Abstract
The availability of habitat structure across spatial scales can determine ecological organization and resilience. However, anthropogenic disturbances are altering the abundance and composition of habitat-forming organisms. How such shifts in the composition of these organisms alter the physical structure of habitats across ecologically important scales remains unclear. At a time of unprecedented coral loss and homogenization of coral assemblages globally, we investigate the inherent structural complexity of taxonomically distinct reefs, across five ecologically relevant scales of measurement (4–64 cm). We show that structural complexity was influenced by coral species composition, and was not a simple function of coral cover on the studied reefs. However, inter-habitat variation in structural complexity changed with scale. Importantly, the scales at which habitat structure was available also varied among habitats. Complexity at the smallest, most vulnerable scale (4 cm) varied the most among habitats, which could have inferences for as much as half of all reef fishes which are small-bodied and refuge dependent for much of their lives. As disturbances continue and species shifts persist, the future of these ecosystems may rely on a greater concern for the composition of habitat-building species and prioritization of particular configurations for protection of maximal cross-scale habitat structural complexity.
Introduction
The physical structure of habitats is integral to the organization, function, and resilience of ecosystems1–3, and therefore the provision of ecosystem goods and services. The diversity and abundance of taxa such as birds, small mammals, lizards, and fish, commonly correlate with the structural complexity of habitats across a range of ecosystems4. Specifically, the availability of microhabitats over a range of spatial scales provides associated organisms of different sizes with refuge from predation, allows for greater niche differentiation and can facilitate other species by mediating competition, and reducing environmental conditions to tolerable levels5. Animals often use their environment at spatial scales relative to their body-size, for example spatial refugia from predators3. However, habitat structural complexity at one scale of measurement is not necessarily synonymous with complexity at other scales (e.g. ref. 6). The availability of fine and coarse scale structural complexity often varies among habitats, with direct implications for the distribution of organisms7, 8, the maintenance of ecosystem processes9, 10, and the resilience of communities2.
The structural complexity of habitats is typically created by communities of living organisms (i.e. habitat-forming organisms such as trees, canopy-forming seaweeds, oysters, wetland grasses, and corals), as well as abiotic features such as the underlying geomorphology, and/or three-dimensional structures of dead organisms6, 11. Importantly, both the abundance and species composition of habitat-forming organisms can have a strong influence on the structural complexity of habitats. For example, the habitat structural complexity of forests varies with tree species composition12, 13; wetland habitats vary with the composition of forbs, grasses and rushes14; and the structure of subtidal temperate reefs is dependent on the species composition of canopy-forming seaweeds15. Similarly on coral reefs, habitat structural complexity is likely underpinned by the relative abundance of component coral species16, and can vary independently of total coral cover17. Corals are structurally diverse taxa, characterized by a range of morphologies (e.g. branching, foliose, massive, or tabulate) that are determined by evolved life history strategies18, genetic variation, and environmental phenotypic plasticity19. Even within these morphological groupings there is considerable variation among species in the size and shape of morphological features (e.g., length and spacing between branches, branching pattern) and hence the interstitial spaces created within, underneath and between colonies20–22.
Globally, pervasive anthropogenic disturbances are reducing species populations, leading to biotic homogenization of communities and changes to the functioning of ecosystems23, 24. On tropical reefs, climate-change induced warm-water anomalies, severe storms, land-based sources of pollution and sedimentation, overfishing, and predation by crown-of-thorns starfish are leading to marked declines in the abundance, and changes in the composition of habitat-forming corals25, 26. Differential susceptibilities to disturbance and variation in life-histories among coral species are causing non-random homogenization of coral assemblages, often dominated by species that are relatively more tolerant to stress, or fast growing and quick to colonize18, 27–29. Some of the most structurally complex corals, such as taxa with branching morphologies, are the most susceptible to a range of disturbances, including storms30, thermal-bleaching31, and crown-of-thorns starfish32. While reductions in the abundance of these structurally complex corals is typically related to reductions in the structural complexity of habitats33, disturbances can also lead to increases in habitat structural complexity, particularly where reefs persist as altered coral-dominated systems34. Consequently, changes in coral composition will likely impact the habitat structural complexity of coral reefs34, with direct implications for the capacity of those reefs to maintain reef functions35, 36, and the provision of coral reef ecosystem goods and services37, 38.
Shifts in the composition of habitat-building coral species are predicted to persist into the future26, 39. Therefore, identifying the structural characteristics of particular coral configurations is critical for the conservation of those systems. However, an understanding of the inherent variation in cross-scale structural complexity of coral reefs is currently lacking. To this end, this study aimed to investigate the influence of coral species composition on cross-scale patterns of habitat structural complexity, at spatial scales of measurement relevant to fish refuge selection (adapted from ref. 8). Cross-scale structural complexity was quantified at randomly selected sites at Lizard Island in the northern Great Barrier Reef, Australia (14°41′S, 145°27′E), following a step-length geometric series using contour distance measuring wheels of different diameters (4–64 cm) along 10-m transects at each site (Fig. 1; see Supplementary Table S1). Specifically, we assessed i) the cross-scale structural complexity of four coral habitats with distinct species configurations and degraded (<10% total coral cover) reef habitat, and ii) cross-scale colony level structural complexity of the dominant coral species at our study location to elucidate the relationship between the complexity of taxa-specific morphologies and colony size.
Figure 1.
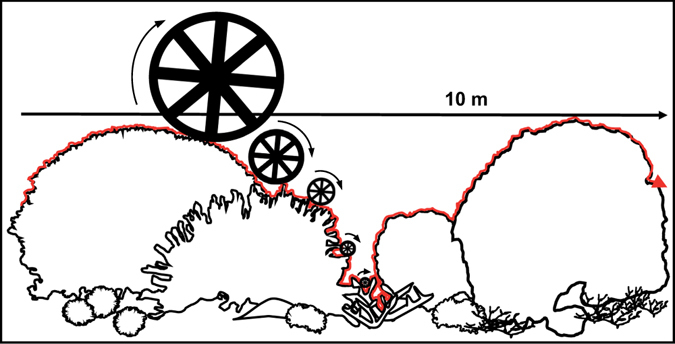
Contour distance estimated with five wheels of different diameters (4–64 cm) along a 10 m transect. Wheels closely follow the surface structure of the benthos and number of rotations are counted. Small wheels fit into more holes than larger wheels and thus estimate greater contour distance.
