Abstract
Objective
Rurally situated African Americans suffer from chronic exposure to stress that may have a deleterious effect on health outcomes. Unfortunately, research on potential mechanisms that underlie health disparities affecting the African American community has received limited focus in the scientific literature. This study investigated the relationship between perceived stress, family resources, and cortisol reactivity to acute stress.
Method
A rural sample of African American emerging adults (N = 60) completed a battery of assessments, the Trier Social Stress Test (TSST), and provided four samples of salivary cortisol: prior to receiving TSST instructions, prior to conducting the speech task, immediately following the TSST, and 15–20 minutes following the TSST.
Results
As predicted, cortisol levels increased in response to a controlled laboratory inducement of acute stress. Moreover, diminished levels of family resources were associated with blunted cortisol reactivity to acute stress. Of note, higher levels of perceived stress over the past month and being male were independently associated with lower levels of cortisol at baseline.
Conclusions
Lack of family resources had a blunting relationship on HPA-Axis reactivity. These findings provide biomarker support for the relationship between family resources – an indicator associated with social determinants of health – and stress physiology within a controlled laboratory experiment. Identifying mechanisms that work toward explanation of within-group differences in African American health disparities is both needed and informative for culturally-informed prevention and intervention efforts.
Keywords: African Americans, HPA-Axis, Cortisol, Stress, Health Disparities
Introduction
African Americans suffer disproportionate rates of disease-related morbidity and mortality. When compared to European Americans, African Americans have a 30% higher mortality for heart disease, 25% higher mortality for cancer, and 41% higher mortality for stroke. African Americans are also eight times more likely to be diagnosed with HIV and at 60% higher risk of developing diabetes (CDC, 2011). The economic burden associated with these health disparities amounts to more than $100 billion annually (LaVeist, Gaskin, & Richard, 2011). Among rural African Americans, stressors such as experiences of discrimination, lack of access to care, marginalization by health care providers, unemployment, and financial strain magnify health risks and further contribute to disease progression (Appel, Harrell & Deng 2002; Downey, 2013; Hartley, 2004). Indeed, recent studies have found health disparities in rural communities that are surpassing what is taking place in urban settings (Cossman, James, Cosby, & Cossman, 2010; Singh & Siahpush, 2014). Given this alarming public health problem, a growing body of literature has implicated stress-related allostatic load mechanisms in disease vulnerability (see Juster, McEwen, & Lupien, 2010, for review). The present study aims to characterize how two social determinants of health – perceived stress and low family resources – impact stress reactivity among rural African American emerging adults.
Physiology of Stress
Allostatic load refers to wear and tear on the body’s stress response system (McEwen, 1998) in response to chronic, extreme, or incessant real or perceived threats in the environment, particularly unpredictable and social threats. Physiological activation is adaptive in that it helps the body mobilize a near immediate fight-or-flight response and acute long-lasting (over the course of minutes to hours) adjustment of the Hypothalamic-Pituitary-Adrenal (HPA) axis (Sapolsky et al., 2000). This comprises allostasis, the body’s capacity to effectively recover from stressful experiences (McEwen & Wingfield, 2003), and the HPA axis is particularly well-suited to helping return physiological systems to baseline through counter-regulatory processes (e.g., negative feedback; Koob & Le Moal, 2008). Over prolonged periods of time, incessant exposure to stressors can alter the HPA axis as the individual attempts to achieve allostasis despite the onslaught of stressors and social challenges in their environment. HPA axis dysregulation can be marked by various patterns of biomarker release, including heightened and blunted reactivity, and may be influenced by adaptive capacities inherent to the individual’s experience (Del Guidice, Ellis, & Shirtcliff, 2011; Heim & Nemeroff, 1999). Importantly, putative dysregulation is not physiological maladaptation, but rather adaptation of the individual’s physiology to a difficult or undesirable social environment (Ellis, Del Giudice, & Shirtcliff, 2013). Adaptive does not mean desirable, but rather shifts the focus on the social determinants of health in order to show how the dysregulation of individual’s physiological response unfolds in response to stressful environments (Shirtcliff, Peres, Dismukes, Lee, & Phan, 2014).
Cortisol, the primary hormonal end-product of the HPA axis released from the adrenal gland, is thought of as a stress biomarker. Cortisol release is beneficial as it has been associated with enhancing functioning of the cardiovascular and anti-inflammatory aspect of immune systems, functions to change glucose metabolism, and impacts affective and cognitive function to help the body cope with stress. Because cortisol reaches several biological systems, dysregulations in its output leaves the person at risk for many stress-related diseases (Lupien et al., 2009). African Americans may be at increased risk for HPA dysregulation and subsequent disease vulnerability due to a disproportionate exposure to chronic stressors experienced through racism, discrimination, violence, crime, neighborhood disorganization, unemployment, access to care, financial strain, and low socioeconomic status (Clark et al., 1999; Williams et al., 2010).
