 10.14670/HH-19.915. [DOI] [PubMed] [Google Scholar]
- 209.Das AK, Sato M, Story MD, Peyton M, Graves R, Redpath S, Girard L, Gazdar AF, Shay JW, Minna JD, Nirodi CS. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66:9601–9608. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 210.Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, Nirodi CS. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67:5267–5274. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- 211.Agarwal JP, Gupta T, Kalyani N, Budrukkar A, Laskar SG, Murthy V, Kumar P, Narohna V, Pai P, Chaturvedi P, D’cruz AK. Cetuximab with radiotherapy in patients with loco-regionally advanced squamous cell carcinoma of head and neck unsuitable or ineligible for concurrent platinum-based chemo-radiotherapy: Ready for routine clinical practice? Indian J Cancer. 2011;48:148–153. doi: 10.4103/0019-509X.82872. [DOI] [PubMed] [Google Scholar]
- 212.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 213.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 214.Frampton JE. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck. Drugs. 2010;70:1987–2010. doi: 10.2165/11205010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 215.Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W, Baron AE, Zeng C, Johnson TK, Bunn PA., Jr The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11:795–805. [PubMed] [Google Scholar]
- 216.Blumenschein GR, Jr, Paulus R, Curran WJ, Robert F, Fossella F, Werner-Wasik M, Herbst RS, Doescher PO, Choy H, Komaki R. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–2318. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shin HK, Kim MS, Lee JK, Lee SS, Ji YH, Kim JI, Jeong JH. Combination effect of cetuximab with radiation in colorectal cancer cells. Tumori. 2010;96:713–720. doi: 10.1177/030089161009600513. [DOI] [PubMed] [Google Scholar]
- 218.Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295–308. doi: 10.1007/s00423-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 219.Barker CA, Burgan WE, Carter DJ, Cerna D, Gius D, Hollingshead MG, Camphausen K, Tofilon PJ. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) Clin Cancer Res. 2006;12:2912–2918. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 220.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 221.Matsuoka H, Shibamoto Y, Kubota T, Tsujitani M, Majima T. In vivo efficacy and pharmacokinetics of a new hypoxic cell radiosensitizer doranidazole in SUIT-2 human pancreatic cancer xenografted in mouse pancreas. Oncol Rep. 2000;7:23–26. doi: 10.3892/or.7.1.23. [DOI] [PubMed] [Google Scholar]
- 222.Mizumoto K, Qian LW, Zhang L, Nagai E, Kura S, Tanaka M. A nitroimidazole derivative, PR-350, enhances the killing of pancreatic cancer cells exposed to high-dose irradiation under hypoxia. J Radiat Res. 2002;43:43–51. doi: 10.1269/jrr.43.43. [DOI] [PubMed] [Google Scholar]
- 223.Shibamoto Y, Kubota T, Kishii K, Tsujitani M. Radiosensitivity of human pancreatic cancer cells in vitro and in vivo, and the effect of a new hypoxic cell sensitizer, doranidazole. Radiother Oncol. 2000;56:265–270. doi: 10.1016/s0167-8140(00)00181-x. [DOI] [PubMed] [Google Scholar]
- 224.Yahiro T, Masui S, Kubota N, Yamada K, Kobayashi A, Kishii K. Effects of hypoxic cell radiosensitizer doranidazole (PR-350) on the radioresponse of murine and human tumor cells in vitro and in vivo. J Radiat Res. 2005;46:363–372. doi: 10.1269/jrr.46.363. [DOI] [PubMed] [Google Scholar]
- 225.Karasawa K, Sunamura M, Okamoto A, Nemoto K, Matsuno S, Nishimura Y, Shibamoto Y. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: long-term results of a placebo-controlled randomised study. Radiother Oncol. 2008;87:326–330. doi: 10.1016/j.radonc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 226.Sunamura M, Karasawa K, Okamoto A, Ogata Y, Nemoto K, Hosotani R, Nishimura Y, Matsui K, Matsuno S. Phase III trial of radiosensitizer PR-350 combined with intraoperative radiotherapy for the treatment of locally advanced pancreatic cancer. Pancreas. 2004;28:330–334. doi: 10.1097/00006676-200404000-00023. [DOI] [PubMed] [Google Scholar]
