Abstract
Age-related alterations in immunity have been linked to increased incidence of infections and decreased responses to vaccines in the aging population. Human peripheral blood monocytes are known to promote antigen presentation and antiviral activities; however, the impact of aging on monocyte functions remains an open question. We present an in-depth global analysis examining the impact of aging on classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14dimCD16+) monocytes. Monocytes sorted from non-frail healthy adults (21–40 yrs) and old (≥ 65 yrs) individuals were analyzed after stimulation with TLR4, TLR7/8, and RIG-I agonists. Our data showed under non-stimulated conditions, monocyte subsets did not reveal significant age-related alternations; however, agonist stimulated-monocytes from adults and old subjects did show differences at the transcriptional and functional levels. These alternations in many immune-related transcripts and biological processes resulted in reduced production of IFNα, IFNγ, IL-1β, CCL20, and CCL8, and higher expression of CX3CR1 in monocytes from old subjects. Our findings represent a comprehensive analysis of the influence of human aging on pattern recognition receptors signaling and monocyte functions, and have implications for strategies to enhance the immune response in the context of infection and immunization.
Introduction
The world population is undergoing a rapid expansion of older adults. It is estimated by 2030, 1 in 5 Americans will be 65 years (yrs) or older (1). Age-associated decline of the immune system, referred to as immunosenescence, has been linked to poor responses to vaccines (2) and higher incidence of infections (including influenza and bacterial pneumonia), cancer, and neurodegenerative and cardiovascular diseases that contribute to increased morbidity and mortality in the elderly (3, 4). Many individuals will age without major health problems; however immunosenescence can be associated with chronic low-grade inflammation and a state of increased disease and frailty referred to as inflammaging (5). Further studies are necessary to understand the mechanisms of immunosenescence leading to effective vaccines and improve health outcome for older adults.
Age-related dysfunctions of the immune system include alterations in the distribution and function of cells involved in the communication between the innate and adaptive immune responses. Of the innate cells, peripheral blood monocytes, derived from the bone marrow myeloid precursors, are the most abundant representing 10% of circulating blood leukocytes in human. Monocytes via pattern recognition receptors (PRRs) such as TLRs, MDA5, RLR, and NOD1 are involved in the innate response to a wide range of pathogens (6, 7). They initiate and support the adaptive immunity through virtue of their functions including phagocytosis, production of pro- and anti-inflammatory mediators, transporting antigens to specialized sites of T cells priming, and differentiating into antigen-presenting cells such as macrophages and DCs (8–10). Monocytes represent heterogeneous population with three distinct subsets distinguished by expression of CD14 and CD16. Classical monocytes have high CD14 and no CD16 expression (CD14+CD16−) and are the most abundant (90%). CD14+CD16− monocytes produce high levels of reactive oxygen species (ROS) and IL-6, IL-8, IL-10, and CCL2 in response to pathogens (11). The minor population (10%) is subdivided into two subsets: intermediate monocytes with high CD14 and low CD16 (CD14+CD16+) and non-classical monocytes with low CD14 and high CD16 (CD14dimCD16+). CD14+CD16+ monocytes are low ROS producers but produce higher levels of reactive nitrogen species (RNS), IL-1β and TNFα; whereas CD14dimCD16+ monocytes are involved in “patrolling” the vascular endothelium via Cx3CR1-Cx3CL1 interactions and produce TNFα, IL-1β, and CCL3 in responses to viruses and immune complexes via a proinflammatory TLR7-TLR8-MyD88-MEK pathway (11, 12).
Studies on the impact of aging on the function of human monocytes are limited in number and often yield conflicting results. Disparate outcomes could be due to the use of total monocytes (instead of individual subsets), different enrollment criteria for subject selection (including varying definitions of frailty), and experimental protocols. Using non-frail healthy adults (21–40 yrs of age) and old (≥ 65 yrs of age) subjects, we have previously shown that the frequency and absolute numbers of the three monocyte subsets were unchanged with aging (13). The expression of TLR3, TLR4, and TLR7 on total monocytes was also comparable between age groups. However, we observed after stimulation of PBMCs with PRRs agonists that the gene signal corresponding to monocytes was strongly induced at an early time in PBMCs from adult subjects compared to old individuals (13). We hypothesize that aging could be impacting the function of monocytes at the transcriptional level which remain an open question. In this study, we assessed the transcriptional programs and function of monocyte subsets in response to PRRs ligation. We analyzed transcriptome data, measured cytokine and chemokine production, as well as measured surface expression of key chemokine receptors. This approach allowed us to identify alternations in the PRRs responses in monocyte subsets from adults and old subjects. Our findings represent a comprehensive analysis of the influence of human aging on PRRs signaling and monocyte function, and have implications for strategies to enhance the immune response in the context of infection and immunization.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Board of the Martin Health System (Stuart, Florida). All participants in this study were > 21 yrs of age. Written informed consent was obtained from all subjects.
Patient recruitment and PBMCs isolation
Healthy community-dwelling subjects were enrolled from Martin Health system (Florida) in two groups: adults (24–36 years, n = 11) and non-frail old individuals (67–83 years, n = 11). Using a screening questionnaire, participants were asked about lifestyle, clinical history and medication usage. Subjects were excluded that self-reported comorbid conditions including cancer (within the last 5 yrs for those ≥ 65 yrs), immunocompromising disorders and steroid usage, whereas inclusion criteria included controlled hypertension, occasional/tolerable “aching joints’ from arthritis and not taking daily NSAIDS or acetaminophen, and controlled diabetes. Our definition of non-frail subjects was based on Fried Criteria (14). The Katz index of independence in activities of daily living (ADL) was used to assess the functional impairment of subjects ≥ 65 yrs (15). The index ranks adequacy of performance in bathing, dressing, toileting, transferring, continence, and feeding. Subjects with a score lower than 6 (indicating moderate to severe impairment) were excluded from the study. Old subjects were also assessed for dementia using the Mini-COG assessment instrument for dementia (16). The Mini-Cog combines two simple cognitive tasks (three-item word memory and clock drawing test (CDT)) with an empirical algorithm for scoring. Subjects with a recall score of 1–2 words with a normal CDT or a recall score of 3 words was considered non-demented. Demographic data for the cohort used in this study is described in Table 1. PBMCs were isolated from leukapheresis using Ficoll-Paque (GE Healthcare, NJ USA) density gradient media. PBMCs were frozen in 90% Gibco heat-inactivated bovine serum (ThermoFisher Scientific, CA USA) and 10% DMSO.
Table I.
Demographic data for the cohort used for gene array, cytokine/chemokine production analysis, and surface phenotypic analysis.
| Adults (n = 11) |
Old Subjects (n = 11) |
|
|---|---|---|
|
| ||
| Ave Age (Range) | 30 yrs (24–36) | 73 yrs (67–83) |
|
| ||
| Gender M/F (female %) | 4/7 (64%) | 5/6 (55%) |
|
| ||
| Race | ||
| White (Non-Hispanics) | 9 (82%) | 11 (100%) |
| White (Hispanics) | 2 (18%) | |
|
| ||
| Comorbidities | 1 (9%) | 9 (82%) |
| None | 10 (91%) | 2 (18%) |
| Arthritis | 1 (9%) | 5 (45%) |
| Hypertension | 5 (45%) | |
| Stroke | 1 (9%) | |
| Heart Disease | 1 (9%) | |
|
| ||
| Medications | ||
| Prescription | 1 (9%) | 6 (55%) |
| Over-the-counter | 5 (45%) | 8 (73%) |
Sorting monocyte subsets
Total PBMCs isolated from adults and old subjects were treated with benzonase nuclease (Millipore, Billerica, MA USA) for 30min at 37°C in a 5% CO2-humidified environment. For ex vivo microarray data, monocytes were sorted directly from PBMCs. For stimulation microarray data, PBMCs were washed and total monocytes were enriched by negative selection using human monocyte enrichment kit without CD16 depletion (Stem cell, Vancouver, DC Canada). Monocytes were counted and resuspended at 50 million/ml with sorting buffer (RPMI without phenol red, 2%FBS, and 1M HEPES 1:40 (25mM)) into 5ml polypropylene tubes. Cells were incubated with TruStain FcR block (Biolegend, San Diego, CA USA) for 5 min at room temperature. The following antibody cocktail CD19 PE Cy7 (Biolegend), CD3 APC Cy7 (BD Biosciences, San Jose, CA USA), CD16 Pacific Blue (BD Biosciences, San Jose, CA USA) or AlexFluor 700 (Biolegend), and CD14 PerCP (R&D Systems, Minneapolis, MN USA) were added and cells were incubated for 20 min at 37°C in a 5% CO2-humidified environment. Dead cells were identified using LIVE/DEAD Fixable Aqua Dead Cell Stain Kit for flow cytometry (Life Technologies, Carlsbad, CA USA). Cells were washed and resupsended in sorting buffer at 30 million/ml and filtered using a 5ml polystyrene tube with cell-strainer cap (BD Biosciences). Cells were sorted at 4°C using BD FACSAria cell sorter into 5ml FACS tubes containing 500ul sorting buffer and 500ul FBS. Cells were kept on ice before and after sorting.
