Abstract
The pathogenesis of cerebral/renal salt-wasting syndrome remains unknown. We herein present a case of salt-wasting syndrome with a natural killer-cell neoplasm without cerebral invasion. A 78-year-old man with hemophagocytic syndrome received two cycles of chemotherapy that did not induce tumor lysis syndrome, but repeatedly caused polyuria and natriuresis. The expression of tumor necrosis factor-α in the neoplasm led us to hypothesize that an oncolysis-induced cytokine storm may have caused renal tubular damage and salt wasting. Our theory may explain the pathogenic mechanism of cerebral/renal salt-wasting syndrome associated with other entities, including cerebral disorders, owing to the elevation of cytokine levels after subarachnoid hemorrhage.
Keywords: cerebral salt wasting syndrome, renal salt wasting syndrome, pathophysiology, pathogenesis, tumor necrosis factor-alpha
Introduction
Certain neoplasms, including lymphoma, frequently invade the central nervous system (CNS) (1). Primary or secondary CNS neoplasm may cause hyponatremia because of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) (2) or hypernatremia with polyuria because of diabetes insipidus (3). Conversely, cerebral salt-wasting syndrome (CSWS), which occasionally occurs after a subarachnoid hemorrhage, includes hyponatremia with polyuria (4). Some clinicians assert that renal salt-wasting syndrome (RSWS), rather than CSWS, is a more appropriate term to characterize cases of salt wasting in the absence of cerebral lesions (5,6). The pathogenic mechanism of CSWS/RSWS has been assumed to involve the following two major hypothetical pathways related to natriuretic peptides or renal sympathetic disruptions. 1a) Increased circulating natriuretic peptides suppress the activity of the renin-angiotensin-aldosterone system (RAAS), and RAAS suppression inhibits renal tubular sodium reabsorption. 1b) The natriuretic peptides also directly inhibit sodium reabsorption in the renal tubule and intramedullary collecting-duct (7,8). 2a) The renal sympathoinhibitory action from injured brain or brain natriuretic peptide (9) increases glomerular filtration, 2b) inhibits renal tubular sodium reabsorption, and 2c) suppresses RAAS activity. As in the 1a pathway, RAAS suppression inhibits reabsorption (8,10). However, the pathogenic mechanism of CSWS/RSWS is still not clearly understood, and controversy remains (11,12). We herein report a case of CSWS/RSWS in a patient with a natural killer (NK)-cell neoplasm without CNS invasion. The salt wasting observed in this case may contribute to elucidating the pathogenic mechanism of CSWS/RSWS.
Case Report
A 78-year-old man presented to our hospital after 5 days of continuous high fever (over 39°C) and a low platelet count (4.4×104/μL). Urinalysis showed microhematuria (1+, <20 RBC/high power field), proteinuria (2+, 100 mg/dL), pH of 5.5, specific gravity of 1.024, and no glucose or leukocyte. He had a slightly elevated serum creatinine level (1.2 mg/dL). Findings of clotting tests ruled out disseminated intravascular coagulation. A peripheral blood smear showed no evidence of microangiopathy, such as schistocytes. Although his medical history included infective endocarditis due to Staphylococcus aureus, a relapse of this entity was ruled out based on negative findings on transthoracic echocardiography and blood cultures. Based on the results of bone marrow aspiration, including scattered reticulum cells, but no obvious hemophagocytosis, we first diagnosed the patient to have hemophagocytic syndrome of unknown cause. The high fever was controlled using steroid pulse therapy, but it recurred within 2 weeks. The serum creatinine levels improved to <0.8 mg/dL by hospital day 4 and were consistently maintained at <1.0 mg/dL except during recurrence of the fever. After the pulse therapy, he lost 5 kg in a week along with hyponatremia (<135 mEq/L) and had a urine output of >2,500 mL/day, despite consuming all food provided, free oral fluid intake, and infusions of >1,400 mL/day. A computed tomography scan, conducted when the patient first presented to our hospital, showed swelling of superficial lymph nodes without any