Abstract
Objective
The mortality rate due to disseminated intravascular coagulation (DIC) is higher in patients with lung cancer than in those without. We examined the effect of treatment with thrombomodulin alfa (TM-α) for DIC in lung cancer patients.
Methods
Subjects were 57 patients with DIC (43 men, 14 women; mean age, 71.7 years), comprising 31 with lung cancer and 26 without. DIC patients with or without lung cancer did not differ significantly in their background characteristics.
Results
No significant difference was noted in the mortality rate between patients with lung cancer (61.3%) and those without (57.7%). However, the dose of TM-α was higher for survivors with lung cancer than for non-survivors (473.1 U/kg/day vs. 380.6 U/kg/day; p<0.01). Although no significant difference was noted in the DIC score between these four groups, the serum C-reactive protein level (6.9 mg/dL vs. 11.6 mg/dL; p<0.05) and prothrombin time-international normalized ratio (PT-INR; 1.10 vs. 1.52; p<0.05) were lower in survivors with lung cancer than in the non-survivors with lung cancer. The initial body temperature in non-survivors without lung cancer was lower than that in survivors without lung cancer (37.2℃ vs. 37.9℃, p<0.01), and the platelet count and the time to recovery from DIC in patients without lung cancer showed a significant negative correlation (r2=0.438, p<0.05).
Conclusion
Our findings suggest that although 380 U/kg/day of TM-α is the recommended dose for DIC treatment, a higher dose may reduce the mortality rate of lung cancer patients with DIC. Furthermore, TM-α should be initiated before worsening of DIC parameters.
Keywords: disseminated intravascular coagulation, lung cancer, thrombomodulin alfa
Introduction
Disseminated intravascular coagulation (DIC) is a manifestation of a coagulation system that has been activated by triggers, leading to the excessive production of microthromboses within the intravascular or extravascular spaces (1,2). These thrombi cause organ dysfunction, and increase the risk of death. The triggers of DIC identified thus far are severe infectious diseases, such as sepsis, and severe external wounds. The presence of solid tumors including lung cancer is also a risk factor for DIC (3,4). The mortality rate among lung cancer patients who develop DIC is very high (5).
Thrombomodulin alfa (TM-α), which is used in the treatment of DIC, has been commercially available in Japan since 2008 (6,7). TM binds to thrombin, thereby down-regulating the coagulatory activity of thrombin. The thrombin-TM complexes activate protein C, and this activated protein C normalizes the coagulation system. Thrombin-TM complexes also cleave high-mobility group box 1 (HMGB1) protein, which is a mediator of endotoxin lethality and promotes the development of DIC and inflammation in the systematic circulation (8).
However, the role of TM and DIC in lung cancer patients has not yet been explored. Several reports have been published on the relationship between prognosis and TM expression in lung cancer (9-11). Pathological analyses have shown that patients with TM-negative lung cancer have a poor prognosis. Studies have shown that DIC is triggered by cancer procoagulant or HMGB1, which is released from the tumor or necrotic cells (12,13). Taken together, these findings suggest that TM may be the most suitable treatment for DIC in lung cancer patients. In this study, we sought to elucidate the relationship between TM and DIC in lung cancer patients by comparing the effects of TM-α in patients with or without lung cancer. Moreover, we analyzed more important markers for the mortality of DIC in lung cancer patients than the DIC score.
Materials and Methods
Study design
TM-α, which is available under the trade name Recomodulin™ (Asahi Kasei Corporation, Tokyo, Japan), was introduced for the treatment of DIC in August 2011 at the Department of Respiratory Medicine and Clinical Immunology, Koshigaya Hospital, Dokkyo Medical University. All 57 patients with DIC who were treated with TM-α at our department between August 2011 and December 2014 were enrolled in this study. This study was designed as a retrospective investigation of patient data to compare patients with or without lung cancer and survivors and non-survivors of DIC.
