Abstract
A 61-year-old woman with chronic-type adult T-cell leukemia-lymphoma (ATL) had been taking low-dose oral etoposide for progressive lymphocytosis. After taking this for 3.5 years, she was diagnosed with therapy-related acute myeloid leukemia (t-AML), with a chromosomal translocation of t (6:11) (q27; q23). She thus received remission induction therapy, consolidation therapy, and allogeneic hematopoietic stem cell transplantation. Although both t-AML and ATL were in remissive states, she died of a therapy-related infection within 1 year. We reviewed 12 reported cases of AML complicating ATL to better characterize this unusual disease. We should therefore include t-AML in the differential diagnosis when administering low-dose etoposide for ATL over a long period of time.
Keywords: t-AML, ATL, HTLV-1, allogeneic hematopoietic stem cell transplantation (allo-HSCT)
Introduction
Adult T-cell leukemia-lymphoma (ATL) is a distinct lymphoid neoplasm caused by human T-cell leukemia virus type 1 (HTLV-1). It is classified into four categories: acute, lymphoma, chronic, and smoldering (1). Intensive chemotherapy is usually administered to treat acute lymphoma, and unfavorable chronic types. The reported 4-year survival rates for acute-, lymphoma-, chronic-, and smoldering-type ATL are 5.0%, 5.7%, 26.9%, and 62.8%, respectively (2). Therapy-related acute myeloid leukemia (t-AML) after chemotherapy requires several years to develop; thus, few cases of t-AML with ATL have been reported (3-5). Favorable chronic- and smoldering-type ATL have relatively good prognoses. In Japan, they are typically followed by watchful waiting and such patients are not usually administered systemic chemotherapy. Five cases of AML complicated by chronic- or smoldering-type ATL have been reported; however, only two of these patients received chemotherapy (one was intensive chemotherapy; the other was oral etoposide) before developing AML, thus suggesting that these complications were not necessarily caused by chemotherapy (5).
Topoisomerase II inhibitors such as etoposide and alkylators are known to cause t-AML (6,7). In the last two decades, t-AML caused by the long-term administration of low-dose etoposide has been a problem in several malignancies (8-12). However, only one report (3) and two abstracts (13,14) have so far described t-AML after the administration of low-dose etoposide in ATL. Furthermore, no report has described allogeneic hematopoietic stem cell transplantation (allo-HSCT) for t-AML with ATL, although allo-HSCT is an established therapy for AML (15). We herein report a case of t-AML that arose after the long-term administration of low-dose etoposide for chronic-type ATL, followed by allo-HSCT after a remission of the t-AML.
