Abstract
Wogonin has recently been shown to possess anti-inflammatory and chondroprotective properties and is of considerable interest due to its broad pharmacological activities. The present study highlights that Wogonin bind DNA and exerts chondroprotective effects in vitro. Wogonin showed strong binding with chondrocytes genomic DNA in vitro. The mode of binding of Wogonin to genomic-DNA was assessed by competing Wogonin with EtBr or DAPI, known DNA intercalator and a minor groove binder, respectively. EtBr fluorescence reduced significantly with increase in Wogonin concentration suggesting possible DNA intercalation of Wogonin. Further, in silico molecular docking of Wogonin on mammalian DNA also indicated possible intercalation of Wogonin with DNA. The denaturation and FRET studies revealed that Wogonin prevents denaturation of DNA strands and provide stability to genomic DNA against a variety of chemical denaturants. The cellular uptake study showed that Wogonin enters osteoarthritis chondrocytes and was mainly localized in the nucleus. Wogonin treatment to OA chondrocytes protects the fragmentation of genomic DNA in response to IL-1β as evaluated by DNA ladder and TUNEL assay. Treatment of chondrocytes with Wogonin resulted in significant suppression of IL-1β-mediated induction of ROS. Further, Wogonin exhibited protective potential through potent suppression of extrinsic and intrinsic apoptotic pathways and induction of anti-apoptotic proteins in IL-1β-stimulated osteoarthritis chondrocytes. Our data thus suggest that DNA intercalation by Wogonin may result in the stabilization of genomic DNA leading to protective activity.
Keywords: Wogonin, DNA binding, Chondroprotective effects, Osteoarthritis, Denaturation
Graphical Abstract
Introduction
Wogonin (5,7-Dihydroxy-8-methoxyflavone) is a naturally occurring flavonoid derived from the root extract of traditional Chinese medicine, Scutellaria baicalensis Georgi, [1]. Wogonin possess diverse biological properties including anti-cancer and anti-inflammatory activities [1–3]. Recently, we have shown the chondroprotective effect of Wogonin through effective suppression of MMPs and other catabolic genes in human osteoarthritis (OA) chondrocytes [4, 5]. Our studies demonstrate that Wogonin exerts protective effects through modulation of redox sensitive transcription factors AP-1 and Nrf2 [4, 5]. However, molecular targets of these flavonoids pertinent to wide range of biological activities are subject to intensive research. Recent reports revealed that nucleic acids could also be affected by flavonoids and several studies have been reported showing the interaction of flavonoid with DNA [6, 7]. Flavonoids have been shown to interact with DNA by intercalating or by binding to minor or major groove of the DNA.
A thorough investigation of interaction between flavonoid and DNA permits a better understanding of pharmaceutical properties of flavonoids. Further, these binding studies are useful in understanding the drug-DNA interactions, for discovering and designing novel and effective drugs [7]. The planar molecules have the potential to intercalate with double stranded DNA, however small molecules have been reported to usually bind with the minor groove of DNA [8, 9]. The high binding constant suggest that flavonoids can preferentially bind to the higher order DNA structure [9]. In the recent past, there is a significant interest in characterizing flavonoid-DNA interactions for understanding the bioactivity such as DNA damaging/DNA protecting potential of investigated flavonoids under pathogenic condition [6].
Present study was designed to investigate the potential of Wogonin to bind to genomic DNA from osteoarthritis chondrocytes, examine the mode of interaction and determine the protective potential under pathological conditions. Herein, the interaction of Wogonin with genomic DNA was characterized by spectral methods including UV-visible absorption spectroscopy, fluorescence spectroscopy, in silico molecular docking, FRET and DNA denaturation studies. Further, to understand the intracellular localization of Wogonin in OA chondrocytes, quantitative cellular uptake and sub-cellular fractionation was investigated using highly sensitive method employing LC-MS/MS. Finally, bioactive potential of Wogonin was investigated in terms of its ability to protect fragmentation of genomic DNA and subsequent apoptosis of chondrocytes under pathological conditions. Additionally, molecular targets of Wogonin for the suppression of apoptosis were further investigated. Our results highlight the ability of Wogonin to intercalate with genomic DNA, and exhibits cytoprotection in osteoarthritis chondrocytes in vitro.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from HyClone Laboratories (Logan, UT, USA). Ham’s F12 Medium was purchased from Lonza (Walkersville, MD, USA). Pronase and Collagenase were from Roche Diagnostics (Indianapolis, IN, USA). Wogonin was purchased from Extrasynthese (Genay Cedex, France). Recombinant human IL-1β and FlowTACS Flow Cytometry Apoptosis Detection Kit were from R&D Systems (St Paul, MN, USA). Thiazolyl blue tetrazolium bromide (MTT), and Propidium Iodide (PI) were from Sigma Aldrich Chemicals Inc. (St. Louis, MO, USA). Antibodies specific for Caspase-3, Caspase-8 and Caspase-9 were purchased from Cell Signaling Technology (Beverly, MA) and anti-β-actin antibody, Bcl2, Bcl-xl was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Appropriate horseradish peroxidase conjugated secondary antibodies were from Cell Signaling Technology (#7074) or Santa Cruz Biotechnology (sc-2020).
Preparation of primary human OA chondrocytes
Prior to the initiation of the studies the study protocol was reviewed and approved by the Institutional Review Board (IRB) of Northeast Ohio Medical University, Rootstown, Ohio as a “non-human subject study under 45 CFR” and that no informed consent was needed. All the methods used in this study were carried out in accordance with the approved guidelines and all experimental protocols were approved by the IRB of Northeast Ohio Medical University, Rootstown, Ohio. Discarded and de-identified knee or hip joint cartilage samples were collected through the NIH supported National Disease Research Interchange (NDRI) as per the IRB approved protocol. The unaffected areas of the cartilage were used to prepare OA chondrocytes by enzymatic digestion as described previously [4, 10].
Genomic DNA Isolation
Genomic DNA from the primary human chondrocytes was isolated using DNeasy kit (Qiagen) according to the instructions provided with the kit. Briefly, cells were harvested from a 60-mm dish by scraping and were resuspended in 200 μl of PBS. Cells were incubated at room temperature in 20 μl of proteinase K and 10 μl of 32 mg/ml RNase A (Sigma-Aldrich) for 5 min. Buffer AL was added to the cells and incubated at 56 °C for 10 min. followed by addition of 100% ethanol. The mixture was applied to a spin column, and the bound DNA was washed with buffers AW1 and AW2 and eluted in 200 μl of elution buffer. The concentration and quality of the DNA were determined by UV absorption analysis using NanoDrop spectrophotometer (Thermo Fisher Scientific).
Wogonin and chondrocyte-DNA interaction with absorption spectrophotometry
Absorption spectra of chondrocyte-DNA in the presence or absence of Wogonin were recorded from 200 to 310 nm using NanoDrop spectrophotometer at room temperature. For these studies chondrocyte-DNA concentration was fixed at 100μg/ml and concentration of Wogonin was varied from 10 to 50 μM in a total volume of 100 μl. The binding constant between chondrocyte-DNA and Wogonin was estimated by method described previously [11] using following equation:
The equilibrium/binding constant K for the above equilibrium is given by the equation
For the above equilibrium, assuming n=1 for 1:1 complex formation, the double-reciprocal plot also expressed as the Benesi-Hildebrand equation is written as equation
Here, ΔA is the difference in the absorbance at 260 nm of a solution containing a mixture of chondrocyte-DNA and varying concentrations of WG and the sum of the individual absorbance of chondrocyte-DNA and WG. The Δε260 is the differential molar extinction coefficient at 260 nm due to chondrocyte DNA-WG complex, unbound WG and chondrocyte-DNA.
