Abstract
Polymorphisms in the human leukocyte antigen (HLA) class I genes can cause the rejection of pluripotent stem cell (PSC)-derived products in allogeneic recipients. Disruption of the Beta-2 Microglobulin (B2M) gene eliminates surface expression of all class I molecules, but leaves the cells vulnerable to lysis by natural killer (NK) cells. Here we show that this ‘missing self’ response can be prevented by forced expression of minimally polymorphic HLA-E molecules. We use adeno-associated virus (AAV)-mediated gene editing to knock in HLA-E genes at the B2M locus in human PSCs in a manner that confers inducible, regulated, surface expression of HLA-E single-chain dimers (fused to B2M) or trimers (fused to B2M and a peptide antigen), without surface expression of HLA-A, B or C. These HLA-engineered PSCs and their differentiated derivatives are not recognized as allogeneic by CD8+ T cells, do not bind anti-HLA antibodies, and are resistant to NK-mediated lysis. Our approach provides a potential source of universal donor cells for applications where the differentiated derivatives lack HLA class II expression.
Introduction
Cellular products derived from human pluripotent stem cells (PSCs) have the potential to treat many human diseases, but their clinical use is limited by the host’s rejection of transplanted cells due to expression of the highly polymorphic human leukocyte antigen (HLA) genes. Various solutions have been proposed for this allogeneic rejection problem, including HLA-typed PSC banks1, or the derivation of induced PSCs from each patient2, but these approaches require that multiple cell lines be derived3, differentiated into therapeutic products, and approved for human administration. The development of a single PSC line that can avoid allogeneic rejection would greatly reduce the time and expense required to advance cellular therapies to the clinic
HLA class I molecules are expressed on most cells and play a central role in allogeneic rejection through their presentation of peptide antigens to CD8+ T cells4. Beta-2 Microglobulin (B2M) is a non-polymorphic gene that encodes a common protein subunit required for surface expression of all polymorphic HLA class I heavy chains5. Our group6 and others7–9 have created B2M−/− human PSCs in order to eliminate class I surface expression and prevent the stimulation of allogeneic CD8+ T cells. However, a major limitation of this simple engineering strategy is that HLA class I-negative cells are lysed by natural killer (NK) cells through the “missing self” response10,11. In mice, host NK cells eliminate transplanted B2m−/− donor cells10, and a similar phenomenon occurs in vitro with HLA class I-negative human leukemic lines12. This NK cell-dependent lysis is normally inhibited through interactions with class I molecules13, including the minimalpolymorphic HLA-E protein14–16, which presents peptides derived from the signal sequences of other HLA class I molecules17, and is a ligand for the inhibitory CD94/NGK2A complex expressed on the majority of NK cells14,15,18. Like other HLA class I molecules, HLA-E forms a heterodimer with a B2M subunit so it is not expressed on the surface of B2M−/− cells. We surmised that B2M−/− cells could be engineered to express HLA-E as a single chain protein fused to B2M, and thereby create cells that express HLA- E as their only surface HLA class I molecule.
Here we use recombinant adeno-associated virus (rAAV)-mediated gene editing to create such PSCs. rAAV vectors deliver single-stranded linear DNA genomes that efficiently recombine with homologous chromosomal sequences in up to 1% of infected human cells without the use of potentially genotoxic nucleases19,20, including human PSCs21–23. We show that single chain HLA-E molecules prevent the NK-mediated lysis of B2M−/− cells without stimulating allogeneic T cells, addressing a major problem in the creation of universal donor cells for regenerative medicine applications.
Results
Generation of HLA-E knock-in cells at the B2M locus
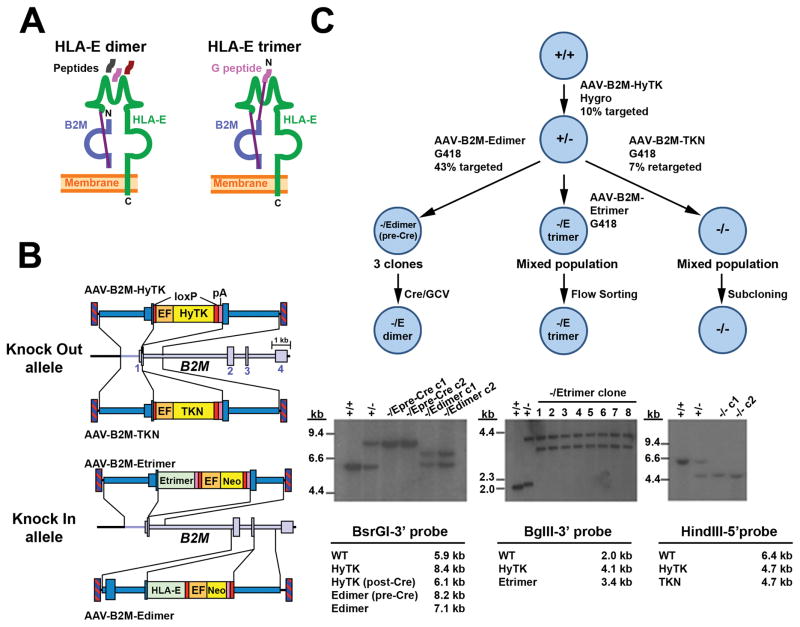
Two types of HLA-E single chain (SC) fusion constructs were designed for our experiments (Fig. 1a). The HLA-E SC dimer consists of an HLA-E heavy chain covalently fused to B2M through a flexible (G4S)4 linker, such that it can bind a normal repertoire of peptides for antigen presentation. The HLA-E SC trimer contains an additional (G4S)3 linker fused to a peptide derived from the signal sequence of HLA-G (another HLA class I molecule), which is a non-polymorphic peptide normally presented by HLA-E that inhibits NK cell-dependent lysis through its binding to CD94/NGK2A15.
Figure 1.
AAV-mediated knock in of HLA-E. (a) Schematic representation of HLA-E single chain (SC) molecules. The HLA-E heavy chain is covalently linked to B2M (by a (G4S)4 linker. In the trimer, the HLA-G signal peptide is covalently attached to the N- terminus by a (G4S)3 linker. (b) AAV knock out and knock in vectors used to edit B2M. Inverted terminal repeats (hatched boxes), B2M exons (large boxes), homology arms (dark blue), loxP signals (red), synthetic poly(A) signals (pink), HyTK or TKN antibiotic resistance cassettes (yellow), human elongation factor-1 alpha (EF-1α) promoter (orange), and HLA-E heavy chain or trimer reading frames (green) are shown. (c) Stepwise targeting strategy to generate B2M-/Edimer, B2M-/Etrimer and B2M−/− ESC lines. Bottom panels show corresponding Southern blots demonstrating accurate gene editing with B2M genotypes of each sample shown, and digests with predicted fragment sizes below (allele maps in Supplementary Fig. 1A).
rAAV gene editing vectors were designed to introduce HLA-E constructs at the B2M locus, such that an HLA-E SC dimer or trimer would be expressed under the control of the B2M promoter (Fig. 1b). In our strategy, the first allele of B2M is edited with a vector that inserts a floxed HyTK fusion gene encoding hygromycin resistance and thymidine kinase at exon 1 to create a null allele (AAV-B2M-HyTK), and the second B2M allele is then edited to express HLA-E. Vector AAV-B2M-Edimer inserts an HLA-E heavy chain reading frame at exon 3 of B2M for HLA-E SC dimer expression, and AAV-B2M-Etrimer inserts a full HLA-E SC trimer gene at exon 1 (including a codon-optimized, wobbled B2M reading frame). Both the dimer and trimer vectors also contain floxed Neo expression cassettes for G418 selection. A second B2M knockout vector was also constructed that inserts a floxed TKNeo fusion gene at exon 1 in order to create B2M−/− control cells (AAV- B2M-TKN).
Human Elf-1 embryonic stem cells (ESCs) were serially infected with these rAAV vectors, and 7 to 43% of selected clones were accurately edited based on screening by PCR with primers in the transgene and outside of the homology arms (Fig. 1c and Table 1). PCR-positive clones infected with AAV-B2M-Edimer were further screened by Southern blot to determine which allele was edited, with 6 of 13 clones edited at the second B2M allele (Table 1). Three of these B2M-/Edimer(pre-Cre) clones were subsequently infected with a non-integrating foamy virus vector to transiently express Cre as described6, and 42 to 46% of ganciclovir-resistant clones had lost both the HyTK and Neo cassettes to produce a B2M-/Edimer genotype. To create cells with a B2M-/Etrimer genotype, a polyclonal population of G418-resistant cells infected with AAV-B2M-Etrimer was flow-sorted for the absence HLA-A,B,C expression, which ensured that the second allele had been edited. Southern blots confirmed that accurate editing occurred (Fig. 1c and Supplementary Fig. 1a) and reprobing showed that none of the edited clones had random integrants (not shown). B2M-/Edimer clones c1 and c2 and B2M-/Etrimer clones c5 and c6 had normal karyotypes (not shown) and were selected for subsequent studies.
Table 1.
