Abstract
Neuronal adaptation to oxidative stress is crucially important in order to prevent degenerative diseases. The role played by the Nrf2/HO-1 system in favoring cell survival of neuroblastoma (NB) cells exposed to hydrogen peroxide (H2O2) has been investigated using undifferentiated or all-trans retinoic acid (ATRA) differentiated SH-SY5Y cells. While undifferentiated cells were basically resistant to the oxidative stimulus, ATRA treatment progressively decreased cell viability in response to H2O2. HO-1 silencing decreased undifferentiated cell viability when exposed to H2O2, proving the role of HO-1 in cell survival. Conversely, ATRA differentiated cells exposed to H2O2 showed a significantly lower induction of HO-1, and only the supplementation with low doses of bilirubin (0,5–1 μM) restored viability. Moreover, the nuclear level of Bach1, repressor of HO-1 transcription, strongly decreased in undifferentiated cells exposed to oxidative stress, while did not change in ATRA differentiated cells. Furthermore, Bach1 was displaced from HO-1 promoter in undifferentiated cells exposed to H2O2, enabling the binding of Nrf2. On the contrary, in ATRA differentiated cells treated with H2O2, Bach1 displacement was impaired, preventing Nrf2 binding and limiting HO-1 transcription. In conclusion, our findings highlight the central role of Bach1 in HO-1-dependent neuronal response to oxidative stress.
Introduction
Cell ability to adapt to stressful conditions is crucial to maintain physiological functions over time. While a severe imbalance between oxidative insults and antioxidant defenses leads to cell damage and death, in presence of functional antioxidants different redox-dependent signaling pathways can be modulated by low amount of reactive oxygen species (ROS), leading to different cell responses, from differentiation to proliferation1, 2.
Due to the high rate of ROS generation, the high content of lipids susceptible to peroxidation, and the relatively low amount of antioxidant defenses, neuronal cells are especially sensitive to oxidative damage in comparison to other cell types3. However, ROS can act as signaling molecules in neuronal cells too, for instance, as far as the differentiation activity of retinoic acid is concerned4–6. Thus, the ability to balance oxidative insults is crucial for neuronal cell survival.
Among the inducible antioxidant defenses heme oxygenase 1 (HO-1) plays a key role7. Indeed, HO-1 is the inducible form of HO system, which carries out the degradation of the iron-containing molecule heme and generates free iron (Fe2+), carbon monoxide and biliverdin. Free iron is quickly quenched by ferritin, which is synthesized in parallel with HO-1 induction8, and biliverdin is further converted into bilirubin by the activity of biliverdin reductase9. Overall ferritin, carbon monoxide and bilirubin exert strong antioxidant, antiapoptotic and anti-inflammatory activities8, 10–12.
HO-1 transcription is induced by multiple redox dependent-signaling pathways such as MAPK, PI3K/AKT kinases, STAT3, AP-1 and especially by the nuclear factor erythroid 2-related factor 2 (Nrf2)13. Nfr2, indeed, drives the adaptive responses of cells under electrophylic or oxidative stimuli. Under stressed conditions, it is released from its negative regulator Kelch-like ECH-associated protein 1 (Keap-1) and moves from the cytosol into the nucleus14. The binding to the Antioxidant Response Element (ARE) sequences in the promoter region of target genes enables the transcription of a plethora of antioxidant and protective genes15, 16.
However, a few number of repressors of HO-1 transcription have been identified, namely Keap1 which favors Nrf2 proteasomal degradation in unstressed conditions17, and Bach1 which prevents Nrf2 binding to the ARE sequences18. Moreover, Bach1 is directly involved in heme homeostasis thus playing a specific role in the induction of HO-119.
We previously showed that retinoic acid-induced neuroblastoma (NB) differentiation increases the generation of anion peroxide from the coordinated activation of PKC delta and NADPH oxidase favoring neurite elongation5. However, we also provided evidence that, after retinoic acid induced differentiation, cells become more sensitive to the oxidative stress induced by advanced glycation end-products (AGEs)20.
In this work we show that NB cell differentiation induced by retinoic acid modifies the activation of Nrf2 and HO-1, impairing the ability to counteract oxidative stress.
Results
ATRA-differentiated cells are more sensitive to H2O2 than undifferentiated ones
The effect of 24 h exposure to increasing concentrations of H2O2 (from 100 μM to 500 μM) on undifferentiated or differentiated SH-SY5Y neuroblastoma (NB) cell viability has been tested. In previous papers we showed that cell differentiation with all-trans retinoic acid for 4 or 7 days (4d-ATRA and 7d-ATRA) increases the number and the length of neurites, slows down the cell cycle and increases the expression of MAP2 as neurite marker5, 21. In the present work, the up-regulation of MAP2 and NeuroD122 have been routinely checked by using RT-PCR to confirm differentiation (Fig. 1a and b).
Figure 1.
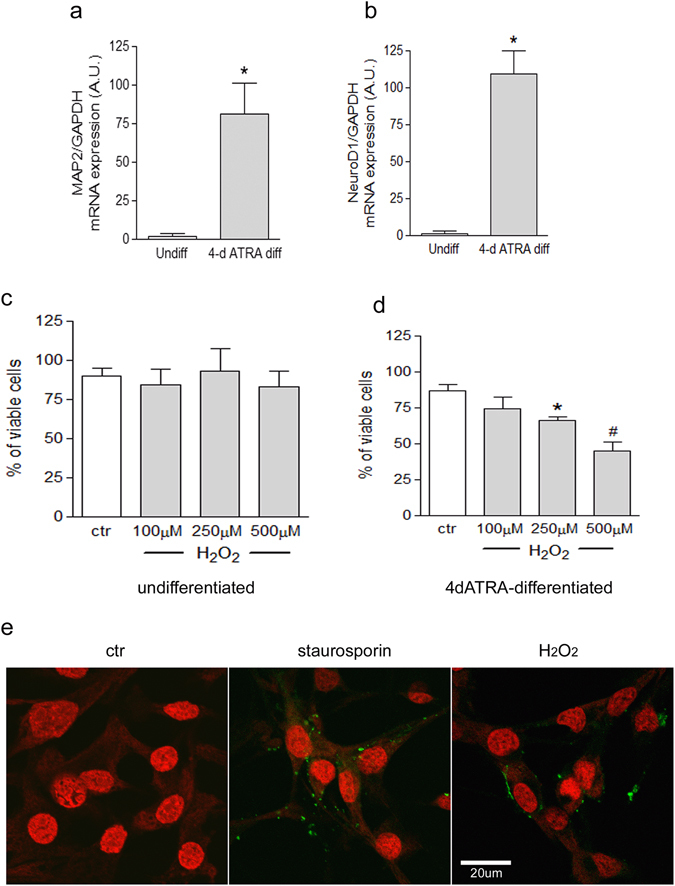
ATRA-induced differentiation increases sensitivity to H2O2, favoring the onset of apoptosis. (a and b) Cell differentiation is checked by RT-PCR analysis of MAP2 and NeuroD1. Statistical analysis: n = 3, *p < 0.05 vs undiff. (c and d) The number of viable cells have been analyzed by using Trypan blue dye after 24 h exposure to H2O2 and expressed as a percentage of viable cells. Statistical analysis: n = 4, *p < 0.05 and #p < 0.01 vs control cells. (e) Positivity to Annexin V-FITC (green staining) of 4d-ATRA differentiated cells has been checked as a marker of early apoptosis after 24 h treatment with 500 µM H2O2 and appears as a spotted green membrane fluorescence. Treatment with 100 nM staurosporin has been used as positive control. Nuclei are counterstained by To-Pro3 iodide as detailed in Materials and Methods. Scale bar = 20 µm.