EtBr competitive binding and DAPI displacement assay
To examine the mode of chondrocytes-DNA and Wogonin interaction, EtBr competitive binding and DAPI displacement assay was performed as described previously [11]. Ethidium bromide (EtBr) competitive binding studies were performed by recording emission spectrum of solutions containing different concentrations of Wogonin (0–50 μM), chondrocytes-DNA (20 μg) and EtBr (10 μM) in 10 mM phosphate buffer (pH 7.4) at room temperature using 480 nm excitation wavelength. Similarly, DAPI displacement studies were performed at room temperature using different concentrations of Wogonin and 15 μM DAPI using 338 nm excitation wavelength using the Synergy H1 multi-mode plate reader.
Molecular docking study
An in silico molecular docking was performed to examine the mode of Wogonin-chondrocytes DNA interaction. An energy minimised three dimensional structure of Wogonin compatible for docking has been used throughout the docking process. The optimized structure of Wogonin was docked with B-DNA (PDB CODE: 1Z3F) using Glide tool in Schrödinger Maestro suite and OPLS3 force field, which has parameters for nucleic acid has been used for docking Wogonin with DNA. The Glide docking output contains multiple docking combinations ranked according to Glide gscore, docking score, binding energy, and other properties. Further, to examine the molecular target of Wogonin in the apoptotic pathways, we performed an in silco molecular docking of pro-apoptotic protein Bax (PDB: 4S0O) with Wogonin (ZINC00899093) using the automated web-based SwissDock program [12]. The docking was performed using the default parameters and results were visualized in UCSF Chimera.
Fluorescence resonance energy transfer (FRET) assay
FRET study was performed with classic FRET pair of FAM and TAMRA with the following complementary oligonucleotides (55 mers) sequences (IDT, CA, USA).
Donor: 5′-CGATACGCTCAAAGTCAAAATAATCAGCGTGACATTCAGAAGGGTAATAAGAACG-3′
Acceptor: 5′-CGTTCTTATTACCCTTCTGAATGTCACGCTGATTATTTTGACTTTGAGCGTATCG-3′
Acceptor was labeled with TAMRA™ at 3′ end, and donor was labeled with FAM at 5′ end [11]. The peak absorbance wavelength for FAM is 494 nm with a peak emission wavelength at 518 nm whereas absorption and fluorescence maxima of TAMRA are at 565 and 580 nm. If FAM and TAMRA are tethered at the 5′ and 3′ ends respectively of a 55-mer oligonucleotide for example, during renaturation reaction and this construct is excited at 494 nm, emission will be at 580 nm and not at 518 nm due to FAM transferring its energy to TAMRA. Once the oligonucleotide is disrupted by, for example, during denaturation reaction, excitation at 494 nm will result in emission at 518 nm. FRET study was performed using method described earlier with double-helical DNA prepared from renaturation of donor (25 μM) and acceptor (25 μM) strands [11]. This double-helical DNA was incubated with DMSO (5M) in presence and absence of Wogonin (10–50μM) at 37°C for 5 minutes in a total reaction volume of 100 μL in phosphate buffer (pH 7.4). DMSO was used as DNA denaturing agent [11]. The change in the emission intensity due to FAM as a result of FRET was measured at 518 nm after excitation at 494 nm.
DNA denaturation study
DNA denaturation experiments were carried out by monitoring the absorption spectra of DNA in presence and absence of the chemical denaturants. Three different denaturating agents-urea (8M), guanidine hydrochloride (GuHCl, 6M), and NaOH (25 mM) were added to DNA solution for 3 h at room temperature. Denaturation was studied by DNA-unwinding by monitoring the absorbance of DNA at 260 nm.
Treatment of primary human OA chondrocytes with Wogonin and IL-1β
Primary human OA chondrocytes (1×106/well of 6-well plate) were seeded in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin, and 100 mg/ml streptomycin for 2–3 days after plating. At about 80% confluence, OA chondrocytes were serum starved overnight and were then treated with different concentration of Wogonin (10–50μM) for 2 h followed by stimulation with IL-1β (1 ng/ml). Chondrocytes treated with 0.1% DMSO served as control.
Cellular uptake of Wogonin
Cellular uptake of Wogonin in human OA chondrocytes was monitored using LC-MS/MS analysis [5]. The cell lysate for mass spectrometric analysis for Wogonin was prepared as described earlier [5]. Briefly, after the treatment with Wogonin (50–100 μM) for 4 or 24h, OA chondrocytes were washed three times with phosphate-buffered saline, lysed with lysis buffer (10 mM Tris-HCl (pH 9.6), 1% Triton X-100,150 mM NaCl, 5 mM EDTA) and deproteinized with an equivalent amount of acetonitrile. After centrifugation for 5 minutes at 10,000 × g, the supernatant was used for MRM- analysis using UHPLC system connected to a triple quadrupole mass spectrometer (LC-MS 8040; Shimadzu, Kyoto, Japan) equipped with an electrospray ionization source.
The uptake was quantitated using the ESI ion source in multiple reaction monitoring and negative ion mode (MRM-) by monitoring transition pairs m/z 283.00 (precursor ion)/162.9 (product ion). The following instrument settings were used for MRM analysis: heat block temperature, 400 °C; DL temperature, 250 °C; nebulizing gas (N2), 3L/min; drying gas (N2), 15L/min; collision energy, 35.0; dwell time, 100 msec. A calibration curve was prepared using purified Wogonin dissolved in methanol at the concentrations of 0.001, 0.005, 0.01, 0.1 and 1mg/ml.
Sub-cellular fractionation study
Chondrocytes grown in 100 cm2 dish were treated with 100 μM Wogonin for 4 h at 37°C and washed with 1X-PBS three times and resuspended in 100 μl of ice-cold lysis buffer (1 mM DTT, 1 mM PMSF, 10 mM KCl, and 10 mM MgCl2, pH 7.5) for 15 min. The cells were pelleted by centrifugation and again resuspended in 40 μl of ice-cold lysis buffer. The cell membranes were lysed by 10 strokes of a 28-gauge syringe. The resulting suspension was centrifuged at 11,000 × g for 20 minutes and the cytosolic fraction of the cells was collected as supernatant. The pellet was resuspended in 40 μl of extraction buffer (1 mM DTT, 1 mM PMSF, 1.5 mM MgCl2, 0.2 M EDTA, 0.42 M NaCl, and 25% glycerol, pH 7.9) and lysed with 10 strokes of a 28-gauge syringe. The lysate was shaken at 1,000 rpm for 45 min at 4 °C and then centrifuged at 20,000 × g for 10 min. at 4°C. The supernatant was collected as the nuclear fraction. Both cytosolic and nuclear fraction were deproteinized with an equivalent amount of acetonitrile and the supernatant obtained through centrifugation was used for MRM- analysis using UHPLC system connected to a triple quadrupole mass spectrometer (LC-MS 8040; Shimadzu, Kyoto, Japan) as described above.
Reactive oxygen species (ROS) measurement
For the measurement of IL-1β induced ROS, chondrocytes were treated with Wogonin (50 μM) for 2h and then stimulated with IL-1β (1 ng/ml) for 5 minutes and then labeled with CellROX® Green Reagent for 0.5 h and ROS levels were estimated by monitoring fluorescence using Synergy H1 multi-mode plate reader at excitation and emission at 485 and 525 nm respectively.
