Abstract
A 35-year-old male who had not previously suffered any major illnesses was admitted to our hospital because of general fatigue, fever, headache, vomiting, consciousness disturbance, and seizures. A neurological examination showed that he was in a semi-comatose state and exhibited neck stiffness. Brain magnetic resonance imaging detected high-intensity areas in the bilateral hippocampi and periventricular white matter. A cerebrospinal fluid examination revealed mononuclear pleocytosis, an elevated protein level, and positivity for human herpesvirus-7 (HHV-7) DNA. The patient's condition improved after the administration of methylprednisolone, intravenous immunoglobulins, and acyclovir. This is the first known case of limbic encephalitis associated with HHV-7 in an immunocompetent Japanese adult.
Keywords: encephalitis, human herpesvirus-7, adult, immunocompetent
Introduction
Human herpesvirus-7 (HHV-7) is a ubiquitous virus belonging to the β-herpesvirinae subfamily. Most people are initially infected with HHV-7 in childhood, causing an asymptomatic latent infection or exanthema subitum (1,2). Neurological complications of HHV-7 infection, including meningitis, encephalitis (3,4), cerebellitis (5), or myelitis (6), can occur in rare cases. Such complications mainly occur in children or immunocompromised hosts (7). Neurological involvement in healthy adults has only been reported in 4 cases (8-10). We herein report the first confirmed case of limbic encephalitis associated with HHV-7 in an immunocompetent adult in Japan.
Case Report
The patient was a 35-year-old male with no history of major illness or family history of immunodeficiency. He had been doing well until he developed general fatigue, low-grade fever, and vomiting 2 weeks before this admission. Four days before admission, he went to a general hospital because of a persistent headache and a high fever of 39℃. Nasopharyngeal swab tests for influenza A and B were both negative, and he was given ibuprofen. At midnight on the day of admission, he suddenly groaned, foamed at the mouth, and lost consciousness, and his wife witnessed his eyes turn upward. He was taken to hospital by ambulance and suffered 5 or 6 generalized seizures. Diazepam and phenobarbital were administered, and he finally was transferred to our hospital.
On physical examination, the patient was found to have a body temperature of 38.9℃, a pulse rate of 94 beats/min, and a blood pressure of 131/81 mmHg, and marked hyperhidrosis and dark reddish skin eruptions were noted on the right forehead and scalp. No abnormalities of the chest, abdomen, or extremities were observed. A neurological examination revealed consciousness disturbance (Japan Coma Scale score: 100; Glasgow Coma Scale score: 6 [E1V1M4]), neck stiffness, and hyporeflexia of the bilateral lower extremities.
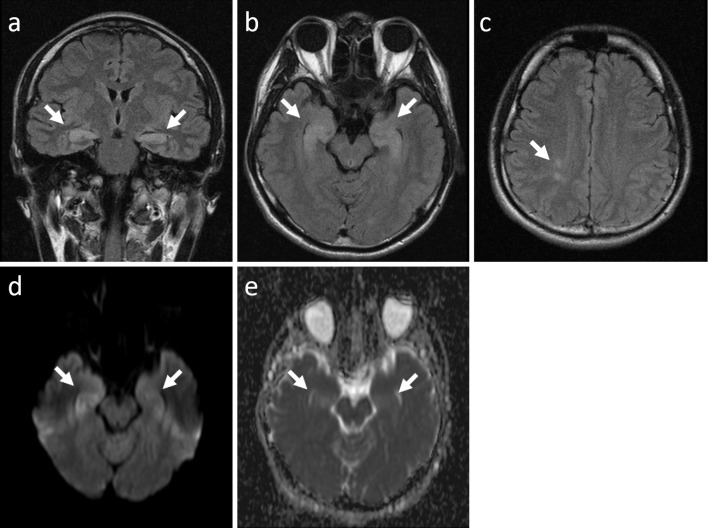
A chest X-ray and electrocardiogram demonstrated normal results. Brain magnetic resonance imaging (MRI) showed high-intensity lesions in the bilateral hippocampi and periventricular white matter on fluid-attenuated inversion recovery images (Fig. 1a-c). Diffusion-weighted imaging and apparent diffusion coefficient mapping showed high-intensity signals emanating from the lesions, which were suggestive of vasogenic edema (Fig. 1d and e). Contrast-enhanced brain MRI did not show any abnormal enhancement (data not shown).
Figure 1.
Brain magnetic resonance imaging showed high-intensity areas in the bilateral hippocampi (a and b, arrows) and periventricular white matter (c, arrow). Diffusion-weighted imaging (d) and apparent diffusion coefficient mapping (e) showed high-intensity signals (arrows), which were suggestive of vasogenic edema.