The mean value of viability of untreated undifferentiated cells was 90% and no modifications were induced by H2O2 treatments (Fig. 1c). The mean value of viability of untreated 4d-ATRA differentiated cells was 86% and was reduced to 66% and 45% after cell exposure to 250 μM and 500 μM H2O2, respectively (Fig. 1d). Moreover, confocal microscopy analysis of Annexin V positivity showed that 4d-ATRA differentiated cells exposed to 500 μM H2O2 increased the membrane expression of phosphatidylserine. The same pattern of expression has been observed in 4d-ATRA differentiated cells treated with staurosporin used as positive control of early apoptosis. On the contrary, untreated cells did not show any membrane staining (Fig. 1e).
When 7d-ATRA differentiated cells have been used, the number of viable cells was further decreased to 57% and 44% by the exposure to 250 μM and 500 μM H2O2 (Supplementary Fig. 1). However, in the following experiments, a single dose of 500 μM H2O2 has been used on undifferentiated and 4d-ATRA differentiated cells.
HO-1 mRNA is differently expressed in undifferentiated and differentiated cells treated with H2O2
RT-PCR analysis showed a significant induction of HO-1 in both undifferentiated and 4d-ATRA differentiated cells exposed to 500 μM H2O2 or to the positive control 50 μM tBHQ for 6 h. However, while the extent of HO-1 induction is highly similar in undifferentiated cells after H2O2 or after tBHQ treatments, in 4d-ATRA differentiated cells instead, the expression of HO-1 was significantly lower after H2O2 treatment than after tBHQ exposure (Fig. 2a).
Figure 2.
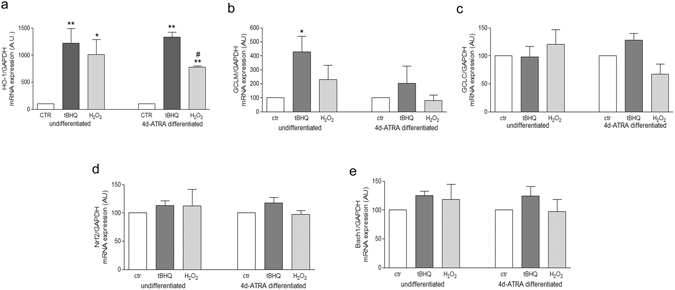
HO-1 mRNA expression is differently regulated in response to oxidative stress before and after cell differentiation. RT-PCR analysis of HO-1(a), GCLM (b) and GCLC (c), Nrf2 (d) and Bach1 (e) in undifferentiated and 4d-ATRA differentiated cells treated for 6 h with 500 μM H2O2 or 50 µM tBHQ - positive control of HO-1 induction - as indicated. Statistical analysis: n = 3 *p < 0.05 vs control; **p < 0.01 vs control; #p < 0.01 vs tBHQ.
Moreover, no significant differences in the expression of the two subunits of γ-glutamyl-cisteine ligase (modulatory, GCLM and catalytic, GCLC) were observed among undifferentiated and differentiated cells exposed to H2O2 in comparison to tBHQ-treated cells or to untreated cells (Fig. 2b and c).
In addition, the mRNA levels of Nrf2 and Bach1, the two main regulators of HO-1 expression, have been analyzed in undifferentiated and differentiated cells exposed to 500 µM H2O2 or 50 µM tBHQ and no significant differences have been observed (Fig. 2d and e).
HO-1 protein expression in response to oxidative stress occurs mainly in undifferentiated cells and favors cell survival
24 h exposure to 500 μM H2O2 clearly increased HO-1 protein expression in undifferentiated SH-SY5Y cells, as shown by confocal microscopy analysis of specific immunofluorescence. 4d-ATRA differentiated cells showed no significant HO-1 immunoreactivity in the same experimental condition. Both undifferentiated and differentiated cells were exposed to tBHQ, a positive control of HO-1 induction, which effectively increased HO-1 expression (Fig. 3a). Moreover, WB analysis confirmed that HO-1 protein level was significantly lower in 4-ATRA differentiated cells than in undifferentiated cells exposed to 500 µM H2O2 (−82% in H2O2 treated 4d-ATRA differentiated cells vs H2O2-treated undifferentiated cells). The expression of HO-1 was under the limit of detection in untreated cells and highly up-regulated by tBHQ treatment both in undifferentiated and in differentiated cells. Thus, the expression level of HO-1 in undifferentiated cells treated with tBHQ has been used as reference (100% of HO-1 expression, Fig. 3b).
Figure 3.