obstruction of the urinary tract nor any bulky mass. Real-time polymerase chain reaction and Southern blotting for Epstein-Barr virus DNA in his blood revealed monoclonal proliferation (3.9×105 copies/106 cells). Analysis of a subcutaneous brachial lymph node biopsy specimen revealed an NK-cell neoplasm with lymphoma-associated hemophagocytic syndrome (Fig. 1). Both the cardiac function (ejection fraction, 60% at admission) and the brain natriuretic peptide level (14.6 pg/mL on hospital day 16) were normal before the initiation of chemotherapy. Chemotherapy using a CHOP-like regimen (cyclophosphamide, pirarubicin, vincristine, prednisolone, and etoposide) was initiated on hospital day 17, after which there was a temporary improvement in his fever and platelet count. However, polyuria with natriuresis (>5,500 mL/day; fractional excretion of sodium [FENa], >4%) developed with a lactate dehydrogenase surge after the chemotherapy was started. Despite only one injection of furosemide (t1/2 <0.5 hour) on the day chemotherapy was initiated (15), polyuria with natriuresis continued for longer than 1 week after that (Fig. 2). Despite massive infusions of solutions containing large amounts of sodium (>300 mEq/day), the serum sodium level hovered around 130 mEq/L because of the natriuresis. The continuous polyuria caused sustained hypouricemia (<2.0 mg/dL), after transient initial hyperuricemia because of tumor lysis. A negative fluid balance (urine volume exceeding fluid intake) and negative sodium balance (urinary sodium excretion exceeding sodium intake) indicated volume depletion and salt wasting (Fig. 2), with a detectable vasopressin level in a hypoosmotic state (0.9 pg/mL in estimated 275 mOsm/L on hospital day 20). An analysis of changes in the body weight also excluded overhydration and dilutional hyponatremia, apart from those conditions on the day chemotherapy was initiated. Thyroid function tests ruled out overt hypothyroidism (thyroid-stimulating hormone 2.5 μIU/mL, free T3 2.2 pg/mL, free T4 1.0 ng/dL). Steroid maintenance therapy given after the pulse therapy ruled out hypocortisolism (with prednisolone 5 mg/day, cortisol level 16.2 μg/dL on hospital day 32). Despite no obvious abnormalities on brain imaging, these clinical and laboratory findings were consistent with a diagnosis of CSWS rather than SIADH (5). Creatinine clearance levels were not elevated along with the polyuria until a sole peak on hospital day 22 (Fig. 2). Excessive urination persisted even at the onset of postchemotherapy neutropenic sepsis. In anticipation of septic renal failure, the infusion volume was reduced to 60 mL/h, but the patient unexpectedly experienced transient hypovolemic shock on hospital day 27. (Enterococcus faecalis was eventually isolated from blood cultures.) Massive infusions of crystalloid solutions (>3,000 mL/day) were required almost daily for 1 month, but did not cause congestive heart failure (brain natriuretic peptide, <130 pg/mL on hospital day 33). The creatinine clearance levels were maintained around 100 mL/min throughout the septic episode. The infection was treated with antibiotics, including amikacin and vancomycin. After the transient shock, FENa fell to <1%, below what is considered “adequate FENa,” that is, the value of FENa necessary to maintain a constant serum sodium concentration appropriate to sodium intake and with no extrarenal sodium loss (16). The fluid balance and the sodium balance also became positive (urine output less than intake), resulting in a normalized sodium concentration (138 mEq/L on hospital day 30) and weight gain (5 kg over 9 days), despite the patient's febrile state. The second cycle of chemotherapy (initiated on hospital day 37) exacerbated the polyuria and natriuresis, again resulting in negative fluid balance and relapse of hyponatremia (125 mEq/L). The patient lost weight (8.5 kg over 13 days) despite total parenteral nutrition (Fig. 2). He remained in a chemorefractory state and had an elevated ferritin level (>1.0×105 ng/mL). On hospital day 56, he died from massive bleeding from diffuse rectal mucosal detachment and ulcers, which could not be treated with endoscopic hemostasis.
