Abstract
Background
In the past few years, increased resistance of microorganisms towards antimicrobial agents become a serious health problem, so there is a need for the discovery of new antimicrobial agents. On the other hand, bis-pyrimidines possess various types of biological activity. In view of this, in the present study we have designed and synthesized a new series of bis-pyrimidine acetamides by Claisen–Schmidt condensation and screened for its in vitro antimicrobial and anticancer activities.
Results
The synthesized bis-pyrimidine acetamide derivatives were confirmed by IR, 1H/13C-NMR, Mass spectral studies as well C, H, N analyses. The synthesized compounds were evaluated for their in vitro antimicrobial potential against Gram positive (Staphylococcus aureus and Bacillus subtilis); Gram negative (Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica) bacterial and fungal (Candida albicans and Aspergillus niger) strains by tube dilution technique and the minimum inhibitory concentration (MIC) recorded in µmol/mL was comparable to reference drugs, cefadroxil (antibacterial) and fluconazole (antifungal). The in vitro anticancer activity (IC50 value) determined against human colorectal carcinoma (HCT116) cancer cell line by Sulforhodamine B (SRB) technique and 5-fluorouracil used as reference drug.
Conclusions
The biological study demonstrated that compounds 3, 13, 16, 17 and 18 were found to be most active antimicrobial agents with best MIC values than the cefadroxil (antibacterial) and fluconazole (antifungal) and compounds 12, 16 and 18 found to have better anticancer activity against human colorectal carcinoma (HCT116) cancer cell line with best IC50 value than the 5-fluorouracil (anticancer).
Graphical abstract.
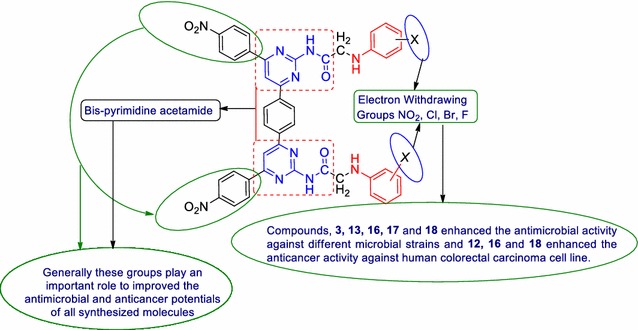
SAR of bis-pyrimidine acetamides
Keywords: Bis-pyrimidines, Claisen–Schmidt condensation, Antimicrobial, Anticancer, SAR
Background
The treatment of bacterial infections remains a challenging therapeutic problem because of emerging infectious diseases as well the increasing number of multidrug resistant microbial pathogens. Despite the many antimicrobial and chemotherapeutic agents are available in market, the emergence of old and new antibiotic resistant bacterial species in the last decade lead to a substantial need for the discovery of new classes of antimicrobial compounds [1]. Cancer is one of the most serious health problems all over the world and one of the leading causes of death. Thus, in the past for several decades, researchers have been struggling to find effective clinical approaches for the treatment of cancer and search for novel anticancer agents [2]. Among a wide variety of heterocyclic compounds that have been explored for developing medicinally important molecules, nitrogen containing heterocyclic pyrimidine derivatives occupies an important place in the field of medicinal chemistry [3]. The presence of a pyrimidine base in thymine, cytosine and uracil, which are the essential binding blocks of nucleic acids, DNA and RNA is one possible reason for their biological and therapeutical activities. Number of marketed drugs (Fig. 1) contains pyrimidine nucleus i.e. nilotinib (i) and capecitabine (ii) as anticancer; proquazone (iii) as anti-inflammatory; idoxuridine (iv) and trifluoridine (v) as antiviral; zidovudine (vi) and stavudine (vii) as anti-HIV; trimethoprim (viii) and sulphamethiazine (ix) as antibacterial; pyrimethamine (x) as antimalarial; minoxidil (xi) and ketanserin (xii) as antihypertensive [4]. The literature survey indicated that a wide range of pharmacological activities are exhibited by the compounds encompassing pyrimidine nucleus i.e. antibacterial [5], antifungal [6], antileishmanial [7], antimycobacterial [8], antimicrobial [9], anti-inflammatory [10], anticancer [11], antiviral [12], antitubercular [13], antimalarial [14], antioxidant activity [15], central nervous system (CNS) depressant and calcium channel blocker [8]. Based on the literature survey and biological profile of bis-pyrimidine acetamides summarized in Fig. 2 and in continuation of our efforts in searching novel antimicrobial and anticancer agents [16–18], in the present work, we hereby report the synthesis, antimicrobial and anticancer activities of a series of bis-pyrimidine acetamide derivatives.
Fig. 1.
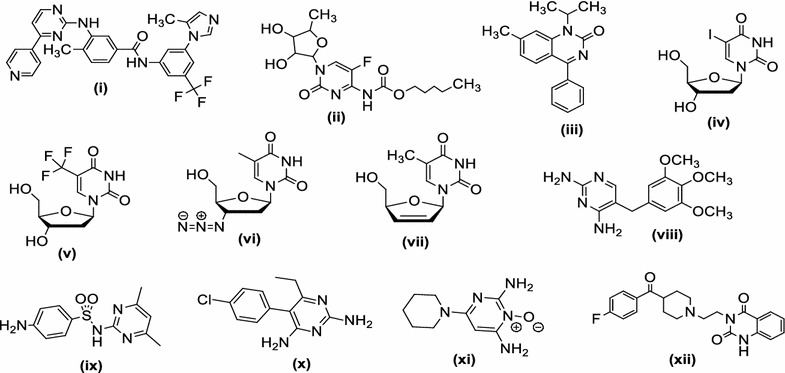
Some marketed drugs contains pyrimidine moiety
Fig. 2.
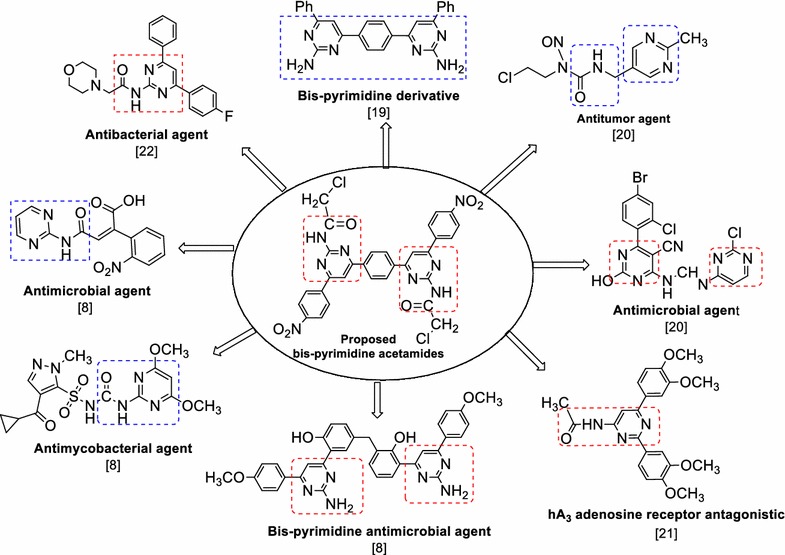
Biological profile of pyrimidine acetamide derivatives found in the resent literature
Results and discussion
Chemistry
A general approach to synthesize the designed bis-pyrimidine acetamide derivatives based on Claisen–Schmidt condensation is outlined in Scheme 1. Initially, the bis-chalcone (I) was synthesized by the reaction of 1-(4-nitrophenyl)ethanone and terephthalaldehyde. The cyclization of a bis-chalcone into the corresponding bis-pyrimidine (II) was accomplished by the reaction of the bis-chalcone with guanidine nitrate. The reaction of bis-pyrimidine (II) with 2-chloroacetyl chloride resulted in the formation of III {N,N′-(6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-chloroacetamide)} which on reaction with corresponding substituted aniline yielded the title compounds (1–20). The chemical structures of the synthesized compounds were established by the determination of their physicochemical and spectroscopic data (FT-IR, 1H-NMR, 13C-NMR, Mass) and elemental analyses. The structure of the bis-chalcone was confirmed by the corresponding IR (KBr pellets, cm−1). The IR spectrum of bis-chalcone (I) showed the characteristic band at 1690 cm−1 which indicated the presence of a –C=O group and characteristic bands at 3106 and 1517 cm−1 indicated the presence of C–H and C=C group in aromatic ring respectively. The existence of Ar–NO2 group in bis-chalcone (I) was displayed by the existence of symmetric Ar–NO2 stretches in the scale of 1341 cm−1 and characteristic bands at 2906 and 1598 cm−1 indicated the presence of C–H and C=C group in alkyl chain respectively. Bis-pyrimidine (II) showed the characteristic bands at 3106 and 1518 cm−1 for the presence of C–H and C=C group in aromatic