Case Report
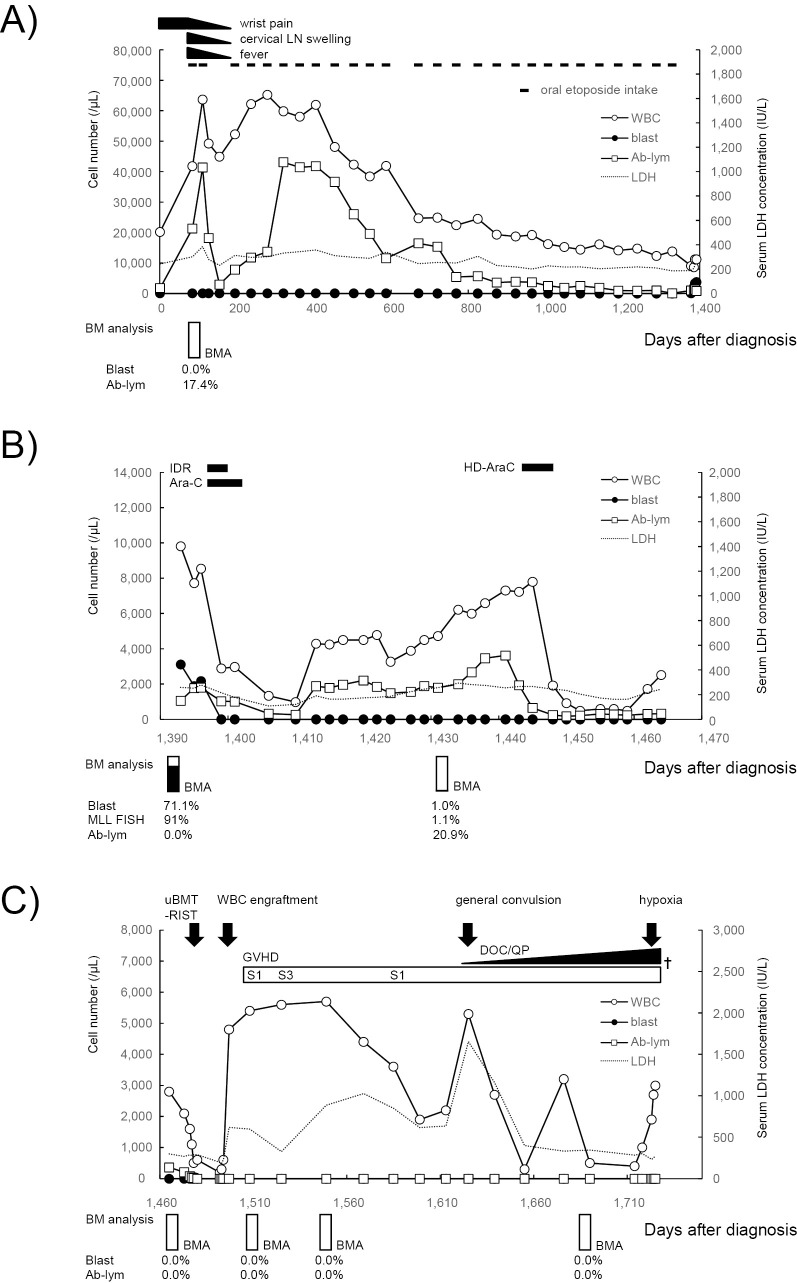
In May 2007, a 61-year-old Japanese woman presented with bilateral wrist pain, and leukocytosis (12×109/L) was discovered. Antibiotics did not improve her symptoms. In June 2007, she was diagnosed with chronic-type ATL (day 1) based on lymphocytosis (white blood cells 20.2×109/L; lymphocytes 69.6%, abnormal lymphocytes 8.6%) with the T-cell receptor (TCR) Cbeta1 gene rearrangement, anti-HTLV-1 antibody (+), and elevated lactate dehydrogenase (LDH), within twice the normal limit. Progressive lymphocytosis and LDH elevation occurred with fever and cervical lymph node swelling, as well as bilateral wrist pain near day 80. Her Eastern Cooperative Oncology Group performance status (PS) was 0 at this time. Oral etoposide was started on day 85 at 25 mg/day for 21 days at 7 week intervals in the first two courses and for 14 days at 6- to 8-week intervals after the third course. ATL cells were detected in the peripheral blood and bone marrow (BM) at the start of oral etoposide intake. After the second course, the physical symptoms, including the bilateral wrist pain, improved in line with the decrease in abnormal lymphocytes (Fig. 1A). Peripheral ATL cells decreased immediately after the second course of oral etoposide, but increased again after day 195. Thus, the intermittent administration of oral etoposide with a short interval was considered (Fig. 1A). ATL cells were well controlled by oral etoposide and decreased gradually over a long time period. Thus, the oral etoposide efficacy was considered to show a partial response. No severe adverse events were observed [common terminology criteria for adverse events (CTCAE) grade: hematological Grade 0, nausea Grade 0, vomiting Grade 0, anorexia Grade 0, alopecia Grade 0]. There was insufficient information about fever, cervical lymph node swelling, and bilateral wrist pain for CTCAE grading. The oral etoposide administration continued until day 1,343 (total, 9,800 mg in 27 courses). Cytomegalovirus (CMV) antigenemia was negative during the oral etoposide therapy.