Despite clear disparities in health and stress exposure among rural African Americans, the stress physiology of this population remains understudied. Previous research has identified dysregulations in basal and diurnal cortisol as well as cortisol awakening response (CAR) among African Americans (Bennett, Merrit, & Wolin, 2004; Cohen et al., 2006; DeSantis et al., 2007; Martin, Bruce & Fisher, 2012; Obasi, Shirtcliff, Brody, et al., 2015; Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011), but cortisol reactivity to acute stress in this population remains largely understudied (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014).
Dysregulations in acute stress reactivity occur secondary to allostatic states by demonstrating an inability to elicit short-term biological changes in order to meet the demands of the proximal social context. The present study therefore aims to characterize acute stress reactivity among rural African American emerging adults. Because activation of stress responses is ultimately rooted in individual appraisals of threat (Lupien et al., 2009; McEwen, 1998), this study will also investigate the relationship between social determinants of health and acute stress reactivity, in order to elucidate individual differences in risk and resilience among this group. Specifically, this study will focus on the unique and collective contributions of perceived stress and family resources.
Perceived Stress and Acute Stress Reactivity
According to allostatic load models, physiological stress activation occurs secondary to the brain’s interpretation or appraisal of an environmental stressor (Juster, McEwen, & Lupien, 2010; Lupien et al., 2009). Subjective perception of stress is therefore equally, if not more, important than objective indicators of stress in enacting physiological response. Consistent with such models, Clark and colleagues (2007) found that perceived stress, above objective environmental stress, was positively correlated with higher allostatic load primary mediators. Goldman and colleagues (2005) further demonstrated that both current and longitudinal perceived stress were positively associated with an index of allostatic load (a measure of cumulative physiological dysregulation) among older adults. Moderate associations between reported perceived stress and altered diurnal cortisol levels have also been consistently evidenced in diverse samples (e.g., Bennett et al., 2004; O’Brien et al., 2013). Despite these findings, the impact of ongoing perceived stress on physiological reactivity to acute stress is understudied among African American emerging adults, whose vulnerability to chronic stress exposure is further compounded by age-related transitional stressors. Identifying changes in acute stress reactivity as a function of perceived stress may help indicate subtle, yet meaningful, allostatic dysregulations that are only detectable when the system is challenged (Kudielka & Wust, 2010).
Impact of Family Resources on Stress
The availability of resources such as food, shelter, financial stability, positive relationships, and quality time with family are integral components to healthy development. Growing up in environments low on these resources can activate prolonged stress responses that have negative ramifications for physical and mental health through adulthood (Bradley & Corwin, 2002; Hostinar & Gunnar, 2013) through two main theorized mechanisms. First, diminished family resources can act as a source of stress. For instance, self-reported low social class is linked to lower cortisol reactivity to acute experimental stressors (Kirstenson et al., 2001). Low SES is also associated with lower basal cortisol, flatter diurnal rhythms, and higher cortisol awakening response (CAR) among adults, suggesting that the effects of low family resources carry over into adulthood and evidence themselves in daily regulatory mechanisms of cortisol output (Cohen et al., 2006; Gustafsson et al., 2010; Steptoe et al., 2003). Individuals with high familial adversity show diminished cortisol and heart rate responses to psychosocial stress, even when diurnal cortisol curves are normal (Lovallo et al., 2012). Second, family-level social support can also affect stress physiology such that social support can attenuate HPA axis reactivity to acute stress, particularly among females (Heinrichs et al. 2003; Kirschbaum et al. 1995a). The presence of family resources may help provide the individual with the internal and external resources needed to cope with stressors, allowing their family to buffer them from a physiological stress response. Interestingly, Uhart and colleagues (2006) found heightened cortisol reactivity in European American, but not African American, participants who grew up in a stressful family context. A recent study has found waking cortisol levels buffered by positive parenting primarily within European American emerging adults more so than African American youth (Shirtcliff, Skinner, Obasi, & Haggerty, in press). This finding suggests important ethnic group differences that further highlight the need to assess acute stress physiology among African Americans.
The Present Study
In context of these variable findings and noted gaps in the literature, this study sought to characterize the trajectory of acute stress reactivity among a vulnerable and understudied population, rural African American emerging adults. The present study also aims to identify psychosocial contributors that are associated with physiological reactivity to acute stress exposure, namely perceived stress and family resources. Based on previous research findings, the following hypotheses were made: (1) Rural African American emerging adults will exhibit blunted cortisol levels when experiencing higher levels of perceived stress; and (2) HPA-axis reactivity to acute stress will be lower among participants with low family resources. When designing this study, an a priori power analysis was conducted based on a meta-analysis (Dickerson & Kemeny, 2004) that found fewer than 50 participants were needed to obtain 80% power in obtaining statistical significance in cortisol reactivity to acute stress (α = 0.05) from the Trier Social Stress Test (TSST). Of note, we utilized a conservative approach to HLM statistical power by basing the sample size on the Level 2 units of analyses (i.e., number of participants) as opposed to Level 1 (i.e., total number of saliva samples collected).
