Abstract
The impact of age on the neural correlates of familiarity-driven recognition memory has received relatively little attention. Here, the relationships between age, the neural correlates of familiarity, and memory performance were investigated using an associative recognition test in young, middle-aged and older participants. Test items comprised studied, rearranged (items studied on different trials) and new word pairs. fMRI ‘familiarity effects’ were operationalized as greater activity for studied test pairs incorrectly identified as ‘rearranged’ than for correctly rejected new pairs. The reverse contrast was employed to identify ‘novelty’ effects. Estimates of familiarity strength were slightly but significantly lower for the older relative to the younger group. With the exception ofoneregion indorsal medial prefrontal cortex, fMRI familiarity effects (which were identified in medial and lateral parietal cortex, dorsal medial and left lateral prefrontal cortex, and bilateral caudate among other regions) did not differ significantly with age. Age-invariant ‘novelty effects’ were identified in the anterior hippocampus and the perirhinal cortex. When entered into the same regression model, familiarity and novelty effects independently predicted familiarity strength across participants, suggesting that the two classes of memory effect reflect functionally distinct mnemonic processes. It is concluded that the neural correlates of familiarity-based memory judgments, and their relationship with familiarity strength, are largely stable across much of the healthy adult lifespan.
Keywords: Aging, associative recognition, hippocampus, perirhinal cortex
1 Introduction
It is well established that long-term memory declines with increasing age (Nyberg et al., 2012). Age-related memory decline is especially prominent when performance depends upon recollection of associative information about a specific episode as, for example, in tests of source memory or associative recognition (e.g.,Bender et al., 2010; see Old and Naveh-Benjamin, 2008, for review). By contrast, memory judgments that can be supported by undifferentiated information, such as an acontextual sense of familiarity, are less affected by age. Indeed, null or relatively small effects of age on familiarity-based recognition memory have been reported innumerous studies (see Koen and Yonelinas, 2014; Yonelinas, 2002, for reviews; see also Henson et al., 2016). Here, we examine neural correlates of familiarity-based memory judgments as a function of age. In doing so, we add to what is currently a sparse literature on this topic, generalizing and extending prior findings by operationalizing familiarity in the context of an associative recognition task, and sampling participants from across the adult lifespan rather than just from its extremes.
Recollection and familiarity are supported by functionally dissociable memory signals (Ingram et al., 2012; Wixted and Mickes, 2010; Yonelinas, 2002) that depend upon distinct neural regionsand networks (Aggleton and Brown, 2006; Eichenbaum et al., 2007; Skinner and Fernandez, 2007). Notably, studies employing fMRI have identified largely non-overlapping patterns of neural activity associated with recollection- and familiarity-based memory judgments (e.g.,Johnson et al., 2013; Montaldi et al., 2006; for reviews, see Kim, 2010, 2013). When recollection is operationalized by the contrast between correctly recognized memory test items for which recollection succeeded or failed, enhanced activity is evident in a characteristic brain network (the ‘core recollection’ network) that includes the hippocampus and medial prefrontal, posterior cingulate, middle temporal and ventral parietal cortex (Rugg and Vilberg, 2013) (The reverse contrast, identifying where activity is greater for familiar than recollected items, has frequently been employed to study the neural correlates of ‘retrieval monitoring’; see de Chastelaine et al., 2016a, and Wang et al., 2016, for recent examples). Familiarity (operationalized, for example, by the contrast between recognized but unrecollected items and unstudied items) is associated with enhanced activity in a different set of regions, including the intra-parietal sulcus (IPS), precuneus, lateral and anterolateral prefrontal cortex and caudate nucleus (Kim, 2010, 2013).
When contrasted with unstudied (‘novel’)items, familiar items are also associated with reductions in neural activity. Familiarity-related reductionsare especially prominent in the anterior medial temporal lobe (MTL), including the perirhinal cortex (e.g., Henson et al., 2003; Staresina et al., 2012; Wang et al., 2014; see Diana et al., 2007, for review of early studies) and the anterior hippocampus (e.g., Daselaar et al., 2006a; Staresina et al., 2012; see Kim, 2013, Nyberg, 2005, and Rugg et al., 2012,for reviews). Perirhinal ‘novelty effects’ are of particular significance in light of findings from studies of experimental animals indicating that perirhinal cortex is necessary for familiarity-based recognition memory (Aggleton and Brown, 2006; Winters et al., 2008), and that single neuron activity (e.g., Xiang and Brown, 1998) and immediate early gene expression (e.g.,Zhu et al., 1995; see Aggleton et al., 2012, for review) in the region are lower for neural activity elicited by familiar rather than novel stimulus events. In light of this evidence, perirhinal cortex has been proposed as a neural region crucial for familiarity-based memory in humans also (Diana et al., 2007; see Bowles et al., 2007, for supporting lesion evidence).
Analogous fMRI effects in anterior hippocampus have typically not been interpreted as neural correlates of a familiarity signal, however, but rather as reflecting the engagement of the region in novelty-driven memory encoding (Johnson et al., 2008; Köhler et al., 2005; Nyberg, 2005; Stark and Okado, 2003). There is however currently little direct evidence to suggest that familiarity-related reduction in fMRI BOLD responses (or, equivalently, novelty-related enhancement of activity) in perirhinal cortex and anterior hippocampus are functionally dissociable (although see Staresina et al., 2012, for electrophysiological evidence that memory-related effects can have different time-courses in the two regions). By contrast, there is some evidence that familiarity-driven activity enhancements and reductions (henceforth, respectively, ‘familiarity’ and ‘novelty’ effects)are functionally dissociable. Note that we have adopted this terminology for the sake of clarity, and not to imply that the effects necessarily reflect functionally distinct processes or mechanisms (although see Daselaar et al., 2006a and Kafkas and Montaldi, 2014, for evidence that the two classes of effect are indeed functionally distinct). In a study in which participants rated their confidence that a recognition test item was old or new (on a scale varying from high confidence ‘old’ to high confidence ‘new’), Daselaar et al. (2006a) reported that neural correlates of novelty (operationalized as activity that was positively correlated with confidence that an item was new) were localized primarily to anterior hippocampus and ‘rhinal’ cortex, whereas correlates of familiarity (activity that correlated positively with confidence that an item was ‘old’) were identified in, among other regions, parahippocampal and lateral parietal cortex and the precuneus. These two classes of effects were independently predictive of recognition memory performance, suggesting that they reflect processes with distinct mnemonic roles. We return to this issue in the Discussion.
As alluded to previously, there is a substantial behavioral literature examining the effects of age on recollection- and familiarity-based memory judgments. Motivated by the vulnerability of recollection to increasing age (see above), several studies have been reported where fMRI was employed to contrast recollection-related neural activity according to age (see de Chastelaine et al., 2016a, and Wang et al., 2016, for two recent examples, and Wang and Cabeza, 2016, for review). There are fewer reports describing age effects on neural correlates of familiarity-based memory judgments (Angel et al., 2013; Daselaar et al., 2006b; Duarte et al., 2010; Wang and Giovanello, 2016), and only a handful of fMRI studies that have examined the influence of age on novelty processing more generally (Bowman and Dennis, 2015; Moriguchi et al., 2011; Wang et al., 2015). The findings for familiarity-related enhancements of cortical activity range from null effects of age in one study (Daselaar et al., 2006b), to broadly similar effects across age groups in two others (Angel et al., 2013; Duarte et al., 2010), albeit in combination with the identification of familiarity-sensitive regions where effects were attenuated (Angel et al., 2013; Duarte et al., 2010) or enhanced (Duarte et al., 2010) in older adults. The findings of Angel et al.(2013) and Duarte et al.(2010) converged in identifying inferolateral and dorsal medial prefrontal cortex (mPFC) as regions where familiarity effectsare attenuated with increasing age.
The balance of the evidence from the aforementioned studiessuggests that MTL novelty effects are largely preserved with increasing age (see Bowman and Dennis, 2015, for an exception).Indeed, age-related enhancement of novelty effects was reported in two studies: in perirhinal cortex in Daselaar et al., 2006b, and in the hippocampus in Wang et al.(2015). The age differences in perirhinal effects were accounted for by the proposal that older individuals are more reliant on familiarity than are younger individuals when making recognition memory judgments. The finding for the hippocampus was interpreted as an example of age-related ‘de-differentiation’ (e.g., Li et al., 2006), reflecting a breakdown in functional segregation between the hippocampus and perirhinal cortex.