Figure 1.
Schematic drawing of the treatment procedure and the changes in hematological parameters. The clinical course is shown separately for the treatment with oral etoposide (A) to ATL, remission induction and consolidation therapies for t-AML (B), and allo-HSCT (C). WBC: white blood cell, Blast: myeloblast, Ab-lym: abnormal lymphocyte, LDH: lactate dehydrogenase, IDR: idarubicin, Ara-C: cytarabine, BM: bone marrow, BMA: bone marrow aspiration, uBMT (RIST): unrelated bone marrow transplantation (reduced intensity stem cell transplantation), GVHD: graft-versus-host disease, S: skin, DOC/QP: disturbance of consciousness and quadriplegia, †: date of death
In April 2011 (day 1,378), the platelet count fell rapidly and she was thus referred to a Research Hospital, The Institute of Medical Science, The University of Tokyo for further examination (day 1,393). Physical examination revealed anemic conjunctiva, no superficial lymph node swelling, and no skin eruptions. On computed tomography, splenomegaly was detected, but no systemic lymph node enlargement. Laboratory tests showed the following: white blood cells 9.8×109/L (myeloblasts 31.7%, abnormal lymphocytes 10.7%), hemoglobin 112 g/L, platelets 45×109/L, LDH 258 IU/L, and soluble interleukin-2 receptor 1,640 U/mL. No hypercalcemia was observed. In the peripheral blood, both abnormal lymphocytes and myeloblasts were observed (Fig. 2A). The monoclonal integration of HTLV-1 DNA was also detected in her peripheral blood mononuclear cells by a Southern blot analysis. On hospitalization, 87% of the nucleated cells in the BM were myeloblasts (Fig. 2B), which were positive for peroxidase staining, and CD13+CD14-CD33+ human leukocyte antigen (HLA)-DR+ by flow cytometry, but negative for nonspecific esterase staining. We diagnosed t-AML according to the WHO classification (2008) and AML with myelocytic maturation (AML M2) according to the French-American-British (FAB) classification. She was PS 0 at the time of t-AML diagnosis, which was 50 days after the last oral etoposide intake (day 1,393). Chromosomal translocation involving the myeloid/lymphoid leukemia (MLL) gene was identified by fluorescence in situ hybridization. The karyotype analysis revealed 46, XX, t (6; 11) (q27;q23) in 17 of 20 cells analyzed. With remission induction therapy using idarubicin plus cytarabine, the level of peripheral myeloblasts decreased immediately and the t-AML entered complete remission, but the number of ATL cells remained and increased again after day 1,410 (Fig. 1B). After administering consolidation therapy using high-dose cytarabine, the number of peripheral ATL cells decreased from 3,600 /μL (day 1,441) to 320 /μL (day 1,464; Fig. 1B). Thus, although the efficacy of remission induction therapy to ATL was considered to indicate progressive disease, consolidation therapy resulted in a partial response of the ATL. CMV antigenemia turned positive on day 1,462, but no symptoms due to CMV infection were observed at that time.
Figure 2.