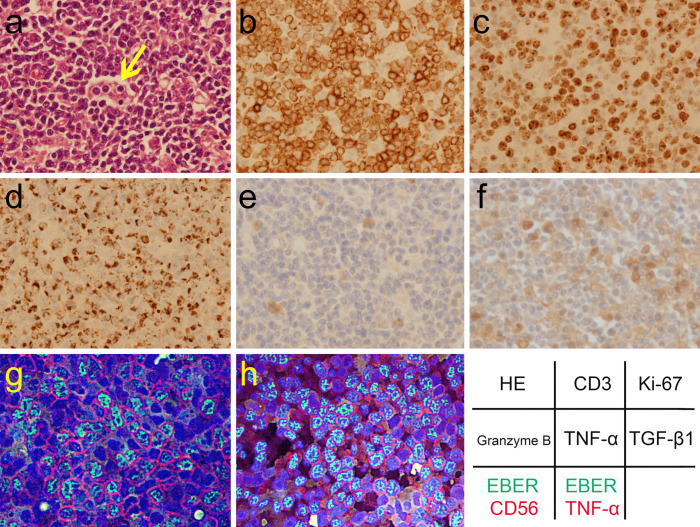
Figure 1.
The pathology of the NK-cell neoplasm with lymphoma-associated hemophagocytic syndrome from a resected lymph node. There are an increased number of atypical lymphoid cells with hemophagocytosis (arrow) on Hematoxylin and Eosin (HE) staining (a, ×40 of objective lens magnification). Immunohistochemical assay showed the immunophenotypes of the neoplasm: CD3+(b, ×40), CD4+, CD8−, CD20−, CD30+, and CD56+. The Ki-67 index was >90% (c, ×40). The neoplastic cells contained granzyme B (d, ×40). Immunohistochemical analysis of the stored biopsy samples also revealed expression of TNF-α+ (e, ×40) and TGF-β1+ (f, ×40), using TNFα (52B83) antibody (1:100 dilution; sc-52746; Santa Cruz Biotechnology, Dallas, USA), and TGFβ1 (V) antibody (1:200 dilution; sc-146; Santa Cruz Biotechnology), respectively (13, 14). Hybrid fluorescence imaging of immunostaining and Epstein-Barr virus-encoded small RNA (EBER)in situ hybridization precisely confirmed the immunophenotypes: green indicates EBER; red indicates CD56 (g, ×60) and TNF-α (1: 100 dilution; sc-52746; h, ×60) in each image; blue indicates DAPI-stained DNA. The component fluorescence images were taken with a fluorescence microscope BZ-8100 (Keyence, Osaka, Japan) by the second author.
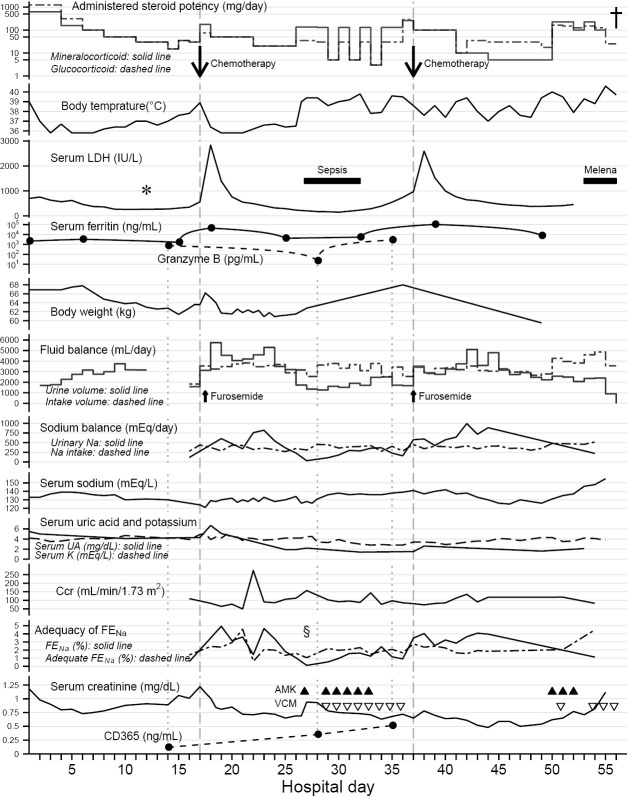
Figure 2.