The somewhat modest effects of age on fMRI familiarity and novelty effects seemingly align well with the relatively weak influence of age on familiarity-based memory judgments (see above). Nonetheless, reliable age effects were identified in five of the above-cited studies (six, if one also includes the finding that age influenced the time-course of novelty effects in the amygdala in one other study; Moriguchi et al., 2011). Here, we further examine the question of whether familiarity or novelty effects are age-sensitive. We took advantage of a previously described large dataset (N = 136; de Chastelaine et al., 2016a) to examine the neural correlates of familiarity and novelty processing with participant samples that spanned the adult lifespan more continuously than was the case in prior studies (all of which employed extreme age group designs), and that provided statistical power sufficient to allow a sensitive assessment of whether these correlates co-vary with memory performance.
As we have discussed in detail elsewhere (de Chastelaine et al., 2016a, 2016b; Rugg, 2016), examination of brain-behavior relationships as a function of age is of considerable theoretical interest. A relationship between neural activity and performance that is constant across age groups (an age-invariant relationship) is consistent with the idea that performance is similarly constrained across the lifespan by the functional capacity of the region or regions manifesting the activity. By contrast, a relationship that is stronger, or only evident, in older individuals (an age-dependent relationship) suggests that the relevant region plays an increasingly important role in mediating performance with advancing age, perhaps reflecting individual differences in the vulnerability of the region to age-related degradation (de Chastelaine et al., 2016a; 2016b).
We examine these issues here through further analysis of data acquired in the study of associative recognition that was first reported by de Chastelaine et al. (2016a). In our original report we focused on the neural correlates of successful recollection and post-retrieval monitoring, and did not describe the outcomes of contrasts that identified familiarity or novelty effects. In the present paper, we describe these effects, contrast them according to age, and examine their relationship with memory performance. On the basis of prior findings (see above), we expected to find little evidence of age differences in novelty effects, along with differences in familiarity effects that, if present, are confined to dorsal medial and left lateral prefrontal cortex. Whether either class of effect demonstrates age-invariant or age-dependent relationships with performance is an open question.
2 Materials and methods
More detailed descriptions of the Materials and Methods can be found in three prior publications where either complementary analyses of the present fMRI data set were reported (de Chastelaine et al., 2016a), or where data from the encoding phase of the experiment were described (de Chastelaine et al., 2015; 2016b). The fMRI findings reported below have not been described previously.
2.1 Participants
Thirty six young (18-29 yrs; M = 22 yrs; SD = 3.0 yrs; 17 female), 36 middle-aged(43-55 yrs; M = 49 yrs; SD = 3.4 yrs; 19 female) and 64 older(63-76 yrs; M = 68 yrs; SD = 3.6 yrs; 35 female) adults participated in the experiment having met the inclusion criteria (see below).Data collected from 12 additional individuals were excluded because of technical problems during scanning (1 middle-aged), abnormalities in their scan (2 older), inadequate behavioral performance either at study or test (2 young, 1 middle-aged and 2 older), or insufficient trial numbers (i.e., less than 10 trials) for fMRI analysis in one of the critical conditions (3 young and 1older).
Participants were recruited from the University of Texas at Dallas and the surrounding communities; all were in general good health, had no history of psychiatric, neurological or cardiovascular disease, and were not taking central nervous system-active medication. Participants were right-handed, had normal or corrected-to-normal vision, and were fluent in English by age 5.Exclusion criteria based on neuropsychological test scores are described below. Participants gave informed consent in accordance with the UT Dallas and UT Southwestern Institutional Review Boards, and were compensated at the rate of $30 per hour.
2.2 Neuropsychological testing
All participants completed our standard neuropsychological test battery (see de Chastelaine et al., 2015 for a detailed description of the battery) on a separate day prior to the experimental MRI session. The battery assessed a range of cognitive functions known to either decline or be maintained with age. Participants were screened for dementia with the Mini-Mental State Examination (MMSE), for which a nominal cutoff score of 27/30 was adopted. Exclusion criteria included scores > 1.5 SDs below the age-appropriate norm on either or both long-term memory tests (CVLT and WMS) or on any two of the other neuropsychologcal tests, or an estimated full-scale IQ < 100 as indexed by performance on the WTAR.
2.3 Experimental stimuli
Stimuli were320 semantically-unrelated visually-presented word pairs that were randomly divided into four lists of 80 pairs. The four lists were rotated such that, across each set of yoked participants (1 young, 1 middle-aged and 1 or 2 older participants), each list provided stimuli for each of three experimental word-pair categories: intact, rearranged and new (see below). For each set of yoked participants, word pairs from three of the lists were pseudo-randomly ordered to form the study list. Null events (when only a white fixation cross was present) were included during both study and test sessions to introduce jitter between experimental trials in order to improve the efficiency of the estimation of item effects. The test list comprised 320 critical word pairs, including 160 that had been presented at study (intact pairs), 80 pairs of studied words that had been re-paired from study (rearranged pairs), and 80 unstudied pairs (new pairs). Words comprising the rearranged pairs were presented in the same position at test as they had been at study. Critical test pairs were intermixed with 106 null trials. A 30s break occurred halfway through each test block and intervals between blocks lasted approximately 2 mins. Two buffer pairs (i.e., word pairs that were modeled as events of no interest and intended to minimize primacy effects on the BOLD responses) were presented in succession at the start and immediately after the rest breaks. Word pairs were pseudo-randomly ordered such that the same word pair category did not occur more than three times in succession. Practice study and test lists were formed from items additional to those included in the experimental lists proper.
2.4 Procedure
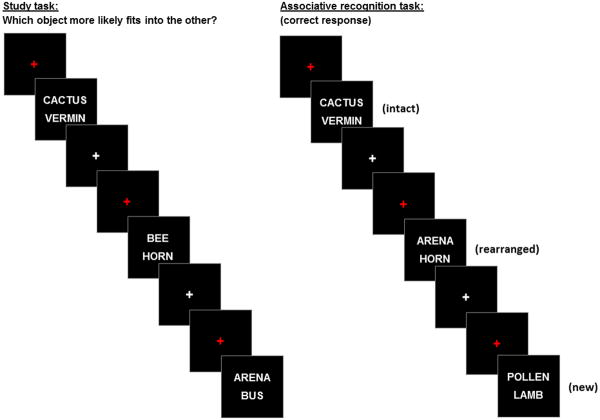
Participants were given instructions and practice sessions for both the study task and the memory test prior to scanning and were thus aware that their memory for the study items would be tested. The study and test phases of the experiment were administered in separate scanning sessions. Figure 1 gives a schematic overview of the study and test procedures. During the first session, the study items were presented in two blocks separated by a short rest interval. For the study task, participants were required to indicate with a button press which of the two objects denoted by the words was more likely to fit into the other. After completing the study task, participants exited the scanner for 15 mins before returning for the test phase. During this second session, participants undertook an associative memory test administered in three consecutive blocks that were separated by short rest intervals. For the memory test, participants were required to press one of three keys to indicate whether a test pair was intact, rearranged or new. An ‘intact’ response was required when participants recognized both words and had a specific memory of the two words being presented together at study. A ‘rearranged’ response was required when both words were recognized without any memory of the words having previously been presented together. A ‘new’ response was required when neither word, or only one of the words, could be recognized from study. The following terms will be used throughout the paper to describe the behavioral responses: ‘associative hits’ to denote correctly endorsed intact items, ‘associative misses’ for intact items incorrectly identified as rearranged, ‘rearranged hits’ for correctly endorsed rearranged items, and ‘CRs’ to denote correctly rejected new items. The second scanning session concluded with diffusion tensor (DTI) and high resolution T1-weighted scans. Study and test instructions emphasized the need for both accuracy and speed.
Figure 1.
Schematic overview of the study and test procedures.
In both the study and test phases, word pairs were presented for a duration of 2 s, one word above and one word below a central fixation character. The words were presented in white uppercase Helvetica 30 point font against a black background. Test pairs were preceded by a red fixation cross that was presented for 0.5 s and were followed by a white fixation cross for 2 s. Responses were accepted from300 ms after word pair onset until 2.5 s after the pair's offset. Null trials consisted of the presentation of a white fixation cross against a black background for 4.5 s.