Methods
Participants
Participants (N = 60) consisted of rural African Americans between the ages of 18 and 22 (X = 20.0, SD = 1.1). The majority of the participants were female (n = 39, 63.9%), unmarried (n = 58, 95.1%), and self-identified as 5th generation in response to immigrant status (n = 54, 88.5%). In response to highest level of education obtained, 1.7% had some high school education, 30.0% graduated from high school, 55.0% had some college or technical classes, 11.7% had a college degree, and 1.7% had some professional training. Moreover, 62.3% of the participants were currently a homemaker, student, or unemployed. Each participant was asked: “Have you ever used professional services that were provided by a psychologist?” Ninety-five percent (n = 57) reported no and 5.0% (n = 3) reported yes.
Procedures
Participants were enrolled into this study after previously participating in a control group from the Adults in the Making (AIM) project. This group represents a random sample of African Americans who reside in rural counties throughout the state of Georgia. Participants who agreed to participate in this study were greeted by a graduate research assistant when they arrived at the laboratory at 2:45 PM. After obtaining informed consent, participants were asked to: (1) provide a breath alcohol sample and urine sample to test for the presence of alcohol, THC, cocaine, opioids, methamphetamine, benzodiazepines, barbiturates, and oxycontin. Participants who tested positive for a licit or illicit substance were not permitted to continue in this session (n = 2) and were not included in the participant data presented above. Following a negative drug screen, the participants were asked to complete a battery of assessments, TSST, and provide saliva samples. Finally, the participants were debriefed and financially compensated $175 for their participation in this study and to offset travel expenses. This study was approved by the university Institutional Review Board.
Trier social stress test (TSST)
The TSST was used to induce the participants into a state of acute stress. Studies have repeatedly demonstrated that cortisol shows reactivity to the TSST (De Wit, Soderpalm, Nikolayev, & Young, 2003; Uhart et al., 2006). During the speech component, participants were instructed to introduce themselves to the “committee” in a free speech (5 min) and convince them why they should be hired for a job vacancy during these tough economic times. This “committee” was introduced as being experts in nonverbal behavior and consisted of an African American and European American confederate. The participants were given 5 minutes to prepare their speech. Once the speech task began, the “committee” asked standardized questions if the participants paused for more than 20 sec prior to the expiration of the speech period. Next, during the arithmetic component, participants were asked to serially subtract the number 13 from 1,022 as quickly and as accurately as possible (5 min). On every failure, participants were prompted to restart at 1,022 with a “committee” member interfering, “Stop. 1,022”.
Salivary cortisol
Four samples of salivary cortisol were collected during the TSST: Sample #1 was collected prior to receiving the TSST instructions after arrival at the laboratory and completing some computerized assessments, sample #2 was collected prior to conducting the speech task, sample #3 was collected immediately following the TSST, and sample #4 was collected 15–20 minutes following sample #3. Peak cortisol levels are found in saliva 20–30 minutes after the experience of an acute stressor so sample #4 was designed to represent the peak cortisol response (PCR) to the TSST.
Measures
Salivary cortisol
All saliva samples were stored immediately in an ultracold laboratory freezer (−30°C), then shipped overnight frozen with dry-ice pellets to the Middleton Research Biodiagnostics Lab (Madison, WI). On the day of assay, samples were thawed and cortisol was assayed in duplicate using a well-established enzyme-linked immunosorbent assay (ELISA) kit specifically designed for use with saliva (Salimetrics, State College, PA). Samples were reanalyzed if the CV for the duplicate measurements were >20%. Samples from the same individual were all assayed on the same run. To normalize distributions, extreme values of raw cortisol were winsorized.
Perceived stress
Stress was measured by the Perceived Stress Scale (PSS; Cohen & Williamson, 1983). The PSS is a 14-item self-report measure that assesses individuals’ perception of situations in their lives that they deem stressful over the past month. The PSS is rated on a 5-point Likert scale ranging from “Never” to “Very Often”. Summary scores for the PSS range between 0 and 56; with higher scores indicating more stress. Each item on the PSS assesses one’s perceived stress within the last month. In previous research, scores on the PSS demonstrated adequate internal consistency and test-retest reliability (Reis, Hino, & Anez, 2010). The Cronbach’s alpha for scores produced by the PSS was .75 in this sample.
Family resources
Family resources was measured by the Family Resource Scale (FRS; Dunst & Lee, 1987). The FRS is a 30-item self-report measure that assesses an individual’s perceived adequacy of concrete resources in the household. Resources include physical necessities and shelter (8 items), growth and support (9 items), necessities and health (7 items), interfamily support (2 items), child care (2 items), and personal resources (2 items). The FRS is rated on a 5-point Likert-type scale ranging from “not at all adequate” to “almost always adequate”. A response of “does not apply” was also permitted. Lower scores on the FRS indicate lower perceived adequacy of resources in the childhood home. In previous research, scores on the FRS demonstrated adequate internal consistency, split-half reliability, and criterion-related validity (Brannon et al., 2006; Dunst & Lee, 1987). The Cronbach’s alpha for scores produced by the family resources scale was .95 in this sample.