B2M editing by different AAV vectors in H1 and Elf-1 ESCs.
| Vector | Comments | ESC line | Editing step | Percent edited CFUa | Total # PCR+ CFU analyzedb | Targeted at 1st allele | Targeted at 2nd allele | Retargeted at 1st allele | Transiently targeted at 2nd allelec |
|---|---|---|---|---|---|---|---|---|---|
| AAV-B2M-HyTK | Total knockout | H1 | First | 16.5 | 15 | 15 | NA | NA | NA |
| AAV-B2M-HyTK | Total knockout | Elf-1 | First | 10.1 | 2 | 2 | NA | NA | NA |
| AAV-B2M-Edimer | HLA-E+ | Elf-1 | First | 33.3 | 8 | 8 | NA | NA | NA |
| AAV-B2M-Edimer | HLA-E+ | Elf-1 | Second | 42.7 | 13 | NA | 6 | 7 | 0 |
| AAV-B2M-TKN | Total knockout | H1 | Second | 5.2 | 5 | NA | 0 | 3 | 2 |
| AAV-B2M-TKN | Total knockout | Elf-1 | Second | 7.2 | 8 | NA | 0 | 8 | 0 |
| AAV-B2M-TKwN | Total knockout, wobbled | H1 | Second | 6.5 | 2 | NA | 0 | 0 | 2 |
| AAV-B2M-TKwN | Total knockout, wobbled | Elf-1 | Second | 9.3 | 8 | NA | 0 | 7 | 1 |
| AAV-B2M-ETKNpAd | Leaky knockout | H1 | First | 30.0 | 9 | 9 | NA | NA | NA |
| AAV-B2M-EHyTKpAd | Leaky knockout | H1 | Second | 10.0 | 6 | NA | 2 | 4 | 0 |
(Edited CFU/Resistant CFU)*100
CFU identifed by primers for targeted alleles
PCR+ Colonies that were mixed and subsequently lost the portion of cells targeted at the 2nd allele
These AAV vectors were used to create our initial B2M KO model in H1 cells
Generation of B2M−/− control cells
We attempted to establish B2M−/− control cells by infecting B2M+/− cells with the second knockout vector AAV-B2M-TKN (Fig. 1), but we consistently obtained G418- resistant cells that had been retargeted at the allele previously edited by the AAV-B2M- HyTK vector, in contrast to the approximately 50:50 allele ratio obtained with the AAV- B2M-Edimer vector (Table 1). In addition, the rare G418-resistant colonies that appeared to be targeted at the second allele by PCR were mixed populations when examined by flow cytometry, and upon further subcloning, we were unable to obtain pure populations that lacked B2M expression and maintained a normal karyotype (Supplementary Fig. 1b). Ultimately, we were able to isolate two pure B2M−/− clones by repeated subcloning and flow cytometry screening of two independently edited cell populations (Supplementary Fig. 1c), but in both cases the clones had abnormal karyotypes (Supplementary Fig. 1d).
These results suggested that euploid B2M−/− Elf-1 ESCs grow poorly, which was surprising given that we had previously generated euploid H1 ESCs with both B2M alleles edited by the nearly identical knockout vectors AAV-B2M-EHyTKpA and AAV-B2M- ETKNpA (Table 1)6. This was not a unique property of Elf-1 cells, since we also failed to generate B2M−/− H1 ESCs with the newer vectors shown in Figure 1 (Table 1). Another possible explanation was that the identical TK sequences present in both vectors were preferentially promoting recombination at the previously edited allele, but when the TK reading frame was wobbled to reduce homology, we were still unable to isolate ESCs edited at both alleles (Table 1). These failures led us to re-examine the euploid B2M−/− H1 ESCs edited with our original vectors, and we found that low levels of class I proteins could be detected by flow cytometry after IFN-γ induction of HLA gene expression (Supplementary Fig. 2a), suggesting that these edited alleles were leaky. This was confirmed by RT-PCR analysis, which detected an mRNA from the edited alleles generated by read-through transcription beyond the pA signal into an open reading frame with an ATG initiation codon in a loxP site and a novel signal sequence in frame with downstream B2M sequences (Supplementary Fig. 2b). This leakiness was not a feature of alleles edited by the newer vectors AAV-B2M-HyTK and AAV-B2M-TKN, which contained additional stop codons downstream from the pA signal to block B2M protein production (Supplementary Fig. 2C). Taken together, these results suggest that a complete lack of B2M expression slows the growth of normal, euploid ESCs. Importantly, this was not a feature of ESCs that uniquely expressed HLA-E SC dimers or trimers, which grew well and had normal karyotypes.
HLA expression in edited cells
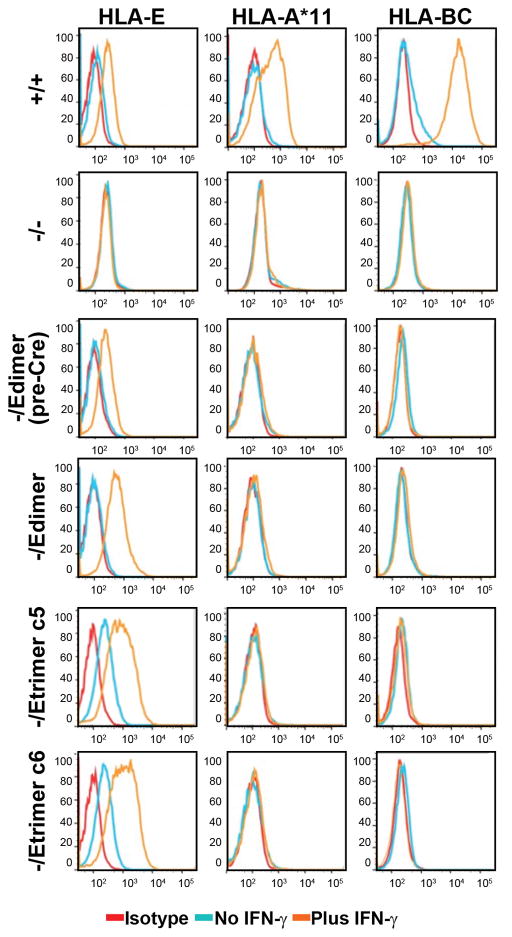
Our editing strategy produces HLA-E SC genes that are expressed from the B2M promoter, so they should be regulated appropriately in response to cytokines. Undifferentiated ESCs with the B2M genotypes from Figure 1 were cultured with and without IFN-γ and the levels of cell surface HLA class I molecules were determined by flow cytometry (Fig. 2). In the absence of IFN-γ, B2M+/+ ESCs expressed nearly undetectable amounts of both HLA-E and polymorphic class I molecules (HLA-A, B and C), all of which were upregulated significantly in the presence of IFN-γ. In contrast, B2M−/− ESCs had no surface expression of HLA class I molecules, even in the presence of IFN-γ, confirming that these cells have a complete knockout of B2M (although they acquired an abnormal tetraploid karyotype).
Figure 2.
Inducible HLA-E expression without other class I molecules. Flow cytometry of HLA-E, HLA-A*11 (present in WT Elf-1 cells) and HLA-BC expression in the presence (orange) or absence (light blue) of IFNγ treatment. Isotype control tracings are shown in red. Single cell suspensions of Elf-1 ESCs with each indicated B2M genotype were derived by trypsin digestion of IFN-γ-treated ESCs. See Supplementary Table 2 for full HLA genotypes.
B2M-/Edimer(preCre) cells expressed low levels of HLA-E upon IFN-γ induction, which explains why these clones could be easily obtained by gene editing, even though they did not express HLA-A, B or C molecules. A similar pattern was observed with B2M-/Edimer cells after Cre-mediated removal of the Neo cassette, but with higher HLA-E SC dimer expression. B2M-/Etrimer cells were distinct in that they also expressed HLA-E without IFN-γ induction, as observed for two independent clones, as well as higher levels upon IFN-γ induction. It is possible that in the absence of IFN-γ, the wild-type HLA-E and HLA-E dimer molecules are not effectively loaded with peptides, which reduces surface expression levels. Both B2M-/Edimer and B2M-/Etrimer cells expressed higher than normal levels of HLA-E, reflecting the fact that all the proteins expressed from one edited B2M allele were HLA-E SC molecules, in contrast to WT B2M proteins that are shared by all types of class I heavy chains. To assess whether expression of HLA-E as the only HLA class I surface molecule influenced differentiation, retinal pigmented epithelium (RPE) cells were derived from B2M-/Edimer ESCs in vitro, and the results were similar to those obtained with B2M+/+ ESCs. Both lines resulted in efficient RPE differentiation, as shown by their cuboidal morphology, and staining for PMEL and MITF (Supplementary Fig. 3).
HLA-E SC expression prevents NK-mediated lysis
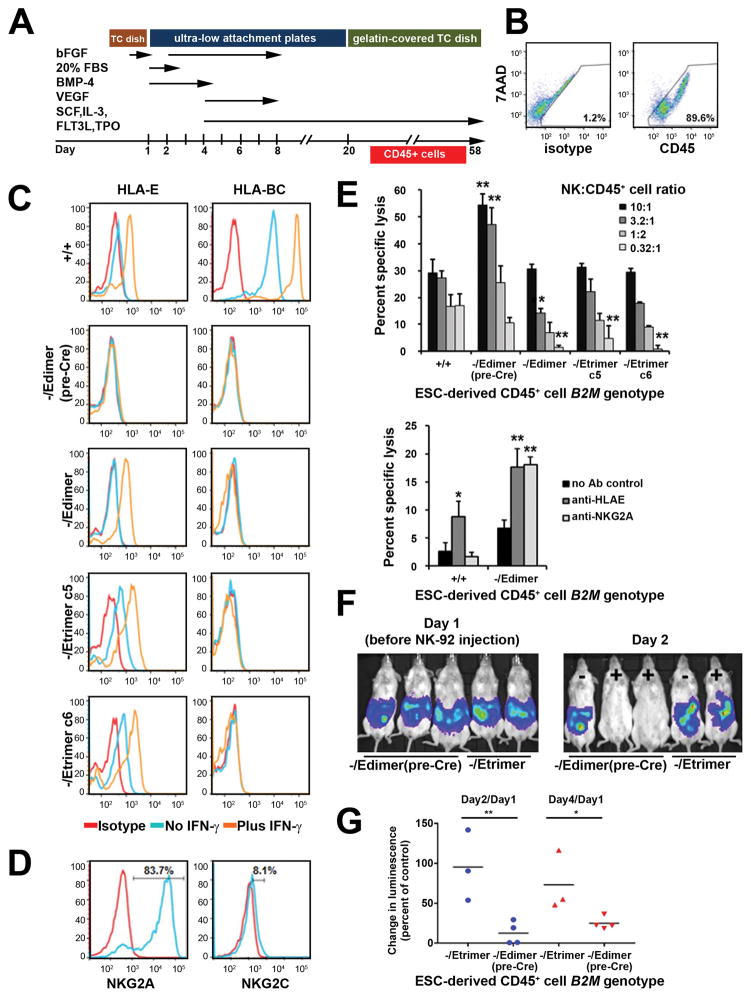
Undifferentiated ESCs have limited immunogenicity and fail to induce NK- mediated lysis24, so we differentiated ESCs into CD45+ hematopoietic derivatives as shown in Fig. 3a before characterizing their immune reactivity. Flow cytometry demonstrated >89% CD45+ hematopoietic cells on day 38 in all edited cell lines except B2M−/− cells (Fig. 3b and Supplementary Fig. 4a), where the total yield of suspension cells (containing CD45+ hematopoietic cells) was approximately 10 times lower (Supplementary Fig. 4B), presumably due to impaired differentiation in these karyotypically abnormal lines. HLA class I expression in ESC-derived CD45+ cells was similar to that observed in undifferentiated ESCs, with IFN-γ inducible HLA-E expression and no other surface class I molecules in B2M-/Edimer and B2M-/Etrimer cells (Fig. 3c). One notable difference was that the B2M-/Edimer(preCre) cells had virtually no detectable HLA-E expression, so for some experiments they were used as class I-negative controls, since B2M−/− CD45+ cells could not be produced in adequate numbers.