The patient's white blood cell count was 12,420/mm3 (neutrophils: 88.7%, lymphocytes: 4.8%, monocytes: 4.9%, eosinophils: 0.1%, basophils: 0.3%, and atypical lymphocytes: 0.0%), and a test for C-reactive protein was negative (0.14 mg/dL). His creatine kinase was elevated (2,225 IU/L), which was considered to have been caused by the general seizures. Other routine blood and urine biochemical analyses did not detect any significant abnormalities.
A cerebrospinal fluid (CSF) analysis performed on admission revealed a normal pressure (16 cmH2O), a colorless appearance, pleocytosis (35/mm3) with a predominance of lymphocytes (94%), a high protein level (76.5 mg/dL), a normal glucose level (113 mg/dL; plasma glucose: 160 mg/dL), and a normal IgG level (4.6 mg/dL; IgG index: 0.50).
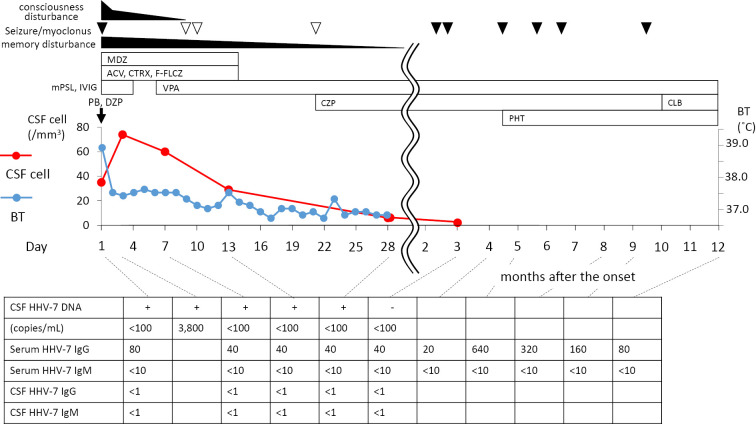
Polymerase chain reaction (PCR)-based tests of the CSF sample produced a positive result for HHV-7 DNA, but negative results for herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, cytomegalovirus (CMV), and HHV-6. Repeated CSF tests confirmed the positive HHV-7 DNA result and the results of PCR were verified with positive and negative controls. A quantitative analysis of the HHV-7 DNA detected 3,800 copies/mL in the CSF specimen obtained on the 3rd hospital day, and a blood sample was also positive for HHV-7 DNA. The patient's serum HHV-7-IgG and IgM levels (fluorescence antibody technique) were 80x and <10x on admission, and his serum HHV-7-IgG level had increased on the follow-up examination (peak: 640x after 5 months), but his serum HHV-7-IgM level remained at <10x. The patient's CSF HHV-7-IgG and IgM levels were <1x and <1x, respectively. All viral DNA assays were performed by a commercial laboratory (SRL, Tokyo, Japan) (Fig. 2).
Figure 2.
Clinical course of the case. The first vertical axis (left) and second axis (right) of the graph show the cell counts of the cerebrospinal fluid and body temperature, while the closed and open triangles indicate fits of seizures and myoclonus, respectively. ACV: acyclovir, BT: body temperature, CLB: clobazam, CSF: cerebrospinal fluid, CTRX: ceftriaxone, CZP: clonazepam, DZP: diazepam, F-FLCZ: fosfluconazole, HHV-7: human herpesvirus-7, IVIG: intravenous immunoglobulin, MDZ: midazolam, mPSL: methylprednisolone, PB: phenobarbital, PHT: phenytoin, VPA: valproate
A culture of the patient's CSF did not show any growth of bacteria, mycobacteria, or fungi. PCR-based tests of the patient's CSF for mycobacterium tuberculosis and avium/intracellulare were negative, and a cytological examination did not show any malignant cells. A serological test for human immunodeficiency virus was negative. The rapid plasma reagin test for syphilis and the Treponema pallidum hemagglutinin test were also negative. Similarly, serological tests for autoantibodies including rheumatoid factor, antinuclear antibodies, and paraneoplastic antibodies (anti-Hu, Yo, and Ri) were all negative.
Tumor marker tests (carcinoembryonic antigen, alpha-fetoprotein, squamous cell carcinoma antigen, cytokeratin 19 fragment, and the soluble interleukin-2 receptor) all produced negative results. Contrast-enhanced chest and abdominal computed tomography (CT) exhibited normal findings. Brain single-photon emission CT (SPECT) and 67Ga-scintigraphy did not show any abnormalities. Electroencephalography (EEG) performed on admission showed slow waves in the frontal area, and the follow-up EEG demonstrated spikes in the left hemisphere.