ring respectively and characteristic bands at 3370, 1600 and 1349 cm−1 for indicated the presence of C–NH2; N=CH str., of pyrimidine and C–N sym stretches of Ar–NO2 group. N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-chloroacetamide) III showed appearance of IR stretching around 3105 and 1519 cm−1 in the spectral data for the presence of C–H and C=C group in aromatic ring respectively and characteristic bands at 1688, 1599, 2928, 761 and 1349 cm−1 for the NH–C=O; N=CH str., of pyrimidine; C–H str., CH2; C–Cl str., (Ar–Cl); C–N sym str. of aromatic NO2 group respectively. The structure of the bis-chalcone and its cyclized products were further confirmed by the corresponding 1H-NMR spectra. The 1H-NMR spectrum of bis-chalcone I showed two doublets at 7.59 ppm (J = 15.1 Hz) and 8.06 ppm (J = 15.1 Hz); indicating that the CH=CH group in the enone linkage is in a trans-conformation. The 1H-NMR spectrum of intermediate-II showed a multiplet signals between 7.43 and 8.40 δ, ppm confirming the cyclisation of the bis-chalcone to give bis-pyrimidine ring. The 1H-NMR spectrum of compound intermediate-II showed a sharp singlet at 6.99 δ, ppm due to the NH2 protons and it also showed a sharp singlet at 7.86 δ, ppm due to HC=C group, which confirmed the cyclization of the bis-chalcone into a bis-pyrimidine ring. Intermediate (III) showed the multiplet signals between 7.54 and 8.93 δ, ppm in 1H-NMR spectra which is indicative of aromatic proton and also exhibited sharp singlet at 7.42, 8.10, 3.66 δ, ppm due to the presence of HC=C; –NH; –CH2 respectively confirmed the conversion of the bis-pyrimidine into bis-pyrimidine acetamide. The appearance of IR stretching around 1575–1569, 3086–2851 and 1580–1600 cm−1 in the spectral data of synthesized derivatives (1–20) specified the existence of C=N group of pyrimidine ring; C–H and C=C group respectively. The IR absorption band in the scale of 1106–1105 cm−1 corresponds to the C–F stretching of aromatic-fluoro compounds (11 and 12) and 697–536 cm−1 corresponds to the C–Br stretching of aromatic-bromo derivative (16). The existence of an arylalkyl ether category (C–O–C, Ar–OCH3) in derivatives, 4, 19 and 20 are established by the existence of an IR symmetric stretches around 1107–1088 cm−1. Further, the existence of halogen group in compounds (6, 7, 9, 10, 13, 17 and 18) is indicated by the existence of Ar–Cl stretching vibrations at 761–700 cm−1. The impression of IR stretching at 1692–1620 cm−1 in the spectra of specified the existence of NH–C=O group of synthesized derivatives. The multiplet signals between 6.74 and 9.08 δ, ppm in 1H-NMR spectra is indicative of aromatic proton of synthesized derivatives (1–20). The compounds, 4, 19 and 20 showed singlet at 3.38–3.75 δ, ppm due to the presence of OCH3 of Ar–OCH3 in their structure. All compounds showed singlet at 7.47–7.91 δ, ppm due to the existence of N=CH in pyrimidine ring. Synthesized compounds showed appearance of IR stretching around 1349 cm−1 which indicated the presence of C–N sym str. of aromatic NO2 group. Compounds, 5, 14 and 15 showed singlet at 2.09–2.51 δ, ppm due to existence of –CH3 at ortho and para position. All compounds showed singlet at 3.34–3.38 δ, ppm and 8.00–8.08 δ, ppm due to the existence of –CH2– and –NH– groups respectively. The elemental analysis results of the synthesized bis-pyrimidine acetamide derivatives were found within ±0.4% of the theoretical results. Finally, the 13C-NMR spectra of the bis-chalcone and the cyclized bis-pyrimidine were recorded in DMSO-d 6 and the spectral signals were in good agreement with the proposed molecular structure of the synthesized compounds. 13C-NMR spectra details of all compounds are given in the experimental part. Characteristic molecular ion peaks were observed in the mass spectra of the bis-chalcone and the cyclized bis-pyrimidine and final bis-pyrimidine acetamide derivatives.
Scheme 1.
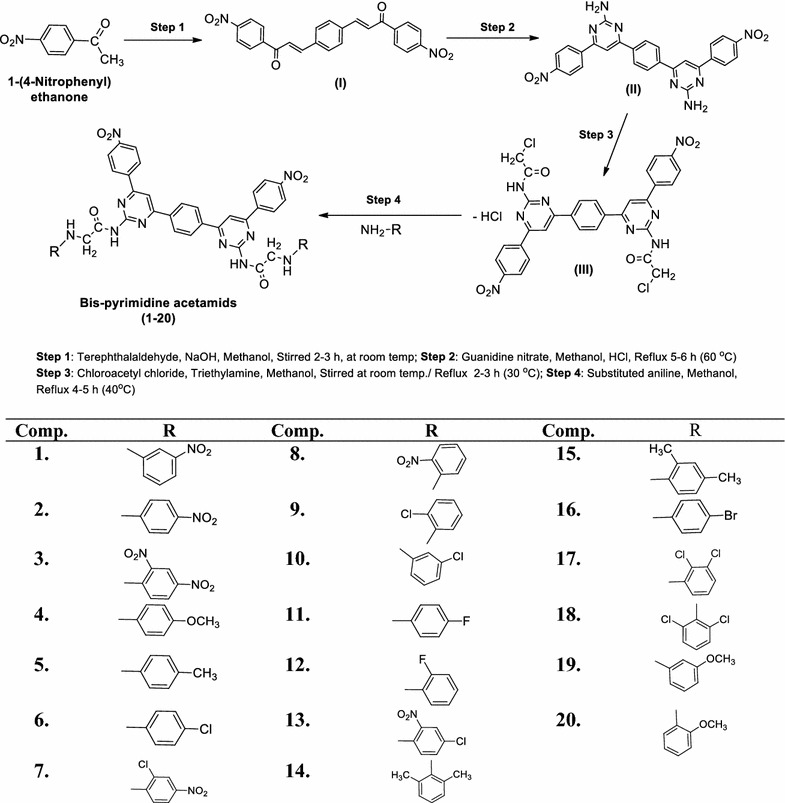
Synthesis of N, N′-(6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl)) bis(2-chloroacetamide) analogues
Antimicrobial activity
The synthesized compounds were screened for their in vitro antimicrobial activity by tube dilution method. The investigation of antimicrobial screening revealed that some of the synthesized compounds showed moderate to good antimicrobial activity against Gram positive bacterial species: Staphylococcus aureus, Bacillus subtilis and Gram negative bacterial species: Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica and fungal species: Aspergillus niger and Candida albicans. Particularly, compounds 3, 13, 16, 17 and 18 have shown more promising antimicrobial activity as compared to standard drugs cefadroxil (antibacterial) and fluconazole (antifungal). The remaining compounds displayed average to poor activity against all seven microbial species. In vitro antimicrobial activity results of synthesized compound are summarized in Table 1. Antibacterial screening results (Fig. 3) against Gram positive bacterial species demonstrated that compound 16 has showed good antibacterial activity against S. aureus and B. subtilis respectively. Compounds, 13 and 17 have sensible activity against S. aureus. The MIC values of 13 nearly close to MIC value of standard drugs cefadroxil while 16 and 17 have more active than standard drugs. Antibacterial screening results (Fig. 4) indicated that compound 3 possessed excellent activity against S. enterica. Compound 16 exhibited promising activity against P. aeruginosa and E. coli. Compound 17 was most active against P. aeruginosa. The MIC values of compounds 16 and 17 was more than the MIC values of standard drug cefadroxil Gram negative bacterial species. Antifungal screening results (Fig. 5) indicated that compound 3 and 18 were found to be most effective ones against A. niger and C. albicans respectively. The rest of the compounds of the series exhibited average to poor antifungal activity. The antimicrobial activity results of synthesized bis-pyrimidine acetamide derivatives indicated that the synthesized compounds are more active than the standard drugs and may be taken as a lead compound to discover novel antimicrobial agents.
Table 1.