- 227.Reichert ZR, Wahl DR, Morgan MA. Translation of Targeted Radiation Sensitizers into Clinical Trials. Semin Radiat Oncol. 2016;26:261–270. doi: 10.1016/j.semradonc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 228.Chang BW, Saif MW. Combining epidermal growth factor receptor inhibitors and radiation therapy in pancreatic cancer: small step or giant leap? JOP. 2009;10:231–236. [PubMed] [Google Scholar]
- 229.Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, Shields JM, Sartor CI. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16:912–923. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Munter M, Timke C, Abdollahi A, Friess H, Jaeger D, Heeger S, et al. Final Results of a Phase II Trial [PARC-Study ISRCTN56652283] for Patients With Primary Inoperable Locally Advanced Pancreatic Cancer Combining Intensity-Modulated Radiotherapy (IMRT) With Cetuximab and Gemcitabine. J Clin Oncol. 2008;26:4613. [Google Scholar]
- 232.Duffy A, Kortmansky J, Schwartz GK, Capanu M, Puleio S, Minsky B, Saltz L, Kelsen DP, O’Reilly EM. A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma. Ann Oncol. 2008;19:86–91. doi: 10.1093/annonc/mdm441. [DOI] [PubMed] [Google Scholar]
- 233.Maurel J, Martin-Richard M, Conill C, Sanchez M, Petriz L, Gines A, Miquel R, Gallego R, Cajal R, Ayuso C, Navarro S, Marmol M, Nadal C, Auge JM, Fernandez-Cruz L, Gascon P. Phase I trial of gefitinib with concurrent radiotherapy and fixed 2-h gemcitabine infusion, in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2006;66:1391–1398. doi: 10.1016/j.ijrobp.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 234.Demols A, Mahin C, Marechal R, Delaunoit T, Borbath I, Hendlisz A, et al. Cetuximab Plus Chemoradiation Combined Therapy for Locally Advanced Inoperable Pancreatic Adenocarcinoma: a Phase I Study. J Clin Oncol. 2008;26:4629. [Google Scholar]
- 235.Robertson JM, Margolis J, Jury RP, Balaraman S, Cotant MB, Ballouz S, Boxwala IG, Jaiyesimi IA, Nadeau L, Hardy-Carlson M, Marvin KS, Wallace M, Ye H. Phase I Study of Conformal Radiotherapy and Concurrent Full-Dose Gemcitabine with Erlotinib for Unresected Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):e187–192. doi: 10.1016/j.ijrobp.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 236.Czito BG, Willett CG, Bendell JC, Morse MA, Tyler DS, Fernando NH, Mantyh CR, Blobe GC, Honeycutt W, Yu D, Clary BM, Pappas TN, Ludwig KA, Hurwitz HI. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol. 2006;24:656–662. doi: 10.1200/JCO.2005.04.1749. [DOI] [PubMed] [Google Scholar]
- 237.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouche O, Shannon J, Andre T, Mineur L, Chibaudel B, Bonnetain F, Louvet C. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 238.Hirsh V. Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. BioDrugs. 2015;29:167–183. doi: 10.1007/s40259-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hurvitz SA, Shatsky R, Harbeck N. Afatinib in the treatment of breast cancer. Expert Opin Investig Drugs. 2014;23:1039–1047. doi: 10.1517/13543784.2014.924505. [DOI] [PubMed] [Google Scholar]
- 240.Modjtahedi H, Cho BC, Michel MC, Solca F. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:505–521. doi: 10.1007/s00210-014-0967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 242.Huguet F, Fernet M, Giocanti N, Favaudon V, Larsen AK. Afatinib, an Irreversible EGFR Family Inhibitor, Shows Activity Toward Pancreatic Cancer Cells, Alone and in Combination with Radiotherapy, Independent of KRAS Status. Target Oncol. 2016;11(3):371–381. doi: 10.1007/s11523-015-0403-8. [DOI] [PubMed] [Google Scholar]