Clinical course and laboratory findings in this case. The results of the lymph node biopsy (*) after methylprednisolone pulse therapy supported the use of chemotherapy for steroid-resistant high fever. The CHOP-like chemotherapies (down arrows) on hospital days 17 and 37 (vertical dashed lines) induced persistent polyuria and natriuresis after transient lactate dehydrogenase (LDH) surges in conjunction with the elevation of serum ferritin levels, with administration of corticosteroids with physiological mineralocorticoid potency. Negative fluid balance, negative sodium (Na) balance and body weight loss indicated volume depletion and salt wasting along with the polyuria. The low serum uric acid (UA) levels persisted even after recovery from the hyponatremia. Potassium (K) levels were managed with intravenous supplementation. The decreasing of granzyme B level on hospital days 28 reflected tumor burden; the simultaneous (as shown by vertical dotted lines) increase in CD365 levels indicated renal tubular damage. Fractional excretion of sodium (FENa) exceeded adequate FENa during polyuria, except during fever after the transient hypovolemic shock (§). This excess also suggested tubular dysfunction rather than tubular adaptation (16). Urine volume and urinary sodium excretion were independent of creatinine clearance (Ccr) levels, apart from sole spike of Ccr on hospital day 22. The administered steroid doses are shown to be equivalent to glucocorticoid and mineralocorticoid potencies of prednisolone by the following conversion coefficients: hydrocortisone, ×0.25 in glucocorticoid potency (dashed line), ×1.25 in mineralocorticoid potency (solid line); prednisolone, ×1.0, ×1.0; methylprednisolone, ×1.25, ×0.625; betamethasone, ×6.25, ×0.0 (17-19). Fluid intake includes oral fluids (negligible since hospital day 34), infused solutions, and transfusions. Sodium intake includes dietary salt (negligible since hospital day 24) and infused sodium. These were calculated from his dietary intake and infused dose, respectively, on the observation chart recorded during nursing. The urinary sodium excretion [mEq/day] was estimated by urine volume [mL/day] × urinary sodium concentration [mEq/mL] (sampled from pooled urine in a collection bag, but not 24-hour pooled urine). The Ccr [mL/min per body surface area 1.73 m2] was estimated by the following formula: urine creatinine concentration [mg/dL] (same samples as for urinary sodium excretion) × urine volume [mL/min] ×1.73/(serum creatinine concentration [mg/dL] × a constant value of patient’s body surface area [m2]). The FENa [%] was calculated by the following formula: 100× urinary sodium concentration [mEq/L] × serum creatinine concentration [mg/dL]/(serum sodium concentration [mEq/L] × urinary creatinine concentration [mg/dL]). The adequate FENa [%] was calculated by the following formula (16): sodium intake [mEq/day] / (0.0144× Ccr [mL/min] × serum sodium concentration [mEq/L]). The dagger (†) represents the patient’s death. The black triangle (▲) represents amikacin (AMK) and the open inverted triangle (▽) represents vancomycin (VCM). This figure was drawn using the ggplot2 software package, version 2.0.0 in R, version 3.2.2.