Data regarding the baseline white blood cell (WBC) count and serum C-reactive protein (CRP) at the time of the diagnosis of DIC were obtained in addition to the data on the baseline levels of blood coagulation factors, including the platelet count, prothrombin time-international normalized ratio (PT-INR), fibrin, serum fibrinogen degradation products (FDP), and serum D-dimer. The relationship between these factors and the mortality rate was analyzed. The study protocol complied with the guidelines of the Ethics Committee of Dokkyo Medical University.
Subjects
The subjects were 57 patients (43 men, 14 women; mean age, 71.7±8.6 years) diagnosed with DIC according to the criteria specified by the Japanese Association for Acute Medicine (2). Thirty-one of these patients had lung cancer, while 26 patients did not. Among the total 57 patients, 23 had survived to 3 points or lower on the DIC scoring system, while 34 had died before recovering to a DIC score of 3 points or lower. Background characteristics of the patients are shown in Table 1. Chi-square test revealed no significant differences between patients with lung cancer and those without in terms of the mortality rate (61.3% and 57.7%, respectively) and background characteristics. Patients were divided into four groups, depending on whether they were survivors or non-survivors and whether they did or did not have lung cancer. None of the patients developed adverse events related to TM-α treatment.
Table 1.
Background of Patients.
| No. of patients (%) | |||
|---|---|---|---|
| Total | 57 | (100.0) | |
| Sex | |||
| Male | 43 | (75.4) | |
| Female | 14 | (24.6) | |
| Lung cancer group | 31 | (54.4) | |
| Adenocarcinoma | 16 | (28.1) | |
| Small cell carcinoma | 8 | (14.0) | |
| Squamous cell carcinoma | 3 | (5.3) | |
| Histological type unknown | 2 | (3.5) | |
| Complex type | 2 | (3.5) | |
| Stage | |||
| IA | 1 | (1.8) | |
| IB | 1 | (1.8) | |
| IIA | 1 | (1.8) | |
| IIIA | 1 | (1.8) | |
| IIIB | 1 | (1.8) | |
| IV | 26 | (45.6) | |
| Non-lung cancer group | 26 | (45.6) | |
| Interstitial pneumonia | 7 | (12.3) | |
| Pneumonia | 7 | (12.3) | |
| COPD | 3 | (5.3) | |
| Heart failure | 2 | (3.5) | |
| Pneumoconiosis | 1 | (1.8) | |
| Myocardial infarction | 1 | (1.8) | |
| Renal cancer | 1 | (1.8) | |
| Subarachnoid hemorrhage | 1 | (1.8) | |
| Microscopic arteritis | 1 | (1.8) | |
| Multiple organ failure | 1 | (1.8) | |
| Cholecystitis | 1 | (1.8) | |
Statistical analysis
All statistical analysis was performed using Microsoft ExcelⓇ and JMPⓇ statistical software. Differences between two independent samples were examined by the chi-square test or Wilcoxon's signed-rank test. Differences at p<0.05 were considered significant.
Results
Relationship between the DIC score and clinical outcome of DIC in patients with or without lung cancer
Table 2 shows the relationship between the DIC score and the clinical outcome of DIC in patients with or without lung cancer (2). Patients with a DIC score of 7 and 8 points were combined, because they were few in number and separate statistical analyses could not be performed. No significant differences were found between the four groups by the chi-square test.
Table 2.
The Relationship between the DIC Score and DIC Mortality.