- 243.Gupta AK, Li B, Cerniglia GJ, Ahmed MS, Hahn SM, Maity A. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia. 2007;9:271–278. doi: 10.1593/neo.07124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 245.Brunner TB, Geiger M, Grabenbauer GG, Lang-Welzenbach M, Mantoni TS, Cavallaro A, Sauer R, Hohenberger W, McKenna WG. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–2706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 246.Konsoula Z, Velena A, Lee R, Dritschilo A, Jung M. Histone deacetylase inhibitor: antineoplastic agent and radiation modulator. Adv Exp Med Biol. 2011;720:171–179. doi: 10.1007/978-1-4614-0254-1_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Deorukhkar A, Shentu S, Park HC, Diagaradjane P, Puduvalli V, Aggarwal B, Guha S, Krishnan S. Inhibition of radiation-induced DNA repair and prosurvival pathways contributes to vorinostat-mediated radiosensitization of pancreatic cancer cells. Pancreas. 2010;39:1277–1283. doi: 10.1097/MPA.0b013e3181dd63e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chan E, Arlinghaus LR, Cardin DB, Goff L, Berlin JD, Parikh A, Abramson RG, Yankeelov TE, Hiebert S, Merchant N, Bhaskara S, Chakravarthy AB. Phase I trial of vorinostat added to chemoradiation with capecitabine in pancreatic cancer. Radiother Oncol. 2016;119:312–318. doi: 10.1016/j.radonc.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rich TA, Winter K, Safran H, Hoffman JP, Erickson B, Anne PR, Myerson RJ, Cline-Burkhardt VJ, Perez K, Willett C. Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco Targets Ther. 2012;5:161–70. doi: 10.2147/OTT.S33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lerner EC, Zhang TT, Knowles DB, Qian Y, Hamilton AD, Sebti SM. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15:1283–1288. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]
- 251.Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J Biol Chem. 1997;272:14093–14097. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 252.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 253.Martin NE, Brunner TB, Kiel KD, DeLaney TF, Regine WF, Mohiuddin M, Rosato EF, Haller DG, Stevenson JP, Smith D, Pramanik B, Tepper J, Tanaka WK, Morrison B, Deutsch P, Gupta AK, Muschel RJ, McKenna WG, Bernhard EJ, Hahn SM. A phase I trial of the dual farnesyltransferase and geranylgeranyltransferase inhibitor L-778,123 and radiotherapy for locally advanced pancreatic cancer. Clin Cancer Res. 2004;10:5447–5454. doi: 10.1158/1078-0432.CCR-04-0248. [DOI] [PubMed] [Google Scholar]
- 254.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 255.Sasabe E, Zhou X, Li D, Oku N, Yamamoto T, Osaki T. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- 256.Schwartz DL, Bankson JA, Lemos R, Jr, Lai SY, Thittai AK, He Y, Hostetter G, Demeure MJ, Von Hoff DD, Powis G. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther. 2010;9:2057–2067. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schwartz DL, Powis G, Thitai-Kumar A, He Y, Bankson J, Williams R, Lemos R, Oh J, Volgin A, Soghomonyan S, Nishii R, Alauddin M, Mukhopadhay U, Peng Z, Bornmann W, Gelovani J. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther. 2009;8:947–958. doi: 10.1158/1535-7163.MCT-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Williams TM, Flecha AR, Keller P, Ram A, Karnak D, Galban S, Galban CJ, Ross BD, Lawrence TS, Rehemtulla A, Sebolt-Leopold J. Cotargeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol Cancer Ther. 2012;11:1193–1202. doi: 10.1158/1535-7163.MCT-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, Arumugarajah S, Hylander-Gans L, Morosini D, Simeone DM, Canman CE, Normolle DP, Zabludoff SD, Maybaum J, Lawrence TS. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–4981. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Engelke CG, Parsels LA, Qian Y, Zhang Q, Karnak D, Robertson JR, Tanska DM, Wei D, Davis MA, Parsels JD, Zhao L, Greenson JK, Lawrence TS, Maybaum J, Morgan MA. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res. 2013;19:4412–4421. doi: 10.1158/1078-0432.CCR-12-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vance S, Liu E, Zhao L, Parsels JD, Parsels LA, Brown JL, Maybaum J, Lawrence TS, Morgan MA. Selective radiosensitization of p53 mutant pancreatic cancer cells by combined inhibition of Chk1 and PARP1. Cell Cycle. 2011;10:4321–4329. doi: 10.4161/cc.10.24.18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chalmers AJ. The potential role and application of PARP inhibitors in cancer treatment. Br Med Bull. 2009;89:23–40. doi: 10.1093/bmb/ldp005. [DOI] [PubMed] [Google Scholar]