Results
Habitat classification
Benthic composition varied among the twelve sites, with five distinct habitat groups identified by MDS and hierarchical clustering of benthic composition (Fig. 2). PERMANOVA supported these groupings with significant differences in benthic composition among the five groups (Pseudo-F = 11.22, df = 4, P = 0.0001; all pairwise comparisons P = 0.0001; see Supplementary Table S2). SIMPER analysis indicated dominant taxa and substrate types (i.e., Porites cylindrica, massive Porites – mostly P. lutea, Pocillopora damicornis, soft coral, dead coral and macroalgae) consistently contributed to average similarity within, or dissimilarity between groups (see Supplementary Table S2). Cover of these dominant coral taxa (including soft coral) ranged from 51.5–90.1% of total live coral in coral-dominated sites (mean total coral cover 51.3% ± 4.6 SE). Conversely, the grouping characterized by dead coral and pavement, rubble, and macroalgae (79.4% ± 1.2 SE benthic cover), had significantly less live coral cover (10.5% ± 1.8 SE; lme, F 4, 7 = 25.83, P = 0.0003; Tukey, all P ≤ 0.03). Among the coral-dominated groupings, total live coral cover was higher at sites dominated by Porites cylindrica than those characterized by P. lutea, Pocillopora damicornis, or soft coral which had comparable cover (lme, F 4,7 = 25.83, P = 0.0003). Sites were classified by habitat groupings according to dominant substrata as follows: Porites cylindrica (hereafter ‘branching Porites’; 3 sites), P. lutea (hereafter ‘massive Porites’; 2 sites), Pocillopora damicornis (hereafter ‘Pocillopora’; 1 site), soft coral (3 sites), and degraded (3 sites) for all subsequent analyses.
Figure 2.
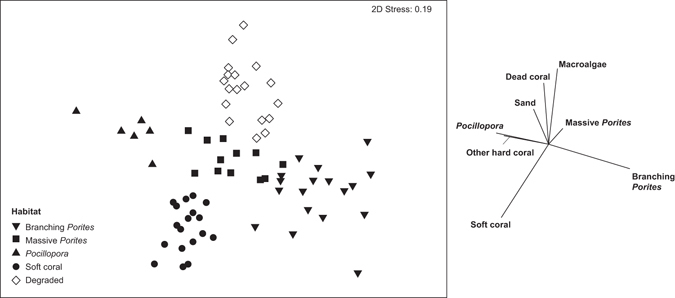
Non-metric multidimensional scaling analysis showing variation in benthic composition among surveyed reef habitats at Lizard Island, using transect level square root transformed data. The relative contribution of benthic categories to the observed variation in benthic composition are illustrated (>0.2 Pearson correlation).
Habitat structural complexity
The structural complexity of habitats quantified using distance measuring wheels of different diameters (4–64 cm) that followed the reef surface contour along four 10-m transects at each site changed with scale of measurement, and varied among habitats with similar levels of coral cover (Fig. 3; see Supplementary Tables S1 and S3). Modelling multi-scale contour distance with coral cover and benthic composition indicated that at the smallest scales (4–8 cm), total coral cover was a significant predictor of contour distance, but variation in benthic composition (habitat type) was also in the top models with a relative importance of 0.27 (4 cm scale) and 0.17 (8 cm scale). Total coral cover was not present in the top models for structural complexity at larger scales (16–64 cm), indicating that benthic composition better predicted variation in contour distance measured among sites (Table 1). Null models featured in the top models for structural complexity at the 8, 16, and 64 cm scales, indicating high variability among transects (scales 8 and 16 cm) and/or sites (64 cm) (see extracted variance components in Supplementary Table S1). Broadly, structural complexity varied significantly among habitats at all scales except 8 cm, though inter-habitat differences were not consistent among scales. Branching Porites and massive Porites habitats were generally more complex than soft coral, Pocillopora and degraded habitats at the small and intermediate scales (4–16 cm). The structural complexity of branching Porites habitats reduced to intermediate levels at the 32 cm scale, comparable with degraded habitats; and massive Porites and degraded habitats were most complex at the largest scale (64 cm) (Fig. 3; see Supplementary Table S3).
Figure 3.
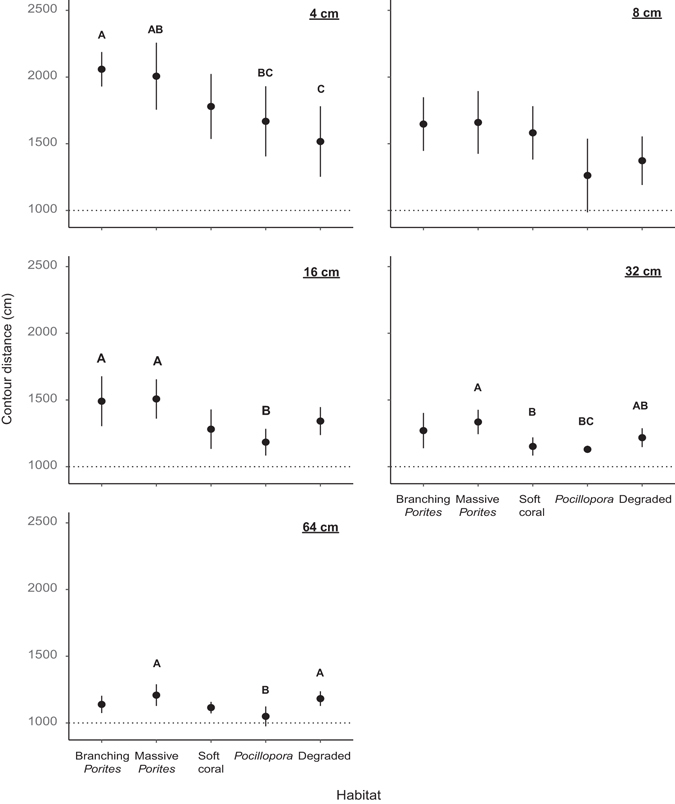
Modelled contour distance (±95% confidence intervals) measured along 10 m transects at scales 4–64 cm with measuring wheels (wheel diameters, cm: 3.99; 7.97; 15.95; 31.89; 63.79), in different coral reef habitats (n = 4–12 per habitat). Significant differences between habitats revealed by post hoc Tukey pair-wise comparisons are illustrated by the pairing of letters (P < 0.05).
Table 1.