| DIC score | 4 points | 5 points | 6 points | 7 and 8 points | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence % (n) |
RR (95%CI) | Prevalence % (n) |
RR (95%CI) | Prevalence % (n) |
RR (95%CI) | Prevalence % (n) |
RR (95%CI) | χ2 (p value) |
Subject | |
| Did not recover from DIC | ||||||||||
| 1. Non-Lung Cancer | 6.67 (1/15) | 20.0 (3/15) | 20.0 (3/15) | 53.3 (8/15) | ||||||
| 2. Lung Cancer | 21.1 (4/19) | 0.32 (0.04-2.55) | 21.1 (4/19) | 0.95 (0.25-3.61) | 31.6 (6/19) | 0.63 (0.19-2.12) | 26.3 (5/19) | 2.02 (0.83-4.93) | 3.21 (0.361) | vs. "1" |
| Recovered from DIC | ||||||||||
| 3. Non-Lung Cancer | 36.4 (4/11) | 0.18 (0.02-1.42) | 27.3 (3/11) | 0.73 (0.18-2.97) | 18.2 (2/11) | 1.10 (0.22-5.51) | 18.2 (2/11) | 2.93 (0.77-11.2) | 5.11 (0.164) | vs. "1" |
| 4. Lung Cancer | 41.7 (5/12) | 0.16 (0.02-1.19) | 25.0 (3/12) | 0.80 (0.20-3.27) | 16.7 (2/12) | 1.20 (0.24-6.06) | 16.7 (2/12) | 3.20 (0.83-12.4) | 6.21 (0.102) | vs. "1" |
| 1.39 (0.707) | "2" vs. "3" | |||||||||
| 2.06 (0.559) | "2" vs. "4" | |||||||||
| 0.07 (0.995) | "3" vs. "4" | |||||||||
| 9.35 (0.405) | "1" ~ "4" | |||||||||
RR: relative risk, CI: confidence interval
Relationship between dose of TM-α and clinical outcome of DIC in patients with or without lung cancer
The dose of TM-α in each group was calculated per kilogram body weight per day. Analysis of the relationship between the dose and survival revealed that the dose of TM-α in survivors with lung cancer (473.1±81.6 U/kg/day) was greater than that in the other groups (non-survivors with lung cancer, 380.6±69.9 U/kg/day, p<0.01; survivors without lung cancer, 394.8±130.1 U/kg/day, not statistically significant [N.S.]; and non-survivors without lung cancer, 344.8±81.9 U/kg/day, p<0.01; Fig. 1).
Figure 1.
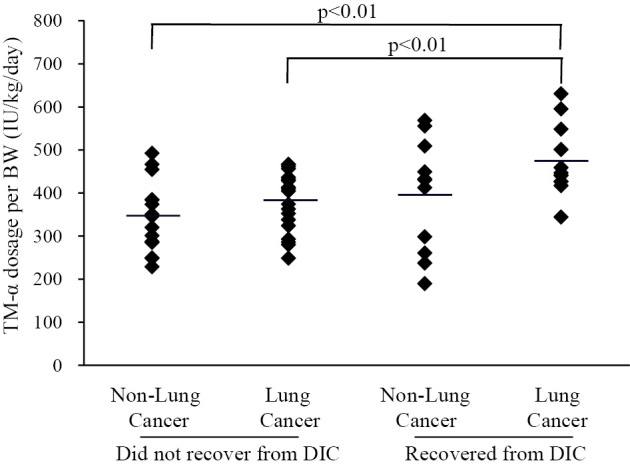
Relationship between dose of thrombomodulin alfa (TM-α) and clinical outcome of disseminated intravascular coagulation (DIC) in patients with or without lung cancer. The dose of TM-α per kilogram body weight (BW) was calculated. A significant difference was observed between the survivors and non-survivors with lung cancer.
To clarify the difference, we performed additional analyses. The high dose group received over 380 U/kg/day, which is the recommended dose for the treatment of DIC in Japan, and the low dose group received less than 380 U/kg/day. In lung cancer patients, the recovery rates from DIC were 52.4% in the high dose group and 10.0% in low dose group by the chi-square test (p<0.05). In the non-lung cancer patients, the recovery rates from DIC were 58.3% and 28.6% in the high dose and low dose groups, respectively. This was not statistically significant. A significant difference was found between the low dose group without lung cancer and the high dose group with lung cancer (p<0.05), but no significant difference was found in other comparisons. Also, no significant differences in vital signs or laboratory data were found between the high and low dose groups with or without lung cancer.