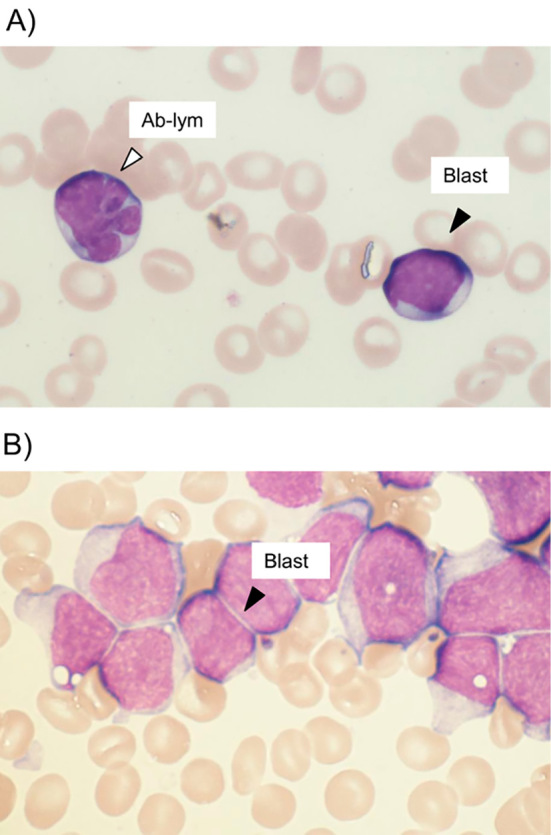
(A) Myeloblasts and abnormal lymphocytes in the peripheral blood at day 1,404 (May-Giemsa ×1,000). The black arrowheads indicate myeloblasts and the white arrowhead indicates an abnormal lymphocyte. (B) Myeloblasts in the bone marrow at day 1,393 (May-Giemsa ×1,000). The black arrowheads indicate myeloblasts. Blast: myeloblast, Ab-lym: abnormal lymphocyte
When the t-AML was in hematological remission on day 1,479 after ATL diagnosis, allo-HSCT was performed from an HLA-matched unrelated donor using a reduced-intensity conditioning regimen with fludarabine 180 mg/m2 intravenously (iv), busulfan 8 mg/kg iv, and 2 Gy of total body irradiation. White blood cell engraftment was identified on day 1,495. Thrombotic thrombocytopenic purpura or thrombotic microangiopathy was suspected as a cause of the LDH elevation after day 1,497. Acute graft versus host disease (GVHD) was observed on day 1,512 as skin lesions of stage 1-2 and reached a maximum on day 1,517 as skin lesions of stage 3. The skin GVHD was managed by steroids for internal and external use (Fig. 1C). GVHD was not observed in the liver or gastrointestinal system. Methicillin-resistant Staphylococcus aureus sepsis occurred on day 1,555 and interstitial pneumonia was observed on day 1,610. CMV antigenemia persisted after allo-HSCT and did not turn negative throughout her clinical course, probably because sufficient doses of foscarnet or ganciclovir could not be used due to BM failure and renal dysfunction. A general convulsion occurred on day 1,625 and encephalomyelitis, caused by CMV, was suspected. Additionally, her disturbance of consciousness and quadriplegia progressed and she fell into hypoxia on day 1,724. She died from respiratory failure on day 1,726. The t-AML was in a remissive state and no ATL cells were detected in the peripheral blood or BM after allo-HSCT in her clinical course. Computed tomography scanning and magnetic resonance imaging did not show any ATL-like lesions. No abnormal lymphocytes were detected in the cerebrospinal fluid obtained by lumbar puncture. The efficacy of allo-HSCT with regard to ATL was considered represent a complete response. An autopsy revealed CMV pneumonia as well as CMV encephalitis and cerebral ventriculitis. ATL cells and myeloblasts were not detected in the autopsy. The direct cause of her death was presumed to be respiratory failure, due to CMV pneumonia.
Discussion
This was a case of t-AML caused by etoposide used to treat chronic-type ATL, in a patient who underwent allo-HSCT for t-AML. The most important finding is that low-dose oral etoposide triggered t-AML as a single agent after long-term administration. Most reports on t-AML caused by etoposide have described the intravenous administration of etoposide and a combination of other cytotoxic drugs (16). The second important finding is that both t-AML and ATL maintained remissive states after allo-HSCT.
Chemotherapy for chronic-type ATL is considered at the time of disease progression and in cases with unfavorable prognostic factors. In the present case, the patient had an unfavorable prognostic factor when diagnosed. No transformation to acute-type ATL was observed despite LDH elevation (Fig. 1A). Although intensive chemotherapy followed by allo-HSCT, instead of oral etoposide, was also possible, which might have presented the onset of t-AML thus leading to a better prognosis, the patient did not choose this until t-AML had already occurred. Tsukasaki et al. (5) described a possible association between ATL and AML regardless of chemotherapy; however, we diagnosed our case to have t-AML based on the history of oral etoposide intake. Our case had etoposide-induced AML with unique characteristics, which usually occurs as M4 or M5 carrying a translocation involving 11q23, and develops within 3 years (6,16). Typically, alkylating agent-induced AML has the M2 phenotype and a latent period of longer than 4 years (16). The oral administration of etoposide, rather than intravenous, might have contributed to the unique characteristics observed in our case (12).