Methylprednisolone (1 g/day, 3 days), intravenous immunoglobulins (IVIG) (5 g/day, 3 days), ceftriaxone (4 g/day, 7 days), acyclovir (1,500 mg/day, l4 days), glycerol (400 mL/day, 14 days), midazolam (20 mg/day, 14 days), and fosfluconazole (100 mg/day, 6 days) were administered. The patient's seizures and fever improved on the 2nd hospital day, and his consciousness gradually became clear. However, he demonstrated a severe memory disturbance. His revised Hasegawa Dementia Scale (HDS-R) score [a modified simple dementia scale in Japan, which is closely compatible with the Mini-Mental State Examination (MMSE)] was 12 points (full score: 30 points) on the 3rd hospital day and he could not recall any of 3 objects even after hints were given. We started to administer valproate (400 mg/day) on the 6th day. On the 9th day, myoclonus appeared; therefore, we added clonazepam (0.5 mg/day) and obtained a good response. His memory disturbance had disappeared by the 31st day [HDS-R score 29/30; MMSE 29/30; Wechsler adult intelligence scale-revised (WAIS-R) intelligent quotient (IQ) 86 verbal IQ (VIQ) 81, performance IQ (PIQ) 96)], and he left our hospital on the 37th hospital day. The patient's CSF cell counts normalized, and the HHV-7 DNA disappeared three months later (Fig. 2).
Since he suffered several seizures after being discharged, phenytoin and clobazam were added serially (Fig. 2). Carbamazepine was also used, but it was terminated because of a drug-induced lip eruption. At present, he is independent in terms of his daily life and ability to work. He has not shown any emotional or behavioral disturbances. He has been followed for 7 years at our clinic without any recurrence of his encephalitis. He is in good health and does not exhibit any signs of an immunocompromised state or autoimmune disorders.
Brain MRI showed a gradual improvement of the high-intensity areas in the hippocampus (data not shown). Blood tests for HHV-7 DNA continued to produce positive results throughout the follow-up period.
Discussion
This is the first reported case of encephalitis associated with HHV-7 in an immunocompetent Japanese adult, and the fifth such case in the world. It was diagnosed based on the detection of HHV-7 DNA in the patient's CSF and significant changes in his serum IgG titer to HHV-7. The absence of an elevated serum HHV-7-IgM level indicates that this case involved HHV-7 re-activation.
There have been a few reports about the neurological complications of HHV-7 in HIV-positive patients (6,11) or transplant recipients (3,12-16), but such complications are very rare in immunocompetent adults (8-10). Of these cases, one case was considered to involve a primary infection (8), and another was considered to involve a previous primary infection and CMV co-infection (9). The type of infection was not specified in the other cases (10). Our patient displayed positivity for HHV-7-IgG on admission, which suggests that the present case involved HHV-7 reactivation. While our patient demonstrated persistent positivity for HHV-7 DNA in his blood after he had recovered, such findings are also seen in healthy individuals (17).
It is unclear why our patient, an immunocompetent adult, developed HHV-7-associated encephalitis. We did not perform whole genome sequencing of HHV-7 in this case; therefore, we do not know if some genotypic variation or mutations played a role. We have not detected any genetic or environmental susceptibility factors in our patient.
We cannot completely exclude the possibility of concomitant causes (i.e., HHV-7 co-activation induced by other causes), but our long-term follow-up did not identify any other potential causes of the patient's illness. HHV-7 is sometimes co-activated in the presence of CMV (9,15,19,20), but our case was not complicated by a co-infection with CMV.
Antiviral treatment is usually unnecessary for HHV-7 infections because most infections are asymptomatic or self-limiting. No standard treatment for HHV-7-associated neurological complications has been established, but our patient exhibited a good clinical recovery after the administration of methylprednisolone and IVIG. Acyclovir was also used as a treatment for possible herpes simplex encephalitis, but this approach remains controversial because acyclovir and other thymidine kinase-dependent drugs are only marginally effective against HHV-7 (21). Alternative treatments include ganciclovir, foscarnet, and cidofovir (18), but these drugs can cause serious side effects including bone marrow suppression and renal dysfunction. Further investigation is necessary to establish an effective treatment for HHV-7-associated neurological complications.