Top candidate models selected to describe the relationship between habitat structural complexity across scales (4–64 cm), with total coral cover and habitat type (benthic composition).
| Scale (cm) | Model rank | AICc | df | logLik | ΔAICc | wAICc | Total coral cover (%) | Habitat | Model output (lme) |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 1 | 665.26 | 8 | −322.68 | 0.00 | 0.73 | X | F 1,33 = 23.06, P < 0.0001 | |
| 2 | 667.29 | 11 | −318.76 | 2.03 | 0.27 | X | F 4,7 = 6.73, P = 0.02 | ||
| 8 | 1 | 668.63 | 8 | −324.37 | 0.00 | 0.57 | X | F 1,33 = 5.03, P = 0.03 | |
| 2 | 670.16 | 7 | −326.60 | 1.53 | 0.26 | Null model | F 1,34 = 1112.41, P < 0.0001 | ||
| 3 | 671.05 | 11 | −320.64 | 2.42 | 0.17 | X | F 4,7 = 3.32, P = 0.08 | ||
| 16 | 1 | 634.33 | 11 | −302.28 | 0.00 | 0.80 | X | F 4,7 = 6.18, P = 0.02 | |
| 2 | 637.14 | 7 | −310.09 | 2.80 | 0.20 | Null model | F 1,34 = 1187.59, P < 0.0001 | ||
| 32 | 1 | NA | 11 | −255.26 | NA | NA | X | F 4,7 = 8.81, P = 0.01 | |
| 64 | 1 | 517.18 | 11 | −243.71 | 0.00 | 0.69 | X | F 4,7 = 4.34, P = 0.04 | |
| 2 | 518.80 | 7 | −250.93 | 1.62 | 0.31 | Null model | F 1,34 = 4792.91, P < 0.0001 | ||
Models are ranked by Akaike’s information criteria (AICc), with all models within ΔAICc < 3 of the top ranked model. The relative weight of evidence for each model is indicated by Akaike weight (wAICc), and the variables present in each model are indicated with an X. Null models refer to variance explained by site or transect level sampling Outputs are presented for each model, tested using Site as a random effect, and fitted with a constant variance structure to allow for heterogeneity at all scales.
Contour distance travelled along transects declined with increasing wheel size in all habitats, however, the magnitude of change and cross-scale patterns of structural complexity varied among habitats (Fig. 4). Branching Porites and massive Porites had the greatest variation in complexity across scales, while degraded habitat had the least (Fig. 4; see Supplementary Table S4). Habitat structural complexity was identified at four distinct scales in the massive Porites and branching Porites habitats, three in Pocillopora, and at two distinct scales in the soft coral and degraded habitats. In soft coral, massive- and branching-Porites habitats, structural complexity at the two smallest scales (4 and 8 cm) was significantly greater than structural complexity at the two largest scales (32 and 64 cm). However, in the Pocillopora and degraded habitats these distinctions between scales were less apparent. Within the Pocillopora habitat, structural complexity at the smallest scale (4 cm) was greater than at the remaining scales (8–64 cm), while in the degraded habitat structural complexity was similar across all but the largest scale (64 cm).
Figure 4.
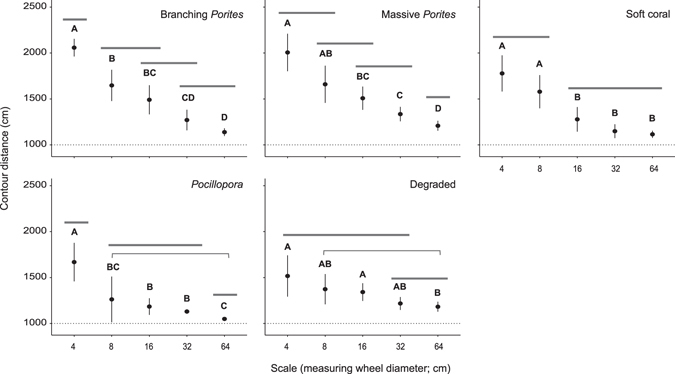
Modelled contour distance (±95% confidence intervals) measured using wheels representing scales 4–64 cm (wheel diameters, cm: 3.99; 7.97; 15.95; 31.89; 63.79), within each habitat. Significant differences between habitats revealed by linear mixed effects modelling and post hoc Tukey pair-wise comparisons are illustrated by the pairing of letters (P < 0.05). Grey bars across scales further illustrate similarities in structural complexity across scales of measurement in each habitat. Thin grey bars in Pocillopora and Degraded habitats denote similarities in contour distance over scales at either end of the bar (i.e. non-consecutive sales).
Colony level structural complexity
Colony level analyses revealed strong linear relationships between the structural complexity of massive Porites, P. cylindrica and Pocillopora damicornis and colony size for each taxa, with constant relationships between contour distance and colony diameter at all five scales (correlation coefficients ranged from 0.96–0.97, 0.93–0.97, and 0.68–0.91, respectively) (Fig. 5, see Supplementary Table S5). Visual inspection of regression slopes suggests that both Porites taxa were more structurally complex than Pocillopora damicornis across their size ranges at all scales. Porites cyclindrica colonies appear more structurally complex than massive Porites colonies of the same size at the 4 cm scale, and to a lesser degree at the 8 cm scale. Conversely, massive Porites colonies appear more complex at the 16–64 cm scales (Fig. 5, see Supplementary Table S5).
Figure 5.
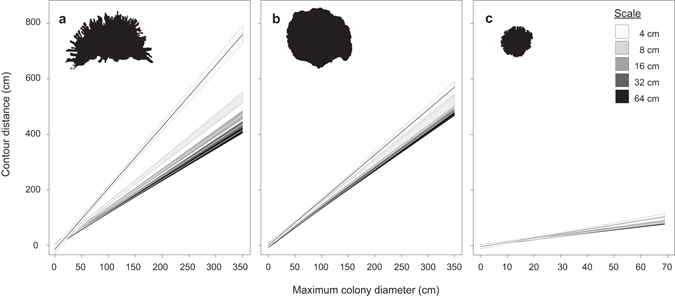
Relationships between maximum colony diameter (cm) and contour distance travelled by measuring wheels of diameters 4–64 cm (±95% confidence intervals): (a) Porites cylindrica (R2 = 0.93–0.97); (b) massive Porites (R2 = 0.95–0.97); and (c) Pocillopora damicornis (R2 = 0.68–0.91).
Discussion
Disturbance induced biotic homogenization threatens the architecture of habitats at ecologically relevant scales and the resilience of ecosystems12, 24. Previous studies have described the physical flattening of habitats associated with the loss of key organisms such as trees, kelp, and corals, and the often profound consequences for biodiversity and related ecosystem services12, 33, 40. Here, we show that the habitat structural complexity of the studied reefs was inextricably tied to the identity of constituent habitat-building species, and was not shaped solely by the absolute cover of corals. Importantly, the structural complexity of habitats changed with scale of measurement. The greatest differentiation in habitat structural complexity was at the smallest (4 cm) and most vulnerable scale of measurement41. Furthermore, cross-scale structural complexity varied among all habitats, evident at four distinct scales in both Porites habitats, three in Pocillopora habitat, and two in soft coral and degraded habitats. It should be noted that the less rigid biota such as soft corals and large macroalgae likely provide elements of structural complexity that may not be effectively captured by the methods used in this study. These findings have substantial implications for the relative suitability of coral reef habitats for associated organisms that are refuge dependent, including other reef invertebrates and small-bodied reef fishes41, 42.
