Abstract
Abnormal aggregation of β-amyloid (Aβ) peptides is a major hallmark of Alzheimer’s disease (AD). In spite of numerous attempts to prevent the β-amyloidosis, no effective drugs for treating AD have been developed to date. Among many candidate chemicals, methylene blue (MB) has proved its therapeutic potential for AD in a number of in vitro and in vivo studies; but the result of recent clinical trials performed with MB and its derivative was negative. Here, with the aid of multiple photochemical analyses, we first report that photoexcited MB molecules can block Aβ42 aggregation in vitro. Furthermore, our in vivo study using Drosophila AD model demonstrates that photoexcited MB is highly effective in suppressing synaptic toxicity, resulting in a reduced damage to the neuromuscular junction (NMJ), an enhanced locomotion, and decreased vacuole in the brain. The hindrance effect is attributed to Aβ42 oxidation by singlet oxygen (1O2) generated from photoexcited MB. Finally, we show that photoexcited MB possess a capability to disaggregate the pre-existing Aβ42 aggregates and reduce Aβ-induced cytotoxicity. Our work suggests that light illumination can provide an opportunity to boost the efficacies of MB toward photodynamic therapy of AD in future.
Introduction
Methylene blue (MB) is a member of the phenothiazine family and has been utilized in pharmacology for more than a century. Since it was applied to malaria in 18911, MB has been studied as a therapeutic agent for treating various diseases, proving its effectiveness against diseases such as methemoglobinemia and vasoplegic syndrome2–4. Owing to its ability to cross the blood-brain barrier (BBB) in addition to its high solubility in aqueous media and low toxicity, MB can target brain disorders in the central nervous system (CNS), such as ifosfamide-induced encephalopathy and Huntington’s disease, for which no effective cure exists yet5, 6. Recent studies reported that MB possesses a high potential for treating another common CNS disorder, Alzheimer’s disease (AD)7. MB was highlighted as a potential AD drug after TauRx Pharmaceuticals Ltd. presented successful results during phase II clinical trial performed with mild-to-moderate AD patients8. In addition, studies conducted with mouse AD models demonstrated that the treatment of MB not only reduces amyloid deposition but also improves behavior impairments including learning and memory defects by reducing amyloid plaque deposition in the brain9, 10. However, in spite of the encouraging results of the phase II clinical trial and in vivo studies performed with animal models, leuco-methylthioninium-bis(hydromethanesulfonate), a derivative of MB, failed to slow down the progression of AD in the phase III clinical trial, indicating a critical need for improved therapeutic options11.
AD is the most prevalent neurodegenerative disease among people aged over 65, and the number of patients living with AD is growing in a high rate12. AD causes a gradual and irreversible decline in the patient’s cognitive ability and memory, which is characterized by abnormal accumulation of β-amyloid (Aβ) peptides of 39–43 amino acids13. Decades of studies have revealed that Aβ aggregation is a central pathological hallmark of AD, but the original function of Aβ and the mechanism by which Aβ self-assembly induces neurotoxicity have not been clearly elucidated14. Previous studies have shown that the aggregation of Aβ into β-sheet-rich oligomers or fibrils is a key pathogenic event in the onset of AD15. In this regard, the prevention of the self-assembly of Aβ monomers into aggregate states has been deemed vital for the treatment of AD. Over the years, researchers have made numerous efforts to screen small molecules that can inhibit Aβ aggregation16. Recently, photosensitizing chemicals have been explored for light-induced inhibition of Aβ assembly17, 18. For example, photosensitized riboflavin and water-soluble porphyrin molecules significantly suppressed Aβ aggregation by oxidizing the peptides in the early stage of Aβ assembly17, 19. MB is also known for its excellent photosensitizing property and has been extensively used for photodynamic treatment of cancer cells and microbes due to its high quantum yield of 1O2 generation (ϕΔ ~ 0.5) under red light20, 21. Based on the photochemical property of MB, here we explore light-induced inhibition of Aβ42 aggregation by MB in vitro as well as the suppression of synaptic toxicity in Drosophila AD model under light illumination, as depicted in Fig. 1. Furthermore, we investigated the possibility of disintegrating pre-formed Aβ42 aggregates by photo-excited MB molecules. One of the remarkable merits of MB as a photo-induced therapeutic agent for treating neurodegenerative diseases is its ability to cross BBB, which is regarded as a major difficulty for the development of brain-targeting drugs22. Furthermore, MB can be excited upon the absorption of red light (>630 nm), of which tissue penetration is better than that of green or blue light23. The higher tissue penetration depth of red light is a potential advantage of MB over previously reported, light-driven anti-amyloid aggregation agents of metal oxides and organic compounds, the absorption maxima of which lie at much lower wavelengths17, 19, 24.
