Abstract
Background
The objective of this study was to determine whether hatha yoga is an efficacious adjunctive intervention for individuals with continued depressive symptoms despite antidepressant treatment.
Methods
We conducted a randomized controlled trial of weekly yoga classes (n = 63) vs. health education classes (Healthy Living Workshop, or HLW; n = 59) in individuals with elevated depression symptoms and antidepressant medication use. HLW served as an attention-control group. The intervention period was 10 weeks, with follow-up assessments 3 and 6 months afterwards. The primary outcome was depression symptom severity assessed by blind rater at 10 weeks. Secondary outcomes included depression symptoms over the entire intervention and follow-up periods, social and role functioning, general health perceptions, pain, and physical functioning.
Results
At 10 weeks, we did not find a statistically significant difference between groups in depression symptoms (b=−0.82, SE=0.88, p=0.36). However, over the entire intervention and follow-up period, when controlling for baseline, yoga participants showed lower levels of depression than HLW participants (b = −1.38, SE = 0.57, p = 0.02). Fifty-one percent of yoga participants demonstrated a response (≥ 50% reduction in depression symptoms) at 6 month-follow-up, compared to 31% of HLW participants (OR = 2.31; p = 0.04). Yoga participants showed significantly better social and role functioning and general health perceptions over time.
Conclusions
Although we did not see a difference in depression symptoms at the end of the intervention period, yoga participants showed fewer depression symptoms over the entire follow-up period. Benefits of yoga may accumulate over time.
Introduction
The goal of depression treatment is to help individuals achieve symptomatic remission and normal functioning. However, partial and non-response to existing treatments, such as antidepressant medications, remains a significant problem. For example, in a large, naturalistic study of depression treatment in which 77% of participants received “adequate” or “aggressive” pharmacotherapy, 46% of participants were classified as non-responders after 6 months, and 32% were considered to have responded but not remitted (Corey-Lisle et al., 2004). Similarly, in the Sequenced Treatment Alternatives to Relieve Depression trial, the remission rate after initial antidepressant treatment was 37%, and the cumulative remission rate with up to four successive treatment steps was 67% (Rush et al., 2006). Thus, there is a need to test innovative adjunctive interventions to further improve outcomes in this group of patients who do not fully respond to traditional treatments.
Yoga is an ancient Indian system of philosophy and practice (Iyengar, 1993). Approximately 5% of U.S. adults practice yoga (Barnes et al., 2004). Most practice hatha yoga, which involves training the body with the ultimate goal of promoting good physical and mental health. Hatha yoga includes breath control (pranayama), physical postures (asanas), and meditation (dhyana). Hatha yoga can combine mindfulness practice (i.e., non-judgmental attention to present-moment experience) and physical activity in a way that is internally consistent. Increased mindfulness may improve depression by reducing rumination, increasing self-compassion, or promoting the view that thoughts and feelings are transitory cognitive contents rather than self-defining (i.e., meta-cognitive awareness) (van der Velden et al., 2015). Exercise has been demonstrated helpful for depression as well (Cooney et al., 2013).
A recent meta-analysis of 12 randomized controlled trials (RCTs) of yoga for clinical depression reported yoga was significantly better than usual care, relaxation exercises, or aerobic exercise in decreasing depressive symptoms (Cramer et al., 2013). Yoga may also improve physical health outcomes comorbid with depression, particularly pain. However, studies of yoga for depression have numerous methodologic limitations, including small sample sizes, lack of assessment of instructor fidelity to the yoga protocol, lack of outcome assessment by blind raters, lack of intent-to-treat analyses, and insufficient documentation of randomization procedures (Uebelacker et al., 2016).
The purpose of this study is to examine whether hatha yoga is efficacious for depression when used as an adjunct to antidepressant treatment. We enrolled individuals with current or recent major depression who were receiving antidepressant medication and continued to have elevated depressive symptoms. We randomized participants to participate in yoga classes vs. an attention control group (i.e., a health education class entitled Health Living Workshop; HLW). We encouraged all participants to continue to take their antidepressant medications. The intervention phase lasted 10 weeks; participants were then followed for 6 months afterwards. We hypothesized that yoga participants would show lower depression severity over time as assessed by the Quick Inventory of Depression Symptomatology – Clinician Rating (QIDS) (Rush et al., 2003), as well as better social and role functioning, better general health perceptions and physical functioning, and less physical pain relative to the control group. A priori, the 10-week QIDS assessment was designated as the primary outcome.
Methods
The Institutional Review Board of Butler Hospital approved the study. All study activities occurred at the hospital, which is a psychiatric hospital in Providence, RI. We recruited participants from the greater Providence area from July 2011–June 2014, and some participants were active in the study until March 2015. Participants were not required to be receiving any other psychiatric treatment at the hospital, and most were not. Advertisements presented study interventions with equipoise, using the rationale that both yoga and HLW were designed to promote good physical and mental health.
Interested individuals underwent a telephone screen and, if potentially eligible, attended an in-person interview (Baseline 1) where they provided written informed consent, completed interviews to determine eligibility, and completed other assessments. Subsequently, study staff requested medical clearance from the participant’s primary care provider. Once staff received clearance, a research assistant called the participant (Baseline 2), administered a QIDS by telephone, and randomized the participant.
We randomized participants to groups using a 1:1 ratio with a computer program that employed urn randomization (Stout et al., 1994). We stratified participants on three variables: depression severity (QIDS ≤ 10 vs. QIDS ≥ 11), current psychotherapy with visits more often than once per month (yes or no), and gender. Study staff had no way of knowing to which arm the next participant would be randomized.
Subsequent to randomization, participants were enrolled in the intervention phase of the study for 10 weeks and then in the follow-up phase of the study for 6 months. During the intervention phase, participants were invited to attend classes in their assigned arm. The follow-up phase included assessment only. Staff attempted to contact all participants for all assessments regardless of whether they were attending classes.