A few reports have described secondary AML in ATL (Table). Of the 12 reported cases, 3 had received no treatment before the onset of AML. Therefore, these AMLs were caused by factors other than chemotherapy. Another four cases had been given oral etoposide first (1 chronic, 1 lymphoma, and 2 acute type). Of these four cases, three had received intensive chemotherapy in addition to oral etoposide, while one (chronic type) received only oral etoposide for 3 months before the onset of AML. We believe that this last case is important as a candidate for t-AML caused by oral etoposide; however, the authors were unable to conclude whether the AML was due to ATL or oral etoposide because there was no earlier report of AML M4 with BM eosinophilia (M4Eo) as a comorbidity with ATL and the duration of oral etoposide therapy was short (13). Recently, Owatari et al. reported secondary AML that occurred 5 years after ATL diagnosis (3). Their patient received intensive chemotherapy for ATL and additional oral etoposide therapy for 29 months. It is therefore difficult to determine precisely which therapy played a critical role in the onset of secondary AML. However, based on the findings of our case, we deem it necessary to consider secondary AML when low-dose etoposide is administered in ATL for a long time.
Table.
Clinical Features of Concurrent ATL and AML; Review of the Literature.
| Patient No. |
Age/ gender |
Subtype of ATL |
Subtype of AML |
Karyotype of AML | Latent Period (m) |
Preceding Oral VP-16 | Other chemotherapy before AML | Survival (m) |
Refs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No | dose/ period (m) |
|||||||||
| 1 | 49/M | Smoldering | M1 | not evaluable | 0 | No | - | none | 6 | [5], [21] |
| 2 | 64/M | Acute | M5 | 47,XY,+8 | 17 | No | none | DXR, VP-16, CY, MTX | 18 | [5], [22] |
| 3 | 37/M | Chronic | M2 | 46,XY,t(9;11)(p22;q23) | 23 | No | - | VCR, CY, DXR, THP-ADM, MCNU, VCM, VP-16, CBDCA | 24 | [5], [23] |
| 4 | 36/F | Smoldering | M4 | 46, XX, t(15;17)(q22;q21) | 0 | No | - | none | 30+ | [5], [24] |
| 5 | 74/F | Chronic | M4Eo | 46,XY, inv(16)(p13q22) | 17 | Yes | nr/3 | none | 18 | [5], [13] |
| 6 | 51/M | Acute | M2 | 46,XY,7q- | 24 | Yes | 20,000 mg/16 | Polypharmacy including high dose VP-16 | nr | [5], [14] |
| 7 | 81/F | Chronic | M4 | 47,XX,+8,2p-,10p+ | 2 | No | - | none | 29+ | [5] |
| 8 | 69/M | Acute | M2 | 46,XY,7q-,18p+ | 26 | No | - | CY, DXR, MCNU, THP-ADM, VP-16, CBDCA | 39 | [5] |
| 9 | 63/F | Lymphoma | M4 | 46,XX,t(8;16)(p11;p13) | 23 | No | - | CY, DXR, VCR, MIT, MTX, VP-16 | 27 | [5] |
| 10 | 63/F | Acute | M4 | 46,XX,inv(9)(p11q13)c, inv(16)(p13q22) |
23 | Yes | nr/nr | VCR, CY, DXR, MCNU, VCM, VP-16, CBDCA, Ara-C, MTX | 30 | [4] |
| 11 | 64/M | Acute | M2 | 46,XY,t(8;21)(q22;q22) | 21 | No | - | VCR, CY, DXR, MCNU, VCM, VP-16, CBDCA, Ara-C, MTX, MST-16 | nr | [4] |