Figure 1.
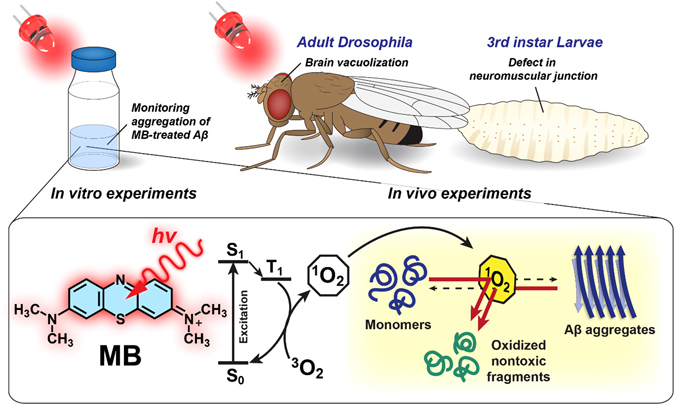
Schematic description of Aβ42 aggregation inhibition and the dissociation of pre-formed aggregates by photo-excited MB. The in vitro and in vivo experiments performed with the Drosophila AD model were conducted under the illumination of red LED light. The binding interaction of MB to Aβ42 aggregates and the photo-oxidation of the peptides induce disruption in the structural conformation, thereby blocking (or reversing) the progress of aggregation.
The delivery of light into the brain tissue through the skull has been a major obstacle for the application of light in neuroscience and neuroengineering fields. Recent progresses in optogenetics, a technology to control a specific neural activity in the brain circuit using light25, facilitate the delivery of light to the target brain areas much feasible. To activate (or silence) a specific neural circuit, the researches illuminate the confined area using light guides such as fiber optics26. The optic fibers allows the light to be transferred to the deep brain areas, retaining its power density; they can easily be implanted in the head of freely moving animals. Moreover, recently development of wireless, implantable microLED platforms provide a minimal restriction in the behavior27. We envision that these recent advances in the implantable optoelectronic devices may lower the existing barrier in future applications of phototherapies to the neurodegenerative disorders.
Results
MB inhibits Aβ42 aggregation under light
To observe the inhibitory effect of MB on Aβ42 aggregation under light, we performed multiple photochemical analyses. As shown in Fig. 2a, circular dichroism (CD) spectrum of Aβ42 incubated without MB under dark presented a positive and a negative band at 195 nm and 216 nm, respectively, which corresponds well to the typical profile of the β-sheet secondary structure. In the case of Aβ42 monomers (40 μM) incubated with MB (10 μM) under dark conditions, CD profile showed a negligible change, while the peaks completely disappeared in the presence of MB under light illumination. This result indicates that photosensitized MB molecules strongly affect the conversion of Aβ42 monomers into β-sheet rich aggregates. CD spectra of Aβ42 recorded at different times show that the secondary structure of the peptides are changed from random coil structure to β-sheet structure. (Figure S1) The diminished CD peaks monitored in Aβ42 treated with photo-excited MB implies that the unstructured Aβ42 monomers were remained after the incubation. Thioflavin T (ThT) fluorescence assay and atomic force microscope (AFM) analysis also support the photo-induced inhibitory effect of MB. MB-treated Aβ42 under dark conditions showed an insignificant decrease in ThT fluorescence compared to the native Aβ42 (Fig. 2b). While dense networks of mature fibrils were observed in the AFM images after incubation of Aβ42 monomers for 24 hours (Fig. 2d,e), numerous short Aβ42 fibrils were found when MB was treated (Fig. 2f). The short length of the fibrils is attributed to the accelerated rate of nucleation and fibril formation28. According to the previous study29, MB promotes the progress of Aβ42 fibrillation by stabilizing pre-nuclear intermediates that favor Aβ42 nucleation. In contrast, upon light illumination, substantially decreased ThT emission was monitored in the presence of MB, and only a limited number of aggregates were observed (Fig. 2g). The effect of photo-excited MB on the result of ThT assay was negligible