Abstract
rag1 −/− zebrafish have been employed in immunological research as a useful immunodeficient vertebrate model, but with only fragmentary evidence for the lack of functional adaptive immunity. rag1-null zebrafish exhibit differences from their human and murine counterparts in that they can be maintained without any specific pathogen-free conditions. To define the immunodeficient status of rag1 −/− zebrafish, we obtained further functional evidence on T- and B-cell deficiency in the fish at the protein, cellular, and organism levels. Our developed microscale assays provided evidence that rag1 −/− fish do not possess serum IgM protein, that they do not achieve specific protection even after vaccination, and that they cannot induce antigen-specific CTL activity. The mortality rate in non-vaccinated fish suggests that rag1 −/− fish possess innate protection equivalent to that of rag1 +/− fish. Furthermore, poly(I:C)-induced immune responses revealed that the organ that controls anti-viral immunity is shifted from the spleen to the hepatopancreas due to the absence of T- and B-cell function, implying that immune homeostasis may change to an underside mode in rag-null fish. These findings suggest that the teleost relies heavily on innate immunity. Thus, this model could better highlight innate immunity in animals that lack adaptive immunity than mouse models.
Introduction
Recombination-activating gene 1 (rag1) plays an essential role in the rearrangement and recombination of immunoglobulins and T-cell receptor (TCR) genes. Rag1-deficient animals lack functional T- and B-cells in adaptive immunity, and thus, it is possible to investigate innate immunity that is not affected by T- and B-cells in this model1–3. As Rag deficiency results in severe combined immunodeficiency (SCID) in mice and humans, they must be maintained in specific-pathogen free (SPF) conditions. Thus, mammals are thought to depend heavily on adaptive immunity.
Teleost fish are primitive vertebrates with an adaptive immune system, and they possess T- and B-cell functionality equivalent to that in mammals4–9. Rag genes have been identified in many fish species10–14, and it has been confirmed that the antigenic diversity is attributable to the TCRs and immunoglobulins of teleost fish8, 15. In 2002, rag1-deficient zebrafish were established using the targeting induced local lesions in genomes method (TILLING)16. Thus far, several groups have utilized this fish for immunological studies and demonstrated their functions in some pathogenic models17–19. The observations in these studies and our findings suggest that the rag1-null zebrafish possess more robust rag1-independent immunity than mammalian models. For instance, this fish never develops SCID, and it can be maintained for a long period (at least 2 years) under conventional conditions (non-SPF environment)16 (our unpublished information). Furthermore, they display antigen-specific protection against intracellular bacterial infection, implicating that they have immunological memory17. Therefore, we examined whether the rag1-null fish completely lacks functional T- and B-cells.
Previous studies reported evidence of T- and B-cell deficiency in rag1-null fish. The lymphocyte population in the deficient animals was significantly smaller than that in wild-type fish20. In addition, the V segments of TCR and immunoglobulin mRNA were not found in the null animals. Although these findings indicate that the rag1-null fish exhibits one of the typical phenotypes of rag1-deficient animals, it remains unclear whether they actually lack the ability to produce immunoglobulin, whether they are protected by vaccination, and whether they exhibit specific cell-mediated immunity, such as cytotoxic T cell function. Thus, additional inspections at the protein, cellular, tissue, and organism levels are necessary to confirm immunodeficiency in the fish strain. The present study sought to establish assays for analyzing these questions at the protein and cellular levels for small fish, validating T- and B-cell deficiency, investigating the expression profiles of cytokines in rag1-null fish, and discussing differences in the balance of adaptive and innate immunity between mammals and teleosts.
Results
Detection of IgM protein in serum and IgM-positive cells in kidneys and blood
Sera from rag1 −/− and wild-type zebrafish were fractionated by gel filtration and analyzed for IgM expression by western blotting with chemiluminescent detection. The elution profiles exhibited marked differences in the high-molecular-weight fractions corresponding to tetrameric IgM, with lower protein peaks in rag −/− fish than in wild-type fish (Fig. 1A). In Western blotting of high-molecular-weight fractions (#1–5), the 85-kDa band of IgM heavy chain, which was evident in wild-type fish, was undetectable even by chemiluminescent detection in rag1 −/− fish (Fig. 1B). These results indicate that IgM is absent in serum from rag1 −/− fish.
Figure 1.
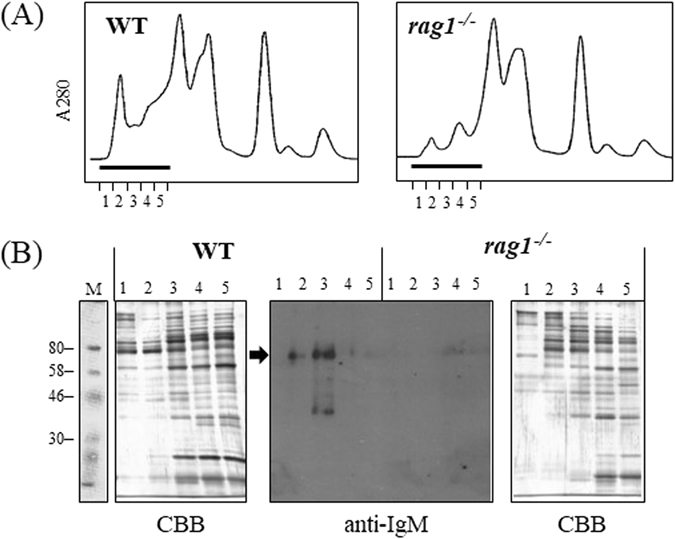
Validations of absence of IgM protein in serum. (A) Elution profiles in gel filtration chromatography of serum from wild-type (left) and rag1 −/− zebrafish (right). Bars indicate the fractions that were applied to western blotting. Fraction numbers are indicated on the X-axis. (B) Detection of IgM in serum from wild-type (left) and rag1 −/− fish (right) by western blotting. The arrow denotes the heavy chain of IgM (85 kDa). The numbers on the lane correlate to the fraction numbers in Fig. 1A.