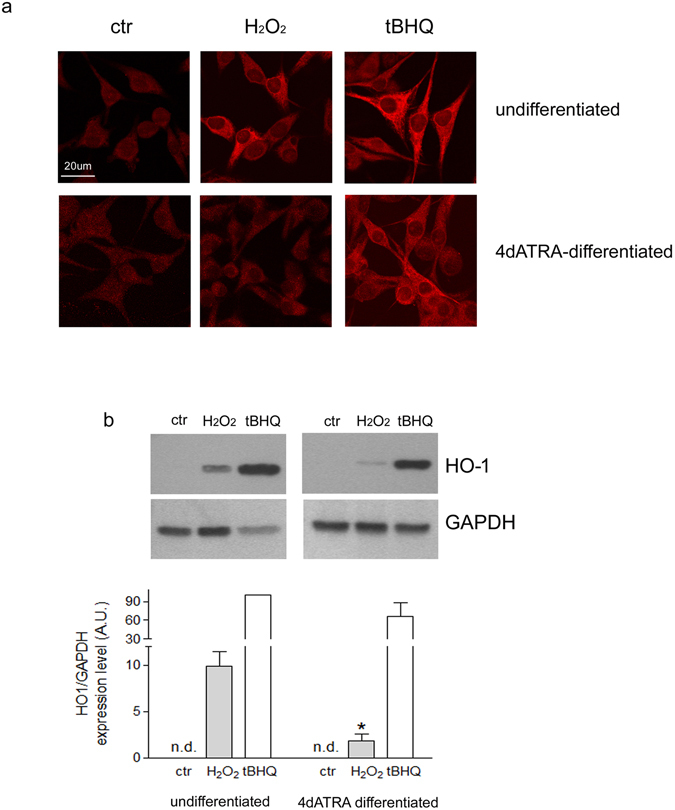
HO-1 protein expression is differently regulated in response to oxidative stress before and after differentiation. (a) Confocal microscopy analysis of HO-1 specific immunofluorescence in undifferentiated and 4d-ATRA differentiated cells exposed for 24 h to 500 μM H2O2 and 50 µM tBHQ, a positive control of HO-1 induction. The panels report one representative experiment of three. Scale bar: 20 μm. (b) As shown by Western Blot analysis, HO-1 expression is not detectable in both undifferentiated and 4d-ATRAdifferentiated untreated cells. For this reason, the expression of HO-1 in undifferentiated cells treated with tBHQ has been considered as reference (100% of HO-1 expression). The expression of GAPHD has been used as loading control to normalize the expression of HO-1. The bands show one representative experiment of three. Statistical analysis: n = 3, *p < 0.05 vs H2O2-treated undifferentiated cells. Full-length blots are presented in supplementary information.
Furthermore, HO-1 silencing, which completely abolished H2O2-dependent HO-1 up-regulation (Fig. 4a), decreased the viability of undifferentiated cells exposed to H2O2 of about 50% in comparison to untreated cells, confirming the involvement of HO-1 in undifferentiated cells resistance to oxidative stress (Fig. 4b). Then, 4d-ATRA differentiated cells supplemented with low doses of bilirubin (0.5 and 1 μM) increased resistance against H2O2, indirectly proving that the lack of HO-1-derived bilirubin was responsible for ATRA-differentiated cells sensitivity to H2O2 (Fig. 4c).
Figure 4.
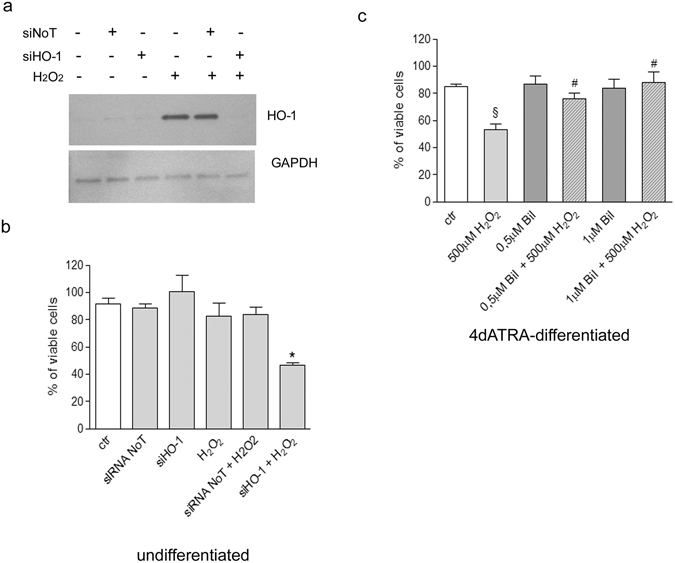
HO-1 expression is responsible for undifferentiated cell resistance to oxidative stress. Bilirubin supplementation restores viability in differentiated cells exposed to H2O2. (a) WB analysis of HO-1 in undifferentiated cells exposed for 24 h to 500 μM H2O2 and silenced for HO-1. GAPDH expression have been used as loading control. The bands show one representative experiment of three. (b) % of viability in undifferentiated cells exposed for 24 h to 500 μM H2O2 and silenced for HO-1. Some samples have been treated with a scramble siRNA (siNoT) to exclude aspecific cell responses. Statistical analysis: n = 3, *p < 0.05 vs H2O2. (c) 4d-ATRA differentiated cells, have been co-treated with 500 μM H2O2 and bilirubin (0.5 µM or 1 µM) in order to prevent cell death induced by cell exposure to H2O2 alone. The % of viable cells is shown. Statistical analysis: n = 3, §p < 0.01 vs ctr; #p < 0.05 vs H2O2. Full-length blots are presented in supplementary information.
Differentiation modifies Nrf2/Bach1 nuclear ratio in response to H2O2
HO-1 induction is regulated by the displacement of Bach1 from the ARE sequences in the two enhancers of HO-1 promoter. Bach1 displacement enables the binding of Nrf2 and the following HO-1 transcription23. Thus, WB analysis of Bach1 and Nrf2 expression levels has been performed in undifferentiated and differentiated cells exposed to H2O2 or tBHQ.
Bach1 protein level was under the limit of detection in the cytosolic compartment of both undifferentiated and differentiated cells. Its nuclear level strongly decreased in undifferentiated cells treated with H2O2 in comparison to control cells (Fig. 5a). On the contrary, in 4d-ATRA differentiated cells the treatment with H2O2 did not modify Bach1 nuclear level (Fig. 5b).
Figure 5.