according to the control experiment (Fig. S2). Native gel electrophoresis results revealed that the contents of Aβ42 monomers (4.5 kDa) increased significantly with photo-excited MB, implying that the considerable amount of monomers did not assemble into the aggregates of high molecular weight (Fig. 2c). Note that effect of MB on the reduction of Ag+ ion during the silver staining was negligible. The results obtained from sedimentation assay also demonstrate that the insoluble aggregates of Aβ42 were significantly reduced when the monomers were incubated with MB under light. (Figure S3) We further verified that the degree of photo-induced inhibition increased with the increasing MB concentration (Figs S4, S5). These photochemical analysis results clearly show that MB effectively suppressed the self-assembly of Aβ42 monomers into neurotoxic, β-sheet-rich aggregates under light. Further studies to investigate the effect of photo-excited MB on the oligomerization of Aβ42 or the equilibrium between various intermediates are needed. According to the literature, the distribution of Aβ42 oligomers at the certain time point can be assessed using photoinduced cross-linking of unmodified proteins (PICUP), which provides “snapshots” of the size distribution of various intermediates existing during the assembly30.
Figure 2.
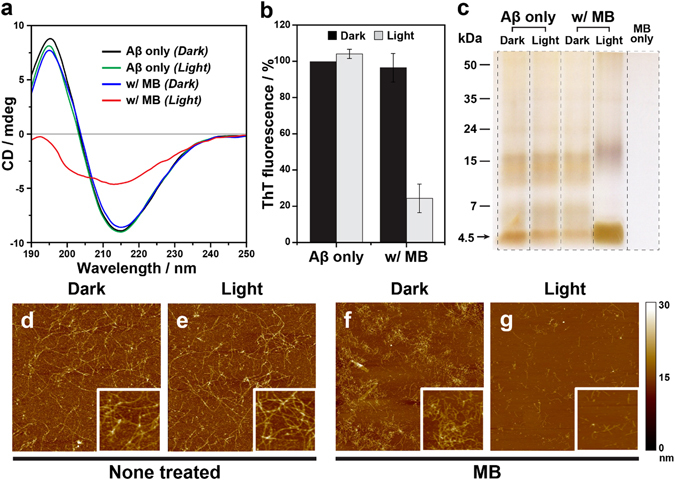
Light-induced suppression of Aβ42 self-assembly by MB. (a) CD spectra of Aβ42 aggregates incubated under various conditions. Two characteristic peaks in the CD spectrum presenting the β-sheet structure disappeared in the MB (10 μM)-treated Aβ42 (40 μM) under light illumination. (b) ThT fluorescence assay to measure the formation of amyloid fibrils. Significant decrease in ThT fluorescence indicates that the aggregation of Aβ42 monomers was substantially suppressed. (c) Silver-stained native gel electrophoresis showing that the monomeric contents was highly increased in MB-treated Aβ42 under light illumination. The arrow indicates a 4.5 kDa molecular mass that corresponds to the monomers of Aβ42. (d–g) Representative AFM images of Aβ42 incubated with or without MB under dark and light conditions. Only fragmented fibrils were observed in MB-treated Aβ42 (see insets enlarged from panels).
We further monitored the photo-induced Aβ42 aggregation inhibition effect by changing light wavelength, power density, and illumination time. We investigated the effect of the light wavelength using red (λmax = 630 nm), green (λmax = 520 nm), and blue (λmax = 450 nm) LEDs. The maximum degree of inhibition was observed under red light, and the effect decreased with LEDs that had shorter light wavelengths (Fig. S6a). We attribute the result to the unique optical property of MB; as shown in Fig. S6b, the absorbance spectrum of MB overlaps mostly with the emission spectrum of red LED, but MB shows only a weak absorption in the shorter wavelength region (<550 nm). Moreover, we verified that the hindrance effect of photo-excited MB correlates with the power density of the light source (Fig. S7). In addition, when we shortened the illumination time from 24 h to 15 min, we could observe almost a similar degree of the inhibition effect on Aβ42 aggregation (Fig. S8), which indicates that light illumination for a very brief period is sufficient to induce a full capacity of photo-excited MB against Aβ42 aggregation. These results show that the efficacy of light-induced inhibition of MB can be easily controlled by the modulation of the light illumination system.
