Abstract
A 56-year-old woman, without any immunocompromising diseases, was referred to our hospital because of a recurrence of pyogenic spondylitis. Computed tomography revealed multiple osteolytic changes in the whole body. Vertebral magnetic resonance imaging revealed osteomyelitis and spondylitis. Mycobacterium scrofulaceum was detected in sputum cultures, in abscesses from the right knee, and in a subcutaneous forehead abscess. Therefore, the patient was diagnosed with disseminated Mycobacterium scrofulaceum infection. The patient was treated with rifampicin, ethambutol, and clarithromycin, which resulted in symptomatic relief and radiological improvement. We herein report a rare case of disseminated Mycobacterium scrofulaceum infection in an immunocompetent host.
Keywords: Mycobacterium scrofulaceum, osteomyelitis, pyogenic spondylitis
Introduction
Nontuberculos mycobacteria (NTM) are widely found in the environment, such as in dust, soil, and water and more than 150 species have been identified. Mycobacterium scrofulaceum is a slow-growing NTM and classified in the Runyon group II. M. scrofulaceum infections are rare and account for around 2.2% of NTM infections (1). There are only a few reports describing disseminated mycobacterial infections due to M. scrofulaceum (2-10). We herein report a rare case of disseminated M. scrofulaceum infection in an immunocompetent host.
Case Report
A 56-year-old woman suffering from fever and general fatigue visited a neighboring hospital and was examined. During her hospitalization, pyogenic spondylitis complicated by an iliopsoas abscess, due to an infection with Mycobacterium species, was diagnosed. However, the species causing the condition was not identified. An infection with Mycobacterium tuberculosis was suspected, and isoniazid (INH), rifampicin (RFP), pyrazinamide, and ethambutol (EB) were prescribed for 2 months from January 2011. Following the initial treatment, INH and RFP were prescribed for 7 additional months. However, the patient's fever persisted, and the iliopsoas abscess gradually worsened. Therefore, in June 2011, for a period of 2 months, a daily dose of 500 mg levofloxacin (LVFX) was added to the INH and RFP regimen, and the abscess improved. In April 2014, the patient was referred to our hospital for further examination and treatment because of a recurrence of pyogenic spondylitis.
The results of the physical examination were as follows: body temperature, 36.8℃; pulse rate, 68 beats per minute; blood pressure, 93/75 mmHg; respiratory rate, 16 breaths per minute; and oxygen saturation, 97% on room air. Respiratory sounds and cardiac and abdominal examination findings were normal. There was a purulent region on the left sinciput (Fig. 1) and her right knee was slightly red, swollen, and painful.
Figure 1.
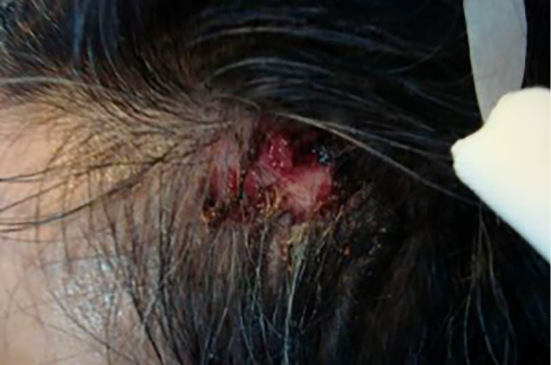
There was a purulent region on the left sinciput.
The white blood cell count was 12,100 /μL with 73.4% neutrophils, 14.2% lymphocytes, 4.2% monocytes, 7.7% eosinophils, and 0.5% basophils. The levels of C-reactive protein, alkaline phosphatase, and soluble interleukin-2 receptor were elevated to 18.25 mg/dL (normal range: 0-0.3 mg/dL), 988 U/L (normal range: 110-340 U/L), and 4,459 U/mL (normal range: 127-582 U/mL), respectively. Tests for serum antibodies to T-cell leukemia virus and immunodeficiency virus yielded negative results. An interferon-gamma (IFN-γ) release assay, QuantiFERONⓇ-TB Gold (QFT-G) was indeterminate (M. tuberculosis antigen response: <0.05 IU/mL; negative control: <0.05 IU/mL; mitogen response: <0.05 IU/mL).