Figure 3.
Protection of HLA-E-expressing cells from NK cell-mediated lysis. (a) Schematic representation of the hematopoietic differentiation protocol used for Elf-1 ESCs. Hematopoietic cells were harvested from day 24 to day 58. (b) Representative CD45 expression on day 38 suspension cells produced by B2M-/Etrimer ESC clone c5 as measured by flow cytometry (y axis, 7AAD; x axis, isotype control or CD45). Results for other B2M-edited lines are shown in Supplementary Figure 4A. (c) Flow cytometry of HLA-E and HLA-BC expression in ESC-derived CD45+ cells with the indicated B2M genotypes. (d) Expression of inhibiting and activating receptors NKG2A and NKG2C on NK cells derived from a healthy donor (donor 1). Isotype and specific antibody tracings shown in red and blue respectively. Percents shown are calculated by subtracting corresponding isotype control frequencies. (e) Chromium release assay results obtained with ESC-derived CD45+ cells of the indicated B2M genotype and normal NK cells (donor 1) at the indicated ratios (mean + SD, n=3). The p values (one-way ANOVA test) were <0.002 at all cell ratios. Asterisks indicate pair-wise comparisons with B2M+/+ cells that had p<0.05(*) or <0.01(**) after applying the post hoc Tukey HSD test. (f) Chromium release assays performed as in (e) in the presence of neutralizing antibodies against either HLA-E or NKG2A at a NK/CD45+ cell ratio of 10:1 (mean + SD, n=3). P values were calculated using the one-way ANOVA followed by Tukey HSD test; asterisks indicate statistical significant differences in regard to no Ab controls. (g) Luciferase imaging of 5 representative mice containing ESC-derived CD45+ cells of B2M-/Edimer(pre- Cre) (serves as class I-negative control) or B2M-/Etrimer genotypes, some of which also received NK-92 cells (− or + labels in the right panel). Results are shown for the same mice before NK-92 injection (Day 1) and one day afterwards (Day 2). (h) Quantitation of luciferase-expressing CD45+ cell levels in mice treated as in (g). The change in luminescence between Day 1 (pre NK-92 cell injection) and Day 2 or Day 4 (1 and 3 days after NK-92 cell injection) was measured for individual mice and divided by the average of control mice that did not receive NK-92 cells (n = 3 for both B2M-/Etrimer and B2M-/Edimer (pre-Cre) control groups). Horizontal black bars indicate the mean for each group. P values were calculated using the unpaired Student’s t test. For all panels, ** = p<0.01, and * = p<0.05.
HLA-E binds to both the inhibiting and activating receptors NKG2A and NKG2C, respectively25, but NKG2A is expressed in most human NK cells18 so we expected that the overall effect of HLA-E should be inhibitory. Allogeneic peripheral blood CD56+ NK cells isolated from normal donors were incubated with 51Cr-labeled ESC-derived CD45+ cells and lysis was measured in chromium release assays. A typical NK donor with 83.7% NKG2A+ cells and 8.1% NKG2C+ cells (Fig. 3d) showed increased lysis of class I- negative B2M-/Edimer(preCre) CD45+ cells, confirming that the missing self response observed in mice is also important for human hematopoietic cells (Fig. 3e). This increased lysis was completely reversed by expression of HLA-E SC molecules in B2M-/Edimer and B2M-/Etrimer CD45+ cells, and at lower NK:CD45 cell ratios, HLA-E SC expression reduced lysis to below that of B2M+/+ CD45+ cells, perhaps due to their higher than normal HLA-E levels, or a lack of interactions with activating Killer-cell Immunoglobulin-like Receptors (KIRs). Blocking antibodies against HLA-E or NKG2A increased the lysis of B2M-/Edimer cells ~2.5 fold, confirming that the HLA-E/NKG2A interaction was responsible (Fig. 3f). NK cells from a donor with unusually low NKG2A expression (49.86% NKG2A+, 3.66% NKG2C+, Supplementary Fig. 4c) also preferentially lysed class I-negative ESC-derived CD45+ cells, and this was partly reversed by HLA-E expression in B2M-/Edimer cells (Supplementary Fig. 4d). Control experiments confirmed that both B2M−/− and B2M-/Edimer(pre-Cre) CD45+ cells were lysed at equivalent levels by NK cells, and that HLA-E expression also prevented lysis by NK cells grown at lower IL-2 concentrations (Supplementary Fig. 4e and 4f). As in vivo proof of concept, we monitored the survival of luciferase-expressing ESC-derived CD45+ cells and teratomas in immunodeficient mice and showed that HLA-E expression improved survival in mice that also received NK-92 cells, an NK cell line that expresses NKG2A26,27 (Fig. 3g, 3h and Supplementary Fig. 4g–h). Taken together, these experiments demonstrate that HLA-E SC expression prevents NK-mediated lysis in otherwise HLA class I-negative cells.
HLA-E SC expressing cells are not recognized as allogeneic
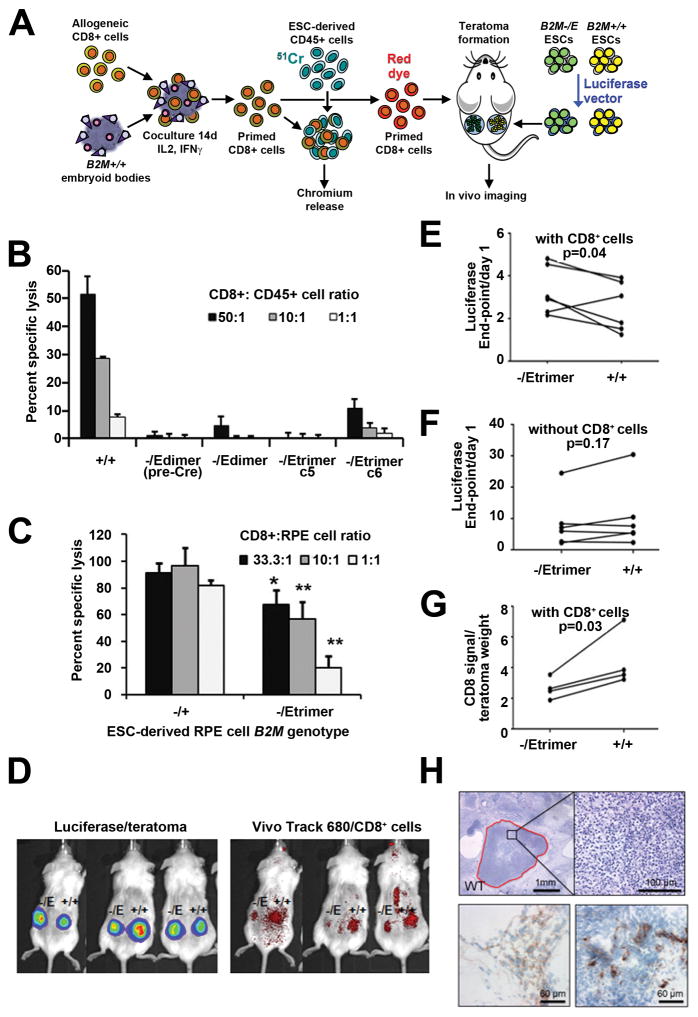
We used both in vitro and in vivo models to determine if allogeneic T cells recognize and respond to B2M-/Edimer and B2M-/Etrimer cells (Fig. 4a). Normal, human CD8+ T cells were first cocultured with embryoid bodies (EBs) derived from B2M+/+ Elf-1 ESCs, to enrich for T cells that recognize Elf-1 class I molecules. EBs contain several types of cells, and express high levels of HLA class I molecules, but not HLA class II (e.g., HLA-DR) (Supplementary Fig. 5A). The costimulatory molecules CD40 (45%) and CD54 (97%) were also present on EB cells, but not CD86, CD80, CD83 or CD275 (Supplementary Fig. 5b). These EB-primed T cells were then incubated with 51Cr-labeled ESC-derived CD45+ cells in chromium release assays to detect T cell-mediated cytotoxicity. The CD45+ cells robustly expressed CD40, CD54 and CD86 costimulatory molecules (Supplementary Fig. 5C). While EB-primed T cells efficiently lysed B2M+/+ CD45+ cells, they did not kill B2M-/Edimer(preCre), B2M-/Edimer, and B2M-/Etrimer CD45+ cells (Fig. 4b), and a similar protection from lysis was observed for ESC-derived RPE cells expressing HLA-E (Fig. 4c), demonstrating both that cytotoxic T cell responses can be prevented by eliminating surface expression of polymorphic class I molecules, and that non- polymorphic HLA-E SC dimers and trimers do not stimulate lysis. These experiments also show that indirect HLA class I antigen presentation as modeled by priming CD8+ T cells with wild-type EBs does not stimulate responses against B2M-/Edimer and B2M-/Etrimer cells, which contain intracellular polymorphic heavy chains.