Estimation of apoptosis using DNA ladder assay
Fragmentation of genomic DNA is the hallmark of apoptosis and assay was performed as reported earlier with slight modification [13]. In brief, Chondrocytes were treated as above were harvested at 48 h post IL-1β addition. The cells were washed with 1X-PBS, pellet down by centrifugation and suspended in 100 μl lysis buffer (10 mM Tris-HCl, pH8.0, 10 mM EDTA, 100 mM NaCl and 0.5% SDS, 0.1 mg/ml proteinase K) and incubated at 55 °C for 16 h. RNase (50 μg/ml) was added to the digested lysates and further incubated at 55° C for 1h. The proteinase K was heat inactivated by raising temperature to 65 °C for 0.5h and mixed with gel loading buffer. DNA sample were electrophoresed in 1.8% agarose gel in TBE buffer (pH 8.0) containing ethidium bromide and bands were visualized and photographed under UV light using Geldoc imaging system (Syngene, Frederick, MD).
Estimation of apoptosis using TUNEL assay
To measure IL-1β induced apoptosis in Wogonin treated chondrocytes, TUNEL assay was performed according to the manufacturer’s protocols. The assays use an optimized terminal transferase (TdT) to label free 3′OH ends in genomic DNA with fluorescein-dUTP. Apoptotic death was estimated using a BDAccuri C6 Flowcytometer r by measuring the fluorescence of fluorescein-dUTP-labeled DNA nicks and percent apoptotic cells were determined by analyzing FITC positive cells using FlowJo software [13].
Cell viability assay
Primary human OA chondrocytes (40,000 cells/well of 96-well plate), were serum starved overnight, and then treated with Wogonin (10–50 μM) for 2 h followed by stimulation with IL-1β (10 ng/ml) in serum free medium and cultured for 48 h at 37° C in 5% CO2. At end of incubation, 20 μl of MTT from freshly prepared 5 mg/ml stock in 1X-PBS was added to cells and incubated for 3 h at 37° C in CO2 incubator. Finally, 100 μl of DMSO was added in each well and absorbance was recorded at 570 nm using Synergy H1 multi-mode plate reader. Cell viability was calculated as percentage of untreated control using method described earlier [10].
Estimation of apoptosis by PI staining
Chondrocytes (1×106/well of 6-well plate) were treated with Wogonin (10–50 μM) for 2 h and then stimulated with IL-1β (10 ng/ml) for 48 h at 37° C. At end of incubation, chondrocytes were harvested and single cell suspension was prepared using 1mM EDTA containing 1X-PBS, washed twice with IX-PBS and stained with PI solution. A total of 20,000 cells were acquired in BD Accuri C6 Flowcytometer and percent apoptotic cells were determined by analyzing a sub-G1 population (<2 n DNA content) using FlowJo software [13].
Western Immunoblotting
Chondrocytes treated with Wogonin (50 μM) under stimulation with IL-1β (10 ng/ml, 48 h) were harvested, washed with cold PBS and lysed in ice-cold RIPA buffer and lysate were prepared as described previously [5]. Equivalent amounts of lysate protein (35 μg) were resolved by 10% SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, USA) and the blots were incubated with primary antibodies diluted in 2% blocking buffer for overnight at 4°C. Blots were then incubated with horse radish peroxidase-conjugated secondary antibody, followed by washing with TBST. Blot were developed using Luminata Western HRP substrate (EMS Millipore) and the antibody reactive proteins were visualized by chemiluminescence and imaged using the Pxigel imaging system (Syngene, Frederick, MD).
Statistical Analyses
The values are presented as the Mean±SD and the statistically significant difference between the experimental groups and controls were analyzed using one-way ANOVA followed by post hoc analyses using the Tukey test. Unless otherwise noted, each experiment was repeated three times using three independent samples. P<0.05 was considered to be statistically significant.
Results
Wogonin exhibited binding with genomic DNA isolated from human OA chondrocytes
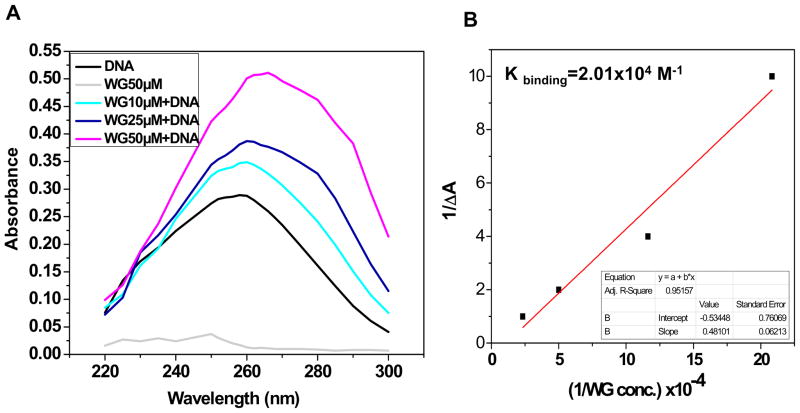
The binding of small molecules to DNA has been characterized by absorption spectroscopy and hypochromic or hyperchromic effects are observed in the absorption spectra of small molecules if they intercalate with DNA. Therefore, interaction of Wogonin with chondrocytes-DNA was initially studied using UV-vis spectrophotometry. Results shown in Fig. 1A, depicts the absorption spectra of chondrocytes-DNA in presence or absence of Wogonin (10–50 μM) in 10 mM phosphate buffer (pH 7.4). A significant increase in the absorption at 260 nm was observed with the increasing concentration of Wogonin indicated that Wogonin interacted with chondrocytes-DNA. The difference in the absorbance at 260 nm (ΔA) was calculated by subtracting the sum of the individual absorbances due to Wogonin and chondrocytes-DNA from that of the mixture of chondrocytes-DNA and Wogonin. Benesi-Hildebrand equation was used for quantitative estimation of the binding constant, K. The ratio of intercept to the slope of 1/ΔA versus 1/[Wogonin] gave a binding constant, K which was estimated to be 2.01 × 104 M−1 (Fig. 1B). The calculated binding constant (log K = 4) suggest the significant affinity of Wogonin toward genomic DNA in vitro.
Fig. 1. Wogonin (WG) binds to intercalate with genomic DNA isolated from chondrocytes.
(A) Absorption spectra of chondrocytes-DNA in presence or absence of various concentrations of Wogonin (10–50 μM). An intense interaction was observed at A260 nm between chondrocyte-DNA and Wogonin with a hyperchromic shift. (B) Binding constant, K was calculated using Benesi-Hildebrand equation. Ratio of intercept to the slope of 1/ΔA versus 1/[Wogonin] gave value of K, 2.01× 104 M−1. Representative spectra are shown and three such independent experiments were performed. (C) Fluorescence emission spectra of DAPI (15 μM), λ (Ex)-338 nm and DAPI (15 μM)-chondrocyte-DNA (20 μg) mixture in presence of increasing concentration of Wogonin (10–50 μM) in 10 mM phosphate buffer (pH 7.4). (D) Fluorescence emission spectra of EtBr (10 μM), λ (Ex):480 nm and EtBr (10 μM)-chondrocyte-DNA (20 μg) mixture in the presence of increasing concentration of Wogonin (10–50 μM) in 10 mM phosphate buffer (pH 7.4). Two such independent experiments were performed.