2.5 MRI data acquisition
A Philips Achieva 3T MR scanner (Philips Medical System, Andover, MA USA) equipped with a 32 channel head coil was used to acquire functional and anatomical images. A 3D MP-RAGE pulse sequence (FOV= 256×224, voxel size 1×1×1 mm, 160 slices, sagittal acquisition) was employed for T1-weighted anatomical image acquisition. Functional scans were acquired with a T2*–weighted echo-planar image (EPI) (TR 2 s, TE 30 ms, flip angle 70°, FOV 240×240, matrix size 80×78). Each functional volume comprised 33slices (3 mm thickness, 1 mm inter-slice gap) with an in-plane resolution of 3×3 mm. Slices were oriented parallel to the AC-PC line, acquired in ascending order, and positioned for full coverage of the cerebrum and most of the cerebellum. Functional data were acquired with a sensitivity encoding (SENSE) reduction factor of 2.fMRI data were acquired during both the study and test phases (351 volumes for each test block). The first five volumes of each block were discarded to allow tissue magnetization to achieve a steady state. For the general linear model (GLM) analyses, test sessions were concatenated to form a single time-series prior to model estimation.
2.6 MRI data analysis
We report fMRI analyses for the test phase only. The analyses were conducted with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK), run under Matlab R2008a (MathWorks). Functional images were motion and slice-time corrected, realigned, and spatially normalized using a sample-specific template based on the MNI reference brain (Cocosco et al. 1997) (see de Chastelaine et al., 2015, for further details). Normalized volumes were resampled into 3 mm isotropic voxels and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. T1 anatomical images were normalized with a procedure analogous to that applied to the functional images, and were employed to define anterior hippocampal and perirhinal regions of interest (ROIs; see below), and tolocalize and depict the functional results. For the functional data, item-elicited neural activity was modeled in each participant by a delta function and convolved with 2 hemodynamic response functions (HRFs). These functions consisted of a canonical HRF (Friston et al., 1998) and an orthogonalized, delayed HRF (Andrade et al. 1999), the latter generated by shifting the canonical HRF one TR (2s) later in time. The results obtained for the late HRF added little of theoretical significance to the findings obtained with the canonical function and are not reported here.
fMRI data were analyzed in two stages. First, a separate GLM was constructed for each participant. The four events of interest were associative hits, associative misses, rearranged hits and CRs – respectively, the median number (and range) of trials for these events were101 (42-114), 35 (12-85), 53 (20-76) and 54 (25-77) for the young adults, 102 (30-134), 41 (14-78),45 (18-63) and 51 (25-76) for the middle-aged adults, and 97 (25-142), 38 (11-97), 38 (11-68) and 50 (17-77) for the older adults. Intact pairs wrongly judged as new were also separately modeled. All other events, including incorrect responses to rearranged items, false alarms to new pairs, fillers, and trials where a response was not given, were modeled as events of no interest. Additionally, the three 30s breaks interposed during the test list were modeled. Six regressors representing motion-related variance (three for rigid-body translation and three for rotation), and constants representing means across each scan session, were also included. Data from volumes showing a transient displacement > 1mm or > 1 degree in any direction were eliminated by assigning them as covariates of no interest when estimating item-related effects. The time series in each voxel were high-pass filtered to 1/128 Hz and scaled within session to a grand mean of 100 across voxels and scans. In the second stage of the fMRI analysis, participant-specific parameter estimates for the four events of interest (associative hits, associative misses, rearranged hits and CRs) were taken forward to a 3 (age group) × 4 (item type) mixed-design ANOVA model as implemented within SPM8 (and hence employing a single pooled error term).
In line with previous fMRI investigations that compared activity for familiar with that for novel items(e.g., Henson et al., 1999), fMRI effects associated with familiarity were operationalized by greater BOLD signal for recognized but unrecollected word pairs(associative misses) than for correctly endorsed unstudied pairs (CRs). Associative misses are assumed to have been wrongly endorsed as rearranged on the basis of an above-criterion familiarity signal accompanied by a weak or absent recollection signal (see also de Chastelaine et al. 2016a). By contrast, CRs are assumed to be associated with absent recollection and low (sub-criterion) familiarity strength. There verse contrast (CRs > associative misses) was employed to operationalize novelty effects (see Introduction). A trial type that might be thought to be as, or more, appropriate for these contrasts are the rearranged hits, which can also be correctly identified on the basis of familiarity alone. As we previously argued, however(de Chastelaine et al., 2016a), correct identification of rearranged pairs can be supported by a recollection-based, ‘recall-to-reject’ strategy (Rotello and Heit, 2000; Yonelinas and Parks, 2007). Recall-to-reject has been reported to be more prevalent in young than in older participants (Cohn et al., 2008), raising the possibility of a confound between age and the employment of this strategy.
A concern with the contrast we selected to identify familiarity effects (associative hits > CRs) is that the contrast might also capture activity associated with successful recollection. This is because even when recollection of the word pair association failed, it is possible that other aspects of the study episode were nonetheless recollected. We examined this issue by exclusively masking the main effect of familiarity with a contrast selective for recollection (associative hits > associative misses). Even at a liberal mask threshold of p < .05 uncorrected, the results of the familiarity contrast were almost unchanged and, crucially, the effects documented in Table 3 and below were unmodified. These findings provide reassurance that the present familiarity effects are ‘contaminated’ by recollection to only a minor extent, if at all.
Table 3.
Peak voxels and corresponding Brodmann Areas (BAs) of the across-group main effect of familiarity, exclusively masked by the 2-sided age group by item type contrast.
| Coordinates | Peak Z | No. of above-threshold voxels | Region | BA | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| -48 | 26 | 28 | Infinite | 2308 | Left lateral anterior PFC | 46 |
| -12 | 5 | 4 | 7.69 | 143 | Left caudate nucleus | |
| -3 | -31 | 28 | 5.96 | 31 | Left posterior cingulate cortex | 23 |
| -60 | -52 | -17 | 5.49 | 38 | Left inferior temporal gyrus | 20 |
| -36 | -58 | 40 | Infinite | 1793 | Left IPS | 40 |
| 0 | -25 | -29 | 4.96 | 11 | Pons | |
| 51 | 32 | 22 | 5.05 | 10 | Right lateral anterior PFC | 46 |
| 15 | 14 | -2 | 7.72 | 101 | Right caudate nucleus | |
| 33 | 8 | 55 | 5.74 | 76 | Right superior frontal gyrus | 6 |
| 45 | -46 | 40 | 5.51 | 187 | Right IPS | 40 |
| 30 | -67 | -35 | 7.66 | 44 | Right cerebellum | |
To examine fMRI novelty effects in MTL regions that were of interest a priori (see Introduction), we relied primarily on the averaging of parameter estimates across voxels falling within anatomically defined regions of interest (ROIs)that encompassed the perirhinal cortex and, separately, theanterior hippocampus, in each hemisphere. The ROIs (see Figure 2) were manually traced on the across-group average T1 anatomical template (see above) using the protocols specified by Insausti et al. (1998) for perirhinal cortex and the EADC-ADNI harmonized Protocol for Hippocampal Segmentation (Boccardi et al., 2015) for the hippocampus. In accord with the proposal of Poppenk et al. (2013), the anterior hippocampus was defined as the portion of the hippocampus anterior to y = -21 in MNI space. The perirhinal and hippocampal masks were traced conservatively so as to eliminateoverlap between the respective ROIs. Importantly, we ensured that, after the ROIs had been resliced and smoothed to approximate the smoothness of the functional data, no voxels were shared between the perirhinal and hippocampal masks. The data from each ROI were included in a 3 (age group) × 2 (region) × 2 (hemisphere) × 2 (item type) ANOVA to assess whether novelty effects interacted with age group and whether the profile of novelty effects across the two MTL regions or hemispheres differed. Using the difference between the parameter estimates (CR - associative misses), we also conducted a multiple regression analysis to examine the relationship between novelty effects and memory performance. To minimize the number of such analyses, we averaged the parameter estimates across the four ROIs (see Inline Supplementary Table 1 for results for the individual ROIs). The initial regression model included item memory performance (‘Pr’ – see section 3.2 ‘Behavioral results’ below) as the dependent variable, and age group, RT differences between associative misses and CRs (see section 3.2 ‘Behavioral results’), the fMRI novelty effect, and the age group × fMRI novelty effect interaction term as predictor variables. The interaction term was far from statistically significant, and therefore it was dropped from the model reported below. For the sake of brevity, we report the outcome of this regression analysis, and also the analogous analysis of the familiarity effects (see below), solely interms of the relationship between the respective fMRI effects and memory performance. We use the partial correlation coefficient as a measure of the strength of the relationship.
