Abstract
The hippocampus and amygdala exhibit sensitivity to stimulus novelty that is reduced in participants with inhibited temperament, which is related to trait anxiety. Although the bed nucleus of the stria terminalis (BNST) is highly connected to the amygdala and is implicated in anxiety, whether the BNST responds to novelty remains unstudied, as well as how trait anxiety may modulate this response. Additionally how novelty, stimulus negativity and trait anxiety interact to affect activity in these areas is also unclear. To address these questions, we presented participants with novel and repeated, fearful and neutral faces, while measuring brain activity via fMRI, and also assessed participants’ self-reported trait anxiety. As the small size of the BNST makes assessing its activity at typical fMRI resolution difficult, we employed high resolution 7 Tesla scanning. Our results replicate findings of novelty sensitivity that is independent of valence in the hippocampus. Our results also provide novel evidence for a BNST novelty response toward neutral, but not fearful faces. We also found that the novelty response in the hippocampus and BNST was blunted in participants with high trait anxiety. Additionally, we found left amygdala sensitivity to stimulus negativity that was blunted for high trait anxiety participants. These findings extend past research on the response to novel stimuli in the hippocampus and amygdala at high resolution, and are the first to demonstrate trait anxiety modulated novelty sensitivity in the BNST that is dependent on stimulus valence.
The detection of novel objects and individuals is an important function of the brain, because these stimuli represent a potential source of threat or reward. Sensitivity toward novelty has been found in both the hippocampus (Daselaar, Fleck & Cabeza, 2006; Grunwald, Lehnertz, Heinze, Helmstaedter & Elger, 1998; Kirwan, Shrager & Squire, 2009; Lever et al., 2010; Menon, White, Eliez, Glover & Reiss, 2000; Tulving, Markowitsch, Craik, Habib & Houle, 1996) and amygdala (Balderston, Schultz & Helmstetter, 2011; Blackford, Buckholtz, Avery, & Zald, 2010; Ousdal, Andreassen, Server & Jensen, 2014; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Wright et al., 2003; Wright et al., 2008). Although the bed nucleus of the stria terminalis (BNST) is heavily connected to the amygdala, both structurally and functionally (Avery et al., 2014; Torrisi et al., 2015), and plays a role in responding to threat (Alvarez, Chen, Bodurka, Kaplan & Grillon, 2011; Somerville et al., 2013) and anxiety-related hypervigilance (Somerville et al., 2010), no studies have specifically investigated whether the BNST is sensitive to stimulus novelty.
While early studies on stimulus novelty focused on the hippocampus (Daselaar et al., 2006; Grunwald et al., 1998; Kirwan et al., 2009; Lever et al., 2010; Menon et al., 2000; Tulving, et al., 1996), a growing body of literature has established that the amygdala responds to the novelty of a variety of stimuli, including objects (Blackford et al., 2010), faces (Balderston et al., 2013; Ousdal et al., 2014; Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2008) and other images of humans (Balderston et al., 2011). Balderston et al. (2011; 2013) posited that while the hippocampus plays a general role in novelty detection, novelty detection in the amygdala complements the amygdala involvement in threat detection. This is compatible with conceptualizations of the amygdala that emphasize its role in vigilance (Davis & Whalen, 2001), as vigilance toward potential threat may involve identifying and evaluating novel stimuli.
While no studies have specifically investigated how stimulus novelty affects activity in the BNST, past findings about BNST activity suggest that it may exhibit a response to novelty. The BNST is closely connected to the amygdala (Avery et al., 2014; Torrisi et al., 2015), and is considered a part of the extended amygdala (Davis & Whalen, 2001). Additionally, like the amygdala, BNST activity responds to threat (Choi, Padmala & Pessoa, 2012; Klumpers et al., 2015; Mobbs et al., 2010) and has been implicated in vigilance related behaviors, specifically anxiety-related hypervigilance (Somerville, Whalen & Kelley, 2010). These observations suggest that the BNST may work with the amygdala in maintaining vigilance toward threat. If novelty sensitivity in the amygdala is related to its role in threat detection (Balderston et al., 2011; Balderston et al., 2013), the BNST is likely to exhibit a similar novelty sensitivity.
One factor that may modulate novelty sensitivity in the amygdala is trait anxiety. Blackford et al. (2011; 2013) found that both the amygdalar and hippocampal response to novelty is altered by inhibited temperament – a personality trait that is closely related to anxiety. They found that participants high in inhibited temperament exhibit reduced novelty sensitivity due to sustained activation to familiar faces. These results suggest that high trait anxiety may similarly predict reduced novelty sensitivity in the both the hippocampus and amygdala.
If the BNST plays a role in novelty detection that is similar to the amygdala, BNST novelty sensitivity may also be modulated by trait anxiety. Although no one has tested this hypothesis, past studies have found that trait anxiety alters BNST activity. Trait anxiety is associated with increased BNST reactivity to negatively-valenced stimuli (Mobbs et al., 2010; Choi et al., 2012; Grupe et al., 2013; Klumpers et al., 2015; Haufler, Nagy & Paré, 2013; Jennings, Sparta, Stamatakis, Ung & Pleil, 2013; Meloni, Jackson, Gerety, & Carlezon, 2006), as is the case in the amygdala (Etkin et al., 2004; Fakra et al., 2009; Hariri, 2009; Hariri et al., 2009; Laeger et al., 2012; Stein, Simmons, Feinstein & Paulus, 2007). Additionally, high trait anxiety predicts a greater BNST response to uncertainty in participants with generalized anxiety disorder (Yassa, Hazlett, Stark & Hoehn-Saric, 2012). In rodents BNST-amygdala connectivity is related to anxiety-like behaviors (Cai, Bakalli & Rinaman, 2012), and BNST activity has been implicated in the avoidance of novel conspecifics (Khoshbouei, Cecchi & Morilak, 2002; Lungwitz et al., 2012). Based on these past findings, trait anxiety may be expected to modulate novelty sensitivity in the BNST.