Competition with EtBr and DAPI confirmed the chondrocyte-DNA intercalating ability of Wogonin
The mode of interaction of Wogonin with chondrocytes-DNA was monitored using competition assay with EtBr and DAPI. EtBr is a well-known DNA intercalator, and its fluorescence intensity increases considerably after binding with DNA due to intercalation [11]. In contrast, DAPI is also a fluorogenic probe whose fluorescence intensity increases upon interaction with DNA through minor groove binding [14]. In the presence of another molecule, there would be competition between the new probe and EtBr or DAPI for binding to DNA. Such competitive binding studies would help in identifying the site of binding of the molecules. Therefore, to examine the mode of DNA interaction, EtBr or DAPI was added to chondrocytes DNA solution in presence or absence of different concentrations of Wogonin (10–50 μM). As shown in Fig 1C no significant decrease in fluorescence intensity of DAPI bound to chondrocytes-DNA is observed even at the highest concentration of Wogonin, indicating the absence of competition between DAPI and Wogonin to bind with chondrocytes-DNA. However, a significant decrease in the fluorescence intensity of EtBr bound to chondrocytes-DNA was observed in the presence of increasing concentration of Wogonin (Fig. 1D) indicating that Wogonin has the ability to displace EtBr, but not DAPI. Therefore, Wogonin is likely to be an intercalator of DNA but not the minor groove binder.
In silico molecular docking of Wogonin with DNA
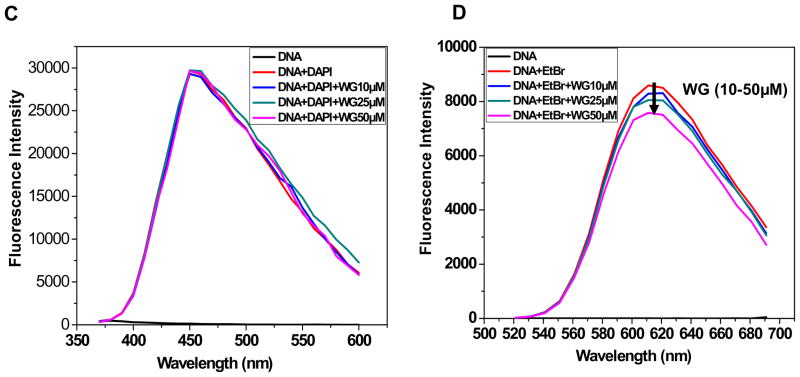
An in silico molecular docking of Wogonin was performed using mammalian DNA obtained from the protein data bank (PDB CODE: 1Z3F) using Glide tool in Schrödinger Maestro suite. The docking studies of Wogonin with DNA revealed that Wogonin binds and intercalates the DNA (Fig. 2A). The intercalation was found between guanine and cytosine base pairs of the DNA. Hydrogen bonding has been formed in between hydroxyl groups of Wogonin and guanine and cytosine residues of DNA (Fig. 2B). In silico binding studies also supported DNA intercalating ability of Wogonin.
Fig. 2. Molecular docking of Wogonin with DNA.
(A) Interaction of Wogonin with DNA a mammalian DNA (PDB CODE: 1Z3F) by intercalation. (B) Hydrogen bonding between hydroxyl groups of Wogonin and guanine and cytosine residues of DNA.
FRET studies indicated Wogonin inhibits DNA denaturation
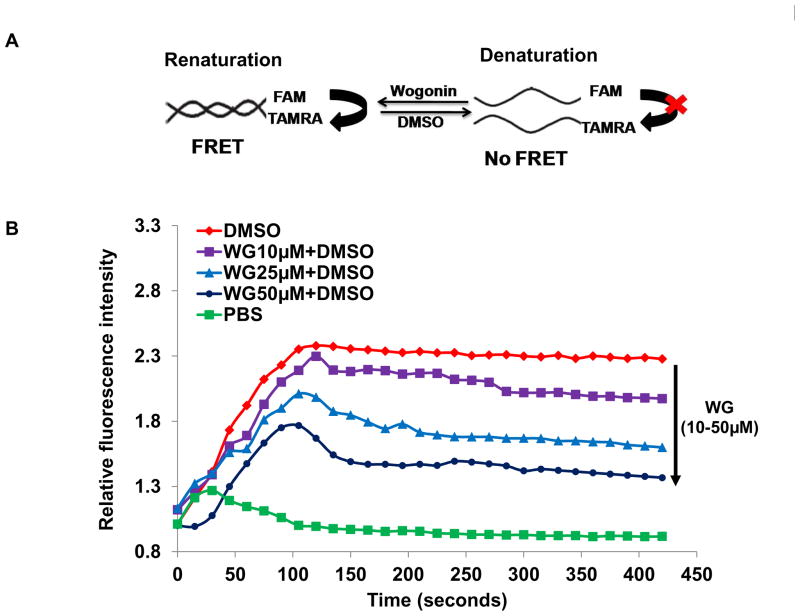
Two complementary oligonucleotides (donor and acceptor), were labeled with FAM and TAMRA at their 5′ and 3′ ends, respectively. The assay is based on non-radiative resonance energy transfer between donor (FAM) and acceptor (TAMRA). FRET, being dependent on the distance between donor and acceptor moieties, has been used in many studies to investigate the site of binding of the drugs to macromolecules [11, 15]. The design of the FRET experiment is summarized in Figure 3A. FAM and TAMRA are good FRET pair, where donor molecule FAM is initially excited at 494 nm, which emits fluorescence at 518 nm. Due to energy transfer to the acceptor molecule TAMRA, loss of its emission intensity at 518 nm is observed. During renaturation of the two strands, the donor and acceptor molecules come closer and the energy transfer causes decrease in the emission intensity at 518 nm. During denaturation, the separation of these duplex strands causes loss of FRET and thereby results in increase in the emission intensity (Fig. 3A). Since DMSO is a well known DNA denaturant (25), therefore, DMSO was added to FRET pair and time dependent denaturation upto 120 seconds was observed as monitored by intensity of the fluorescence emission at 518 nm (Fig. 3B). Interestingly, when Wogonin (10 to 50 μM) was added along with DMSO to the same system, a concentration dependent inhibition of denaturation was recorded (Fig. 3B). These results confirmed that Wogonin inhibited the DMSO mediated denaturation of DNA, most likely through intercalating with DNA strand and thereby preventing the separation of the two strands.
Fig. 3. Interaction with Wogonin provided stability of DNA against chemical denaturants.
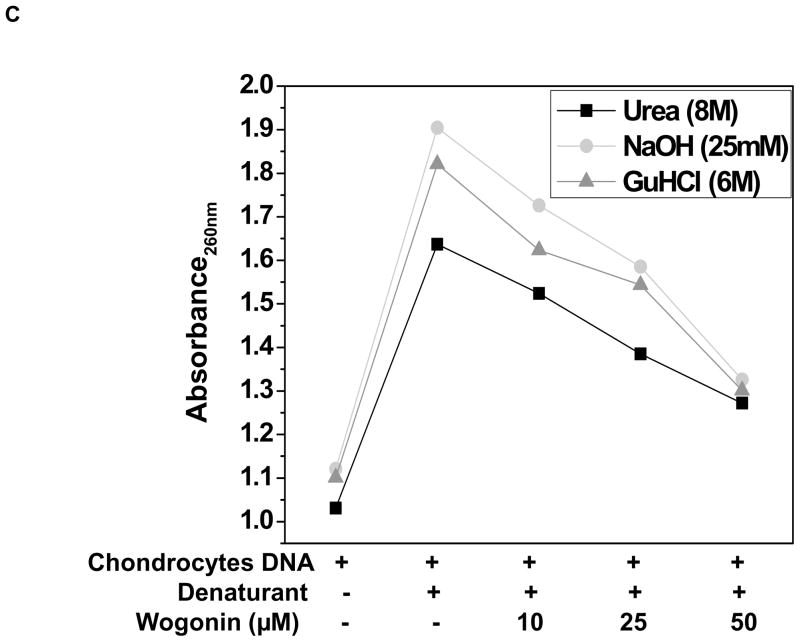
(A) Schematic representation of the FRET assay indicating the denaturation reaction mediated by DMSO in the presence and absence of Wogonin. (B) Fluorescence intensity of FAM during DMSO mediated denaturation of FRET pairs in the presence and absence of Wogonin (10–50μM) are plotted against time. The emission was monitored at measured at 518 nm after excitation at 494 nm using Synergy H1 multi-mode plate reader. (C) Denaturation of genomic DNA was initiated by chemical denaturants Urea (8M), GuHCl (6 M) and NaOH (25 mM) in the presence or absence of Wogonin (50μM) as monitored by absorption spectrophotmetry. Two such independent experiments were performed. *p≤0.05, as compared to untreated DNA; # ≤0.05, as compared to chondrocytes-DNA treated with chemical denaturants.