Membrane IgM (mIgM)-positive cells were detected in the kidneys and blood of rag +/− fish (28.3% and 15.5%, respectively). On the contrary, leukocytes in kidneys and blood from rag −/− fish contained less than 1.0% mIgM-positive cells, indicating that rag −/− fish lack mature IgM-positive B-cells (Fig. 2).
Figure 2.
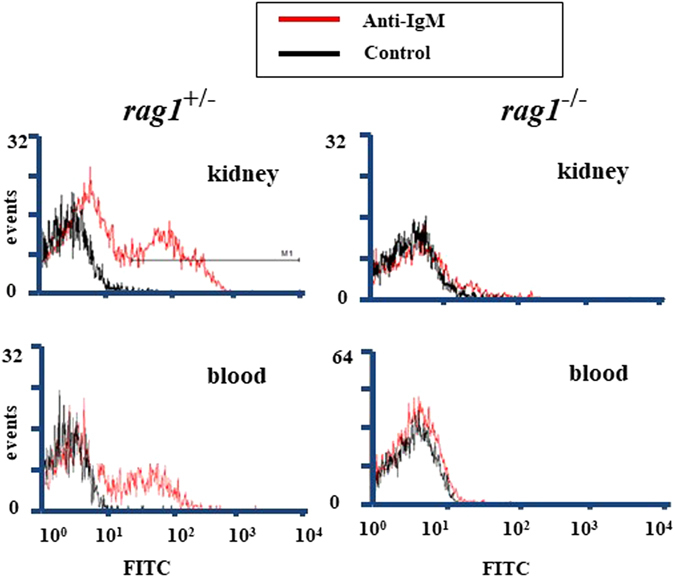
Flow cytometry analysis of IgM-positive leukocytes in kidneys and blood from rag1 +/− and rag1 −/− fish. Gray and black lines show anti-IgM–positive and anti-IgG–negative controls. Three individual samples were independently analyzed, and representative data are shown.
Detection of antibody titers in vaccinated fish
To examine specific antibody production, the agglutinating antibody titer of serum from vaccinated fish was investigated in rag1 +/− and rag1 −/− animals. All sera from vaccinated rag1 +/− fish displayed antibody titers of 32–320, whereas those of rag1 −/− fish were below the detection limit (titer <16) (Table 1). The results clearly illustrate that rag1 −/− fish completely lack specific antibody production.
Table 1.
Antibody titer against vibrio in rag−/− and rag+/− zebrafish.
| Fish No. | RAG1 genotype | Antibody titer |
|---|---|---|
| 1 | +/− | 320 |
| 2 | +/− | 256 |
| 3 | +/− | 40 |
| 4 | +/− | 64 |
| 5 | +/− | 32 |
| 6 | +/− | 32 |
| 7 | −/− | ND* |
| 8 | −/− | ND |
| 9 | −/− | ND |
| 10 | −/− | ND |
| 11 | −/− | ND |
| 12 | −/− | ND |
*Antibody titers were less than 16.
Protective effect of vaccination following infection with bacteria
Bath infection with Vibrio anguillarum caused symptoms of vibriosis and mortality in both strains of fish (Fig. 3). The fish began to die 12–48 h post-infection. Petechial hemorrhages were observed in on the skin and gills of dead fish in both groups (data not shown). The cumulative mortality rate of vaccinated rag1 +/− fish was significantly lower than that of non-vaccinated fish (Fig. 3A). By contrast, no benefit of vaccination was detected in rag1 −/− fish (Fig. 3B). These results indicated that rag1 −/− fish did not engage adaptive immune responses against Vibrio infection. There was no significant difference in mortality between the strains for non-vaccinated fish, suggesting that the innate immunity of rag1 −/− fish against Vibrio infection is comparable to that of rag1 +/− fish.
Figure 3.
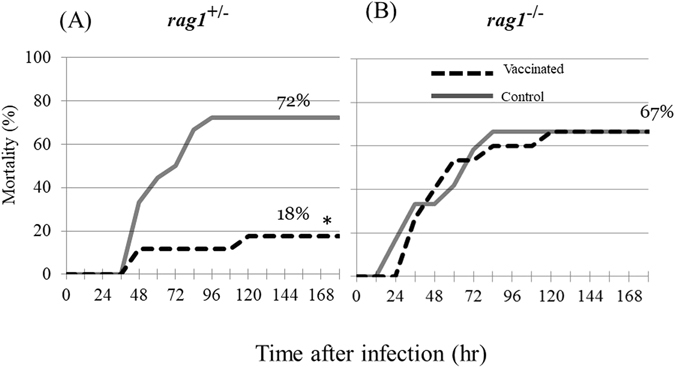
Cumulative mortality of rag1 +/− and rag1 −/− fish after Vibrio infection. The mortalities of vaccinated and unvaccinated (control) fish are shown as solid and dashed lines, respectively. The numbers in the graph indicate the final mortality rate as percentages. Asterisk (*) indicates a significant difference when compared with the control group.
Histological observation of the thymus in rag1+/− and rag1−/− fish
Histological observation revealed significant differences in thymus morphology between rag1 −/− and rag +/− fish. A mature thymus, which contains a lymphocyte clump, was observed in all rag +/− fish (n = 5) (Fig. 4A). On the contrary, two of five rag1 −/− fish lacked a thymus. The thymus in rag1 −/− fish was atrophic and smaller than that in rag1 +/− fish (Fig. 4B). Only a small lymphocyte clump was observed in the atrophic thymus. These findings illustrated that rag1 −/− fish lack a mature thymus. No significant difference was observed in other organs between rag1 −/− and rag +/− fish (data not shown).
Figure 4.