Figure 2.
Representative sagittal and coronal sections of the across-participants mean T1-weighted structural image showing manual tracings of the anterior hippocampus (red ROIs) and perirhinal cortex (blue ROIs).
In addition to the ROI analyses described above, we conducted whole brain analyses to identify familiarity (associative misses > CRs) and novelty (CRs > associative misses) effects common to the three age groups, as well as to identify regions where either of these classes of effects differed between groups. Common effects were identified by exclusively masking the across-group main effects of familiarity and novelty, each thresholded at p < 0.05 after FWE correction, with the two-sided(associative misses versus CRs) age group x fMRI interaction contrast, liberally thresholded at p < 0.05 uncorrected. Regions demonstrating age-related differences in familiarity or novelty effects were sought in two ways. First, with the (2-sided) age group × fMRI effect interaction contrast from the aforementioned ANOVA model, thresholded at p < 0.05 after FWE correction. Second, we performed an analysis targeted on regions that demonstrated a main effect of novelty or familiarity. This was achieved by using the main effects (thresholded at p < .05, FWE corrected) of familiarity and novelty as masks, and performing a small volume correction (p < 0.05, FWE corrected) within the mask for the group x item interaction effect, thus identifying voxels where the interaction was reliable. For all contrasts, we applied acluster extent threshold of k > 9 to remove clusters deemed too small to be of theoretical interest. Note that because these analyses were corrected for FWE at the voxel-wise level, the cluster threshold plays no formal role in establishing the statistical significance of the findings.
We also assessed whether fMRI familiarity effects covaried with item memory performance, and whether any of these relationships varied according to age group. Parameter estimates were extracted from the across-group main effect of familiarity (thresholded at p < 0.05, FWE correction) within regions that have consistently been reported previously as being sensitive to similar contrasts (Kim, 2010, 2013; see Introduction).These regions comprised left IPS, left precuneus, left lateral anterior PFC, left dorsal PFC and bilateral caudate(see Table 1 for a list of the regions along with their respective coordinates).For each participant, mean parameter estimates were extracted across all voxels within a 5 mm radius of the cluster peak from every region except for the caudate, where data were extracted from voxels within a 3 mm radius of the cluster peaks. As for the MTL ROIs, the data were collapsed across the 6 familiarity-sensitive regions (the results of regression analyses conducted on the data from each individual region are reported in the Inline Supplementary Table 2). The regression model included Pr as the dependent variable, and age group, RT differences, the fMRI familiarity effect, and the age group × familiarity effect interaction term as predictor variables. As was the case for the analyses of the MTL novelty effects described above, the interaction term was not significant and was dropped from the final model.
Table 1.
Peak coordinates and corresponding Brodmann Areas (BAs) of the 6 familiarity-sensitive ROIs described in the main text that were selected to assess whether fMRI familiarity effects covaried with item memory performance.
| Coordinates | Region | BA | ||
|---|---|---|---|---|
|
| ||||
| x | y | z | ||
| -6 | 29 | 43 | Left dorsal mPFC | 8 |
| -48 | 29 | 22 | Left lateral anterior PFC | 46 |
| -12 | 8 | 4 | Left caudate | |
| -36 | -58 | 40 | Left IPS | 40 |
| -6 | -76 | 43 | Left Precuneus | 7 |
| 12 | 11 | 1 | Right caudate | |
Since each of the two principal multiple regression analyses yielded three partial correlations (one each for the variables of age group, RT differences, and the fMRI effect of interest) we Bonferroni corrected for a total of six comparisons to ensure a family-wise error rate of p < 0.05 (corresponding to a correlation-wise significance level of p < 0.008).
2.7 Visualization of fMRI findings
Caret software (Van Essen et al., 2001) was used to map fMRI effects of interest on to inflated fiducial brains derived from the PALS-B12 atlas (Van Essen 2002, 2005) in SPM5 space. Results were also presented on sections from the across-groups averaged T1 structural image.
3 Results
3.1 Neuropsychological data
Full details of the demographic and neuropsychological data for the three age groups can be found in previous publications (de Chastelaine et al., 2015, 2016a; 2016b). The pattern of significant and non-significant age effects on the test scores was typical of that reported in numerous previous studies employing similar participant samples. Specifically, equivalent performance for the 3 age groups was found on tests that are typically preserved with age (Digit Span, Letter and Category Fluency, and the WTAR, a measure of crystallized intelligence), while composite recall of word lists was significantly lower in the older, but not in the middle-aged group, compared to the younger participants. Older participants also demonstrated poorer performance on tests of speeded cognition relative to both the younger and middle-aged groups. Performance of both older and middle-aged participants was lower than that for younger participants on a test of fluid intelligence (Raven's Matrices), while performance on a test of crystallized intelligence (WTAR) was age-invariant. Older adults had significantly more years of education than younger adults (15.5 vs 17.1 yrs, respectivcly), but years of education did not differ significantly for the middle-aged and either of the two other age groups. The single age-group difference in years of education likely reflects the fact that a high proportion of the young group had not yet completed college.
3.2 Behavioral results
A summary of performance on the associative recognition test for each age group is shown in Table 2. As reported previously (de Chastelaine et al., 2015, 2016a, 2016b; see also King et al., 2015), our measure of recollection accuracy was the difference between the proportion of intact test pairs correctly endorsed as intact (associative hits) and the proportion of rearranged test pairs incorrectly judged intact (associative false alarms). Mean (SD) recollection scores were 0.48 (0.19), 0.39 (0.14) and 0.31 (0.15) for the young, middle-aged and older groups, respectively, and a one way ANOVA revealed that these means differed significantly (F2, 135 = 12.80, p <0.001). Pair-wise contrasts (t-tests, equal variances not assumed) revealed a graded decline with age: young participants were more accurate than both middle-aged (t64 = 2.25, p = 0.028) and older participants (t59 = 4.50, p <0.001), and middle-aged participants were more accurate than the older participants (t76 = 2.60, p = 0.012).
Table 2.
Mean proportions (SD) of intact, rearranged, and new test pairs given intact, rearranged, and new responses in each age group. Correct responses are highlighted in bold.
| Young | Middle-aged | Older | ||
|---|---|---|---|---|
| Intact responses | ||||
| Intact pairs | 0.63 (0.17) | 0.63 (0.14) | 0.56 (0.16) | |
| Rearranged pairs | 0.15 (0.11) | 0.24 (0.12) | 0.25 (0.13) | |
| New pairs | 0.03 (0.06) | 0.06 (0.07) | 0.07 (0.07) | |
| Rearranged responses | ||||
| Intact pairs | 0.26 (0.12) | 0.27 (0.11) | 0.29 (0.13) | |
| Rearranged pairs | 0.64 (0.16) | 0.55 (0.15) | 0.49 (0.16) | |
| New pairs | 0.29 (0.15) | 0.30 (0.16) | 0.30 (0.16) | |
| New responses | ||||
| Intact pairs | 0.11 (0.09) | 0.10 (0.09) | 0.14 (0.09) | |
| Rearranged pairs | 0.20 (0.11) | 0.21 (0.13) | 0.26 (0.12) | |
| New pairs | 0.68 (0.17) | 0.64 (0.19) | 0.63 (0.18) | |
We computed two estimates of familiarity-based discrimination. The first of these, pF, was derived from the independence assumption underlying dual-process theories of recognition memory (Yonelinas and Jacoby, 1995) and was estimated as: (p associative miss)/(1- p associative hit) - (p rearranged response to new items)/(1 - p intact response to new items). While pF has been argued to be a relatively ‘process pure’ measure of familiarity strength when the independence assumption holds, the measure can be unstable when the proportions of recollection- and familiarity-based responses are highly unequal (Yonelinas, 2002). This was the case in the present experiment for a substantial number of our participants and, indeed, it led to the exclusion of one of our older participants who had a markedly outlying score (>3 SDs from the group mean). Our other familiarity estimate (Pr) was a simple measure of item memory that indexed discriminability of old and new word pairs, and reflected the difference between the probability of endorsing any test pair containing studied items as intact or rearranged and the probability of incorrectly endorsing a new pair as either intact or rearranged (for both measures, chance performance is 0). Compared to pF, Pr is a less well-motivated measure from a theoretical standpoint, but it is a more stable one given that it is derived from all categories of test trials and does not depend for its estimation on probability ratios. While we report behavioral analyses of both pF and Pr, regression analyses investigating the relationships between fMRI effects and memory performance reported below focused on Pr. These analyses gave rise to very similar, albeit slightly weaker, results when pF was employed instead (see Inline Supplementary Tables 3-4). This is unsurprising given that the two estimates of familiarity strength were strongly correlated (r = 0.861, p <0.001).