Interaction with Wogonin protected chondrocyte DNA against chemical denaturants
To further confirm the FRET results and monitor the potency of Wogonin to inhibit DNA denaturation, we used variety of chemical denaturants and examined the effect of Wogonin on the stability of genomic DNA against these denaturants. For this study, we used guanidine hydrochloride (GuHCl), urea and NaOH because these have been shown to be more effective in DNA unfolding than thermal unfolding [16]. To examine the effect of Wogonin on stability of DNA against denaturants, urea or GuHCl or NaOH were added to DNA or Wogonin-DNA solution for 3h at room temperature. Absorption spectroscopic data, as shown in Fig. 4 depicts that treatment of genomic DNA with urea or GuHCl or NaOH results in significant unfolding of DNA helix as evaluated by increased absorbance at 260 nm. However, addition of these denaturants to Wogonin-DNA solution did not cause any significant increase in DNA undwinding indicating that interaction with Wogonin provides stability of DNA against chemical denaturation (Fig. 3C). The stabilization of the Wogonin-DNA complexes may be due to a hydrophobic interaction between the hydrophobic segment of Wogonin and the intercalation site, which would permit this segment to penetrate the DNA helix and to arrange its planar structure parallel to the adjacent planes of the nitrogenous bases. Furthermore, hydrogen bond formed between Wogonin and DNA as observed in our docking study provides additional stability.
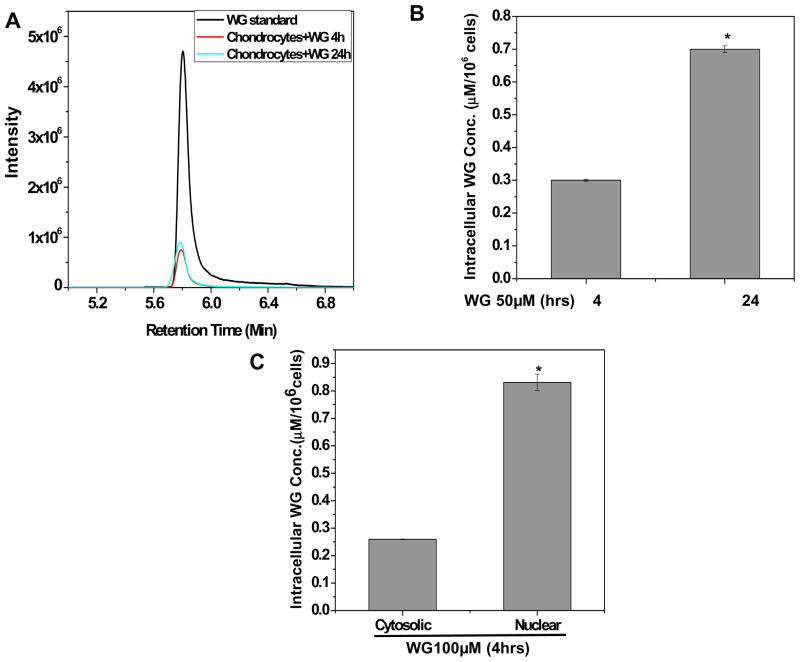
Fig. 4. Wogonin (WG) was found to be localized in the nucleus of osteoarthritis chondrocytes.
OA chondrocytes were treated with WG (50 μM) for 4 or 24h. Cell lysates were prepared and subjected to LC-MS/MS analysis using the ESI ion source in multiple reaction monitoring and negative ion mode (MRM-) by monitoring transition pairs m/z 283.00 (precursor ion)/162.9 (product ion) as described in method section. (A) MS chromatogram of WG uptake in OA chondrocytes (B) Quantification of Wogonin in the cellular milieu of OA chondrocytes was calculated by four point calibration curves of Wogonin using MRM- analysis (C) Distribution of WG in cytosolic and nuclear fraction of chondrocytes. Bar graph represents mean±SD from two subjects. *p≤0.01, as compared to Wogonin (50μM, 4h) treated cells or as compared to cytosolic fraction.
Wogonin was found to be localized in the nucleus
To monitor the cellular uptake, osteoarthritis chondrocytes were treated with Wogonin (50 μM) for 4 and 24 h at 37 °C and lysates were prepared to examine the uptake using MRM (−) analysis. Results as depicted in Figure 4A–B showed a time dependent increase in the intensity of Wogonin in the cell lysates (Fig. 4A–B). The intracellular concentration of Wogonin in OA chondrocytes was calculated as 0.3±0.003 μM/106 cells upon treatment with 50 μM of Wogonin for 4 h and it was increased to 0.7±0.01 μM/106 cells in 24 h of incubation. Further, sub-cellular fractionation was performed to prepare the nuclear and cytosolic lysates and MRM (−) analysis was performed. Results showed that Wogonin was preferentially found in the nuclear fraction of treated chondrocytes suggesting the nuclear localization of Wogonin in osteoarthritis chondrocytes (Fig. 4C).
Wogonin inhibited ROS generation and DNA fragmentation in IL-1β stimulated OA chondrocytes
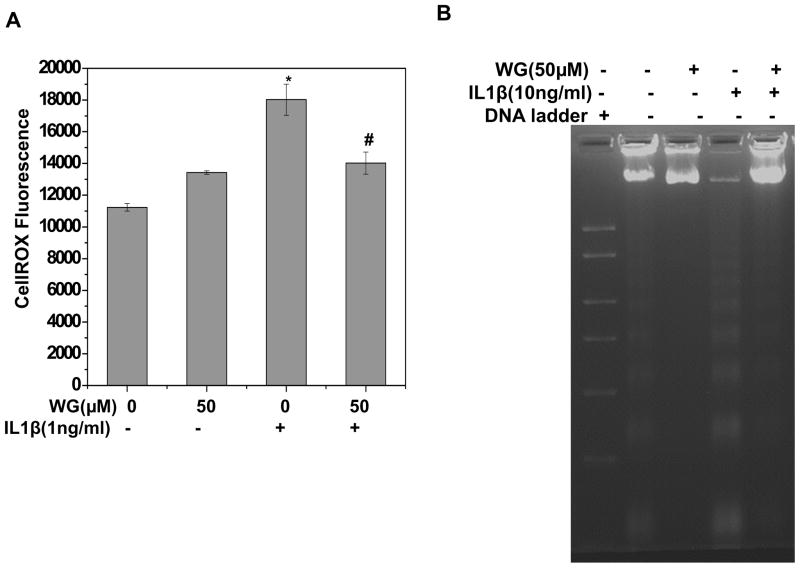
Since IL-1β is an important pro-inflammatory cytokine involved in OA pathogenesis, we have used IL-1β as stimulus to induce OA pathogenic condition in vitro. To examine the effect of Wogonin on ROS generation, OA chondrocytes treated with Wogonin (10–50μM, 2h) were stimulated with IL1β for 5 minutes and ROS were measured using Cell ROX® assay. Result showed that IL-1β treatment significantly increased the ROS production in OA chondrocytes whereas, pretreatment of OA chondrocytes with Wogonin significantly suppressed the IL-1β-mediated generation of ROS in a dose dependent manner (Fig. 5A).