- 263.Hirai T, Shirai H, Fujimori H, Okayasu R, Sasai K, Masutani M. Radiosensitization effect of poly(ADP-ribose) polymerase inhibition in cells exposed to low and high liner energy transfer radiation. Cancer Sci. 2012;103:1045–1050. doi: 10.1111/j.1349-7006.2012.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Karnak D, Engelke CG, Parsels LA, Kausar T, Wei D, Robertson JR, Marsh KB, Davis MA, Zhao L, Maybaum J, Lawrence TS, Morgan MA. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin Cancer Res. 2014;20:5085–5096. doi: 10.1158/1078-0432.CCR-14-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Estrada-Bernal A, Chatterjee M, Haque SJ, Yang L, Morgan MA, Kotian S, Morrell D, Chakravarti A, Williams TM. MEK inhibitor GSK1120212-mediated radiosensitization of pancreatic cancer cells involves inhibition of DNA double-strand break repair pathways. Cell Cycle. 2015;14:3713–3724. doi: 10.1080/15384101.2015.1104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, Morgan MA. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res. 2013;19:4422–4432. doi: 10.1158/1078-0432.CCR-13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies MW, Muschel RJ, Brunner TB. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Prevo R, Fokas E, Reaper PM, Charlton PA, Pollard JR, McKenna WG, Muschel RJ, Brunner TB. The novel ATR inhibitor VE-821 increases sensitivity of pancreatic cancer cells to radiation and chemotherapy. Cancer Biol Ther. 2012;13:1072–1081. doi: 10.4161/cbt.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 270.Jia L, Bickel JS, Wu J, Morgan MA, Li H, Yang J, Yu X, Chan RC, Sun Y. RBX1 (RING box protein 1) E3 ubiquitin ligase is required for genomic integrity by modulating DNA replication licensing proteins. J Biol Chem. 2011;286:3379–3386. doi: 10.1074/jbc.M110.188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, Smith PG, Morgan MA, Sun Y. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72:282–293. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Niida H, Katsuno Y, Sengoku M, Shimada M, Yukawa M, Ikura M, Ikura T, Kohno K, Shima H, Suzuki H, Tashiro S, Nakanishi M. Essential role of Tip60-dependent recruitment of ribonucleotide reductase at DNA damage sites in DNA repair during G1 phase. Genes Dev. 2010;24:333–338. doi: 10.1101/gad.1863810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shafaee Z, Schmidt H, Du W, Posner M, Weichselbaum R. Cyclopamine increases the cytotoxic effects of paclitaxel and radiation but not cisplatin and gemcitabine in Hedgehog expressing pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;58:765–770. doi: 10.1007/s00280-006-0227-4. [DOI] [PubMed] [Google Scholar]
- 274.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038–1048. doi: 10.1158/1535-7163.MCT-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Masui T, Hosotani R, Ito D, Kami K, Koizumi M, Mori T, Toyoda E, Nakajima S, Miyamoto Y, Fujimoto K, Doi R. Bcl-XL antisense oligonucleotides coupled with antennapedia enhances radiation-induced apoptosis in pancreatic cancer. Surgery. 2006;140:149–160. doi: 10.1016/j.surg.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 279.Guoan X, Hanning W, Kaiyun C, Hao L. Adenovirus-mediated siRNA targeting Mcl-1 gene increases radiosensitivity of pancreatic carcinoma cells in vitro and in vivo. Surgery. 2010;147:553–561. doi: 10.1016/j.surg.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 280.Dote H, Cerna D, Burgan WE, Camphausen K, Tofilon PJ. ErbB3 expression predicts tumor cell radiosensitization induced by Hsp90 inhibition. Cancer Res. 2005;65:6967–6975. doi: 10.1158/0008-5472.CAN-05-1304. [DOI] [PubMed] [Google Scholar]