Mean (SD) pF was 0.43 (0.18), 0.41 (0.16) and 0.35 (0.16) for the young, middle-aged and older groups respectively. One-way ANOVA indicated that these means did not differ significantly (F2, 135 = 2.98, p = 0.054).Nonetheless, in view of our a priori interest in the effects of age on this memory index, we conducted pair-wise t-tests(equal variances not assumed) between the means. These tests indicated that young participants had significantly higher scores than older participants (t68 = 2.20, p = 0.031), whereas the scores of the middle-aged participants did not differ from those of either the young or older groups (both ps > 0.09). Mean (SD) Prwas0.54 (0.17), 0.50 (0.16) and 0.45 (0.16) for the young, middle-aged and older groups respectively, and a one-way ANOVA indicated that these means differed significantly (F2, 135 = 3.49, p = 0.026).Follow-up pair wise tests (equal variances not assumed) indicated that young participants were more accurate than older participants (t68 = 2.49, p =0.015), where as middle-aged participants did not differ in their Prscores from either the young or older groups (both ps >0.1).
RTs for associative misses and CRs were subjected to a 3 (age group) × 2 (item type) mixed design ANOVA. This revealed a main effect of item type (F1,133 = 122.93, p < 0.001), indicating faster responses to CRs than to associative misses. Collapsed across age group, mean RTs (SDs) were 1998 (401) ms and 2235 (398) ms, respectively. There were no effects of age group, nor was there an age group by item type interaction (Fs< 1.2).Importantly, the RT difference between associative misses and CRs correlated positively across participants with Pr(after controlling for age group, partial r = 0.441, p < 0.001). Therefore, to remove possible confounding effects of RT differences on relationships between fMRI familiarity/novelty effects, age and Pr, we included RT differences cores as a covariatein each of the multiple regression analyses reported below. The difference score for one young participant was an extreme outlier, (> 4 SDs from the group mean), and was replaced by the mean score of the remaining participants.
3.3 fMRI data
3.3.1 MTL: Anatomical ROI analyses
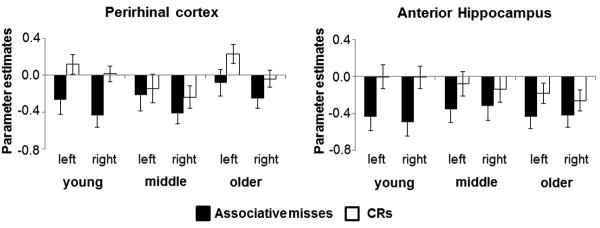
Figure 3 illustrates the mean parameter estimates from bilateral perirhinal and anterior hippocampa lROIs (see section 2.6 ‘MRI data analysis’)for associative misses and CRs according to age group. A 3 (age group) × 2 (region) ×2 (hemisphere) × 2 (item type) mixed design ANOVA revealed a main effect of item type (F1,133 = 22.11, p < 0.001), indicating greater activity for CRs relative to associative misses (‘novelty effects’). The ANOVA failed to reveal any significant interactions involving the factors of age-group and item type (max F = 1.98, p = 0.142). An ANCOVA conducted on the same data, controlling for the effects of Pr, also failed to reveal any interactions involving the age-group and item type (max F = 1.42, p = 0.244).1
Figure 3.
Parameter estimates (arbitrary units) and standard errors for associative misses and CRs from perirhinal and anterior hippocampal ROIs.
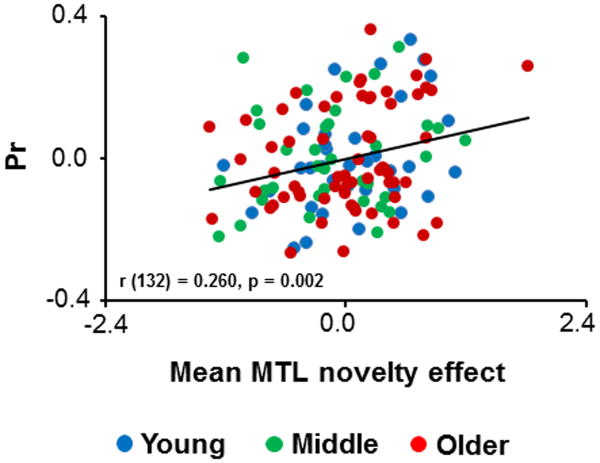
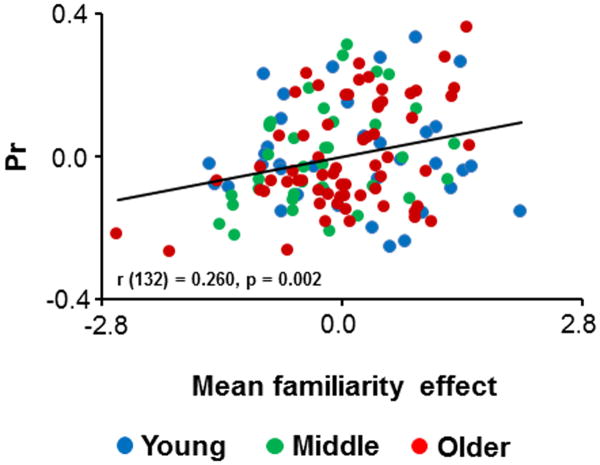
Multipleregression revealed a significant positive relationship between MTL fMRI novelty effects and Pr(partial r = 0.260, p = 0.002). The partial plotillustrating this relationship is depicted in Figure 4.
Figure 4.
Partial plot showing the relationships across participants between mean MTL novelty effects and Pr.
3.3.2 Whole brain analyses
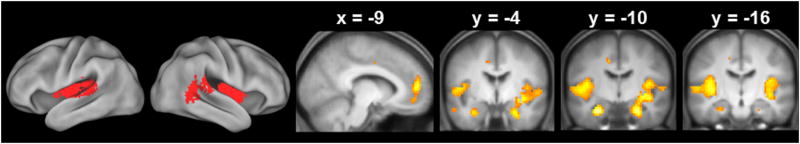
The across-group main effect of familiarity (associative misses>CRs), masked exclusively by the age group × item type interaction contrast, identified effects common to the three age groups in several regions previously identified as familiarity-sensitive (Figure 5 and Table 3). These regions included the IPS, precuneus, lateral anterior PFC, dorsal mPFC and caudate nucleus.
Figure 5.
Clusters demonstrating familiarity effects common to the 3 age groups. The effects in this and the following figures are superimposed on the bilateral surfaces of a standardized brain (PALS-B12) atlas using Caret 5, and on sections of the across-groups mean T1-weighted structural image.
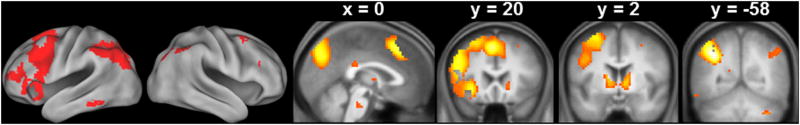
As Figure 6 shows, the across-group main effect of novelty (CRs > associative hits), exclusively masked with the age group × item type interaction contrast, identified robust age-invariant novelty effects bilaterally within the MTL (including anterior hippocampus and perirhinal cortex, consistent with the ROI analyses reported above), as well as in the temporoparietal junction (TPJ) and insula (see Table 4 for MNI coordinates of the peak voxels).
Figure 6.
Clusters demonstrating novelty effects common to the 3 age groups.
Table 4.