Antimicrobial and anticancer screening results of synthesized compounds
| Compounds No. | Minimum inhibitory concentration (MIC = µmol/mL) | IC50 (µmol/mL) Cancer cell line |
||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial species | Fungal species | |||||||
| Gram positive | Gram negative | |||||||
|
S. aureus
MTCC-3160 |
B. subtilis
MTCC-441 |
P. aeruginosa
MTCC-3542 |
S. enterica
MTCC-1165 |
E. coli
MTCC-443 |
C. albicans
MTCC-227 |
A. niger
MTCC-281 |
HCT-116 | |
| 1. | 0.72 | 0.36 | 1.45 | 0.72 | 1.45 | 1.45 | 1.45 | 3.86 |
| 2. | 0.72 | 0.72 | 0.72 | 0.72 | 1.45 | 0.72 | 1.45 | 3.86 |
| 3. | 1.31 | 0.33 | 0.66 | 0.66 | 1.31 | 0.66 | 1.31 | 3.50 |
| 4. | 0.75 | 0.19 | 0.75 | 0.75 | 1.50 | 0.75 | 1.50 | 4.00 |
| 5. | 0.78 | 0.78 | 0.78 | 1.56 | 1.56 | 0.78 | 1.56 | 4.16 |
| 6. | 0.74 | 0.37 | 0.74 | 1.48 | 1.48 | 1.48 | 1.48 | 3.96 |
| 7. | 0.67 | 0.67 | 1.34 | 0.67 | 1.34 | 1.34 | 1.34 | 3.58 |
| 8. | 0.72 | 1.45 | 1.45 | 0.72 | 1.45 | 0.72 | 1.45 | 3.86 |
| 9. | 0.74 | 0.74 | 0.74 | 1.48 | 1.48 | 0.74 | 1.48 | 1.98 |
| 10. | 0.74 | 0.19 | 0.74 | 1.48 | 1.48 | 0.74 | 1.48 | 3.96 |
| 11. | 0.39 | 0.39 | 0.77 | 0.77 | 0.77 | 0.77 | 1.55 | 1.52 |
| 12. | 0.39 | 0.39 | 0.77 | 0.77 | 1.55 | 0.77 | 1.55 | 0.74 |
| 13. | 0.17 | 0.34 | 0.67 | 0.67 | 1.34 | 0.67 | 1.34 | 3.58 |
| 14. | 1.51 | 0.75 | 0.38 | 1.51 | 1.51 | 0.75 | 1.51 | 4.02 |
| 15. | 0.38 | 0.38 | 0.75 | 0.75 | 0.75 | 0.75 | 1.51 | 2.17 |
| 16. | 0.17 | 0.17 | 0.34 | 0.67 | 0.67 | 0.67 | 1.34 | 0.98 |
| 17. | 0.17 | 0.34 | 0.34 | 0.69 | 1.37 | 0.69 | 1.37 | 1.22 |
| 18. | 0.34 | 0.69 | 0.69 | 0.69 | 1.37 | 0.34 | 1.37 | 0.73 |
| 19. | 0.75 | 0.38 | 0.75 | 0.75 | 1.50 | 0.75 | 1.50 | 4.00 |
| 20. | 0.19 | 0.38 | 0.75 | 0.75 | 1.50 | 0.75 | 1.50 | 2.16 |
| DMSO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Cefadroxil | 1.72 | 0.86 | 1.72 | 1.72 | 1.72 | – | – | – |
| Fluconazole | – | – | – | – | – | 1.02 | 2.04 | – |
| 5-Fluorouracil | – | – | – | – | – | – | – | 7.67 |
Std. drugs: cefadroxil—Antibacterial; fluconazole—Antifungal; 5-fluorouracil—Anticancer
Fig. 3.
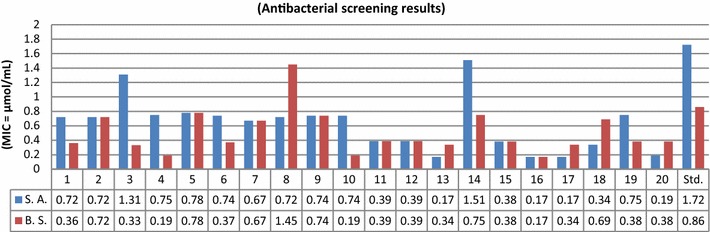
Antibacterial screening results against Gram positive species
Fig. 4.
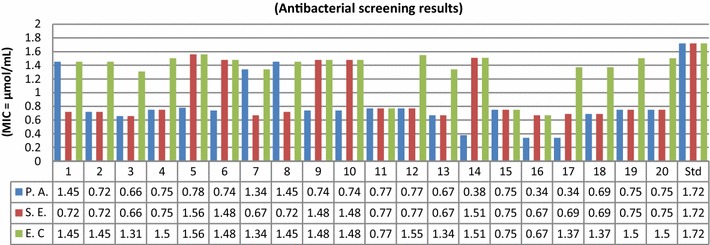
Antibacterial screening results against Gram negative species
Fig. 5.
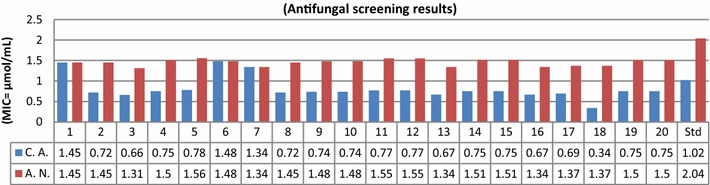
Antifungal screening results against fungal species
Anticancer activity
The anticancer screening (IC50 = µmol/mL) of the synthesized bis-pyrimidine acetamide derivatives was determined against human colorectal carcinoma [HCT-116 (ATCC CCL-247)] cancer cell line by Sulforhodamine B (SRB) technique [28] using 5-fluorouracil as reference drug and the results are presented in Table 1. Anticancer screening results (Fig. 6) revealed that in general bis-pyrimidine acetamides exhibited good anticancer potential against human colorectal cancer cell line, especially, Compounds, 12, 16 and 18 displayed more anticancer activity than the reference drug 5-fluorouracil (IC50 = 7.67 µmol/mL) with IC50 values of 0.74, 0.98 and 0.73 µmol/mL respectively.
Fig. 6.
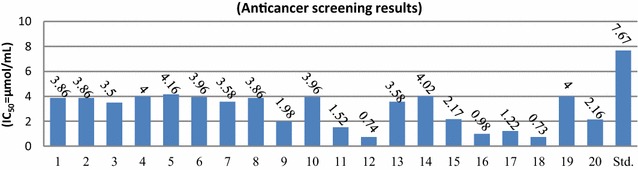
Anticancer screening results of the synthesized compounds
The structure–activity relationship of the synthesized bis-pyrimidine acetamides indicated that the compounds bearing electron withdrawing group at different position of phenyl group plays an important role in enhancing the antimicrobial and anticancer potentials (Fig. 7).
Fig. 7.
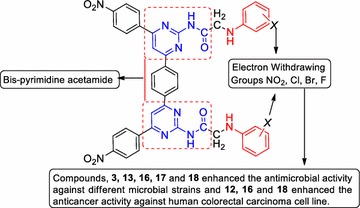
Structure activity relationship of bis-pyrimidine acetamides
Experimental part
Preparatory materials for the research work were obtained from commercial sources and were used without further purification. All reactions were monitored by thin-layer chromatography on 0.25 mm silica gel (Merck) plates, using benzene as mobile phase and spots were observed by exposure to iodine vapours or visualized with UV light. Melting points of synthesized compounds was determined in open capillary tube. An infrared spectrum was recorded (KBr-pellets) in Bruker 12060280, Software: OPUS 7.2.139.1294 spectrometer. 1H-NMR and 13C-NMR were recorded at 600 and 150 MHz, respectively on Bruker Avance III 600 NMR spectrometer by appropriate deuterated solvents. The results are conveyed in parts per million (δ, ppm) downfield from tetramethyl silane (internal standard). 1H-NMR spectral details of the synthesized derivatives are represented with multiplicity like singlet (s); doublet (d); triplet (t); multiplet (m) and the number hydrogen ion. Elemental analysis of the new synthesized compounds was obtained by Perkin–Elmer 2400 C, H and N analyzer. All the compounds gave C, H and N analysis within ±0.4% of the theoretical results. Mass spectra were taken on Waters Micromass Q-ToF Micro instrument. The synthesized compounds were characterized by the determination of their physicochemical and spectral characteristics.
General procedure of the synthesized bis-pyrimidine acetamide derivatives (1–20)
Step i: Synthesis of 3,3′-(1,4-phenylene)bis(1-(4-nitrophenyl)prop-2-en-1-one) (I)
A mixture of 1-(4-nitrophenyl)ethanone (0.02 mol) and terephthalaldehyde (0.01 mol) in methanol (5–10 mL) was stirred with drop wise addition of 40% sodium hydroxide solution (10 mL) at room temperature till a dark yellow mass was obtained (2–3 h). Then reaction mixture was allowed to stand overnight at room temperature and then was poured into icecold water and acidified with hydrochloric acid and the precipitated 3,3′-(1,4-phenylene)bis(1-(4-nitrophenyl) prop-2-en-1-one) was filtered, dried and recrystallized from methanol [23, 24].
Step ii: Synthesis of 6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidin-2-amine) (II)
The solution of 3,3′-(1,4-phenylene)bis(1-(4-nitrophenyl)prop-2-en-1-one) (0.01 mol) (synthesized in previous step-i) in methanol (80 mL) was added with 0.01 mol of potassium hydroxide and 40 mL of 0.50 M solution of guanidine nitrate and refluxed for 5–6 h. The reaction mixture was then cooled and acidified with few drops of hydrochloric acid (20 mL of 0.5 M solution) and the resultant precipitate, 6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl) pyrimidin-2-amine) was separated dried and recrystallized from methanol [23, 24].