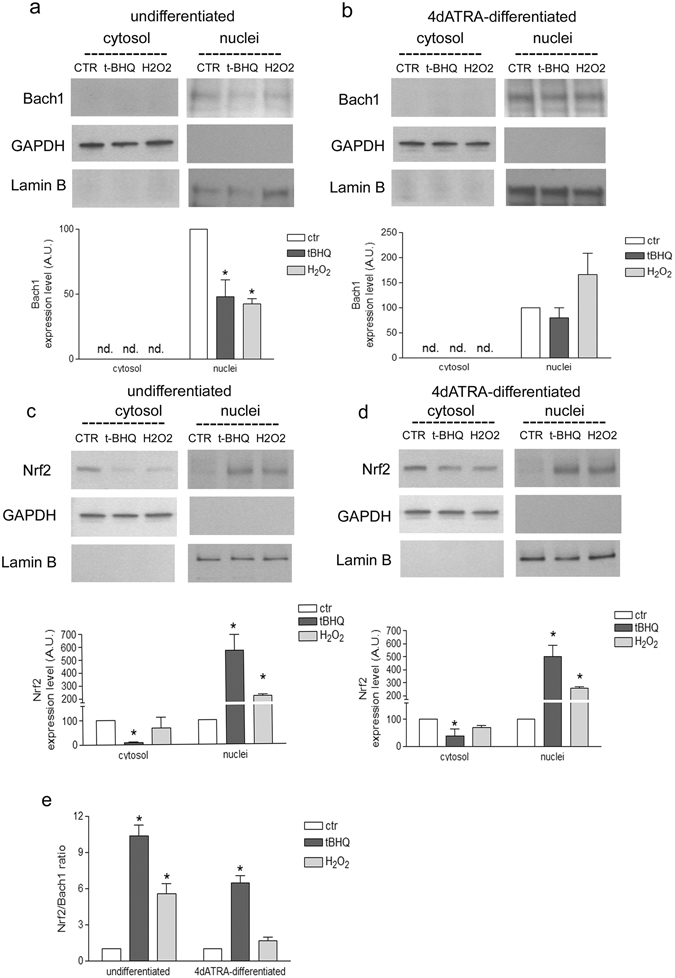
Differentiation modified the nuclear ratio between Nrf2 and Bach1 in cell treated with H2O2. (a and b) Western Blotting analysis of Bach1 cytosolic and nuclear levels of undifferentiated or 4d-ATRA differentiated cells treated for 3 h with 500 µM H2O2 or 50 µM tBHQ, as indicated. Statistical analysis: n = 3, *p < 0.05 vs ctr. (c and d) WB analysis of Nrf2 cytosolic and nuclear levels of undifferentiated or 4d-ATRA differentiated cells treated with 500 µM H2O2 or 50 µM tBHQ, as indicated. Statistical analysis: n = 3, *p < 0.05 vs ctr. The expression of GAPDH and Lamin B was checked in all the experimental conditions to verify the purity of cell fractioning and then used as loading control to normalize protein expression. (e) Nrf2/Bach1 ratio has been calculated to emphasize the different behavior in the two experimental conditions. Statistical analysis: n = 3, *p < 0.05 vs ctr. Full-length blots are presented in supplementary information.
The analysis of Nrf2 protein expression in cytosol and nuclei, however, revealed no differences between undifferentiated and differentiated cells. In fact, Nrf2 is mainly expressed in the cytosol in untreated cells and moves from the cytosol into the nuclei after the exposure to H2O2 in both undifferentiated (Fig. 5c) and 4d-ATRA differentiated cells (Fig. 5d). Cell have been exposed to tBHQ as positive control of Nrf2 activation. The ratio between Nrf2 and Bach1 protein levels in the nuclei has been calculated to highlight and clearly show the different behavior of differentiated cells compared with undifferentiated ones (Fig. 5e).
Differentiation impairs Bach1 displacement form HO-1 promoter in H2O2 treated cells
Bach1 and Nrf2 binding to ARE sequence in the enhancer 1 (E1) of HO-1 has been checked by ChIP analysis. In undifferentiated cells Bach1 binding to ARE decreased (−41, 2% vs control, Fig. 6a) and Nrf2 binding increased (+100% vs control, Fig. 6b) in response to oxidative stress, while in 4d-ATRA differentiated cells Bach1 binding did not decrease and Nrf2 binding did not significantly increase after the exposure to H2O2. tBHQ was always used as positive control able to displace Bach1 allowing Nrf2 to bind (Fig. 6). Normal IgGs have been used as control (Fig. 6c).
Figure 6.
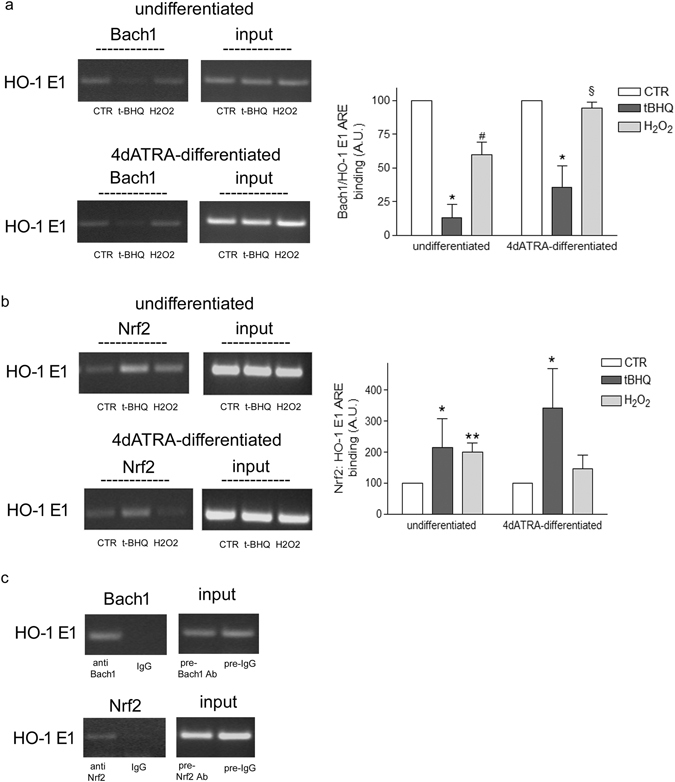
Differentiation impairs Bach1 displacement from the promoter region of HO-1 in response to H2O2. Undifferentiated and 4d-ATRA differentiated cells have been treated for 3 h with 500 μM H2O2. t-BHQ (50 μM) has been used as positive control able to displace Bach1, allowing Nrf2 to bind. (a) ChIP analysis of Bach1 binding to ARE sequences in the E1 promoter of HO-1. Statistical analysis: n = 3, *p < 0.01vs control; #p < 0.05 vs control; §p < 0.05 vs H2O2 undiff. (b) ChIP analysis of Nrf2 binding to ARE sequences in the E1 promoter of HO-1. Statistical analysis: n = 3, *p < 0.05 and **p < 0.01 vs control. (c) Negative control using IgG. As indicated no bands are detected in samples immunoprecipited with normal rabbit/goat IgG in comparison to samples immunoprecipitated by using rabbit Anti Nrf2 or goat Anti Bach1 antibodies. The intensity of PCR products amplified from immunoprecipitated samples are normalized on the intensity of bands obtained from the amplification of pre-cleared DNA (input). The bands show one representative experiment of three. Full-length gels are presented in supplementary information.