Relationship between vital signs and clinical outcome of DIC in patients with or without lung cancer
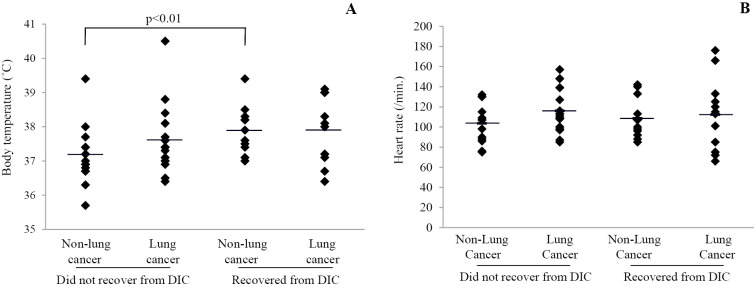
Body temperature and heart rate at diagnosis of DIC were analyzed in each of the four groups (Fig. 2). The initial body temperature (Fig. 2A) in non-survivors without lung cancer was lower than that in survivors without lung cancer (37.2±0.9℃ vs. 37.9±0.7℃, p<0.01). No significant differences were noted between the other groups (survivors with lung cancer, 37.9±1.0℃; non-survivors with lung cancer, 37.6±0.9℃). With respect to the heart rate at diagnosis of DIC (Fig. 2B), no significant differences were noted between the four groups (survivors with lung cancer, 112.3±35.2/min; non-survivors with lung cancer, 116.0±21.2/min; survivors without lung cancer, 108.5±20.8/min; non-survivors without lung cancer, 103.7±19.1/min).
Figure 2.
Relationship between vital signs and clinical outcome of DIC with or without lung cancer. The initial body temperature and heart rates at the time of diagnosis of DIC are shown in A and B, respectively. A significant difference in body temperature was observed between the survivors and non-survivors without lung cancer.
Relationship between laboratory data and clinical outcome of DIC in patients with or without lung cancer
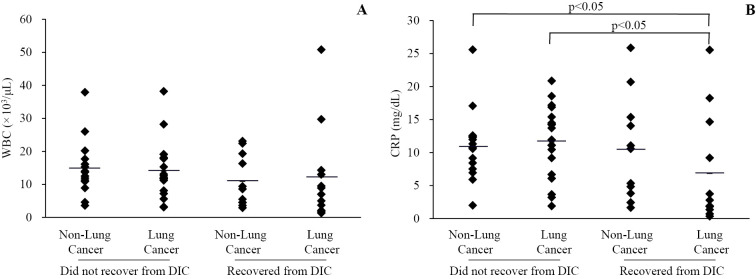
The WBC count and serum CRP level at the time of the diagnosis of DIC were analyzed in each of the four groups (Fig. 3). No significant differences were noted between the four groups in terms of the initial WBC count (Fig. 3A) (survivors with lung cancer, 12,250±14,465 /μL; non-survivors with lung cancer, 14,063±8,462 /μL; survivors without lung cancer, 10,918±7,853 /μL; non-survivors without lung cancer, 14,973±8,474 /μL). However, the initial serum CRP level (Fig. 3B) in survivors with lung cancer (6.9±8.3 mg/dL) was lower than that in the other groups (non-survivors with lung cancer, 11.6±5.4 mg/dL, p<0.05; survivors without lung cancer, 10.5±7.9 mg/dL, N.S.; non-survivors without lung cancer, 11.0±5.4 mg/dL, p<0.05).
Figure 3.
Relationship between laboratory data and clinical outcome of DIC with or without lung cancer. The initial white blood cell (WBC) count and serum C-reactive protein (CRP) level at the time of diagnosis of DIC are shown in A and B, respectively. A significant difference in CRP was observed between the survivors and non-survivors with lung cancer and between the survivors with lung cancer and non-survivors without lung cancer.