Photo-excited MB suppresses Alzheimer’s Defects in Drosophila
We further investigated the in vivo efficacy of photosensitized inhibition of Aβ42 aggregation by MB using Drosophila AD model. Animal models of human diseases are vital for understanding pathogenesis and for developing potential therapeutic agents. Drosophila melanogaster is one of most popular animal models due to its well-studied anatomical features that enable quantitative analysis of various phenotypes31. The AD model of Drosophila achieved by the overexpression of Aβ42 shows several neurodegeneration phenotypes, such as morphological defects of neuromuscular junction (NMJ), locomotion defects, and brain vacuolization32, 33. Here, we tested the suppression of the Aβ42-induced phenotypes by feeding MB to the Drosophila AD model under red LED light. The postsynaptic overexpression of Aβ42 (Mhc > Aβ42) leads to a significant loss in the synaptic bouton number34. We found that the number of bouton was reduced by ~30% in Mhc > Aβ42 larvae compared to the control (Mhc-GAL4/+) according to the confocal images of muscle 6/7 of abdominal segment 3 (Fig. 3a,e). A negligible improvement was monitored with the treatment of MB under dark or light illumination without MB. (Fig. 3f,g) The defect, however, was significantly rescued in the NMJ of larvae treated with 100 nM MB as well as light. (Fig. 3h,i) Reduced NMJ synaptic connection in the larvae is known to cause defective movement of the muscles related to the crawling behavior of the larvae and brain vacuolization32, 35. Figure 3j,k show that the larvae with postsynaptic overexpression of Aβ42 exhibited the reduction in crawling distance by more than 30% compared to the Mhc-GAL4/+ control. In contrast, when MB was fed to Mhc > Aβ42 larvae and was illuminated by red light, a significant improvement in the crawling behavior was observed in a dose-dependent manner (Fig. 3l), which coincides with the NMJ analysis results. When cultured under dark conditions, however, we could monitor only slight increase in the locomotion even when a highly concentrated MB was treated. We attribute the mild recovery of the phenotype to the therapeutic activity of MB itself without the aid of light, as reported previously7, 9. Yet, the photo-excited MB showed notably higher efficacy in a low dosage than static MB. We also found that photo-excited MB can reduce Aβ42 toxicity-induced brain vacuolization in the fly’s brain. In the brain of 30-days-old flies, overexpression of Aβ42 (elav > Aβ42) showed severe brain vacuolization in the cell body region compared to the elav-GAL4 control (Figs 4a, and S9). In contrast, the number of vacuoles of photo-excited-MB-treated elav > Aβ42 was reduced compared to that of non-MB-treated elav > Aβ42 fly brains. The lost area of the photo-excited-MB-treated elav > Aβ42 fly brains were reduced by ~20% compared to non-treated flies (Fig. 4b). This significant restoration of Aβ42-induced toxicity in the elav > Aβ42 fly brains coincided with the results of the locomotion defect experiment. Taken together, these results suggest that photo-excited MB can suppress defects of NMJ morphology, locomotion defects, and Aβ42-induced toxicity in the Drosophila AD model.
Figure 3.