- 281.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66:9211–9220. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- 282.Milanovic D, Firat E, Grosu AL, Niedermann G. Increased radiosensitivity and radiothermosensitivity of human pancreatic MIA PaCa-2 and U251 glioblastoma cell lines treated with the novel Hsp90 inhibitor NVP-HSP990. Radiat Oncol. 2013;8:42. doi: 10.1186/1748-717X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cordes N, Frick S, Brunner TB, Pilarsky C, Grutzmann R, Sipos B, Kloppel G, McKenna WG, Bernhard EJ. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–6862. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 284.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother Oncol. 2009;92:362–370. doi: 10.1016/j.radonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 285.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER, Hammer GD, Lawrence TS, Ben-Josef E. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12:357–365. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sahu RP, Epperly MW, Srivastava SK. Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation therapy. Front Biosci (Elite Ed) 2009;1:568–576. doi: 10.2741/e55. [DOI] [PubMed] [Google Scholar]
- 287.Veeraraghavan J, Natarajan M, Lagisetty P, Awasthi V, Herman TS, Aravindan N. Impact of curcumin, raspberry extract, and neem leaf extract on rel protein-regulated cell death/radiosensitization in pancreatic cancer cells. Pancreas. 2011;40:1107–1119. doi: 10.1097/MPA.0b013e31821f677d. [DOI] [PubMed] [Google Scholar]
- 288.Choudhuri R, Degraff W, Gamson J, Mitchell JB, Cook JA. Guggulsterone-mediated enhancement of radiosensitivity in human tumor cell lines. Front Oncol. 2011;1:19. doi: 10.3389/fonc.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pruitt R, Sasi N, Freeman ML, Sekhar KR. Radiosensitization of cancer cells by hydroxychalcones. Bioorg Med Chem Lett. 2010;20:5997–6000. doi: 10.1016/j.bmcl.2010.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chung HW, Wen J, Lim JB, Bang S, Park SW, Song SY. Radiosensitization effect of STI-571 on pancreatic cancer cells in vitro. Int J Radiat Oncol Biol Phys. 2009;75:862–869. doi: 10.1016/j.ijrobp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 291.Bence AK, Adams VR, Crooks PA. L-Canavanine as a radiosensitization agent for human pancreatic cancer cells. Mol Cell Biochem. 2003;244:37–43. [PubMed] [Google Scholar]
- 292.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, Iovanna JL, Tomasini R, Vasseur S. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dholakia AS, Chaudhry M, Leal JP, Chang DT, Raman SP, Hacker-Prietz A, Su Z, Pai J, Oteiza KE, Griffith ME, Wahl RL, Tryggestad E, Pawlik T, Laheru DA, Wolfgang CL, Koong AC, Herman JM. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:539–546. doi: 10.1016/j.ijrobp.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 298.Coleman MC, Asbury CR, Daniels D, Du J, Aykin-Burns N, Smith BJ, Li L, Spitz DR, Cullen JJ. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 299.Galuska D, Zierath J, Thorne A, Sonnenfeld T, Wallberg-Henriksson H. Metformin increases insulin-stimulated glucose transport in insulin-resistant human skeletal muscle. Diabete Metab. 1991;17:159–163. [PubMed] [Google Scholar]
- 300.Fasih A, Elbaz HA, Huttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182:50–59. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Shimura T, Noma N, Sano Y, Ochiai Y, Oikawa T, Fukumoto M, Kunugita N. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol. 2014;112:302–307. doi: 10.1016/j.radonc.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 302.Du J, Cieslak JA, III, Welsh JL, Sibenaller ZA, Allen BG, Wagner BA, Kalen AL, Doskey CM, Strother RK, Button AM, Mott SL, Smith B, Tsai S, Mezhir J, Goswami PC, Spitz DR, Buettner GR, Cullen JJ. Pharmacological Ascorbate Radiosensitizes Pancreatic Cancer. Cancer Res. 2015;75:3314–3326. doi: 10.1158/0008-5472.CAN-14-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol. 2014;42:247–251. doi: 10.1016/j.exphem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 306.Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS, Kwon TK. beta-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014;5:e1230. doi: 10.1038/cddis.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Planchon SM, Pink JJ, Tagliarino C, Bornmann WG, Varnes ME, Boothman DA. beta-Lapachone-induced apoptosis in human prostate cancer cells: involvement of NQO1/xip3. Exp Cell Res. 2001;267:95–106. doi: 10.1006/excr.2001.5234. [DOI] [PubMed] [Google Scholar]