To examine how trait anxiety affects the novelty response in the hippocampus, amygdala and BNST, we measured the blood-oxygen-level dependent (BOLD) signal of participants who were presented with images of novel and repeated faces, and collected participants’ self-reported trait anxiety scores. To examine whether stimulus negativity interacts with stimulus novelty to affect activity in these regions, we included fearful and neutral face stimuli. Given the small size of the BNST, it is difficult to distinguish activity in this area from other medial basal forebrain structures at typical functional magnetic resonance imaging (fMRI) resolutions. To increase our ability to confidently assess BNST-specific activation, we used high resolution 7-Tesla imaging to collect fMRI images with a 1 mm × 1 mm × 1.5 mm resolution. Coverage of the fMRI scan was limited to our areas of interest, in order to maintain small slice thickness. As such, our analysis focuses on testing a priori predictions concerning activity in the BNST, amygdala and hippocampus, rather than employing a whole-brain approach.
We expected to replicate past findings of a novelty response in the hippocampus (Daselaar et al., 2006; Grunwald et al., 1998; Kirwan et al., 2009; Lever et al., 2010; Menon et al., 2000; Tulving et al., 1996) and amygdala (Blackford et al., 2010; Balderston et al., 2011; Balderston et al., 2013; Ousdal et al., 2014; Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2008), and that this novelty response would be independent of stimulus negativity in both of these areas (Balderston et al., 2011; Balderston et al., 2013). We did not have strong predictions about whether stimulus novelty and negativity would interact in the BNST. On one hand, given that the BNST plays a role in threat detection, one might expect novelty and negativity to interact in the BNST. On the other hand, this same logic applies to the amygdala, but past studies that have directly tested this have indicated that novelty and negativity affect amygdala activity independently (Balderston et al., 2011; Balderston et al., 2013).
Based on past studies (Etkin et al., 2004; Fakra et al., 2009; Hariri, 2009; Hariri et al., 2009; Laeger et al., 2012; Stein, Simmons, Feinstein & Paulus, 2007) we predicted increased amygdala reactivity to stimulus negativity in participants with high trait anxiety. Given that the BNST is sensitive to negatively-valenced stimuli (Choi et al., 2012; Klumpers et al., 2015; Mobbs et al., 2010) and is involved in hypervigilant threat monitoring (Somerville et al., 2010), we predicted that the BNST would also exhibit a trait-anxiety dependent response to stimulus negativity. We did not expect an interaction between negativity and anxiety in the hippocampus.
Based on past findings (Blackford et al., 2013; Blackford et al., 2011), we predicted that participants high in trait anxiety would exhibit a decrease in novelty sensitivity, due to a sustained response across stimulus repetition in both the hippocampus and amygdala. Given that the BNST is closely connected to the amygdala, and BNST activity is altered by trait anxiety, we reasoned that the BNST may also exhibit this pattern.
Method
Participants
Thirty-seven undergraduate students at the University of Wisconsin – Milwaukee participated in the study. Three participants withdrew from the study before completing the fMRI task, three participants were excluded from analysis due to excessive motion during the scan, two participants were excluded due to signal loss during the fMRI scan that affected the BNST region, and one was excluded due to equipment failure. As a result, data from 26 participants (18 female) were included in the analysis.
Trait Anxiety Measure
Participants completed the State-Trait Anxiety Inventory (STAI-Trait), a 20-item measure designed to evaluate trait anxiety (Spielberger, Gorsuch & Lushene, 1970). The STAI-Trait has high internal consistency (α = .89) and test-retest reliability (r = .88; Barnes, Harp & Jung, 2002). Participants’ STAI-Trait scores ranged from 26 to 45 (M = 36.9, SD = 6.2).
Task Design
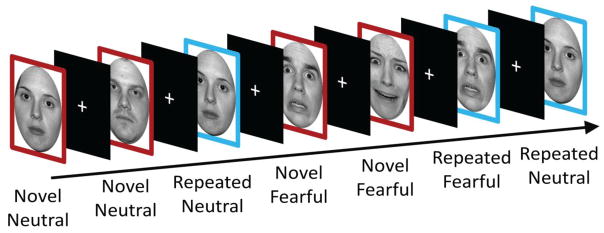
The task used a 2 × 2 factorial design, with participants viewing a series of faces that varied along the factors of Novelty (novel vs. repeated) and Expression (fearful vs. neutral). Ten fearful and ten neutral face stimuli were taken from the NimStim Set of Facial Expressions (Nim et al., 2009) and were converted to gray-scale and luminance equated. For each participant two fearful and two neutral faces were randomly chosen to be repeated five times (in addition to the initial presentation), with the rest of the images only presented once. The novel conditions consisted of the presentation of the eight non-repeating images, as well as the initial presentations of the images selected to be repeated, for a given stimulus Expression type. The repeated conditions consisted of the five repetitions of the repeated images with the initial presentation excluded for a given stimulus Expression type. The task design is depicted in Figure 1.
Figure 1.
Task Design. Participants were shown novel (red) and repeated (blue) faces with either neutral or fearful expressions. Images were presented for three seconds each and were followed by an ITI that varied from 1–11 seconds. Note that images that were repeated were counted as novel on their first presentation and counted as repeated on all subsequent presentations.
All images were presented in a single run for 3 seconds each, with an intertrial interval that varied in duration from 1–11 seconds (M = 5). The order of neutral and fearful faces was counterbalanced. The particular images used for each condition type was randomized.
MRI Data Acquisition
MRI data were acquired on a 7 Tesla MR950 General Electric (GE Healthcare, Waukesha, WI) scanner. High-resolution T1-weighted whole-brain anatomical images were acquired using BRAVO, a 3D gradient echo sequence with IR preparation (inversion time/repetition time/echo time/flip angle/field of view/matrix/slice thickness: 1050 ms/7.972 ms/3.776 ms/5°/220 mm/276 × 276 mm/.8 mm).