A brain autopsy, performed with the consent of his family, and immunohistochemistry confirmed that there was no cerebral invasion by the neoplasm, even in the pituitary gland. The granzyme B level by enzyme-linked immunosorbent assay (ELISA) kit (human granzyme B, 850.790.048, Diaclone, Besançon, France) in the cerebrospinal fluid was <31.3 pg/mL, as compared with the serum level of 4.6×104 pg/mL, which also confirmed the absence of cerebral invasion. The neoplasm expressed tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β1 (Fig. 1). We measured these cytokines levels in stored platelet-poor plasma collected before and after the chemotherapy (on hospital day 14, and hospital days 28 and 35, respectively), using the Quantikine ELISA kits (human TNF-α, DTA00C; human TGF-β1, DB100B; R&D Systems, Minneapolis, USA). However, the measured values in the samples neither increased with chemotherapy nor exceeded the normal limits of reported healthy controls (TNF-α<40 pg/mL, TGF-β1<1.2×104 pg/mL) (20,21). The plasma granzyme B level returned to a normal level once after chemotherapy (<31.3 pg/mL on hospital day 28, compared with levels of <52.4 pg/mL reported in healthy controls) (22), reflecting the activity of hemophagocytic syndrome and the tumor burden. The levels of plasma kidney injury molecule-1 (KIM-1, synonyms: T-cell immunoglobulin mucin domain 1, TIM-1; hepatitis A virus cellular receptor 1, HAVCR1; recently defined as CD365) (23), which has been shown to correlate with renal tubular damage, was continuously elevated in the patient's samples (>0.078 ng/mL, the upper limit of normal reported in healthy controls) (24), quantified using human TIM-1 (HAVCR1) ELISA kit (EHHAVCR1, ThermoFisher Scientific, Waltham, USA). Therefore, the elevated CD365 levels, which were independent of the granzyme B levels, demonstrated renal tubular damage, even though CD365 has been reported to be expressed not only on proximal tubular epithelial cells (25), but also on NK-cells (26) and lymphoma cells (27) (Fig. 2). An analysis of stored samples was conducted based on informed consent signed while the patient was alive, in accordance with the Helsinki Declaration, and with the approval of the hospital ethics committee.
Discussion
This case indicates a potential previously unknown mechanism in the pathogenesis of CSWS/RSWS (4). According to the leading hypothesis, the release of natriuretic factors, particularly brain natriuretic peptide from the damaged brain, induces CSWS/RSWS (10). However, some studies have found no correlation between brain natriuretic peptide and CSWS/RSWS (11,12). The rare occurrence of CSWS/RSWS (28) and its similarity to SIADH (6) have thus made it difficult to elucidate the pathogenesis of CSWS/RSWS. In our case, negative fluid balances, even without including insensible water loss or sweat loss (29), and hypovolemic shock episode demonstrated volume depletion, which distinguishes CSWS/RSWS from SIADH including chemotherapy-induced SIADH (4), even though both SIADH and CSWS/RSWS have relatively high vasopressin levels in a hypoosmotic state (30). The prolonged hypouricemia after recovery from hyponatremia was also consistent with CSWS/RSWS, in which increased fractional excretion of uric acid (FEUA) persists, as opposed to SIADH, in which FEUA returns to normal (5,6). Even if the observed body weight loss after the chemotherapy or pulse therapy included lysed tumor weight, SIADH could be excluded by the fact that the weight decreased in the presence of hyponatremia. Because, the actual weight lost exceeded the tumor weight of advanced acute leukemia (3 kg of 3×1012 cells) in reported estimates (31). To the best of our knowledge, there has been no similar report of CSWS/RSWS occurring after chemotherapy for a lymphoid neoplasm without CNS lesions. There has only been one case report of CSWS/RSWS associated with a CNS lymphoma (32) and a few case reports of CSWS/RSWS in a non-CNS lymphoma following hematopoietic stem cell transplantation (33,34).