Step iii: Synthesis of N,N′-(6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis (2-chloroacetamide) (III)
In ethanol (30 mL), chloroacetyl chloride (0.02 mol) and 2–3 drops of triethylamine were added and the mixture was stirred in water bath for 10 min after that the solution of 6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidin-2-amine) (synthesized in previous step-ii) (0.01 mol) in ethanol (80 mL) was added drop wise and refluxed for 2–3 h. The reaction mixture was then cooled and poured into icecold water and resultant precipitate was filtered and washed with water, dried and recrystallized from alcohol [25].
Step iv: Synthesis of final (1–20) bis-pyrimidine acetamide derivatives
The reaction mixture of N,N′-(6,6′-(1,4-phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-chloroacetamide) (0.01 mol) and substituted aniline (0.02 mol) in ethanol was refluxed for 4–5 h. The reaction progress was monitored by thin layer chromatography. After completion of reaction, the reaction mixture was poured into icecold water and the precipitated title compound was filtered, dried and recrystallized from methanol.
Spectral data interpretation of the synthesized compounds (Fig. 8)
Fig. 8.
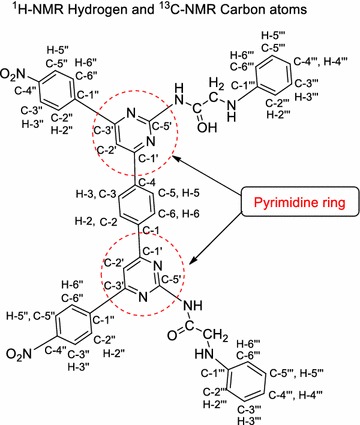
Number of hydrogen and carbon atoms is present in the synthesized compounds
3,3′-(1,4-Phenylene)bis(1-(4-nitrophenyl)prop-2-en-1-one) (I)
IR (KBr pellets, cm−1): 3106 (C–H str., phenyl nucleus), 1517 (C=C str., phenyl nucleus), 1690 (C=O str.), 1598 (C=C str., alkyl chain), 2906 (C–H str., alkyl chain), 1341 (C–N sym str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 6.94–9.08 {m, 12H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2}, 7.59 {d, 4H, (CH)2, J = 15.1 Hz), 8.06 (d, 4H, (CH)2, J = 15.1 Hz of CH2 = CH2}; 13C-NMR (δ, DMSO-d 6): 129.8 (C-2, C-3, C-5, C-6), 133.4 (C-1, C-4); 148.0 (C-1′), 121.3 (C-2′), 164.7 (C = O); 145.1 (C-1″), 130.0 (C-2″, C-6″), 124.9 (C-3″, C-5″), 162.5 (C-4″); Elem. Anal. Calcd. C24H16N2O6: C, 67.29; H, 3.76; N, 6.54; Found: C, 67.26; H, 3.75; N, 6.50; MS ES+(ToF): m/z 429 [M+ +1].
6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidin-2-amine) (II)
IR (KBr pellets, cm−1): 3107 (C–H str., phenyl nucleus), 1518 (C=C str., phenyl nucleus), 3370 (C–NH2 str.), 1600 (N=CH str., of pyrimidine), 1349 (C–N sym str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 7.43–8.40 {m, 12H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2}, 7.86 (s, 2H, (CH)2 of pyrimidine), 6.97 (s, 2H, (NH)2); 13C-NMR (δ, DMSO-d 6): 128.5 (C-2, C-3, C-5, C-6), 134.6 (C-1, C-4); 157.8 (C-1′, C-3′, C-5′), 102 (C-2′), 149.7 (C-5′) pyrimidine; 141.5 (C-1″), 126.4 (C-2″, C-6″), 124.4 (C-3″, C-5″), 148.3 (C-4″); Elem. Anal. Calcd. C26H18N8O4: C, 61.66; H, 3.58; N, 22.12; Found: C, 61.64; H, 3.51; N, 22.15; MS ES + (ToF): m/z 507 [M++1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-chloroacetamide) (III)
IR (KBr pellets, cm−1): 3105 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1688 (NH–C=O str.), 1599 (N=CH str., of pyrimidine), 2928 (C–H str., CH2), 761 (C–Cl str., Ar–Cl), 1349 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 7.54–8.93 {m, 12H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2}, 7.42 (s, 2H, (CH)2 of pyrimidine), 8.10 (s, 2H, (NH)2), 3.66 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.5 (C-2, C-3, C-5, C-6), 136.6 (C-1, C-4); 162.0 (C-1′, C-3′), 108.0 (C-2′), 149.7 (C-5′) pyrimidine; 141.0 (C-1″), 126.6 (C-2″, C-6″), 124.5 (C-3″, C-5″), 149.8 (C-4″), 188.0 (C = O), 44.3(CH2); Elem. Anal. Calcd. C30H20Cl2N8O6: C, 54.64; H, 3.06; N, 16.99; Found: C, 54.60; H, 3.00; N, 16.92; MS ES + (ToF): m/z 660 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((3-nitrophenyl) amino)acetamide) (1)
Brown crystals; Yield: 70.12%; M.p.: 252–254 °C; Rf value: 90.12; IR (KBr, cm−1): 3085 (C–H str., phenyl nucleus), 1528 (C=C str., phenyl nucleus), 1679 (NH–C=O str.), 1572 (N=CH str., of pyrimidine), 2863 (C–H str., CH2), 1217 (C–NH–str.), 1347 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 7.63–8.25 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-4‴,H-5‴, H-6‴) × 2}, 7.63 (s, 2H, (CH)2 of pyrimidine), 8.08 (s, 2H, (NH)2), 4.0 (s, 2H, (NH)2), 3.40 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.3, 128.4 (C-2, C-3, C-5, C-6), 136.5 (C-1, C-4); 165.0 (C-1′, C-3′), 109 (C-2′), 149.7 (C-5′) pyrimidine; 141.0 (C-1″), 126.5 (C-2″, C-6″), 124.5 (C-3″, C-5″), 149.6 (C-4″); 149.5 (C-1‴, C-3‴), 106.9 (C-2‴), 129.9 (C-5‴), 122.1 (C-6‴); 170.2 (C = O), 48.0 (CH2); Elem. Anal. Calcd. C42H30N12O10: C, 58.47; H, 3.50; N, 19.48; Found: C, 58.42; H, 3.49; N, 19.46; MS ES + (ToF): m/z 864 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-nitrophenyl) amino)acetamide) (2)
Dark brown crystals; M.p.: 262–264 °C; Rf value: 0.32; Yield: 92.45%; IR (KBr, cm−1): 3086 (C–H str., phenyl nucleus), 1530 (C=C str., phenyl nucleus), 1679 (NH–C = O str.), 1573 (N=CH str., of pyrimidine), 2929 (C–H str., CH2), 1219 (C–NH– str.), 1348 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 6.74–9.09 (m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.04 (s, 2H, CH of pyrimidine), 8.01 (s, 2H, (NH)2), 3.37 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 127.3 (C-2, C-3, C-5, C-6), 138.9 (C-1, C-4); 164.0 (C-1′, C-3′), 112 (C-2′), 148.3 (C-5′) pyrimidine; 126.3 (C-2″, C-6″), 121.5 (C-3″, C-5″), 147.6 (C-4″); 152.5 (C-1‴), 115.3 (C-2‴, C-6‴), 127.5 (C-3‴, C-5‴), 135.6 (C-4‴), 168.2 (C=O), 45.0 (CH2); Elem. Anal. Calcd. C42H30N12O10: C, 58.47; H, 3.50; N, 19.48; Found: C, 58.41; H, 3.48; N, 19.46; MS ES + (ToF): m/z 864 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2,4-dinitrophenyl) amino)acetamide) (3)
Brown crystals; M.p.: 257–259 °C; Rf value: 0.41; Yield: 92.67%; IR (KBr pellets, cm−1): 3211 (C–H str., phenyl nucleus), 1529 (C=C str., phenyl nucleus), 1620 (NH–C=O str.), 1675 (N=CH str., of pyrimidine), 3086 (C–H str., CH2), 1274 (C–NH– str.), 1345 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 6.95–8.90 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-5‴, H-6‴) × 2}, 7.87 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.40 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.6 (C-2, C-3, C-5, C-6), 135.05 (C-1, C-4); 164.0 (C-1′, C-3′), 102 (C-2′), 149.7 (C-5′) pyrimidine; 141.0 (C-1″), 127.3 (C-2″, C-6″), 124.9 (C-3″, C-5″), 148.6 (C-4″); 152.5 (C-1‴), 135.6 (C-2‴), 119.7 (C-3‴), 138.9 (C-4‴), 130.2 (C-5‴); 168.2 (C=O), 51.0 (CH2); Elem. Anal. Calcd. C42H28N14O14: C, 52.95; H, 2.96; N, 20.58; Found: C, 52.85; H, 2.46; N, 20.48; MS ES + (ToF): m/z 954 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-methoxyphenyl) amino)acetamide) (4)