Differential habitat structural complexity across scales emphasizes the role of species composition in shaping the physical architecture of reef ecosystems. For example, we show that at small to mid-scales (4–16 cm), habitat structural complexity was greatest in massive and branching Porites habitats, relative to Pocillopora, soft coral and degraded habitats, while at larger scales (32–64 cm), the greatest structural complexity was in massive Porites and degraded habitats. Similar differences were evident in colony level complexity for both Porites taxa versus Pocillopora. Notably, the greatest differentiation in habitat structural complexity was evident at the smallest scales of measurement (4–8 cm). Structural complexity at these scales is largely determined by variation in the surface morphology of individual coral colonies (see Fig. 5), and likely provides the most benefit to small bodied and/or juvenile fishes subject to high risk of predation41. Branching Porites colonies were notably more structurally complex at the 4 cm scale, relative to massive Porites colonies of the same size, though relatively high contour distances were also observed in the latter (Fig. 5; Supplementary Table S5). Branching Porites species such as P. cylindrica create intricate and discrete interstitial spaces between their branches, whilst large colonies of massive Porites species can form fine scale corrugations or crevices in their otherwise relatively planar surfaces thereby providing small-scale microhabitats for small-bodied reef organisms43, 44. Despite greater complexity of branching Porites colonies, the differentiation in complexity between Porites taxa was lost at the transect level possibly due to variable size distributions of colonies in these habitats. Overall, the high contour distances measured in these Porites habitats likely reflect the typically large size of the branching and massive Porites colonies, as well as the undercut areas beneath and the vertical relief between colonies.
Whilst Pocillopora habitat was the least structurally complex of the four coral-dominated habitats across all scales, at scales finer than those considered in this study (i.e. <4 cm), P. damicornis is structurally intricate, and likely provides important refugia for many reef fishes45. However, due to its small size, brooding reproduction and fast growth rates46, Pocillopora dominated reefs can be characterised by multiple, tightly-aggregated colonies of similar sizes, offering little relief between them that might otherwise provide greater structural relief across all scales, but particularly at an inter-colony scale of approximately 8–32 cm.
The role of live coral in providing structurally complex tropical reef habitats has received much attention33, 47. However, the absolute cover of live corals alone does not capture all of the inherent variation in habitat structural complexity17, 34, 48, 49. We found that total coral cover was a good predictor of structural complexity at the two smallest scales (4–8 cm), but the inclusion of habitat composition further increased the predictive capacity of the models. At the larger scales (16–64 cm), the relationship between total coral cover and structural complexity of the habitat broke down. Our findings are consistent with studies from the Caribbean showing that whilst the fine-scale habitat structural complexity of reefs (0.7 cm scale) increases with coral cover, much of the variance in complexity at high levels of coral cover results from the dominance of particular corals16. It is important to note that despite differences in structural complexity among habitats at the larger scales (i.e. 8–64 cm), variation in the underlying reef structure, as well as the likely contribution of other benthic organisms, introduced substantial variation in complexity at the transect and site sampling levels. The contribution of the underlying substrata (geomorphological structure and dead reef matrix) to the structural complexity of reef habitats was further highlighted by the greater structural complexity of the degraded habitat found at larger scales (32–64 cm). This supports previous findings comparing multiscale complexity of coral-, and macroalgal-dominated habitats8.
The availability of habitat structural complexity across a range of scales is important for maintaining the organisation of associated organisms, including body-size distributions, food web structure and ecosystem functioning2, 50. We found that cross-scale habitat structural complexity varied with coral composition, with multi-scale structure most distinguished in branching and massive Porites habitats, relative to Pocillopora and soft coral habitats. These coral-dominated habitats all contrasted with the low relief degraded reef habitats across all scales. As reef habitats degrade, they become flatter and more structurally homogenous33, providing fewer potential refuges at different scales8, though large stands of macroalgae also contribute to elements of structural complexity51. Broadly, the structural complexity of coral reef habitats is evident both within and between colonies, and at larger scales that capture the corrugations of the underlying substratum6. More specifically however, our results reveal that cross-scale habitat structural complexity is influenced by the composition of coral species, with habitats providing structure ranging from just two scales measured in this study (e.g. soft coral and degraded habitats), to four scales (e.g. massive-, and branching Porites habitats). Interestingly, whilst the Porites habitats both displayed structural complexity at four distinct scales of measurement, cross-scale complexity varied between them. For example, structural complexity at the 4 cm scale differed to complexity at the 8 cm scale in branching Porites habitats, but not in massive Porites habitats. This was supported by colony level analyses indicating greater complexity of branching Porites at the 4 cm scale relative to massive Porites resulting from the interstitial spaces created between branches of P. cylindrica. Similarly, structural complexity at the 64 cm scale was distinct from complexity at the smaller scales in massive Porites habitats, but not branching Porites habitats, likely due to the overhangs often created by large colonies of massive Porites. The only shared variation in structural complexity occurred between the 8–16 cm and 16–32 cm scales resulting from the similar overall colony surface structures of branching and massive Porites at these intermediate scales.
Soft coral habitat structural complexity was unexpectedly high, particularly at the smallest scales (4–8 cm), and surprising given that the study method likely underestimates structural complexity of less rigid biota such as soft coral. While the relative contribution of soft corals to reef structural complexity are apparent due to their physical presence when alive, quantification of their structural complexity is complex due to their only partially calcified structures, and has received little attention (though see refs 52, 53). Despite this, structural complexity of these soft coral habitats was found at two distinct scales of measurement (4–8 cm and 16–64 cm), as the two smallest wheels were able to fit in between adjoining colonies often reaching the substratum below, whereas the larger wheels rolled over the surface of colonies suggesting less relief at larger scales. Similarly, there was little medium- to large-scale structure (16–64 cm) in Pocillopora habitat due to the small colony sizes and limited space between them, resulting in the larger wheels remaining on the reef surface. Building upon empirical and modelling studies of Caribbean reefs16, 17, 48, our findings show that not only is the identity of constituent corals an important driver of habitat structural complexity, but this occurs across scales. Moreover, the size and number of scales of measurement at which structure is available varies substantially among habitats.