| 12 | 50/F | Lymphoma | M2 | 46, X, t(X;10)(p11.2;p11.2), t(5;12)(q31;p13), inv(9)(p12q13) |
63 | Yes | 10,000 mg/29 | VCR, CY, DXR, MCNU, VCM, VP-16, CBDCA, Ara-C, MTX | 66 | [3] |
| Present case | 61/F | Chronic | M2 | 46,XY,t(6;11)(q23,q27) | 47 | Yes | 9,800 mg/41 | none | 58 | |
VP-16: etoposide, VCR: vincristine, CY: cyclophosphamide, DXR: doxorubicin, MCNU: ranimustine, VDS: vindesine, CBDCA: carboplatin, Ara-C: cytarabine, MTX: methotrexate: THP-ADM: pirarubicin, MST-16: sobuzoxane, nr: not reported, m: months, refs: references
Secondary leukemia including t-AML is less responsive to chemotherapy than de novo leukemia and the median reported survival time is 7-8 months (17,18). Although the prognosis of t-AML can be improved by allo-HSCT, it is still worse than other subtypes of AML (19,20). Our patient achieved a complete remission of t-AML with remission induction therapy and remained in remission after one course of consolidation therapy. Tsukasaki et al. (5) also reported that 4 of 7 AML on ATL cases experienced complete remission. They described that the onset of secondary AML on ATL could be explained by such mechanisms as immune dysfunction and cytokine production caused by ATL, rather than chemotherapy or radiotherapy. Given that this was true in our case, t-AML on ATL occurred more readily and harbored less DNA damage than t-AML with typical etiology, which could explain the high sensitivity to chemotherapy of t-AML on ATL. Idarubicine plus cytarabine showed anti-tumor efficacy only for AML in this case; however, the high-dose cytarabine used for consolidation therapy suppressed the growth of both ATL and AML cells. Complete remission of t-AML was maintained up to autopsy. Furthermore, both peripheral and BM ATL cells disappeared after allo-HSCT and were not detected throughout the rest of her clinical course, including the autopsy survey. Although both t-AML and ATL were in remissive states, the patient died of a therapy-related infection within 1 year after allo-HSCT. Her age (>50 years) and status other than a complete response to ATL were risk factors that she had for allo-HSCT for ATL, and these are associated with lower survival rates (2), which might also be related to her prognosis. Our case is also the first reported one to receive allo-HSCT for t-AML on ATL. More such cases are needed to evaluate the therapeutic efficacy of allo-HSCT in this situation.
In conclusion, we herein reported a case of t-AML complicated with chronic-type ATL. We should therefore consider t-AML in the differential diagnosis when administering low-dose etoposide for ATL for a long period of time.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Dr. Koji Miyazaki of Kitasato University School of Medicine (Sagamihara, Japan), Dr. Kiyosumi Ochi, and Ms. Etsuko Nagai of The Institute of Medical Science, The University of Tokyo (Tokyo, Japan) for valuable assistance with the preparation of the manuscript.