Figure 4.
Allogeneic CD8+ T cells do not recognize HLA-engineered cells. (a) Schema for priming allogeneic CD8+ T cells against Elf-1 HLA antigens. (b) Chromium release assays measuring the cytotoxicity of allogeneic CD8+ T cells towards ESC-derived hematopoietic cells with the indicated B2M genotypes (mean + SD, n=3; p values determined by one-way ANOVA were <0.001 and by HSD Tukey test p<0.01, when comparing all the HLA-engineered lines to B2M+/+ cells at all cell ratios). For representation purposes, negative specific lysis values were plotted as zero. (c) Analogous chromium release assays towards ESC-derived RPE cells with the indicated B2M genotypes (mean + SD, n=3 for each cell ratio, p values determined by unpaired Student’s t-test, * = p<0.05 and ** = p<0.01). (d) Representative examples of teratoma and CD8+ cell imaging in live animals. (e) Fold change of luciferase expression by B2M-/Etrimer and B2M+/+ teratomas from day 1 of CD8+ cell infusion to endpoint (day 12, n=4 or day 16, n=2), with each line representing one mouse. P-value was determined by paired Student t- test (n=6 mice). (f) Same as (e) but luciferase measurements of teratomas were made in control mice that did not receive CD8+ T cells (n=6 mice per group). (g) Infiltration of teratomas with allogeneic CD8+ T cells as measured by fluorescent signal divided by weight of teratomas harvested at the time of sacrifice (n=4 mice, P-value determined by paired Student t- test). (h) Representative hematoxylin and eosin staining of a B2M+/+ teratoma harvested from mice that received allogeneic CD8+ T cells. Necrotic areas with abundant polymorphonuclear cells are indicated in red. The two bottom photos show immunohistochemistry staining of CD8+ T cells infiltrating B2M+/+ teratomas.
In vivo responses were examined by growing luciferase-expressing B2M+/+ and B2M-/Etrimer ESC-derived teratomas in immunodeficient mice, and transplanting primed allogeneic CD8+ T cells labeled with VivoTrack 680 (examples in Fig. 4d). Serial luciferase measurements showed that B2M-/Etrimer teratomas grew more than their B2M+/+ counterparts in 5 of 6 mice after CD8+ cell infusion (Fig. 4e and Supplementary Fig. 6a), but this did not occur in the absence of CD8+ cells (Fig. 4f). Similar results were obtained in separate experiments with three different CD8+ T cell donors (Supplementary Fig. 6b). CD8+ cells also preferentially localized to B2M+/+ teratomas as measured by in vivo tracking (Fig. 4g). Histological examination demonstrated multiple areas of necrosis in the B2M+/+ teratomas as well as the presence of human CD8+ cells at higher levels than found in B2M-/Etrimer teratomas (Fig. 4h and Supplementary Fig. 6c).
We also examined possible B cell responses against ESC-derived CD45+ cells by performing a complement-dependent cytotoxicity (CDC) assay with human serum samples containing defined anti-HLA antibodies (Table 2). Cytotoxicity was detected when B2M+/+ cells were exposed to 14 of 16 sera samples with antibodies against Elf-1 HLA-A or B alleles (A*11, A*24, B*35 and B*55), but not after exposure to 17 samples with antibodies against other HLA-A or B alleles, demonstrating the accuracy of the assay. No cytotoxicity was observed for B2M-/Edimer (pre-Cre), B2M-/Edimer or B2M-/Etrimer cells, except for one single sample reacting against B2M-/Etrimer c6, which could reflect contaminating antibodies against non-HLA class I antigens, including possible class II alleles that may be expressed in some CD45+ cells.
Table 2.
Human anti-HLA antibodies do not recognize HLA-E expressing cells.
| B2M genotype of ESC-derived CD45+ cells | |||||
|---|---|---|---|---|---|
|
| |||||
| Serum anti-HLA type | +/+ | -/Edimer (pre-Cre) | -/Edimer | -/Etrimer c5 | -/Etrimer c6 |
| Positive Control | ++++ | − | − | − | − |
| A1 | − | − | − | − | − |
| A1, 9, 11, 24, 25, 26, 36, 66, 6802 | ++++ | − | − | − | − |
| A1, 11, 26, 34, 36, 6601 | +++ | − | − | − | − |
| A1, 11, 36, 80 | − | − | − | − | − |
| A2 | − | − | − | − | − |
| A2, 68, 69 | − | − | − | − | − |
| A3 | − | − | − | − | − |
| A10, 28, 33 | − | − | − | − | − |
| A11 | ++ | − | − | − | − |
| A11, 66 | + | − | − | − | − |
| A23 | − | − | − | − | − |
| A23, 24 | ++++ | − | − | − | − |
| A24 | ++++ | − | − | − | − |
| A68, 69 | − | − | − | − | − |
| B5, 18, 35, 49, 53 | ++++ | − | − | − | − |
| B7 | − | − | − | − | − |
| B7, 13, 41, 48, 60, 61 | − | − | − | − | − |
| B7, 27, 42, 67 | − | − | − | − | − |
| B7, 42 | − | − | − | − | − |
| B7, 42, 55, 56, 67 | ++++ | − | − | − | − |
| B7, 48 | − | − | − | − | − |
| B12, 35, 50, 62, 70, 75, 76, 77 | − | − | − | − | − |
| B13, 27, 47, 60, 61 | − | − | − | − | − |
| B35 | ++++ | − | − | − | ++ |
| B35, 53 | ++ | − | − | − | − |
| B35, 49, 50, 53, 62, 70, 75, 77 | ++++ | − | − | − | − |
| B35, 75 | +++ | − | − | − | − |
| B41, 45, 50 | − | − | − | − | − |
| B54, 55, 56 | ++++ | − | − | − | − |
| B55 | +++ | − | − | − | − |
| B60 | − | − | − | − | − |
| B60 | − | − | − | − | − |
Discussion
In this report we show that HLA-E single chain dimer and trimer expression in human cells lacking polymorphic HLA class I surface molecules can prevent the ‘missing self’ response. Most human NK cells express NKG2A18, an inhibitory receptor that binds HLA-E, and this interaction was enough to reduce NK-mediated lysis of CD45+ cells that uniquely expressed HLA-E to a level below that of wild-type cells, and to protect cells from NK-92 cell-mediated lysis in vivo. This level of host NK cell population inhibition may also be adequate to protect transplanted cells in clinical trials, given that cumulative NK signaling can dictate the outcome28, and in mice NK cells can be tolerized to B2m−/− cells over time29. If necessary, the subset of host NK cells that are NKG2A-negative could be inhibited with additional gene editing steps, such as expressing single chain HLA-G proteins13,30 and/or other class I molecules targeting inhibitory KIR31 or other NK receptors25, especially in recipients with fewer NKG2A+ NK cells.
We were surprised that B2M−/− human ESCs completely lacking surface HLA class I proteins grew poorly and failed to differentiate robustly into CD45+ cells, given the relative health of B2m−/− mice32,33. Other groups have described B2M knockouts in human cells7–9,34, with at least one report showing that the editing frequencies were lower than expected in normal T cells as compared to HEK293 cells34. These findings suggest that B2M-edited cells should be carefully screened for low level or inducible class I surface expression and/or compensatory mutations. The two independent B2M−/− lines that we eventually isolated by repeated subcloning had common cytogenetic abnormalities (tetraploidy, add20(q13), −15 and −22) that may have improved their survival. While the basis for the poor growth of B2M−/− human cells is not understood, it is not a feature of cells that express HLA-E as their sole class I surface protein, which grew normally and produced hematopoietic cells and RPE cells as efficiently as wild-type cells.
B2M-/Edimer and B2M-/Etrimer ESCs and their differentiated derivatives were not lysed by CD8+ T cells, and they were not recognized by human anti-HLA antibodies. Thus these cells were not only resistant to NK-mediated lysis, but they were not recognized as allogeneic, and their expression of single chain HLA-E molecules was not immunogenic. These in vitro findings were confirmed by in vivo studies showing that allogeneic CD8+ cells invaded B2M+/+ teratomas more than B2M-/Etrimer teratomas, reducing their growth rate and producing necrosis. Our results support the use of HLA-E-expressing PSCs as a source of first generation, universal donor cells, at least in applications where the transplanted cell type does not express HLA class II molecules. Additional engineering steps could be used to prevent class II expression, such as knocking out the genes responsible for Bare Lymphocyte Syndrome35. While HLA-engineering should minimize the rejection of many types of PSC-derived cell products, especially relevant clinical applications include hematopoietic cell transplantation where HLA mismatches profoundly affect engraftment, and autoimmune or genetic diseases where the presentation of autoantigens and neoantigens would otherwise cause rejection.
Reduced HLA expression also raises specific safety concerns. Class I-deficient mice and humans are not tumor-prone36,37, but class I peptide presentation may contribute towards the elimination of malignant cells formed by other mechanisms, and it plays a role in clearing infections. The HLA-E dimer molecule could provide some protection in these settings, since HLA-E can present pathogen-specific peptides that are recognized by cytotoxic T cells38,39, and possibly tumor-specific peptides as well40. HLA-independent mechanisms should also limit infections based on the partial immunity of class I-deficient humans37 and mice41–43. In addition, many regenerative medicine applications will involve the treatment of recipients with normal immune systems, delivery to internal sites sequestered from pathogens, and the transplantation of terminally differentiated and possibly irradiated PSC-derived cells that are resistant to transformation. Ultimately, the safety of these cell products may be enhanced by the inclusion of suicide genes such as the TK gene used in our editing steps, and the extensive pre-clinical testing that the use of a single universal donor cell line allows, which may not be practical when characterizing cellular products derived from multiple autologous iPSCs or HLA-typed PSC banks.