Partial-brain functional scans were obtained using a single-shot, T2*-weighted gradient-echo echo-planar image (EPI) sequence (repetition time/echo time/flip angle/number of excitations/field of view/matrix: 2775 ms/23.6 ms/73°/1/220 mm/224 × 224; 34 × 1.5-mm axial slices; gap: 0 mm; 121 volumes) with voxel resolution of 1 mm × 1 mm × 1.5 mm. The scan coverage was determined for each participant by positioning the most anterior edge of the coverage just anterior to the amygdala, and then checking that coverage spanned at least 5 millimeters anterior to the anterior commissure, to ensure coverage of the BNST. After the task, an additional single-volume EPI scan with reverse phase encode polarity was collected and used for susceptibility-related distortion correction.
Anatomical ROIs
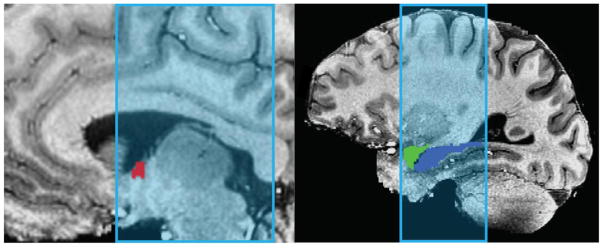
Because our hypotheses concerned how the amygdala, hippocampus and BNST respond to novelty, our analysis focused on anatomically defined ROIs for these regions. After N4 bias field correction (Advanced Normalization Tools 2.1; Avants, Tustison & Song, 2009) was applied to anatomical images, the amygdala and hippocampus ROIs were created for each subject using Freesurfer image analysis suite’s subcortical segmentation (v. 5.3, Martinos Center for Biomedical Imaging, Harvard-MIT, Boston, USA; Fischl et al., 2002). Volumes generated by Freesurfer were aligned to native space using Analysis of Function NeuroImages version 16.0 (AFNI; Cox, 1996). These ROIs were then visually inspected to ensure accurate segmentation and alignment. BNST ROIs were traced by hand in AFNI using the anatomical boundaries detailed by Avery et al. (2014; see Figure 2). These anatomical ROIs (.8 mm isotropic) were down-sampled to the resolution of the EPI images (1 mm × 1 mm × 1.5 mm), with EPI voxels being considered part of the ROI if there was more than 50% overlap with the anatomical ROI. The ROIs were then checked by overlaying them on the EPI data and adjusted if necessary, for example if the ROI encroached onto lateral ventricle. On average, the left BNST ROI consisted of 58.6 EPI voxels, while the right consisted of 55.3 voxels.
Figure 2.
Example of BNST (red), Amygdala (green) and Hippocampus (blue) ROIs used to sample BOLD activity, after downsampling to EPI resolution. ROIs are overlaid onto anatomical scan, with coverage of partial brain EPI scan highlighted in light blue. BNST ROIs were drawn for each subject, according to the anatomical boundaries detailed by Avery et al. (2014). Amygdala and hippocampus ROIs were created using Freesurfer (v. 5.3.0, Martinos Center for Biomedical Imaging, Harvard-MIT, Boston, USA; Fischl et al., 2002).
FMRI Data Analysis
FMRI data was analyzed using the Analysis of Functional NeuroImages software package (AFNI; Cox, 1996). The first 3 volumes were discarded to allow for spins to achieve a steady state and volumes with excessive motion were censored (Euclidean norm > .3). Remaining EPI volumes were slice time corrected and motion corrected. To create a distortion correction template, the third volume from the task EPI data and the third volume of the reverse polarity EPI scan were aligned to each participant’s anatomical scan, and warped together using the “plusminus” option in AFNI’s 3dQwarp. EPI task data was aligned to the anatomical image, non-linearly warped to the distortion correction template, and then to the anatomical image. These three transformations were calculated, and applied in a single step to reduce the number of times the data were interpolated. EPI data were converted to percent signal change. Single subject BOLD responses at stimulus onset were modeled using GLM and a 13.875 second tent function, with six tents. Regressors for each of the four condition types (novel neutral, repeated neutral, novel fearful and repeated fearful) were included, as well as nuisance regressors for low-frequency drift (linear, quadratic and cubic) and motion (L/R, A/P, S/I, roll, pitch, yaw, and their derivatives). An average of tents 2–5 (representing peak activation) for each condition type was computed for each voxel.
Statistical Analysis
We initially extracted mean percent signal change for each participant for each ROI and condition. However, we found that our BNST ROIs appeared to be more susceptible to outliers and showed greater variability, likely because they are relatively small, and border on ventricle. As such, we decided to extract median percent signal change for each participant for each condition and ROI, because median is more robust to outliers and such variability. The data were fitted with a linear mixed-effects model with Novelty (novel vs. repeated), Expression (fearful vs. neutral), ROI (amygdala vs. BNST vs. hippocampus), Hemisphere (left vs. right) and Anxiety (continuous) as fixed factors, and participant as a random factor using the R (R Core Team, 2016) software package lme4 (Bates, Maechler, Bolker & Walker, 2015). Significance of main and interaction effects were assessed using Type II Wald Chi-squared tests using the R package car (Fox & Weisberg, 2011). Follow-up tests were obtained using contrasts of adjusted means with the R package phia (De Rosario-Martinez, 2015).
In order to test a priori hypotheses about how the amygdala, hippocampus and BNST respond to novelty and stimulus negativity, we also ran Novelty × Expression × Hemisphere × Anxiety mixed model analyses for each of these ROIs.