References
- 1. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol 79: 428-437, 1991. [DOI] [PubMed] [Google Scholar]
- 2. Tanosaki R, Tobinai K. Adult T-cell leukemia-lymphoma: Current treatment strategies and novel immunological approaches. Expert Rev Hematol 3: 743-753, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Owatari S, Arai A, Tsuruta T, Haraguchi K, Otsuka M, Hanada S. Acute myeloid leukemia diagnosed 5 years after adult t-cell leukemia/lymphoma. J Clin Exp Hematop 55: 29-31, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Owatari S, Otsuka M, Uozumi K, Takeshita T, Hanada S. Two cases of secondary acute myeloid leukemia accompanying adult T-cell leukemia/lymphoma. Int J Hematol 85: 32-35, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Tsukasaki K, Koba T, Iwanaga M, et al. Possible association between adult T-cell leukemia/lymphoma and acute myeloid leukemia. Cancer 82: 488-494, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Smith MA, Rubinstein L, Ungerleider RS. Therapy-related acute myeloid leukemia following treatment with epipodophyllotoxins: Estimating the risks. Med Pediatr Oncol 23: 86-98, 1994. [DOI] [PubMed] [Google Scholar]
- 7. Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: Susceptibility and incidence. Haematologica 92: 1389-1398, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Katato K, Flaherty L, Varterasian M. Secondary acute myelogenous leukemia following treatment with oral etoposide. Am J Hematol 53: 54-55, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Stine KC, Saylors RL, Sawyer JR, Becton DL. Secondary acute myelogenous leukemia following safe exposure to etoposide. J Clin Oncol 15: 1583-1586, 1997. [DOI] [PubMed] [Google Scholar]
- 10. Okamoto T, Okada M, Wakae T, Mori A, Takatsuka H, Kakishita E. Secondary acute promyelocytic leukemia in a patient with non-Hodgkin's lymphoma treated with VP-16 and MST-16. Int J Hematol 75: 107-108, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Schiavetti A, Varrasso G, Maurizi P, Castello MA. Two secondary leukemias among 15 children given oral etoposide. Med Pediatr Oncol 37: 148-149, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Kikuta T, Shimazaki C, Hirai H, et al. Three cases of secondary acute myeloid leukemia after long-term treatment with oral etoposide. Rinsho Ketsueki 37: 1276-1282, 1996. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 13. Osato M, Asou N, Takatsuki K, Takada N, Kawano F. A case of chronic type adult T-cell leukemia complicated with acute myelomonocytic leukemia (M4Eo) [abstract]. Int J Hematol 61: 77, 1995. (in Japanese).7734715 [Google Scholar]
- 14. Satoh M, Hatsumi N, Itoh K, Sakura T, Shiozaki H, Miyawaki S. Acute myeloid leukemia in a patient treated with etoposide for adult T-cell leukemia [abstract]. Jpn J Clin Hematol 36: 994, 1995. (in Japanese). [Google Scholar]
- 15. Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood 127: 53-61, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitlock JA, Greer JP, Lukens JN. Epipodophyllotoxin-related leukemia: Identification of a new subset of secondary leukemia. Cancer 68: 600-604, 1991. [DOI] [PubMed] [Google Scholar]
- 17. Smith SM, LeBeau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood 102: 43-52, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Olney HJ, Mitelman F, Johansson B, Mrózek K, Berger R, Rowley JD. Unique balanced chromosome abnormalities in treatment-related myelodysplastic syndromes and acute myeloid leukemia: Report from an international workshop. Genes Chromosomes Cancer 33: 413-423, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Witherspoon RP, Deeg HJ, Storer B, Anasetti C, Storb R, Appelbaum FR. Hematopoietic stem-cell transplantation for treatment-related leukemia or myelodysplasia. J Clin Oncol 19: 2134-2141, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Litzow MR, Tarima S, Pérez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood 115: 1850-1857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohtsu T, Tobinai K, Minato K, et al. Concurrent adult T-cell leukemia and acute myeloblastic leukemia. Jpn J Clin Oncol 18: 33-41, 1988. [DOI] [PubMed] [Google Scholar]
- 22. Tokioka T, Shimamoto Y, Funai N, et al. Coexistence of acute monoblastic leukemia and adult T-cell leukemia: Possible association with HTLV-I infection in both cases? Leuk Lymphoma 8: 147-155, 1992. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura H, Ishizaki T, Itoyama T, et al. Acute myeloid leukaemia with t(9;11)(p22;q23) in a patient treated for adult T cell leukaemia. Br J Haematol 86: 222-224, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Tsukasaki K, Fujimoto T, Hata T, Yamada Y, Kamihira S, Tomonaga M. Concomitant complete remission of APL and smoldering ATL following ATRA therapy in a patient with the two diseases simultaneously. Leukemia 9: 1797-1798, 1995. [PubMed] [Google Scholar]