Previous work has shown that broad-scale habitat structural complexity, determined by coral composition and reef condition, can drive the taxonomic and functional diversity of reef fish assemblages22, 54–56. More specifically however, the range of scales at which habitat structure is available likely regulates how species organisation is partitioned and ecological processes are maintained1, 3. Evidence suggests that ecosystems are strongly influenced by processes operating over different scales, and their resilience is determined by diverse, though overlapping, functions at and across those scales2, 50. For example, herbivory, a critical process on coral reefs, is mutually reinforced when reef fishes with shared functions can operate across multiple spatial scales, thereby minimising competition between fishes of similar body-sizes10. Therefore, a loss of habitat structural complexity at specific scales may compromise resilience3, even where habitat-building organisms remain present and appear intact, but have undergone species shifts57.
The homogenisation of habitats characterized by increasingly monospecific assemblages of habitat-building species therefore has broader implications than simply the habitat structural complexity of ecosystems. Conservation practitioners responsible for maintaining coral reef ecosystem services are therefore advised to consider changes in the composition of coral assemblages, and not simply total coral cover on reefs17. Studies suggest that total coral cover alone is a poor surrogate for habitat structural complexity17, the organization of reef associated species58, ecological function59, or reef recovery60, as it does not capture sufficient variation in structural complexity driven by benthic composition. Some coral habitats might warrant relatively greater protection as their inherent variation in habitat structural complexity may support enhanced ecosystem resilience (e.g. Acropora and Orbicella reefs in the Caribbean, refs 36, 61, 62). The strong linear relationships between structural complexity and the dimension of individual colonies of massive Porites, P. cylindrica, and P. damicornis suggest that data on the composition of habitat-building species may prove to be a useful proxy for cross-scale habitat structural complexity. In this way, a refined surrogate for habitat structural complexity that combines coral cover and composition may offer a more effective resilience indicator, thereby improving the likelihood of success of important and costly conservation initiatives61, 63. Mechanistic models of structural complexity have been developed to describe the structure of Caribbean reefs at a broad scale in relation to shifts in benthic communities, using simplified colony shapes of explicit volumes48, or finer scale estimates of coral structural complexity standardised by colony size36. Similar models could be developed for Indo-Pacific reefs using emerging low-cost, effective techniques such as photogrammetry34, 64, allowing predictions of cross-scale structural dynamics resulting from shifts in dominance patterns of corals in the region.
The likely outcomes of continued coral loss on the structural complexity of coral reef habitats will be largely dependent on the nature, frequency and severity of future disturbances, and the capacity for different coral taxa to adapt to changing conditions26, 39. Among those habitats considered here, massive Porites generally provided the most structurally complex habitat at each scale, arguably due to the sheer size of colonies. Massive Porites are relatively slow-growing and tolerant to stressors such as warm-water anomalies18, poor water quality65, and large storms30, and as such is among those taxa predicted to persist into the future18. Similarly, branching Porites was structurally complex across scales, but is relatively fast-growing18, and exhibits varied levels of sensitivity to thermal stress66. This may afford some optimism for future reefs despite escalating anthropogenic disturbance, as persistent corals that can offer refugia across a range of scales have the potential to mediate predator-prey interactions67, thereby extending fish body-size distributions8, and coral reef food chains58. Conversely, the cross-scale habitat structural complexity of degraded reefs with little remaining coral cover (<10%) highlight both the vulnerability of fine scale structure to disturbance, and the more robust nature of larger scale reef structure. The loss of fine scale structure has important implications for species and life stages of fishes that rely on it for refugia, and is likely to lead to rapid reductions in small bodied fish species, and lagged declines in larger bodied species that rely on fine scale habitat structural complexity as juveniles41, 68.
Our results provide new insight into the cross-scale structural dynamics of taxonomically distinct coral reef habitats across spatial scales of measurement relevant to refuge selection by fishes8, 69. However, the outcomes of assemblage shifts can be diverse and spatially variable, such that quantification of the cross-scale habitat structural complexity of additional coral configurations are warranted. For example, tabular and branching Acropora is structurally distinctive and typically dominates large areas of undisturbed coral reef habitats in the Indo-Pacific60, but was not locally abundant at Lizard Island during this study. Furthermore, this study focused on shallow, sheltered reefs only, providing scope for broader investigation. Coral species composition and the morphology of some coral species vary with abiotic conditions (e.g. exposure, depth, water flow, light), biological processes (e.g. recruitment, competition, predation), and disturbance histories39, 70, likely causing variation in the structural complexity of habitats. Similarly, the structural complexity of degraded coral reef habitats can be highly variable, influenced by local disturbance histories (e.g. coral bleaching versus large storms71), the underlying substrate8, and the colonisation of other benthic organisms (e.g. macroalgae51). Finally, the method employed in this study while useful for capturing some aspects of structural complexity (e.g. spaces under overhangs and in non-vertical recesses) that may be underestimated using approaches such as profile gauges and photographic methods72, only captures an estimate of the two-dimensional structural complexity of habitats. Coral reef structures are often multidimensional, with different sized holes and passages throughout the matrix itself. Therefore, in seeking to understand how reef structure relates to the distribution of associated organisms, it would be prudent to consider the specific method used for assessing variation in habitat structural complexity73.
Our results provide evidence that habitat structural complexity can be multifaceted over ecologically relevant scales, and demonstrates the importance of going beyond a consideration of just the presence of habitat-building organisms, to include taxonomic structure in efforts to maintain ecosystems and the provision of associated goods and services37, 58. Coral reefs are among the world’s most biodiverse but threatened ecosystems26. As global conservation increases in response to coral reef degradation74, assessments of reef condition and the identification of priority areas for protection should consider the composition as well as cover of corals and other habitat forming organisms. Moreover, identifying inherent patterns of cross-scale habitat structural complexity typical of likely future configurations of species may prove critical for understanding the ecology and conservation of those coral reef systems.
Methods
Study location
This study was conducted in September 2015 on the reefs surrounding Lizard Island, a granitic island in the northern Great Barrier Reef, Australia (14°41′S, 145°27′E). Benthic composition and cross-scale habitat structural complexity were quantified at twelve randomly selected sites on the leeward side of the island. All sites were shallow (<6-m depth) reef edges (>5-m wide) adjacent to sand. All sites were in areas protected from the prevailing south-east swell, with comparable water clarity and flow, light levels, and geomorphology. Adjacent sites were separated by a minimum of 500 m.
Benthic composition was quantified along six replicate 30-m point-intercept transects at each site, recording the substratum directly beneath the transect line at 25-cm intervals (120 points per transect). Transects were positioned parallel to the reef edge at a depth of 2–6-m, with a minimum of 5 m between adjacent transects. Substratum types included hard (scleractinian) corals (identified to genus or species where possible, and growth form noted), soft (alcyonacean) corals, ‘other sessile invertebrates’ (primarily sponges, giant clams, and ascidians), macroalgae, erect crustose coralline algae, dead coral and pavement, rubble and sand.