Four observations in this case support a new hypothesis regarding a pathogenic mechanism involving chemotherapy-induced excessive urination with hypouricemia (the opposite of what is seen in tumor lysis syndrome). First, the autopsy-proven absence of cerebral invasion confirmed that cerebral lesions are not essential for CSWS/RSWS (5,6). The limited elevation in the brain natriuretic peptide level and the absence of congestive cardiac failure in the clinical course did not support the previously postulated mechanisms involving brain natriuretic peptides (7,8). Second, the sustained polyuria while corticosteroids were administered, the dose of which exceeded a replacement dose for the physiological requirement for mineralocorticoid potency (50 mg prednisolone, 0.32 mg fludrocortisone equivalent) (17,18,35), contradicted the previously postulated mechanisms involving the suppression of RAAS activity (7,8,10). The acceleration of sodium reabsorption (<1% of FENa, less than the adequate FENa) with the transient hypovolemic shock at the febrile state indicated continued functioning of the RAAS and renal sympathetic nervous system. These findings also did not match the concept of mineralocorticoid-responsive hyponatremia of the elderly, which explained hyponatremia by age-related hyporesponsive RAAS (30). Third, the neoplasm expressed TNF-α with aggressive cell proliferation (Ki-67 positive rate, >90%). The fulminant clinical course of the neoplasm was consistent with some Epstein-Barr virus-associated T-cell and NK-cell lymphoproliferative diseases, such as aggressive NK-cell lymphoma or nodal NK-cell lymphoma. These are not distinct disease entities in the World Health Organization classification, 4th edition (36). Cytokine production from Epstein-Barr virus-related neoplasms induces hemophagocytic syndrome (37). An elevation of the circulating TNF-α levels has been reported in Epstein-Barr virus-associated NK-cell lymphomas (38). Although elevated TNF-α levels could not be detected in the stored postchemotherapy plasma because of its short half-life (t1/2 <3 hours) (39), the observed lactate dehydrogenase surges immediately after chemotherapy indicated cytokine storms of TNF-α, resulting from tumor lysis. Fourth, non-platinum-based chemotherapy in this case reproducibly exacerbated polyuria with natriuresis, similar to platinum-based chemotherapy-induced salt wasting via renal tubular damage (40,41). According to another hypothetical pathway proposed in a case report of sepsis-associated renal salt wasting, cytokine-induced glomerular hyperfiltration exceeding renal salt retention capacity causes CSWS/RSWS without kidney damage (42). However, calculations of creatinine clearance in our case demonstrated that the initiation of excessive urination did not occur because of an increased glomerular filtration rate, even if an observed peak of the clearance 5 days after the first chemotherapy could be explained by glomerular hyperfiltration in the diuretic phase of acute kidney injury. Through the oncolysis-induced cytokine storm and antibiotic nephrotoxicity, the plasma CD365 levels were elevated, suggesting renal tubular damage (24), regardless of the normal creatinine clearance levels. The integration of these findings indicated that before administering the nephrotoxic antibiotic infusions, post-oncolytic natriuresis was caused by renal tubular damage in normal glomerular filtration. Epithelial-mesenchymal transformation in renal tubular epithelial cells is caused by cytokines, mainly TNF-α rather than TGF-β1 (43). A recent study concerning cardiorenal syndrome showed a relationship, via TNF-α, between myocardial infarction and renal tubular cell apoptosis in rats (44). Therefore, we concluded that even a short-lived cytokine storm may induce long-term renal tubular dysfunction, causing natriuretic polyuria.
This theory of the pathogenesis of cytokine-induced salt wasting should be adapted to CSWS/RSWS following subarachnoid hemorrhage, because elevations of the cytokine levels in cerebral fluid and plasma after a subarachnoid hemorrhage have been reported in numerous studies (45,46). The pathogenic mechanism of CSWS/RSWS could be confirmed by future case-control studies measuring the serum or plasma cytokine levels in cases of subarachnoid hemorrhage, by comparing these levels between the patients with and without CSWS/RSWS.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank the following people for their effort and support. The treatment was performed under the consultation of the following experts: Aza Mizukami as a nephrologist, Hideyuki Maezawa as a cardiologist, Go Koizumi as an endocrinologist, Natsumi Uehara as a gastroenterologist, and Prof. Toshiyuki Mitsuya as a hematological expert pathologist. Takahiko Tonoike supported the autopsy and prepared the pathological sections. Atsuko Nagasawa supported the ELISA analysis. Prof. Tsuyoshi Nakamaki supervised and advised the ELISA analysis.