Participants
Inclusion criteria were: 1) met criteria for major depressive disorder (MDD) within the prior two years assessed via the Structured Clinical Interview for DSM-IV (SCID; First et al., 2001); 2) QIDS score ≥ 8 (mild depression) and ≤ 17 (moderately severe depression); 3) no history of bipolar disorder, schizophrenia, or psychotic symptoms, assessed via the SCID; 4) no current hazardous drug or alcohol use assessed using the Alcohol Use Disorder Identification Test and Drug Used Disorders Identification Test (Babor et al., 2001, Berman et al., 2005); 5) no suicidal ideation or behavior requiring immediate attention; 6) currently taking an antidepressant at a dose with demonstrated effectiveness per American Psychiatric Association practice guidelines (Work Group on Major Depressive Disorder, 2010) for at least 8 weeks; 7) antidepressant dose had not changed in the previous 4 weeks and no plans to change the dose in the next 10 weeks; 8) if in psychotherapy, therapy frequency had not changed in the past 6 weeks AND no plans to change it in the next 10 weeks; 9) medically cleared for moderate physical activity; 10) not pregnant or planning to become pregnant; 11) no more than 4 yoga, tai chi, Mindfulness Based Stress Reduction or health education classes or home practice sessions in the previous year, no more than 8 yoga classes in the previous 2 years, and had not practiced yoga weekly for 8 weeks or more in the previous 5 years; 12) no weekly meditation practice; 13) fluent in English; and 14) aged 18 or older.
Interventions
Yoga
Based on expert opinion (authors TG and GT; also Weintraub, 2004), feedback from pilot research participants (Uebelacker et al., 2010b), and existing literature, we developed a manualized hatha yoga program. Each participant received an introductory 20–30 minute individual meeting with a yoga instructor. We offered group classes twice per week; participants were expected to attend at least one class per week with the option of attending two per week for 10 weeks. Classes were 80-minutes. Classes included breathing exercises (pranayama) and seated meditation; warm-ups and half sun salutations; standing postures (asanas); seated postures; an inversion and a twist; shavasana (relaxation); and wrap-up and discussion of home practice. Instructors had a list of asanas and other practices from which they chose. Similar to many community classes, classes accommodated rolling admission. Instructors were asked to encourage mindful attention to the present moment throughout class, and to repeatedly guide participants through the connection between breath and movement. Instructors tailored the pace of class to the participants present; generally, the class occurred at a gentle pace. To facilitate home practice, we gave each participant a yoga mat, descriptions of suggested practices, two videos featuring study instructors, and a commercial yoga DVD (LifeForce Yoga® to Beat the Blues – Level1 by Amy Weintraub).
All yoga instructors were Registered Yoga Teachers ® with the Yoga Alliance. Yoga instructors also received study-specific training. Using audiorecordings and a structured tool, yoga supervisors Dr. Tremont and Mr. Gillette rated a subset of 55 classes throughout the duration of the study for instructor manual fidelity. Fidelity was excellent for class content (mean fidelity = 95%) and teaching style (mean fidelity= 94%). Yoga instructors met monthly for peer consultation. Yoga supervisors provided feedback about any observed manual deviations.
Healthy Living Workshop (HLW)
Group HLW classes were concurrent with yoga classes. Instructors used a detailed manual adapted from previous work with psychiatric patients and smokers (Abrantes et al., 2014, Abrantes et al., 2012). HLW included an initial individual orientation meeting between the instructor and participant. Subsequently, participants were invited to attend at least one and up to two HLW classes per week for 10 weeks. Classes were 60 minutes long. Instructors followed a detailed manual. There were 20 different class topics that repeated every 10 weeks. Topics included: alcohol, nicotine, and caffeine; being a smart patient; brain diseases; cancer prevention; diabetes; nutrition (3 classes); germs, colds, and the flu; physical activity (2 classes); sleep; physical pain, prevalence and causes of depression; and protecting your heart. Classes included slides, audio or video clips, and demonstrations. Classes were interactive, but instructors avoided focusing on personal problems of participants. To facilitate home learning, we gave each participant a book about nutrition, handouts at each class, and lists of websites with relevant information. Instructors encouraged participants to read materials each week at home.
HLW instructors were post-doctoral fellows in clinical psychology and a master’s level nurse, with supervision by Dr. Abrantes. Using audiorecordings and a structured tool, Dr. Abrantes and a trained research assistant rated a subset of 53 classes for instructor manual fidelity. Fidelity was excellent for both class content (mean fidelity= 97%) and teaching style (mean fidelity = 95%). HLW instructors met monthly for peer consultation.
Assessments
Assessment schedule
Assessments occurred at Baseline 1 (eligibility assessment), Baseline 2 (randomization), 3.3 weeks, 6.6 weeks, and 10 weeks (endpoint of intervention phase). Baseline 1 and 2 were a mean of 7.6 (SD =4.5) days apart. Follow-up assessments occurred 3 months and 6 months after endpoint. The Baseline 2 assessment and 3- and 6-month follow-up assessments occurred by telephone; participants returned self-report instruments by mail at these assessments.
Diagnoses
To assess psychiatric diagnoses, we used the Structured Clinical Interview for DSM-IV (SCID; First et al., 2001). Raters included PhD level psychologists and a trained research assistant who reviewed ratings with a psychologist.
Primary outcome
We assessed the primary outcome, depression symptom severity, using the Quick Inventory of Depression Symptomatology – Clinician Rating (QIDS; Rush et al., 2003) at all assessment timepoints. Scores of 6–10 reflect mild depression symptoms; 11–15 reflect moderate depression symptoms, and scores 16 or greater reflect severe or very severe symptoms. QIDS interviewers were trained research assistants blind to treatment assignment. Participants were instructed not to reveal their intervention assignment to interviewers. We assessed reliability of interviews by having a second rater rate a random selection of 61 interviews; reliability was excellent (ICC = 0.96). Versions of the QIDS have been administered by telephone in previous trials (Rush et al., 2005, Wang et al., 2007) and the telephone version shows good psychometrics and concordance with self-reports of depression symptoms (Rush et al., 2005)
Secondary outcomes
We used the Patient Health Questionnaire- 9 item (PHQ-9; Kroenke et al., 2001) to assess self-report of depressive symptoms. Scores range from 0–27, with higher scores corresponding to more symptoms. We used the physical functioning, bodily pain, and general health perceptions subscales from the 20-item Short-Form Survey (SF-20; Ware et al., 1993). Scores range from 0–100, with higher scores indicating better health. We administered the PHQ-9 and SF-20 at all timepoints except Baseline 2. We used two subscales from the World Health Organization – Disability Assessment Schedule (WHO-DAS II; World Health Organization, 2004): the “Getting along with people” subscale (social functioning) and “Life activities” subscale (work and role functioning). Scores range from 0–20 on “Getting along with people” and 0–32 on “Life activities,” with higher scores indicating more disability. We administered the WHO-DAS-II at Baseline 1, 10 week intervention endpoint, and 3 and 6 month follow-up timepoints.