Results
Omnibus
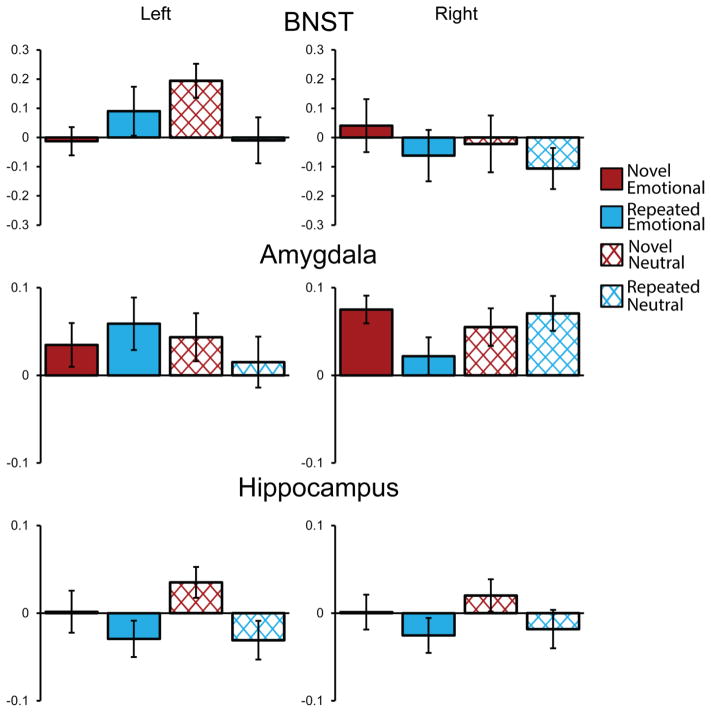
A mixed model was fitted with fixed effects for Novelty, Expression, ROI, Hemisphere and Anxiety and their interactions. The model failed to converge with random slopes for each effect and correlation parameters, however, retaining random slopes for each effect, but removing correlation parameters allowed the model to converge. The means of participant’s median percent signal change for each condition by ROI can be seen in Figure 3.
Figure 3.
BOLD response for novel (red) and repeated (blue), fearful (solid) and neutral faces (cross-hatched) for left and right BNST, amygdalae and hippocampi. With the exception of a significant main effect of Novelty in the hippocampus, there were no main effects or interactions between Novelty and Expression in these areas. Instead, significant effects primarily involved interactions between these variables and anxiety. Error bars depict within-subject variance, computed using the method described by Consineau (2005). BNST = bed nucleus of the stria terminalis.
There were no main effects for Novelty, X2(1) = .87, p = .35, Expression, X2(1) = .32, p = .57, or Anxiety X2(1) = .11, p = .74. There was a main effect of ROI, X2(2) = 11.84, p = .003, with the amygdala exhibiting more activity (M = .047) during the task than the hippocampus (M = −.006), X2(1) =8.98, p = .01, but no difference between BNST and either amygdala or hippocampus (ps = 1; Holm-Bonferroni corrected). There was also a significant interaction between Novelty and Anxiety, X2(1) = 4.84, p = .03, with Anxiety having a larger slope for repeated, b = .008, X2(1) = .65, p = .84, than novel faces, b = −.001, X2(1) = .01, p = .91 (Holm-Bonferroni corrected), although neither of these slopes were significant individually. Thus, high anxious participants exhibited reduced reactivity to novelty, in each ROI.
All of these effects, however, were qualified by a marginally significant Novelty × Expression × Anxiety × ROI interaction, X2(2) = 5.48, p = .06. While only marginally significant, this may suggest differences across regions in how the BOLD response is affected by Novelty, Expression and Anxiety. In order to explore this possibility, and to test our a priori hypotheses, we will also report Novelty × Expression × Hemisphere × Anxiety mixed model analyses for each of these ROIs.
There was also a Novelty × Expression × Side interaction, X2(1) = 4.75, p = .03. For the left hemisphere there was a marginally larger difference for novel minus repeated neutral expressions (M = .1) than for novel minus repeated fearful expressions (M = −.032), X2(1) = 4.82, p = .06. In the right hemisphere there was no difference between novel minus repeated fearful expressions (M = .061) and novel minus repeated neutral expressions (M = .036), X2(1) = .14, p = .71 (Holm-Bonferroni corrected). This suggests a larger effect of Novelty for neutral than fearful faces in ROIs in the left hemisphere. There were no other significant effects in the omnibus analysis (p > .11).
BNST
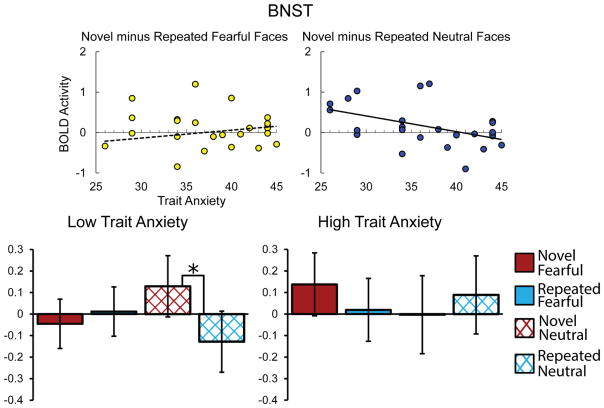
To test a priori hypotheses about how the BNST responds to Novelty and Expression, we ran a Novelty × Expression × Hemisphere × Anxiety mixed model analysis with BNST BOLD activity as the dependent variable. The BNST did not exhibit a significant main effect of Novelty, X2(1) = 1.44, p = .23 or a Novelty × Expression interaction, X2(1) = 1.9, p = .17, There was, however, a significant higher-order Novelty × Expression × Anxiety interaction, X2(1) = 11.37, p < .001, with Anxiety predicting less reactivity to the novelty of neutral, b = −.039, X2(1) = 8.72, p = .01, but not fearful faces, b = .019, X2(1) = 2.22, p = .14 (Holm-Bonferroni corrected). Thus, Anxiety predicted reduced reactivity to the novelty of neutral faces. To frame this Novelty × Expression × Anxiety interaction in the BNST another way, the Novelty × Expression interaction was more pronounced for those with lower anxiety. For example, with anxiety set at 34 (25th percentile), the model shows an effect for novel (M = .129) minus repeated neutral faces (M = −.128), X2(1) = 8.43, p = .02, but not novel (M = −.045) minus repeated fearful faces (M = .012), X2(1) = .42, p = .88. However, with anxiety set at 43 (75th percentile) there is no effect for either novel (M = −.003) minus repeated neutral (M = .089), X2(1) = .66, p = .88, or novel (M = .138) minus repeated fearful faces (M = .019), X2(1) = 1.11, p = .88 (Holm-Bonferroni corrected). For follow-up tests within Novelty condition types and Anxiety set at these same values, we found no effects of Expression for either high or low anxious participants, for either novel or repeated faces (ps > .4; Holm-Bonferroni corrected). These results suggest that trait anxiety predicts reduced reactivity to novelty in the BNST. However, this novelty response is dependent on stimulus negativity, with low anxious participants showing a novelty response to neutral images only. Adjusted means for BNST activation with anxiety set at 25th and 75th percentile scores are depicted in Figure 4. There were no main effects of Expression or Anxiety or other significant interaction effects in the BNST (all ps > .12).