Habitat structural complexity
Habitat structural complexity was estimated at five spatial scales of measurement following a step-length geometric series using distance measuring wheels of different diameters (4–64 cm) along four 10-m transects at each site (adapted from72, following8). The 10-m transects used to quantify structural complexity were positioned within the mid-section (i.e., ~10–20 m) of four of the six 30-m transects used to quantify benthic composition. Adjacent 10-m transects were separated by a minimum of 20 m. The abundance of fishes has been shown to positively correlate with structural complexity relative to fish body-size41, 68, and the aperture diameter of available holes or crevices in the substrate as refuges from predation or environmental stressors69. Therefore, scales of measurement were selected to correspond to the body depths of non-cryptic fish species. The contour distance travelled by each wheel over the reef substratum was estimated by rolling the wheels along the reef surface contour immediately below the length of the taught 10-m transect line, being careful to ensure each wheel followed the detailed surface structure of the benthos (Fig. 1). The number of complete rotations and the proportion of each wheel turned for any incomplete rotations were recorded. The contour distance covered by each wheel was calculated by multiplying the number of rotations by the wheel circumference.
Colony level structural complexity
To assess how the five scales of structural complexity relate to colony size of corals, we quantified the structural complexity of three of the most common hard coral taxa at the study sites. Contour distance travelled by each wheel was estimated across the maximum diameter of individual colonies of Porites cylindrica, massive Porites (mostly Porites lutea), and Pocillopora damicornis (measured to the nearest cm with a tape in situ over the surface of the colony). Structural complexity estimates were acquired across the range of available colony sizes for each taxa (P. cylindrica: 3–350 cm, n = 100; massive Porites: 3–415 cm, n = 100; P. damicornis: 3–69 cm, n = 72), at other sheltered reef edge sites around Lizard Island.
Data analyses
Variation in benthic composition among sites was investigated with non-metric multi-dimensional scaling (nMDS) based on Bray-Curtis similarities of square root transformed benthic cover data in Primer v675. Group-average hierarchical clustering was used to provide an objective assessment of five distinct habitat groups identified with nMDS. Two-way permutational multivariate analysis of variance (PERMANOVA) was used to test the significance of these groupings (9999 permutations), with habitat (fixed; branching Porites, massive Porites, Pocillopora, soft coral, and degraded) and site (random) as factors (PERMANOVA+ add on package). One-way pairwise comparisons between habitat groups were performed with unrestricted permutation of raw data to allow for sufficient permutations to be tested. Similarity Percentage (SIMPER) analysis was used post hoc to identify those benthic categories contributing consistently to average similarity within, and dissimilarity between, habitats with a similarity/dissimilarity test ratio of ≥4.0 or 2.0, respectively75.
Differences in (i) contour distances measured at each scale were compared among habitats (fixed effect), and (ii) differences in contour distances measured were compared across scales (fixed effect) within each habitat, using linear mixed effects models, with lme in nlme in all instances (R version 3.2.3; R Development Core Team 2015). In each analysis, site was treated as a random effect and Tukey multiple comparison tests were used to identify where differences occurred (with the multcomp package). Exploratory graphical analysis of model residuals suggested the data conformed to the assumptions of normality and independence, though there was heterogeneity of variance among habitats at the largest scale. Therefore models were fitted with a constant variance structure to allow for heteroscedasticity at all scales, consequently allowing for cross-scale comparisons. To identify the main sources of variation at each scale, variance components were subsequently extracted using lme4 and the MuMIn package (see Supplementary Table S1).
Multiple linear regression was used to estimate relationships between habitat structural complexity with total coral cover (hard and soft coral) and benthic composition (habitat classification) at each scale. Collinearity between coral cover and habitat type was tested by calculating generalized variance inflation-factors (GVIF^1/2df 76). As GVIF values indicated low levels of collinearity (<377), information-theoretic model selection was used to determine the relative importance of these covariates in predicting variation in habitat structural complexity (MuMIn package). Multi-model inference (including null models) was estimated by ranked changes in AICc <378. To determine the scales where changes in structural complexity occurred within habitats, hierarchical modelling was also used to compare contour distances across scales within each habitat, accounting for site effects, followed by Tukey tests. Due to unequal variance across scales within habitats, models were fitted to allow for heterogeneity as previously described. Only one site was identified to be dominated by Pocillopora, and subsequently contour distance was compared across scales without site effects for this habitat using the gls function of nlme. The relationships between colony size and structural complexity at the same five scales were assessed for massive Porites, P. cylindrica and P. damicornis, using linear regression.
Data availability
The datasets generated and analysed during the current study are available in the James Cook University Tropical Data Hub repository, https://research.jcu.edu.au/researchdata.
Electronic supplementary material
Acknowledgements
This study was funded by the Australian Research Council to ASH (DE130100688) and NAJG (DE130101705). We thank Kirsty Nash for the measuring wheels, Amy Douglas, Jacob Eurich and Lizard Island Research Station staff for field support, Rhondda Jones for statistical advice, and three anonymous reviewers for useful comments.
Author Contributions
The authors contributed equally to developing the question and study design. L.R. conducted the data collection and statistical analyses. All authors outlined the first draft of the manuscript. L.R. wrote the manuscript text and prepared all figures, tables and supplementary material, with significant input from A.H. and N.G. All authors reviewed the manuscript and gave final approval of the submitted version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08109-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levin SA. The problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology. 1992;73:1943–1967. doi: 10.2307/1941447. [DOI] [Google Scholar]
- 2.Peterson G, Allen RC, Holling SC. Ecological resilience, biodiversity, and scale. Ecosystems. 1998;1:6–18. doi: 10.1007/s100219900002. [DOI] [Google Scholar]
- 3.Nash KL, et al. Discontinuities, cross-scale patterns, and the organization of ecosystems. Ecology. 2014;95:654–667. doi: 10.1890/13-1315.1. [DOI] [PubMed] [Google Scholar]
- 4.Huston MA. A general hypothesis of species diversity. Am. Nat. 1979;113:81–101. doi: 10.1086/283366. [DOI] [Google Scholar]
- 5.Stachowicz JJ. Mutualism, facilitation, and the structure of ecological communities. Bioscience. 2001;51:235–246. doi: 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2. [DOI] [Google Scholar]
- 6.Bradbury RH, Reichelt RE, Green DG. Fractals in ecology: methods and interpretation. Mar. Ecol. Prog. Ser. 1984;14:295–296. doi: 10.3354/meps014295. [DOI] [Google Scholar]
- 7.Williams SE, Marsh H, Winter J. Spatial scale, species diversity, and habitat structure: small mammals in Australian tropical rain forest. Ecology. 2002;83:1317–1329. doi: 10.1890/0012-9658(2002)083[1317:SSSDAH]2.0.CO;2. [DOI] [Google Scholar]
- 8.Nash KL, Graham NA, Wilson SK, Bellwood DR. Cross-scale habitat structure drives fish body size distributions on coral reefs. Ecosystems. 2013;16:478–490. doi: 10.1007/s10021-012-9625-0. [DOI] [Google Scholar]
- 9.Yvon-Durocher G, Allen AP. Linking community size structure and ecosystem functioning using metabolic theory. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2012;367:2998–3007. doi: 10.1098/rstb.2012.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash KL, Graham NAJ, Jennings S, Wilson SK, Bellwood DR. Herbivore cross-scale redundancy supports response diversity and promotes coral reef resilience. J. Appl. Ecol. 2016;53:646–655. doi: 10.1111/1365-2664.12430. [DOI] [Google Scholar]
- 11.Jones, C. G., Lawton, J. H. & Shachak, M. Organisms as ecosystem engineers In Ecosystem Management (eds Samson, F. B. & Knopf, F. L.) 130–147 (Springer, 1996).