Materials and Methods
Cell culture
Human Elf-1 ESCs44 were obtained from the University of Washington. H1 and H9 ESCs45 were obtained from the WiCell Research Institute. Human Elf-1, H1 and H9 ESCs were cultured as described23,44 and passaged every 5–6 days. All these ESC lines tested negative for mycoplasma contamination. For RPE differentiation, cells were adapted and maintained in 6 well-plates coated with Matrigel (BD Biosciences, San Diego, CA, USA) in mTeSR1 media (Stem Cell Technologies, Vancouver, BC, Canada). Karyotyping of ESC lines was performed by the Fred Hutchinson Cancer Research Center (FHCRC) cytogenetics facility. EBs were generated by dispase dissociation of ESC cultures, plating 5 x 106 ESCs/per well of an ultra low attachment 6-well plate containing X-VIVO 15™ medium (Lonza) supplemented with 1 mM sodium pyruvate, 1 mM each non-essential amino acid, 50 μM 2-mercaptoethanol and 2 mM L-glutamate. Medium was changed every 3 days and cultures were maintained for 15–20 days. Hematopoietic differentiation of ESCs was performed as described46, with an additional step of plating on 0.1% gelatin- coated dishes on day 20. Suspension cells were collected from day 24 onward for flow cytometry and functional studies. RPE differentiation was performed as described47. Normal human CD56+ NK cells and CD8+ T cells were isolated by positive enrichment (Miltenyi CliniMACS system) of blood cells collected by apheresis from healthy human donors (FHCRC Hematopoietic Cell Processing and Repository Core Facility). NK-92 cells (CRL-2407) were obtained from the American Type Culture Collection (Manassas, VA) and cultured as described in the product sheet.
Viral vectors and genome editing
All the AAV vectors used were serotype 3B and prepared as described20. AAV- B2M-EHyTKpA and AAV-B2M-ETKNpA have been described6. AAV-B2M-HyTK and AAV-B2M-TKN are similar to these vectors respectively, except they contain additional sequences with stop codons upstream of the 3′ homology arm. AAV-B2M-TKwN is the same as AAV-B2M-TKN but with the TK reading frame wobbled to reduce homology. AAV-B2M-Edimer homology arms span B2M exon 3 (chr15:44,715,280–44,717,402 GRCh38/hg38 human assembly) with a (G4S)4 flexible linker coding sequence, HLA-E 01:03 heavy chain coding sequence, and floxed EF1a promoter-Neo-pA cassette inserted at the terminal codon of B2M (chr15:44,716,339). AAV-B2M-Etrimer homology arms span B2M exon 1 (chr15:44,711,007–44,712,332), with coding sequences for the wobbled B2M signal peptide, HLA-G signal peptide VMAPRTLFL, (G4S)3 flexible linker, wobbled B2M mature protein, (G4S)4 flexible linker and HLA-E 01:03 heavy chain followed by a floxed EF1a promoter-Neo-pA cassette. These AAV vectors were used to infect ESCs, and drug-resistant colonies were picked and expanded as described6,20,23. The non-integrating foamy virus vector NIFV-EokCreW was used to express Cre and remove floxed transgenes as described6. Vector sequences are available upon request.
DNA isolation and analysis
Genomic DNA was isolated by using the Puregene DNA purification system (Gentra Systems, Minneapolis, MN). Southern blot analysis, plasmid preparation, and restriction digestion were performed according to standard protocols48. Radiolabeled probes were synthesized by random priming using Rediprime II (GE Healthcare, Piscataway, NJ). The genomic coordinates for the 5′ and 3′ B2M probes were: chr15:44,709,549–44,710,044 and chr15:44,711,550–44,712,205 respectively. For PCR analysis, cells were lysed in colony-PCR lysis buffer that consisted of 105 mM KCl, 14 mM Tris-HCl, pH 8.3, 2.5 mM MgCl2, 0.3 mg/ml gelatin, 0.45% (vol/vol) NP-40, 0.45% (vol/vol) Tween 20 and Proteinase K (100 μg/ml), incubated at 55°C for 1 hour, and heated at 95 °C for 15 minutes to inactivate Proteinase K49. The lysate was clarified by centrifugation on a Mini Strip Spin Microcentrifuge (Thomas Scientific) for 5 minutes and the supernatant was used as a PCR template. The primers and Tm’s used for PCR screening edited clones are described in Supplementary Table 1. PCR was performed under the following conditions: 1X Green GoTaq© Flexi buffer, 0.75 units GoTaq polymerase (Promega, Madison, WI), 5 μl cell lysate (~150 ng genomic DNA), 0.5 μM each primer, 0.25 mM each dNTP and 2 mM MgCl2 in a 25 μl volume for 40 cycles in a PTC-200 Thermo Cycler (Biorad, MJ Research, Hercules, CA).
HLA-E genotypes were determined by sequence-specific PCR (PCR-SSP)50 with two different 5′ primers and a common 3′ primer to distinguish the non-synonymous difference of HLA-E*01:01 and HLA-E*01:03 at amino acid position 107. HLA-A,B,C typing was performed by the Immunogenetics / HLA Laboratory at the Bloodworks Northwest facility.
Flow cytometry
ESCs were harvested with trypsin for 3 min at 37°C and single cell suspensions were obtained by passing through a 70 μm cell strainer. The subsequent staining and washes were performed in phosphate-buffered saline containing 1% bovine serum albumin. A 98 μl suspension of ESCs was preincubated with 2 μl FcR blocking reagent (Miltenyi Biotec, Auburn, CA) for 10 min on ice and then stained with antibodies for 30–60 min on ice. 7-amino-actinomycin D (BD Biosciences, Bedford, MA) was added to identify dead cells. Single suspension cells derived from EBs were analyzed the same way. Samples were analyzed on a LSRII flow cytometer (BD Biosciences) and the data were plotted using FlowJo software (TreeStar, Ashland, OR). The antibodies used were: anti- HLA-E (clone 3D12HLA-E), anti-HLA-BC (clone B1.23.2), anti-HLA-ABC (clone W6/32), isotype controls mouse IgG1 κ, and mouse IgG2b,κ (eBioscience); anti-HLA Class I A11 (4i93, Abcam); IgG(γ) goat anti-mouse (Invitrogen); anti-HLA-DR (clone AC122, MACS Miltenyi Biotec); anti-B2M (clone B2M-01) and isotype control mouse IgG2a,κ (Santa Cruz Biotechnology); anti-NKG2A (clone Z199, Beckman Coulter); anti-NKG2C (clone 134591) and isotype control mouse IgG1 (R&D); anti-CD45 (clone HI30), anti-CD40 (clone 5C3), anti-CD54 (clone HA58), anti-CD80 (clone L307.4), anti-CD86 (clone 2331), anti-CD83 (clone HB15e), anti-CD275 (clone 2D3) and their corresponding isotype controls (BD Biosciences).
When sorting cells infected with AAV-B2M-Etrimer, ESCs were treated for 72 h with 25 ng/ml IFN-γ, harvested by digestion with trypsin (3 min at 37°C) and passed through a 70 μm cell strainer. Single cells (4x106 in 0.1 ml) were preincubated with FcR blocking reagent and stained with 6 μg of the pan-HLA-class I antibody (W6/32, eBioscience). HLA class I-negative cells were sorted on a BD Aria III cytometer. 9,300 class I-negative cells were collected, plated onto 10 cm dishes, and individual colonies were picked for PCR and Southern blot analysis. 4 of 12 colonies analyzed were edited.
Immunocytochemistry
After 14 days of RPE differentiation, cells were plated on 8-chamber glass slides coated with matrigel and kept for an additional 28 days in XVIVO-10 media (Lonza, Basel, Switzerland). The cells were washed with PBS, followed by permeabilization and treatment with blocking buffer (0.5% BSA, 0.05% saponin in PBS) for 45 min. Primary antibodies were prepared in blocking buffer and incubated with the cells for 1 hr at room temperature. After washing with PBS, the cells were incubated with goat anti-mouse Alexa Fluor-488 secondary antibodies (Thermo Fisher Scientific, Carlsbad, CA, USA) for 45 min at room temperature. The glass slide was mounted with Fluoro-Gel II with DAPI (EMS, Hatfield, PA, USA) and images were taken on an Olympus BX51 upright microscope. The primary antibodies used in this study were: mouse anti-PMEL (Abcam, Cambridge, MA, USA; 1:100) and mouse anti-MITF (Millipore, Billerica, MA, USA; 1:100).
Chromium release assays with NK cells
Hematopoietic progenitors were evaluated for their susceptibility to NK cell- mediated lysis by 4 h 51Cr release assay (adapted from 51). 48 h before the assay, NK cells were thawed and cultured in NK medium consisting of RPMI 1640-HEPES with 10% heat-inactivated human AB serum (Valley Biomedical), 400 U/ml IL-2 (100 U/ml IL-2 in the experiment shown in Supplementary Fig. 4F), 2 mM L-glutamate, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 50 U/ml penicillin and 50 μg/ml streptomycin. Hematopoietic cells were treated with 25 ng/ml IFN-γ for 72 h, washed, and 2–5x105 cells were resuspended in 200 μl hematopoietic medium (StemPro34 SFM by Life Technologies, supplemented with 1 mM each non-essential amino acid, 2 mM L- glutamate, 55 μM 2-mercaptoethanol and 50 μg/ml ascorbic acid) and labeled with 50 μCi of 51Cr (Perkin Elmer) for 2 h at 37° C, followed by 3 washes with NK medium lacking IL-2 or IFN-γ. 104 51Cr-labeled cells were plated per well of a 96-U bottom plate (Corning Costar), NK cells were added at different ratios to target cells, and incubated for 4 h at 37°C. The plate was spun at 1,000 rpm for 5 min before and after the coculture. Controls included labeled cells without NK cells (spontaneous release) and labeled cells lysed with 1% Triton X-100 (total lysis). 100 μl of each reaction supernatant was mixed with 1 ml liquid scintillation cocktail (Ultima Gold, Perkin Elmer), counted on the LS6500 counter (Beckman, Indianapolis IN) and the percent specific lysis was calculated as (sample – average spontaneous release)/(average total lysis-average spontaneous release)*100. Samples were done in triplicate.