In vitro stimulation of monocytes
Sorted monocyte subsets were transferred to 5ml polypropylene tubes, spun down and suspended in cultured RPMI medium [RPMI with L-glutamine (Corning Cellgro, Manassas, VA USA) supplemented with 10% FBS and 1X (50U) Penicillin-Streptomycin (Invitrogen, Carlsbad, CA USA)]. Cells were plated at 30,000 cells/well in 96-well V-bottom plates. TLR and RIG-I agonists were added at the following concentrations: LPS-TLR4 (0.5 μg/ml) or CLO97-TLR7/8 (1 μg/ml) or 5′pppRNA-RIG-I (500 ng/ml). Optimal concentrations of different TLR agonists were selected based on median production of IL-6 and IFNα and a ≥ 85% survival rate of monocytes. All PRR ligands were purchased commercially (InvivoGen, San Diego, CA USA) except for 5′-pppRNA, which was custom synthesized, as previously described (13). Monocytes cultured in medium alone were used as a control for LPS and CLO97. Stimulation by 5′-pppRNA required the use of a cationic transfection agent LyoVec (InvivoGen) so medium plus LyoVec alone was used as a control. Monocytes were cultured for up to 24 hrs at 37°C in a 5% CO2-humidified environment.
Microarray and bioinformatics analyses
Agonist-stimulated cells were washed twice with cold PBS and lysed with 100 μl of cold RLT buffer (QIAGEN, Germany) supplemented with 1% beta-mercaptoethanol (BM) (Sigma, St. Louis, MO USA), and transferred to 1.7 ml tubes with 250 μl of cold RLT/BM buffer and quickly stored at −80°C. For ex vivo study, individual monocyte subsets were sorted directly into 1.7ml tube with 500ul of cold RLT/BM buffer and placed on dry ice then transferred to −80°C. 30,000 to 100,000 cells were sorted depending on the subset and donor. Reverse transcription reactions were performed to obtain cDNAs, which were hybridized to Illumina Human HT-12 V4 Expression BeadChips according to the manufacturer′s instruction, and quantified using an Illumina iScan System. The data were collected using Illumina GenomeStudio software. Analysis of the microarray data was conducted using the R statistical language (17) and packages from the community repository of Bioconductor [bioconductor.org] (18). Microarrays displaying unusually low median intensity, low variability, or low correlation relative to the bulk of the arrays were removed as technical outliers from the rest of the analysis. The raw data was preprocessed using Quantile normalization, followed by a log2 transformation using facilities from LIMMA package (19). Subsequently, the LIMMA package was also used to fit a linear model (2 group analysis) to each probe and to perform a (moderated) student’s t test on various differences of interest (20). In addition, the LIMMA package from Bioconductor was used to identify differentially expressed genes (DEGs) between treated versus controls (untreated or LyoVec only) or adult versus old subjects. For data mining and functional analyses, DEGs satisfied a False Discovery Rate (FDR) of 5% with ≥ 1.3 or ≤ −1.3 fold-change. Probes that did not map to annotated RefSeq genes and control probes were removed. The expected proportions of false positives (FDR 5%) were estimated from the unadjusted p-value using the Benjamini and Hochberg method (21). Gene Set Variation Analysis (GSVA) were used for differential pathway analysis, which converts gene expression to pathways using gene sets (22). MsigDB gene sets (23) were employed and results tested with the same LIMMA linear modeling as outlined above. To gain a deeper insight into the biological functions unique to each monocyte subset, unique up-regulated and down-regulated DEGs were analyzed separately for enrichment of Gene Ontology (GO) biological processes using the DAVID functional annotation tool (24, 25). An innate gene filter, derived from Gene Ontology query GO: 0045087, was used to highlight innate immune-related genes within the datasets. Some network analysis was performed with Ingenuity Pathway Analysis (IPA: Ingenuity systems). Illumina experimental data were imported into IPA with gene symbol. DEGs (selected based on a nominal p-value ≤ 0.05 and fold-change ≥ 1.3 or ≤ −1.3) that were associated with a canonical pathway in Ingenuity’s Knowledge Base were used for pathway analysis. The significance of the association between the dataset and the canonical pathway was measured in two ways: (1) a ratio of the number of genes from the dataset that map to the pathway divided by the total number of genes that map to the canonical pathway was displayed; (2) over-representation Fisher’s exact test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway was explained by chance alone. The pathways were ranked by -log p-value. Microarray data is available at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GSE94499) https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ubibcawuvxitbwb&acc=GSE94499.
Cytokine and chemokine analysis
Supernatants collected from stimulated monocytes were analyzed for chemokine/cytokine levels using Bio-Plex Pro magnetic bead assays (Bio-Rad, Hercules, CA USA). The following human chemokine premixed panels was used: I-309 (CCL1), MCP-1 (CCL2), MIP-1α (CCL3), MCP-3 (CCL7), MCP-2 (CCL8), Eotaxin (CCL11), MCP-4 (CCL13), MIP-1δ (CCL15), TARC (CCL17), MIP-3β (CCL19), 6Ckine (CCL21), MIP-3α (CCL20), MDC (CCL22), MPIF-1 (CCL23), Eotaxin-2 (CCL24), TECK (CCL25), Eotaxin-3 (CCL26), CTACK (CCL27), GM-CSF, GRO-α (CXCL1), GRO-β (CXCL2), ENA-78 (CXCL5), GCP-2 (CXCL6), MIG (CXCL9), IP-10 (CXCL10), I-TAC (CXCL11), SDF-1A+β (CXCL12), BCA-1 (CXCL13), SCYB16 (CXCL16), Fractalkine (CX3CL1), MIF, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-16, TNF-α, and IFN-γ. The manufacturer’s protocol was followed. Data was acquired on a Bio-Plex 200 System (using bead regions defined in the Bio-Rad protocol) and analyzed with the Bio-Plex Manager 6.1 software from Bio-Rad. Supernatants collected from stimulated monocytes were analyzed for IFN-α production using an IFN-α ELISA (PBL Assay Science, Piscataway, NJ USA).
CX3CR1 surface receptor expression by flow cytometry
PBMCs were treated with and without agonists for 18hrs. Cells were washed with PBS then stained with the following antibodies: Annexin V FITC, CD3 AlexaFluor 700, CD19 AlexaFluor 700, CD14 BV650, CD16 PECy7, and CX3CR1 PE. All antibodies were from Biolegend. Dead cells were identified using LIVE/DEAD Fixable Aqua Dead cell stain kit. Cells were evaluated on a BD LSR II flow cytometer and frequencies and mean fluorescent intensity (MFI) were gated using FlowJo software.
Statistical analysis
For cytokine production, significant differences between multiple comparisons [LPS or CLO97 treated- versus untreated-monocytes, 5′-pppRNA treated- versus LyoVec only treated-monocytes, and adult versus old subjects] were determined by two-way ANOVA test followed by Bonferroni correction for multiple comparisons (with alpha 0.05, 95% confidence interval). A p value < 0.05 was considered significant. For surface receptor upregulation analysis p values were determined by two-sided parametric t test with Welch’s correction (did not assume equal standard deviations). Data were analyzed and figures generated using GraphPad Prism 6 software.
Results
Ex vivo sorted monocyte subsets revealed distinct transcriptional profiles
Healthy non-frail individuals enrolled into the study were arranged into two groups: adults and old subjects (n = 11 per group). The average age for adults were 30 yrs (range 24–36 yrs), whereas old individuals were 73 yrs (range 67–83 yrs) (Table I). Individuals with comorbid conditions including cancer (within the last 5 yrs for those greater than 65 yrs), immunocompromising disorders, and steroid usage were excluded; whereas inclusion criteria included controlled hypertension, occasional/tolerable “aching joints” from arthritis and not taking daily non-steroidal anti-inflammatory drugs (NSAIDS) or acetaminophen, and controlled diabetes (see Methods). Monocytes were first evaluated under healthy non-stimulus conditions to determine transcriptional differences between the three subsets, and the effects of aging on gene expression. Using CD14 and CD16 conjugated antibodies, monocytes were sorted from frozen PBMCs isolated from adults and old subjects (n = 9 per group) into three subsets: classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14dimCD16+) (Figure 1A). Differential gene expression between monocyte subsets was evaluated by microarray analysis using the Illumina BeadChips platform (see Methods). A multidimensional scaling plot (MDS) was created to visualize the relationship amongst the three monocyte datasets and between adult and old individuals using the relative expression of more than 18,000 transcripts (Figure 1B). Each point on the graph represents a sample from one subset indicated by color from either adults or old individuals. The relative proximity of the samples along the X- and Y-axis reflect the principle components that separate the datasets based on gene expression intensities. It was evident that CD14+CD16− (red/yellow), CD14+CD16+ (blue/green), and CD14dimCD16+ (black/grey) monocytes occupied non-overlapping spaces indicating that sorted subsets are primarily distinct and have unique transcriptional profiles (Figure 1B). Using a hierarchical cluster heat map to analyze the top 100-discriminant genes also showed a distinct separation of monocyte datasets (Figure 1C).
Figure 1. Distinct transcriptional profiles of ex vivo sorted monocyte subsets.