Relationship between blood coagulation factors and clinical outcome of DIC in patients with or without lung cancer
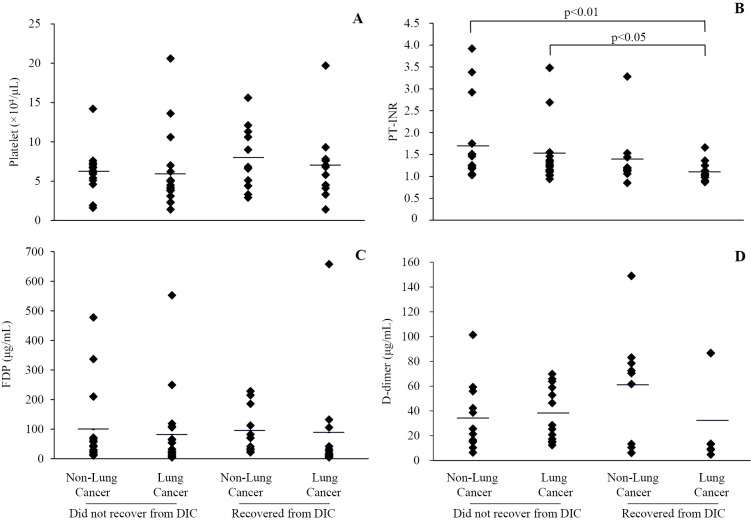
The platelet count, PT-INR, and serum levels of FDP and D-dimer at the time of the diagnosis of DIC were determined for the four groups (Fig. 4). While the initial platelet count (Fig. 4A) showed no significant intergroup difference (survivors with lung cancer, 7.0±4.6×104/μL; non-survivors with lung cancer, 5.9±4.6×104/μL; survivors without lung cancer, 8.0±4.1×104/μL; non-survivors without lung cancer, 6.2±2.8×104/μL), the initial PT-INR (Fig. 4B) in survivors with lung cancer (1.10±0.22) was lower than that in the other groups (non-survivors with lung cancer, 1.52±0.78, p<0.05; survivors without lung cancer, 1.38±0.66, N.S.; non-survivors without lung cancer, 1.71±0.92, p<0.01). However, the initial serum FDP level (Fig. 4C) showed no significant intergroup differences (survivors with lung cancer, 88.9±183.6 μg/mL non-survivors with lung cancer, 82.3±128.0 μg/mL; survivors without lung cancer, 94.9±79.3 μg/mL; non-survivors without lung cancer, 98.9±136.8 μg/mL). The initial serum D-dimer level (Fig. 4D) also showed no significant intergroup differences (survivors with lung cancer, 31.8±37.6 μg/mL; non-survivors with lung cancer, 37.8±22.1 μg/mL; survivors without lung cancer, 60.6±45.5 μg/mL; non-survivors without lung cancer, 34.0±27.5 μg/mL).
Figure 4.
Relationship between blood coagulation factors and clinical outcome of DIC in patients with or without lung cancer. The initial platelet count, prothrombin time-international normalized ratio (PT-INR), and serum levels of fibrinogen degradation products (FDP) and D-dimer at the time of the diagnosis of DIC are shown in A, B, C, and D, respectively. A significant difference in PT-INR was observed between the survivors and non-survivors with lung cancer and between the survivors with lung cancer and non-survivors without lung cancer.
Relationship between blood coagulation factor and time to recovery from DIC in the survivor group
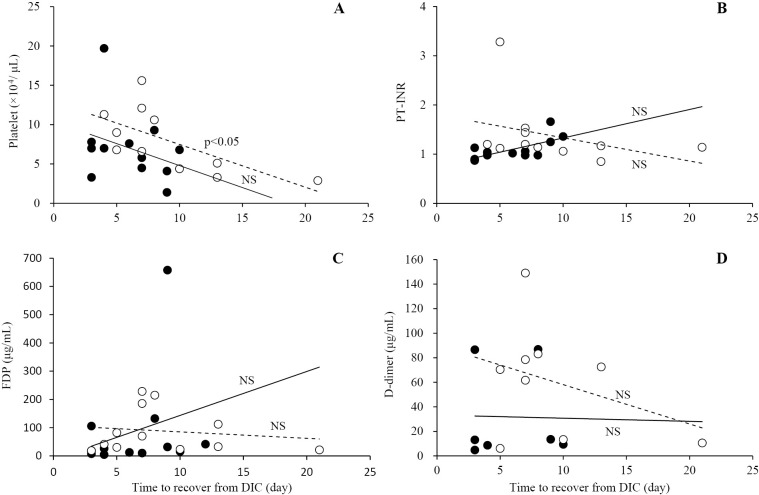
The relationship between the platelet count, PT-INR, and serum levels of FDP and D-dimer at the time of diagnosis of DIC and the recovery time from DIC among the survivors were analyzed (Fig. 5). The initial platelet count showed a significant negative correlation with the absence of lung cancer (r2=0.438, p<0.05), but not with the presence of lung cancer (r2=0.095, N.S.). However, the correlations of PT-INR, serum levels of FDP and D-dimer, and the presence/absence of lung cancer were not significant (presence of lung cancer, r2=0.287, r2=0.070, and r2=0.000; absence of lung cancer, r2=0.127, r2=0.074, and r2=0.127, respectively).