In experiments testing neutralizing antibodies, 51Cr-labeled hematopoietic cells or NK cells were preincubated with 10 μg/ml anti-human HLA-E (clone 3D12HLA-E, eBioscience) or 5 μg/ml anti-NKG2A (clone Z199, Beckman) respectively for 30 min at room temperature before performing the cocultures.
Cytotoxicity assays with CD8+ T cells
Normal, human CD8+ T cells were primed by coculturing with single cells derived from IFN-γ treated WT Elf-1 ESC-derived EBs (2 x106 CD8+ cells and 5x 105 EB cells /2 ml) in RPMI 1640-HEPES supplemented with 10% human AB serum (Valley Biomedical), 50 U/ml IL-2, 25 ng/ml IFN-γ, 1mM sodium pyruvate, 2 mM L-glutamate, 50 μM 2- mercaptoethanol, 50 U/ml penicillin and 50 μg/ml streptomycin. On day 7, cocultures were replenished with 1.75 x 106 fresh single cells from IFN-γ treated EBs (final CD8+/EB cell ratio was 1). On day 14, these primed CD8+ T-cells were collected by centrifugation and used in chromium release assays as described above for NK cells. Costimulatory molecule expression on both the EB cells used for priming and the ESC-derived hematopoietic cells used for lysis was determined by flow cytometry. CD8+ T cells primed by the same method were also used for in vivo studies. Chromium release assays were performed on RPE cultures by adding primed CD8+ T cells to IFN-γ treated adherent RPE cultures on day 171 of differentiation after labeling with 20 μCi/well of 51Cr (Perkin Elmer) overnight at 37° C. The exact number of RPEs was determined by trypsin treatment of control wells that were not 51Cr-labeled. The CD8+ T cell/RPE cocultures were incubated overnight at 37° C and the chromium release was measured the next day, as described above.
Recognition by anti-HLA antibodies
Complement Dependent Cytotoxicity (CDC) assays were performed to determine if human anti-HLA antibodies recognize ESC-derived hematopoietic cells. Assay plates were prepared by adding 1 μl of a specific human serum containing previously characterized anti-HLA antibodies (or control serum) and 5 μl of light mineral oil per well. IFN-γ treated ESC-derived CD45+ cells isolated on day 32 of differentiation were resuspended in RPMI 1640 medium with 15% FBS at a concentration of 1.5x106 cells/ml, and 1 μl of each cell suspension was added per well. The reactions were incubated 30 min at RT and 5 μl of rabbit serum (Immucor GTI Diagnostics, Inc.) was added as a source of complement per well. After 1 h of incubation at RT, 5 μl of acridine orange/ethidium bromide solution (14.3 μg/ml acridine orange, 47.6 μg/ml ethidium bromide, 2.4% v/v India ink in Hanks Balanced Salt Solution) was added per well. Percent cytotoxicity was determined by counting dead and live cells visualized on a fluorescent microscope, subtracting spontaneous lysis values obtained in the absence of anti-HLA antibodies, and scoring with the semi-quantitative scale described by Terasaki et al.52. Each serum sample was derived from a single individual. Sera were obtained from multiparous blood donors or extracted from placentas post-partum. The positive control serum was generated as a pool of sera from 30 high Panel Reactive Antibody individuals. The panel was tested and prepared by Bloodworks Northwest (Seattle, WA).
In vivo reactivity with allogeneic CD8+ T cells and NK-92 cells
All animal experiments were approved by University of Washington’s Institutional Animal Care and Use Committee. “NSG-B2m” mice (NOD.Cg-B2mtm1Unc Prkdcscid Il2rgtm1Wjl/SzJ) were purchased from the Jackson Laboratory. ESCs were stably transduced with the luciferase-expressing Red-FLuc-Puromycin lentivirus vector (CLS96000, PerkinElmer) as per the manufacturer’s protocol, and puromycin-resistant polyclonal populations were used to produce teratomas. Implants containing ~2x106 luciferase-expressing ESCs were made by suspending ESCs in a collagen gel in 0.5 cm diameter Merocel polyvinyl alcohol sponges (Medtronic, Inc., Mystic, CT) as described53. Implants were cultured overnight at 37°C, and on the next day they were inserted subdermally into NSG-B2m mice where they formed tri-lineage teratomas. Recipients were 3–4 month old females. Randomization or blinding of recipients was not used. No recipients were excluded. Experimental group sizes were based on pilot studies.
Allogeneic human CD8+ T cells primed against B2M+/+ Elf-1 EB cells (described above) were labeled with VivoTrack 680 (NEV12001, PerkinElmer) as per the manufacturer’s instructions. Cell labeling was >99% by flow cytometry (not shown). On day 35 after implant placement, 8.5x105 labeled CD8+ T cells were injected IV into each mouse via the retro-orbital sinus. Control mice containing implants did not receive labeled CD8+ cells. The experiments in Supplementary Figures 6B and 6C were performed with CD8+ T cells from three different donors (non-HLA-typed) that were not labeled with VivoTrack 680.
Teratoma size was measured weekly after implant placement by intra-peritoneal injection of 150 mg/kg sterile-filtered D-luciferin substrate in DPBS (Cat. #122799, Perkin Elmer) and recording the peak bioluminescence in a Lumina II IVIS imaging system (Caliper Life Sciences). In vivo tracking of labeled CD8+ T cells was done by recording fluorescence at an excitation wavelength of 675 nm and emission filter of 690–770 nm. Accurate quantitative measurement of CD8+ T cells was difficult in live mice due to background signal from the skin and hair, so CD8+ cell quantitation was determined by imaging harvested teratomas after sacrifice and normalizing for teratoma weights. In all cases, data analysis was done using the Living Image software v. 4.2 (Caliper Life Sciences). Harvested teratomas were also analyzed by hematoxylin and eosin (H&E) staining of paraffin-embedded sections and by anti-human CD8 antibody staining of frozen sections (Cat No. NCL-L-CD8-295, Leica Biosystems) using the Leica Bond Polymer DAB system, with hematoxylin blue counterstain. Histological procedures were conducted in the Histology and Imaging Core / Comparative Pathology Program and the Pathology Research Services Laboratory at the University of Washington. CD8+ T cell infiltration in histological sections was calculated with Image J software (version 1.50i) as a % of area with CD8+ cell color signal. Data were obtained from at least 12 separate non-overlapping fields of the same cross-sectional area for each teratoma gentoype (n = 5 teratomas with each genotype) and are presented as an average for each mouse.
Teratoma studies with NK-92 cells (Supplementary Figs. 4G–H) were performed as described above except that 107 NK-92 cells were delivered IV into each recipient on days 13, 15 and 17 after teratoma placement, and IVIS imaging was performed, before NK-92 injection on day 13 (pre) and on day 19 (post).
In vivo studies of luciferase-expressing, ESC-derived CD45+ cells were performed by intraperitoneal (IP) injection of 2.5x105 CD45+ cells of each B2M genotype into NSG- B2m mice and IVIS-based imaging of pre-NK-92 luciferase levels, followed 4 h later by the IP injection of 6.25x106 NK-92 cells into half of the mice and re-imaging one and three days later. The average change in luminescence in the NK-92 non-injected mice was used to normalize the data shown in Fig. 3h.
Statistics
Statistical analysis was carried out with GraphPad Prism software v. 5.03 and Microsoft Excel 2007. All numerical data shown in the chromium release assays are presented as mean ± standard deviation. Statistically significant results were considered when p< 0.05. One-way ANOVA tests were used to analyze the data derived from NK and CD8 chromium release assays. In Figures 3E, 3F and 3H and Supplementary Fig. 4F, pair-wise comparisons used the post hoc Tukey HSD test. Data shown in Fig. 4C, Fig. 3H and the comparison between groups (with vs. without NK-92 cells) shown in Supplementary Fig. 4G were analyzed by using the unpaired Student’s t-test. Data shown in Figs. 4E, 4F, 4G, the analysis within groups shown in Supplementary Figs. 4G and the data shown in Supplementary Figs. 6B and 6C were analyzed by using the paired Student’s t-test.
Data availability
The DNA sequences of the vectors and plasmids used in this study are available upon request.
Supplementary Material
Supplementary Table 1. PCR primers and conditions.
Supplementary Table 2. HLA types of ESCs, NK cells, and CD8+ T cells.
Supplementary Figure 1. Generation of B2M−/− ESCs. (a) Maps of the B2M alleles in cells with the indicated B2M genotypes. Probes and restriction enzymes used in Southern blots are indicated (H, Hind III; B, Bgl II; G, BsrG I). Dark blue regions show overlap with the gene editing vectors used to produce each allele. (b) B2M flow cytometry of different mixed colonies of ESCs and their subcloning results showing the bias against obtaining pure populations of normal B2M−/− cells. (c) Flow cytometry showing a lack of B2M expression in two B2M−/− ESC clones after IFN-γ treatment. (d) Karyotypic analysis of these same two B2M−/− clones.
Supplementary Figure 2. Trace B2M expression in B2M-edited clones. (a) Pan-HLA- ABC flow cytometry of IFN-γ-treated B2M+/+ and B2M−/− H1 ESCs created with vectors AAV-B2M-EHyTKpA and AAV-B2M-ETKNpA6 (blue tracings) and isotype controls (red tracings) showing low level expression. (b) The sequence of an mRNA isolated from B2M−/− H1 ESCs showing transcription of the edited allele through the pA site, the location of an initiation codon (underlined) in the loxP site, and in-frame continuation of the reading frame into downstream B2M exon 1 sequences. (c) Modifications made in vectors AAV- B2M-HyTK and AAV-B2M-TKN that eliminate trace B2M expression. The B2M gene is shown after Cre-mediated excision of the HyTK or TKN genes present in either vector, with the loxP-encoded ATG start codon shown above, and the downstream stop codons that prevent translation (asterisks) in all three reading frames shown below.