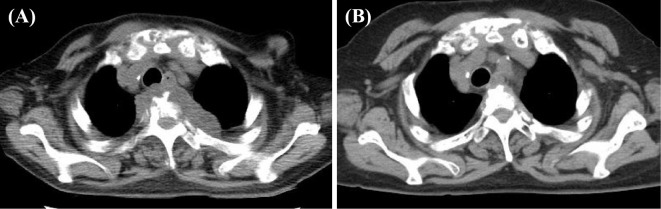
Computed tomography (CT) revealed osteolytic changes in the vertebrae and abscesses adjacent to the T3 vertebral body (Fig. 2A). 18-fluorine-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) examination showed multiple lesions with an intense FDG accumulation in the abscesses adjacent to the T3 vertebral body and various bones, such as the skull, sternum, clavicles, ribs, vertebral columns, scapulae, pelvic bones, bilateral humerus, and thigh bones. These findings of osteolytic changes in the bones of the whole body more strongly suggested the presence of metastatic carcinomas and blood diseases, such as malignant lymphoma and multiple myeloma than the existence of bacterial infections.
Figure 2.
(A) Computed tomography (CT) image showing osteolytic changes in the vertebrae and abscesses adjacent to the T3 vertebral body. (B) A CT image showing an improvement at 21 months after treatment with rifampicin, ethambutol, and clarithromycin.
T2-weighted imaging (WI) and diffusion-WI (DWI) revealed several hyperintense signals in the vertebral bodies, their surroundings, the intervertebral spaces, which was consistent with osteomyelitis and spondylitis (Fig. 3), and in the right thigh bone and tibia (Fig. 4). These hyperintense signals of T2-WI and DWI, in investigated bones more strongly suggested bacterial infections than metastatic carcinomas and blood diseases.
Figure 3.
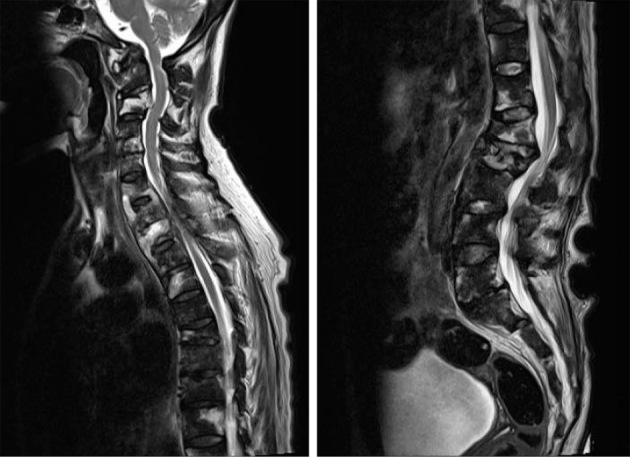
Sagittal T2-weighted image showing several hyperintense signals in the vertebral bodies, their surroundings, and intervertebral spaces.
Figure 4.
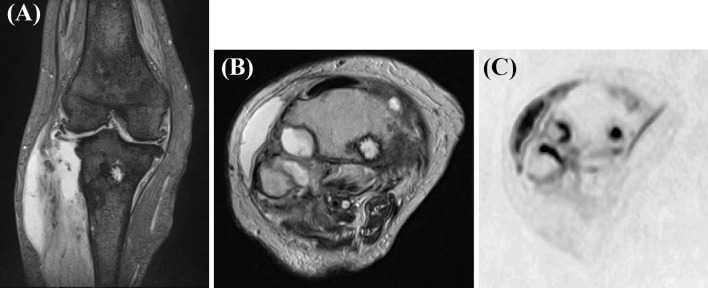
A T2-weighted image (A: coronal image, B: axial image) and diffusion-weighted image (C) of the right knee showing hyperintense signals in the thigh bone and tibia. Abscesses in the lateral condyle of the tibia can be seen extending to the outside of the bone and up to proximal fibula. In addition, the abscesses extended to the joint cavity.