Analytic Strategy
A 2-level hierarchical linear model (HLM) was used to estimate peak cortisol reactivity in response to the TSST. Thus, the within-subject dependent variable included all four samples of salivary cortisol from baseline to 15–20 minutes after the completion of the TSST. To understand how cortisol changed across the session, predictors of cortisol were included at the within-subjects level (i.e., Level 1). A random effect for time since baseline (TSB) was modeled in minutes and captured variation in time associated with the collection of saliva samples across the session. A positive TSB indicates a rise in cortisol across the entire session. Cortisol reactivity, however, is not expected to be a gradual rise. Therefore, the peak cortisol response (PCR) to the TSST was modeled using a dummy variable that was coded 1 for sample #4 and 0 for the remaining samples. A positive PCR indicates a larger rise after the TSST than expected by the change during the rest of the session. Of note, this is a conservative approach to estimating reactivity in which cortisol may be elevated above baseline and above a general laboratory-session related cortisol rise. Lastly, the intercept in this model (β0) indexed cortisol levels (sample #1) at baseline prior to the start of the TSST.
Individual difference predictors were then modeled as cross-level interactions between these cortisol parameters and variables of interest (i.e., perceived stress or family resources). Perceived stress, family resources, age, and sex were entered as main effects on baseline cortisol levels. Moreover, family resources was entered as a main effect on PCR after controlling for perceived stress, family resources, age, and sex at baseline. None of the predictors were found to influence the variation in time associated with the collection of saliva samples (β1) since this took place in a controlled laboratory setting.
| Level 1 | (within-individual) Cortisol = β0 + β1 (TSB) + β2 (PCR) + r |
| Level 2 | (between individual) β0 = γ00 + γ01 (Age) + γ02 (Sex) + γ03 (PSS) + γ04 (FRS) + U0 β1 (TSB) = γ10 + U1 β2 (PCR) = γ20 + γ21 (FRS) + U2 |
Note. TSB = time since baseline, PCR = peak cortisol response, PSS = Perceived Stress Scale, FRS = Family Resource Scale.
Results
Descriptive Statistics
Scores on the PSS ranged from 8 to 38 (M = 24.62; SD = 6.26) and scores on the FRS ranged from 38 to 163 (M = 135.81; SD = 24.92). Moreover, the PSS was not significantly correlated to the FRS (r = −0.16, p = .237). Mean cortisol levels were 0.14 μl/dL (SD = 0.07) at baseline, 0.14 μl/dL (SD = 0.07) prior to starting the TSST, 0.19 μl/dL (SD = 0.12) at the end of the TSST, and 0.25 μl/dL (SD = 0.17) 15–20 minutes following the TSST. Of note, peak cortisol reactivity to acute stress represented a large effect size (d = 1.51) in this study.
Stress and Cortisol Levels
Trait (systematic) cortisol comprised a significant proportion (39.7%) of the total variability in cortisol reactivity (χ2 (57) = 205.47, p < .001). This suggests that rural African American emerging adults have moderately stable salivary cortisol levels in response to acute stress. TSB represented a significant linear slope from the baseline sample to the peak sample (β = .002, t(57) = 4.80, p < .001). On average, cortisol levels increased in response to the controlled laboratory stressor via the TSST. Furthermore, 29.3% of the total variance in cortisol was found to be a function of stable systematic individual differences after accounting for time in the model (χ2(57) = 155.76, p < .001). PCR also represented a significant linear slope to the model (β = .02, t(57) = 2.36, p = .022). This positive relationship indicated that cortisol levels significantly increased in sample #4 which represented the peak cortisol reactivity to the TSST. Moreover, 65.4% of the total variance in cortisol was found to be a function of stable systematic individual differences after accounting for time and the peak cortisol response to the TSST in the model (χ2(56) = 185.11, p < .001).
We tested if some of the variability in cortisol at baseline could be accounted for by age, sex, perceived stress, and family resources. Sex was associated with baseline levels of cortisol (γ02 = 0.043, t(53) = 2.20, p = .032). Indeed, males were found to have lower levels of cortisol at baseline. Additionally, perceived stress had a significant inverse relationship with baseline levels of cortisol (γ03 = −0.003, t(53) = −2.14, p = .037). More specifically, experiencing higher levels of perceived stress over the past month was associated with lower levels of cortisol at baseline. Age and family resources were not associated with levels of cortisol at baseline (see Table 1). Of note, post-hoc analyses found that there were no sex differences in perceived stress or family resources.
Table 1.