Supplementary Figure 3. Retinal pigmented epithelium (RPE) cell differentiation. RPE cells derived from B2M+/+ H9 ESCs (panels a–c) and B2M-/Edimer ESCs (panels d–f) were visualized by bright field microscopy (a, c) and immunofluorescence microscopy for retinal markers PMEL (b, e) and MITF (c, f) after 42 days of differentiation. Bright field images demonstrate the level of pigmentation. PMEL+ and MITF+ cells are shown in green, with DAPI stained nuclei in blue. Scale bar = 50 μm.
Supplementary Figure 4. Hematopoietic potential and NK cell-mediated lysis of ESC- derived CD45+ cells. (a) Flow cytometry analysis of CD45 expression after hematopoietic differentiation of Elf-1 ESCs with the indicated B2M genotypes. Data were acquired from suspension cells on day 38 of differentiation. Results for B2M-/Etrimer c5 shown in Fig. 3B. (b) B2M−/− ESCs produce fewer hematopoietic cells. Kinetics of suspension cell production during hematopoietic differentiation of ESCs with the indicated B2M genotypes. Y-axis denotes number of live suspension cells generated per 5x106 undifferentiated ESCs. The results from two independent differentiation experiments are shown with numbers between parentheses. (c) Flow cytometry analysis of NKG2A and NKG2C receptors on NK cells derived from donor 2. Percents were calculated by subtracting the isotype control frequencies. (d) Chromium release assay with NK cells from donor 2 and ESC-derived CD45+ cells with the indicated B2M genotypes showing B2M-/Edimer expression partially prevents lysis by NK cells with low NKG2A expression levels. Data are represented as mean + SD (n=3). (e) Chromium release assay with NK cells from donor 1 and ESC− derived CD45+ cells showing that B2M−/− and B2M-/Edimer (pre-Cre) cells had similar susceptibility to NK-mediated lysis. Data are represented as mean + SD (n=3). (f) Chromium release assay as in (d) but with NK cells from donor 3 cultured at a low IL-2 dose (100 U/ml). Asterisks indicate p<0.05 for pair-wise comparison between the indicated cells (ANOVA followed by the post hoc Tukey HSD test). (g) Change in luciferase expression in B2M-/Edimer(pre-Cre) (HLA class I-negative control) and B2M-/Etrimer teratomas measured from day 13 to day 19 after implantation, with NK-92 cells administered to half the animals on days 13, 15 and 17. P-values were determined in each group (with or without NK-92 cells) by paired Student’s t-test (n=3 mice for both groups that received NK-92 cells and n=4 for both groups that did not). An unpaired Student’s t-test (p=0.096) was also applied to the same data to compare the relative growth of the B2M-/Etrimer and B2M-/Edimer(pre-Cre) teratomas in mice that received NK-92 cells to their relative growth in mice that did not receive NK-92 cells. (h) Examples of luciferase imaging in mice from (g), half of which received NK-92 cells as noted. “Pre” indicates B2M-/Edimer(pre-Cre) genotype, “-/E” indicates B2M-/Etrimer genotype. Red circles indicate measured areas.
Supplementary Figure 5. HLA molecule and costimulatory receptor expression. (a) Flow cytometry analysis of HLA-ABC and HLA-DR expression in IFN-γ-stimulated B2M+/+ Elf-1 EBs used for priming CD8+ T cells as shown in Figure 4A. (b) Costimulatory receptor profile of B2M+/+ Elf-1 EBs. Isotype controls in red and specific antibodies in blue. (c) Costimulatory receptor profile for ESC-derived CD45+ cells with the indicated B2M genotypes.
Supplementary Figure 6. Differential growth of B2M+/+ and B2M-/Etrimer ESC-derived teratomas when challenged with allogeneic CD8+ T cells in vivo. (a) Luciferase signal measured on day 1 was used to normalize the data. Each graph shows the results from an individual mouse. In all panels, blue and red lines show the growth of B2M-/Etrimer and B2M+/+ teratomas respectively. The three bottom panels show teratoma growth in mice that did not receive CD8+ cells. (b) B2M-/Etrimer and B2M+/+ teratoma growth rates (luciferase measurement increase per day) were measured before and after allogeneic primed CD8+ T cell infusions from three different donors (n=9 teratomas per genotype). Horizontal black bars indicate the means. P values were calculated by paired Student’s t-tests. (c) Quantitative measurement of CD8+ T cells in B2M-/Etrimer and B2M+/+ teratomas (n=5 mice, each with both types of teratoma; each line represents one mouse). Results were calculated as % of CD8 signal in the total area examined. The p value was calculated by paired Student’s t-test.
Acknowledgments
This work was supported by US National Institutes of Health (NIH) grants DK55759 and HL007093 to D.W.R, and HL007093 to A.G.C. as well as California Institute for Regenerative Medicine grants DR3-07438, TG2-01161, TG2-01151, CL1-00521 and FA1-00616 to D.O.C. We also acknowledge support from the Garland Initiative for Vision, The Foundation Fighting Blindness Wynn-Gund Translational Research Acceleration Program, and the UCSB Institute for Collaborative Biotechnologies through grant W911NF-09-0001 from the U.S. Army Research Office. The authors thank Raisa Stolitenko for technical assistance, Dan Geraghty (FHCRC) for providing HLA-E plasmids, Dave McDonald (FHCRC) for cytogenetic analyses, Danny Youngs (Bloodworks Northwest) for help in performing CDC assays, Piper Treuting, Brian Johnson and Ying-Tzang Tien (University of Washington) for histology and immunohistochemistry analysis, Scott Quaratella (Medtronic Inc.) for providing PVA sponges, Maria Gerace and David Yadock (FHCRC) for providing CD8+ T and CD56+ NK cells (funding provided by the Cooperative Centers of Excellence in Hematology: NIH Grant DK106829) ), and Claire Levy (FHCRC) for help with graphing programs. D.W.R. is on the Advisory Board of Horizon Discovery and co-founder of Universal Cells, Inc. D.O.C. is co-founder of Regenerative Patch Technologies, LLC. The other authors declare no conflict of interest.
Footnotes
Authors’ contributions.
G.G.G. and D.W.R. wrote the manuscript. G.G.G. and D.W.R designed the experiments. G.G.G and R.K.H. prepared AAV vectors stocks and performed gene editing. G.G.G. performed flow cytometry, hematopoietic differentiation, chromium release assays, CDC assays and data analysis. S.E.F. conducted in vivo experiments, PCR analysis and Southern blots. D.P. and R.K.H performed flow sorting experiments. G.G.G, S.E.F. and G.M. did molecular cloning. G.M. performed RNA analysis. L.R. designed both the HLA-E dimer and trimer open reading frames, conducted preliminary studies with HLA-E vectors, and optimized chromium release assay protocols. V. S. L., D.O.C. and G.G.G. performed RPE differentiation experiments and immunocytochemistry. L.A.H. and C.T. did flow cytometry to detect costimulatory receptors. A.G.C and L.R. each designed and built one of the AAV vectors. All authors discussed the results and commented on the manuscript.
References
- 1.Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient hla types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa S, Goldstein Ra, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CJ, et al. Banking on human embryonic stem cells: Estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 4.Braciale TJ. Antigen processing for presentation by MHC class I molecules. Curr Opin Immunol. 1992;4:59–62. doi: 10.1016/0952-7915(92)90126-y. [DOI] [PubMed] [Google Scholar]
- 5.Arce-Gomez B, Jones EA, Barnstable CJ, Solomon E, Bodmer WF. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978;11:96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 6.Riolobos L, et al. HLA engineering of human pluripotent stem cells. Mol Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu P, et al. Generating hypoimmunogenic human embryonic stem cells by the disruption of beta 2-microglobulin. Stem Cell Rev. 2013;9:806–13. doi: 10.1007/s12015-013-9457-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Quan Y, Yan Q, Morales JE, Wetsel RA. Targeted Disruption of the β2-Microglobulin Gene Minimizes the Immunogenicity of Human Embryonic Stem Cells. Stem Cells Transl Med. 2015;4:1234–45. doi: 10.5966/sctm.2015-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Q, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Reports. 2014;3:817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bix M, et al. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 11.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Sci (New York, NY) 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 12.Zarcone D, Tilden AB, Friedman HM, Grossi CE. Human leukemia- derived cell lines and clones as models for mechanistic analysis of natural killer cell-mediated cytotoxicity. Cancer Res. 1987;47:2674–2682. [PubMed] [Google Scholar]
- 13.Pazmany L, et al. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274:792–5. doi: 10.1126/science.274.5288.792. [DOI] [PubMed] [Google Scholar]
- 14.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 15.Lee N, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilienfeld BG, Crew MD, Forte P, Baumann BC, Seebach JD. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14:126–134. doi: 10.1111/j.1399-3089.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller JD, et al. Analysis of HLA-E peptide-binding specificity and contact residues in bound peptide required for recognition by CD94/NKG2. J Immunol. 2003;171:1369–75. doi: 10.4049/jimmunol.171.3.1369. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz A, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell DW, Hirata RK. Human gene targeting by viral vectors. Nat Genet. 1998;18:325–330. doi: 10.1038/ng0498-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan IF, Hirata RK, Russell DW. AAV-mediated gene targeting methods for human cells. Nat Protoc. 2011;6:482–501. doi: 10.1038/nprot.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain JR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 22.Li LB, et al. Trisomy Correction in Down Syndrome Induced Pluripotent Stem Cells. Cell Stem Cell. 2012;11:615–619. doi: 10.1016/j.stem.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan IF, et al. Engineering of human pluripotent stem cells by AAV-mediated gene targeting. Mol Ther. 2010;18:1192–9. doi: 10.1038/mt.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichiryu N, Fairchild PJ. Immune privilege of stem cells. Methods Mol Biol. 2013;1029:1–16. doi: 10.1007/978-1-62703-478-4_1. [DOI] [PubMed] [Google Scholar]