Figure 5.
Relationship between blood coagulation factor and time to recovery from DIC in the recovery group. The initial platelet count, PT-INR, and serum levels of FDP and D-dimer at the time of the diagnosis of DIC are shown in A, B, C, and D, respectively. Open and closed circles represent the non-lung cancer and lung cancer groups, respectively. With the regression line, broken and solid lines represent the non-lung cancer and lung cancer groups, respectively. A significant negative correlation in the platelet count was observed in patients without lung cancer.
Discussion
In this study, we analyzed the effects of TM-α in DIC patients with or without lung cancer. The dose of TM-α recorded for surviving lung cancer patients was significantly greater than that for the non-survivors with or without lung cancer. Furthermore, the initial serum CRP level and PT-INR were significantly lower in survivors with lung cancer than in non-survivors with lung cancer. These markers may be more important than the DIC score regarding mortality for patients with lung cancer.
TM-α, Recomodulin™, is sold in doses of 12,800 U/vial, and 380 U/kg/day for 7 days is the recommended dose and regimen for the treatment of DIC in Japan. Thus, for example, 1.5 vials are used for a patient with a body weight of 50 kg (5). Although the ideal dose of TM-α to be administered is 380 U/kg/day, doses that may be slightly less or more than required are administered using 1 vial, 1.5 vials, or 2 vials, as appropriate [e.g., a person weighing 40 kg who requires 1.19 vials (380 U/kg/day ×40 kg /12,800 U/vial =1.19) is administered 1 vial]. Therefore, different doses of TM-α per kilogram body weight are used among patients. Dose-response effects of Recomoculin™ in DIC patients were obtained between doses of 38 and 380 U/kg/day, as per the reports of the Phase IIb study conducted in Japan. Accordingly, a dose of 380 U/kg/day is officially recommended for the treatment of DIC; however, doses of more than 380 U/kg/day have not yet been evaluated. Our findings in this study indicate that the mortality of DIC improved after the administration of higher doses of TM-α, which was 473.1 U/kg/day in patients with lung cancer. The same trend was observed in patients without lung cancer, although the effect was not significant. On calculating the cut-off values for the survivors and non-survivors, the values obtained were 412.2 [area under curve (AUC), 0.701], 416.9 (AUC, 0.785), and 412.2 (AUC, 0.618) for all patients, lung cancer patients, and non-lung cancer patients, respectively. Although our study had a small sample population, our findings suggest that the recommended dose of TM-α warrants further investigation.
Regarding the expression of TM without lung cancer, the TM level in plasma is lower in chronic obstructive pulmonary disease (COPD) patients than in healthy controls (14). However, in idiopathic pulmonary fibrosis (IPF), the TM level in plasma is higher in patients with acute exacerbation than in stable patients (15). TM positive endothelial microparticles are increased in patients with severe sepsis, compared with healthy controls. IPF with acute exacerbation and infection will contribute TM expression (16). In Lung cancer, the relationship between TM and lung cancer has been frequently examined. Pathological studies have shown that TM expression is higher in squamous cell carcinoma than in adenocarcinoma or other types of lung cell carcinomas (9,17). Further, the percentage of patients with TM-positive serum among patients with stage IV metastatic lung cancer is higher than that among patients with stage I-IIIB localized lung adenocarcinoma (18). TM expression is also higher in mesothelioma than in adenocarcinoma (19,20). TM expression has been shown to influence the prognosis of lung cancer, with TM-negative lung cancer having a poorer prognosis than TM-positive lung cancer of the same stage (9,10,21). Therefore, pathological examination for TM expression may help assess the prognosis of the patients with lung cancer. TM is thought to suppress cell growth in lung cancer (22). Additionally, detection of plasma TM has been associated with pulmonary toxicity secondary to early-phase radiation for lung cancer (23). Thus, TM has several effects in lung cancer.