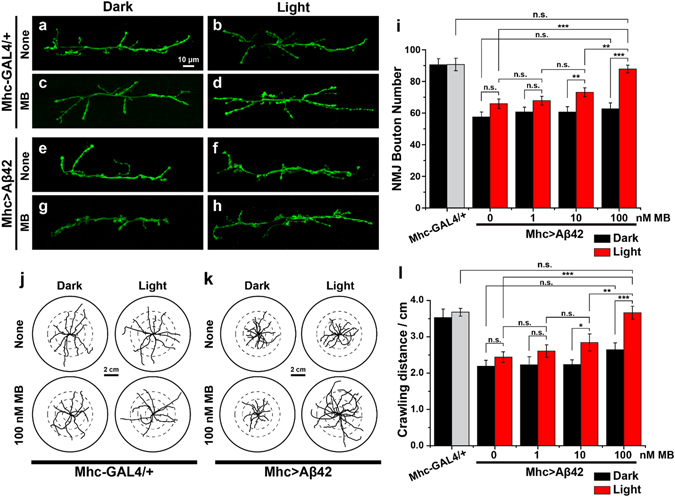
Photo-excited MB restores the phenotypes of Aβ42 toxicity in vivo model system. (a–h) The images of the NMJ boutons on muscle 6/7 of A3. Indicated genotype flies were incubated with or without 100 nM MB treatment under dark and red LED light. NMJ boutons were observed by HRP immunostaining. (a,c) NMJ of the Mhc-GAL4/+ control and (e,g) NMJ of the Mhc > Aβ42 under dark condition; (b,d) NMJ of the Mhc-GAL4/+ control and (f,h) NMJ of the Mhc > Aβ42 treated with or without 100 nM MB under red LED light. (h) NMJ morphology phenotype caused by Aβ42 overexpression is rescued by photoexcited-MB. Scale bar: 10 μm. (i) Effect of various concentration of MB on the total number of NMJ boutons on muscle 6/7 of A3. Indicated genotype flies were incubated with 0, 1, 10, and 100 nM concentration of MB under dark and red LED light conditions. (j,k) The diagram of the crawling path of the larvae on the plate. Diameter of inner-circles are 1.0 cm, 2.0 cm and 3.0 cm, respectively. (j) The crawling path of the Mhc-GAL4/+ control and (k) Mhc > Aβ42 with or without 100 nM MB treatment under dark and red LED light. The locomotor phenotype in Aβ42 overexpression is rescued by photoexcited MB. Scale bar: 2 cm (l) Quantification of crawled distance of larvae within 90 seconds. Indicated genotype flies were incubated with 0, 1, 10, and 100 nM concentration of MB under dark and red LED light conditions.
Figure 4.
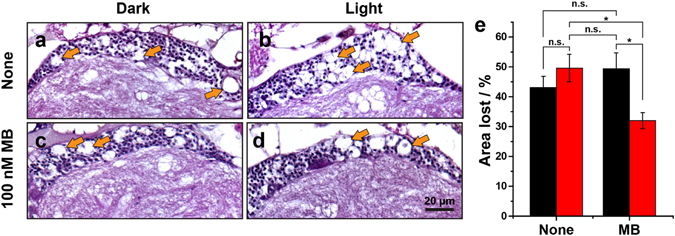
Photo-excited MB reduces the brain vacuolization in adult Drosophila. (a–d) Representative haematoxylin and eosin staining of adult head sections in AD model flies (elav > Aβ42) with or without 100 nM MB treatment under dark and red LED light conditions. Arrows indicate vacuole phenotypes in aged fly head. Scale bar: 20 μm. (e) Quantification of the vacuole size in adult head sections in AD model flies (elav > Aβ42) with or without MB treatment under dark and red LED light conditions. Percentage of the area lost in the cell body areas are shown as the averages s.e.m. (n = 5–7 hemispheres). The error bars represent means s.e.m. Experiments were performed at least three times. *P < 0.05, **P < 0.01, ***P < 0.001. n.s. not significant.
Photo-excited MB dissociate the pre-exisiting aggregates
While numerous studies have focused on the inhibition of the Aβ42 assembly pathway, recent studies have attempted to reverse the progress by dissociating pre-formed Aβ42 aggregates36–38. Previous studies have demonstrated that the clearance of pre-existing amyloid deposits could reverse AD pathology, including behavioral deficits, in transgenic mouse models36, 39. To examine the possibility of disassembling Aβ42 aggregates by photo-excited MB, we incubated pre-formed aggregates with MB under dark or light conditions and monitored the changes in ThT fluorescence, morphology, and cytotoxicity. For the experiment, Aβ42 monomers were incubated for 48 h to produce fully-grown, fibrillar aggregates. According to the ThT assay result (Fig. 5a), ThT fluorescence was drastically diminished (~50%) when photo-excited MB was applied, while MB under dark conditions caused a negligible decrease. The corresponding AFM images also confirmed that the density of the fibril networks decreased in the presence of photo-excited MB (Fig. 5f). Both the results of the ThT assay and the AFM images clearly indicate that light triggers disassembly of existing Aβ42 aggregates when incubated with MB. Additional researches such as size-exclusion chromatography (SEC) and in vivo studies using the brain of mouse AD models are required to further investigate the efficacy of photo-excited MB against pre-formed aggregates.