Fig. 5. Wogonin (WG) inhibited ROS generation and DNA fragmentation in IL-1β stimulated OA chondrocytes.
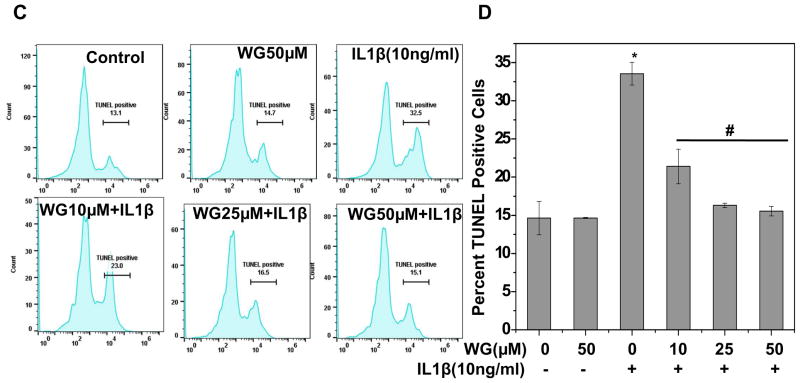
Human OA chondrocytes were treated with Wogonin (50 μM) for 2 h, stimulated with IL-1β (1ng/ml) for 5 minutes at 37°C and stained with Cell ROX ® for 0.5h at 37°C and ROS levels were estimated by monitoring fluorescence using Synergy H1 multimode plate reader at excitation and emission at 485 and 525 nm respectively. (B–D) Human OA chondrocytes were treated with Wogonin (50 μM) for 2 h, stimulated with IL-1β (10ng/ml) for 48 h at 37°C and processed for (B) DNA fragmentation assay and (C–D) TUNEL assay as described in method section. The data were expressed as a mean±SD of two replicates from a representative experiment and two such independent experiments were carried out. *p≤0.01, as untreated control; # ≤0.05, as compared to IL-1β treated cells.
IL-1β mediated ROS generation caused the activation of downstream catabolic events leading to fragmentation of genomic DNA and subsequent apoptosis of chondrocytes. DNA fragmentation was measured using DNA ladder assay in the control and experimental groups. Results showed that treatment of chondrocytes with IL-1β caused a significant fragmentation of genomic DNA, however pretreatment with Wogonin significantly inhibited IL-1β mediated fragmentation of chondrocytes DNA (Fig. 5B). To confirm these results, TUNEL assay was performed which is an established method for detecting DNA fragments. Data in Fig. 5C, D represent flowcytometric histograms and corresponding bar graphs showing TUNEL positive cells, showed that IL-1β induced fragmentation of DNA was significantly inhibited by treatment of chondrocytes with Wogonin (Fig. 5C, D). Taken together, these results suggest that Wogonin inhibited IL-1β mediated generation of ROS and fragmentation of genomic DNA in OA chondrocytes.
Wogonin inhibited IL-1β mediated extrinsic and intrinsic apoptotic pathways and up-regulated anti-apoptotic proteins in osteoarthritis chondrocytes
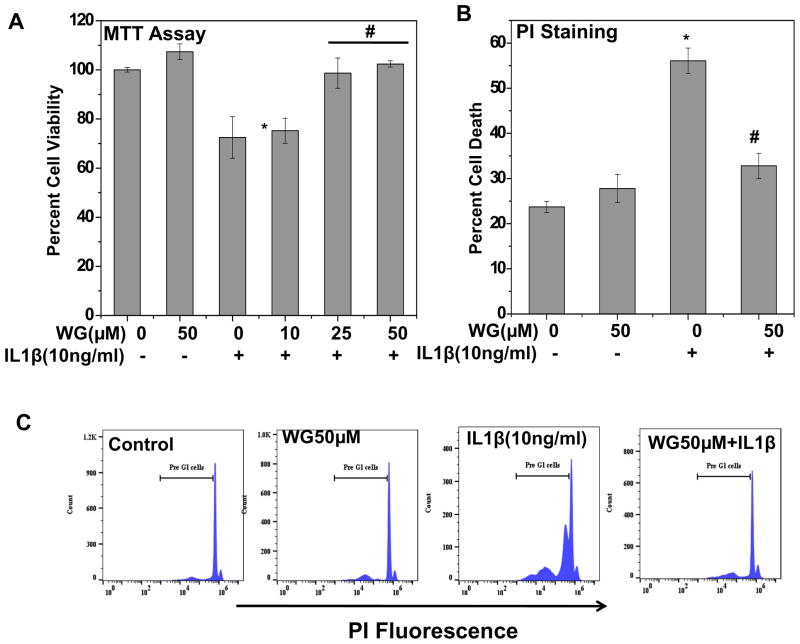
Since fragmentation of genomic DNA leads to apoptosis and loss of viability, we monitored the effect of Wogonin on IL-1β induced loss of chondrocytes viability at 48 h by MTT assay. Results showed that IL-1β treatment led to a significant loss of chondrocytes viability, whereas pretreatment with Wogonin significantly inhibited the loss of viability (Fig. 6A). Further to confirm these results, % sub G1 (apoptotic cells) population was enumerated using propidium iodide (PI) staining in OA chondrocytes. Fig. 6B–C represent flowcytometric histograms and their corresponding bar graphs showing sub G1 population in osteoarthritis chondrocytes treated with Wogonin and IL-1β. These results were in agreement with MTT assay results. There was a significant increase in sub G1 population in IL-1β treated group, which was significantly inhibited Wogonin treatment (Fig. 6B, C).
Fig. 6. Wogonin (WG) inhibited IL-1β mediated chondrocytes apoptosis.
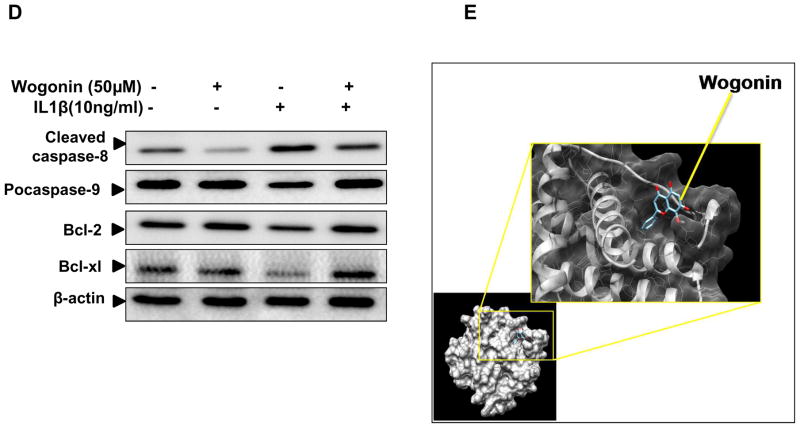
(A) Human OA chondrocytes (0.04×106/well of 96-well plate or 1×106/well of 6-well plate) were treated with Wogonin (10–50 μM) for 2h then stimulated with IL-1β (10 ng/ml) for 48 h at 37°C and cell viability was measured by MTT assays. Chondrocytes treated with 0.1% DMSO served as control. Viability was expressed relative to control cells. (B–C) Flow cytometric histograms and the corresponding bar chart showing % sub G1 population in propidium iodide stained OA chondrocytes. Twenty thousand cells in each group were acquired using a flowcytometer and percent sub G1 population representing apoptosis was calculated using flow Jo software and expressed as a mean ± SD of two replicates from a representative experiment and two such independent experiments were carried out. *p≤0.01, as untreated control; # ≤0.05, as compared to IL-1β treated cells. (D) Wogonin (50 μM) inhibited IL-1β (10 ng/ml, 48 h) mediated activation of caspase-8 and caspase-9 and the suppression of Bcl-2 and Bcl-xl in OA chondrocytes. Activation of Caspase-8, 9 and levels of Bcl-2, Bcl-xl was investigated by immunoblotting using antibodies against indicated protein. β-actin was used as a control for equal loading. (E). In silico molecular docking of Wogonin with Bax. Wogonin (ZINC ID: 00899093) binds to Bax (PDB ID: 4S0O) in top score position from SwissDock with highest Full Fitness Score -1022.28Kcal/mol, ΔG −6.80Kcal/mol.