Peak voxels and corresponding Brodmann Areas (BAs) of the across-group main effect of novelty, exclusively masked by the 2-sided age group by item type contrast.
| Coordinates | Peak Z | No. of above-threshold voxels | Region | BA | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| -9 | 53 | 13 | 6.54 | 298 | Left ventral mPFC | 10 |
| -12 | -7 | 43 | 5.28 | 13 | Left middle cingulate cortex | 24 |
| -27 | -10 | -23 | 7.45 | 72 | Left hippocampus | |
| -18 | -31 | 43 | 5.06 | 14 | Left posterior cingulate cortex | 31 |
| -60 | -34 | 16 | Infinite | 777 | Left TPJ | 42 |
| -42 | -34 | 22 | 6.81 | sub-peak | Left insula | 13 |
| 24 | -10 | -23 | 7.40 | 887 | Right hippocampus | |
| 36 | -7 | -5 | 6.89 | sub-peak | Right insula | 13 |
| 60 | -37 | 19 | 6.83 | sub-peak | Right TPJ | 46 |
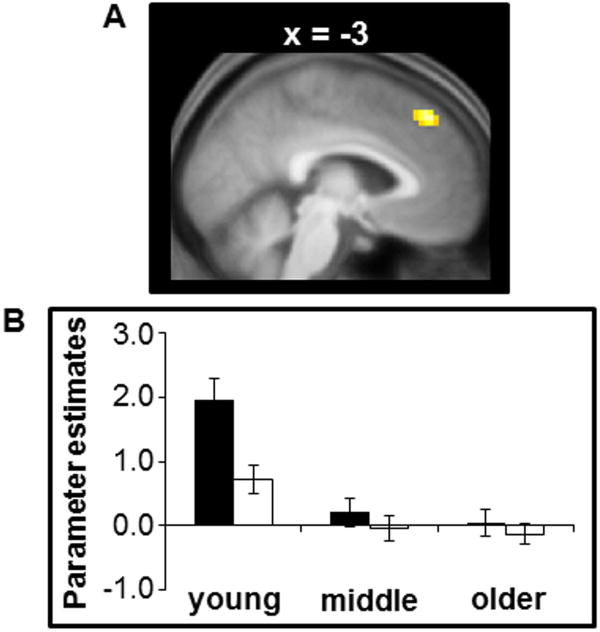
The whole-brain age group by item type interaction contrast failed to identify any significant voxels. There were also null effects when the interaction contrast was masked by the main effect of novelty and the threshold adjusted accordingly (see section 2.6 ‘MRI data analysis’). However, when the interaction contrast was masked by the main effect of familiaritya single cluster was identified in dorsal mPFC (MNI coordinates: -3, 41, 46; peak Z = 4.14; Figure 7). Parameter estimates for associative misses and CRs were extracted from a spherical ROI around the peak voxel and subjected to a 3 (group) × 2 (item type) ANCOVA model to determine if the group × item type interaction remained after controlling for individual differences in Pr; the interaction remained significant (F2,132 = 8.91, p < 0.001). Follow up ANCOVAs indicated that familiarity effects in the dorsal mPFC were larger in the young group relative to both the middle-aged (F1,69 = 12.83, p < 0.001) and the older groups (F1,97 = 12.57, p < 0.001), while there were no significant differences between the middle-aged and older groups (p > 0.8). Further analyses indicated that familiarity effects in this region were significantly different from zero in the young group (t35 = 5.69, p < 0.001) but not in the 2 older groups (ps > 0.09).2
Figure 7.
(A) Cluster within the dorsal mPFC demonstrating a group by item interaction. (B) Mean of the parameter estimates (arbitrary units) for associative misses and CRs averaged across all voxels within a 5 mm radius of the peak voxel of the illustrated cluster.
3.3.3 Relationships between fMRI familiarity effects and Pr
As was described in the section 2.6 ‘MRI data analysis’, to investigate the relationships between fMRI familiarity effects, age, and item memory (Pr) mean parameter estimates for associative misses and CRs were extracted from ROIs centered on selected regions identified by the across-group main effect of familiarity (Table 1). Multiple regression identified a positive relationship between the mean of these fMRI familiarity effects and Pr(partial r = 0.260, p = 0.002; see Figure 8).
Figure 8.
Partial plot showing the relationships across participants between mean familiarity effects and Pr.
At the request of a reviewer, the parameter estimates from the dorsal mPFC region where a group by item type interaction was identified (see above) were also employed in a regression analysis. As for the previous regression analyses, the interaction term for this region was not significant and was therefore dropped from the model. The final regression model revealed a positive relationship between the fMRI effects and Pr(partial r = 0.208, p = 0.016).
3.3.4 Independence of the relationship between fMRI familiarity and novelty effects and Pr
The analyses reported above indicate that, after controlling for age group and RT differences, anterior MTL novelty effects and familiarity effects in the neocortex and striatum correlated positively with Pr. In a final regression analysis, we examined whether these two classes of effects accounted for independent fractions of the variance in item memory performance. Thus, we included Pr as the dependent variable, and entered age group, RT differences and mean novelty and familiarity effects as predictor variables. The novelty and familiarity effects independently accounted for significant proportions of the variance (partial r = 0.312, p < 0.001, in both cases).
4 Discussion
4.1 Behavioral findings
As was described in the Results section, we computed two estimates of familiarity-based discrimination. One of these, pF, was based on the independence assumption underlying dual-process theories of recognition memory (Yonelinas and Jacoby, 1995), and arguably is a ‘process pure’ measure of familiarity strength when that assumption holds. The other estimate of familiarity (Pr) was a simple measure of item memory that indexed discriminability between old and new item pairs. pF and Pr (which were strongly correlated across participants) were both lower in older than in young participants, as was an estimate of associative recollection, pR. In keeping with the prior literature (see Introduction), the effect size for age on pF was modest (eta squared = 0.043), and almost four times smaller than the effect size for recollection (eta squared = 0.162). The effect size for Pr (0.050) was very similar to that for pF. These findings suggest that pF and Pr both reflect a memory signal that, unlike associative recollection, is only modestly affected with increasing age. We propose that, like pF, Pr is primarily a reflection of familiarity strength.
It is noteworthy that differences in RTs between associative misses and correct rejection judgments correlated positively across participants not only with fMRI familiarity effects, but also with memory performance (as indexed either by Pr, partial r = 0.441,or pF, partial r = 0.293). These observations motivated us to employ these RT differences as a covariate in regression models examining relationships across participants between fMRI effects and performance, obviating the possibility that there lationships were mediated by the RT effects. It is noteworthy that the correlation between RT differences and familiarity strength was mirrored by an equally strong positive correlation between RT differences and the correct rejection rate (r = 0.455, p <0.001). We take this as evidence that apossible determinant of familiarity-driven memory performance in the present study was the cognitive resources and processing time allocated to the detection of new (novel) test pairs.
4.2 fMRI findings
4.2.1 Perirhinal and hippocampal novelty effects
As was demonstrated both by analysis of fMRI activity in anatomically defined ROIs, and by whole brain analyses, robust novelty effects were evident in bilateral anterior hippocampus and perirhinal cortex. The magnitude of the effects did not significantly vary across age groups (although see Footnote 1), adding weight to prior evidence that, as indexed with fMRI, novelty responses in the anterior MTL are preserved with increasing age (Angel et al., 2013; Daselaar et al., 2006b; Duarte et al., 2010; Moriguchi et al., 2011; Wang and Giovanello, 2016; Wang et al., 2015). In addition, the across participants correlation that was identified between item memory performance and MTL novelty effects was also age-invariant. Therefore, as in the case of the familiarity effects discussed below, it appears that any mnemonic contributions of the processes reflected by these effects are largely stable across the lifespan.
The nature of these contributions is uncertain. According to one perspective (e.g., Johnson et al., 2008; Nyberg, 2005), novelty effects in perirhinal cortex reflect the role of the region in supporting item familiarity, while effects in the hippocampus reflect its role in the encoding of novel item-context associations. However, the finding that perirhinal and hippocampal novelty effects were similarly correlated across participants with Pr (see Inline Supplementary Table 1) could be taken as evidence that the effects in the two regions reflect a common process, such as detection of experimentally novel items (a process that would obviously benefit the ability to discriminate between studied and unstudied test pairs). It is important to note however that the hippocampus is very likely functionally heterogeneous along its longitudinal axis; whereas anterior hippocampus demonstrates strong functional and anatomical connectivity with perirhinal cortex, this is not the case for more posterior hippocampal regions, where connectivity with parahippocampal cortex predominates (see Strange et al., 2014 and Poppenk et al., 2013, for reviews). Thus, the present findings of seemingly shared functional properties between anterior hippocampal and perirhinal novelty effects may not extend to mid- and posterior hippocampus. In support of this possibility it is noteworthy that, even at a very liberal threshold (p < 0.05 uncorrected), hippocampal novelty effects were undetectable in middle and posterior hippocampus (that is, posterior to MNI coordinate y = -21, which has been proposed as the border between anterior and mid-hippocampus; Poppenk et al., 2013).