- 308.Chakrabarti G, Moore ZR, Luo X, Ilcheva M, Ali A, Padanad M, Zhou Y, Xie Y, Burma S, Scaglioni PP, Cantley LC, DeBerardinis RJ, Kimmelman AC, Lyssiotis CA, Boothman DA. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by ss-lapachone. Cancer Metab. 2015;3:12. doi: 10.1186/s40170-015-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ahn KJ, Lee HS, Bai SK, Song CW. Enhancement of radiation effect using beta- lapachone and underlying mechanism. Radiat Oncol J. 2013;31:57–65. doi: 10.3857/roj.2013.31.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li LS, Reddy S, Lin ZH, Liu S, Park H, Chun SG, Bornmann WG, Thibodeaux J, Yan J, Chakrabarti G, Xie XJ, Sumer BD, Boothman DA, Yordy JS. NQO1-Mediated Tumor-Selective Lethality and Radiosensitization for Head and Neck Cancer. Mol Cancer Ther. 2016;15:1757–1767. doi: 10.1158/1535-7163.MCT-15-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Souchek JJ, Baine MJ, Lin C, Rachagani S, Gupta S, Kaur S, Lester K, Zheng D, Chen S, Smith L, Lazenby A, Johansson SL, Jain M, Batra SK. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br J Cancer. 2014;111:1139–1149. doi: 10.1038/bjc.2014.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Harada T, Chelala C, Crnogorac-Jurcevic T, Lemoine NR. Genome-wide analysis of pancreatic cancer using microarray-based techniques. Pancreatology. 2009;9:13–24. doi: 10.1159/000178871. [DOI] [PubMed] [Google Scholar]
- 313.Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, Chen JH, Zhang JT. Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol. 2011;2:89–98. [PMC free article] [PubMed] [Google Scholar]
- 314.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Daemen A, Peterson D, Sahu N, McCord R, Du X, Liu B, Kowanetz K, Hong R, Moffat J, Gao M, Boudreau A, Mroue R, Corson L, O’Brien T, Qing J, Sampath D, Merchant M, Yauch R, Manning G, Settleman J, Hatzivassiliou G, Evangelista M. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci U S A. 2015;112:E4410–E4417. doi: 10.1073/pnas.1501605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Regine WF, Winter KW, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, MacDonald JS, Willett CG, Rich TA. RTOG 9704 a Phase III Study of Adjuvant Pre and Post Chemoradiation 5-FU Vs. Gemcitabine for Resected Pancreatic Adenocarcinoma. J Clin Oncol. 2004;24:4007. [Google Scholar]
- 319.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, Benson AB., III Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol. 2016;39:302–313. doi: 10.1097/COC.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 321.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK, Kee S, Trueblood W, Yang G, Bastidas JA. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 322.Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J, Yang GP. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 323.Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, Nellemann H, Kiil BA, Eberholst F, Engelholm SA, von der MH. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 324.Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, Fisher GA, Quon A, Desser TS, Norton J, Greco R, Yang GP, Koong AC. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 325.Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, Fisher GA, Kunz PL, Van DJ, Quon A, Desser TS, Norton J, Hsu A, Maxim PG, Xing L, Goodman KA, Chang DT, Koong AC. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 326.Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 327.Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547–1559. doi: 10.1007/s11605-010-1323-7. [DOI] [PubMed] [Google Scholar]
- 328.Verma V, Lazenby AJ, Zheng D, Bhirud AR, Ly QP, Are C, Sasson AR, Lin C. Dosimetric Parameters Correlate With Duodenal Histopathologic Damage After Stereotactic Body Radiotherapy for Pancreatic Cancer: Secondary Analysis of a Prospective Clinical Trial. Radiotherapy and Oncology, Radiother Oncol. 2017 doi: 10.1016/j.radonc.2016.12.030. http://dx.doi.org/10.1016/j.radonc.2016.12.030. [DOI] [PMC free article] [PubMed]