M. scrofulaceum was detected in a sputum culture, the abscesses of the right knee, and the subcutaneous forehead abscess. In vitro, the bacterial isolate was resistant to INH, EB, and para-aminosalicylic acid (PAS), but it was sensitive to streptomycin (SM), RFP, cycloserine (CS), kanamycin (KM), enviomycin (EVM), ethionamide (ETH), and LVFX (Vite Spectrum SR, Kyokuto Pharmaceutical Industrial, Tokyo, Japan). The minimum inhibitory concentrations (MICs) reported for RFP, EB, and SM were 40 μg/mL, 2.5 μg/mL, and 10 μg/mL, respectively (Broth MIC NTM, Kyokuto Pharmaceutical Industrial, Tokyo, Japan). The patient was prescribed a combination antibiotic therapy with RFP, 300 mg daily; EB, 500 mg daily; clarithromycin (CAM), 800 mg daily; and SM, 500 mg 3 times a week, from July to August 2014. After completing the first treatment regime, she was prescribed additional treatment with 3 drugs (RFP, EB, and CAM) from September 2014 to August 2016, which resulted in symptomatic relief and a radiological improvement (Fig. 2B).
Discussion
The name M. scrofulaceum derives from “scrofula”, an infection of the cervical lymph nodes, and it is relatively well known that M. scrofulaceum is associated with cervicofacial lymphadenitis in pre-school children (12). Pulmonary infections due to this organism are relatively rare. Lung M. scrofulaceum infections account for around 0.7% of nontuberculous lung mycobacteriosis in Japan (13). The characteristic of pulmonary lesions is slowly-progressive cavernous pneumonia (1). Disseminated M. scrofulaceum infections are rarely reported (2-10). In the present case, a disseminated mycobacterial infection, caused by M. scrofulaceum, in an immunocompetent host was successfully treated with combination therapy (RFP, CAM, and EB) over a 2-year period.
To our knowledge, only 10 cases, including this case, with detailed descriptions of disseminated M. scrofulaceum infection in multiple organs, or even a single internal organ, have been published in the English literature since 1971 (Table) (2-10). Four cases involved children, whereas 6 cases involved adults, comprising 8 men and 3 women. The mean age of these 10 patients was 25 years (age range: 2-56 years). For the pediatric cases, the identified underlying cause of the disease were advanced systemic amyloidosis and IFN-γ receptor 1 (IFN-γR1) deficiency (2-4). In the case of amyloidosis, the relationship between secondary amyloidosis and immunity was uncertain, and Dustin et al. concluded that the prolonged stimulation of the immune system by the weakly antigenic M. scrofulaceum appeared to be the main cause of amyloidosis (2). Regarding the adult cases, 1 patient had an underlying immunodeficiency due to AIDS (5). The patient diagnosed with AIDS had a CD4 lymphocyte count of 13/mm3 and presented with chronic ulcerative and nodular skin lesions with probable cavitary lung involvement (5). Two patients had leukemia-related underlying diseases (6,7). There was no known immunodeficiency in the two cases, including the present case (8). The patient presented herein had slight lymphopenia and a low mitogen response in the QFT-G assay. A low mitogen response may occur because of an insufficient number of lymphocytes or the presence of IFN-γ antibodies (14). In our case, lymphopenia or the hypofunction of the lymphocytes might be related to disseminated M. scrofulaceum infection.
Table.
Features of Disseminated Mycobacterium Scrofulaceum Infection.