- 25.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, Maki G, Klingemann H. Characterization of a human cell line (NK- 92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 27.Brooks AG, Posch PE, Scorzelli CJ, Borrego F, Coligan JE. NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J Exp Med. 1997;185:795–800. doi: 10.1084/jem.185.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodin P, Kärre K, Höglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, et al. NK cells play a critical role in the regulation of class I-deficient hemopoietic stem cell engraftment: evidence for NK tolerance correlates with receptor editing. J Immunol. 2005;175:3753–61. doi: 10.4049/jimmunol.175.6.3753. [DOI] [PubMed] [Google Scholar]
- 30.Rouas-Freiss N, Gonçalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A. 1997;94:11520–5. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan JE. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc Natl Acad Sci U S A. 1997;94:6313–8. doi: 10.1073/pnas.94.12.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science (80-) 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 33.Zijlstra M, et al. Beta 2-microglobulin deficient mice lack CD4–8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 34.Mandal PK, et al. Efficient Ablation of Genes in Human Hematopoietic Stem and Effector Cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–652. doi: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeSandro A, Nagarajan UM, Boss JM. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am J Hum Genet. 1999;65:279–86. doi: 10.1086/302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Street SEA, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–84. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadola SD, Moins-Teisserenc HT, Trowsdale J, Gross WL, Cerundolo V. TAP deficiency syndrome. Clin Exp Immunol. 2000;121:173–8. doi: 10.1046/j.1365-2249.2000.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietra G, et al. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:10896–901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen SG, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–20. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietra G, Romagnani C, Manzini C, Moretta L, Mingari MC. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J Biomed Biotechnol. 2010;2010:907092. doi: 10.1155/2010/907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–80. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–25. [PubMed] [Google Scholar]
- 43.Spriggs MK, et al. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A. 1992;89:6070–4. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ware CB, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:4484–9. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson JA. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science (80- ) 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 46.Kotini AG, et al. Functional analysis of a chromosomal deletion associated with myelodysplastic syndromes using isogenic human induced pluripotent stem cells. Nat Biotechnol. 2015;33:646–55. doi: 10.1038/nbt.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leach LL, Buchholz DE, Nadar VP, Lowenstein SE, Clegg DO. Canonical/β-catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Invest Ophthalmol Vis Sci. 2015;56:1002–13. doi: 10.1167/iovs.14-15835. [DOI] [PubMed] [Google Scholar]
- 48.Molecular Cloning: A Laboratory Manual (3 Volume Set): 9780879693091: Medicine & Health Science Books @ Amazon.com. at <http://www.amazon.com/Molecular-Cloning-Laboratory-Manual-Volume/dp/0879693096>
- 49.Papapetrou EP, Sadelain M. Generation of transgene-free human induced pluripotent stem cells with an excisable single polycistronic vector. Nat Protoc. 2011;6:1251–73. doi: 10.1038/nprot.2011.374. [DOI] [PubMed] [Google Scholar]
- 50.Lauterbach N, Voorter CEM, Tilanus MGJ. Molecular typing of HLA-E. Methods Mol Biol. 2012;882:143–58. doi: 10.1007/978-1-61779-842-9_8. [DOI] [PubMed] [Google Scholar]
- 51.Aicheler RJ, Stanton RJ. Functional NK cell cytotoxicity assays against virus infected cells. Methods Mol Biol. 2013;1064:275–87. doi: 10.1007/978-1-62703-601-6_20. [DOI] [PubMed] [Google Scholar]
- 52.Teraski PI, McClelland JD. Mirodroplet assay of human serum cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 53.Vernon RB, et al. Reversal of diabetes in mice with a bioengineered islet implant incorporating a type I collagen hydrogel and sustained release of vascular endothelial growth factor. Cell Transplant. 2012;21:2099–110. doi: 10.3727/096368912X636786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. PCR primers and conditions.
Supplementary Table 2. HLA types of ESCs, NK cells, and CD8+ T cells.
Supplementary Figure 1. Generation of B2M−/− ESCs. (a) Maps of the B2M alleles in cells with the indicated B2M genotypes. Probes and restriction enzymes used in Southern blots are indicated (H, Hind III; B, Bgl II; G, BsrG I). Dark blue regions show overlap with the gene editing vectors used to produce each allele. (b) B2M flow cytometry of different mixed colonies of ESCs and their subcloning results showing the bias against obtaining pure populations of normal B2M−/− cells. (c) Flow cytometry showing a lack of B2M expression in two B2M−/− ESC clones after IFN-γ treatment. (d) Karyotypic analysis of these same two B2M−/− clones.
Supplementary Figure 2. Trace B2M expression in B2M-edited clones. (a) Pan-HLA- ABC flow cytometry of IFN-γ-treated B2M+/+ and B2M−/− H1 ESCs created with vectors AAV-B2M-EHyTKpA and AAV-B2M-ETKNpA6 (blue tracings) and isotype controls (red tracings) showing low level expression. (b) The sequence of an mRNA isolated from B2M−/− H1 ESCs showing transcription of the edited allele through the pA site, the location of an initiation codon (underlined) in the loxP site, and in-frame continuation of the reading frame into downstream B2M exon 1 sequences. (c) Modifications made in vectors AAV- B2M-HyTK and AAV-B2M-TKN that eliminate trace B2M expression. The B2M gene is shown after Cre-mediated excision of the HyTK or TKN genes present in either vector, with the loxP-encoded ATG start codon shown above, and the downstream stop codons that prevent translation (asterisks) in all three reading frames shown below.
Supplementary Figure 3. Retinal pigmented epithelium (RPE) cell differentiation. RPE cells derived from B2M+/+ H9 ESCs (panels a–c) and B2M-/Edimer ESCs (panels d–f) were visualized by bright field microscopy (a, c) and immunofluorescence microscopy for retinal markers PMEL (b, e) and MITF (c, f) after 42 days of differentiation. Bright field images demonstrate the level of pigmentation. PMEL+ and MITF+ cells are shown in green, with DAPI stained nuclei in blue. Scale bar = 50 μm.
Supplementary Figure 4. Hematopoietic potential and NK cell-mediated lysis of ESC- derived CD45+ cells. (a) Flow cytometry analysis of CD45 expression after hematopoietic differentiation of Elf-1 ESCs with the indicated B2M genotypes. Data were acquired from suspension cells on day 38 of differentiation. Results for B2M-/Etrimer c5 shown in Fig. 3B. (b) B2M−/− ESCs produce fewer hematopoietic cells. Kinetics of suspension cell production during hematopoietic differentiation of ESCs with the indicated B2M genotypes. Y-axis denotes number of live suspension cells generated per 5x106 undifferentiated ESCs. The results from two independent differentiation experiments are shown with numbers between parentheses. (c) Flow cytometry analysis of NKG2A and NKG2C receptors on NK cells derived from donor 2. Percents were calculated by subtracting the isotype control frequencies. (d) Chromium release assay with NK cells from donor 2 and ESC-derived CD45+ cells with the indicated B2M genotypes showing B2M-/Edimer expression partially prevents lysis by NK cells with low NKG2A expression levels. Data are represented as mean + SD (n=3). (e) Chromium release assay with NK cells from donor 1 and ESC− derived CD45+ cells showing that B2M−/− and B2M-/Edimer (pre-Cre) cells had similar susceptibility to NK-mediated lysis. Data are represented as mean + SD (n=3). (f) Chromium release assay as in (d) but with NK cells from donor 3 cultured at a low IL-2 dose (100 U/ml). Asterisks indicate p<0.05 for pair-wise comparison between the indicated cells (ANOVA followed by the post hoc Tukey HSD test). (g) Change in luciferase expression in B2M-/Edimer(pre-Cre) (HLA class I-negative control) and B2M-/Etrimer teratomas measured from day 13 to day 19 after implantation, with NK-92 cells administered to half the animals on days 13, 15 and 17. P-values were determined in each group (with or without NK-92 cells) by paired Student’s t-test (n=3 mice for both groups that received NK-92 cells and n=4 for both groups that did not). An unpaired Student’s t-test (p=0.096) was also applied to the same data to compare the relative growth of the B2M-/Etrimer and B2M-/Edimer(pre-Cre) teratomas in mice that received NK-92 cells to their relative growth in mice that did not receive NK-92 cells. (h) Examples of luciferase imaging in mice from (g), half of which received NK-92 cells as noted. “Pre” indicates B2M-/Edimer(pre-Cre) genotype, “-/E” indicates B2M-/Etrimer genotype. Red circles indicate measured areas.
Supplementary Figure 5. HLA molecule and costimulatory receptor expression. (a) Flow cytometry analysis of HLA-ABC and HLA-DR expression in IFN-γ-stimulated B2M+/+ Elf-1 EBs used for priming CD8+ T cells as shown in Figure 4A. (b) Costimulatory receptor profile of B2M+/+ Elf-1 EBs. Isotype controls in red and specific antibodies in blue. (c) Costimulatory receptor profile for ESC-derived CD45+ cells with the indicated B2M genotypes.
Supplementary Figure 6. Differential growth of B2M+/+ and B2M-/Etrimer ESC-derived teratomas when challenged with allogeneic CD8+ T cells in vivo. (a) Luciferase signal measured on day 1 was used to normalize the data. Each graph shows the results from an individual mouse. In all panels, blue and red lines show the growth of B2M-/Etrimer and B2M+/+ teratomas respectively. The three bottom panels show teratoma growth in mice that did not receive CD8+ cells. (b) B2M-/Etrimer and B2M+/+ teratoma growth rates (luciferase measurement increase per day) were measured before and after allogeneic primed CD8+ T cell infusions from three different donors (n=9 teratomas per genotype). Horizontal black bars indicate the means. P values were calculated by paired Student’s t-tests. (c) Quantitative measurement of CD8+ T cells in B2M-/Etrimer and B2M+/+ teratomas (n=5 mice, each with both types of teratoma; each line represents one mouse). Results were calculated as % of CD8 signal in the total area examined. The p value was calculated by paired Student’s t-test.
Data Availability Statement
The DNA sequences of the vectors and plasmids used in this study are available upon request.