Hierarchical linear models of the relationship between perceived stress, family resources, and cortisol reactivity to acute stress.
| Model | Fixed Effect | Coefficient | SE | t | df | p-value |
|---|---|---|---|---|---|---|
| 1 | γ00 (Baseline) | 0.175 | 0.011 | 15.275 | 57 | < 0.001 |
|
| ||||||
| 2 | γ00 (Baseline) | 0.128 | 0.009 | 13.973 | 57 | < 0.001 |
| γ10 (TSB) | 0.002 | 4.03E-04 | 4.803 | 57 | < 0.001 | |
|
| ||||||
| 3 | γ00 (Baseline) | 0.133 | 0.010 | 14.012 | 57 | < 0.001 |
| γ10 (TSB) | 0.001 | 4.16E-04 | 3.067 | 57 | 0.003 | |
| γ20 (PCR) | 0.045 | 0.019 | 2.357 | 57 | 0.022 | |
|
| ||||||
| 4 | γ00 (Baseline) | 0.304 | 0.130 | 2.330 | 53 | 0.024 |
| γ01 (age) | −0.009 | 0.006 | −1.465 | 53 | 0.149 | |
| γ02 (sex) | 0.043 | 0.020 | 2.199 | 53 | 0.032 | |
| γ03 (PSS) | −0.003 | 0.001 | −2.143 | 53 | 0.037 | |
| γ04 (FRS) | −4.65E-04 | 3.01E-04 | −1.546 | 53 | 0.128 | |
| γ10 (TSB) | 0.001 | 4.15E-04 | 3.076 | 57 | 0.003 | |
| γ20 (PCR) | 0.045 | 0.019 | 2.395 | 57 | 0.02 | |
|
| ||||||
| 5 | γ00 (Baseline) | 0.304 | 0.130 | 2.333 | 53 | 0.024 |
| γ01 (age) | −0.009 | 0.006 | −1.469 | 53 | 0.148 | |
| γ02 (sex) | 0.043 | 0.020 | 2.203 | 53 | 0.032 | |
| γ03 (PSS) | −0.003 | 0.001 | −2.158 | 53 | 0.035 | |
| γ04 (FRS) | −3.59E-04 | 2.93E-04 | −1.225 | 53 | 0.226 | |
| γ10 (TSB) | 0.001 | 4.18E-04 | 3.084 | 57 | 0.003 | |
| γ20 (PCR) | 0.045 | 0.019 | 2.430 | 56 | 0.018 | |
| γ21 (FRS) | 0.001 | 4.61E-04 | 2.295 | 56 | 0.025 | |
Note. PSS = Perceived Stress Scale, FRS = Family Resource Scale, TSB = time since baseline, PCR = peak cortisol response.
Family Resources and Peak Cortisol Reactivity to Acute Stress
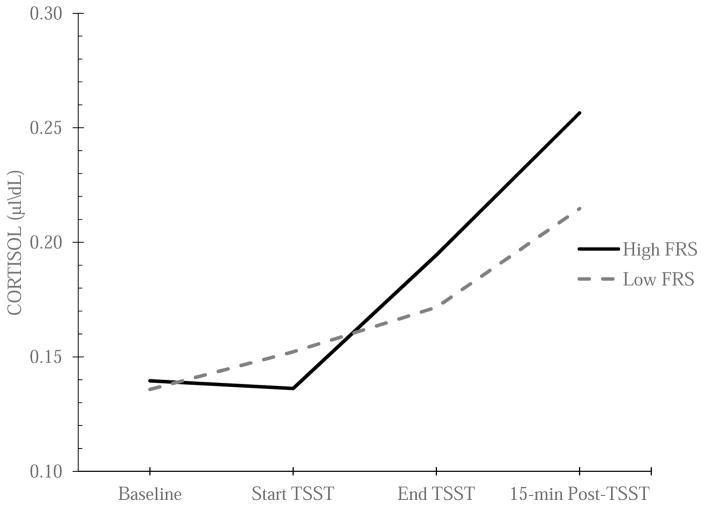
We tested whether some of the variability in PCR could be accounted for by family resources after controlling for age, sex, perceived stress, and family resources at baseline. Family resources had a significant positive relationship with PCR (γ21 = .001, t(56) = 2.30, p = .025). More specifically, coming from a family with diminished levels of basic resources was associated with a blunted peak cortisol response to acute stress (see Figure 1). Of note, a post-hoc analysis found that perceived stress was not associated with the peak cortisol response to acute stress.
Figure 1.
Relationship between family resources and mean cortisol reactivity to acute stress at each time point during the TSST.
Note. TSST = Trier Social Stress Test, FRS = Family Resource Scale.