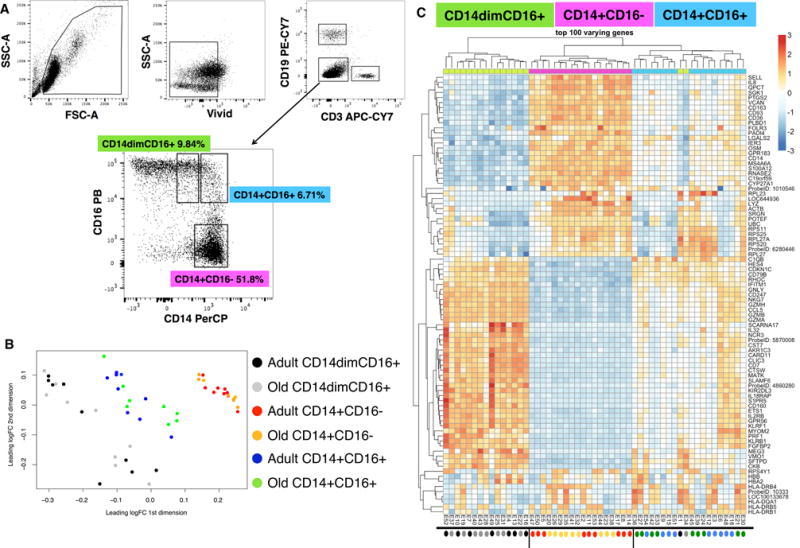
(A) Flow cytometry gating scheme used to sort CD14dimCD16+, CD14+CD16+, and CD14+CD16− monocyte subsets. (B) Multidimensional scaling plot (MDS) analysis was used to visualize the relationship amongst the three subsets using the relative expression of more than 18,000 transcripts. Each point represents a sample from one subset indicated by color (CD14+CD16− (red/yellow), CD14+CD16+ (blue/green), and CD14dimCD16+ (black/grey) from adult or old subjects (n = 9 per age group). The relative proximity of the samples along the X- and Y-axis reflect the principle components that separate the datasets based on gene expression intensities. (C) Hierarchical clustering analysis heat map of the top 100-discriminant genes (listed on the right) for each monocyte (CD14dimCD16+ (green), CD14+CD16− (purple), and CD14+CD16+ (blue) dataset (age groups were combined for each dataset). Scale is provided. Red denotes increased gene expression and blue denotes decreased gene expression. The colored circles under the heat map correspond to colors used in the MDS plot to indicate adults and old subjects.
The developmental relationship between monocyte subsets remains unclear but CD16+ monocytes are considered to be in a more advanced stage of differentiation with CD14+CD16+ monocytes exhibiting an intermediate phenotype when compared to classical and non-classical monocytes (26). Our clustering analysis showed that the CD14+CD16− and CD14+CD16+ monocyte subsets were closely clustered and are distant from the CD14dimCD16+ subset (Figure 1C). Interestingly, when looking at the intensity of gene expression (red depicts high gene expression and blue depicts low gene expression), we observed that the intensity of gene expression for CD14+CD16+ monocyte subset was in between those of CD14+CD16− and CD14dimCD16+. This suggests that the CD14+CD16+ monocyte subset might represent a transitional differentiation state between CD14+CD16− and CD14dimCD16+. This gene expression pattern was previously noted in other monocyte profiling studies further supporting the idea that CD14+CD16+ monocytes have a more intermediate differentiated phenotype (27, 28). Overall, these results demonstrated that sorted ex vivo monocyte subsets are transcriptionally distinct.
Unique gene expression of ex vivo CD16+ and CD16− monocytes
Using the MDS and hierarchical cluster analysis (Figure 1B–C), we did not observe a tight aggregation in ex vivo unstimulated monocyte subsets based on age groups. Therefore, transcriptome analysis was performed independent of age while focusing on the transcriptional difference between subsets. Complied microarray data from 18 subjects (n = 9/age group) for one subset was contrasted against the remaining two subsets (for example, CD14dimCD16+ versus CD14+ CD16− and CD14+ CD16+). A total of 18,615 transcripts were detected across the three contrasts (Supplementary Table 1). Significantly differentially expressed genes (DEGs) were denoted with an adjusted p value < 0.05. Profiles of DEGs consisted of 6204 distinct transcripts for CD14+CD16− monocytes, 5289 transcripts for CD14dimCD16+, and 1731 transcripts for CD14+CD16+ monocytes (Figure 2A). This lower expression of significant genes for CD14+CD16+ monocytes is consistent with our cluster analysis (Figure 1C), which showed a higher gene expression in non-classical and classical subsets.
Figure 2. Differential expressed genes of ex vivo monocyte subsets.
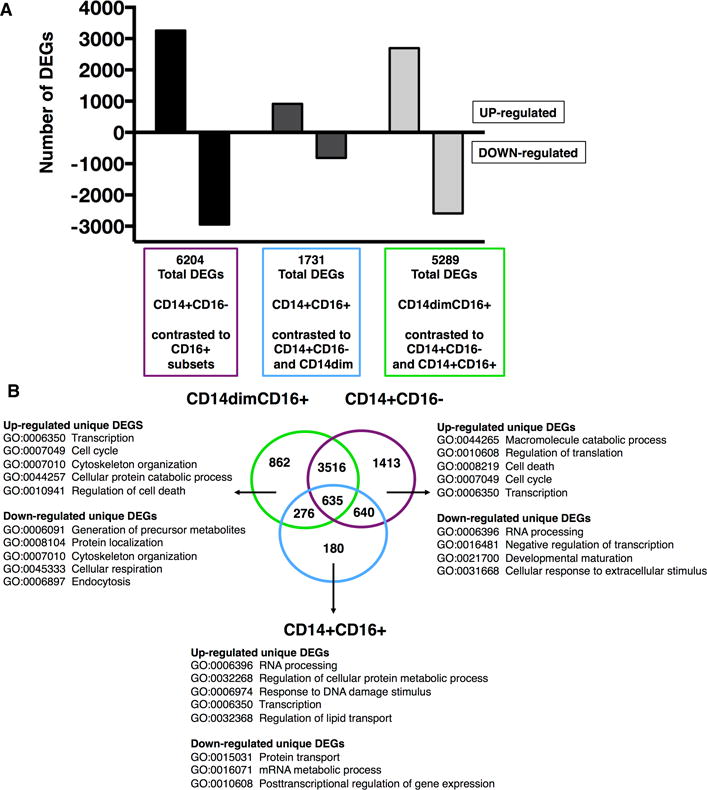
Compiled microarray data analysis from 18 samples (n = 9 per age group) for one subset was contrasted against the remaining two subsets. A total of 18, 615 transcripts were detected across the three contrasts. (A) Graph depicts the total number of differentially expressed genes (DEGs) (adjusted p value < 0.05) subdivided into up-regulated (positive fold-change (FC)) and down-regulated (negative FC) for each subset. (B) Venn diagram analysis of total DEGs revealed unique and common DEGs for each monocyte subset. Listed for each subset are selected top Gene Ontology (GO) biological processes related to unique up- and down-regulated DEGs.
Using Venny, an interactive tool for comparing lists by Venn diagrams (29), profiles of DEGs for each subset were compared to identify unique and common transcripts (Figure 2B). CD14+CD16− monocytes had the most unique genes at 1413, whereas CD14dimCD16+ and CD14+CD16+ had 862 and 180 unique genes, respectively (Supplementary Table 1). We focused our analysis on immune responses using a gene filter derived from Gene Ontology (GO), which included cytokines and chemokines, co-stimulatory factors, transcriptional factors, adhesion molecules, and metabolism related genes. Genes up-regulated for CD16+ monocytes (but down-regulated in CD16− monocytes) included Cx3CR1, FCGR3A (CD16), ITGAL, SOD1, RARA, IFITM1/2/3, CD115 (CSF1R), SIGLEC10, IFNG, and TNF (Table II). The expression of the TNF transcript indicates that CD16+ monocytes are more pro-inflammatory compared to classical CD14+CD16− monocytes. Immune related up-regulated genes for CD16− monocytes (but down-regulated in CD16+ monocytes) included SELL (CD62L), CD93 (C1qR1), CD36, CD114 (CSF3R), CCR1/2, IL4R, IL6R, IL1B, MyD88, FPR1/2, HIF1A, and TLR2/4/5/6/8 (Table II). The up-regulation of multiple TLR transcripts indicates that CD14+CD16− monocytes could have a better ability to respond to various pathogens. Genes shared between CD14+CD16− and CD14+CD16+ monocytes (but down-regulated in CD14dimCD16+) included CD14, ALDH2, CD1A/B, CD63, and MHC class II molecules HLA-DMA/DMB/DRA/DRB3. CD14+CD16+ monocytes also up-regulated class II molecules HLA-DOA, HLA-DPB1, HLA-DQB1, and HLA-DRB6, whereas CD14+CD16− monocytes up-regulated MHC class I molecules HLA-B, HLA-F, and HLA-H (Table II). This down-regulation of MHC class II and I transcripts suggest CD14dimCD16+ monocytes have less antigen presentation potential compared to classical and intermediate monocytes. The results pertaining to CD16+ and CD16− monocytes were in line with other published transcriptional profile data providing initial validation of microarray analysis (26, 27, 30). For example, classical monocytes express CD62L and CCR2 and migrate in response to CCL2, whereas patrolling CD14dimCD16+ monocytes express Cx3CR1 and migrate into tissues expressing Cx3CL1.
Table II. The expression pattern of selected monocyte-related DEGs.