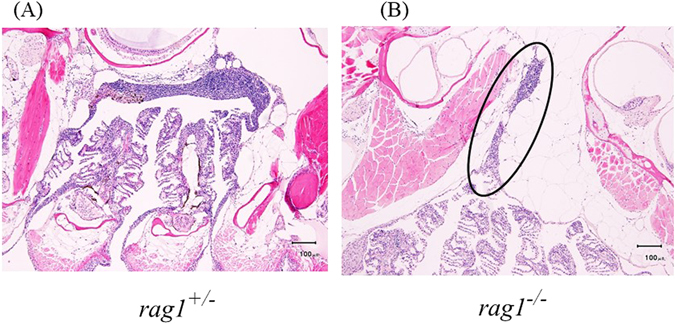
Histological observation of the hematoxylin–eosin-stained thymus in rag1 +/− (left) and rag1 −/− fish (right). The thymus in rag1 −/− fish is representative of five independent samples. The thymus in rag1 +/− fish is representative of three independent samples. A thymus was not found in two rag1 +/− fish. The circle shows atrophic lymphocyte clump.
In vivo rejection of allogeneic erythrocytes
Donor fish, which were bled three times by pricking the caudal peduncle, could be kept alive during the immunization period. In flow cytometry, the peak of 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled and transferred cells was clearly detected (Fig. 5A). The percentages of transferred erythrocytes in immunized rag1 −/− fish were significantly higher than those in immunized rag1 +/− fish (Fig. 5B). Conversely, there was no significant difference in the survival of transferred erythrocytes in non-immunized fish between the fish groups (Fig. 5B). The results indicated that alloantigen-specific rejection was induced as a result of immunization with allogeneic cells in rag1 +/− fish but totally impaired in rag1 −/− fish, suggesting that rag1 +/− fish lack alloantigen-specific CTL.
Figure 5.
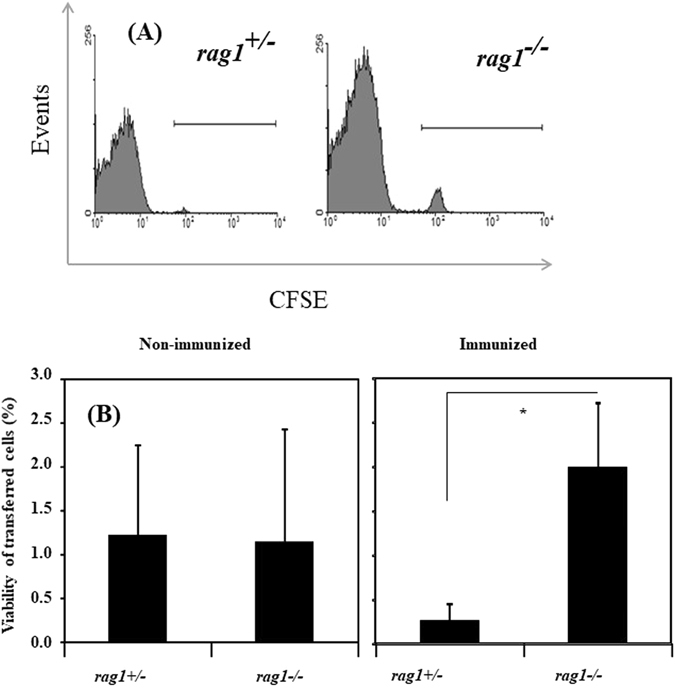
Rejection of allogeneic erythrocytes in rag1 +/− and rag1 −/− fish. Flow cytometry histograms show the transferred positive cells in rag1 +/− (left) or rag1 −/− recipients (right) (A). Horizontal bars in the histogram indicate the 5(6)-carboxyfluorescein diacetate N-succinimidyl ester-positive region. The data are representative of three independent samples. The percentage of variable transferred cells in recipients is shown in non-immunized and immunized fish (B). Asterisk (*) indicates a significant difference between the two strains (p < 0.05).
Expression profiles of cytokines after poly(I:C) stimulation
To reveal differences in immune homeostasis between rag1 +/− and rag1 −/− fish, the mRNA expression of cytokines was analyzed in the kidneys, spleen, and hepatopancreas. There was no significant difference in mRNA expression between the fish under non-stimulated conditions (data not shown). In rag1 +/− fish, the expression of several cytokines (IL-4, IL-10, TNF-α, IFN-γ1) was significantly enhanced by poly(I:C) stimulation, whereas no enhancement was observed in the hepatopancreas. By contrast, in rag1 −/− fish, the transcription of four cytokines (IL-4, IL-10, IL-17AF2, and IL-17AF3) was significantly increased in the hepatopancreas but not in the spleen (Fig. 6 and Fig. S2). No significant difference was observed in the kidneys. The results indicate that the production site of cytokines differs between rag1 +/− and rag1 −/− fish.
Figure 6.
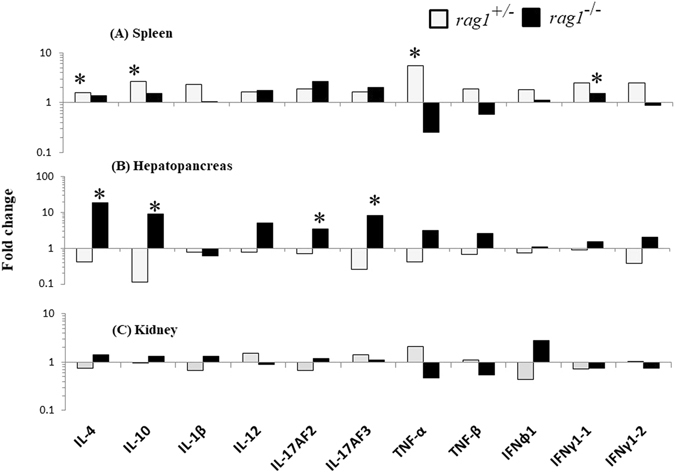
Expression of cytokines in the spleen (A), hepatopancreas (B) and kidney (C) of rag1 +/− and rag1 −/− fish. Data from the three individual experiments are shown as the mean fold change in mRNA expression relative to that in control fish. In all quantitative real-time PCR experiments, melting curve analyses were performed, and single specific melting peaks were observed, indicating amplification specificity. Statistical comparisons between stimulated and control fish were made using an unpaired t-test. Asterisks indicate significant differences at P < 0.05.