Discussion
This study sought to extend the existent literature by investigating the relationship between psychosocial determinants of health, specifically family resources and perceived stress, and HPA reactivity to acute stress by utilizing a controlled laboratory experiment. To our knowledge, this is the first study to investigate within-group variation in psychosocial determinants of health that utilized a controlled laboratory assessment of acute stress with a community sample of rural African American emerging adults. The results were consistent with previous research studies that investigated perceived stress as an indicator of allostatic load (Clark et al., 2007; Goldman et al., 2005). More specifically, participants who reported greater levels of perceived stress over the past month had blunted levels of cortisol at baseline. Previous research has also demonstrated a link between blunted diurnal cortisol output and chronic stress among rural African Americans, suggesting perceived stress has deleterious consequences for stress physiology across both acute and chronic paradigms (Obasi et al., 2015). High exposure to such unmitigated stressors may put this population at increased risk for allostatic ‘wear and tear’ that contributes to negative health outcomes over time. To this end, a previous longitudinal study found that lower cortisol reactivity to acute stress was associated with a greater incidence of obesity, poorer self-rated health, poorer lung function, and lower cognitive function 5 years later (de Rooij, 2013). It is important to note that sex was also associated with baseline levels of cortisol. More specifically, African American males tended to have a more blunted cortisol level at baseline in comparison to their female counterparts. Unfortunately, it is difficult to explain this finding given the fact that there were no sex differences in perceived stress or family resources.
This study extends the current literature by also investigating the relationship between social determinants of health (i.e., family resources) and HPA functioning. Participants who reported lower consistency of having access to adequate family resources in the household had a blunted peak cortisol reactivity to acute stress. This is consistent with previous research demonstrating blunted cortisol reactivity to acute stress in individuals with childhood chronic stress exposure (Andreotti et al., 2015). Higher rates of reported exposure to psychosocial stressors within this population undergird the need to investigate causal mechanisms underlying the relationship between psychosocial determinants of health and negative health outcomes. While we emphasize the role of the social environment in blunting physiological responses to acute stress, previous research has also found that a higher perceived sense of social control was positively related to more robust cortisol reactivity in acute laboratory stressors (Barrington et al., 2014). Although beyond the scope of this study, such findings collectively suggest that one’s sense of personal control over their social environment, in addition to their social environment itself, may be a key component in either exacerbating or mitigating against the effects of stress.
Theoretical models are increasingly taking a dynamic and functional view of stress biomarkers that allows for high (or low) physiological arousal to have both costs and benefits (Hostinar & Gunnar, 2013). High cortisol appears adaptive when being open to the environment allows for greater encoding of positive social information (Del Giudice, Ellis, & Shirtcliff, 2013). For example, pharmacological administration of cortisol or a rise in cortisol after awakening are associated with energy, productivity, and reduced symptoms of fatigue (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Chida & Steptoe, 2009; Lovas & Husebye, 2003). Within acute stressors like the TSST, social memory for salient cues is enhanced within stress-reactive individuals (Wiemers, Sauvage, Schoofs, Hamacher-Dang, & Wolf, 2013) and pharmacologically blocking a cortisol response is linked with greater self-reports of distress (Andrews, D’Aguiar, & Pruessner, 2012). The costs of an “open” or sensitive profile are expected to be minimal in low stress environments. Physiological attunement, including the HPA axis, allows individuals to benefit from stress buffering provided by a supportive individual (Gunnar & Hostinar, 2015; Heinrichs et al, 2003) or psychosocial resources bolstered by a supportive environment (Taylor et al, 2008; Taylor et al, 2010).
In more challenging environments, however, the costs outweigh benefits of social sensitivity, and it is adaptive instead to be internally buffered from the onslaught of daily hassles and difficulties. For African Americans, this “buffered” profile may be common given, at minimum, experiences with racism and discrimination (DeSantis et al., 2007; Skinner et al., 2011). Within the present study, this buffered profile was further apparent within African Americans with greater stress exposure and fewer family resources. A buffered profile has benefits in that social threat and uncontrollability exert a diminished impact on the individual, yet there are also potential costs – many of which take years to accumulate a wear and tear on the individual (Koob & Le Moal, 2008). For example, the anti-inflammatory and glucose metabolism functions of cortisol may leave the low-cortisol individual at heightened risk for immune and insulin-related health problems (Miller, Cohen, & Ritchey, 2002; Raison & Miller, 2003). Importantly, this calibration of the body’s stress response system is in response to the individual’s social environment and stressor exposure (Voellmin et al., 2015), calling attention to the importance of social determinants of health particularly within the African American community.
Taken together, findings from the present study suggest that ongoing perceived stress, in addition to diminished family resources, can profoundly impact stress physiology. Having greater insight into these causal mechanisms of stress dysregulation is critically important given the growing body of evidence linking stress dysregulation to drug use vulnerability and other poor health outcomes. Stress management is a psychological phenomenon that has been long studied in the social and behavioral sciences. While the elimination of social inequities is likely the most robust strategy for eradicating known health disparities in the African American community, creating and disseminating culturally-informed stress management and family-strengthening prevention and intervention efforts could also help mitigate this public health problem.