Figure 4.
Graphs depicting Novelty x Valence interaction in BNST. Scatter plots (top) depict BNST BOLD activity for novel minus repeated fearful (yellow) and neutral (blue) faces on the vertical axis and self-reported trait anxiety on the horizontal axis, with trend line depicting simple linear regression (for novel minus repeated neutral faces: y = −0.039x + 1.58, R2 = .22). Bold trend lines indicate a significant relationship, based on follow-up tests, p<.05 (Holm-Bonferroni corrected). Bar graphs (bottom) depict adjusted means for novel (red) and repeated (blue) fearful (solid) and neutral (cross-hatched) faces, with anxiety set at a low (25th percentile; left) and high (75th percentile; right) value. Low anxious participants exhibit a novelty response for neutral, but not fearful faces, while high anxious participants did not exhibit a novelty response for either neutral or fearful faces. Asterisk denotes a significant comparison, based on follow-up tests, p<.05 (Holm-Bonferroni corrected). Error bars represent standard error of the adjusted means.
Amygdala
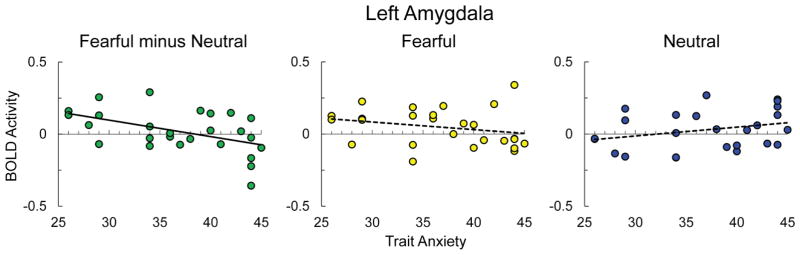
To test a priori hypotheses about how the amygdala responds to Novelty and Expression, we ran a Novelty × Expression × Hemisphere × Anxiety mixed model analysis with amygdala BOLD activity as the dependent variable. The amygdala did not exhibit a main effect of Novelty, X2(1) = .17, p = .68, or a Novelty × Anxiety interaction, X2(1) = 2.06, p = .15. There was an Expression × Anxiety × Hemisphere interaction, X2(1) = 5.88, p = .02, with Anxiety predicting less activity to fearful than neutral faces in the left, b = −.011, X2(1) = 7.37, p = .01, but not right amygdala, b = .002, X2(1) = .12, p = .73 (Holm-Bonferroni corrected). Follow up tests in the left amygdala show that the slope for anxiety was negative for fearful faces, b = −.005, X2(1) = 1.62, p = .25, and positive for neutral faces, b = .006, X2(1) = 2.38, p = .25, but neither of these slopes were significant on their own (Holm-Bonferroni corrected, see Figure 5). This suggests reduced sensitivity to negativity in participants with high anxiety. There was also a marginal trend toward a Novelty × Expression × Side interaction, X2(1) = 3.61, p = .06. The right amygdala exhibited a greater response to novel faces when they were fearful (M = .075) vs. neutral (M =.055), while exhibiting a greater response to repeated faces when they were neutral (M =.071) vs. fearful (M =.022). Meanwhile, the left amygdala exhibited the opposite pattern with a greater response to novel faces when they were neutral (M =.044) vs. fearful (M =.035), while exhibiting a greater response to repeated faces when they were fearful (M =.059) vs. neutral (M =.015), although none of these comparisons reached significance (ps = 1, Holm-Bonferroni corrected). There was no main effect of Expression or Anxiety, and no other significant interaction effects in the amygdala (ps > .12).
Figure 5.
Scatter plot depicting amygdala BOLD activity for fearful minus neutral (green), fearful (yellow), and neutral (blue) faces on the vertical axis and self-reported trait anxiety on the horizontal axis, with trend line depicting simple linear regression (for fearful minus neutral: y = −0.011x + 0.439, R2 = .24). Bold trend lines indicate a significant relationship based on follow-up tests, p<.05 (Holm-Bonferroni corrected).
Hippocampus
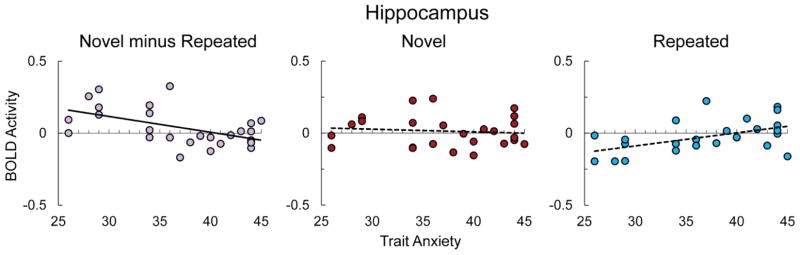
To test a priori hypotheses about how the hippocampus responds to Novelty and Expression, we ran a Novelty × Expression × Hemisphere × Anxiety mixed model analysis with hippocampus BOLD activity as the dependent variable. There was a significant Novelty effect in the hippocampus, X2(1) = 3.9, p = .048, that was qualified by a Novelty × Anxiety interaction, X2(1) = 10.58, p = .001. There was a significant difference between the slope of anxiety for novel and repeated images (b = −.011), although neither of these slopes were significant when tested individually (novel: b = −.002, X2(1) = .18, p = .67, repeated: b = .009, X2(1) = 2.98, p = .17; Holm-Bonferroni correction). This Novelty × Anxiety interaction resulted in a significant novelty response for low anxious participants (Anxiety set at 25th percentile), b = .072, X2(1) = 10.17, p = .003, and no novelty response for high anxious participants (Anxiety set at 75th percentile), b = −.026, X2(1) = .81, p = .37 (Holm-Bonferroni corrected). This suggests that the reduction in the hippocampal response to novel stimuli is absent in participants who are high in trait anxiety (see Figure 6). There was no main effect of Expression or Anxiety, and no other significant interaction effects in the hippocampus (ps > .21).