| No | Reference | Age/Sex | Lesion | Treatment | Outcome | Underlying disease |
|---|---|---|---|---|---|---|
| 1 | [7] | 37/F | Liver | INH, SM, EB | Death | Leukemia |
| 2 | [10] | 6/M | Skin, Bone marrow | INH, SM, Operation | Recurrence | NR |
| 3 | [2] | 9/M | Skin, Lymph node, Lung, Liver, Testis, Epididymis, Kidney | INH, RFP, SM, PAS, CS, ETH, CLF, DDS, TAC | Death | Amyloidosis |
| 4 | [9] | 39/M | Liver | INH, RFP, CS | Cure | NR |
| 5 | [6] | 38/F | Lung, Skin | INH, RFP, EB, ETH, CS, MINO, Operation | Recurrence | Leukemia |
| 6 | [5] | 32/M | Lung, Skin | INH, SM, RFP, EB, PZA | Death | AIDS |
| 7 | [8] | 27/M | Lung, Lymph node, Liver, Bone marrow, Skin, Kidney | INH, EB, RFP, OFLX | Cure | Nothing |
| 8 | [3] | 2/M | Bone marrow, Lymph node | RFP, RBT, CAM | Recurrence | IFN-γ deficiency |
| 9 | [4] | 2/M | Bone marrow | RFP, RBT, CAM | Recurrence | IFN-γ deficiency |
| 10 | This case | 56/F | Bone marrow, Skin, Lung | RFP, EB, CAM, SM | Cure | Nothing |
CAM: clarithromycin, CLF: clofazimine, CS: cycloserine, DDS: dapsone, EB: ethambutol, ETH: ethionamide, IFN-γ: interferon-gamma, INH: isoniazid, MINO: minocycline, NR: not reported, OFLX: ofloxacin, PAS: para-aminosalicylic acid, PZA: pyrazinamide, RBT: rifabutin, RFP: rifampicin, SM: streptomycin, TAC: thiacetazone
The areas of the body with lesions, due to disseminated M. scrofulaceum infection, included the skin (6 cases), bone marrow (5 cases), lung (5 cases), and liver (4 cases). In the four cases with liver involvement alone and IFN-γR1 deficiency, the course of invasion was unclear (3,4,7,9). The 5 patients with associated lung lesions were thought to have been infected via the lung (2,5,6,8). Lincoln and Gilbert reported that the onset of osteomyelitis due to M. scrofulaceum began with small, swollen, reddened areas of the skin overlying affected bones (10). These findings suggest that these bacteria can invade the skin and spread from there to other organs.
In this case, the MICs for M. scrofulaceum were determined using the Middlebrook 7H9 broth microdilution system based on clinical and laboratory standards institute 2003. In this method, the in vitro susceptibilities for many NTM do not correlate well with clinical response to anti-tuberculous drugs, except for CAM. Therefore it is considered that in vitro susceptibility data of NTM isolates other than CAM should be used as a reference (15). A few reports have described the sensitivity of M. scrofulaceum towards anti-tuberculous drugs. M. scrofulaceum is one of the NTMs which tends to demonstrate antibiotic resistance. Some strains of M. scrofulaceum were reported to be resistant to INH, PAS, and KM whereas other strains were sensitive to RFP, rifabutin, EB, SM, CS, amikacin, and ETH (1). Some studies showed that the MIC for 90% of the strains (MIC90) for CAM was ≤0.5 μg/mL (16), for roxithromycin was ≤1.0 μg/mL (17), and for sparfloxacin was 1.0 μg/mL (18). Thus, these drugs may be useful for the treatment of M. scrofulaceum infections.