We defined depression “response” as a decrease of 50% or greater on the QIDS from Baseline 1 to follow-up. We defined “remission” as a QIDS score of ≤ 5 (no depression symptoms). We assessed response and remission at 10 weeks, 3 months, and 6 months.
Other assessments
We assessed demographics via self-report. For race, we asked participants to choose as many as applied from a list of options. We assessed amount of physical activity with the International Physical Activity Questionnaire (IPAQ; Craig et al., 2003). IPAQ data were used to calculate metabolic equivalent of task (MET) minutes per week (IPAQ Research Committee, 2005) over the previous week. We added questions to the IPAQ to assess amount of time spent in yoga practice either in class or at home; these were not used in the MET score calculation. We administered the modified IPAQ at all assessment points except Baseline 2. We assessed adverse events every 3 weeks during the intervention phase with a single question about injuries related to study participation, and at 10 weeks with the Systematic Assessment of Treatment-Emergent Events, General Inquiry (SAFTEE-GI; Levine & Schooler, 1986).
Statistical methods
Baseline characteristics, class attendance, minutes of yoga per week, and other treatment during study participation
We summarized variables using descriptive statistics, and compared differences between treatment groups (Yoga vs. HLW) using either a X2 test or t-test.
Continuous outcome variables
As a general strategy, we included outcome assessments from all randomized participants in linear mixed effects (LME) models assessing continuous outcomes with a dummy coded index included in each model to represent treatment assignment. Because participants with missing covariates (n =5) or less than two follow-up assessments for the primary outcome (n = 10) would be removed from LME models, we used a multiple imputation approach (van Buuren & Groothuis-Oudshoorn, 2011) to ensure inclusion of all allocated cases (n= 122). Our data analytic strategy involved multiple imputation (m= 50) of missing values with multivariate imputation by chained equations using all independent, dependent, and covariate terms in the multiple estimations of missing values
The baseline value for the outcome variable was not included as part of the dependent variable; rather, the baseline value was included as a covariate. Thus, a significant main effect for the treatment assignment variable indicates significant differences between groups in average score on the outcome variable across all non-baseline timepoints (i.e., weeks 3.3., 6.6., 10 (endpoint), and 3 and 6-month followup), demonstrating that treatment assignment (Yoga vs. HLW) has a significant impact on that outcome while controlling for the baseline value of that outcome. We also tested models that included a group × time interaction. Planned covariates included age, gender, and time. We included as covariates baseline values of general health perceptions and level of physical activity given the potential for influence on physical aspects of yoga practice.
Our a priori hypotheses for group differences in QIDS at the end of treatment was tested using multiple imputation and a regression model with 10-week QIDS score as the dependent variable and covariates including baseline QIDS and other covariates described above. As an index of effect size, we computed between-group effect sizes in standard deviation units (Feingold, 2009).
Consistent with previous studies, limitations in physical functioning (i.e. the SF-20 physical functioning scale) were counted regardless of duration and were scored to reflect the number of limitations present (Stewart et al., 1981). Thus, for this variable, we used a negative binomial mixed effects model because it provided the best fit to the skewed data.
Categorical outcome assessments
We included outcome assessments from all participants with relevant follow-up data (i.e., with data at 10 weeks, 3 months, or 6 months) in linear mixed effects (LME) models assessing continuous outcomes and generalized LME assessing categorical outcomes (i.e. response, remission). We included baseline QIDS as a covariate. We did not use multiple imputation for these models.
Statistical package
All analyses were conducted using R 3.2 (R Core Team, 2015) and tools within the nlme (Pinheiro et al., 2015), lme4 (Bates et al., 2015), and mice (van Buuren & Groothuis-Oudshoorn, 2011) packages.
Power
Power analyses were based on a priori determinations of minimally clinically significant differences. Using recommendations on depression from the National Institute for Clinical Excellence in the UK (National Institute for Health and Clinical Excellence, 2007) and descriptions of effect size (Cohen, 1988), we set a minimum clinically significant difference for depression treatments at an effect size of d = .40 (approximately 2–3 points difference on the QIDS). Using an empirical power analysis, we found that, assuming a group size of 75 (including a 20% dropout rate), and setting alpha at .05, we would have >80% power to detect an effect of d= .40 at endpoint. However, we were ultimately only able to recruit 122 participants during the funding period.
Results
Cohort
We recruited 122 participants and allocated 63 participants to yoga and 59 to HLW (Figure 1). Table 1 shows demographic and clinical characteristics at baseline. Almost two thirds of our sample had chronic major depression. Average depression severity was in the moderate range. Participants reported, on average, a moderate level of physical activity (IPAQ Research Committee, 2005). Table 1 also provides information about baseline values of outcome variables, including means and standard deviations. There were no significant differences between groups on baseline variables.
Figure 1.
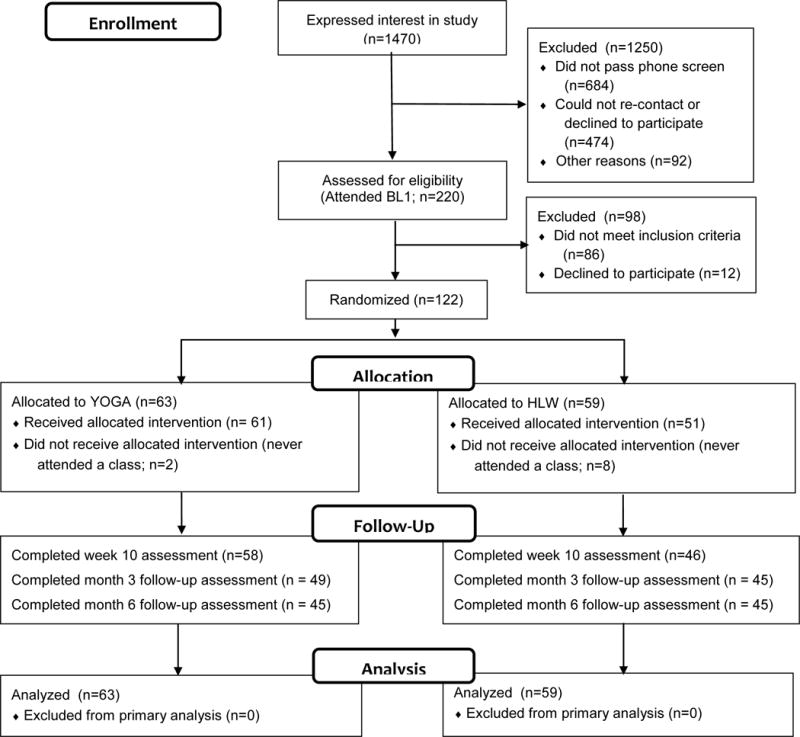
Consort Flow Diagram
Table 1.