Figure 6.
Scatter plot depicting BOLD activity for novel minus repeated (purple), novel (red), and repeated (light blue) faces on the vertical axis and self-reported trait anxiety on the horizontal axis, with trend line depicting simple linear regression (for novel minus repeated: y = −.011x + .445, R2 =27). Bold trend lines indicate a significant relationship based on follow-up tests, p<.05 (Holm-Bonferroni corrected).
Discussion
Past studies have found a novelty response in both the amygdala (Blackford et al., 2010; Balderston et al., 2011; Balderston et al., 2013; Ousdal et al., 2014; Schwartz et al., 2003; Wright et al., 2003; Wright et al., 2008) and hippocampus (Daselaar, Fleck & Cabeza, 2006; Grunwald, Lehnertz, Heinze, Helmstaedter & Elger, 1998; Kirwan, Shrager & Squire, 2009; Lever et al., 2010; Menon, White, Eliez, Glover & Reiss, 2000; Tulving, Markowitsch, Craik, Habib & Houle, 1996). In two studies, Blackford et al. (2011; 2013) found that the novelty response in the amygdala and hippocampus is dependent on levels of inhibited temperament – a trait closely related to trait anxiety. Our results partially replicated this finding at high spatial resolution. For the hippocampus, we found that low trait anxiety predicted a larger novelty response, such that for participants with low (25th percentile) trait anxiety there was a significant novelty effect, but no novelty response for high (75th percentile) trait anxiety participants. This result is consistent with those of Blackford et al. (2011; 2013). By using techniques optimized for investigating BNST activity, we found that the BNST exhibited a similar pattern of activation, with highly anxious participants exhibiting reduced novelty sensitivity. In the BNST, however, this pattern was specific to neutral faces. The high resolution imaging employed in the current study affords greater confidence that these results are, in fact, being driven by BNST activity. The current work extends past research by characterizing hippocampal and amygdala responses to novelty at high resolution and demonstrates a previously unstudied aspect of BNST function – a valence dependent response to novelty that is modulated by trait anxiety. This study extends Blackford et al.’s (2011, 2013) findings by showing that the reduced novelty sensitivity associated with high trait anxiety in the hippocampus is consistent for faces with both neutral and negative expressions. We have also extended Blackford et al.’s (2011, 2013) findings by using high resolution imaging to demonstrate that the BNST also exhibits sustained activation across repetition, but only for neutral faces.
Blackford et al. (2013) suggested that sustained hippocampus and amygdala responses across repetition of face stimuli may underlie “shy and cautious” behaviors that accompany inhibited temperament, and may underlie risk for social anxiety. As the STAI-trait correlates with measures of social anxiety (Blankstein, 1976), this aspect of trait anxiety may have driven the sustained activation across repetition of face stimuli found in hippocampus and BNST in the current study. This could suggest that the BNST is a component of the circuitry that underlies altered social behaviors in individuals with high trait anxiety, and related personality traits, and that altered BNST activity may be involved in social anxiety disorders.
Although our omnibus analysis suggested reduced sensitivity to novelty in high anxious participants, regardless of ROI, our amygdala specific analysis did not replicate Blackford et al.’s (2011, 2013) finding in the amygdala. There are several methodological differences that may account for this. Blackford et al. (2011; 2013) examined individual differences in inhibited temperament, while we assessed trait anxiety. Moreover, Blackford et al. (2011; 2013) used an extreme groups approach in examining inhibited temperament. It may be that differences in amygdala activation toward novelty associated with anxiety-related traits emerge – or are more robust – when comparing extreme groups. Finally, participants in our study viewed neutral and fearful faces, while Blackford et al. (2011; 2013) used only neutral faces. Some have posited that the amygdala detects salient stimuli (Davis & Whalen, 2001). If this is the case, it may be that the salience of the fear faces included in our study reduced the salience of stimulus novelty.
Contrary to our hypotheses, we did not find a main effect of novelty in the amygdala, but found a marginal trend toward a Novelty × Expression × Side interaction in the amygdala. While further study is needed to investigate whether this trend is a robust result, this may suggest that the amygdala response to the novelty of face stimuli depends on stimulus valence. This would run counter to Balderston et al.’s (2011, 2013) finding that the effects of novelty and valence are independent in the amygdala. However, Balderston et al. manipulated valence by presenting participants with images of neutral humans and mutilated bodies in one study (2011), and to snakes and flowers in another (2013). As facial expressions are a unique and evolutionarily relevant signal of valence, neural processing of the novelty and valence of facial expressions may be specialized, and may therefore differ from that of other stimulus categories.
We hypothesized that trait anxiety would predict increased reactivity to stimulus negativity in the amygdala and BNST. However, we found that high trait anxiety was associated with decreased reactivity to stimulus negativity in the left amygdala. While past studies more commonly find increased amygdala reactivity for high trait anxious participants (Etkin et al., 2004; Fakra et al., 2009; Hariri, 2009; Hariri et al., 2009; Laeger et al., 2012; Stein et al., 2007), this association is dependent on a number of factors. For example, Etkin et al. (2004) found that trait anxiety modulated amygdala reactivity only for masked faces. Another study found that the correlation between trait anxiety and amygdala reactivity only exists for participants who are low in perceived social support (Hyde, Gorkac, Manucka & Hariri, 2011), and a study using an adolescent sample found that amygdala reactivity to facial expressions only correlated with social dimensions of trait anxiety (Killgore & Yurgelun-Todd, 2005). Dickie and Armony (2008) found that trait anxiety negatively predicted the amygdala response to attended (vs. unattended) fear faces in females, while males exhibited the opposite pattern. These studies suggest that a variety of factors, including the characteristics of the participants and method of presentation of the stimuli, can alter the relationship between trait anxiety and amygdala reactivity.