- 12.Ellison AM, et al. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005;3:479–486. doi: 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2. [DOI] [Google Scholar]
- 13.Huston, M. A. Biological diversity: the coexistence of species (Cambridge University Press, 1994).
- 14.Brose U. Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia. 2003;135:407–413. doi: 10.1007/s00442-003-1222-7. [DOI] [PubMed] [Google Scholar]
- 15.Wernberg T, Thomsen MS, Tuya F, Kendrick GA. Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. J. Exp. Mar. Biol. Ecol. 2011;400:264–271. doi: 10.1016/j.jembe.2011.02.017. [DOI] [Google Scholar]
- 16.Alvarez-Filip L, Dulvy NK, Côté IM, Watkinson AR, Gill JA. Coral identity underpins architectural complexity on Caribbean reefs. Ecol. App. 2011;21:2223–2231. doi: 10.1890/10-1563.1. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Filip L, Côté IM, Gill JA, Watkinson AR, Dulvy NK. Region-wide temporal and spatial variation in Caribbean reef architecture: is coral cover the whole story? Glob. Change Biol. 2011;17:2470–2477. doi: 10.1111/j.1365-2486.2010.02385.x. [DOI] [Google Scholar]
- 18.Darling ES, McClanahan TR, Côté IM. Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 2013;19:1930–1940. doi: 10.1111/gcb.12191. [DOI] [PubMed] [Google Scholar]
- 19.Todd PA. Morphological plasticity in scleractinian corals. Biol. Rev. 2008;83:315–337. doi: 10.1111/j.1469-185X.2008.00045.x. [DOI] [PubMed] [Google Scholar]
- 20.Luckhurst B, Luckhurst K. Analysis of the influence of substrate variables on coral reef fish communities. Mar. Biol. 1978;49:317–323. doi: 10.1007/BF00455026. [DOI] [Google Scholar]
- 21.Gratwicke B, Speight M. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish Biol. 2005;66:650–667. doi: 10.1111/j.0022-1112.2005.00629.x. [DOI] [Google Scholar]
- 22.Harborne AR, Mumby PJ, Ferrari R. The effectiveness of different meso-scale rugosity metrics for predicting intra-habitat variation in coral-reef fish assemblages. Environ. Biol. Fish. 2012;94:431–442. doi: 10.1007/s10641-011-9956-2. [DOI] [Google Scholar]
- 23.Ellis EC, et al. Used planet: A global history. Proc. Nat. Acad. Sci. 2013;110:7978–7985. doi: 10.1073/pnas.1217241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dornelas M, et al. Assemblage time series reveal biodiversity change but not systematic loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- 25.De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Nat. Acad. Sci. 2012;109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 27.Pratchett M, Trapon M, Berumen M, Chong-Seng K. Recent disturbances augment community shifts in coral assemblages in Moorea, French Polynesia. Coral Reefs. 2011;30:183–193. doi: 10.1007/s00338-010-0678-2. [DOI] [Google Scholar]
- 28.Bento R, Hoey AS, Bauman AG, Feary DA, Burt JA. The implications of recurrent disturbances within the world’s hottest coral reef. Mar. Pollut. Bull. 2016;105:466–472. doi: 10.1016/j.marpolbul.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Guest JR, et al. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE. 2012;7:e33353. doi: 10.1371/journal.pone.0033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harmelin-Vivien ML. The effects of storms and cyclones on coral reefs: a review. J. Coast. Res. 1994;12:211–231. [Google Scholar]
- 31.Marshall P, Baird A. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163. doi: 10.1007/s003380000086. [DOI] [Google Scholar]
- 32.Baird AH, Pratchett MS, Hoey AS, Herdiana Y, Campbell SJ. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs. 2013;32:803–812. doi: 10.1007/s00338-013-1025-1. [DOI] [Google Scholar]
- 33.Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 2009;276:3019–3025. doi: 10.1098/rspb.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari R, et al. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob. Chang. Biol. 2016;22:1965–1975. doi: 10.1111/gcb.13197. [DOI] [PubMed] [Google Scholar]
- 35.Done TJ. Coral community adaptability to environmental change at the scales of regions, reefs and reef zones. Am. Zool. 1999;39:66–79. doi: 10.1093/icb/39.1.66. [DOI] [Google Scholar]
- 36.Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R. Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 2013;3:3486. doi: 10.1038/srep03486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999;29:215–233. doi: 10.1016/S0921-8009(99)00009-9. [DOI] [Google Scholar]
- 38.Hicks CC, Cinner JE. Social, institutional, and knowledge mechanisms mediate diverse ecosystem service benefits from coral reefs. Proc. Nat. Acad. Sci. 2014;111:17791–17796. doi: 10.1073/pnas.1413473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. Projecting coral reef futures under global warming and ocean acidification. Science. 2011;333:418–422. doi: 10.1126/science.1204794. [DOI] [PubMed] [Google Scholar]
- 40.Steneck RS, et al. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 2002;29:436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- 41.Wilson SK, et al. Habitat degradation and fishing effects on the size structure of coral reef fish communities. Ecol. App. 2010;20:442–451. doi: 10.1890/08-2205.1. [DOI] [PubMed] [Google Scholar]
- 42.Stella J, Pratchett MS, Hutchings P, Jones G. Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. 2011;49:43–104. [Google Scholar]
- 43.Wellington G, Victor B. El Niño mass coral mortality: a test of resource limitation in a coral reef damselfish population. Oecologia. 1985;68:15–19. doi: 10.1007/BF00379466. [DOI] [PubMed] [Google Scholar]
- 44.Gardiner NM, Jones GP. Synergistic effects of habitat preference and gregarious behaviour on habitat use in coral reef cardinalfish. Coral Reefs. 2010;29:845–856. doi: 10.1007/s00338-010-0642-1. [DOI] [Google Scholar]