Baseline demographic and clinical characteristics, and baseline values of outcome variables
| Variable | Overall (n=122)
|
Yoga (n=63)
|
HLW (n=59)
|
Between Group Differences
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | X2/t | df | p | |
| Demographics | |||||||||
|
| |||||||||
| Gender | 0.02 | 1 | 0.88 | ||||||
| Male | 19 | 15.6% | 9 | 14.3% | 10 | 16.9% | |||
| Female | 103 | 84.4% | 54 | 85.7% | 49 | 83.1% | |||
| Agea | 46.5 | 12.16 | 46.78 | 12.27 | 46.2 | 12.13 | −0.26 | 119.6 | 0.80 |
| Marital Status | 0.55 | 1 | 0.46 | ||||||
| Married/Cohabiting | 44 | 36.4% | 25 | 40.3% | 19 | 32.2% | |||
| Single/Divorced/Separated/Widow | 77 | 63.6% | 37 | 59.7% | 40 | 67.8% | |||
| Missing/No Answer | 1 | 1 | 0 | ||||||
| Race | 0.02 | 2 | 0.99 | ||||||
| White or Caucasian | 103 | 84.4% | 53 | 84.1% | 50 | 84.7% | |||
| Black or African American | 4 | 3.3% | 2 | 3.2% | 2 | 3.4% | |||
| Other or Multiracialb | 15 | 12.3% | 8 | 12.7% | 7 | 11.9% | |||
| Ethnicity | 0.3 | 1 | 0.58 | ||||||
| Not Latino | 112 | 94.9% | 60 | 96.8% | 52 | 92.9% | |||
| Latino | 6 | 5.1% | 2 | 3.2% | 4 | 7.1% | |||
| Missing/No Answer | 4 | 1 | 3 | ||||||
| Education | 0.34 | 2 | 0.84 | ||||||
| High School or Less | 20 | 16.5% | 10 | 15.9% | 10 | 17.2% | |||
| Some College | 30 | 24.8% | 17 | 27% | 13 | 22.4% | |||
| College or Graduate Degree | 71 | 58.7% | 36 | 57.1% | 35 | 60.3% | |||
| Missing/No Answer | 1 | 0 | 1 | ||||||
| Income | 3.45 | 3 | 0.33 | ||||||
| $0–25000 | 38 | 32.8% | 17 | 28.3% | 21 | 37.5% | |||
| $25000–49,999 | 36 | 31% | 23 | 38.3% | 13 | 23.2% | |||
| $50000–99999 | 31 | 26.7% | 14 | 23.3% | 17 | 30.4% | |||
| >$100 | 11 | 9.5% | 6 | 10% | 5 | 8.9% | |||
| Missing/No Answer | 6 | 3 | 3 | ||||||
| Employment | 1.88 | 4 | 0.76 | ||||||
| Employed Full-time or Part-time | 50 | 41.7% | 27 | 42.9% | 23 | 40.4% | |||
| Student | 6 | 5% | 2 | 3.2% | 4 | 7% | |||
| Unemployed | 27 | 22.5% | 15 | 23.8% | 12 | 21.1% | |||
| Receiving Disability | 24 | 20% | 11 | 17.5% | 13 | 22.8% | |||
| Homemaker/Full-time Parent/Retired | 13 | 10.8% | 8 | 12.7% | 5 | 8.8% | |||
| Missing/No Answer | 2 | 0 | 2 | ||||||
|
| |||||||||
| Clinical Characteristics | |||||||||
|
| |||||||||
| Has Chronic Depressionc | 75 | 64.7% | 43 | 70.5% | 32 | 58.2% | 1.42 | 1 | 0.23 |
| Engaged in Psychotherapy | 49 | 40.2% | 29 | 46% | 20 | 33.9% | 1.4 | 1 | 0.24 |
| Number of weeks antidepressants unchanged | 23.05 | 36.20 | 17.86 | 30.71 | 28.69 | 40.87 | 1.66 | 119 | 0.10 |
| Age onset first MDE | 22.55 | 18.03 | 24.21 | 22.18 | 20.78 | 12.07 | −1.06 | 95.49 | 0.29 |
| Physical Activity, in METS, Not Including Yoga (IPAQ) | 1436 | 2000 | 1300 | 2016 | 1574 | 1991 | 0.75 | 117 | 0.46 |
|
| |||||||||
| Baseline Values of Outcome Variables | |||||||||
|
| |||||||||
| Level of Depressive Symptoms (QIDS) | 12.87 | 2.78 | 12.92 | 2.9 | 12.81 | 2.67 | −0.21 | 120 | 0.83 |
| Level of Depressive Symptoms (PHQ-9) | 14.28 | 4.72 | 14.28 | 4.23 | 14.29 | 5.24 | 0.01 | 107.7 | 0.99 |
| Getting along with people (WHO-DAS) | 7.16 | 3.91 | 6.75 | 3.65 | 7.58 | 4.16 | 1.08 | 100.8 | 0.28 |
| Life activities (WHO-DAS) | 15.27 | 6.34 | 15.25 | 6.37 | 15.28 | 6.41 | 0.02 | 62 | 0.98 |
| Physical Pain (SF-20) | 62.08 | 26.12 | 61.11 | 27.48 | 63.16 | 24.72 | 0.43 | 118 | 0.67 |
| General Health Perceptions (SF-20) | 49.28 | 26.55 | 46.11 | 27.42 | 52.79 | 25.32 | 1.39 | 118 | 0.17 |
| Physical Functioning (SF-20) | 67.92 | 33.41 | 69.31 | 34.39 | 66.37 | 32.66 | −0.48 | 118 | 0.63 |
Note. IPAQ = International Physical Activity Questionnaire. MDD = major depressive disorder. MDE = major depressive episode. METS = metabolic equivalent of task minutes per week. PHQ-9 = Patient Health Questionnaire, 9 item. QIDS = Quick Inventory of Depression Symptomatology. SF-20 = 20-item Short-Form Survey. WHO-DAS = World Health Organization Disability Assessment Schedule.