While this study makes a significant contribution to the health disparities literature, it is not without limitations. First, this representative community sample of rural African Americans was small and recruited from the southeast United States. Extending these finding to other rural – and potentially urban – communities should be done with caution. Additionally, while this study was funded to collect data on peak cortisol reactivity to acute stress, we were unable to collect and assay subsequent cortisol samples that would provide information regarding how efficiently cortisol was downregulated after exposure to a controlled laboratory stressor. Future research should be conducted to investigate these relationships in other geographical regions, while also collecting additional cortisol samples to effectively model both the up- and down-regulation of cortisol reactivity to acute stress in this population. Future research may also investigate the down-stream effects of such dysregulation in cortisol reactivity to acute stress as a function of sex. Cortisol, and the HPA-axis more broadly, can affect multiple biological systems and is recognized to have meaningful cross-talk with other neuroendocrine mechanisms (Marceau et al, 2014). For example, our focus on family resources dovetails well with emerging literature on oxytocin as a protective biomarker (Hostinar, Sullivan, & Gunnar, 2014) which is altered within individuals with life stress exposure (Seltzer, Ziegler, Connolly, Prososki, & Pollak, 2014). However, the impact of sex and such dysregulation on other regulatory functions in the acute phase is not well understood.
Despite some limitations, this study reinforces the need for future research to identify between-group and within-group variation in how psychosocial determinants of health can get “under the skin” and develop different health profiles across time. Identifying explanatory mechanisms linked to health disparities will better position researchers to create culturally informed prevention and intervention strategies aimed at reducing – and ultimately eliminating – health disparities in the African American community.
Acknowledgments
Funding.
This project was supported in part by the National Institutes of Health, National Institute on Drug Abuse (R03DA027481, R01DA034739, PI: EM Obasi). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the project supporters.
Support: This research was supported by funds from the NIH National Institute on Drug Abuse (R03DA027481, R01DA034739, PI: Obasi).
Footnotes
Conflicts of Interest: None
Compliance with Ethical Standards
Conflicts of Interest. The authors declare that they have no conflict of interest.
Ethical Approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent. Informed consent was obtained from all individual participants included in the study.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Garrard P, Venkatraman SL, Compas BE. Stress-related changes in attentional bias to social threat in young adults: Psychobiological associations with the early family environment. Cognitive Therapy & Research. 2015;39(3):332–342. doi: 10.1007/s10608-014-9659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, D’Aguiar C, Pruessner JC. The combined dexamethasone/TSST paradigm--a new method for psychoneuroendocrinology. PLoS One. 2012;7(6):e38994. doi: 10.1371/journal.pone.0038994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SJ, Harrell JS, Deng S. Racial and socioeconomic differences in risk factors for cardiovascular disease among Southern rural women. Nursing Research. 2005;51(3):140–147. doi: 10.1097/00006199-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Barrington WE, Stafford M, Hamer M, Beresford SA, Koepsell T, Steptoe A. Neighborhood socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health & Place. 2014;27:120–126. doi: 10.1016/j.healthplace.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethnicity & Health. 2004;9(4):337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Brannan AM, Manteuffel B, Holden EW, Heflinger CA. Use of the family resource scale in children’s mental health: Reliability and validity among economically diverse samples. Administration and Policy in Mental Health and Mental Health Services Research. 2006;33(2):182–197. doi: 10.1007/s10488-006-0032-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. CDC. Summary health statistics for U.S. adults. National Health Interview Survey 2011. 2011 Retrieved from http://www.cdc.gov/minorityhealth/CHDIR/2011/CHDIR2011.html.
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychology. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54(10):805. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cossman JS, James WL, Cosby AG, Cossman RE. Underlying causes of the emerging nonmetropolitan mortality penalty. American Journal of Public Health. 2010;100(8):1417–1419. doi: 10.2105/AJPH.2009.174185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij SR. Blunted cardiovascular and cortisol reactivity to acute psychological stress: A summary of results from the Dutch famine birth cohort study. International Journal of Psychophysiology. 2013;90(1):21–27. doi: 10.1016/j.ijpsycho.2012.09.011. [DOI] [PubMed] [Google Scholar]
- De Wit H, Soderpalm AHV, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcoholism: Clinical & Experimental Research. 2003;27(8):1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. Making sense of stress: An evolutionary-developmental framework. In: Laviola G, Macri S, editors. (Mal)adaptive aspectives of developmental stress. New York: Springer; 2013. pp. 23–44. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Franz CE, Prom-Wormley E, Eaves LJ, Mendoza SP, Hellhammer DH, … Jacobson KC. Negative emotionality, depressive symptoms and cortisol diurnal rhythms: Analysis of a community sample of middle-aged males. Hormones & Behavior. 2011;60(2):202–209. doi: 10.1016/j.yhbeh.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey LH. Rural populations and health: Determinants, disparities, and solutions. Preventing Chronic Disease. 2013;10:E104. [Google Scholar]
- Dunst CJ, Leet HE. Measuring the adequacy of resources in households with young children. Child: Care, Health & Development. 1987;13(2):111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Shirtcliff EA. Beyond allostatic load: The stress response system as a mechanism of conditional adaptation. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 2. Hoboken, NJ: Wiley; 2013. pp. 251–284. [Google Scholar]