Brown crystals; M.p.: 215–217 °C; Rf value: 0.53; Yield: 89.44%; IR (KBr pellets, cm−1): 3084 (C–H str., phenyl nucleus), 1529 (C=C str., phenyl nucleus), 1680 (NH–C=O str.), 1572 (N=CH str., of pyrimidine), 1221 (C–NH– str.), 1347 (C–N sym. str., Ar–NO2), 1088 (C–O–C str., –OCH3); 1H-NMR (δ, DMSO-d 6): 7.04–9.09 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.75 (s, 2H, (CH)2 of pyrimidine), 8.0 (s, 2H, (NH)2), 3.38 (s, 6H, (OCH3)2); 13C-NMR (δ, DMSO-d 6): 127.3 (C-2, C-3, C-5, C-6), 138.5 (C-1, C-4); 164.0 (C-1′, C-3′), 108 (C-2′), 148.7 (C-5′) pyrimidine; 142.0 (C-1″), 127.5 (C-2″, C-6″), 124.9 (C-3″, C-5″), 149.6 (C-4″); 138.5 (C-1‴), 115.3 (C-2‴, C-6‴), 115.0 (C-3‴, C-5‴), 171.2 (C=O), 51.0 (CH2), 53.0 (p-OCH3); Elem. Anal. Calcd. C44H36N10O8: C, 63.46; H, 4.36; N, 16.82; Found: C, 63.44; H, 4.37; N, 16.85; MS ES + (ToF): m/z 834 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-(p-tolylamino) acetamide) (5)
Brown crystals; M.p.: 250–252 °C; Rf value: 0.30; Yield: 85.43%; IR (KBr pellets, cm−1): 3084 (C–H str., phenyl nucleus), 1529 (C=C str., phenyl nucleus), 1681 (NH–C=O str.), 1569 (N=CH str., of pyrimidine), 2866 (C–H str., CH2), 1217 (C–NH– str.), 1348 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 6.99–8.56 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.48 (s, 2H, (CH)2 of pyrimidine), 8.03 (s, 2H, (NH)2), 2.51 (s, 6H, (CH3)2); 13C-NMR (δ, DMSO-d 6): 128.8, 128.4 (C-2, C-3, C-5, C-6), 136.8, (C-1, C-4); 168.0 (C-1′, C-3′), 108 (C-2′), 149.7 (C-5′) pyrimidine; 141.0 (C-1″), 126.4 (C-2″, C-6″), 123.8 (C-3″, C-5″), 149.7 (C-4″); 144.5 (C-1‴), 117.0 (C-2‴, C-6‴), 129.6 (C-3‴, C-5‴), 188.0 (C=O), 48.0 (CH2), 20.5 (p-CH3); Elem. Anal. Calcd. C44H36N10O6: C, 65.99; H, 4.53; N, 17.49; Found: C, 65.92; H, 4.49; N, 17.40; MS ES + (ToF): m/z 802 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-chlorophenyl) amino)acetamide) (6)
Brown crystals; M.p.: 168–170 °C; Rf value: 0.42; Yield: 87.58%; IR (KBr pellets, cm−1): 3084 (C–H str., phenyl nucleus), 1530 (C=C str., phenyl nucleus), 1687 (NH–C=O str.), 1603 (N=CH str., of pyrimidine), 2866 (C–H str., CH2), 1215 (C–NH– str.), 1348 (C–N sym. str., Ar–NO2), 739 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 6.35–9.08 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.49 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.36 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.4 (C-2, C-3, C-5, C-6), 134.5 (C-1, C-4); 164.0 (C-1′, C-3′), 106 (C-2′), 148.3 (C-5′) pyrimidine; 127.4 (C-2″, C-6″), 124.9 (C-3″, C-5″), 147.6 (C-4″); 145.5 (C-1‴), 115.1 (C-2‴, C-6‴), 129.9 (C-3‴, C-5‴), 126.1 (C-4‴); 165.2 (C=O), 53.0 (CH2); Elem. Anal. Calcd. C42H30Cl2N10O6: C, 59.94; H, 3.59; N, 16.64; Found: C, 59.90; H, 3.51; N, 16.60; MS ES + (ToF): m/z 843 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2-chloro-4-nitro phenyl)amino)acetamide) (7)
Brown yellow crystals; M.p.: 170–172 °C; Rf value: 0.60; Yield: 87.12%; IR (KBr pellets, cm−1): 3085 (C–H str., phenyl nucleus), 1530 (C=C str., phenyl nucleus), 1688 (NH–C=O str.), 1603 (N=CH str., of pyrimidine), 2869 (C–H str., CH2), 1215 (C–NH– str.), 1348 (C–N sym. str., Ar–NO2), 740 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 6.48–9.08 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-5‴, H-6‴) × 2}, 7.87 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.37 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 129.3 (C-2, C-3, C-5, C-6), 135.8 (C-1, C-4); 163.0 (C-1′, C-3′), 113 (C-2′), 148.7 (C-5′) pyrimidine; 126.3 (C-2″, C-6″), 124.9 (C-3″, C-5″), 149.3 (C-4″); 151.3 (C-1‴), 124.3 (C-2‴), 138.9 (C-4‴), 125.1 (C-3‴), 122.1 (C-5‴); 166.2 (C=O), 51.0 (CH2); Elem. Anal. Calcd. C42H28Cl2N12O10: C, 54.15; H, 3.03; N, 18.04; Found: C, 54.11; H, 3.00; N, 18.12; MS ES + (ToF): m/z 933 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2-nitrophenyl) amino)acetamide) (8)
Light Brown crystals; M.p.: 163–165 °C; Rf value: 0.34; Yield: 84.23%; IR (KBr pellets, cm−1): 3085 (C–H str., phenyl nucleus), 1530 (C=C str., phenyl nucleus), 1688 (NH–C=O str.), 1603 (N=CH str., of pyrimidine), 2868 (C–H str., CH2), 1215 (C–NH– str.), 1348 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 7.03-8.25 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴, H-6‴) × 2}, 7.79 (s, 2H, (CH)2 of pyrimidine), 8.04 (s, 2H, (NH)2), 3.38 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 127.4 (C-2, C-3, C-5, C-6), 135.6 (C-1, C-4); 162.0 (C-1′, C-3′), 105 (C-2′), 148.7 (C-5′) pyrimidine; 141.0 (C-1″), 126.5 (C-2″, C-6″), 124.3 (C-3″, C-5″), 149.6 (C-4″); 147.5 (C-1‴), 130.1 (C-2‴), 125.9 (C-3‴), 119.1 (C-4‴), 136.2 (C-5‴); 164.2 (C=O), 50.0 (CH2); Elem. Anal. Calcd. C42H30N12O10: C, 58.47; H, 3.50; N, 19.48; Found: C, 58.42; H, 3.45; N, 19.41; MS ES + (ToF): m/z 864 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-(2-chlorophenyl) acetamide) (9)
Canary yellow crystals; M.p.: 150–152 °C; Rf value: 0.58; Yield: 75.25%; IR (KBr pellets, cm−1): 3075 (C–H str., phenyl nucleus), 1521 (C=C str., phenyl nucleus), 1690 (NH–C=O str.), 1598 (N=CH str., of pyrimidine), 2851 (C–H str., CH2), 1210 (C–NH– str.), 1344 (C–N sym. str., Ar–NO2), 757 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 7.80–8.25 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴, H-6‴) × 2}, 7.80 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.37 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.6 (C-2, C-3, C-5, C-6), 135.5 (C-1, C-4); 163.0 (C-1′, C-3′), 107 (C-2′), 149.7 (C-5′) pyrimidine; 141.0 (C-1″), 127.5 (C-2″, C-6″), 123.5 (C-3″, C-5″), 149.8 (C-4″); 134.5 (C-1‴), 129.1 (C-2‴, C-3‴, C-4‴), 127.9 (C-5‴), 133.1 (C-6‴); 156.2 (C=O), 39 (CH2); Elem. Anal. Calcd. C42H30Cl2N10O6: C, 59.94; H, 3.59; N, 16.64; Found: C, 59.90; H, 3.53; N, 16.61; MS ES + (ToF): m/z 843 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-(2-chlorophenyl) acetamide) (10)