To further determine the molecular events of suppression of apoptosis, we examined the effects of Wogonin on inhibition of extrinsic and intrinsic apoptotic pathways in IL-1β stimulated OA chondrocytes. Extrinsic apoptotic pathways are mediated by activation of caspase-8, whereas Caspase-9 occupies central role in intrinsic apoptosis [17]. Our results as shown in Fig. 6D denote that treatment of OA chondrocytes with Wogonin inhibited IL-1β induced activation of caspase-8, and caspase-9 suggesting that Wogonin suppressed both the extrinsic and intrinsic apoptotic pathways. Next, we examined the effect of Wogonin on levels of anti-apoptotic and pro-apoptotic proteins in IL-1β stimulated OA chondrocytes. Results depicted in Figure 6D showed that Wogonin inhibited the IL-1β-mediated suppression of anti-apoptotic proteins-Bcl2 and Bcl-xl in human osteoarthritis chondrocytes. Further, our in silico docking study of Wogonin with pro-apoptotic proteins revealed that Wogonin binds to Bax (PDB ID: 4S0O) suggesting that pro-apoptotic protein Bax might be a molecular target of Wogonin mediated apoptosis inhibition (Fig. 6E). Altogether, these data suggest that Wogonin inhibited IL-1β mediated apoptosis through effective suppression of extrinsic and intrinsic apoptotic pathways and significant up-regulation of anti-apoptotic proteins in human OA chondrocytes.
Discussion
Present study was designed to investigate the interaction of natural flavonoid Wogonin with genomic DNA isolated from human osteoarthritis chondrocytes. We have recently shown that Wogonin is an activator of redox sensitive transcription factor Nrf2 and is pharmacologically effective against pathogenic events involved in OA and therefore, a potentially attractive molecule for the management of OA [4, 5]. Our spectrophotometric data suggests that classic intercalation is dominant binding mode of interactions of Wogonin with chondrocytes genomic DNA. DNA binding ability of other natural flavonoids such as baicaelin, baicalin, and quercetin have been extensively studied using calf thymus DNA as model system [9]. These small molecules bind to DNA by intercalation or binding at minor groove. The intercalation occurs through insertion of planar molecules in between DNA base pairs which results in lengthening and twisting of DNA helix. Ours is the first study to perform such analyses using genomic DNA of a cell directly implicated in a disease, namely OA.
We observed that Wogonin binds to chondrocytes genomic DNA through intercalation with moderate binding efficiency (log Ks ~ 4) (Fig. 1B). It has been shown that association constants in the range of 105–1011 M−1 is mediated though hydrogen bonding, hydrophobic interactions, ionic and van der waal’s interactions [18]. The observed affinity of interaction is biologically relevant, suggesting that between Wogonin/DNA complexes are stable under experimental conditions. These data were in nice agreement with earlier reported results for Wogonin-calf thymus DNA interaction [7, 19]. To envisage the Wogonin-DNA interaction, competitive binding assay with a known intercalator, EtBr, and with a known groove binder, DAPI, was performed. Accordingly, the addition of Wogonin decreased the fluorescence of EtBr bound to DNA but not of DAPI bound to DNA indicating the intercalation as major binding mode (Fig 1C, D). We further used in silico binding approach by docking the Wogonin molecule on a B-DNA isoform which also predicted the possibility of interaction through DNA intercalation with minimal enthalpy (Fig. 2A, B). A recent report supports similar observation of DNA intercalating ability of Wogonin through formation of H-bonding [19].
Our FRET studies also revealed that Wogonin indeed binds to DNA and prevents denaturation of complimentary strands (Fig. 3B). Further denaturation studies confirmed that Wogonin prevents denaturation of DNA strands thereby providing stability to genomic DNA against variety of chemical denaturants including urea, GuHCl and NaOH (Fig. 3C). Further, it was examined whether Wogonin enters in the cellular organelle that contains genomic DNA, when added to cells. Our localization studies revealed that Wogonin enters in osteoarthritis chondrocytes and localized mainly in the nucleus whereby its intercalation with genomic DNA could result in protective activity (Fig. 4C). The observed nuclear localization of Wogonin might be due to its transport to nucleus via binding to nuclear proteins, which needs to be validated experimentally. These data indicate the possibility that Wogonin might have potential to stabilize and protect chondrocytes DNA against pathogenic conditions. To test the hypothesis, we determined whether Wogonin had any effect on fragmentation of genomic DNA in response to stimulation with IL-1β in osteoarthritis chondrocytes. Our studies involving DNA ladder and TUNEL assay showed that Wogonin treatment resulted in potent suppression of IL-1β-mediated fragmentation of genomic DNA (Fig. 5B–D).
Oxidative stress due to excess generation of ROS plays a crucial role in the pathogenesis of OA though regulation of a number of catabolic events in chondrocyte such as induction of apoptosis and activation of matrix degrading proteases [20]. Therefore, we determined whether Wogonin suppress IL-1β-induced oxidative stress in OA chondrocytes by using oxidation-sensitive dye Cell ROX® and our data revealed that Wogonin treatment significantly suppressed the generation of ROS (Fig. 5A). IL-1β mediated generation of ROS lead to the activation of apoptotic pathways resulting into loss of chondrocytes viability [21]. Treatment of chondrocytes with Wogonin resulted in significant suppression of IL-1β mediated induction apoptotic cell death (Fig. 6A–C). Chondrocyte apoptosis is hallmarks of osteoarthritic cartilage and is primarily mediated through activation of both extrinsic and intrinsic apoptotic pathways [22, 23]. An earlier report demonstrate that activation of Fas (CD95) mediated pathway contribute to major cell death in primary OA chondrocytes suggesting the involvement of extrinsic apoptosis in chondrocytes death [22]. Additional studies demonstrating the impairment of mitochondrial membrane potential and loss of mitochondrial ATP production in IL-1 β-stimulated chondrocytes suggest the possible involvement of intrinsic apoptosis in chondrocytes death [23]. Our data shown in Fig 6D demonstrate that treatment of Wogonin suppressed IL-1β-mediated activation of caspase-8 and caspase-9 suggesting that both the extrinsic and intrinsic apoptotic pathways are inhibited by Wogonin in human OA chondrocytes. Further Wogonin mediated upregulation Bcl2 and Bcl-xl in IL-1β stimulated OA chondrocytes suggest that inhibition of apoptosis was mediated at least in part through up regulation of anti-apoptotic proteins in human OA chondrocytes. Altogether, these results indicate that Wogonin treatment reduced oxidative and apoptotic response in human OA chondrocytes and thereby may have the potential to exhibit chondroprotective effects in vitro. Our studies on Wogonin showing potent anti-apoptotic activity against IL-1β are encouraging and may find application in the management of OA.