Continuous variables are in italics and differences between groups are compared using a t-test. Variables not in italics are categorical.
Other or multiracial included people who were multiracial (n = 5), Hispanic/Latino (n =4), Cape Verdean (n = 3), Indian (n = 1), Native American (n = 1), and chose not to respond (n=1).
All participants had a current episode of major depression or an episode in the previous 2 years. To assess whether their depression would be considered chronic, after assessing the depressive episode, we asked whether they had experienced depressed mood and other symptoms discussed for at least 2 years, more than half the days, with no more than 2 months of feeling okay.
Other treatment during the study
At the 10-week, 3-month, and 6-month assessment timepoints, 95%–100% of participants reported that they continued to take an antidepressant medication, and approximately 40% reported engaging in psychotherapy. There were no significant differences between intervention arms on either variable (all p-values > 0.25). Please see Supplementary Table 1.
Adherence and yoga “dosage”
Yoga participants attended a mean of 8.9 classes (SD = 5.1) throughout the 10 week intervention phase; HLW participants attended an average of 7.0 classes (SD = 5.7; t(120) = 1.88, p = .06). As a manipulation check, we examined minutes per week engaged in yoga (either in a class or at home) in both arms in the intervention phase. As expected, the average total time practicing yoga was significantly higher in the yoga arm than in the HLW arm during the intervention phase (i.e., at weeks 3.3, 6.6, and 10; all p-values < 0.001). In the yoga arm, mean minutes practicing yoga per week ranged from 113–123 (SDs ranging from 76–170 minutes), whereas in the HLW arm, mean minutes practicing yoga per week ranged from 1–5 (SDs ranging from 5–27). There was a consistent difference between groups during the follow-up phase as well. At 3 months, average minutes of yoga practice per week was 36 mins (SD =74 mins) and 2 mins (SD =10 mins) in the yoga arm and HLW arm, respectively (t(91) =−2.98, p = .004). At 6 months, average minutes of yoga practice per week was 34 mins (SD =64 mins) and 2 mins (SD =11 mins) in the yoga arm and HLW arm, respectively (t(89) = −3.29, p = .001).
Outcomes
First, we conducted an analysis of group differences in change in the primary outcome (QIDS) at week 10. There was no significant difference between groups at this assessment point (b=−0.89, SE=0.85, p=0.30). Average change in QIDS was −3.15 (SD=5.09) and −3.93 (SD=3.90) for HLW and Yoga conditions respectively. To estimate effect size, we divided the difference between groups in average change in QIDS by the raw standard deviation of the baseline QIDS. This yielded an effect size of 0.29, favoring the yoga arm. This is a small-to-medium effect size (Cohen, 1988).
When we examined QIDS scores over the entire intervention and follow-up period while adjusting for baseline QIDS, we did observe a significant difference between groups, with yoga participants demonstrating lower depression severity than HLW participants (Table 2). On average across all (non-baseline) timepoints, the difference between groups was approximately one half of one standard deviation– a medium effect size (Cohen, 1988). The treatment by time interaction was not significant (b= −0.041, SE=0.03, p=0.17), indicating that there was no significant difference between the non-baseline timepoints in the magnitude of the difference between groups. See figure 2 for a graphical depiction of QIDS scores over time.
Table 2.
Regression model results comparing Yoga and HLW on primary and secondary outcomes.
| Parameter Estimate | 95% confidence interval | SE | p | |
|---|---|---|---|---|
| Depression Symptoms (QIDS) | ||||
|
| ||||
| Baseline Depression Symptoms (QIDS) | 0.74 | (0.54, 0.94) | 0.10 | 0.00 |
| Gender: Female vs Male | −0.50 | (−1.99, 1.00) | 0.76 | 0.51 |
| Age | 0.06 | (0.02, 0.10) | 0.02 | 0.01 |
| Baseline Physical Activity (IPAQ) | 0.43 | (−0.08, 0.93) | 0.26 | 0.10 |
| General Health Perceptions (SF-20) | −0.03 | (−0.05, −0.01) | 0.01 | 0.00 |
| Month of Assessment | −0.05 | (−0.09, −0.02) | 0.02 | 0.00 |
| Group: Yoga vs HLW | −1.42 | (−2.42, −0.43) | 0.51 | 0.01 |
|
| ||||
| Depression Symptoms (PHQ-9) | ||||
|
| ||||
| Baseline PHQ-9 | 0.38 | (0.23, 0.52) | 0.07 | 0.00 |
| Gender: Female vs Male | −1.54 | (−3.39, 0.30) | 0.94 | 0.10 |
| Age | 0.01 | (−0.05, 0.06) | 0.03 | 0.80 |
| Baseline Physical Activity (IPAQ) | 0.00 | (−0.63, 0.63) | 0.32 | 1.00 |
| General Health Perceptions (SF-20) | −0.04 | (−0.07, −0.02) | 0.01 | 0.00 |