- 329.Koukourakis MI. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Br J Radiol. 2012;85:313–330. doi: 10.1259/bjr/16386034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Grdina DJ, Shigematsu N, Dale P, Newton GL, Aguilera JA, Fahey RC. Thiol and disulfide metabolites of the radiation protector and potential chemopreventive agent WR-2721 are linked to both its anti-cytotoxic and anti-mutagenic mechanisms of action. Carcinogenesis. 1995;16:767–774. doi: 10.1093/carcin/16.4.767. [DOI] [PubMed] [Google Scholar]
- 331.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 332.Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002;13:181–209. doi: 10.1097/00001813-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 333.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res. 2007;167:465–474. doi: 10.1667/RR0758.1. [DOI] [PubMed] [Google Scholar]
- 334.Makinde AY, Luo-Owen X, Rizvi A, Crapo JD, Pearlstein RD, Slater JM, Gridley DS. Effect of a metalloporphyrin antioxidant (MnTE-2-PyP) on the response of a mouse prostate cancer model to radiation. Anticancer Res. 2009;29:107–118. [PubMed] [Google Scholar]
- 335.Tong Q, Weaver MR, Kosmacek EA, O’Connor BP, Harmacek L, Venkataraman S, Oberley-Deegan RE. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic Biol Med. 2016;94:185–94. doi: 10.1016/j.freeradbiomed.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Onconova Therapeutics, Inc. [Last accessed 23 january 2017];2017 Available from: http://www.onconova.com/product-pipeline/recilisib.php.
- 338.Ghosh SP, Perkins MW, Hieber K, Kulkarni S, Kao TC, Reddy EP, Reddy MV, Maniar M, Seed T, Kumar KS. Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Radiat Res. 2009;171:173–179. doi: 10.1667/RR1367.1. [DOI] [PubMed] [Google Scholar]
- 339.Morgan MA, Lawrence TS. Molecular Pathways: Overcoming Radiation Resistance by Targeting DNA Damage Response Pathways. Clin Cancer Res. 2015;21:2898–2904. doi: 10.1158/1078-0432.CCR-13-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yu HA, Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers. J Natl Compr Canc Netw. 2013;11:161–169. doi: 10.6004/jnccn.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di CS, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 342.Chun SG, Zhou W, Yee NS. Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer. Cancer Biol Ther. 2009;8:1328–1339. doi: 10.4161/cbt.8.14.8633. [DOI] [PubMed] [Google Scholar]
- 343.Dain A, Kerkut GA, Smith RC, Munday KA, Wilmshurst TH. The interaction of free radicals in protein and melanin. Experientia. 1964;20:76–78. doi: 10.1007/BF02151249. [DOI] [PubMed] [Google Scholar]
- 344.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 345.Schulman SG. Fundamentals of interaction of ionizing radiations with chemical, biochemical, and pharmaceutical systems. J Pharm Sci. 1973;62:1745–1757. doi: 10.1002/jps.2600621102. [DOI] [PubMed] [Google Scholar]
- 346.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381:244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zheng W, Skowron KB, Namm JP, Burnette B, Fernandez C, Arina A, Liang H, Spiotto MT, Posner MC, Fu YX, Weichselbaum RR. Combination of radiotherapy and vaccination overcome checkpoint blockade resistance. Oncotarget. 2016;7(28):43039–43051. doi: 10.18632/oncotarget.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Seifert L, Werba G, Tiwari S, Giao Ly NN, Nguy S, Alothman S, Alqunaibit D, Avanzi A, Daley D, Barilla R, Tippens D, Torres-Hernandez A, Hundeyin M, Mani VR, Hajdu C, Pellicciotta I, Oh P, Du K, Miller G. Radiation Therapy Induces Macrophages to Suppress T-Cell Responses Against Pancreatic Tumors in Mice. Gastroenterology. 2016;150:1659–1672. doi: 10.1053/j.gastro.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 352.Klinkenbijl JH, Jeekel J, Sahmoud T, van PR, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 354.Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL, Laheru DA, Farnell MB, Swartz MJ, Gunderson LL, Miller RC. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Haller DG. Chemotherapy for advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;56:16–23. doi: 10.1016/s0360-3016(03)00448-6. [DOI] [PubMed] [Google Scholar]