Light yellow crystals; M.p.: 138–140 °C; Rf value: 0.31; Yield: 80.22%; IR (KBr pellets, cm−1): 3074 (C–H str., phenyl nucleus), 1521 (C=C str., phenyl nucleus), 1690 (NH–C=O str.), 1597 (N=CH str., of pyrimidine), 2854 (C–H str., CH2), 1210 (C–NH– str.), 1344 (C–N sym. str., Ar–NO2), 757 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 7.82 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-4‴, H-5‴, H-6‴) × 2}, 7.80 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.38 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.3 (C-2, C-3, C-5, C-6), 135.5 (C-1, C-4); 165.0 (C-1′, C-3′), 108 (C-2′), 149.8 (C-5′) pyrimidine; 142.0 (C-1″), 127.5 (C-2″, C-6″), 123.5 (C-3″, C-5″), 149.6 (C-4″); 149.5 (C-1‴), 110 (C-2‴), 134.9 (C-3‴), 120.4 (C-5‴); 163.2 (C=O), 56.0 (CH2); Elem. Anal. Calcd. C42H30Cl2N10O6: C, 59.94; H, 3.59; N, 16.64; Found: C, 59.90; H, 3.52; N, 16.67; MS ES + (ToF): m/z 843 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-fluorophenyl) amino)acetamide) (11)
Yellow crystals; M.p.: 123–125 °C; Rf value: 0.33; Yield: 85.63%; IR (KBr pellets, cm−1): 3073 (C–H str., phenyl nucleus), 1518 (C=C str., phenyl nucleus), 1665 (NH–C=O str.), 1596 (N=CH str., of pyrimidine), 2852 (C–H str., CH2), 1210 (C–NH– str.), 1338 (C–N sym. str., Ar–NO2), 1105 (C–F str., Ar–F); 1H-NMR (δ, DMSO-d 6): 7.25–8.69 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.39 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.34 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 129.3 (C-2, C-3, C-5, C-6), 136.7 (C-1, C-4); 161.0 (C-1′, C-3′), 105 (C-2′), 149.8 (C-5′) pyrimidine; 142.0 (C-1″), 126.4 (C-2″, C-6″), 124.5 (C-3″, C-5″), 147.4 (C-4″); 144.5 (C-1‴), 115.9 (C-3‴, C-5‴), 119.0 (C-2‴, C-6‴), 159.8 (C-5‴); 169.2 (C=O), 68.0 (CH2); Elem. Anal. Calcd. C42H30F2N10O6: C, 62.37; H, 3.74; N, 17.32; Found: C, 62.33; H, 3.72; N, 17.35; MS ES + (ToF): m/z 810 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2-fluorophenyl) amino)acetamide) (12)
Gold Yellow crystals; M.p.: 170–172 °C; Rf value: 0.25; Yield: 77.12%; IR (KBr pellets, cm−1): 3108 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1665 (NH–C=O str.), 1600 (N=CH str., of pyrimidine), 2934 (C–H str., CH2), 1211 (C–NH– str.), 1340 (C–N sym. str., Ar–NO2), 1106 (C–F str., Ar–F); 1H-NMR (δ, DMSO-d 6): 7.91–839 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴, H-6‴) × 2}, 7.90 (s, 2H, (CH)2 of pyrimidine), 3.38 (s, 4H, (CH2), 8.00 (s, 2H, (NH)2); 13C-NMR (δ, DMSO-d 6): 129.3 (C-2, C-3, C-5, C-6), 135.5 (C-1, C-4); 162.0 (C-1′, C-3′), 105 (C-2′), 149.7 (C-5′) pyrimidine; 127.5 (C-2″, C-6″), 124.7 (C-3″, C-5″), 147.6 (C-4″); 130.5 (C-1‴), 153.2 (C-3‴), 116.9 (C-3‴), 121.9 (C-4‴); 188.2 (C=O), 49.0 (CH2); Elem. Anal. Calcd. C42H30F2N10O6: C, 62.37; H, 3.74; N, 17.32; Found; C, 62.34; H, 3.67; N, 17.28; MS ES + (ToF): m/z 810 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-chloro-2-nitro phenyl)amino)acetamide) (13)
Light yellow crystals; M.p.: 175–177 °C; Rf value: 0.16; Yield: 85.25%; IR (KBr pellets, cm−1): 3107 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1665 (NH–C=O str.), 1600 (N=CH str., of pyrimidine), 2936 (C–H str., CH2), 1247 (C–NH– str.), 1341 (C–N sym. str., Ar–NO2), 761 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 7.05–8.39 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-5‴, H-6‴) × 2}, 7.48 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.66 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.9 (C-2, C-3, C-5, C-6), 135.5 (C-1,C-4); 165.0 (C-1′, C-3′), 110 (C-2′), 149.8 (C-5′) pyrimidine; 141.0 (C-1″), 126.4 (C-2″, C-6″), 124.0 (C-3″, C-5″), 149.9 (C-4″); 143.5 (C-1‴), 132.2 (C-2‴), 126.5 (C-3‴), 122.9 (C-4‴), 134.1 (C-5‴), 118.1 (C-6‴); 188.2 (C=O), 52.5 (CH2); Elem. Anal. Calcd. C42H28Cl2N12O10: C, 54.15; H, 3.03; N, 18.04; Found: C, 54.13; H, 3.00; N, 18.01; MS ES + (ToF): m/z 933 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2,6-dimethyl phenyl)amino)acetamide) (14)
Gold yellow crystals; M.p.: 158–160 °C; Rf value: 0.38; Yield: 82.33%; IR (KBr pellets, cm−1): 3074 (C–H str., phenyl nucleus), 1520 (C=C str., phenyl nucleus), 1692 (NH–C=O str.), 1601 (N=CH str., of pyrimidine), 2853 (C–H str., CH2), 1211 (C–NH– str.), 1343 (C–N sym. str., Ar–NO2);1H-NMR (δ, DMSO-d 6): 7.94–8.39 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴) × 2}, 7.91 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.39 (s, 4H, (CH2)2), 2.09 (s, 6H, (CH3)2); 13C-NMR (δ, DMSO-d 6): 128.6 (C-2, C-3, C-5, C-6), 135.5 (C-1, C-4); 162.0 (C-1′, C-3′), 108 (C-2′), 149.8 (C-5′) pyrimidine; 141.0 (C-1″), 126.5 (C-2″, C-6″), 123.5 (C-3″, C-5″), 149.0 (C-4″); 145.5 (C-1‴), 119.0 (C-4‴), 127.2 (C-2‴, C-6‴), 127.9 (C-3‴, C-5‴); 170.2 (C=O), 52.5 (CH2), 17.8 (2,6-CH3); Elem. Anal. Calcd. C46H40N10O6: C, 66.66; H, 4.86; N, 16.90; Found: C, 66.63; H, 4.81; N, 16.87; MS ES + (ToF): m/z 830 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2,4-dimethyl phenyl)amino)acetamide) (15)
Gold yellow crystals; M.p.: 135–137 °C; Rf value: 0.45; Yield: 89.63%; IR (KBr pellets, cm−1): 3074 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1664 (NH–C=O str.), 1597 (N=CH str., of pyrimidine), 2917 (C–H str., CH2), 1208 (C–NH– str.), 1339 (C–N sym. str., Ar–NO2); 1H-NMR (δ, DMSO-d 6): 6.58-8.40 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-5‴, H-6‴) × 2}, 7.47 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.36 (s, 4H, (CH2)2), 2.51(s, 6H, (CH3)2); 13C-NMR (δ, DMSO-d 6): 128.9 (C-2, C-3, C-5, C-6), 136.5 (C-1, C-4); 163.0 (C-1′, C-3′), 104 (C-2′), 149.8 (C-5′) pyrimidine; 141.0 (C-1″), 126.4 (C-2″, C-6″), 124.5 (C-3″, C-5″), 149.8 (C-4″); 143.6 (C-1‴), 126.5, 126.2 (C-2‴, C-5‴), 133.9 (C-3‴), 136.9 (C-4‴), 116.1 (C-6‴); 188.2 (C=O), 52.0 (CH2), 18.2 (o-CH3), 21.2 (p-CH3); Elem. Anal. Calcd. C46H40N10O6: C, 66.66; H, 4.86; N, 16.90; Found: C, 66.63; H, 4.87; N, 16.95; MS ES + (ToF): m/z 830 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((4-bromophenyl) amino)acetamide) (16)
Gold yellow crystals; M.p.: 138–140 °C; Rf value: 0.23; Yield: 87.77%; IR (KBr pellets, cm−1): 3107 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1665 (NH–C=O str.), 1599 (N=CH str., of pyrimidine), 2933 (C–H str., CH2), 1209 (C–NH– str.), 1341 (C–N sym. str., Ar–NO2), 697 (C–Br str., Ar–Br); 1H-NMR (δ, DMSO-d 6): 6.51–8.69 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-3‴, H-5‴, H-6‴) × 2}, 7.55 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.37 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.2 (C-2, C-3, C-5, C-6), 136.7 (C-1, C-4); 160.0 (C-1′, C-3′), 104 (C-2′), 149.8 (C-5′) pyrimidine; 141.5 (C-1″), 126.4 (C-2″, C-6″), 123.5 (C-3″, C-5″), 149.7 (C-4″); 145.5 (C-1‴), 115 (C-2‴, C-6‴), 132.0 (C-3‴, C-5‴), 118.8 (C-5‴); 188.2 (C = O), 52.5 (CH2); Elem. Anal. Calcd. C42H30Br2N10O6: C, 54.21; H, 3.25; N, 15.05; Found: C, 54.18; H, 3.20; N, 15.00; MS ES + (ToF): m/z 933 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2,3-dichlorophenyl) amino)acetamide) (17)