The numbers of patients with squamous cell carcinoma, adenocarcinoma, and small cell carcinoma in this study were 3, 16, and 8, respectively, and the corresponding numbers treated at our hospital during the study period were 191, 378, and 78. The percentage of squamous cell carcinoma patients with DIC was significant low (chi-square test; x2=9.328, p<0.01). This is probably because a high percentage of squamous cell carcinoma patients express TM, which may inhibit the development of DIC, although TM expression in infection or IPF is not clear.
Few studies have investigated the relationship between lung cancer and TM in DIC. One study has shown that the percentage of survival from DIC was 59.1% among lung cancer patients (24). In our study, 38.7% of patients with lung cancer recovered from DIC, which is lower that the abovementioned percentage. The exact reason for this difference is unknown, but it may be attributed to the fact that the previous study involved analysis of the background and other parameters for all solid tumors, including stomach, breast, and pancreas, and was not focused on lung cancer only. In our study, the survival rate was significantly higher in patients with lung cancer when DIC treatment was started in the presence of low serum CRP level and low PT-INR. We did not obtain data of DIC parameters before the development of DIC, and we could not find any relevant references for these values in lung cancer. Before the development of DIC in cases of solid tumors, the platelet count is low (9.3×104/μL); PT-INR is normal (1.13); and serum levels of FDP and D-dimer are high (23.3 and 12.0, respectively) (25). The fluctuation in the PT-INR may be associated with the progress of DIC in lung cancer because PT-INR is the only pertinent parameter reported to be normal before the development of DIC.
Lung cancer and its associated comorbid conditions such as infections are triggering factors for DIC. Although we did attempt to identify the trigger for DIC, it was not possible in all cases. All patients were prescribed antibiotics with TM-α because the doctors could not completely rule out the possibility of infection. Also, it was very difficult to differentiate between pneumonia and progression of lung cancer in non-survivors without anatomical pathology on examination by imaging only. To clarify these problems, we are currently conducting another study in which we will elucidate the triggers of DIC.
An important consideration in our study was whether TM-α should be used in the treatment of cases of DIC patients with advanced lung cancer, since such patients are expected to eventually succumb to the cancer and TM-α is very expensive. In such cases, we fully explained the treatment of DIC to the patients and/or their families and TM-α therapy was initiated once consent was obtained. Almost all the patients and their family members appreciated the delay of death. Therefore, we believe that it is meaningful to treat DIC in patients with advanced-stage lung cancer if they desire the treatment.
In conclusion, TM-α should be the first choice for the treatment of DIC in lung cancer patients, considering that TM improves the prognosis of lung cancer. The cut-off values noted in this study suggest that although the current recommended dose of TM-α is 380 U/kg/day for DIC treatment, a dose of more than 410 U/kg/day may be beneficial in the management of lung cancer patients who develop DIC. Furthermore, we noted that in lung cancer patients with DIC, serum CRP level and PT-INR were correlated with the mortality rate and the time to recovery from DIC; therefore, we suggest that TM-α be used before substantial worsening of these markers.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Ms. Seiko Sekiguchi, Ms. Kuniko Okuyama, and Ms. Natsumi Suzuki at Dokkyo Medical University Koshigaya Hospital for technical assistance.