The main lesion in this case is thought to be limbic system and its involvement sometimes shows severe memory impairment, seizure and psycho-behavioral symptoms. The determinants of prognosis for HHV-7 encephalitis are unknown, but it is possible that the relatively good clinical course observed in this case could have been due to his youth, a negative finding for HIV and no other concomitant illnesses.
In conclusion, this is the first reported Japanese adult case of HHV-7-associated encephalitis in an immunocompetent patient. This case suggests the neurotropic and pathogenic potential of HHV-7, and it is recommended that a systematic survey of herpesviruses should be performed in patients presenting with meningitis/limbic encephalitis, even in immunocompetent adults, to ensure an accurate diagnosis and the use of appropriate medication, and to increase our understanding of the pathophysiology of HHV-7.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J Clin Virol 32: 183-193, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Ward KN. Human herpesviruses-6 and -7 infections. Curr Opin Infect Dis 18: 247-252, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Chan PK, Peiris JS, Yuen KY, et al. Human herpesvirus-6 and human herpesvirus-7 infections in bone marrow transplant recipients. J Med Virol 53: 295-305, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Pohl-Koppe A, Blay M, Jäger G, Weiss M. Human herpes virus type 7 DNA in the cerebrospinal fluid of children with central nervous system diseases. Eur J Pediatr 160: 351-358, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Hacohen Y, Niotakis G, Aujla A, et al. Acute life threatening cerebellitis presenting with no apparent cerebellar signs. Clin Neurol Neurosurg 113: 928-930, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Escobar-Villalba A, Sainz de la Maza S, Pérez Torre P, et al. Acute myelitis by human herpes virus 7 in an HIV-infected patient. J Clin Virol 77: 63-65, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Clark DA. Human herpesvirus 6 and human herpesvirus 7: emerging pathogens in transplant patients. Int J Hematol 76 (Suppl 2): 246-252, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Ward KN, Kalima P, MacLeod KM, Riordan T. Neuroinvasion during delayed primary HHV-7 infection in an immunocompetent adult with encephalitis and flaccid paralysis. J Med Virol 67: 538-541, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Ginanneschi F, Donati D, Moschettini D, et al. Encephaloradiculomyelitis associated to HHV-7 and CMV co-infection in immunocompetent host. Clin Neurol Neurosurg 109: 272-276, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Miranda CM, Torres JPT, Larrañaga CL, Acuña GL. Meningomyelitis associated with infection by human herpes virus 7: report of two cases. Rev Med Chil 139: 1588-1591, 2011. (in Spanish, Abstract in English). [PubMed] [Google Scholar]
- 11. Broccolo F, Iuliano R, Careddu AM, et al. Detection of lymphotropic herpesvirus DNA by polymerase chain reaction in cerebrospinal fluid of AIDS patients with neurological disease. Acta Virol 44: 137-143, 2000. [PubMed] [Google Scholar]
- 12. Ward KN, White RP, Mackinnon S, Hanna M. Human herpesvirus-7 infection of the CNS with acute myelitis in an adult bone marrow recipient. Bone Marrow Transplant 30: 983-985, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Chan PK, Chik KW, To KF, et al. Case report: human herpesvirus 7 associated fatal encephalitis in a peripheral blood stem cell transplant recipient. J Med Virol 66: 493-496, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Ljungman P. Beta-herpesvirus challenges in the transplant recipient. J Infect Dis 186 (Suppl 1): S99-S109, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Razonable RR, Paya CV. The impact of human herpesvirus-6 and -7 infection on the outcome of liver transplantation. Liver Transpl 8: 651-658, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Holden SR, Vas AL. Severe encephalitis in a haematopoietic stem cell transplant recipient caused by reactivation of human herpesvirus 6 and 7. J Clin Virol 40: 245-247, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Kidd IM, Clark DA, Ait-Khaled M, Griffiths PD, Emery VC. Measurement of human herpesvirus 7 load in peripheral blood and saliva of healthy subjects by quantitative polymerase chain reaction. J Infect Dis 174: 396-401, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Clark DA, Emery VC, Griffiths PD. Cytomegalovirus, human herpesvirus-6, and human herpesvirus-7 in hematological patients. Semin Hematol 40: 154-162, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Singh N. Human herpesviruses-6, -7 and -8 in organ transplant recipients. Clin Microbiol Infect 6: 453-459, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Dockrell DH, Paya CV. Human herpesvirus-6 and -7 in transplantation. Rev Med Virol 11: 23-36, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Yoshikawa T. Human herpesvirus-6 and -7 infections in transplantation. Pediatr Transplant 7: 11-17, 2003. [DOI] [PubMed] [Google Scholar]