Discussion
In this work we demonstrated that SH-SY5Y neuroblastoma (NB) cell differentiation induced by all-trans retinoic acid (ATRA) increases cell sensitivity to oxidative stress through the impairment of Bach1-depedent HO-1 induction.
Alteration of cell ability to counteract oxidative stress plays a key role not only in age-related degenerative diseases, which can be favored by the loss of adaptive responses, but also in the gain of resistance of cancer cells which progressively increase their adaptability.
We showed that undifferentiated SH-SY5Y cells are basically resistant to a medium-high degree of oxidative stress induced by cell exposure to 100–500 μM H2O2 for 24 h. However, cells differentiated by the exposure to ATRA progressively decrease viability when exposed to oxidative stress. ATRA is known to induce differentiation towards the neuronal lineage, proved by the increased expression of different neuronal markers22, confirmed in our experimental model as well. The acquisition of neuronal features is dependent on the generation of a controlled amount of reactive oxygen species (ROS) and the modulation of specific redox sensitive signaling pathways5, 6. In addition, an increased sensitivity to stress has been already demonstrated in differentiated SH-SY5Y NB cells in comparison to undifferentiated cells by our group20 and by others24. In addition, we showed that H2O2 favors the onset of apoptosis of differentiated cells, demonstrated by the expression of phosphatdylserine on the outer membrane, in agreement with other works performed in similar experimental conditions25.
However, in this context NB cell ability to counteract ROS generation preventing oxidative damage has not been extensively investigated.
We considered the antioxidant mechanisms regulated by Nrf2 focusing on HO-1 which are together recognized of primary importance in favoring cell survival10. However, among the Nrf2-dependent antioxidant responses, we also analysed the main enzymes involved in the synthesis of glutathione, namely γ-glutamyl-cisteine ligase (GCL). No differences between undifferentiated and differentiated cells exposed to oxidative stress have been observed as far as the regulation of the two subunits of GCL is concerned. In agreement with our result, the inability of neurons to up-regulate GSH synthesis has been already proved in response to nitric oxide exposure26. As a consequence, the level of glutathione is not modified in our experimental conditions (data not shown). Heme oxygenase 1 (HO-1), instead, is differently regulated: its expression is induced significantly more in undifferentiated cells treated with H2O2 than in differentiated cells in the same experimental conditions. Heme oxygenase (HO) is one of the most important cytoprotectant systems and its over-expression is crucial in the adaptive response to stress27. HO-1 and HO-2 are the two main isoforms in human cells. HO-2 is constitutively expressed in neuronal cells but it has been shown to be especially regulated in response to glucocorticoids28 or drugs like atorvastatin29. The inducible form HO-1, instead, has been shown to be up-regulated in response to ROS, heat shock, ischemia and it is also induced by its substrate heme playing a pivotal role in response to acute neuronal damage30 and, for this reason, we only considered HO-1. It is interesting to note that, in our experimental model, HO-1 mRNA level is detectable in control cells both before and after differentiation, while its protein level is under the limit of detection with a standard WB technique. Conceivably, even if there are still no evidence in SH-SY5Y cells, some microRNA can be involved in the regulation of HO-1 protein expression, as already shown in podocytes (miRNA 218)31 or in bone-marrow derived macrophages (miRNA 183)32. In addition, we cannot exclude that the post-transcriptional modification of HO-1 can play a crucial role in the different modulation of HO-1 expression that we observed in SH-SY5Y cells exposed to oxidative stimuli before and after differentiation, but this aspect has not been investigated yet.
In our experimental model, then, HO-1 appears to be responsible for undifferentiated cell resistance to oxidative stress. Indeed, HO-1 silencing in undifferentiated cells increases sensitivity to oxidative stress, proving the role played by HO-1 in favoring cell survival. The role of HO-1 in protecting cells from oxidative damage is ascribed to the three products of its activity, ferritin, carbon monoxide and bilirubin33. We showed that differentiated cells treated with low doses of bilirubin increase their resistance to oxidative stress, pointing out the lack of HO-1 derived bilirubin as a crucial mechanism of differentiated cell sensitivity to H2O2.
Bilirubin is receiving increasing attention for its powerful antioxidant activity, already recognized in vitro 34 but now well demonstrated in vivo, especially in the cardiovascular system35. Indeed, a mild increase of plasma bilirubin improves endothelial function preventing or reducing the severity of cardiovascular pathologies36 and metabolic diseases37 and, also, is protective against neuronal death induced by ischemia/reperfusion38. Moreover, the endogenous generation of bilirubin has been shown to be crucial in cell adaptation to oxidative stress39, not only in endothelial cells40, 41 but also in vascular muscle cells42 and in neuronal cells43, 44.
It is also important to note that bilirubin is constantly recycled in the bilirubin/biliverdin cycle by the activity of Biliverdin Reductase (BRV), allowing bilirubin to exert its antioxidant activity even at very low concentration. In fact, it has been demonstrated that 10 nM bilirubin can protect against 10000 higher concentration of hydrogen peroxide, acting complementary to GSH45.
Furthermore, it is worth underlining that our experimental model consists of a neuroblastoma cell line and that the endogenous generation of bilirubin from cancer cells has been recently proposed as one of the mechanisms involved in tumor progression, for instance as far as melanoma aggressiveness is concerned46. However, further studies are needed to understand the role of bilirubin as mediator of cancer cell survival and gain of resistance.