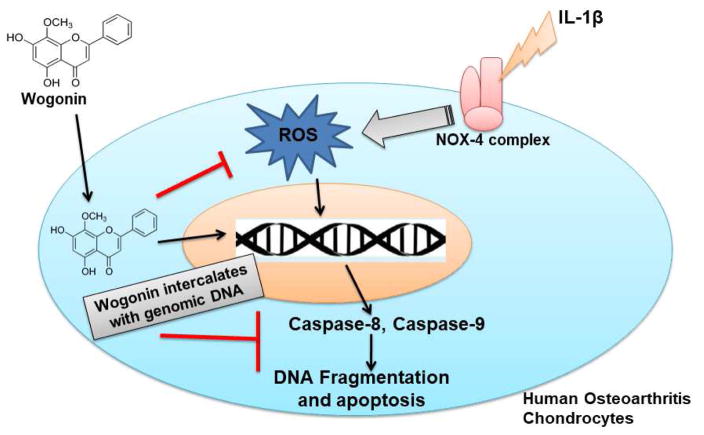
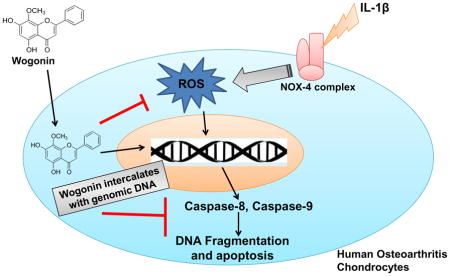
In conclusion, Wogonin binds chondrocytes DNA through an intercalation mechanism. It was found to be localized in the nucleus and exhibited protective effects in osteoarthritis chondrocytes by inducing the anti-apoptotic proteins and suppressing the IL-1β mediated induction of ROS, DNA fragmentation and extrinsic and intrinsic apoptotic pathways (Figure 8).
Fig. 7. Schematic representation of proposed mechanism of Wogonin mediated protective effects in human OA chondrocytes.
Wogonin exhibited chondrocytes DNA binding ability through intercalation mechanism and found to be localized in the nucleus and exhibited protective effects in osteoarthritis chondrocytes by suppressing the IL-1β mediated induction of ROS, DNA fragmentation, and extrinsic and intrinsic apoptotic pathways.
Highlights.
Wogonin exhibited intercalating ability with genomic DNA isolated from chondrocytes.
Wogonin was found to be localized in the nucleus human osteoarthritis chondrocytes.
Wogonin protected fragmentation of genomic DNA under pathological condition.
Wogonin stabilized genomic DNA against chemical denaturants.
Wogonin exhibited protective effect under osteoarthritis pathogenic condition.
Acknowledgments
This work was supported in part by National Institute of Health grants (RO1-AT-005520; RO1-AT-007373; RO1-AR- 067056; R21-AR- 064890) and funds from the Northeast Ohio Medical University to TMH.
List of abbreviation
- IL-1β
Interleukin-1β
- ROS
Reactive oxygen species
- EtBr
Ethidium bromide
- DAPI
4′,6-Diamidino-2-Phenylindole, Dihydrochloride
- AP-1
Activator protein-1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- MMP
Matrix metalloproteinase
- OA
Osteoarthritis
- GuHCl
Guanidine hydrochloride
- TUNEL
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling
- PI
Propidium iodide
- FRET
Fluorescence resonance energy transfer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen SC, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production. Eur J Pharmacol. 2002;446:187–194. doi: 10.1016/s0014-2999(02)01792-2. [DOI] [PubMed] [Google Scholar]
- 2.Chi YS, Lim H, Park H, Kim HP. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol. 2003;66:1271–1278. doi: 10.1016/s0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 3.Yao J, Zhao L, Zhao Q, Zhao Y, Sun Y, Zhang Y, Miao H, You QD, Hu R, Guo QL. NF-kappaB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. doi: 10.1038/cddis.2014.221. doi:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan NM, Haseeb A, Ansari MY, Haqqi TM. A wogonin-rich-fraction of Scutellaria baicalensis root extract exert chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Scientific Reports. 2017;7:43789. doi: 10.1038/srep43789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan NM, Haseeb A, Ansari MY, Devarapalli P, Hyanie S, Haqqi TM. Wogonin, a plant derived small molecule exerts potent anti-inflammatory and chondroprotective effects through activation of ROS/ERK/Nrf2 signaling pathways in human OA chondrocytes. Free Radic Biol Med. 2017;106:288–301. doi: 10.1016/j.freeradbiomed.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusak G, Piantanida I, Masic L, Kapuralin K, Durgo K, Kopjar N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem Biol Interact. 2010;188:181–189. doi: 10.1016/j.cbi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Bi S, Song D, Qiao C, Mu D, Zhang H. Study on the interaction mechanism between DNA and the main active components in Scutellaria baicalensis Georgi. Sensors and Actuators B: Chemical. 2008;129:799–810. doi: 10.1016/j.snb.2007.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodek P, Hanustiak P, Krizkova J, Mikelova R, Krizkova S, Stiborova M, Trnkova L, Horna A, Beklova M, Kizek R. Toxicological aspects of flavonoid interaction with biomacromolecules. Neuro Endocrinol Lett. 2006;27:14–17. [PubMed] [Google Scholar]
- 9.Ragazzon PA, Iley J, Missailidis S. Structure-activity studies of the binding of the flavonoid scaffold to DNA. Anticancer Res. 2009;29:2285–2293. [PubMed] [Google Scholar]
- 10.Khan NM, Ansari MY, Haqqi TM. Sucrose, But Not Glucose, Blocks IL1-beta-Induced Inflammatory Response in Human Chondrocytes by Inducing Autophagy via AKT/mTOR Pathway. J Cell Biochem. 2017;118:629–639. doi: 10.1002/jcb.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunwar A, Simon E, Singh U, Chittela RK, Sharma D, Sandur SK, Priyadarsini IK. Interaction of a curcumin analogue dimethoxycurcumin with DNA. Chem Biol Drug Des. 2011;77:281–287. doi: 10.1111/j.1747-0285.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 12.Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan NM, Poduval TB. Bilirubin augments radiation injury and leads to increased infection and mortality in mice: molecular mechanisms. Free Radic Biol Med. 2012;53:1152–1169. doi: 10.1016/j.freeradbiomed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Zaitsev EN, Kowalczykowski SC. Binding of double-stranded DNA by Escherichia coli RecA protein monitored by a fluorescent dye displacement assay. Nucleic Acids Res. 1998;26:650–654. doi: 10.1093/nar/26.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajanikant C, Kumbhakar M, Pal H, Rao BJ, Sainis JK. DNA strand exchange activity of rice recombinase OsDmc1 monitored by fluorescence resonance energy transfer and the role of ATP hydrolysis. Febs J. 2006;273:1497–1506. doi: 10.1111/j.1742-4658.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- 16.Devaraj KB, Kumar PR, Prakash V. Comparison of activity and conformational changes of ficin during denaturation by urea and guanidine hydrochloride. Process Biochemistry. 2011;46:458–464. [Google Scholar]
- 17.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Palchaudhuri R, Hergenrother PJ. DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action. Curr Opin Biotechnol. 2007;18:497–503. doi: 10.1016/j.copbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Tu B, Chen ZF, Liu ZJ, Cheng LY, Hu YJ. Interaction of flavones with DNA in vitro: structure-activity relationships. RSC Advances. 2015;5:33058–33066. [Google Scholar]
- 20.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Wei L, Sun XJ, Wang Z, Chen Q. CD95-induced osteoarthritic chondrocyte apoptosis and necrosis: dependency on p38 mitogen-activated protein kinase. Arthritis Res Ther. 2006;8:R37. doi: 10.1186/ar1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]