Transcriptome analysis was generated by contrasting one ex vivo sorted subset with the remaining two subsets. Positive FC values (+Log2FC) indicate up-regulated genes, whereas negative FC values (−Log2FC) indicate down-regulated genes. Blank boxes indicate genes were not significantly expressed for that subset.
| Genes | CD14+CD16− (Log2FC) | CD14+CD16+ (Log2FC) | CD14dimCD16+ (Log2FC) |
|---|---|---|---|
| CCR1 | 2.176 | −2.441 | |
| CCR2 | 1.462 | −0.496 | −0.967 |
| CD14 | 3.180 | 0.676 | −3.856 |
| CD163 | 3.133 | −3.191 | |
| CD1D | 1.738 | −1.894 | |
| CD36 | 3.177 | −2.961 | |
| CD93 | 2.720 | −2.959 | |
| CD97 | −0.417 | ||
| CSF1R (CD115) | −1.484 | 0.713 | 0.771 |
| CSF3R | 2.465 | −2.595 | |
| CX3CR1 | −0.901 | 0.604 | |
| FCGR1A (CD64) | 1.148 | 0.465 | −1.613 |
| FCGR3A (CD16) | −0.627 | 0.683 | |
| FPR1 | 2.239 | −2.433 | |
| HIF1A | 1.667 | −0.520 | −1.146 |
| HLA-DMA | 0.551 | 0.993 | −1.544 |
| HLA-DMB | 0.590 | 0.684 | −1.273 |
| HLA-DOA | −0.342 | 1.078 | −0.736 |
| HLA-DRA | 0.761 | 0.468 | −1.230 |
| HLA-DRB3 | 0.631 | 0.620 | −1.250 |
| ICAM3 | 0.425 | −0.439 | |
| ICAM2 | −1.339 | 0.696 | |
| ICAM4 | −1.617 | 1.802 | |
| ICAM5 | 0.149 | ||
| IFITM1 | −3.640 | 0.887 | 2.753 |
| IFITM2 | −0.996 | 0.421 | 0.575 |
| IFITM3 | −1.147 | 0.666 | |
| IFNG | −0.554 | 0.773 | |
| ITGAL | −1.262 | 1.054 | |
| LYN | −0.315 | ||
| LYZ | 2.863 | −2.185 | |
| MAFB | 1.434 | −1.037 | |
| MNDA | 0.992 | −1.012 | |
| MPO | 1.060 | −1.062 | |
| MYD88 | 0.614 | −0.446 | |
| PLBD1 | 2.882 | −3.045 | |
| RARA | −0.521 | 0.390 | |
| S100A12 | 3.614 | −3.377 | |
| S100A8 | 0.628 | 0.792 | −1.420 |
| S100A9 | 0.930 | 0.613 | −1.543 |
| SELL (CD62L) | 2.795 | −0.761 | −2.034 |
| SIGLEC10 | −1.811 | 0.658 | 1.153 |
| SOD1 | −0.843 | 0.753 | |
| SOD2 | 0.949 | −0.690 | |
| TLR2 | 0.488 | −0.186 | −0.303 |
| TLR4 | 0.669 | −0.558 | |
| TLR5 | 0.778 | −1.046 | |
| TLR6 | 0.312 | −0.268 | |
| TLR7 | −0.549 | ||
| TLR8 | 0.454 | −0.537 | |
| TNF | −1.248 | 0.537 | 0.711 |
| TREM1 | 2.018 | −2.261 |
To gain a deeper insight into the biological functions unique to each monocyte subset, unique up-regulated and down-regulated DEGs were analyzed separately for enrichment of GO biological processes using the DAVID functional annotation tool (Supplementary Table 1) (31). We observed that top GO biological processes (with significant p value < 0.05) were related to transcription, cell cycle, metabolic processes, and cell death suggesting ex vivo monocyte subsets are in a steady state of differentiation (Figure 2B). Interestingly, the non-classical subset is known to have highly motile patrolling behavior in vivo, which support the unique expression of transcripts related to cytoskeleton organization. Overall, our transcriptional profiling of the three monocyte subsets revealed distinct functional characteristics and more importantly the transcriptional profiles of ex vivo unstimulated monocytes sorted from healthy non-frail old subjects appear not to be affected by aging.
Transcriptional profiles of agonist-stimulated monocyte subsets
We then determined the transcriptional responses of monocyte subsets following stimulation with various PRRs agonists. Monocyte subsets were sorted from adult and old subjects (n = 8/age group) and stimulated for 24 hours with LPS (TLR4), CLO97 (TLR7/8), or 5′pppRNA complexed with LyoVec (a cationic transfection reagent that facilitates intracellular delivery) (RIG-I) (see Methods). MDS were again used to visualize the relationship amongst the three monocyte datasets and between treatment and negative controls. Each point on the graph represents a treated subset indicated by color and shape: CLO97, LPS, and negative control (no treatment) on Figure 3A, and 5′pppRNA-LyoVec and negative control (LyoVec only) on Figure 3B. It was evident from the analysis that each monocyte subset occupied non-overlapping spaces and that stimulation with CLO97, LPS, and 5′pppRNA produced unique transcriptional profiles compared to negative controls.
Figure 3. Distinct transcriptional profiles for agonist-stimulated monocyte subsets.
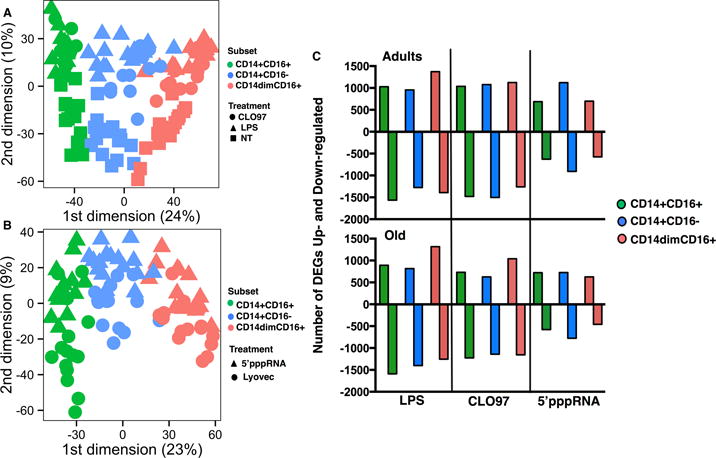
Multidimensional scaling plot (MDS) analysis was used to visualize the relationship amongst the three subsets treated with (A) CLO97 (circle), LPS (triangle), and negative control No Treatment (square), and (B) 5′pppRNA-conjugated LyoVec (triangle) and negative control LyoVec only (circle). Color depicts individual subsets: CD14dimCD16+ (pink), CD14+CD16+ (green), and CD14+CD16− (blue). (C) Transcriptional profiles were generated by normalizing individual treatments to their respective negative controls. Graph depicts the total number of DEGs (fold-change (FC) ≥ +1.3 or ≤ −1.3 and p value < 0.05) subdivided into up-regulated (positive FC) and down-regulated (negative FC) for adults (top) and old subjects (bottom). n = 8 per age group.
Figure 5. Adult contrasted to old subjects revealed altered biological functions in response to agonists.
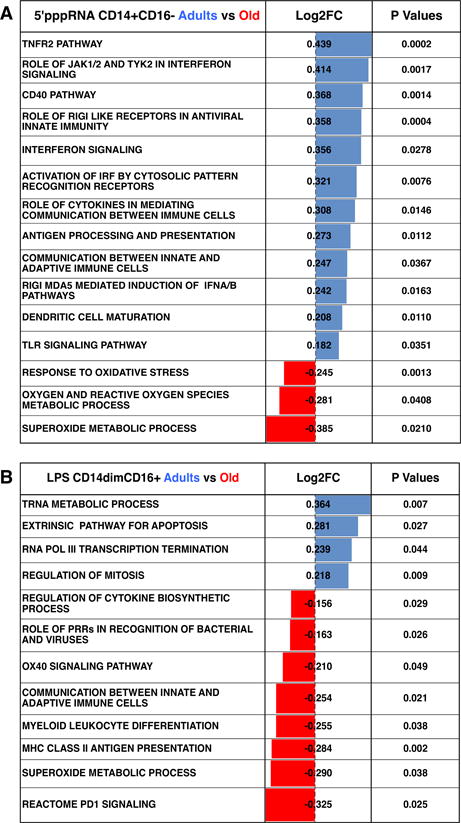
Tables depict significantly enriched functional pathways (p value < 0.05) for 5′-pppRNA treated CD14+CD16− monocytes (A) and LPS treated CD14dimCD16+ monocytes (B). Positive fold-change (Log2FC) values (blue) indicate pathways that are significantly enriched in adults, whereas negative Log2FC values (red) indicate pathways that are significantly enriched in old subjects.
We preformed transcriptome analysis by normalizing LPS and CLO97 stimulated-monocyte datasets to untreated monocytes, whereas 5′pppRNA/LyoVec was normalized to LyoVec only treated monocyte subsets. DEGs were selected based on fold-change (FC) ≥ +1.3 or ≤ −1.3 and p value < 0.05 (Supplementary Table 2). These criteria were chosen based on our own validation scheme used for other published studies (13, 32). Figure 3C shows the number of up- and down-regulated DEGs induced after treatment for all three subsets sorted from adult and old subjects. In comparison to 5′pppRNA, LPS and CLO97 treatment of all three subsets induced a higher number of total DEGs with majority of these transcripts being down-regulated in comparison to untreated monocytes. Focusing on transcriptional profiles for adults, we assessed the impact of each treatment on individual monocyte subsets by evaluating the elicited chemokine and cytokine responses. Table III shows the top five up- and down-regulated chemokine and cytokine transcripts for each treatment condition. Interestingly, we observed 5′pppRNA induced a high up-regulation of antiviral interferon IFNα and IFNβ transcripts in CD14+CD16− and CD14+CD16+ monocytes, whereas CLO97 and LPS induced a high up-regulation of IFNγ transcript in CD14+CD16+ and CD14dimCD16+ monocytes (Table III). It is worth noting that the expression levels of IFNα and IFNβ transcripts were two-fold higher in CD14+CD16− than in CD14+CD16+ monocytes, as well as IFNγ expression levels was two-fold higher in CLO97-treated CD14+CD16+ monocytes. Other examples of unique responses included the monocyte chemoattractant transcript CXCL10 was induced by 5′pppRNA in all three subsets, whereas LPS and CLO97 treatment down-regulated CXCL10 transcript expression in subsets. LPS and CLO97 treatment did induced expression of pro-inflammatory cytokine IL-6 in all three subsets. Overall, this data revealed that treatment of individual monocyte subsets induced distinct transcriptional patterns of interferons, cytokines, and chemokines due to the different array of PRRs found on monocyte subsets.