Changes of phagocytic activity and cell composition after poly(I:C)-stimulation
The percentage of macrophage/granulocyte was significantly increased in hepatopancreas from rag1 −/− fish by poly(I:C)-stimulation, while there is no significant difference in hepatopancreas from rag1 +/+ fish. The representative scatter plots was shown in Fig. S3. The percentage of lymphocyte was decreased in spleen from rag1 −/− fish after the stimulation. In contrast, the macrophage/granulocyte population was increased in the spleen (Fig. 7). Since morphological observation indicated that macrophages or granulocytes are dominant cells in large cell population from hepatopancreas or from kidney, respectively, suggesting that macrophage/granulocyte fraction mainly consists of macrophages in hepatopancreas, and granulocytes in kidney (data not shown).
Figure 7.
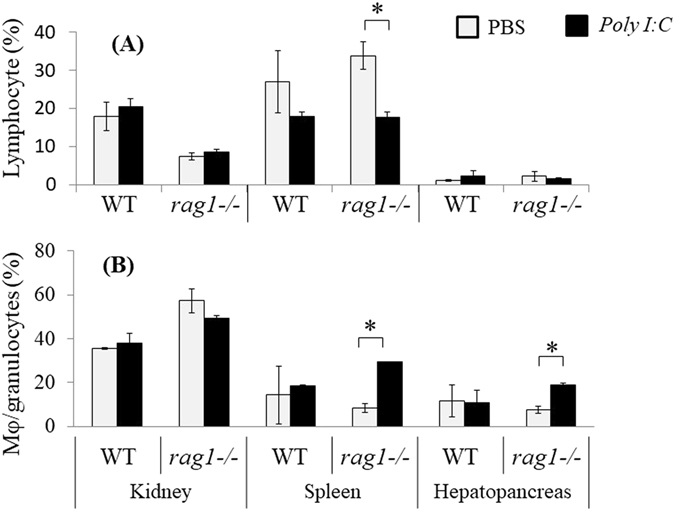
Leukocyte composition in the kidney, spleen, and hepatopancreas of wild-type (WT) (A) and rag1 −/− fish (B) after poly(I:C) stimulation. Data from the three individual experiments are shown as the mean percentages of the lymphocyte and macrophage/neutrophil fractions. Error bars indicate standard errors. Statistical comparisons between stimulated and control fish were made using an unpaired t-test. Asterisks indicate significant differences at P < 0.05.
Discussion
Rag-null mice and humans develop SCID as a result of T- and B-cell deficiency and thus require pathogen-free environments for survival21, 22. This phenomenon is evidence that the clonal diversity of T and B cells is essential for maintaining the life of higher vertebrates. By contrast, rag1-null fish can survive under non-SPF conditions for at least for 2 years, and they exhibited immunological memory-like responses to intracellular bacterial infection17. These findings raise fundamental questions regarding whether rag1-null zebrafish actually lack T- and B-cell function. If this is true, then it can be hypothesized that teleosts intrinsically rely more heavily on Rag-independent immunity than mammals.
Although previous studies suggested that rag1-deficient fish lack the ability to generate antibody diversity at a genetic aspect16, 20, there was no direct evidence that they lack immunoglobulins and functional B-cells. As zebrafish are not of suitable size for collecting sufficient amounts of blood, it was difficult to detect the target proteins in their organs or blood. To overcome this disadvantage, we pooled blood from several fish and concentrated proteins of similar molecular weights as IgM via gel filtration chromatography. Highly sensitive chemiluminescent Western blotting did not detect IgM in serum from rag1 −/− fish, suggesting that they do not produce IgMs including natural antibodies. In addition, agglutinating antibody was also undetectable in rag1 −/− fish after repeated immunization with a Vibrio vaccine, suggesting that they cannot develop a functional antibody response. A previous report described that rag1 −/− fish had a smaller lymphocyte population than rag1 +/− fish based on flow cytometry scattergrams of peripheral and kidney leukocytes20. This was confirmed by the present findings that rag1 −/− fish do not possess IgM-positive lymphocytes. Although the remote possibility that IgZ-positive cells exist in rag1 −/− fish remains, we concluded that rag1 −/− fish lack mature B-cells and cannot produce immunoglobulins.
It is known that crosstalk between thymocytes and thymic epithelial cells preserves the thymic architecture, and in fact, several types of Rag mutations result in defective thymic stromal maturation in mouse models3. Although previous work observed no remarkable abnormalities of the intestine, kidneys, and liver in rag1 −/− fish, no information on thymus development has been reported in the null animal23. In the present study, we clearly observed atrophy of the thymus in rag1 −/− fish. These results infer that intercellular communication malfunction similar to that in mammals occurred in the rag1 −/− zebrafish thymus, thereby suggesting evolutionary conservation of the mechanism of T-cell maturation and education in the thymus.
Allograft rejection experiments represent a typical assay for evaluating antigen-specific and MHC-restricted cellular immunity. Several studies revealed that the alloantigen-specific immune response is executed by CTL in teleost fish4, 5, 24, 25. In fish, grafting of allogeneic skin or scales has been employed as a simple test for examining allograft rejection by the cellular immune response, but the methodology is not easily applied to a wide variety of fish species, especially small fish such as zebrafish. In vivo rejection utilizing erythrocytes as an allograft has previously been demonstrated in rainbow trout, suggesting that CTL recognized MHC class I molecules as a major determinant for rejecting allogeneic erythrocytes26. Thus, we considered that allogeneic erythrocyte rejection is a useful modality for evaluating CTL activity in vivo, and we attempted to apply this assay to zebrafish, a small fish species. Immunized rag1 +/− recipients rejected allogeneic erythrocytes from donor fish, whereas rag1 −/− fish could not reject donor cells. This finding suggests that rag1 −/− fish lack CTL activity.