Next, we investigated the molecular mechanisms involved in HO-1 transcription, starting from the evaluation of its principal negative regulator Bach1. Bach1 is fundamental in the physiological adaptation to oxidative stress23. Through its binding to ARE sequences Bach1 represses HO-1 transcription, preventing Nrf2 binding47. Under stressed conditions, Bach1 is displaced from HO-1 promoter and degraded by the activity of proteasome48 and Nrf2 can move from the cytosol into the nucleus inducing HO-1 transcription10. This is completely consistent with what we have observed in undifferentiated cells exposed to oxidative stimuli.
On the contrary, in differentiated cells Bach1 is not displaced from HO-1 promoter and Nrf2 is not allowed to bind, although maintaining its ability to sense H2O2 moving into the nucleus.
In addition, in our experimental conditions, Bach1 and Nrf2 mRNA levels were not modified by oxidative stress further corroborating the hypothesis that the main regulation of both Bach1 and Nrf2 occurs at post-transcriptional level.
For the best of our knowledge, this is the first piece of evidence on the involvement of Bach1 in human neuroblastoma cell response to oxidative stress. In fact, it has been reported that Bach2 contributes to the differentiation of a murine NB cell line which, however, does not express Bach149. Moreover, in the work there is no evidence concerning cell response to oxidative stress. Nonetheless, the importance of Nrf2/Bach1 ratio in the regulation of oxidative response has been highlighted in rat cortical neurons50. Importantly, it has also been shown that Bach1 is significantly up-regulated in the brain of subjects with Down Syndrome increasing oxidative stress and favoring the onset of Alzheimer’s disease51.
Thus, Bach1 might play an important role in the regulation of antioxidant responses in neuronal cells. Our work shed a new light on the involvement of retinoic acid as regulator of Bach1-dependent HO-1 induction and this is the first evidence concerning these aspects of neuronal antioxidant responses. In our experimental system, NB cells are able to differentiate toward neuronal features when treated with ATRA but this stimulus eventually impairs cell ability to counteract a new oxidative challenge. ATRA exerts its differentiating effects through the activation of its nuclear receptors RAR and RXR. It has been shown that the activation of RAR impairs Nrf2 binding to ARE52, but there is no evidence on a possible involvement of RAR or RXR in the modulation of Bach1 activity.
Finally, it is also important to consider the role played by different miRNA in ATRA-induced NB cell differentiation53 and in redox adaptation54 as well. Indeed, miR-15555 and miR-19656, are considerably important in the processing of Bach1 mRNA and, notably, miR-155 is dramatically reduced after retinoic acid differentiation57. Even though in our experimental conditions we did not find any significant modulation of Bach1 mRNA level before and after cell differentiation we cannot exclude that Bach1 stabilization on the promoter region of HO-1 is due to a modulation of other co-factors involved in Bach1 binding to ARE, for instance MafG or other related proteins. In fact, Bach1 binding to DNA is strictly dependent on its dimerization with Maf proteins47 and it has been recently highlighted MafG role in favoring the Bach1/DNA binding in melanoma cells, for instance58. Yet, the role of Maf proteins in Bach1 regulation, during neuroblastoma cell differentiation and in the response to oxidative stress is still completely unexplored. Further investigations are needed to better clarify these aspects of Bach1 regulation.
Methods
Cell culture, differentiation and treatments
SH-SY5Y neuroblastoma (NB) cells were cultured in RPMI 1640 medium (Euroclone, Italy) plus FBS (10%, from Euroclone), glutamine (2 mM, from Sigma-Aldrich, Italy), amphotericin B (1% Sigma-Aldrich), penicillin/streptomycin (1%, Sigma-Aldrich). Cells were split at 1:5 every 5 days and maintained in 5% CO2 humid atmosphere. Cells were differentiated by growth in the same medium supplemented with 10 μM all-trans retinoic acid (ATRA) (Sigma-Aldrich) for 4 and 7 days. Undifferentiated, 4 days (4d-ATRA) and 7 days (7d-ATRA) differentiated cells were exposed to increasing concentration of H2O2 (100–250–500 μM) for 24 h and the number of viable cells was measured by using Trypan Blue exclusion test and expressed as percentage. In the following experiments undifferentiated and 4d-ATRA differentiated cells were treated with 500 μM H2O2 or with 50 μM tBHQ, used as positive control of Nrf2/HO-1 induction, for 3 h (for the analysis of nuclear protein translocation and ChIP), 6 h (for the analysis of mRNA target genes) or 24 h (for the analysis of HO-1 protein expression). Some of the 4d-ATRA differentiated samples were treated with 100 nM staurosporin as a positive control of early apoptosis or exposed to 0.5 μM and 1 μM bilirubin (Sigma- Aldrich) alone or in combination with 500 μM H2O2 for 24 h.
Evaluation of early apoptotic cells
Confocal microscopy detection of phosphatidylserine exposure on the outer membrane - marker of early apoptosis - has been performed by using the Annexin V-FITC kit (Biovision). SH-SY5Y cells were seeded on 8-well Lab-Tek II chamber slides (Nalge Nunc International) (15 × 103 cells per well) and differentiated for 4 days with 10 µM retinoic acid as described before. After 24 h of treatment with 500 µM H2O2 or 100 nM staurosporin, cells were washed with PBS and incubated in the dark with Annexin V-FITC diluted 1:100 in the given binding buffer. Nuclei were counterstained with 3ng/ml To-Pro3 iodide (Invitrogen) to enable cell visualization. All the cell nuclei appear red and only early apoptotic cells show a green spotted membrane staining. Images were collected by using a three-channel TCS SP2 laser scanning confocal microscope (Leica Mycrosystems, Germany).
HO-1 silencing
HO-1 mRNA has been silenced in undifferentiated cells exposed for 24 h to 500 μM H2O2 in 6 well plates using 120pmoles of a specific pool of oligonucleotides against human HO-1 (siHO-1, On-TargetPlus SMART pool human heme oxygenase 1; Dharmacon, USA). A scrambled pool of oligonucleotides (siRNA NoT, On-TargetPlus siControl non targeting pool; Dharmacon) has been also used to exclude aspecific cell responses. The oligonucleotides have been transfected using Polyplus - Transfection Interferin (Euroclone) as already described59, following manufacturer instructions.