Table III. The top five up- and down-regulated chemokine and cytokine transcripts for each treatment condition.
Profiles were generated by contrasting treatment against negative controls. Positive FC indicates up-regulation, whereas negative (−) FC indicates down-regulation. Gray shaded boxes highlight the expression pattern of IFNα and IFNγ, whereas bold font highlights CXCL10 and IL-6 expression.
| 5′pppRNA-LyoVec (vs LyoVec only)
| |||||
|---|---|---|---|---|---|
| CD14+CD16− | FC | CD14+CD16+ | FC | CD14dimCD16+ | FC |
| CXCL10 | 12.80 | IFNA14 | 4.16 | CCL8 | 4.43 |
| IFNA14 | 9.32 | IFNA16 | 4.12 | CXCL10 | 2.18 |
| IFNA16 | 8.49 | IFNB1 | 3.64 | IL1RN | 2.04 |
| IFNA2 | 8.30 | CXCL10 | 3.31 | CCR7 | 2.01 |
| IFNB1 | 7.64 | IL27 | 3.05 | IFNB1 | 1.93 |
|
| |||||
| CCL24 | −1.92 | CCL24 | −1.72 | CCL24 | −1.50 |
| IL1B | −2.16 | IL1A | −1.92 | CCL1 | −1.61 |
| IL1A | −2.32 | IL1B | −1.96 | IL3RA | −1.74 |
| CXCL6 | −2.55 | CXCL5 | −2.17 | CXCL8 | −1.76 |
| CXCL5 | −3.53 | CCL1 | −2.67 | IL1B | −2.39 |
|
LPS (vs NT) | |||||
| CD14+CD16− | FC | CD14+CD16+ | FC | CD14dimCD16+ | FC |
|
| |||||
| IL23A | 6.76 | IL23A | 12.98 | IFNG | 12.59 |
| CCL22 | 4.96 | IFNG | 10.01 | IL2RA | 6.90 |
| IL24 | 4.43 | CCL20 | 6.55 | IL6 | 5.08 |
| IL6 | 4.22 | IL24 | 6.17 | IL1B | 3.93 |
| CCL3 | 3.30 | IL6 | 4.77 | CCR7 | 3.66 |
|
| |||||
| IL3RA | −3.13 | CXCL16 | −4.15 | IL18BP | −8.19 |
| CCL7 | −3.36 | CCL13 | −5.41 | CXCL10 | −8.41 |
| CCR1 | −4.33 | CCL2 | −6.19 | CXCL16 | −9.86 |
| CCL8 | −7.99 | CCL8 | −8.80 | CCL8 | −10.88 |
| CCL13 | −10.45 | CXCL10 | −9.39 | IL13RA1 | −14.11 |
|
CLO97 (vs NT) | |||||
| CD14+CD16− | FC | CD14+CD16+ | FC | CD14dimCD16+ | FC |
|
| |||||
| CCL22 | 4.34 | IFNG | 25.98 | IFNG | 12.69 |
| IL6 | 3.80 | IL18RAP | 5.44 | IL6 | 10.39 |
| CXCL2 | 3.09 | IL6 | 4.41 | CCL19 | 6.08 |
| CCL20 | 2.58 | CXCR4 | 3.57 | CCR7 | 5.38 |
| CCL3 | 2.54 | IL36G | 3.47 | IL2RA | 4.79 |
|
| |||||
| CCR1 | −2.78 | IL7R | −2.78 | IL6R | −3.59 |
| CCL23 | −3.00 | IL13RA1 | −2.93 | IL3RA | −3.70 |
| IL18BP | −3.48 | IL18BP | −2.96 | IL18BP | −4.77 |
| CXCL10 | −4.14 | CCL13 | −3.03 | CXCL16 | −5.30 |
| CCL13 | −4.21 | CCL23 | −7.18 | IL13RA1 | −6.88 |
Agonists elicited distinct transcription responses between adults and old subjects
We then sought to determine the impact of aging on PRRs-induced responses in sorted monocyte subsets. To assess the enrichment of DEGs in both age groups, transcriptional profiles for monocyte subsets from adults were contrasted to old subjects (Supplementary Table 3). DEGs were selected based on fold-change (FC) ≥ +1.3 or ≤ −1.3 and p value < 0.05. Figure 4A depicts total number of enriched DEGs for each treated subset. Positive fold-change (+FC) values indicated DEGs enriched in adults, whereas negative fold-change (−FC) values indicate DEGs enriched in old subjects.
Figure 4. Adult contrasted to old subjects revealed distinct enriched transcripts in response to agonists.
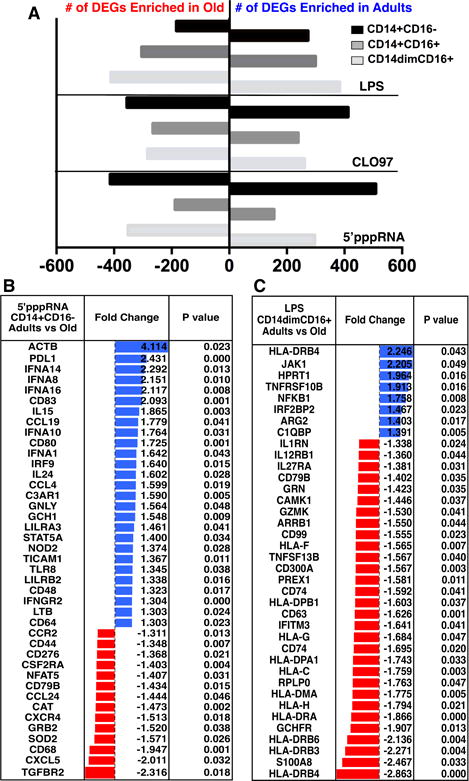
Transcriptional profiles for monocyte subsets from adults were contrasted to old subjects (n = 8 per age group). Differentially expressed genes were selected based on fold-change (FC) ≥ +1.3 or ≤ −1.3 and p value < 0.05. (A) Graph depicts the total number of DEGs for adults and old subjects for each treatment. (B–C) Tables show the FCs and p-values for selected enriched immune-related genes for 5′-pppRNA treated CD14+CD16− (B) and LPS treated CD14dimCD16+ (C) monocytes. Positive FC values (blue) indicate genes that are significantly enriched in the adults, whereas negative FC values (red) indicate genes that are significantly enriched in the old subjects.
For adults, we observed the highest number of enriched DEGs for 5′pppRNA-treated CD14+CD16− (508 DEGs) and CLO97-treated CD14+CD16− (412 DEGs) monocytes, whereas for old subjects 5′pppRNA-treated CD14+CD16− (413 DEGs) and LPS-treated CD14dimCD16+ (411 DEGs) monocytes had the highest enrichment of DEGs. We focused our analysis on immune genes and observed a wide range of immune-related transcripts for both age groups. For example, 5′pppRNA-treated classical CD14+CD16− monocytes had a significant enrichment of interferon transcripts IFNA1/8/10/14/16, IFNGR2, and IRF9 in adults compared to old subjects. Also, adults had a higher enrichment of co-stimulatory molecules (PDL1, CD80, and CD83), phagocytosis Fc receptor (FCGR2A), TLR8, monocyte activation marker (CD48), and cytokines (CCL19, CCL4, IL15, and IL24). Old subjects had an enrichment of chemokines (CCR2, CCL24, CXCR4, and CXCL5), TGFBR2, superoxide dismutase (SOD2), and co-stimulatory molecule CD276 (B7-H3) (Figure 4B).
Enriched DEGs for adult and old subjects were further evaluated using the Ingenuity Pathway Analysis (IPA) software. Supplementary Table 3 lists the log 2-fold-change (Log2FC) of statistical significantly (p value < 0.05) expressed pathways for each treated monocyte subset. Positive FC values indicate pathways significantly enriched in adults, whereas negative FC values indicate pathways significantly enriched in old subjects. Focusing on 5′pppRNA-treated CD14+CD16− monocytes, adults had a higher enrichment of immune response pathways compared to old subjects including TLR signaling pathway, RIGI like receptor signaling pathway, antigen processing and presentation, dendritic cell maturation, regulation of IFNA signaling, and communication between innate and adaptive immune cells (Figure 5A). CD14+CD16− isolated from old individuals had enrichment of superoxide metabolic process, oxygen and reactive oxygen species metabolic process, and response to oxidative stress pathways (Figure 5A). These results demonstrate that 5′pppRNA stimulated classical CD14+CD16− monocytes from adults display a qualitatively stronger antiviral transcriptional response when compared to those from old individuals.