References
- 1. Venugopal A. Disseminated intravascular coagulation. Indian J Anaesth 8: 603-608, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wada H, Matsumoto T, Hatada T. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. Expert Rev Hematol 5: 643-652, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Feinstein DI. Disseminated intravascular coagulation in patients with solid tumors. Oncology (Williston Park) 29: 96-102, 2015. [PubMed] [Google Scholar]
- 4. Carlson KS, DeSancho MT. Hematological issues in critically ill patients with cancer. Crit Care Clin 26: 107-132, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Voulgaris E, Pentheroudakis G, Vassou A, et al. . Disseminated intravascular coagulation (DIC) and non-small cell lung cancer (NSCLC): report of a case and review of the literature. Lung Cancer 64: 247-249, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Saito H, Maruyama I, Shimazaki S, et al. . Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 5: 31-41, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Yamakawa K, Ogura H, Fujimi S, et al. . Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med 39: 644-652, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito T, Kawahara K, Okamoto K, et al. . Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 28: 1825-1830, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Tamura A, Hebisawa A, Hayashi K, et al. . Prognostic significance of thrombomodulin expression and vascular invasion in stage I squamous cell carcinoma of the lung. Lung Cancer 34: 375-382, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Ogawa H, Yonezawa S, Maruyama I, et al. . Expression of thrombomodulin in squamous cell carcinoma of the lung: its relationship to lymph node metastasis and prognosis of the patients. Cancer Lett 149: 95-103, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Hamatake M, Ishida T, Mitsudomi T, et al. . Prognostic value and clinicopathological correlation of thrombomodulin in squamous cell carcinoma of the human lung. Clin Cancer Res 2: 763-766, 1996. [PubMed] [Google Scholar]
- 12. De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol 50: 187-196, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Hatada T, Wada H, Nobori T, et al. . Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost 94: 975-979, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Cella G, Sbarai A, Mazzaro G, et al. . Plasma markers of endothelial dysfunction in chronic obstructive pulmonary disease. Clin Appl Thromb Hemost 7: 205-208, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Collard HR, Calfee CS, Wolters PJ, et al. . Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 299: L3-L7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsumoto H, Yamakawa K, Ogura H, et al. . Enhanced expression of cell-specific surface antigens on endothelial microparticles in sepsis-induced disseminated intravascular coagulation. Shock 43: 443-449, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tolnay E, Wiethege T, Müller KM. Expression and localization of thrombomodulin in preneoplastic bronchial lesions and in lung cancer. Virchows Arch 430: 209-212, 1997. [DOI] [PubMed] [Google Scholar]
- 18. Tamura A, Komatsu H, Hebisawa A, et al. . Is thrombomodulin useful as a tumor marker of a lung cancer? Lung Cancer 15: 189-195, 1996. [DOI] [PubMed] [Google Scholar]
- 19. Ordóñez NG. The value of antibodies 44-3A6, SM3, HBME-1, and thrombomodulin in differentiating epithelial pleural mesothelioma from lung adenocarcinoma: a comparative study with other commonly used antibodies. Am J Surg Pathol 21: 1399-1408, 1997. [DOI] [PubMed] [Google Scholar]
- 20. Collins CL, Ordonez NG, Schaefer R. Thrombomodulin expression in malignant pleural mesothelioma and pulmonary adenocarcinoma. Am J Pathol 141: 827-833, 2010. [PMC free article] [PubMed] [Google Scholar]
- 21. Liu PL, Tsai JR, Chiu CC, et al. . Decreased expression of thrombomodulin is correlated with tumor cell invasiveness and poor prognosis in nonsmall cell lung cancer. Mol Carcinog 49: 874-881, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Fujiwara M, Jin E, Ghazizadeh M, et al. . Antisense oligodeoxynucleotides against thrombomodulin suppress the cell growth of lung adenocarcinoma cell line A549. Pathol Int 52: 204-213, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Hauer-Jensen M, Kong FM, Fink LM, et al. . Circulating thrombomodulin during radiation therapy of lung cancer. Radiat Oncol Investig 7: 238-242, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Tamura K, Saito H, Asakura H, et al. . Recombinant human soluble thrombomodulin (thrombomodulin alfa) to treat disseminated intravascular coagulation in solid tumors: results of a one-arm prospective trial. Int J Clin Oncol 20: 821-828, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Kawasugi K, Wada H, Hatada T, et al. . Japanese Society of Thrombosis Hemostasis/DIC Subcommittee. Prospective evaluation of hemostatic abnormalities in overt DIC due to various underlying diseases. Thromb Res 128: 186-190, 2011. [DOI] [PubMed] [Google Scholar]