Horn and Hanson (2012) reported that re-infection with the intracellular bacterium Edwardsiella ictaluri provides rag1 −/− fish immunological memory-like protection, suggesting that NK-like cells might play an important role as innate memory cells17. By contrast, the present study did not identify any memory-enhancing effect of vaccination in rag1 −/− fish against Vibrio anguillarum challenge. The contradiction between these findings is presumably attributed to differences in the intracellular and extracellular bacteria. Because NK-like cells or innate lymphoid cells (ILCs) may not efficiently eliminate extracellular bacteria such as Vibrio anguillarum and a specific antibody was not secreted in rag1 −/− fish, it appears that they did not receive any benefit from vaccination against Vibrio infection. It is noteworthy that no difference in mortality was observed between non-vaccinated rag1 −/− and rag1 +/− fish, inferring that rag1 −/− fish possess protective ability equivalent to that of rag1 +/− fish without any significant contribution from T and B cells to host defense.
The present study uncovered a significant difference between adaptive immunity-deficient and normal fish. Although the spleen is a secondary lymphoid organ harboring functional T- and B-cells in vertebrates, cytokine mRNA expression was not increased after poly(I:C) stimulation in the spleen from rag1 −/− fish. On the contrary, the hepatopancreas is inferred to be an important site of cytokine production in adaptive immunity-lacking fish. Although absolute level of the cytokines expression in hepatopancreas was lower than that in spleen (Fig. S2), significant upregulation of the cytokines in rag1 −/− was detected in only hepatopancreas by poly(I:C) stimulation. This finding suggests that hepatopancreas compensates the roles of secondary immune organs by lacking adaptive immunity. In mammals, the primary site of ILC development is the liver in the fetus, and liver-resident NK cells play important roles in immune homeostasis27–29. Flow cytometry revealed that the numbers of macrophages in the hepatopancreas in rag1 −/− fish were increased by stimulation, whereas they were not increased in wild-type fish. Thus, macrophage in primitive vertebrates, but not ILC, may compensate for the functions of T and B cells in the liver or hepatopancreas.
The zebrafish is widely employed in immunological and hematological studies as a vertebrate model, contributing to both fish immunology and human medical science22, 30–38. The present and preceding studies illustrated that teleosts rely more heavily on rag-independent immunity than mammals and suggest that zebrafish can serve as a better animal model for exploring the potential of innate immunity than mice. At present, it remains unclear which factors compensate, at least in part, to enable the long-term survival of fish under non-SPF rearing conditions. Jima et al. (2009) reported that the expression of genes encoding components of the complement and coagulation systems was enhanced in rag1 −/− fish23. However, we did not detect significant differences in the serum concentration of complement component C3 protein between rag1 −/− and rag1 +/− fish (data not shown). In addition, although we did not find rag1 −/− fish-specific upregulation of several cytokine mRNA under normal (uninfected) conditions, poly(I:C) stimulation induced the differential expression of cytokine mRNAs between rag1 −/− and rag1 +/− fish. Thus, enhanced immune factors that could support the innate defense of rag1 −/− fish independently from B- and T-cell–mediated adaptive immunity should be investigated for various pathogens with different infection routes. These rag1 −/− fish appear to possess more efficient innate immune defenses than mammals, and thus, they may provide insight into a novel methodology to enhance the innate defense of immune-compromised mammals, such as patients with terminal cancer and other adaptive immune deficiencies.
Materials and Methods
Fish
The rag1 −/− line (rag1t26683) was previously generated at the Hubrecht Institute16 and provided by the Tübingen 2000 Screen Consortium. rag1 −/− fish were reproduced at the National Research Institute of Aquaculture and Kyushu University. rag1 +/− fish were generated by crossing the null strain with the normal wild-type or albino strain. They were genotyped using previously described PCR methods16. Adult fish (0.1–0.5 g, 5 weeks–6 month old) were used in the experiments. rag1 +/− or wild-type (rag1 +/+) fish were used as controls. All animal experiments were performed in accordance with the guidelines of the Animal Experiments Committee at Kyushu University, and all experimental protocols were endorsed by the committee.
Gel filtration, SDS-PAGE, and Western blotting
Fish (wild-type and rag1 −/−) were anesthetized in 20 ppm quinaldine and bled by caudal amputation into a hematocrit capillary tube. The blood was allowed to clot at 4 °C for 2 h and centrifuged at 3000 rpm for 5 min. The tubes were cut at the boundary between serum and blood cells to collect serum, which was cleared by centrifugation at 12,000 rpm for 10 min. Nine microliters of pooled serum were diluted in 180 μl of 20 mM sodium phosphate buffer containing 0.9% NaCl (pH 7.4). The diluted serum was passed through a Superdex 200 gel filtration column (1 × 30 cm) equilibrated with same buffer and eluted at 0.5 ml/min. The eluted protein was precipitated with 17% (w/v) trichloric acid, washed with acetone, and dissolved in a 1/10 volume of SDS sample buffer containing 5% 2-mercaptoethanol.
Protein samples from the fractions (#1–5 in Fig. 1A) were subjected to SDS-PAGE on 10% gel under reducing conditions. For Western blotting, the separated proteins were electroblotted onto a nitrocellulose membrane (Hybond-C Extra, GE Healthcare Life Sciences, Little Chalfont, UK) in a transfer buffer composed of 100 mM Tris, 192 mM glycine, 20% methanol, and 0.02% SDS. After blocking with 5% skim milk in PBS (SM-PBS), the membrane was treated with anti-ayu (Plecoglossus altivelis) IgM rabbit serum (a gift from Dr. Masakazu Kondo, National Fisheries University, Shimonoseki, Japan; 1/200 diluted in SM-PBS) and then with peroxidase-conjugated goat anti-rabbit IgG (diluted 1/2,000 in SM-PBS; Cappel, MP Biomedicals, Santa Ana, CA, USA). This primary antibody was selected from three candidates of anti-teleost IgM antibodies by Western blotting (data not shown). Between each step, the membrane was washed gently with 0.05% Tween-PBS. Chemiluminescent detection was performed using ECL Western Blotting Detection Reagents (Amersham, GE Healthcare BioScience Corporate, Piscataway, NJ, USA) followed by exposure to Fuji UR X-ray film, which was developed with GBX Development and Fixation solutions (Kodak Co., Rochester, NY, USA).