References
- 1.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 22: 1-6, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Teshima T, Akashi K, Shibuya T, et al. . Central nervous system involvement in adult T-cell leukemia/lymphoma. Cancer 65: 327-332, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Habu M, Tokimura H, Hirano H, et al. . Pituitary metastases: current practice in Japan. J Neurosurg 123: 998-1007, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Oh JY, Shin JI. Syndrome of inappropriate antidiuretic hormone secretion and cerebral/renal salt wasting syndrome: similarities and differences. Front Pediatr 2: 146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int 76: 934-938, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol 4: 309-315, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendes E, Walter M, Cullen P, et al. . Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 349: 245-249, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Momi J, Tang CM, Abcar AC, Kujubu DA, Sim JJ. Hyponatremia-what is cerebral salt wasting? Perm J 14: 62-65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol 37: 1221-1227, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cerdà-Esteve M, Cuadrado-Godia E, Chillaron JJ, et al. . Cerebral salt wasting syndrome: review. Eur J Intern Med 19: 249-254, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Costa KN, Nakamura HM, Cruz LR, et al. . Hyponatremia and brain injury: absence of alterations of serum brain natriuretic peptide and vasopressin. Arq Neuropsiquiatr 67: 1037-1044, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Kojima J, Katayama Y, Moro N, Kawai H, Yoneko M, Mori T. Cerebral salt wasting in subarachnoid hemorrhage rats: model, mechanism, and tool. Life Sci 76: 2361-2370, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama S, Yokote T, Hirata Y, et al. . TNF-α expression in tumor cells as a novel prognostic marker for diffuse large B-cell lymphoma, not otherwise specified. Am J Surg Pathol 38: 228-234, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Ho CL, Sheu LF, Li CY. Immunohistochemical expression of angiogenic cytokines and their receptors in reactive benign lymph nodes and non-Hodgkin lymphoma. Ann Diagn Pathol 7: 1-8, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Rupp W. Pharmacokinetics and pharmacodynamics of Lasix. Scott Med J 19 (Suppl 1): 5-13, 1974. [DOI] [PubMed] [Google Scholar]
- 16.Schück O. Fractional sodium excretion in patients with chronic renal failure with respect to the therapy. Nephron 30: 95-96, 1982. [DOI] [PubMed] [Google Scholar]
- 17.Liddle GW. Clinical pharmacology of the anti-inflammatory steroids. Clin Pharmacol Ther 2: 615-635, 1961. [DOI] [PubMed] [Google Scholar]
- 18.Travis RH, Sayers G. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 3rd ed. Goodman LS, Gilman A, Eds. Macmillan, New York, 1966: 1608-1648. [Google Scholar]
- 19.Forsham PH. The adrenals. In: Textbook of Endocrinology. 3rd ed. Williams RH, Ed. W.B. Saunders, Philadelphia, PA, 1962: 282-394. [Google Scholar]
- 20.Himmerich H, Fulda S, Linseisen J, et al. . TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw 17: 196-201, 2006. [PubMed] [Google Scholar]
- 21.Wakefield LM, Letterio JJ, Chen T, et al. . Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clin Cancer Res 1: 129-136, 1995. [PubMed] [Google Scholar]
- 22.Kamata Y, Kimura U, Matsuda H, et al. . Relationships among plasma granzyme B level, pruritus and dermatitis in patients with atopic dermatitis. J Dermatol Sci 84: 266-271, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Engel P, Boumsell L, Balderas R, et al. . CD Nomenclature 2015: Human Leukocyte Differentiation Antigen Workshops as a Driving Force in Immunology. J Immunol 195: 4555-4563, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Sabbisetti VS, Waikar SS, Antoine DJ, et al. . Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177-2186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichimura T, Bonventre JV, Bailly V, et al. . Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135-4142, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Kim HS, Lee CW, Chung DH. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol 184: 4095-4106, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto W, Nishikori M, Arima H, et al. . Expression of Tim-1 in primary CNS lymphoma. Cancer Med 5: 3235-3245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbalis JG. Hyponatremia with intracranial disease: not often cerebral salt wasting. J Clin Endocrinol Metab 99: 59-62, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Cox P. Insensible water loss and its assessment in adult patients: a review. Acta Anaesthesiol Scand 31: 771-776, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa S, Saito T, Fukagawa A, et al. . Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J Clin Endocrinol Metab 86: 1665-1671, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Rubinow SI, Lebowitz JL. A mathematical model of the acute myeloblastic leukemic state in man. Biophys J 16: 897-910, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochazka V, Kubova Z, Raida L, Papajik T, Paucek B, Indrak K. Cerebral salt wasting syndrome in a patient with primary CNS lymphoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 153: 219-220, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Jeon YJ, Lee HY, Jung IA, Cho WK, Cho B, Suh BK. Cerebral salt-wasting syndrome after hematopoietic stem cell transplantation in adolescents: 3 case reports. Ann Pediatr Endocrinol Metab 20: 220-225, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuda S, Mori T, Kato J, et al. . Sodium-losing nephropathy caused by tacrolimus after allogeneic hematopoietic stem cell transplantation. Rinsho Ketsueki (Jpn J Clin Hematol) 54: 2187-2191, 2013. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 35.Gupta P, Bhatia V. Corticosteroid physiology and principles of therapy. Indian J Pediatr 75: 1039-1044, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Ko YH. Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol 41: 29-39, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Chuang HC, Lay JD, Hsieh WC, Su IJ. Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to Epstein-Barr virus-associated T-cell lymphoma: nuclear factor-kappa B pathway as a potential therapeutic target. Cancer Sci 98: 1281-1287, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori A, Takao S, Pradutkanchana J, Kietthubthew S, Mitarnun W, Ishida T. High tumor necrosis factor-alpha levels in the patients with Epstein-Barr virus-associated peripheral T-cell proliferative disease/lymphoma. Leuk Res 27: 493-498, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi T. Phase I study of recombinant human tumor necrosis factor (rHu-TNF:PT-050). Cancer Detect Prev 12: 561-572, 1988. [PubMed] [Google Scholar]
- 40.Hamdi T, Latta S, Jallad B, Kheir F, Alhosaini MN, Patel A. Cisplatin-induced renal salt wasting syndrome. South Med J 103: 793-799, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Seyberth HW. Pathophysiology and clinical presentations of salt-losing tubulopathies. Pediatr Nephrol 31: 407-418, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Saleh M. Sepsis-associated renal salt wasting: how much is too much? BMJ Case Rep 2014. pii: bcr2013201838. doi: 10.1136/bcr-2013-201838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adachi T, Arito M, Suematsu N, et al. . Roles of layilin in TNF-α-induced epithelial-mesenchymal transformation of renal tubular epithelial cells. Biochem Biophys Res Commun 467: 63-69, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Cho E, Kim M, Ko YS, et al. . Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant 28: 2766-2778, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins SJ, McMahon CJ, Singh N, et al. . Cerebrospinal fluid and plasma cytokines after subarachnoid haemorrhage: CSF interleukin-6 may be an early marker of infection. J Neuroinflammation 9: 255, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Park DH, Back DB, et al. . Comparison of cerebrospinal fluid biomarkers between idiopathic normal pressure hydrocephalus and subarachnoid hemorrhage-induced chronic hydrocephalus: a pilot study. Med Sci Monit 18: PR19-PR25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]