- 359.John M, Pajak T, Flam M, Hoffman J, Markoe A, Wolkov H, Paris K. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92–08. Cancer J Sci Am. 1996;2:205–211. [PubMed] [Google Scholar]
- 360.Overgaard J, Hjelm-Hansen M, Johansen LV, Andersen AP. Comparison of conventional and split-course radiotherapy as primary treatment in carcinoma of the larynx. Acta Oncol. 1988;27:147–152. doi: 10.3109/02841868809090334. [DOI] [PubMed] [Google Scholar]
- 361.Fletcher GH. Textbook of Radiotherapy. 3. Philadelphia, Pa: Lea & Febiger; 1980. pp. 330–363. [Google Scholar]
- 362.Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31:353–359. doi: 10.1016/0360-3016(94)00354-N. [DOI] [PubMed] [Google Scholar]
- 363.Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas HO, Jr, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 364.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 365.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 366.Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, de GA, Louvet C. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 367.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, Bosset JF, Aparicio T, Mineur L, Azzedine A, Hammel P, Butel J, Stremsdoerfer N, Maingon P, Bedenne L. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 368.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 369.Heinemann V. Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. Semin Oncol. 2002;29:9–16. doi: 10.1053/sonc.2002.37372. [DOI] [PubMed] [Google Scholar]
- 370.Ogawa K, Utsunomiya T, Mimori K, Tanaka F, Haraguchi N, Inoue H, Murayama S, Mori M. Differential gene expression profiles of radioresistant pancreatic cancer cell lines established by fractionated irradiation. Int J Oncol. 2006;28:705–713. [PubMed] [Google Scholar]
- 371.Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang PF, Li C, Peng F, Tang CE, Li JL, Chen ZC, Xiao ZQ. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res. 2010;70:3450–3462. doi: 10.1158/0008-5472.CAN-09-4099. [DOI] [PubMed] [Google Scholar]
- 372.Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Xu QY, Gao Y, Liu Y, Yang WZ, Xu XY. Identification of differential gene expression profiles of radioresistant lung cancer cell line established by fractionated ionizing radiation in vitro. Chin Med J (Engl) 2008;121:1830–1837. [PubMed] [Google Scholar]
- 374.Yang HJ, Kim N, Seong KM, Youn H, Youn B. Investigation of radiation-induced transcriptome profile of radioresistant non-small cell lung cancer A549 cells using RNA-seq. PLoS One. 2013;8:e59319. doi: 10.1371/journal.pone.0059319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Young A, Berry R, Holloway AF, Blackburn NB, Dickinson JL, Skala M, Phillips JL, Brettingham-Moore KH. RNA-seq profiling of a radiation resistant and radiation sensitive prostate cancer cell line highlights opposing regulation of DNA repair and targets for radiosensitization. BMC Cancer. 2014;14:808–814. doi: 10.1186/1471-2407-14-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Suetens A, Moreels M, Quintens R, Chiriotti S, Tabury K, Michaux A, Gregoire V, Baatout S. Carbon ion irradiation of the human prostate cancer cell line PC3: a whole genome microarray study. Int J Oncol. 2014;44:1056–1072. doi: 10.3892/ijo.2014.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Suetens A, Moreels M, Quintens R, Soors E, Buset J, Chiriotti S, Tabury K, Gregoire V, Baatout S. Dose- and time-dependent gene expression alterations in prostate and colon cancer cells after in vitro exposure to carbon ion and X-irradiation. J Radiat Res. 2015;56:11–21. doi: 10.1093/jrr/rru070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wei F, Liu Y, Guo Y, Xiang A, Wang G, Xue X, Lu Z. miR-99b-targeted mTOR induction contributes to irradiation resistance in pancreatic cancer. Mol Cancer. 2013;12:81. doi: 10.1186/1476-4598-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]