Agglutinating antibody titer
A commercial Vibrio vaccine (Kyoritsu Seiyaku co., Ltd. Tokyo, Japan) and an emulsion of ISA 763 A (Seppic, France) were mixed at a 1:3 ratio. rag1 +/− and rag1 −/− fish (6 fish per strain) were injected with 10 µl of the mixture. A second vaccination was performed 2 weeks after the primary vaccination. The vaccinated fish were bled by caudal amputation using a heparinized hematocrit capillary. The collected blood was centrifuged at 11,000 rpm for 3 min, and 4.0–5.0 µl of plasma were obtained. The plasmas were serially diluted with PBS in a U-bottom microtiter plate (initial dilution 1:8). Ten microliters of the vaccine suspension in PBS were added in to well containing 10 µL of the plasma dilution. The plate was mixed for 15 min at room temperature and centrifuged at 1000 rpm. Agglutination was observed, and the antibody titers were scored as the highest dilution giving positive agglutination.
Histological observation of organs
At five weeks post-hatching, both strains of fish were anesthetized and dissected, and tissue surrounding the thymus, brain, muscle, spine, kidneys, and hepatopancreas was isolated. The organs were fixed in Davidson’s solution for 24 h. The fixed organs were dehydrated through an ethanol series, treated with xylene, and embedded in paraffin wax. The paraffin-embedded tissues were sectioned at 3 μm and stained with hematoxylin–eosin. The tissue section was observed by light microscopy.
Flow cytometry of IgM-positive cells
Fish were anesthetized and bled using heparinized hematocrit capillaries as described previously to separate peripheral blood cells, and then they were dissected to isolate the trunk kidney. The kidney cells were isolated by pressing the tissues through a 150-gauge mesh stainless steel sieve in RPMI-1640 medium (Nissui Pharmaceutical, Tokyo, Japan). The dispersed cells were treated with anti-ayu IgM rabbit serum (diluted 1/500) and then with FITC-conjugated goat anti-rabbit IgG (diluted 1/50; Cappel, MP Biomedicals) for 40 min on ice. Normal rabbit serum was used as a negative control. Between each step, the cells were washed with RPMI-1640 by centrifuging at 300 × g. IgM+ cells in the lymphocyte gate were detected by flow cytometry (EpicsXL, Beckman Coulter).
Protective effect of vaccination against Vibrio anguillarum infection
It is known that Vibrio anguillarum is useful for bacterial infection model on zebrafish39, and vaccine against V. anguillarum are available in many fish species40. rag1 +/− (n = 17) and rag1 −/− (n = 15) zebrafish were vaccinated with formalin-killed V. anguillarum VR775 by intraperitoneal injection (1.0 × 106 cells/5 μl of PBS per fish fish). The second and third immunizations were performed at 7-day intervals. Control fish (rag1 +/−, n = 18; and rag1 −/−, n = 12) were injected with the same volume of PBS. At two weeks after the final vaccination and PBS-injection, the vaccinated and control fish were challenged by immersion with the same strain of V. anguillarum (1.0 × 107 cells/ml) in 200-ml beakers for 1.5 h at 28 °C. Subsequently, they were transferred to 2-l tanks (5–8 fish/tank) and kept at 28 °C. The number of dead fish was recorded daily for 7 days. The water in each tank was changed every 2 days.
In vivo rejection of transferred erythrocytes
To evaluate adaptive cell-mediated immunity, we established in vivo allograft rejection experiments in zebrafish (Fig. S1) in which rag1 +/− and rag1 −/− fish were employed as recipients, and wild-type fish (rag1 +/+) served as donor fish. Donors were anesthetized and pricked with a 27-gauge needle just ventral to the lateral line of the caudal peduncle. The wound with a few microliters of bleeding was dipped in a Petri dish with D-MEM (Invitrogen) containing 1% heparin, and the blood cells were then suspended in the medium. The suspension was centrifuged at 300 × g for 5 min, and erythrocytes were collected for use as allogeneic immunogens. The donors could be kept alive and maintained during the experiment for repeated blood cell collection. The recipient fish (weighting approximately 0.3 g) were intraperitoneally injected with the donor erythrocytes (1 × 105 cells in 10 μl of PBS/fish). The second and third immunizations were performed with the same doses of cells freshly prepared from the same donor at 10-day intervals. At 10 days after the final immunization, the recipient fish were bled from the tail using the previously described method. The blood was suspended in D-MEM, and the erythrocytes were precipitated by centrifugation at 300 × g for 5 min. The erythrocytes were incubated with 0.3 μg/ml CFSE (Sigma-Aldrich) for 20 min at 25 °C and washed twice in D-MEM. The recipient fish received 1 × 106 of the CFSE-labeled erythrocytes from the same donor. The erythrocytes from the recipients were harvested at 4 days post-transplantation by the aforementioned method, and the percentage of surviving CFSE-positive cells was measured by flow cytometry. Three sets of donors and recipients were tested. In addition, allograft rejection by non-immunized fish was investigated using the same procedure.
To confirm allogenecity between donors and recipients, the genotypes of the donor/recipient pairs were investigated by PCR using primers for the U-lineage of MHC class I41. All pairs exhibited different genotypes of the U-lineage (data not shown).