- Fishbein DH, Lozovsky D, Jaffe JH. Impulsivity, aggression, and neuroendocrine responses to serotonergic stimulation in substance abusers. Biological Psychiatry. 1989;25(8):1049–1066. doi: 10.1016/0006-3223(89)90293-x. [DOI] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Seplaki C, Liu IW, Weinstein M. Perceived stress and physiological dysregulation in older adults. Stress: The International Journal on the Biology of Stress. 2005;8(2):95–105. doi: 10.1080/10253890500141905. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE. The social buffering of the Hypothalamic-Pituitary-Adrenocortical Axis in humans: Developmental and experiential determinants. Social Neuroscience. 2015;10(5):479–488. doi: 10.1080/17470919.2015.1070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Socioeconomic status over the life course and allostatic load in adulthood: results from the Northern Swedish Cohort. Journal of Epidemiology & Community Health. 2011;65(11):986–992. doi: 10.1136/jech.2010.108332. [DOI] [PubMed] [Google Scholar]
- Hartley D. Rural health disparities, population health, and rural culture. American Journal of Public Health. 2004;94(10):1675–1678. doi: 10.2105/ajph.94.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. The developmental effects of early life stress: An overview of current theoretical frameworks. Current Dirrections in Psychological Science. 2013;22(5):400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, Adam EK. Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology. 2014;50:155–166. doi: 10.1016/j.psyneuen.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- King RJ, Jones J, Scheuer JW, Curtis D, Zarcone VP. Plasma cortisol correlates of impulsivity and substance abuse. Personality & Individual Differences. 1990;11(3):287–291. [Google Scholar]
- Kirschbaum C, Bartussek D, Strasburger CJ. Cortisol responses to psychological stress and correlations with personality traits. Personality & Individual Differences. 1992;13(12):1353–1357. [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Wüst S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress: The International Journal on the Biology of Stress. 2009;13(1):1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- LeBlanc J, Ducharme MB. Influence of personality traits on plasma levels of cortisol and cholesterol. Physiology & Behavior. 2005;84(5):677–680. doi: 10.1016/j.physbeh.2005.02.020. [DOI] [PubMed] [Google Scholar]
- LaVeist TA, Gaskin D, Richard P. Estimating the economic burden of racial health inequalities in the United States. International Journal of Health Services. 2011;41(2):231–238. doi: 10.2190/HS.41.2.c. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma family health patterns project. Biological Psychiatry. 2012;71(4):344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovas K, Husebye ES. Replacement therapy in Addison’s disease. Expert Opinion on Pharmacotherapy. 2003;4(12):2145–2149. doi: 10.1517/14656566.4.12.2145. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones & Behavior. 2012;61(5):661–668. doi: 10.1016/j.yhbeh.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones & Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Rabin BS, Skoner DP, Doyle WJ. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain, Behavior, And Immunity. 1999;13(2):109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Tronick EZ, Moore CL. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress & Health. 2013;29(4):337–344. doi: 10.1002/smi.2475. [DOI] [PubMed] [Google Scholar]
- Obasi EM, Shirtcliff EA, Brody GH, MacKillop J, Pittman D, Cavanagh L, Philibert RA. The relationship between alcohol consumption, perceived stress, and CRHR1 genotype on the hypothalamic-pituitary-adrenal axis in rural African Americans. Frontiers in Psychology. 2015;6:832. doi: 10.3389/fpsyg.2015.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57(5):460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Phillips CD, McLeroy KR. Health in rural America: remembering the importance of place. American Journal of Public Health. 2004;94(10):1661–1663. doi: 10.2105/ajph.94.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22(8):615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Ricketts TC, editor. Rural health in the United States. Oxford University Press; New York, NY: 1999. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, Pollak SD. Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Development. 2014;85(2):501–512. doi: 10.1111/cdev.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Hormones: Commentary: Riding the physiological roller coaster: Adaptive significance of cortisol stress reactivity to social contexts. Journal of Personality Disorders. 2014;28(1):40. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Skinner ML, Obasi EM, Haggerty KP. Positive parenting predicts calibration of cortisol functioning six years later in young adults. Developmental Science. doi: 10.1111/desc.12461. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Siahpush M. Widening rural–urban disparities in life expectancy, US, 1969–2009. American Journal of Preventive Medicine. 2014;46(2):e19–e29. doi: 10.1016/j.amepre.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development & Psychopathology. 2011;23(04):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine. 2003;65(3):461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality & Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality & Social Psychology. 2010;98(1):47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Van Horn ML, Bellis JM, Snyder SW. Family Resource Scale – Revised: Psychometrics and validation of a measure of family resources in a sample of low-income families. Journal of Psychoeducational Assessment. 2001;19:54–68. [Google Scholar]
- Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, … Bader K. Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology. 2015;51:58–67. doi: 10.1016/j.psyneuen.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT. What we remember from a stressful episode. Psychoneuroendocrinology. 2013;38(10):2268–2277. doi: 10.1016/j.psyneuen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]