Extraction of RNA and Reverse Trancription-PCR
The extraction of total RNA was performed using TRIZOL (Life Techonologies, USA) by following the suggested protocol. RNA reverse transcription into cDNA was carried out by the SuperScriptTM II Reverse Transcriptase (Life-Techonologies) using random hexamer primers. cDNA amplification was achieved by using PCR Master Mix 2X (Fermentas-Dasit, Italy) and specific primers for human MAP-2, NeuroD1, GCLC, GCLM, HO-1, Nrf2, Bach1 and GAPDH. All the primer sequences used have been synthesized at Tib Mol Biol, Italy and listed in supplementary table 1. After separation on 2% agarose gel, a densitometric analysis of PCR products, stained with ethidium bromide and visualized under UV light, has been performed using a GelDoc apparatus (Bio-Rad, Italy). The expression of all the genes analyzed have been normalized on the expression of GAPDH.
Preparation of total cell lysates and subcellular fractioning
Total protein extractions were performed using RIPA buffer while cytosolic and nuclear subcellular fractions were prepared using HEPES/EDTA buffer as previously described60. Protein content was measured using the BCA assay (Pierce, Thermo Scientific, USA).
Immunoblot analysis
Proteins from total cell lysates or nuclear fractions were denatured in Laemmli buffer and then subjected to SDS-polyacrylamide gel electrophoresis (200 Volt for 50 min, Mini Protean precast TGX Gel - percentage of acrylamide is specified in supplementary information - Bio-Rad, Milan, Italy), followed by electroblotting (100 Volt for 50 min) on PVDF membrane (GE Healthcare, Amersham Place, UK). Immunodetection was performed using rabbit anti Nrf2 (1:2000, Cell Signaling), mouse anti Bach1 (1:1000, Santa Cruz Biothec) and rabbit anti HO-1 (1:2000, Origene). After incubation with specific secondary antibodies (GE Healthcare), the bands were detected by means of an enhanced chemiluminescence system (GE Healthcare). The membranes were stripped using Re-blot plus solution (Chemicon International, CA, USA) and re-probed with rabbit anti GAPDH (loading control for total lysates or cytosolic marker, 1:10000, Santa Cruz Biotech) or mouse anti lamin B (loading control for nuclear proteins, 1:1000, AbCam). Developed films were analysed using a specific software (GelDoc; Bio-Rad).
Immunofluorescence assay
To study HO-1 expression, SH-SY5Y cells were grown as wild type or differentiated in 8-well chamber slides and then exposed to 500 μM H2O2 or 50 μM tBHQ for 24 h. By means of a standard technique of immunofluorescence (fixing in cold methanol), HO-1 expression was detected by using anti HO-1 (10 μg/ml rabbit anti HO-1, Origene) and ALEXA 633 (anti rabbit 1:400, Life-Technologies). Images were collected by using a three-channel TCS SP2 laser scanning confocal microscope (Leica Mycrosystems).
Chromatin immunoprecipitation assay
Nrf2 and Bach1 binding to ARE sequences in the enhancer E1 in the promoter region of HO-1, was assessed by chromatin immunoprecipitation (ChIP) by using rabbit anti Nrf2 C-20 and goat anti Bach1 C-20 (Santa Cruz) antibodies, as already described61. Normal rabbit and goat IgG (Merk Millipore) has been employed as non specific IgG control. The sequences of the primers used for the amplification of E1 HO-1 promoter region are listed in Supplementary Table 1.
Statistical analysis
By using GraphPad Prism software (San Diego,USA) the mean value ± SEM of the results derived from 3 or more experiments was calculated. The statistical analysis of the differences was then performed by using t-test to compare two groups or one-way ANOVA followed by Dunnett’s post-test to compare more groups.
Data availability statement
All the data supporting this study are provided in full in the result section or as supplementary information.
Electronic supplementary material
Acknowledgements
Grants from MIUR-PRIN 2012 (Ministero dell’Istruzione, dell’Università e della Ricerca - Progetto di Ricerca di Interesse Nazionale 20125S38FA) (to MN) and by Genoa University (FRA2015 to MN).
Author Contributions
S.P., A.L.F. and M.N. conceived and designed the experiments. S.P., A.L.F., L.B. and M.P. conducted the experiments. S.P., A.L.F. and M.N. analysed the results. M.P., U.M.M., M.A.P. and M.N. contributed reagents/materials/analysis. S.P., U.M.M. and M.N. wrote the paper. All the authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08095-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–21. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 2.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016;8:205–15. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvis AM, McCormick ML, Spitz DR, Kiningham KK. Redox balance influences differentiation status of neuroblastoma in the presence of all-trans retinoic acid. Redox Biol. 2016;7:88–96. doi: 10.1016/j.redox.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitti M, et al. PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell Signal. 2010;22:828–35. doi: 10.1016/j.cellsig.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Kunzler, A. et al. Changes in Cell Cycle and Up-Regulation of Neuronal Markers During SH-SY5Y Neurodifferentiation by Retinoic Acid are Mediated by Reactive Species Production and Oxidative Stress. Mol Neurobiol (2016). [DOI] [PubMed]
- 7.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. Faseb J. 1988;2:2557–68. [PubMed] [Google Scholar]
- 8.Cheng HT, et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta. 2015;1850:2506–17. doi: 10.1016/j.bbagen.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Foresti, R., Green, C. J.,Motterlini, R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp 177–92 (2004). [DOI] [PubMed]
- 10.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–47. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loboda A, Jozkowicz A, Dulak J. HO-1/CO system in tumor growth, angiogenesis and metabolism - Targeting HO-1 as an anti-tumor therapy. Vascul Pharmacol. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–94. doi: 10.1016/S0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 13.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234-235:249–63. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–78. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–94. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davudian S, Mansoori B, Shajari N, Mohammadi A, Baradaran B. BACH1, the master regulator gene: A novel candidate target for cancer therapy. Gene. 2016;588:30–7. doi: 10.1016/j.gene.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Girvan HM, Munro AW. Heme sensor proteins. J Biol Chem. 2013;288:13194–203. doi: 10.1074/jbc.R112.422642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitti M, et al. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neurosci Lett. 2007;416:261–5. doi: 10.1016/j.neulet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Piras S, et al. Monomeric Abeta1-42 and RAGE: key players in neuronal differentiation. Neurobiol Aging. 2014;35:1301–8. doi: 10.1016/j.neurobiolaging.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 23.Warnatz HJ, et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem. 2011;286:23521–32. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster JI, et al. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J Biomol Screen. 2016;21:496–509. doi: 10.1177/1087057115625190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashabi G, Ahmadiani A, Abdi A, Abraki SB, Khodagholi F. Time course study of Abeta formation and neurite outgrowth disruption in differentiated human neuroblastoma cells exposed to H2O2: protective role of autophagy. Toxicol In Vitro. 2013;27:1780–8. doi: 10.1016/j.tiv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Gegg ME, et al. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J Neurochem. 2003;86:228–37. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- 27.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. lClin Geriatr Med. 2004;20:329–59. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Raju VS, McCoubrey WK, Jr., Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta. 1997;1351:89–104. doi: 10.1016/S0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield DA, et al. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15:981–7. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 30.Chen J. Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci. 2014;25:269–80. doi: 10.1515/revneuro-2013-0046. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Wang Q, Li S. MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophys Res Commun. 2016;471:582–8. doi: 10.1016/j.bbrc.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Ke K, Sul OJ, Rajasekaran M, Choi HS. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone. 2015;81:237–46. doi: 10.1016/j.bone.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, Otterbein LE. Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal. 2014;20:1709–22. doi: 10.1089/ars.2013.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 35.Gazzin S, Vitek L, Watchko J, Shapiro SM, Tiribelli C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol Med. 2016;22:758–68. doi: 10.1016/j.molmed.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Maruhashi T, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation. 2012;126:598–603. doi: 10.1161/CIRCULATIONAHA.112.105775. [DOI] [PubMed] [Google Scholar]
- 37.Dekker D, et al. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:458–63. doi: 10.1161/ATVBAHA.110.211789. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura Y, et al. Hyperbilirubinemia protects against focal ischemia in rats. J Neurosci Res. 2003;71:544–50. doi: 10.1002/jnr.10514. [DOI] [PubMed] [Google Scholar]
- 39.Takeda TA, Mu A, Tai TT, Kitajima S, Taketani S. Continuous de novo biosynthesis of haem and its rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci Rep. 2015;5:10488. doi: 10.1038/srep10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He M, et al. Heme oxygenase-1-derived bilirubin protects endothelial cells against high glucose-induced damage. Free Radic Biol Med. 2015;89:91–8. doi: 10.1016/j.freeradbiomed.2015.07.151. [DOI] [PubMed] [Google Scholar]
- 41.Calay D, Mason JC. The multifunctional role and therapeutic potential of HO-1 in the vascular endothelium. Antioxid Redox Signal. 2014;20:1789–809. doi: 10.1089/ars.2013.5659. [DOI] [PubMed] [Google Scholar]
- 42.Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348(Pt 3):615–9. doi: 10.1042/bj3480615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Tu Y, Moon C, Nagata E, Ronnett GV. Heme oxygenase-1 and heme oxygenase-2 have distinct roles in the proliferation and survival of olfactory receptor neurons mediated by cGMP and bilirubin, respectively. J Neurochem. 2003;85:1247–61. doi: 10.1046/j.1471-4159.2003.01776.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–13. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- 45.Sedlak TW, et al. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA. 2009;106:5171–6. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Biase S, et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell. 2016;30:136–46. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Wu H, Zhao M, Chang C, Lu Q. The Bach Family of Transcription Factors: A Comprehensive Review. Clin Rev Allergy Immunol. 2016;50:345–56. doi: 10.1007/s12016-016-8538-7. [DOI] [PubMed] [Google Scholar]
- 48.Su C, et al. Tetrachlorobenzoquinone induces Nrf2 activation via rapid Bach1 nuclear export/ubiquitination and JNK-P62 signaling. Toxicology. 2016;363-364:48–57. doi: 10.1016/j.tox.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Shim KS, Rosner M, Freilinger A, Lubec G, Hengstschlager M. Bach2 is involved in neuronal differentiation of N1E-115 neuroblastoma cells. Exp Cell Res. 2006;312:2264–78. doi: 10.1016/j.yexcr.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Zhang DX, Zhang LM, Zhao XC, Sun W. Neuroprotective effects of erythropoietin against sevoflurane-induced neuronal apoptosis in primary rat cortical neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed Pharmacother. 2017;87:332–341. doi: 10.1016/j.biopha.2016.12.115. [DOI] [PubMed] [Google Scholar]
- 51.Di Domenico F, et al. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-a system: insights for transition to Alzheimer’s disease. J Alzheimers Dis. 2015;44:1107–20. doi: 10.3233/JAD-141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci USA. 2007;104:19589–94. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith B, et al. Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PLoS One. 2010;5:e11109. doi: 10.1371/journal.pone.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng X, Ku CH, Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 55.Pulkkinen KH, Yla-Herttuala S, Levonen AL. Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med. 2011;51:2124–31. doi: 10.1016/j.freeradbiomed.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Go H, et al. MiR-196a regulates heme oxygenase-1 by silencing Bach1 in the neonatal mouse lung. Am J Physiol Lung Cell Mol Physiol. 2016;311:L400–11. doi: 10.1152/ajplung.00428.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Culpan D, Kehoe PG, Love S. Tumour necrosis factor-alpha (TNF-alpha) and miRNA expression in frontal and temporal neocortex in Alzheimer’s disease and the effect of TNF-alpha on miRNA expression in vitro. Int J Mol Epidemiol Genet. 2011;2:156–62. [PMC free article] [PubMed] [Google Scholar]
- 58.Fang M, Hutchinson L, Deng A, Green MR. Common BRAF(V600E)-directed pathway mediates widespread epigenetic silencing in colorectal cancer and melanoma. Proc Natl Acad Sci USA. 2016;113:1250–5. doi: 10.1073/pnas.1525619113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furfaro AL, et al. Resistance of neuroblastoma GI-ME-N cell line to glutathione depletion involves Nrf2 and heme oxygenase-1. Free Radic Biol Med. 2012;52:488–96. doi: 10.1016/j.freeradbiomed.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Furfaro AL, et al. Impaired synthesis contributes to diabetes-induced decrease in liver glutathione. Int J Mol Med. 2012;29:899–905. doi: 10.3892/ijmm.2012.915. [DOI] [PubMed] [Google Scholar]
- 61.Furfaro AL, et al. Role of Nrf2, HO-1 and GSH in Neuroblastoma Cell Resistance to Bortezomib. PLoS One. 2016;11:e0152465. doi: 10.1371/journal.pone.0152465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting this study are provided in full in the result section or as supplementary information.