Expression analysis of cytokines by real-time quantitative PCR
The fish were intraperitoneally injected with 5 μl of 0.8 μg/μl poly(I:C) solution and sampled 24 h post-stimulation. The fish were anesthetized and dissected to isolate the trunk kidney, spleen, and hepatopancreas. Control fish were injected with the same amount of PBS. Total RNA was extracted from these organs using ISOGEN Reagent (Nippon Gene, Tokyo). First-strand cDNA was synthesized from total RNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Life Technologies, Carlsbad, CA, USA) with an oligo (dT) primer according to the manufacturer’s instructions. Eleven cytokines related to inflammation and anti-inflammation (IL-1β, IL-17AF2, IL-17AF3, TNFα-1, TNFα-2, IFNφ1, IFNγ1-1, IFNγ1-2, IL-10, and IL-4) were selected in this study. The primers used for real-time PCR are listed in Table S1. The internal control for normalization was EF-1α. The sequences or primer sets of IFN-γ, perforin, and EF-1α are indicated in the reference articles. Quantitative real-time PCR was performed in duplicates using an Mx 3000 P System (Stratagene, La Jolla, CA, USA) in 16-µl reaction mixtures containing 2 μl of template cDNA, 0.5 μM primers, and other reagent components from the Fast Start DNA Master SYBR Green (Roche Applied Science, Mannheim, Germany). Thermal cycling was performed using a two- or three-step thermal cycling mode composed of initial denaturation for 1 min at 95 °C followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C (IL-1, IL-4, IL-10, IL-17AF3, TNFα1, IFNγ1-1, IFNγ1-2, IFNφ1, and EF-1α) or 40 cycles of 10 s at 95 °C, 15 s at 60 °C, and 30 s at 72 °C (IL-12, IL-17AF2, TNFα2). The relative quantitative value of each gene was calculated according to the standard curve from a serial dilution of a reference cDNA in the same plates and normalized by the level of EF1α.
Leukocyte composition assay via flow cytometry
The fish were injected with poly(I:C) and dissected to isolate the trunk kidney, spleen, and hepatopancreas as described previously. The cells were isolated by pressing the tissues through a 150-gauge mesh stainless steel sieve in RPMI-1640 medium (Nissui Pharmaceutical). The erythrocytes were lysed with erythrocyte lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA), and the leukocytes were washed twice with PBS.
3,3-Dihexyloxacarbocyanine (DiOC6, Molecular Probes, Eugene, OR, USA) staining was used to enhance erythrocyte fluorescence and side scatter according to a previously described method for fish42. The isolated cells were treated with DiOC6 (10 μg/ml), incubated for 10 min at room temperature, and washed twice with PBS. The lymphocyte and macrophage/granulocytes fractions were gated according to the methods for zebrafish20.
Electronic supplementary material
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI Grant Number 16H04982, 50212080 (to MN). We thank the Tübingen 2000 Screen Consortium* for providing rag1 mutants. *Tübingen 2000 Screen Consortium. Max-Planck-Institut fur Entwicklungsbiologie, Tübingen: Busch-Nentwich E, Dahm R, Frank, O, Frohnhofer H-G, Geiger H, Gilmour D, Holley S, Hooge J, Jülich D, Knaut H, Maderspacher F, Maischein H-M, Neumann C, Nicolson T, Nüsslein-Volhard C, Roehl H, Schonberger U, Seiler C, Sollner C, Sonawane M, van Bebber, F, Wehner A, Weiler C, Exelis Deutschland GmbH: Erker P, Habeck H, Hagner U, Hennen Kaps C, Kirchner A, Koblizek T, Langheinrich U, Loeschke C, Metzger C, Nordin R, Odenthal J, Pezzuti M, Schlombs K, deSantana-Stamm J, Trowe T, Vacun G, Walderich B, Walker A, Weiler C. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author Contributions
Y.T. contributed to the idea and design of the study and performed the main experimental work. M.S. contributed to the infectious experiment and in vivo allograft rejection assay. R.S. contributed to the cytokine expression study. Y.Y. maintained the mutant strain and contributed to the design of the study. I.K. performed histological assays and interpreted the tissue sections. M.O. contributed to the agglutinating antibody titer experiment. M.N., T.N., and T.S. contributed to the design of the study, supervision of the experimental work, interpretation of results and data, design of the figures, and writing of the main body of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08000-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu. Rev. Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 2.Peaudecerf L, Krenn G, Goncalves P, Vasseur F, Rocha B. Thymocytes self-renewal: a major hope or a major threat? Immunol. Rev. 2016;271:173–184. doi: 10.1111/imr.12408. [DOI] [PubMed] [Google Scholar]
- 3.Marrella V, Poliani PL, Notarangelo LD, Grassi F, Villa A. Rag defects and thymic stroma: lessons from animal models. Front. Immunol. 2014;5:259. doi: 10.3389/fimmu.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakanishi T, Toda H, Shibasaki Y, Somamoto T. Cytotoxic T cells in teleost fish. Dev. Comp. Immunol. 2011;35:1317–1323. doi: 10.1016/j.dci.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi T, Shibasaki Y, Matsuura YT. Biology (Basel) 2015. Cells in Fish; pp. 640–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye J, Kaattari IM, Ma C, Kaattari S. The teleost humoral immune response. Fish Shellfish Immunol. 2013;35:1719–1728. doi: 10.1016/j.fsi.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Somamoto T, Koppang EO, Fischer U. Antiviral functions of CD8(+) cytotoxic T cells in teleost fish. Dev. Comp. Immunol. 2014;43:197–204. doi: 10.1016/j.dci.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Bengten E, Wilson M. Antibody Repertoires in Fish. Results Probl. Cell Differ. 2015;57:193–234. doi: 10.1007/978-3-319-20819-0_9. [DOI] [PubMed] [Google Scholar]
- 9.Magadan S, Sunyer OJ, Boudinot P. Unique Features of Fish Immune Repertoires: Particularities of Adaptive Immunity Within the Largest Group of Vertebrates. Results Probl. Cell Differ. 2015;57:235–264. doi: 10.1007/978-3-319-20819-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenhalgh P, Steiner LA. Recombination activating gene 1 (Rag1) in zebrafish and shark. Immunogenetics. 1995;41:54–55. doi: 10.1007/BF00188438. [DOI] [PubMed] [Google Scholar]