Gold yellow crystals; M.p.: 195–197 °C; Rf value: 0.16; Yield: 72.01%; IR (KBr pellets, cm−1): 3108 (C–H str., phenyl nucleus), 1520 (C=C str., phenyl nucleus), 1666 (NH–C=O str.), 1599 (N=CH str., of pyrimidine), 2934 (C–H str., CH2), 1211 (C–NH– str.), 1341 (C–N sym. str., Ar–NO2), 700 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 7.93–8.39 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-4‴, H-5‴, H-6‴) × 2}, 7.91 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.38 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 128.9 (C-2, C-3, C-5, C-6), 135.1 (C-1, C-4); 162.0 (C-1′, C-3′), 104.1 (C-2′), 149.0 (C-5′) pyrimidine; 126.4 (C-2″, C-6″), 123.8 (C-3″, C-5″), 149.6 (C-4″); 145.3 (C-1‴), 123.6 (C-2‴), 133.9 (C-3‴), 122.9 (C-4‴), 129.1 (C-5‴), 113.1 (C-6‴); 188.2 (C=O), 52.6 (CH2); Elem. Anal. Calcd. C42H28Cl4N10O6: C, 55.40; H, 3.10; N, 15.38; Found: C, 55.38; H, 3.07; N, 15.33; MS ES + (ToF): m/z 912 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((2,6-dichlorophenyl) amino)acetamide) (18)
Gold yellow crystals; M.p.: 129–131 °C; Rf value: 0.33; Yield: 75.30%; IR (KBr pellets, cm−1): 3107 (C–H str., phenyl nucleus), 1520 (C=C str., phenyl nucleus), 1666 (NH–C = O str.), 1599 (N=CH str., of pyrimidine), 2936 (C–H str., CH2), 1211 (C–NH– str.,), 1342 (C–N sym. str., Ar–NO2), 700 (C–Cl str., Ar–Cl); 1H-NMR (δ, DMSO-d 6): 7.04–9.09 {m, 18H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴) × 2}, 7.75 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.38 (s, 4H, (CH2)2); 13C-NMR (δ, DMSO-d 6): 127.3 (C-2, C-3, C-5, C-6), 134.5 (C-1, C-4); 164.0 (C-1′, C-3′), 102 (C-2′), 148.3 (C-5′) pyrimidine; 126.4 (C-2″, C-6″), 124.5 (C-3″, C-5″), 148.6 (C-4″); 144.5 (C-1‴), 123.5 (C-2‴, C-6‴), 128.5 (C-3‴, C-5‴), 121.1 (C-6‴); 165.2 (C=O), 53.0 (CH2); Elem. Anal. Calcd. C42H28Cl4N10O6: C, 55.40; H, 3.10; N, 15.38; Found: C, 76.13; H, 4.24; N, 11.12; MS ES + (ToF): m/z 912 [M+ + 1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-((3-methoxyphenyl) amino)acetamide) (19)
Gold yellow crystals; M.p.: 198–200 °C; Rf value: 0.21; Yield: 75.30%; IR (KBr pellets, cm−1): 3074 (C–H str., phenyl nucleus), 1519 (C=C str., phenyl nucleus), 1666 (NH–C=O str.), 1598 (N=CH str., of pyrimidine), 2836 (C–H str., CH2), 1210 (C–NH– str.), 1341 (C–N sym. str., Ar–NO2), 1105 (C–O–C str., Ar–OCH3); 1H-NMR (δ, DMSO-d 6): 5.22–8.69 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-2‴, H-4‴, H-5‴, H-6‴) × 2}, 7.41 (s, 2H, (CH)2 of pyrimidine), 8.01 (s, 2H, (NH)2), 3.73 (s, 4H, (CH2)2, 3.38 (s, 6H, (OCH3)2); 13C-NMR (δ, DMSO-d 6): 128.3 (C-2, C-3, C-5, C-6), 136.7 (C-1, C-4); 160.0 (C-1′, C-3′), 106 (C-2′), 149.8 (C-5′) pyrimidine; 141.5 (C-1″), 126.4 (C-2″, C-6″), 123.5 (C-3″, C-5″), 148.3 (C-4″); 149.7 (C-1‴), 106.4 (C-2‴, C-6‴), 113.3 (C-5‴); 188.2 (C=O), 54.4 (CH2), 55.1 (OCH3); Elem. Anal. Calcd. C44H36N10O8: C, 63.46; H, 4.36; N, 16.82; Found: C, 63.39; H, 4.30; N, 16.86; MS ES + (ToF): m/z 834 [M+ +1].
N,N′-(6,6′-(1,4-Phenylene)bis(4-(4-nitrophenyl)pyrimidine-6,2-diyl))bis(2-(2-methoxyphenyl) acetamide (20)
Canary yellow crystals; M.p.: 179–180 °C; Rf value: 0.32; Yield: 89.23%; IR (KBr pellets, cm−1): 3076 (C–H str., phenyl group), 1519 (C=C str., phenyl group), 1666 (NH–C=O str.), 1599 (N=CH str., of pyrimidine), 2842 (C–H str., CH2), 1211 (C–NH– str.), 1340 (C–N sym. str., Ar–NO2), 1107 (C–O–C str., Ar–OCH3); 1H-NMR (δ, DMSO-d 6): 7.14-8.39 {m, 20H, Ar = H-2, H-3, H-5, H-6, (H-2″, H-3″, H-5″, H-6″) × 2, (H-3‴, H-4‴, H-5‴, H-6‴) × 2}, 7.47 (s, 2H, (CH)2 of pyrimidine), 8.00 (s, 2H, (NH)2), 3.82 (s, 4H, (CH2)2), 3.75 (s, 6H, (OCH3)2); 13C-NMR (δ, DMSO-d 6): 128.9 (C-2, C-3, C-5, C-6), 136.7 (C-1, C-4); 164.0 (C-1′, C-3′), 110.0 (C-2′), 149.8 (C-5′) pyrimidine; 141.5 (C-1″), 126.4 (C-2″, C-6″), 124.5 (C-3″, C-5″), 149.7 (C-4″); 126.5 (C-1‴), 158.0 (C-2‴), 55.5 (OCH3), 113.3 (C-3‴), 128.9 (C-4‴), 121.3 (C-5‴), 133.2 (C-6‴); 188.2 (C=O), 39.0 (CH2); Elem. Anal. Calcd. C44H36N10O8: C, 63.46; H, 4.36; N, 16.82; Found: C, 63.40; H, 4.31; N, 16.85; MS ES + (ToF): m/z 834 [M+ +1].
Biological study (antimicrobial and anticancer)
The antimicrobial activity i.e. The minimum inhibitory concentration (MIC) of the synthesized compounds (1–20) was determined by tube dilution method [26] using cefadroxil (antibacterial) and fluconazole (antifungal) as reference drugs against Gram-positive [S. aureus, MTCC-3160 (Microbial Type Culture Collection); B. subtilis, MTCC-441] and Gram-negative bacteria (E. coli, MTCC-443; P. aeruginosa, MTCC-3542; S. enterica, MTCC-1165). The antifungal activity was assayed against yeast (C. albicans, MTCC-227) and mould (A. niger, MTCC-281). Serial dilutions of the test compounds and reference drugs were prepared in double strength nutrient broth I.P. (bacteria) or sabouraud dextrose broth I.P. (fungi) [27]. The stock solution of the test compounds and reference drugs was prepared in dimethyl sulfoxide (DMSO). Further progressive dilutions were done to obtain final concentrations of 50, 25, 12.5, 6.25, 3.125 and 1.562 µg/mL. The samples were incubated at 37 ± 1 °C for 24 h (bacteria), at 25 ± 1 °C for 7 days (A. niger) and at 37 ± 1 °C for 48 h (C. albicans) respectively and the results were recorded in terms of MIC. The MIC was the lowest concentration of the tested compound that yields no visible growth of microorganisms in the tube. To ensure that the solvent had no effect on the bacterial growth, a control was performed with the test medium supplemented with DMSO at the same dilutions as used in the experiments and DMSO had no effect on the microorganisms in the concentrations studied. The anticancer screening (IC50 = µmol/mL) of synthesized compounds was determined against human colorectal carcinoma [HCT-116 (ATCC (American Type Culture Collection) CCL-247)] cancer cell line using sulforhodamine-B (SRB) assay. In this study, the culture material was fixed with trichloroacetic acid and then stained for 30 min with 0.4% (w/v) sulforhodamine B mixed with 1% acetic acid. Unbound dye was discarded by five washes of 1% acetic acid solution and protein-bound dye was extracted with 10 mM Tris base [tris(hydroxymethyl) aminomethane] for confirmation of optical density in a computer-interfaced, 96-well microtiter plate reader [28].