Figure 5.
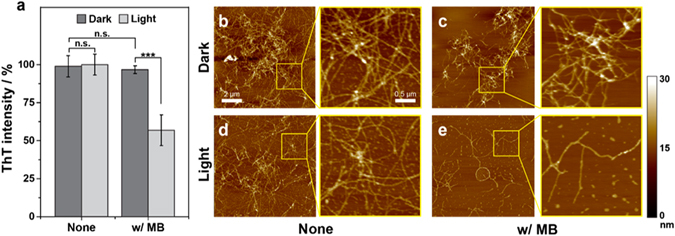
Disassembly of pre-formed Aβ42 aggregates by photoexcited MB (10 μM). The fully-grown Aβ42 aggregates were formed by the incubation of monomeric Aβ42 for 48 hrs at 30 °C. (a) ThT fluorescence of Aβ42 aggregates after the 6 hrs of treatment with MB under dark and light conditions. (b–e) AFM images of fully-grown Aβ42 aggregates after 6 hrs of incubation in the absence or presence of 10 μM MB under dark and light conditions. The fully-grown aggregates were produced by incubation of Aβ42 monomers for 42 h at 30 °C.
Discussion
We attribute the light-induced hindrance effect of MB to its high binding affinity to Aβ42 and oxidative stress generated from photochemical reactions. To investigate the interaction between MB and Aβ42, we monitored the change of MB fluorescence in the presence of Aβ42. We observed enhanced fluorescence of MB with a blue shift with an increasing concentration of Aβ42 peptides (Fig. 6a). According to the literature40, the fluorescence enhancement can be attributed to the reduction in the non-radiative decay of photo-excited MB due to the suppressed rotation and vibration upon binding to other chemicals. The blue shift of the fluorescence of fluorophore occurs when a dye exists in a more non-polar environment because the energy difference between the excited and ground state increases41, 42. This implies that MB may bind to the hydrophobic C terminus of Aβ42 monomers43. Further studies, including computational simulations, are required to predict the exact binding site of MB to Aβ42. The binding constant (K d) of MB to Aβ42, estimated from the changes in the fluorescence intensity at 670 nm for various MB/Aβ42 ratios, was 48.7 ± 3.6 μM (Fig. 6b). This is comparable to the K d of curcumin, a well-known small molecular inhibitor of Aβ42 aggregation (K d = 46 μM)44.
Figure 6.
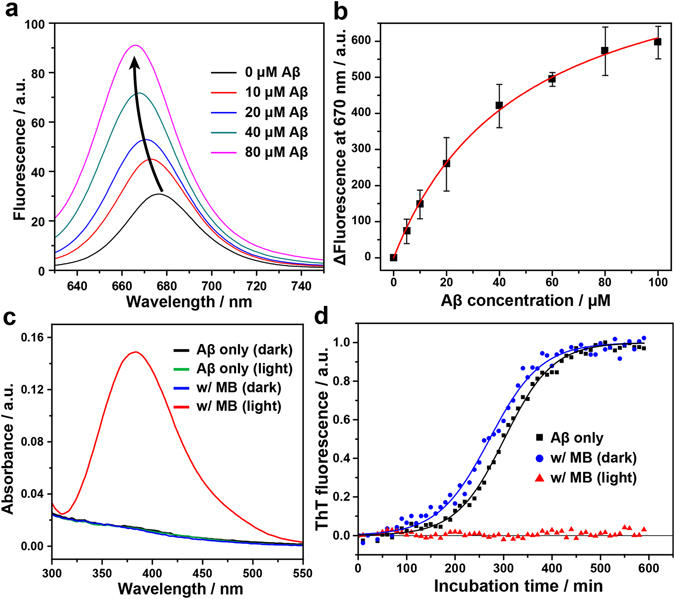
Photo-excited MB inhibits the Aβ42 aggregation by means of the binding affinity of MB and the photo-oxidation of Aβ42. (a) Change in fluorescence spectra of 0.2 μM MB upon addition of various concentrations of the Aβ42 monomer. (b) Fluorescence binding affinity assay between Aβ42 and MB. The fluorescence of MB (0.2 μM) at 670 nm was measured with increasing concentration of Aβ42 from 0 to 100 μM. Binding constant Kd was derived from the fitted curve. (c) DNPH assay to monitor a carbonyl content in the Aβ42 peptide. DNPH reacts with the carbonyl groups in oxidized peptides, resulting in the formation of a DNP hydrazone product, which shows an absorption maximum of near 380 nm. (d) The kinetics of Aβ42 fibril formation monitored at 30 °C by ThT fluorescence in the absence and presence of MB under dark or light conditions. For the light condition, the MB-treated Aβ42 samples were irradiated with LED light for 30 min at 4 °C before the measurement. Each point is an average of the fluorescence signal of at least four wells containing the same solutions. Lines indicate fits of a sigmoidal growth curve.