Another example of altered immune responses was LPS- treated CD14dimCD16+ monocytes, we observed that old subjects had a higher enrichment of MHC class I and II transcripts including HLA-DRB4, HLA-DRB6, HLA-C, and HLA-G which corresponded to an enrichment of functional pathways related to communication such as antigen processing and presentation and communication between innate and adaptive immune cells (Figure 4C and 5B). There was also an enrichment of other immune-related functional pathways including PD1 signaling, superoxide metabolic process, myeloid leukocyte differentiation, and role of PRRs in recognition of bacteria and viruses, whereas monocytes sorted from adults had an enrichment of pathways related to transcription/RNA metabolic processes and regulation of cell cycle. We observed that untreated non-classical CD14dimCD16+ monocytes expressed low levels of MHC class I and II transcripts (Table II); therefore, the enrichment of these transcripts in response to LPS suggests a possible differentiation of non-classical monocytes into macrophages or DCs. Overall, our data revealed a wide range of potential age-associated alternations in PRRs signaling in individual monocyte subsets sorted from both adults and old subjects indicating monocytes undergo complex cellular differentiations in response to different pathogens.
Stimulated monocytes from adults produced higher levels of inflammatory cytokines, IFNs and chemokines
To assess whether the observed transcriptional immune response in monocytes translated into functional production of interferons, cytokines, and chemokines, we profiled supernatants harvested from agonist-stimulated monocyte subsets from both age groups using multiplex immunoassays. Production of analytes was considered significantly (p value < 0.05) increased or decreased by comparing to negative controls: no treatment (for LPS and CLO97) and LyoVec only (for 5′pppRNA-LyoVec) (Supplementary Table 4). Focusing on adults at 24 hrs, we observed LPS and CLO97 stimulation of all three subsets elicited the most significant changes (compared to no treatment) in cytokines and chemokines production. Interestingly, 5′pppRNA stimulation induced significant changes (compared to LyoVec only) in analytes’ production only in CD14+CD16− and CD14+CD16+ monocyte subsets. In response to LPS treatment, distinct analytes were produced including Cx3CL1, IL-1β, and IL-6 for classical CD14+CD16− monocytes, CCL26 and IL-4 for CD14+CD16+ monocytes, and IFNγ, CCL1, CCL11, CCL23, and CXCL13 for CD14dimCD16+ monocytes (Figure 6). CLO97 treatment of monocyte subsets elicited unique analytes CCL7, CXCL1, CXCL5, and CXCL6 for classical CD14+CD16− monocytes, CXCL10, CXCL11, and IL-10 for intermediate CD14+CD16+ monocytes, and IFNγ, CXCL12, CCL22, and CXCL13 for CD14dimCD16+ monocytes. Unique analytes induced by 5′pppRNA included CCL7 and CXCL6 for classical CD14+CD16− monocytes and CXCL10, CXCL11, CCL24, and CXCL13 for intermediate CD14+CD16+ monocytes (Figure 6). Interestingly, we observed higher production of TNFα by CD14+CD16+ monocytes treated by LPS (8717 pg/ml versus 5216 pg/ml for CD14+CD16− and 3116 pg/ml for CD14dimCD16+) and by CLO97 (7699 pg/ml versus 5099 pg/ml for CD14+CD16− and 2680 pg/ml for CD14dimCD16+) (Supplementary Table 4), which is in line with CD14+CD16+ monocytes having more of a pro-inflammatory phenotype. Differential expressed chemokine and cytokine transcripts were observed for 5′pppRNA-treated CD14dimCD16+ monocytes (Table III), which did not correlate with our multiplex analysis for both age groups. We did however observed up-regulation of IFNγ (LPS- and CLO97-treated CD14dimCD16+) and IFNα (5′pppRNA-treated CD14+CD16− and CD14+CD16+) transcripts (Table III) that corresponded to significant production in these subsets (Figure 6). We also observed the decreased production of CXCL10 after treatment with CLO97 and LPS but increased production in response to 5′pppRNA (Figure 6) that related to CXCL10 transcript expression (Table III). Overall, we observed distinct production of chemokines and cytokines for each monocyte subset in response to PRR agonists.
Figure 6. Distinct chemokine and cytokine production by individual agonists.
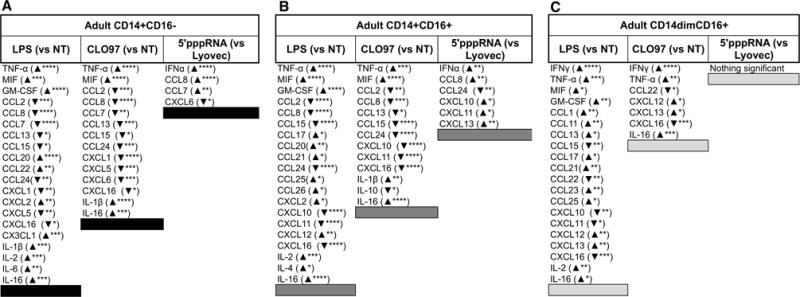
Table lists the significant production of chemokines and cytokines elicited from agonist-treated CD14+CD16− (A), CD14+CD16+ (B), and CD14dimCD16+ (C) monocytes sorted from adults. An upward arrow indicates significantly higher production compared to negative controls (NT or LyoVec), while a downward arrow indicates significantly lower production compared to negative controls. Asterisks indicate statistical significance (****) p value < 0.0001, (***) p value < 0.001, (**) p value < 0.01, and (*) p value < 0.05) between treatment and negative control using two-way ANOVA followed by Bonferroni correction for multiple comparisons. n = 8 per age group.
A comparison of adults to old subjects revealed age-related alternations in the production of IFNγ that was higher in CD14dimCD16+ monocytes sorted from adults treated with LPS (1359 versus 594 pg/ml, p value < 0.001) or CLO97 (1412 versus 729 pg/ml, p value < 0.001) for up to 24 hrs (Figure 7A). CD14+CD16− monocytes from adults produced higher IL-1β in response to CLO97 (21295 versus 11616 pg/ml, p value < 0.05); and higher CCL20 in response to LPS (1666 versus 1163 pg/ml, p value < 0.05) (Figure 7B–C). Also, classical CD14+CD16− monocytes from adults produced higher IFNα (34 versus 19 pg/ml, p value < 0.01) and CCL8 (2271 versus 1237 pg/ml, p value < 0.01) in response to 5′pppRNA (Figure 7D–E). The kinetics of expression or biological factors, such as mRNA degradation, could affect the correlation between protein and transcript levels; however, we did observed 5′pppRNA elicited a higher IFNα transcriptional response in classical monocytes from adults compared to old subjects (Figure 4B). Overall, monocytes from old subjects had a decreased production of anti-viral interferons and pro-inflammatory cytokines and chemokines.
Figure 7. Age-related alternations in cytokine and chemokine production.
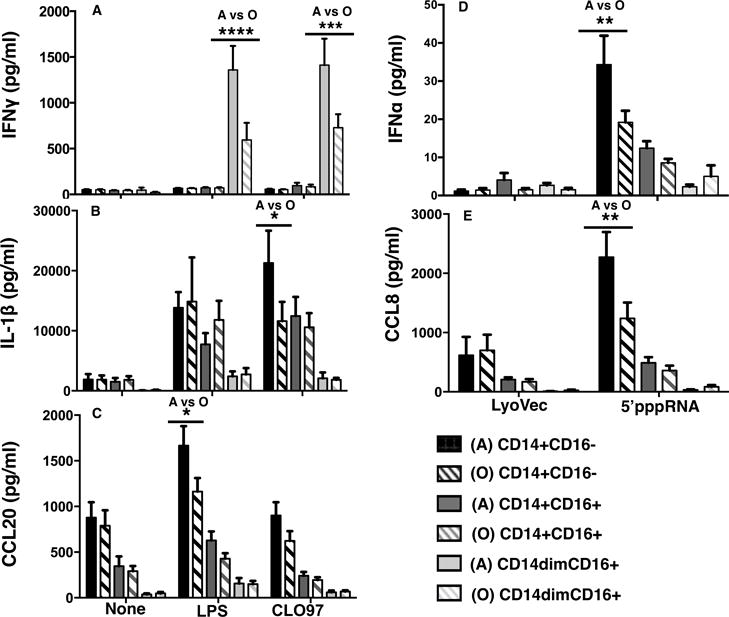
(A–C) Graphs depict the means ± SEM of IFNγ, IL-1β, and CCL20 concentrations in response to stimulation with LPS and CLO97 for up to 24 hrs for adult and old subjects. (D–E) Graphs depict the means ± SEM of IFNα and CCL8 concentrations in response to stimulation with 5′-pppRNA for up to 24 hrs for adult and old subjects. All concentrations were measured using the human chemokine 40-plex from Bio-Rad except for IFNα measured by ELISA. Asterisks indicate statistical significance (****) p value < 0.0001, (***) p value < 0.001, (**) p value < 0.01, and (*) p value < 0.05) between adults and old subjects using two-way ANOVA followed by Bonferroni correction for multiple comparisons. n = 8 per age group.
Expression of fractalkine receptor Cx3CR1 on monocyte subsets
Cx3CR1 interaction with its ligand fractalkine Cx3CL1 has been shown to be critical for monocyte survival by reducing ROS and trafficking into Cx3CL1 tissues including the gut and heart under both steady-state and inflammatory conditions (33, 34). This strongly suggests that higher expression of Cx3CR1 in old subjects could lead to enhance recruitment of monocytes to inflammatory sites and might contribute to pathogenic effects in the heart and other organs observed in the elderly. We thus validate the expression of this molecule at the protein level in our monocyte subsets. CD14dimCD16+ monocytes showed the highest expression of Cx3CR1 compared to intermediate and classical monocytes (Figure 8). Interestingly, we observed under non-stimulated conditions significantly (p value < 0.05) higher expression of Cx3CR1 on CD14+CD16− and CD14dimCD16+ monocytes from old subjects compared to adults (Figure 8) indicating a possible higher number of Cx3CR1+ monocytes in Cx3CL1 expressing tissue in old individuals. Overall, we observed age-related alternations in fractalkine receptor Cx3CR1 that could be associated with age-mediated pathogenesis in the elderly.