- 11.Boschi I, et al. Transcription of T cell-related genes in teleost fish, and the European sea bass (Dicentrarchus labrax) as a model. Fish Shellfish Immunol. 2011;31:655–662. doi: 10.1016/j.fsi.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Mao MG, Lei JL, Alex PM, Hong WS, Wang KJ. Characterization of RAG1 and IgM (mu chain) marking development of the immune system in red-spotted grouper (Epinephelus akaara) Fish Shellfish Immunol. 2012;33:725–735. doi: 10.1016/j.fsi.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XL, Lu YS, Jian JC, Wu ZH. Cloning and expression analysis of recombination activating genes (RAG1/2) in red snapper (Lutjanus sanguineus) Fish Shellfish Immunol. 2012;32:534–543. doi: 10.1016/j.fsi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Tan X, Zhang PJ, Zhang Y, Xu P. Recombination-activating gene 1 and 2 (RAG1 and RAG2) in flounder (Paralichthys olivaceus) J. Biosci. 2014;39:849–858. doi: 10.1007/s12038-014-9469-1. [DOI] [PubMed] [Google Scholar]
- 15.Castro R, et al. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol. 2011;31:644–654. doi: 10.1016/j.fsi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Wienholds E, Schulte-Merker S, Walderich B, Plasterk RH. Target-selected inactivation of the zebrafish rag1 gene. Science. 2002;297:99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 17.Hohn C, Petrie-Hanson L. Rag1−/− mutant zebrafish demonstrate specific protection following bacterial re-exposure. PLoS One. 2012;7:e44451. doi: 10.1371/journal.pone.0044451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brugman S, et al. T lymphocyte-dependent and -independent regulation of Cxcl8 expression in zebrafish intestines. J. Immunol. 2014;192:484–491. doi: 10.4049/jimmunol.1301865. [DOI] [PubMed] [Google Scholar]
- 19.Saralahti A, et al. Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 2014;42:345–353. doi: 10.1016/j.dci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Petrie-Hanson L, Hohn C, Hanson L. Characterization of rag1 mutant zebrafish leukocytes. BMC Immunol. 2009;10:8-2172–10-8. doi: 10.1186/1471-2172-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobacchi C, Marrella V, Rucci F, Vezzoni P, Villa A. RAG-dependent primary immunodeficiencies. Hum. Mutat. 2006;27:1174–1184. doi: 10.1002/humu.20408. [DOI] [PubMed] [Google Scholar]
- 22.Shultz LD, et al. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014;2014:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jima, et al. Enhanced transcription of complement and coagulation genes in the absence of adaptive immunity. Mol. Immunol. 2009;46:1505–16. doi: 10.1016/j.molimm.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toda H, et al. Allo-antigen specific killing is mediated by CD8 positive T cells in fish. Dev. Comp. Immunol. 2009;33:646–652. doi: 10.1016/j.dci.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Shibasaki Y, et al. Kinetics of lymphocyte subpopulations in allogeneic grafted scales of ginbuna crucian carp. Dev. Comp. Immunol. 2015;52:75–80. doi: 10.1016/j.dci.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Sarder MR, et al. The MHC class I linkage group is a major determinant in the in vivo rejection of allogeneic erythrocytes in rainbow trout (Oncorhynchus mykiss) Immunogenetics. 2003;55:315–324. doi: 10.1007/s00251-003-0632-3. [DOI] [PubMed] [Google Scholar]
- 27.Sun JC, et al. Immunological memory within the innate immune system. EMBO J. 2014;33:1295–303. doi: 10.1002/embj.201387651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberl G, et al. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2016;22:348. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasbender F, et al. Natural Killer Cells and Liver Fibrosis. Front Immunol. 2016;29:7–19. doi: 10.3389/fimmu.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwanami N. Zebrafish as a model for understanding the evolution of the vertebrate immune system and human primary immunodeficiency. Exp. Hematol. 2014;42:697–706. doi: 10.1016/j.exphem.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Svoboda O, et al. Ex vivo tools for the clonal analysis of zebrafish hematopoiesis. Nat. Protoc. 2016;11:1007–1020. doi: 10.1038/nprot.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Wiest DL. Using the Zebrafish Model to Study T Cell Development. Methods Mol. Biol. 2016;1323:273–292. doi: 10.1007/978-1-4939-2809-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AC, et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood. 2010;115:3296–3303. doi: 10.1182/blood-2009-10-246488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y, Martin P. Imaging innate immune responses at tumour initiation: new insights from fish and flies. Nat. Rev. Cancer. 2015;15:556–562. doi: 10.1038/nrc3979. [DOI] [PubMed] [Google Scholar]
- 35.Langenau DM, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 36.Langenau DM, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu JW, et al. Zebrafish as a disease model for studying human hepatocellular carcinoma. World J. Gastroenterol. 2015;21:12042–12058. doi: 10.3748/wjg.v21.i42.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasighaemi P, et al. Zebrafish as a model for leukemia and other hematopoietic disorders. J. Hematol. Oncol. 2015;8:29-015–0126-4. doi: 10.1186/s13045-015-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caruffo M, et al. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front Microbiol. 2015;6:1093. doi: 10.3389/fmicb.2015.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frans I, et al. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis. 2011;34:643–641. doi: 10.1111/j.1365-2761.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 41.de Jong JL, et al. Characterization of immune-matched hematopoietic transplantation in zebrafish. Blood. 2011;117:4234–42. doi: 10.1182/blood-2010-09-307488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue T, et al. A new method for fish leucocyte counting and partial differentiation by flow cytometry. Fish & Shellfish Immunology. 2002;13:379–390. doi: 10.1006/fsim.2002.0413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