Past literature points to some possible mechanisms for decreased amygdala reactivity among participants with high anxiety. Anxiety has been associated with attentional avoidance of negative stimuli, particularly at long stimulus durations (Cisler & Koster, 2010) such as those used in the current study. In line with these findings, Blair et al. (2008) found reduced amygdala reactivity to fear faces in patients with generalized anxiety disorder. Additionally, past studies have found increased amygdala activation to neutral faces in participants with social anxiety compared to healthy controls (Birbaumer et al., 1998; Cooney, Atlas, Joormann, Eugène & Gotlib, 2006). As the STAI-Trait correlates with social aspects of trait anxiety (Blankstein, 1976), a similar effect could be driving our findings of reduced amygdala reactivity to fear vs. neutral faces in participants with high trait anxiety. Future research is needed to investigate the factors that lead to reduced amygdala reactivity to fear faces in anxiety, and whether mechanisms like attentional avoidance and increased sensitivity to neutral faces mediate this effect.
We also predicted an Expression × Anxiety interaction in the BNST, with greater BNST reactivity to fear faces with increasing trait anxiety scores. Instead we found a more complex Novelty × Expression × Anxiety interaction. While the BNST novelty response depended on both Expression and Anxiety, looking within novel and repeated conditions revealed that neither high nor low trait anxiety participants exhibited BNST-sensitivity to expression, regardless of whether the images were novel or repeated. Researchers have proposed that while the central nucleus of the amygdala mediates the response to brief, discrete signals of threat, the BNST mediates the response to prolonged, diffuse threat (Davis, Walker, Miles, & Grillon, 2010). Several imaging studies have supported this distinction (Alvarez et al., 2011; Grupe, Oathes, & Nitschke, 2013; Herrmann, et al., 2016; McMenamin, Langeslag, Sirbu, Padmala & Pessoa, 2014; Sommerville et al., 2013), although these studies may have had limited power in detecting activation specific to the BNST, given the low voxel resolution used in these studies relative to the size of the BNST. We have replicated the finding that the BNST is not sensitive to brief signals of threat using high resolution imaging. Although this finding is consistent with the view that the amygdala is sensitive to brief signals of threat, while the BNST is not, two recent reviews argue that the literature does not support the conclusion of a double dissociation in the types of threat the BNST and amygdala respond to (Gungor & Paré, 2016; Shackman & Fox, 2016), citing several studies finding a BNST response to brief threat stimuli (Mobbs et al., 2010; Choi et al., 2012; Grupe et al., 2013; Klumpers et al., 2015; Haufler et al., 2013; Jennings et al., 2013; Meloni et al., 2006). Further research is needed to specify the circumstances under which the BNST responds to briefly-presented signals of threat.
One limitation to the current study is that we did not manipulate stimulus valence independent of stimulus arousal and did not include positively-valenced stimuli in our study design. As such, we cannot distinguish whether our findings involving negativity were due to valence, or to stimulus arousal or whether the neural novelty response differs for positive vs. neutral or negative stimuli. Future studies are needed to investigate these issues.
Because we did not select participants for trait anxiety, our sample did not contain many extreme trait anxiety scores. As such, our sample illustrates that trait anxiety reduces reactivity to novelty in the BNST and hippocampus within normal ranges of trait anxiety. However, this lack of extreme scores also leaves the possibility that the pattern of results we have reported may not hold for those very high or very low in trait anxiety. Additionally, as our sample was more demographically homogenous than the population in general, they may have displayed less variation in responses to the stimuli than a more diverse sample. As such, the general population may exhibit a different pattern of results than the sample used the current study. Further investigation is needed to determine whether this is the case.
It should be noted that while using median voxel activation reduced the variance of the BOLD signal in the BNST, BNST ROIs still tended to exhibit more variance than the hippocampus or amygdala. This increased variance can be seen in the within subject error bars (Cousineau, 2005) in Figure 3. This may be due to the small size of our BNST ROIs. Additionally, because the BNST borders ventricle it is likely more prone to cerebrospinal pulsation effects (Chang, Raven & Duyn, 2016; Dagli, Ingeholm & Haxby, 1999), which could contribute to increased BOLD signal variance. Thus, effects in the BNST that we otherwise may have detected could have been obscured, as increased variance leads to a loss of power. The size and location of the BNST will continue to pose challenges for adequately assessing its activity via neuroimaging. Future studies should seek to investigate the function of the BNST at high resolution with large sample sizes to compensate for these challenges. In addition, scanning procedures designed to mitigate cerebrospinal effects such as cardiac gating, or a reduced TR time, could improve future researchers’ ability to assess BNST function (Backes & Dijk, 2001; Zhang et al., 2006).
While future research is needed to investigate these questions, the current study contributes several observations to the literature on the neural response to stimulus novelty and negativity. By using high resolution imaging, our study lends support to past studies examining how inhibited temperament affects the neural response to novelty (Blackford et al., 2013; Blackford et al., 2011) by demonstrating a decrease in reactivity to novelty in high trait anxiety in the hippocampus. We have also extended these findings by showing a decrease in BNST novelty reactivity to neutral, but not fearful faces. Additionally, we have found a reduction in sensitivity to fearful faces in the left amygdala of trait anxious participants. We also support past studies suggesting the BNST exhibits a lack of sensitivity to the negativity of briefly presented stimuli, by replicating this result at increased voxel resolution – highlighting a need for further research into the factors that determine whether the BNST exhibits sensitivity to brief threat stimuli. As this is the first study to employ high resolution imaging to examine the novelty response in the BNST, as well as the amygdala and hippocampus, these results suggest further investigation of the role the BNST plays in detecting and responding to novel stimuli, and how alterations in the BNST novelty response may contribute to personality differences like trait anxiety. As altered BNST function has been implicated in anxiety disorders (Grupe & Nitscke, 2013), this line of research may also lead to a better understanding of the mechanisms underlying these disorders.
Highlights.