Figure 8. Age-related alterations in CX3CR1 surface receptor expression.
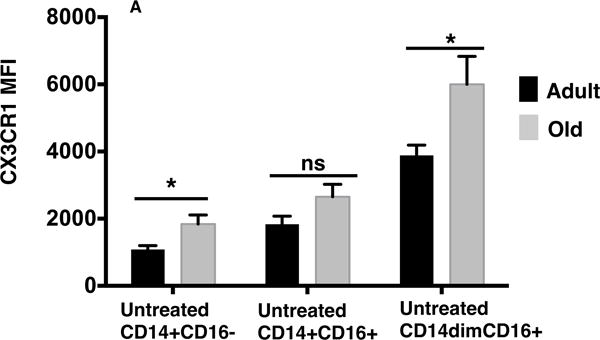
(A) Graph depicts the mean fluorescent intensity (MFI) of CX3CR1 for untreated monocyte subsets from adults (black) and old (gray) subjects. P values ((*) p value < 0.05) were calculated using unpaired t test with Welch’s correction. n = 8 per age group
Discussion
In this study, we have performed a comprehensive and detailed analysis on the impact of aging on the three main monocyte subsets (CD14+CD16−, CD14dimCD16+, and CD14+CD16+) using genomics and functional assays. In particular, we determined whether aging would impact the ability of monocyte subsets to respond to TLR triggering with RIG-I agonist (5′pppRNA), TLR4 agonist (LPS), and TLR7/8 agonist (CL097). To our knowledge, this is the first study to examine the impact of healthy human aging on ex vivo and TLR-stimulated monocyte subsets at both the transcriptional and functional levels. Our data showed under healthy non-stimulus conditions, monocyte subsets did not reveal significant age-related alternations; however, agonist stimulated-monocytes isolated from adults and old subjects did show alternations at transcriptional and functional levels. These included genes and biological processes associated with defense mechanisms against bacteria and viruses, as well as with regulation of adaptive immune response. These alterations could explain some of the defects associated with the increased susceptibility to infections observed in the elderly population.
Monocytes were isolated from healthy subjects selected using an extensive screening procedure including medial history questionnaire and testing of blood for immune cell activation and underline viral infections. For this study, enrollment criteria for older subjects included non-frail, non-dementia, and independent living with control hypertension and arthritis to circumvent older individuals with increased frailty that can be associated with increased co-morbidities and medication usage. This in depth screening process reduces the impact of cofounders in a smaller cohort influencing results. Our previous study Metcalf, T et al 2015, showed that a small cohort of healthy non-frail subjects was able to provide an accurate immune representative of age-associated alternations (13).
Contrasting the three monocyte subsets, we observed that CD14+CD16+ monocytes have a more intermediate transcriptional expression profile compare to CD14+CD16− and CD14dimCD16+ monocytes. The developmental relationship between monocyte subsets remains an unanswered critical question. Using hierarchical clustering analysis, microarray studies have observed a close proximity between intermediate (CD14+CD16+) and non-classical (CD14dimCD16+), whereas classical monocytes (CD14+CD16−) were the most distant (27, 28). In our hands, using a similar hierarchical analysis we observed a closer relationship between CD14+CD16+ and CD14+CD16− monocytes with the CD14dimCD16+ subset being the most distant. This shift in subset clustering could be due to use of a larger sample number (18 subjects) whereas above mention studies contained 3–4 subjects. Despite this discrepancy, all studies uniformly showed that CD14+CD16+ monocytes have an intermediate transcriptional expression profile.
We observed under non-stimulated conditions that CD14+CD16− monocytes had an enrichment of MHC class I and II molecules indicating this subset might have greater ability to trigger the activation of antigen-specific lymphocytes directly or by further differentiation into antigen presenting macrophages or DCs. The high enrichment of TLRs also supports the idea that this subset is most likely the first line of innate immune defense against pathogens. Similar to CD14+CD16− monocytes, intermediate CD16+ monocytes had an enrichment of genes known to be associated with phagocytosis, migration, and MHC class II molecules indicating that they are also predisposed for Ag presentation and serving on the front line of defense against pathogens (35). In line, we observed that both CD14+CD16− and CD14+CD16+ monocytes in response to LPS produced higher level of ROS than CD14dimCD16+ monocytes (data not shown). It is also likely that CD14+CD16− monocytes might be involved in replenishing monocytes in the blood and tissue by serving as the precursor to CD16+ monocytes; as well as studies have showed that CD14+CD16− monocytes are recruited in the tissue to become tissue-resident macrophages under homeostatic conditions (36). This data supports our hierarchical analysis that showed a closer relationship between CD14+CD16+ and CD14+CD16− monocytes.
Non-classical CD14dimCD16+ monocytes up-regulated a high level of transcripts involved in cytoskeleton organization that support their “patrolling” motile function in response to viral antigens (11). In line, we observed transcripts related to cytoskeleton organization, cell adhesion, and locomotion. Cros J et al 2010 also observed that CD14dimCD16+ non-classical monocytes responded to viruses and immune complexes but poorly to surface associated TLR4 stimulation (LPS) compared to classical and intermediate subsets. In our hands, we noted significant production of chemokines and cytokines by CD14dimCD16+ in response to LPS, as well as other studies have observed significant production of TNFα and IL-1β (28, 37). This discrepancy in results could be due to use of different concentrations and stocks of LPS, or the nature of anti-CD14 antibody used in sorting of individual subsets that could block LPS activity. We also observed that CD14dimCD16+ monocytes expressed elevated levels of granzymes transcripts including GZMA and GZMB. Granzymes have been shown to inactivate viruses, cleave surface receptors and extracellular matrix proteins facilitate leukocyte migration, and induced production of pro-inflammatory cytokines and phagocytosis by monocytes (38, 39). This suggests these proteases could play an essential role in the immunoregulation by non-classical monocytes. CD16+ natural killer (NK) cells are also known to express high levels of granzymes and it is possible that the gating scheme used to sort ex vivo CD14dimCD16+ monocytes could have included a small percentage of these cells.
We did not observe significant age-related alternations in transcriptional profiles of non-stimulated monocyte subsets. Therefore, we investigated whether aging would impact the ability of these subsets to respond to TLR ligands as we have previously reported for PBMCs (13). In line, we observed alternations in chemokine and cytokine production, expression in chemokine surface receptor, and the expression of multiple innate immune genes. We observed that 5′pppRNA-treated CD14+CD16− “classical” monocytes sorted from old subjects produced significantly lower levels of IFNα and had lower expression levels of IFNα transcripts. This data is supported by work from Pillai, P et al 2016 that recently showed type I interferon (IFNβ) responses to influenza A virus (IAV) were significantly attenuated in older human monocytes (40). They showed that monocytes and macrophages from old individuals had intact RIG-I signaling to elicit the production of pro-inflammatory cytokines and the inflammasome but had impaired signaling to induce type I IFNs. To see if the alternations in antiviral responses translated to other viruses, we performed preliminary studies looking at infection rate of dengue virus (DENV-2) and IL-6 production of monocyte subsets and observed similar levels between adults and old subjects (data not shown). It is possible that dengue virus does not influence the function of old monocytes similar to influenza virus, which causes a more virulent infection in older individuals.
In addition, we also observed that LPS- and CLO97-treated CD14dimCD16+ “non-classical” monocytes sorted from old subjects also produced lower levels of IFNγ. The ability of monocytes to produce IFNγ suggests an important role of IFNγ in linking innate and adaptive immune responses. IFNγ affects many biological functions primarily related to host defense, cell cycle, apoptosis, and inflammation. It enhances the up-regulation of MHC class I and II molecules and improves antigen presentation by professional antigen-presenting cells (APCs). Additionally, IFNγ elicits leukocyte trafficking, maturation and differentiation (including naïve monocytes into DCs) (41). Overall our data shows that the interferon-mediated anti-viral response in monocyte subsets was significantly impacted with aging, which support the implied immune response to viruses observed in old individuals.
We also observed age-related alternations in IL-1β, CCL8, and CCL20 production by CD14+CD16− monocytes from old subjects treated with LPS, 5′pppRNA, and CLO97, respectively. IL-1β produced as a result of pro-IL-1β transcriptional upregulation via TLR signaling, and a second signal that activate the NLRP3 inflammasome; however, human monocytes are unique in their ability to secret IL-1β by an alternative inflammasome activation using a single LPS stimulation of TLR4 (42). IL-1β is an important mediator for host defense against a wide variety of viruses and bacteria species by eliciting pro-inflammatory signaling pathways, increasing cell adhesion molecules on endothelial cells, and trigging of adaptive immunity such as Th17 response (43, 44). Our results are in line with other studies that shown significantly lowered IL-1β production by LPS-treated monocytes from old subjects (45). CCL8 and CCL20 levels are also reduced in aging subjects. CCL8 is recognized by receptors CCR1, CCR2, and CCR5 that are expressed on different cell types including granuolocytes and mononuclear phagocytes and T lymphocytes (46, 47), whereas CCL20/CCR6 mediates the recruitment of immature DCs and lymphocytes into mucosal-associated tissues (48). Overall, decreased production of these mediators resulting in reduced migration of immune cells to sites of infection such as lung and gastric mucosa could explain why infections such as influenza and bacterial pneumonia have worst outcomes in old subjects.