Furthermore, the required treatment periods for disseminated M. scrofulaceum infection are unknown. It is believed that the treatment period may change depending on the patient's state of immunity. There are currently 2 cases that have been reported to be cured by drugs. Patel et al. described that treatment with INH, RFP, and CS against hepatic granulomatosis for 1 year improved the symptoms and normalized laboratory results (9). In this case an underlying disease was not reported. Hsueh et al. reported that the patient with no underlying disease was given a combination therapy of INH, RFP, EB, and Ofloxacin for 1 year and was cured (8). No long-term follow up with the patients was carried out in either of the reports. In our case, a treatment for 2 months, with 2 antibiotics (RFP, LVFX) to which the organism was found to be sensitive in vitro, caused the disease to recur. A combination therapy with RFP, CAM, and EB over a 2-year period was successful in curing the patient. A treatment regime with more than 2 antibiotics to which the organism is sensitive to over a period of more than 1 year might thus be necessary to cure the disease. In addition, it is necessary to monitor the patient for any recurrent infection. Further investigations into the most appropriate combination therapy for infections with M. scrofulaceum are therefore necessary.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Horowitz EA. Mycobacterium scrofulaceum. In: Tuberculosis and Nontuberculous Mycobacterial Infections. 6th ed. Schlossberg D, Ed. Washington DC, 2001: 601-606. [Google Scholar]
- 2. Dustin P, Demol P, Derks-Jacobovitz D, Cremer N, Vis H. Generalized fatal chronic infection by Mycobacterium scrofulaceum with severe amyloidosis in a child. Pathol Res Pract 168: 237-248, 1980. [DOI] [PubMed] [Google Scholar]
- 3. Marazzi MG, Chapgier A, Defilippi AC, et al. Disseminated Mycobacterium scrofulaceum infection in a child with interferon-gamma receptor 1 deficiency. Int J Infect Dis 14: 167-170, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Taramasso L, Boisson-Dupuis S, Garrè ML, et al. Pineal germinoma in a child with interferon-γ receptor 1 deficiency. case report and literature review. J Clin Immunol 34: 922-927, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Sanders JW, Walsh AD, Snider RL, Sahn EE. Disseminated Mycobacterium scrofulaceum infection: a potentially treatable complication of AIDS. Clin Infect Dis 20: 549, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Gallo JH, Young GA, Forrest PR, Vincent PC, Jennis F. Disseminated atypical mycobacterial infection in hairy cell leukemia. Pathology 15: 241-245, 1983. [DOI] [PubMed] [Google Scholar]
- 7. McNutt DR, Fudenberg HH. Disseminated scotochromogen infection and unusual myeloproliferative disorder. Report of a case and review of the literature. Ann Intern Med 75: 737-744, 1971. [DOI] [PubMed] [Google Scholar]
- 8. Hsueh PR, Hsiue TR, Jarn JJ, Ho SW, Hsieh WC. Disseminated Infection due to Mycobacterium scrofulaceum in an immunocompetent host. Clin Infect Dis 22: 159-161, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Patel KM. Granulomatous hepatitis due to Mycobacterium scrofulaceum: report of a case. Gastroenterology 81: 156-158, 1981. [PubMed] [Google Scholar]
- 10. Lincoln EM, Gilbert LA. Disease in children due to mycobacteria other than Mycobacterium tuberculosis. Am Rev Respir Dis 105: 683-714, 1972. [DOI] [PubMed] [Google Scholar]
- 11. Runyon EH. Identification of mycobacterial pathogens utilizing colony characteristics. Am J Clin Pathol 54: 578-586, 1970. [DOI] [PubMed] [Google Scholar]
- 12. Hautmann G, Lotti T. Diseases caused by Mycobacterium scrofulaceum. Clin Dermatol 13: 277-280, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Tsukamura M, Kita N, Shimoide H, et al. Studies on the nontuberculous lung mycobacteriosis in Japan (report of the study year 1985 of the Mycobacteriosis Research Group of the Japanese National Chest Hospitals). Incidence rate of lung disease caused by Mycobacterium kansasii is still increasing which elevates the incidence rate of nontuberculous lung mycobacteriosis. Kekkaku (Tuberculosis) 62: 319-327, 1987. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 14. Powell RD 3rd, Whitworth WC, Bernardo J, Moonan PK, Mazurek GH. Unusual interferon gamma measurements with QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube tests. PLos One 6: e20061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Brown BA, Wallace RJ Jr, Onyi GO. Activities of clarithromycin against eight slowly growing species of nontuberculous mycobacteria, determined by using a broth microdilution MIC system. Antimicrob Agents Chemother 36: 1987-1990, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rastogi N, Goh KS, Bryskier A. In vitro activity of roxithromycin against 16 species of atypical mycobacteria and effect of pH on its radiometric MICs. Antimicrob Agents Chemother 37: 1560-1562, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yew WW, Piddock LJ, Li MS, Lyon D, Chan CY, Cheng AF. In-vitro activity of quinolones and macrolides against mycobacteria. J Antimicrob Chemother 34: 343-351, 1994. [DOI] [PubMed] [Google Scholar]