4.2.2 Familiarity effects
In a replication of numerous prior studies (see Introduction), robust familiarity effects were evident in medial and lateral parietal cortex (precuneus and IPS), dorsal medial and left lateral PFC, and bilateral caudate, among other regions. Evidence for the influence of age on these effects was sparse: with the exception of effects in a single cluster located in dorsal mPFC, all of the effects were age-invariant at our chosen level of statistical significance. However, in contrast to recollection-related activity in the core recollection network, where age effects appear to be almost entirely absent (de Chastelaine et al., 2016a; Wang et al., 2016), familiarity-related cortical activity does seem to vary across the healthy adult lifespan, albeit in only a single region.
The present findings are largely consistent with those from prior studies (see Introduction). As was noted in the Introduction, null effects of age on familiarity-related activity were reported in one study (Daselaar et al., 2006b), while in two others (Angel et al., 2013; Duarte et al., 2010) familiarity effects were broadly similar between young and older age groups, but were attenuated in older individuals in some regions. A strong point of convergence between the present study and those two prior studies comes from the common findings of age-related attenuation of familiarity effects in dorsal mPFC, albeit with some variability between studies in the precise loci of the effects. As in Angel et al. (2013), here the effects in the dorsal mPFC cluster demonstrating the interaction with age were reliable in the young group only. Age-related attenuation of these effects remained statistically significant after controlling for individual differences in memory performance, suggesting that the influence of age was not secondary to group-wise differences in performance (cf. de Chastelaine et al., 2016a).
The functional significance of the age-related attenuation in dorsal mPFC familiarity effects is uncertain, not least because the role of the region in familiarity-driven memory judgments is unknown. There is some evidence however that the mPFC plays a necessary role in such judgments. Using an ROC (receiver operating characteristic) approach to estimate the relative contributions of familiarity and recollection to recognition memory judgments, MacPherson et al. (2008) reported that both lateral and ‘medial’ PFC lesions (the loci of the lesions were not specified further) were associated with a selective deficit in familiarity strength (see Duarte et al., 2005, for additional lesion evidence implicating PFC in familiarity-driven recognition memory). Thus, in as much as aging is associated with a reduction in familiarity strength (see Introduction and above), the convergence of the lesion evidence and the fMRI findings reported here and by Angel et al. (2013) and Duarte et al. (2010) suggest that age-related decline in dorsal mPFC function might play a role in this reduction.
The present findings also suggest however that this functional decline is not confined to older individuals: relative to the young group, dorsal mPFC familiarity effects were attenuated (and statistically non-significant) in our middle-aged participants also, even though behavioral estimates of familiarity strength in these participants did not significantly differ from those of the young group. The dissociation between these neural and behavioral findings is open to several interpretations. For example, it might indicate that the fMRI familiarity effects evident in this region in young adults do not, in fact, reflect processes necessary for familiarity or item memory. Alternately, it could indicate that dorsal mPFC impairment must be combined with dysfunction in additional regions (not detected in the present study) before a reduction in memory performance is manifest. Finally, the dissociation might have come about merely because our memory measures were insufficiently sensitive to detect a subtle impairment in the middle aged group. Importantly, the finding that the magnitude of dorsal mPFC familiarity effects covaried across participants with Pr in an age-invariant manner suggests that, regardless of the impact of age on the region, the dorsal mPFC continues to play a similar role in familiarity-based recognition memory with increasing age.
The magnitude of the familiarity effects across the six regions that we selected for further analysis correlated across participants with item memory performance (Pr). This finding is consistent with prior evidence that retrieval-related activity in these regions tracks familiarity strength (Daselaar, 2006a; Johnson et al., 2013; Scimeca et al., 2016; Yonelinas et al., 2005). As was already noted during discussion of the dorsal mPFC above, the precise functional roles of these and other familiarity-related regions remains to be established, as does the question of whether they should be considered to belong to a common functional network. Regardless of these issues, it is significant that the relationship between these familiarity effects and memory performance was age-invariant. While caution is required when interpreting null findings, it appears that neural regions that have been implicated in familiarity-driven recognition memory in young adults support processes with a functional relationship to familiarity strength that continues in to later life.
4.2.3 Cortical novelty effects
In addition to the effects in anterior MTL discussed above, robust novelty effects were identified in several other regions, including ventral mPFC and bilateral TPJ and insula (Figure 6). The effects in the ventral mPFC likely reflect its strong functional and structural connectivity with the anterior hippocampus, and are consonant with proposals that the two regions can act synergistically to support mnemonic processing (Preston and Eichenbaum, 2013). By contrast, the TPJ novelty effects seem likely to reflect the well-documented role of the region in ‘bottom-up’ or ‘reflexive’ attentional orienting (Corbetta et al., 2008). In the present study, unstudied words pairs were both relatively rare (25% of all test items) and highly salient, in that their detection obviated the requirement to make an ‘intact/rearranged’ discrimination. Together, these two characteristics likely led new pairs to act as ‘oddball’ stimuli, eliciting enhanced TPJ activity (Corbetta et al., 2008). Insula activity has previously been reported to co-vary negatively with familiarity strength (e.g.,Montaldi et al., 2006; Yonelinas et al., 2005). The functional significance of this result is uncertain, although Montaldi et al. (2006) conjectured that the region might play a role in the subjective experience of novelty.
4.2.4 Functional independence of familiarity and novelty effects
Familiarity and novelty effects robustly and independently explained variance across participants in item memory when the effects were entered as predictors in to a singlemultiple regression model. Consistent with the results reported by Daselaar et al. (2006a; see also Kafkas and Montaldi, 2014), these findings suggest that familiarity and novelty effects reflect functionally distinct processes that make independent contributions to item memory. Further evidence that these effects are functionally distinct comes from the finding that, across participants, the two classes of effects demonstrated strong within-effect correlations (mean correlations of 0.45 for the 4 MTL novelty ROIs, and 0.54 for the 6familiarity ROIs, controlling for age and RT differences), but much weaker correlations between the two classes of effects (mean correlation = -0.08). A clear challenge for future research is to gain a more complete understanding of the mnemonic processes reflected by each class of effect, and equally important, to elucidate the specific functional roles of the different regions manifesting the effects.
4.3 Limitations
The present study has a number of limitations. One of these is a consequence of our fMRI acquisition protocol. Whereas this allowed us to examine memory-related neural activity across the whole brain, the spatial resolution that it afforded (3mm isotropic voxels) is not optimal for separating activity in closely adjacent MTL structures such as anterior hippocampus and perirhinal cortex. While we took considerable care to ensure that anterior hippocampal and perirhinal ROIs did not overlap (see 2.6 ‘MRI data analysis’), we cannot entirely rule out the possibility that the similarity in the functional properties of the novelty effects in the two regions was partially a consequence of a failure to fully separate their respective BOLD responses.
A second caveat stems from our employment of a cross-sectional design to examine the effects of age. Such designs provide no insight into the extent to which age-related differences in memory performance or their neural correlates are a reflection of aging (within-participant decline over time) rather than age-correlated factors such as birth cohort (Baxendale, 2010; Nyberg et al., 2010; Rönnlund and Nilsson, 2009; Rugg, 2016). Thus, our (and others') finding of an age-related decline in dorsal mPFC familiarity effects cannot unambiguously be interpreted as a consequence of aging.
Finally, we note that our conclusion that age has relatively little impact on fMRI familiarity and novelty effects rests on the assumption that the transfer function mediating the relationship between neural activity and the fMRI BOLD signal is age-invariant. There is evidence however that this assumption is invalid. It has been reported that cerebrovascular reactivity (CVR) – an important non-neural determinant of BOLD signal magnitude – declines monotonically with increasing age (e.g., Lu et al., 2011), and that correcting for this decline can modify the outcomes of across-age contrasts (e.g., Liu et al., 2013). It will be important in future studies to incorporate procedures that permit individual differences in CVR to be assessed and corrected. That being said, it should be noted that this issue is unlikely to have impacted our finding of age-invariant relationships between the magnitudes of the fMRI effects and memory performance. Indeed, as was originally noted by de Chastelaine et al. (2016b), if it is assumed that individual variation in CVR is largely uncorrelated with memory performance, correcting for the variation would, if anything, lead to a strengthening of these relationships.