| Month of Assessment | −0.05 | (−0.09, −0.01) | 0.02 | 0.01 |
| Group: Yoga vs HLW | −1.94 | (−3.20, −0.68) | 0.64 | 0.00 |
|
| ||||
| Getting Along with People (WHO-DAS) | ||||
|
| ||||
| Baseline Getting Along with People (WHO-DAS) | 0.59 | (0.45, 0.74) | 0.07 | 0.00 |
| Gender: Female vs Male | −0.69 | (−2.25, 0.86) | 0.79 | 0.38 |
| Age | 0.03 | (−0.02, 0.07) | 0.02 | 0.26 |
| Baseline Physical Activity (IPAQ) | 0.45 | (−0.05, 0.95) | 0.25 | 0.08 |
| General Health Perceptions (SF-20) | −0.03 | (−0.05, −0.01) | 0.01 | 0.02 |
| Month of Assessment | 0.02 | (−0.03, 0.06) | 0.02 | 0.47 |
| Group: Yoga vs HLW | −1.43 | (−2.46, −0.39) | 0.52 | 0.01 |
|
| ||||
| Life Activities (WHO-DAS) | ||||
|
| ||||
| Baseline Life Activities (WHO-DAS) | 0.30 | (0.11, 0.50) | 0.10 | 0.00 |
| Gender: Female vs Male | −0.12 | (−3.23, 2.99) | 1.55 | 0.94 |
| Age | 0.09 | (−0.01, 0.20) | 0.05 | 0.08 |
| Baseline Physical Activity (IPAQ) | 0.51 | (−0.46, 1.49) | 0.49 | 0.30 |
| General Health Perceptions (SF-20) | −0.05 | (−0.09, 0.00) | 0.02 | 0.05 |
| Month of Assessment | 0.05 | (−0.05, 0.14) | 0.05 | 0.36 |
| Group: Yoga vs HLW | −2.64 | (−4.73, −0.55) | 1.05 | 0.01 |
|
| ||||
| General Health Perceptions (SF-20) | ||||
|
| ||||
| General Health Perceptions (SF-20) | 0.71 | (0.61, 0.80) | 0.05 | 0.00 |
| Gender: Female vs Male | 6.14 | (−0.55, 12.83) | 3.40 | 0.07 |
| Age | −0.25 | (−0.45, −0.04) | 0.10 | 0.02 |
| Baseline Physical Activity (IPAQ) | 0.95 | (−1.45, 3.35) | 1.22 | 0.44 |
| Month of Assessment | −0.16 | (−0.28, −0.04) | 0.06 | 0.01 |
| Group: Yoga vs HLW | 7.27 | (2.54, 12.00) | 2.41 | 0.00 |
|
| ||||
| Physical Pain (SF-20) | ||||
|
| ||||
| Baseine Physical Pain (SF-20) | 0.47 | (0.36, 0.58) | 0.06 | 0.00 |
| Gender: Female vs Male | −4.25 | (−11.62, 3.13) | 3.75 | 0.26 |
| Age | −0.24 | (−0.46, −0.03) | 0.11 | 0.03 |
| Baseline Physical Activity (IPAQ) | 1.40 | (−1.35, 4.14) | 1.39 | 0.32 |
| General Health perceptions (SF-20) | 0.24 | (0.13, 0.36) | 0.06 | 0.00 |
| Month of Assessment | −0.20 | (−0.34, −0.05) | 0.07 | 0.01 |
| Group: Yoga vs HLW | 2.79 | (−2.19, 7.78) | 2.54 | 0.27 |
|
| ||||
| Physical Functioning (SF-20)a | ||||
|
| ||||
| Baseline Physical Functioning (SF-20) | 0.46 | (0.30, 0.61) | 0.08 | 0.00 |
| Gender: Female vs Male | 0.18 | (−0.21, 0.57) | 0.20 | 0.37 |
| Age | 0.17 | (0.02, 0.33) | 0.08 | 0.03 |
| Baseline Physical Activity (IPAQ) | −0.02 | (−0.17, 0.13) | 0.08 | 0.81 |
| General Health Perceptions (SF-20) | −0.29 | (−0.46, −0.13) | 0.08 | 0.00 |
| Month of Assessment | 0.01 | (0.01, 0.02) | 0.00 | 0.00 |
| Group: Yoga vs HLW | −0.10 | (−0.37, 0.17) | 0.14 | 0.47 |
Note. Because baseline is included as a covariate and NOT included as part of the dependent variable, the “Group: Yoga vs. HLW” parameter represents differences between groups at all non-baseline timepoints, adjusted for the baseline score. A statistically significant difference on this parameter indicates that the study arm (Yoga vs. HLW) has a significant impact on that outcome. We also tested models that included interactions between time and group for each of the outcome variables. These interaction terms were not statistically significant for any of the outcomes. Thus, the magnitude of the difference between groups was not statistically significantly different across the non-baseline timepoints.
IPAQ = International Physical Activity Questionnaire. PHQ-9 = Patient Health Questionnaire, 9 item. QIDS = Quick Inventory of Depression Symptomatology. SF-20 = 20-item Short-Form Survey. WHO-DAS = World Health Organization Disability Assessment Schedule.
For the physical functioning subscale, we reversed the scale (so that higher values = poorer functioning) prior to analysis in order to fit a negative binomial mixed effects model.
Figure 2. Mean unadjusted QIDS scores for Yoga and HLW participants across assessments.
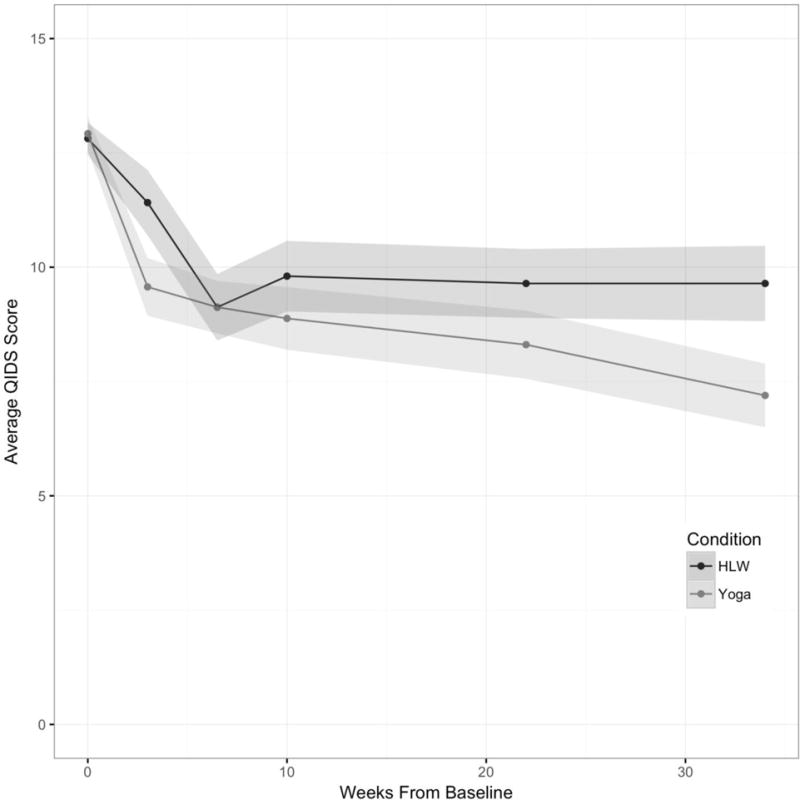
Note: shaded areas represent standard errors.
Similarly, we observed a statistically significant difference between arms across non-baseline timepoints for self-rated depressive symptoms, with yoga participants showing lower PHQ-9 scores (Table 2) on average across the intervention and follow-up phase. Social functioning, work and role functioning, and general health perceptions were also better in the yoga arm across non-baseline timepoints, adjusting for baseline. We did not observe differences between groups in physical functioning or pain. Group X time interactions were not statistically significant and therefore not included in final models.