- 45.Coker DJ, Wilson SK, Pratchett MS. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 2014;24:89–126. doi: 10.1007/s11160-013-9319-5. [DOI] [Google Scholar]
- 46.Darling ES, Alvarez‐Filip L, Oliver TA, McClanahan TR, Côté IM. Evaluating life‐history strategies of reef corals from species traits. Ecol. Lett. 2012;15:1378–1386. doi: 10.1111/j.1461-0248.2012.01861.x. [DOI] [PubMed] [Google Scholar]
- 47.Graham NAJ, Nash KL. The importance of structural complexity in coral reef ecosystems. Coral Reefs. 2013;32:315–326. doi: 10.1007/s00338-012-0984-y. [DOI] [Google Scholar]
- 48.Bozec YM, Alvarez‐Filip L, Mumby PJ. The dynamics of architectural complexity on coral reefs under climate change. Glob. Change Biol. 2015;21:223–235. doi: 10.1111/gcb.12698. [DOI] [PubMed] [Google Scholar]
- 49.Darling ES, et al. Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs. 2017;36:561–575. doi: 10.1007/s00338-017-1539-z. [DOI] [Google Scholar]
- 50.Holling CS. Cross-scale morphology, geometry, and dynamics of ecosystems. Ecol. Monogr. 1992;62:447–502. doi: 10.2307/2937313. [DOI] [Google Scholar]
- 51.Hoey AS, Bellwood DR. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol. Lett. 2011;14:267–273. doi: 10.1111/j.1461-0248.2010.01581.x. [DOI] [PubMed] [Google Scholar]
- 52.Syms C, Jones GP. Soft corals exert no direct effects on coral reef fish assemblages. Oecologia. 2001;127:560–571. doi: 10.1007/s004420000617. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari R. The hidden structure in coral reefs. Coral Reefs. 2017;36:445–445. doi: 10.1007/s00338-017-1540-6. [DOI] [Google Scholar]
- 54.Messmer V, et al. Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology. 2011;92:2285–2298. doi: 10.1890/11-0037.1. [DOI] [PubMed] [Google Scholar]
- 55.Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518:94–97. doi: 10.1038/nature14140. [DOI] [PubMed] [Google Scholar]
- 56.Richardson LE, Graham NAJ, Pratchett MS, Hoey AS. Structural complexity mediates functional structure of reef fish assemblages among coral habitats. Environ. Biol. Fish. 2017;100:193–207. doi: 10.1007/s10641-016-0571-0. [DOI] [Google Scholar]
- 57.Redford KH. The empty forest. Bioscience. 1992;42:412–422. doi: 10.2307/1311860. [DOI] [Google Scholar]
- 58.Alvarez-Filip L, Gill JA, Dulvy NK. Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere. 2011;2:1–17. doi: 10.1890/ES11-00185.1. [DOI] [Google Scholar]
- 59.Cvitanovic C, Hoey AS. Benthic community composition influences within-habitat variation in macroalgal browsing on the Great Barrier Reef. Mar. Freshw. Res. 2010;61:999–1005. doi: 10.1071/MF09168. [DOI] [Google Scholar]
- 60.Johns K, Osborne K, Logan M. Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Coral Reefs. 2014;33:553–563. doi: 10.1007/s00338-014-1148-z. [DOI] [Google Scholar]
- 61.Mumby PJ, et al. Coral reef habitats as surrogates of species, ecological functions, and ecosystem services. Conserv. Biol. 2008;22:941–951. doi: 10.1111/j.1523-1739.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- 62.Harborne AR, et al. Tropical coastal habitats as surrogates of fish community structure, grazing, and fisheries value. Ecol. App. 2008;18:1689–1701. doi: 10.1890/07-0454.1. [DOI] [PubMed] [Google Scholar]
- 63.Hermoso V, Januchowski-Hartley SR, Pressey RL. When the suit does not fit biodiversity: Loose surrogates compromise the achievement of conservation goals. Biol. Conserv. 2013;159:197–205. doi: 10.1016/j.biocon.2012.11.026. [DOI] [Google Scholar]
- 64.Figueira W, et al. Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Rem Sens. 2015;7:15859. doi: 10.3390/rs71215859. [DOI] [Google Scholar]
- 65.Fabricius KE, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011;1:165–169. doi: 10.1038/nclimate1122. [DOI] [Google Scholar]
- 66.McClanahan TR. Changes in coral sensitivity to thermal anomalies. Mar. Ecol. Prog. Ser. 2017;570:71–85. doi: 10.3354/meps12150. [DOI] [Google Scholar]
- 67.Gregor CA, Anderson TW. Relative importance of habitat attributes to predation risk in a temperate reef fish. Environ. Biol. Fish. 2016;99:539–556. doi: 10.1007/s10641-016-0496-7. [DOI] [Google Scholar]
- 68.Graham NA, et al. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007;21:1291–1300. doi: 10.1111/j.1523-1739.2007.00754.x. [DOI] [PubMed] [Google Scholar]
- 69.Hixon MA, Beets JP. Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol. Monogr. 1993;63:77–101. doi: 10.2307/2937124. [DOI] [Google Scholar]
- 70.Williams GJ, et al. Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ. 2013;1:e81. doi: 10.7717/peerj.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NV. Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 2006;12:2220–2234. doi: 10.1111/j.1365-2486.2006.01252.x. [DOI] [Google Scholar]
- 72.Wilding TA, Rose CA, Downie MJ. A novel approach to measuring subtidal habitat complexity. J. Exp. Mar. Biol. Ecol. 2007;353:279–286. doi: 10.1016/j.jembe.2007.10.001. [DOI] [Google Scholar]
- 73.Robson B, Barmuta L, Fairweather PG. Methodological and conceptual issues in the search for a relationship between animal body-size distributions and benthic habitat architecture. Mar. Freshw. Res. 2005;56:1–11. doi: 10.1071/MF04210. [DOI] [Google Scholar]
- 74.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 75.Clarke, K. R. & Warwick, R. M. An Approach to Statistical Analysis and Interpretation: Change in Marine Communities 2 (Plymouth Marine Laboratory, 2001).
- 76.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87:178–183. doi: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- 77.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 78.Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer Science & Business Media, 2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the James Cook University Tropical Data Hub repository, https://research.jcu.edu.au/researchdata.