The BNST responds to the novelty of neutral, but not fearful, faces
The hippocampus exhibits a novelty response that is independent of valence
The novelty response in BNST and hippocampus is blunted by high trait anxiety
The left amygdala response to valence is blunted by high trait anxiety
Footnotes
This research was supported by a Medical College of Wisconsin Daniel M. Soref Research Award (FP8072), and a National Institute of Mental Health grant (MH086809).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55(1):389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS) Insight Journal. 2009;2:1–35. [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014;91(0):311–323. doi: 10.1016/j.neuroimage.2014.01.017. http://dx.doi.org.proxy.lib.mcw.edu/10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes WH, Van Dijk P. Simultaneous sampling of event-related BOLD responses in auditory cortex and brainstem. Magnetic Resonance in Medicine. 2002;47(1):90–96. doi: 10.1002/mrm.10015. [DOI] [PubMed] [Google Scholar]
- Balderston NL, Schultz DH, Helmstetter FJ. The human amygdala plays a stimulus specific role in the detection of novelty. Neuroimage. 2011;55(4):1889–1898. doi: 10.1016/j.neuroimage.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Schultz DH, Helmstetter FJ. The effect of threat on novelty evoked amygdala responses. Plos One. 2013;8(5):e63220. doi: 10.1371/journal.pone.0063220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LLB, Harp D, Jung WS. Reliability generalization of scores on the spielberger state-trait anxiety inventory. Educational and Psychological Measurement. 2002;62(4):603–618. doi: 10.1177/0013164402062004005. [DOI] [Google Scholar]
- Bates D, Maechler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, … Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. http://dx.doi.org/10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive & Affective Neuroscience. 2011;6(5):621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience. 2013;8(2):143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. Neuroimage. 2010;50(3):1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. http://dx.doi.org.proxy.lib.mcw.edu/10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, … Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: Evidence for separate disorders. American Journal of Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein KR. Relationships between Spielberger Trait Anxiety and Lykken Social and Physical Trait Anxiety. Journal of Clinical Psychology. 1976;32(4):781–782. [PubMed] [Google Scholar]
- Cai L, Bakalli H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience. 2012;223:200–208. doi: 10.1016/j.neuroscience.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Raven EP, Duyn JH. Brain–heart interactions: challenges and opportunities with functional magnetic resonance imaging at ultra-high field. Philosophical Transactions Royal Society A. 2016;374(2067):20150188. doi: 10.1098/rsta.2015.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59(2):1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. http://dx.doi.org/10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Research: Neuroimaging. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. http://dx.doi.org/10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1(1):42–45. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dagli MS, Ingeholm JE, Haxby JV. Localization of cardiac-induced signal change in fMRI. Neuroimage. 1999;9(4):407–415. doi: 10.1006/nimg.1998.0424. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96(4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Rosario-Martinez H. R Package Version 0.2–1. 2015. Phia: Post-hoc interaction analysis. [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Research: Neuroimaging. 2008;162(1):51–57. doi: 10.1016/j.pscychresns.2007.08.002. http://dx.doi.org/10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Muñoz KE, Kimak M, … Hariri AR. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Archives of General Psychiatry. 2009;66(1):33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. http://dx.doi.org/10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An R companion to applied regression. 2. Thousand Oaks, CA: Sage; 2011. [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the human hippocampus proper. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex. 2013;23(8):1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D. Functional heterogeneity in the bed nucleus of the stria terminalis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36(31):8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, … Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Netherlands: Elsevier Science; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learning & Memory. 2013;20(11):633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MPI, Tupak SV, Guhn A, Schmidt B, … Straube T. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping. 2016;37(3):1091–1102. doi: 10.1002/hbm.23088. http://dx.doi.org/10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49(4):651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. http://dx.doi.org/10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Morilak DA. Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology. 2002;27(1):25–34. doi: 10.1016/S0893-133X(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Shrager Y, Squire LR. Medial temporal lobe activity can distinguish between old and new stimuli independently of overt behavioral choice. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14617–14621. doi: 10.1073/pnas.0907624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS, … Baas JMP. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry. 2015;78(8):582–589. doi: 10.1016/j.biopsych.2014.07.034. http://dx.doi.org/10.1016/j.biopsych.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Laeger I, Dobel C, Dannlowski U, Kugel H, Grotegerd D, Kissler J, … Zwanzger P. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research. 2012;233(2):508–516. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, Wills TJ, Cacucci F, Burgess N, O’Keefe J. Environmental novelty elicits a later theta phase of firing in CA1 but not subiculum. Hippocampus. 2010;20(2):229–234. doi: 10.1002/hipo.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, … Truitt WA. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiology & Behavior. 2012;107(5):726–732. doi: 10.1016/j.physbeh.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(34):11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA. Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Annals of the New York Academy of Sciences. 2006;1071:538–541. doi: 10.1196/annals.1364.059. http://dx.doi.org/10.1196/annals.1364.059. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL. Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Human Brain Mapping. 2000;11(2):117–129. doi: 10.1002/1097-0193(200010)11:2<117::AID-HBM50>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences. 2010;107(47):20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal OT, Andreassen OA, Server A, Jensen J. Increased amygdala and visual cortex activity and functional connectivity towards stimulus novelty is associated with state anxiety. Plos One. 2014;9(4):e96146. doi: 10.1371/journal.pone.0096146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2013. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants ‘grown up’: Adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS. Contributions of the central extended amygdala to fear and anxiety. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36(31):8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68(5):416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex. 2013;23(1):49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, … Ernst M. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Human brain mapping. 2015;36(10):4076–4088. doi: 10.1002/hbm.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. http://dx.doi.org/10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cerebral Cortex. 1996;6(1):71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer H, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18(3):660–669. doi: 10.1016/S1053-8119(02)00037-X. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42(2):956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46(8):1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WT, Mainero C, Kumar A, Wiggins CJ, Benner T, Purdon PL, … Sorensen AG. Strategies for improving the detection of fMRI activation in trigeminal pathways with cardiac gating. Neuroimage. 2006;31(4):1506–1512. doi: 10.1016/j.neuroimage.2006.02.033. [DOI] [PubMed] [Google Scholar]