Table 3 includes the percentage of participants who met criteria for response or remission. There were significantly higher odds of treatment response for yoga (vs HLW) across 3- and 6-month follow-up assessments. There was not a statistically significant difference in the odds of participants in yoga compared to HLW meeting criteria for full remission.
Table 3.
Percentage of participants in Yoga and HLW classified as Responders or In Remission
| Responders QIDS Reduced by ≥50% |
In Remission QIDS ≤5 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLW | Yoga | HLW | Yoga | |||||||||
|
|
|
|||||||||||
| Assessment | % | n | % | n | OR | p | % | n | % | n | OR | p |
| Week 10 (End of Treatment) |
28.3% | 46 | 36.2% | 58 | 1.44 | 0.54 | 23.9% | 46 | 29.3% | 58 | 1.32 | 0.81 |
| 3-Month Follow-up | 28.9% | 45 | 44.9% | 49 | 2.01 | 0.03 | 24.4% | 45 | 38.8% | 49 | 1.96 | 0.06 |
| 6-Month Follow-up | 31.1% | 45 | 51.1% | 45 | 2.31 | 0.04 | 31.1% | 45 | 42.2% | 45 | 1.62 | 0.16 |
Note. Generalized LME with adjustment for covariates and baseline QIDS supported a significantly higher odds of treatment response for yoga vs HLW (adjusted OR = 2.46, 95%CI=1.12–5.37, p=0.03) across end-of-treatment and 3- and 6-month follow-up assessments. Generalized LME evaluation did not support a statistically significant difference in the odds of participants in yoga compared to HLW meeting criteria for full remission (adjusted OR=1.88, 95%CI=0.88–4.02, p=0.10)
QIDS = Quick Inventory of Depression Symptomatology.
There were no serious adverse events related or possibly related to study procedures in either study arm.
Discussion
A recent meta-analysis demonstrated efficacy of yoga for the treatment of depression (Cramer et al., 2013). We did not replicate the acute treatment effect documented in this meta-analysis. There are several possible reasons. First, much of the prior work was limited by significant methodological weaknesses, as described in the introduction and in previous work (Cramer et al., 2013, Uebelacker et al., 2010a). The current study was designed to address these weaknesses. Second, in the current study, we focused on a potentially difficult to treat group of participants, i.e., people with persistent depressive symptoms despite antidepressant treatment. No prior studies of yoga for depression focused on a comparable group. Third, we employed a control group that was matched for time and controlled for non-specific factors such as scheduled time to focus on one’s own health, an opportunity to leave one’s home, and social support from both an instructor and from peers with depression. This is a more rigorous control than frequently-employed control conditions such as minimal treatment or treatment-as-usual. Consistent with our findings at end-of-intervention (10 weeks), Cramer et al. reported that although they found various styles of yoga to be superior to usual care, relaxation, and aerobic exercise in their meta-analysis, single trials failed to show that yoga was superior to some of the more active control groups such as group therapy, a social support group, massage, or pharmacological treatment.
Although we did not see an acute treatment effect in the current study, we did find that participants who had received yoga showed lower levels of depression, improved general health perceptions, and improved social and work and rule functioning over the entire follow-up period. They were also more likely to show a treatment response at 3-months and at 6-months. That is, it appears that yoga had an enduring effect when compared to health education. This is particularly notable given the rigorous control group and the focus on a difficult-to-treat population. In our yoga intervention, teachers did repeatedly focus on the importance of and options for home practice. This was reinforced by the fact we gave participants materials and tools for home practice. Thus, we hypothesize that yoga taught participants skills for coping with depressed mood and cognitions, and, as they continued to practice these skills even outside of class, the beneficial effects accumulated over time. Similarly, data suggest that cognitive behavioral therapy is more likely to prevent relapse than medications after an acute treatment period, perhaps because the therapy teaches patients a different way to relate to negative cognitive content even when they are no longer in therapy (Bockting et al., 2015).
In this study, at 3-month and 6-month follow-up assessments, the odds ratios for response to yoga vs. HLW were 2.01 and 2.31. These odds ratios compare favorably to those obtained in a meta-analysis of 2–14 weeks of pharmacological augmentation strategies for people with treatment resistant depression (Zhou et al., 2015). Augmentation with aripirazole or quetiapine was demonstrated to be superior to placebo for treatment-resistant depression, with odds ratios of 1.85 and 1.92, respectively, although it is unknown if the response rate be diminished, the same, or increased with a longer-term follow-up more similar to the follow-up in the current study. It is notable that these medications were less well-tolerated than placebo (Zhou et al., 2015), with significant negative side effects. Although there is a risk of physical injury with yoga – as with any type of physical activity – there is no evidence that yoga causes weight gain or other serious side effects associated with atypical antipsychotics. Therefore, hatha yoga may be a more acceptable option for some patients.
A limitation of this study is the predominantly female sample. Although women are more likely than men to experience major depression (Kessler et al., 2003), women are disproportionately represented in our sample. It is possible that the interventions appealed more to women than to men. Women are more likely to engage in mind-body therapies in the community than men (Barnes et al., 2004). Another limitation of this study is that most participants were white and non-Latino. There may be cultural differences in who finds yoga acceptable. However, researchers have had success recruiting non-white and Latino participants (Dutton et al., 2013, Roth & Robbins, 2004) for a related intervention, Mindfulness-Based Stress Reduction. Finally, as with all behavioral interventions, we were unable to keep participants blind to treatment assignment, although we did make efforts to present both arms to participants with equipoise.
There are several areas for future research. One of the key questions is how much yoga each week, and over what period of time, is needed to cause a clinically significant change in depression symptoms. At the dosage we provided in the intervention phase, 10 weeks may not have been enough. However, we did see important differences over longer periods of time. Second, what are the moderators and mediators of the impact of yoga on depression? Understanding of mechanisms may contribute to answering yet another question: what are the active ingredients of yoga that have an impact on depression? There are many styles of hatha yoga available in the community; classes can be gentle or vigorous, can differentially emphasize postures, breathing, and meditation, and can include or not include teaching on yoga philosophy. In this study, we provided a balanced hatha yoga class that emphasized breathing and postures and was suitable for people who may not be physically fit. However, in order to make the recommendation that a depressed patient engage in yoga, clinicians and patients will need to know what to look for in the variety of yoga classes available.