4.4 Concluding comments
The present findings extend prior reports of the effects of age on fMRI familiarity and novelty effects in a several ways. The findings are consistent with several previous reports that hippocampal and perirhinal novelty effects are age-invariant, and extend these reports to an associative recognition paradigm. In addition, we demonstrate that previous findings that dorsal mPFC familiarity effects are attenuated in older participants extend to middle-aged individuals. We also report that the magnitudes of familiarity effects in a variety of cortical regions, as well as anterior MTL novelty effects, co-vary across participants with an index of familiarity strength in an age-invariant manner. Finally, we report that the two classes of effect explain independent fractions of the variance across participants in familiarity strength. Together, the findings suggest that fMRI familiarity and novelty effects are more sensitive to individual differences in familiarity-based memory performance than they are to chronological age.
Supplementary Material
Acknowledgments
We acknowledge the contributions of Hannah Stanton and Kay Moolenijzer for their assistance with participant recruitment and neuropsychological data collection. We also thank the staff of the UTSW Advanced Imaging Center for their assistance with MRI data collection.
Funding: This work was supported by the National Institute on Aging (grant number 1R01AG039103).
Footnotes
The clear absence of age group by item type or age group by item type by region interactions in these ANOVAs indicates that the magnitude of MTL novelty effects does not differ significantly across the age groups. Strictly speaking, these null findings preclude the conduct of follow-up within-group contrasts. Nonetheless, at the request of a reviewer, we conducted these contrasts. Novelty effects were independently significant in all four ROIs in the young group, in the left anterior hippocampus only in the middle-aged group, and in left and right perirhinal cortex, and left anterior hippocampus, in the older group.
We further addressed the question of whether familiarity effects differed according to age after controlling for individual differences in memory performance by including Pr as a covariate in the second level design matrix of the whole brain SPM analysis. Inclusion of the covariate did not alter any of the reported findings.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 2006;10(10):455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW, Albasser MM. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia. 2012;50(13):3141–3155. doi: 10.1016/j.neuropsychologia.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Andrade A, Paradis AL, Rouquette S, Poline JB. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10(4):483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Balteau E, Phillips C, Luxen A, Maquet P, Salmon E, Collette F. Differential effects of aging on the neural correlates of recollection and familiarity. Cortex. 2013;49(6):1585–1597. doi: 10.1016/j.cortex.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Baxendale S. The Flynn effect and memory function. J Clin Exp Neuropsychol. 2010;32(7):699–703. doi: 10.1080/13803390903493515. [DOI] [PubMed] [Google Scholar]
- Bender AR, Naveh-Benjamin M, Raz N. Associative deficit in recognition memory in a lifespan sample of healthy adults. Psychol Aging. 25(2010)(4):940–948. doi: 10.1037/a0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Apostolova LG, Barnes J, Bartzokis G, Corbetta G, DeCarli C, Firbank M, Ganzola R, Gerritsen L, Henneman W. Delphi definition of the EADC-ADNI Harmonized Protocol for hippocampal segmentation on magnetic resonance. Alzheimers Dement. 2015;11(2):126–38. doi: 10.1016/j.jalz.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Köhler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci. 2007;104(41):16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CR, Dennis NA. Age differences in the neural correlates of novelty processing: The effects of item-relatedness. Brain Res. 2015;1612:2–15. doi: 10.1016/j.brainres.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Pike GB, Evans AC. Brain web: Online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:425. [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychol Aging. 2008;23(1):93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006a;96(4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006b;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. Sensitivity of negative subsequent memory and task-negative effects to age and associative memory performance. Brain Res. 2015;1612:16–29. doi: 10.1016/j.brainres.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. The neural correlates of recollection and retrieval monitoring: Relationships with age and recollection performance. Neuroimage. 2016a;138:164–175. doi: 10.1016/j.neuroimage.2016.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. The relationships between age, associative memory performance, and the neural correlates of successful associative memory encoding. Neurobiol Aging. 2016b;42:163–176. doi: 10.1016/j.neurobiolaging.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25(36):8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol Aging. 2010;31(10):1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;200730:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes AN, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13(2):301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Henson RN, Campbell KL, Davis SW, Taylor JR, Emery T, Erzinclioglu S. Multiple determinants of lifespan memory differences. Sci Rep. 2016;6(32527):1–14. doi: 10.1038/srep32527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Muftuler LT, Rugg MD. Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus. 2008;18(10):975–980. doi: 10.1002/hipo.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Suzuki M, Rugg MD. Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Front Hum Neurosci. 2013;7(219):1–15. doi: 10.3389/fnhum.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Two Separate, But Interacting, Neural Systems for Familiarity and Novelty Detection: A Dual-Route Mechanism. Hippocampus. 2014;24:516–527. doi: 10.1002/hipo.22241. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: An Activation Likelihood Estimation Meta-Analysis. Hum Brain Mapp. 2013;34(4):814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. J Neurosci. 2015;35(4):1763–1772. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer's disease on recollection and familiarity: a meta-analytic review. Neuropsychol Rev. 2014;24(3):332–354. doi: 10.1007/s11065-014-9266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and nonrelational information in the hippocampus and the parahippocampal region: a comparison based on eventrelated fMRI. Hippocampus. 2005;15(6):763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Li SC, Brehmer Y, Shing YL, Werkle-Bergner M, Lindenberger U. Neuromodulation of associative and organizational plasticity across the life span: empirical evidence and neurocomputational modeling. Neurosci Biobehav Rev. 2006;30(6):775–790. doi: 10.1016/j.neubiorev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Park DC, Lu H. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage. 2013;78:415–425. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson SE, Bozzali M, Cipolotti L, Dolan RJ, Rees JH, Shallice T. Effect of frontal lobe lesions on the recollection and familiarity components of recognition memory. Neuropsychologia. 2008;46(13):3124–3132. doi: 10.1016/j.neuropsychologia.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16(5):504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, Barrett LF. Differential hemodynamic response in affective circuitry with aging: an FMRI study of novelty, valence, and arousal. J Cogn Neurosci. 2011;23(5):1027–1041. doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L. Any novelty in hippocampal formation and memory? Curr Opin Neurol. 2005;18(4):424–428. doi: 10.1097/01.wco.0000168080.99730.1c. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M NilssonLG. Flynn effects on sub-factors of episodic and semantic memory: Parallel gains over time and the same set of determining factors. Neuropsychologia. 2009;47(11):2174–2180. doi: 10.1016/j.neuropsychologia.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Heit E. Associative recognition: A case of recall-to-reject processing. Mem Cognit. 2000;28(6):907–922. doi: 10.3758/bf03209339. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Sarah SY, Johnson JD, Suzuki M. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. 2012;50(13):3070–3079. doi: 10.1016/j.neuropsychologia.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD. Interpreting age-related differences in memory-related neural activity. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. 4th. Oxford University Press; 2016. [Google Scholar]
- Scimeca JM, Katzman PL, Badre D. Striatal prediction errors support dynamic control of declarative memory decisions. Nat Commun. 2016;7(13061):1–15. doi: 10.1038/ncomms13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45(10):2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nat Neurosci. 2012;15(8):1167–1173. doi: 10.1038/nn.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23(17):6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8(5):443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol. 2002;12(5):574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark-and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Wang WC, Ranganath C, Yonelinas AP. Activity reductions in perirhinal cortex predict conceptual priming and familiarity-based recognition. Neuropsychologia. 2014;52:19–26. doi: 10.1016/j.neuropsychologia.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Dew IT, Cabeza R. Age-related differences in medial temporal lobe involvement during conceptual fluency. Brain Res. 2015;1612:48–58. doi: 10.1016/j.brainres.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Cabeza R. Episodic memory encoding and retrieval in the aging brain. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. 4th. Oxford University Press; 2016. [Google Scholar]
- Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. The effects of age on the neural correlates of recollection success, recollection-related cortical reinstatement, and post-retrieval monitoring. Cereb Cortex. 2016;26:1698–1714. doi: 10.1093/cercor/bhu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Giovanello KS. The role of medial temporal lobe regions in incidental and intentional retrieval of item and relational information in aging. Hippocampus. 2016;26(6):693–699. doi: 10.1002/hipo.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Mickes L. A continuous dual-process model of remember/know judgments. Psychol Rev. 2010;117(4):1025–1054. doi: 10.1037/a0020874. [DOI] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37(4):657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. J Mem Lang. 1995;34(5):622–643. [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995;69(3):821–829. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.