Conclusion
In summary, a series of new bis-pyrimidine acetamide molecules was synthesized in good yields and its chemical structures were confirmed by 1H/13C-NMR, Mass, FT-IR studies and elemental analyses. All the synthesized compounds were tested for their in vitro antimicrobial and anticancer potentials. Among the synthesized compounds, compounds, 3, 13, 16, 17 and 18 exhibited good antimicrobial potential against different microorganism (bacterial species: S. aureus, B. subtilis, E. coli, P. aeruginosa, S. enterica and fungal species: A. niger and C. albicans) than the standard drugs cefadroxil and fluconazole. Similarly, compounds, 12, 16 and 18 were found to be more effective against HCT 116 cancer cell line than the standard drug, 5-fluorouracil.
Authors’ contributions
BN and SK have designed, synthesized and carried out the antimicrobial activity of the bis-pyrimidine acetamide derivatives. KR, SAAS, SML and VM have carried out the spectral analysis, interpretation and anticancer evaluation of synthesized compounds. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, M. D. University, Rohtak for providing necessary facilities to carry out this research work.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sanjiv Kumar, Email: sanjiv.pharmsci@gmail.com.
Siong Meng Lim, Email: stvlsm@gmail.com.
Kalavathy Ramasamy, Email: kalav922@gmail.com.
Mani Vasudevan, Email: vasumpharmacol@gmail.com.
Syed Adnan Ali Shah, Email: benzene301@yahoo.com.
Balasubramanian Narasimhan, Email: naru2000us@yahoo.com.
References
- 1.Keche AP, Hatnapure GD, Tale RH, Rodge AH, Birajdar SS, Kamble VM. Novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg Med Chem Lett. 2012;22:3445–3448. doi: 10.1016/j.bmcl.2012.03.092. [DOI] [PubMed] [Google Scholar]
- 2.Zijian L, Yu W, Lin L, Dazhuang Z, Lihui W, Yanfang Z, Ping G. Design, synthesis and biological evaluation of novel thieno [3,2-d] pyrimidine derivatives containing diaryl urea moiety as potent antitumor agents. Eur J Med Chem. 2014;85:215–227. doi: 10.1016/j.ejmech.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 3.Anupama B, Dinda SC, Prasad YR, Rao AV. Synthesis and antimicrobial activity of some new 2,4,6-trisubstituted pyrimidines. Int J Res Pharm Chem. 2012;2(2):231–236. [Google Scholar]
- 4.Yerragunta V, Patil P, Anusha V, Swamy TK, Suman D, Samhitha T. Pyrimidine and its biological activity: a review. Pharma Tutor. 2013;1(2):39–44. [Google Scholar]
- 5.Holla BS, Mahalinga M, Karthikeyan MS, Akberalib PM, Shettyc NS. Synthesis of some novel pyrazolo [3,4-d] pyrimidine derivatives as potential antimicrobial agents. Bioorg Med Chem. 2006;14:2040–2047. doi: 10.1016/j.bmc.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 6.El-Gaby MSA, Gaber AM, Atalla AA, Al-Wahab KAA. Novel synthesis and antifungal activity of pyrrole and pyrrolo [2,3-d] pyrimidine derivatives containing sulfonamide moieties. Il Farmaco. 2002;57:613–617. doi: 10.1016/S0014-827X(01)01178-8. [DOI] [PubMed] [Google Scholar]
- 7.Pandey S, Suryawanshi SN, Gupta S, Srivastava VML. Synthesis and antileishmanial profile of some novel terpenyl pyrimidines. Eur J Med Chem. 2004;39:969–973. doi: 10.1016/j.ejmech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V, Chitranshi N, Agarwal AK. Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem. 2014;2014:1–31. doi: 10.1155/2014/202784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen PJ, Yang A, Gu YF, Zhang XS, Shao KP, Xue DQ, He P, Jiang TF, Zhang QR, Liu HM. Synthesis, in vitro antimicrobial and cytotoxic activities of novel pyrimidine benzimidazol combinations. Bioorg Med Chem Lett. 2014;24:2741–2743. doi: 10.1016/j.bmcl.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Ashour HM, Shaaban OG, Rizk OH, El-Ashmawy IM. Synthesis and biological evaluation of thieno [2′,3′:4,5] pyrimido [1,2-b][1, 2, 4] triazines and thieno [2,3-d][1, 2, 4] triazolo [1,5-a] pyrimidines as anti-inflammatory and analgesic agents. Eur J Med Chem. 2013;62:341–351. doi: 10.1016/j.ejmech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Song XJ, Shao Y, Gao X. Dong Microwave-assisted synthesis of some novel fluorinated pyrazolo [3,4-d] pyrimidine derivatives containing 1,3,4-thiadiazole as potential antitumor agents. Chin Chem Lett. 2011;22:1036–1038. doi: 10.1016/j.cclet.2011.05.012. [DOI] [Google Scholar]
- 12.Meneghesso S, Vanderlinden E, Stevaert A, McGuigan C, Balzarini J, Naesens L. Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antivir Res. 2012;94:35–43. doi: 10.1016/j.antiviral.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Faldu VJ, Gothalia VK, Shah VH. Characterization and antitubercular activity of synthesized pyrimidine derivatives via chalcones. Indian J Chem. 2015;54B:391–398. [Google Scholar]
- 14.Kumar D, Khan SI, Tekwani BL, Diwan PP, Rawat S. 4-Aminoquinoline-pyrimidine hybrids: synthesis, antimalarial activity, heme binding and docking studies. Eur J Med Chem. 2015;89:490–502. doi: 10.1016/j.ejmech.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Kotaiah Y, Nagaraju K, Harikrishna N, Rao CV, Yamini L, Vijjulatha M. Synthesis, docking and evaluation of antioxidant and antimicrobial activities of novel 1,2,4-triazolo [3,4-b][1, 3, 4] thiadiazol-6-yl) selenopheno [2,3-d] pyrimidines. Eur J Med Chem. 2014;75:195–202. doi: 10.1016/j.ejmech.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Saini M, Kumar P, Kumar M, Kalavathy R, Mani V, Mishra RK, Majeed ABA, Narasimhan B. Synthesis, in vitro antimicrobial, anticancer evaluation and QSAR studies of N′-(substituted)-4-(butan-2-lideneamino) benzohydrazides. Arab J Chem. 2014;7(4):448–460. doi: 10.1016/j.arabjc.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deep A, Kumar P, Narasimhan B, Mishra RK, Mani V, Ramasamy K, Meng LS. 2-Azetidinone derivatives: synthesis, antimicrobial, anticancer evaluation and QSAR studies. Acta Pol Pharm Drug Res. 2016;73:65–78. [PubMed] [Google Scholar]
- 18.Kumar S, Kumar P, Marwaha RK, Narasimhan B. Synthesis, antimicrobial evaluation and QSAR studies of propionic acid derivatives. Arab J Chem. 2017;10:S881–S893. doi: 10.1016/j.arabjc.2012.12.024. [DOI] [Google Scholar]
- 19.Ghoneim AA, El-Farargy AF. Synthesis of some novel fused heterocyclic compounds derivatives from bis-chalcones. Chem Curr Res. 2016;5(1):1–5. [Google Scholar]
- 20.Kharb R, Tyagi M, Sharma AK. Current status and future scenario of pyrimidine derivatives having antimicrobial potential. Der Pharma Chem. 2014;6(4):298–320. [Google Scholar]
- 21.Yaziji V, Rodríguez D, Coelho A, García-Mera X, El Maatougui A, Brea J, Loza MI, Cadavid MI, Gutiérrez-de-Terán H, Sotelo E. Selective and potent adenosine A3 receptor antagonists by methoxyaryl substitution on the N-(2,6-diarylpyrimidin-4-yl) acetamide scaffold. Eur J Med Chem. 2013;59:235–242. doi: 10.1016/j.ejmech.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Kanagarajan V, Thanusu J, Gopalakrishnan M. Synthesis and in vitro microbiological evaluation of an array of biolabile 2-morpholino-N-(4,6-diaryl pyrimidin-2-yl) acetamides. Eur J Med Chem. 2010;45:1583–1589. doi: 10.1016/j.ejmech.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Asiri AM, Khan SA. Synthesis and anti-bacterial activities of a bis-chalcone, derived from thiophene and its bis-cyclized products. Molecules. 2011;16:523–531. doi: 10.3390/molecules16010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parveen H, Hayat F, Mukhtar S, Salahuddin A, Khan A, Islam F, Azam A. Synthesis, characterization and biological evaluation of novel 2,4,6-trisubstituted bis-pyrimidine derivatives. Eur J Med Chem. 2011;46:4669–4675. doi: 10.1016/j.ejmech.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 25.Naik TA, Chikhalia KH. Studies on synthesis of pyrimidine derivatives and their pharmacological evaluation. E-J Chem. 2007;4(1):60–66. doi: 10.1155/2007/507590. [DOI] [Google Scholar]
- 26.Cappuccino JC, Sherman N. Microbiology—a laboratory manual. California: Addison Wesley; 1999. p. 263. [Google Scholar]
- 27.Pharmacopoeia of India, vol Ӏ (2007) Controller of publication, Ministry of Health Department, Govt. of India, New Delhi, pp. 37
- 28.Skehan P, Storeng Scudiero RD, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]