This is the largest study of yoga for depression to date. Using rigorous methodology, we did not see differences between groups at 10 weeks. However, yoga was superior to our control group when we examined outcomes 3- and 6-months after the intervention period, thus suggesting that the impact of participating in yoga may grow and endure over time. Effective and tolerable adjunctive treatments, such as yoga, are clearly needed to address the high symptom burden among individuals with partial response to conventional treatments.
Supplementary Material
Acknowledgments
We would like to acknowledge the assistance of Monica Broughton, BA, Brown University School of Public Health; Morganne Kraines, MA, University of Oklahoma; and Richard Liu Ph.D., Brown University School of Medicine for their assistance with data collection. All were compensated for their work.
Financial support. Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR012005. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT01384916
Conflicts of interest. Dr. Uebelacker’s spouse is employed by Abbvie Pharmaceuticals. Dr. Gaudiano receives book royalties from Oxford University Press and Routledge and has been paid as a consultant by McKesson Health Solutions. Other authors have no conflicts of interest to disclose.
Ethical standards. The authors assert that all procedures contributing to this work comply with standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Abrantes AM, Bloom EL, Strong DR, Riebe D, Marcus BH, Desaulniers J, Fokas K, Brown RA. A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine and Tobacco Research. 2014;16:1094–103. doi: 10.1093/ntr/ntu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, McLaughlin N, Greenberg BD, Strong DR, Riebe D, Mancebo M, Rasmussen S, Desaulniers J, Brown RA. Design and Rationale for a Randomized Controlled Trial Testing the Efficacy of Aerobic Exercise for Patients with Obsessive-Compulsive Disorder. Mental Health and Physical Activity. 2012;5:155–165. doi: 10.1016/j.mhpa.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test Guidelines for use in primary care. 2. World Health Organization; 2001. [Google Scholar]
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002 Advance data from vital and health statistics; no 343. National Center for Health Statistics; Hyattsville, Maryland: 2004. [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker BM, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Berman AH, Bergman H, Palmstierna T, Schlyter F. DUDIT Manual: The Drug Use Disorders Identification Test, Version 1.0. Karolinska Institutet, Department of Clinical Neuroscience; Stockholm: 2005. [Google Scholar]
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review. 2015;41:16–26. doi: 10.1016/j.cpr.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. Exercise for depression. Cochrane Database Systematic Revew. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Lisle PK, Nash R, Stang P, Swindle R. Response, partial response, and nonresponse in primary care treatment of depression. Archives of Internal Medicine. 2004;164:1197–204. doi: 10.1001/archinte.164.11.1197. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Haller H, Dobos G. A systematic review and meta-analysis of yoga for low back pain. The Clinical Journal of Pain. 2013;29:450–460. doi: 10.1097/AJP.0b013e31825e1492. [DOI] [PubMed] [Google Scholar]
- Dutton MA, Bermudez D, Matas A, Majid H, Myers NL. Mindfulness-Based Stress Reduction for Low-Income, Predominantly African American Women With PTSD and a History of Intimate Partner Violence. Cognitive Behavioral Practice. 2013;20:23–32. doi: 10.1016/j.cbpra.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, Research version, Patient edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- IPAQ Research Committee. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms 2005 [Google Scholar]
- Iyengar BKS. Light on the Yoga Sutras of Patanjali. The Aquarian Press; London: 1993. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JL, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin. 1986;22:343–381. [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. Clinical Guideline 23 (amended): Depression: Management of depression in primary and secondary care. National Institute for Health and Clinical Excellence; London, UK: 2007. [Google Scholar]
- Pinheiro JC, Bates DM, DebRoy S, Sarkar D, R Core Team nlme: Linear and nonlinear mixed effects models. 2015 R package version 3.1-120. [Google Scholar]
- R Core Team. R: A language an environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- Roth B, Robbins D. Mindfulness-based stress reduction and health-related quality of life: findings from a bilingual inner-city patient population. Psychosomatic Medicine. 2004;66:113–23. doi: 10.1097/01.psy.0000097337.00754.09. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M. An Evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression Trial Report. Biological Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller M. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Ware JE, Brook RH. Advances in the measurement of functional status: construction of aggregate indexes. Medical Care. 1981;19:473. doi: 10.1097/00005650-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;S12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Uebelacker LA, Epstein-Lubow G, Gaudiano BA, Tremont G, Battle CL, Miller IW. Hatha yoga for depression: critical review of the evidence for efficacy, plausible mechanisms of action, and directions for future research. Journal of Psychiatric Practice. 2010a;16:22–33. doi: 10.1097/01.pra.0000367775.88388.96. [DOI] [PubMed] [Google Scholar]
- Uebelacker LA, Lavretsky H, Tremont G. Yoga therapy for depression. In: Khalsa SB, Cohen L, McCall T, Telles S, editors. The Principles and Practice of Yoga in Health Care. Handspring Publishing; Edinburgh: 2016. pp. 73–90. [Google Scholar]
- Uebelacker LA, Tremont G, Epstein-Lubow G, Gaudiano BA, Gillette T, Kalibatseva Z, Miller IW. Open trial of Vinyasa yoga for persistently depressed individuals: evidence of feasibility and acceptability. Behavior Modification. 2010b;34:247–64. doi: 10.1177/0145445510368845. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- van der Velden AM, Kuyken W, Wattar U, Crane C, Pallesen KJ, Dahlgaard J, Fjorback LO, Piet J. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clinical Psychology Review. 2015;37:26–39. doi: 10.1016/j.cpr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Wang PS, Simon GE, Avorn J, Azocar F, Ludman EJ, McCulloch J, Petukhova MZ, Kessler RC. Telephone screening, outreach, and care management for depressed workers and impact on clinical and work productivity outcomes: a randomized controlled trial. Journal of the American Medical Association. 2007;298:1401–11. doi: 10.1001/jama.298.12.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. New England Medical Center, The Health Institute; Boston, MA: 1993. [Google Scholar]
- Weintraub A. Yoga for depression: a compassionate guide to relieve suffering through yoga. Broadway Books; New York: 2004. [Google Scholar]
- Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder. third. American Psychiatric Association; 2010. [Google Scholar]
- World Health Organization. WHO-DAS II Disability Assessment Schedule Training manual: A guide to administration. World Health Organization; 2004. [Google Scholar]
- Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, Liu Y, Fang Y, da Silva T, Zhang Y, Fang L, Wang X, Xie P. Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. Journal of Clinical Psychiatry. 2015;76:e487–98. doi: 10.4088/JCP.14r09204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