Reactive oxygen and nitrogen species are involved in the regulation of diverse processes including phagocytes, anti-bacteria, cell proliferation, and apoptosis (49, 50). The production of reactive oxygen species serves as a link between cellular senescence and age-associated pathologies and could be involved in accelerating immunesenescence. (51). Our data revealed that agonist-stimulated monocyte subsets sorted from old subjects had an enrichment of transcripts related to reactive oxygen and nitrogen species production. This included elevated levels of SOD2, CCS, NQO1, ARG2, CAT, DDAH2, and GCHFR transcripts, which corresponded to enrichment of the superoxide metabolic process and ROS metabolic pathways. We have observed comparable levels of reactive oxygen species using fluorescent probes, as well as mitochondrial function/oxidative burst using extracellular flux analyzer (Seahorse Bioscience) between untreated and agonist-treated monocytes from adults and old subjects (data not shown). The impact of aging on monocyte mitochondrial function remains an open question, and our data revealed, at the transcriptional level, an impact of aging on the production of reactive oxygen species.
At the transcriptional levels, we observed age-related alterations in co-stimulatory receptors crucial in the development of an effective immune response including 5′pppRNA induced higher expression of CD80, PDL1, and CD83 (DCs maturation) transcripts in CD14+CD16− monocytes from adults, while LPS induced higher CD40 and CD80 expression in CD14+CD16+ monocytes from old subjects. However, we observed that untreated and TLR agonist stimulated CD14+CD16− and total CD16+ monocytes isolated from adults and old subjects up-regulated comparable surface levels of co-stimulatory receptors CD80, CD86, CD40, PDL1, and HLADR after 24 hrs (data not shown). It is possible the surface expression of these receptors could be alternated at earlier or later time points. We did observe the surface expression of fractalkine receptor CX3CR1 to be higher on enriched untreated monocyte subsets CD14+CD16− and CD14dimCD16+ from old individuals. CD14dimCD16+ monocytes expresses the highest surface levels of CX3CR1 and are known to adhere in vitro to endothelial cells via fractalkine CX3CL1-CX3CR1 interaction (34). Aging and the recruitment of monocytes are known risk factors in inflammatory conditions such as the development of atherosclerosis. Therefore, an increased expression of CX3CR1 on monocytes from old individuals could be related to the increased incidents of chronic inflammatory diseases observed in this population.
In conclusion, the phenotypic and functional differences between monocyte subsets during homeostasis and in disease conditions are not fully defined. The vast array of surface molecules and production of pro- and anti-inflammatory molecules allows monocyte subsets to be recruited into different anatomic sites and play distinct functional roles in immunity and disease pathogenesis. We have shown that agonist–treated monocyte subsets sorted from old subjects had an impaired transcriptional and biological response in comparison to adults. The age-related alternations in monocytes support the observed dysfunctions in old subjects including increased susceptibility to infections. The data gathered from this study will help in the elucidation of aging monocyte subsets, which could help in the understanding of innate immune defects and in the design of treatments directed toward old individuals.
Supplementary Material
Acknowledgments
We are deeply thankful to all of our study participants; this study would not have been possible without their continued participation. We wish to acknowledge Dawn Brown (Florida-Martin Memorial Hospital) for subject recruitment and technical assistance, and for facilitating blood draws. We thank the staff of the Applied Functional Genomics Core at Case Western Reserve University (Dr. Cameron) for performing the microarray assays.
This work by the authors was supported primarily by the NIH contract number HHSN272201100017C (NIA/NIAID/N01-A1 00017). Also, in part by the NIH contract HHSN272201400055C.
Abbreviations used in this article
- DEGs
differential expressed genes
- FC
fold-change
- GO
gene ontology
- MDS
multidimensional scaling
- PRR
pattern recognition receptor
- RLR
RIG-I (retinoic acid-inducible gene I)-like receptor
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
EKH, TUM and JNZ conceived the idea. TUM and EKH designed experiments. CC and JBH helped with RIGI experimental design. TUM performed and analyzed all experiments. MJC performed the microarray analysis, PAW performed bioinformatics analysis and GEO submission, and KG performed preliminary bioinformatics analysis. TUM and EKH wrote the manuscript and AMW and JNZ edited the manuscript.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.High KP. Infrastructure and resources for an aging population: embracing complexity in translational research. Translational research: the journal of laboratory and clinical medicine. 2014;163:446–455. doi: 10.1016/j.trsl.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JM, Plowden J, Renshaw-Hoelscher M, Lu X, Tumpey TM, Sambhara S. Immunity to influenza: the challenges of protecting an aging population. Immunologic research. 2004;29:113–124. doi: 10.1385/IR:29:1-3:113. [DOI] [PubMed] [Google Scholar]
- 3.Gavazzi G, Krause KH. Ageing and infection. The Lancet infectious diseases. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 4.Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annual review of immunology. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farina C, Theil D, Semlinger B, Hohlfeld R, Meinl E. Distinct responses of monocytes to Toll-like receptor ligands and inflammatory cytokines. International immunology. 2004;16:799–809. doi: 10.1093/intimm/dxh083. [DOI] [PubMed] [Google Scholar]
- 8.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends in immunology. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunology and cell biology. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 11.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. Journal of leukocyte biology. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 13.Metcalf TU, Cubas RA, Ghneim K, Cartwright MJ, Grevenynghe JV, Richner JM, Olagnier DP, Wilkinson PA, Cameron MJ, Park BS, Hiscott JB, Diamond MS, Wertheimer AM, Nikolich-Zugich J, Haddad EK. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging cell. 2015;14:421–432. doi: 10.1111/acel.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouillon K, Kivimaki M, Hamer M, Sabia S, Fransson EI, Singh-Manoux A, Gale CR, Batty GD. Measures of frailty in population-based studies: an overview. BMC geriatrics. 2013;13:64. doi: 10.1186/1471-2318-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. The Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 16.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. Journal of the American Geriatrics Society. 2003;51:1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 17.R, R. D. C. T. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 18.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43 doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth G, Gentleman R, Carey V, Dudoit S, Irizarry R, et al. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; New York: 2005. Limma: linear models for microarray data; pp. 397–420. [Google Scholar]
- 21.Benjamini YAHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 22.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DWAW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Huang DWAW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidl C, Renner K, Peter K, Eder R, Lassmann T, Balwierz PJ, Itoh M, Nagao-Sato S, Kawaji H, Carninci P, Suzuki H, Hayashizaki Y, Andreesen R, Hume DA, Hoffmann P, Forrest ARR, Kreutz MP, Edinger M, Rehli M, F. consortium Transcription and enhancer profiling in human monocyte subsets. Blood. 2014;123:9. doi: 10.1182/blood-2013-02-484188. [DOI] [PubMed] [Google Scholar]
- 28.Wong KL, Tai JJ, Wong WCC, Han H, Sem X, Yeap WHH, Kourilsky P, Wong SCC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 29.Oliveros J. Venny. An interactive tool for comparing lists with Venn’s diagrams 2007–2015 [Google Scholar]
- 30.Martinez FO. The transcriptome of human monocyte subsets begins to emerge. Journal of biology. 2009;8:99. doi: 10.1186/jbiol206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 32.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, 3rd, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly RP. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. The Journal of experimental medicine. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White GE, McNeill E, Channon KM, Greaves DR. Fractalkine promotes human monocyte survival via a reduction in oxidative stress. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2554–2562. doi: 10.1161/ATVBAHA.114.304717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. The Journal of experimental medicine. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RRR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 36.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Frontiers in immunology. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belge KUU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. Journal of immunology (Baltimore, Md: 1950) 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 38.Romero V, Andrade F. Non-apoptotic functions of granzymes. Tissue antigens. 2008;71:409–416. doi: 10.1111/j.1399-0039.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 39.Sower LE, Froelich CJ, Allegretto N, Rose PM, Hanna WD, Klimpel GR. Extracellular activities of human granzyme A. Monocyte activation by granzyme A versus alpha-thrombin. Journal of immunology (Baltimore, Md: 1950) 1996;156:2585–2590. [PubMed] [Google Scholar]
- 40.Pillai PS, Molony RD, Martinod K, Dong H, Pang IK, Tal MC, Solis AG, Bielecki P, Mohanty S, Trentalange M, Homer RJ, Flavell RA, Wagner DD, Montgomery RR, Shaw AC, Staeheli P, Iwasaki A. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science (New York, NY) 2016;352:463–466. doi: 10.1126/science.aaf3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraaij MD, Vereyken EJ, Leenen PJ, van den Bosch TPP, Rezaee F, Betjes MG, Baan CC, Rowshani AT. Human monocytes produce interferon-gamma upon stimulation with LPS. Cytokine. 2014;67:7–12. doi: 10.1016/j.cyto.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Snodgrass RG, Huang S, Choi IWW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. Journal of immunology (Baltimore, Md: 1950) 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 45.Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Experimental gerontology. 1999;34:959–970. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 46.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nature immunology. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 47.Ruffing N, Sullivan N, Sharmeen L, Sodroski J, Wu L. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cellular immunology. 1998;189:160–168. doi: 10.1006/cimm.1998.1379. [DOI] [PubMed] [Google Scholar]
- 48.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine & growth factor reviews. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 49.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature reviews Microbiology. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 50.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. American journal of respiratory and critical care medicine. 2002;166:8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekaran A, Idelchik MD, Melendez JA. Redox control of senescence and age-related disease. Redox biology. 2016;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