The capacity of MB as a light-driven 1O2 generator has been widely utilized in a number of studies45, 46. Under the irradiation of red light (λmax = 666 nm), MB monomers produce 1O2 through the type II photochemical pathway, in which the energy from triplet state MB (i.e., 3MB+) is transferred to molecular oxygen20, and the generated 1O2 oxidizes organic compounds nearby47. To explore the possible photo-oxidation of Aβ42 by MB, we conducted 2,4-dinitrophenylhydrazine (DNPH) assay, which is one of the most commonly used methods to assess the amount of carbonyl groups formed by oxidative stress48–50. As shown in Fig. 6c, we observed a new absorption band at 380 nm only when Aβ42 was incubated with MB under light illumination, indicating that Aβ42 peptides were oxidized by photo-excited MB. In addition, our ThT assay and CD analysis revealed that the hindrance effect of photo-excited MB on Aβ42 aggregation decreases significantly under anaerobic conditions (Figs S10, S11). These results indicate that the generation of 1O2 is a major cause of light-induced inhibition of Aβ42 assembly by MB. We suppose that the generated oxidative stress induces sulfoxidation of Aβ42’s methionine, which is known to be a most readily oxidizable residue51. According to the literature52, the oxidation of Met35 causes structural alteration in the hydrophobic C-terminus of Aβ42 and impedes the association and self-assembly between monomers. The oxidation of Aβ42 by photo-excited MB was further studied with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Figure S12 shows that the mass of the MB-treated Aβ42 increases when the light was irradiated. We attribute a +14 Da-modification to the oxidation of His13 or His 14 residues, which generates a dehydro-2-imidazolone derivative53. According to the literature, the further increases in the mass of Aβ42 by 16 Da are resulted from the oxidation of Met35 and Tyr1019. We monitored how static and photo-excited MB affect the aggregation kinetics of Aβ42 differentially using ThT fluorescence assay. For the experiment, we pre-incubated MB-treated Aβ42 solution for 30 min at 4 °C under dark or light conditions before the measurement was performed at 30 °C. According to our results (Fig. 6d), while static MB slightly affected the nucleation of Aβ42 peptides with a decreased lag time from 202.0 min to 164.7 min, photo-excited MB completely blocked the progression of Aβ42 aggregation in the early stage. We attribute the hindered aggregation to the oxidation of Aβ42 monomers by localized 1O2 generated during light illumination. The negligible inhibitory activity of pre-illuminated MB supports that the hindrance effect of photo-excited MB was derived from the photodynamic reaction of MB under light. (Figure S13 ) For the therapeutic applications, it is vital to minimize the undesirable oxidative damages to the surrounding by limiting the 1O2 generation sites. Future studies to enhance the Aβ-specific targeting of photosensitizers are essential for the phototherapy of amyloidosis.
In summary, we demonstrated that photo-excited MB molecules exhibit a high degree of inhibition against β-amyloidosis in vitro and in vivo. Our kinetic study revealed that, while static MB accelerates Aβ42 aggregation, MB under illumination thoroughly blocks the progress in the early stage by oxidizing the peptide. We examined the in vivo effect of light-induced inhibition of aggregation by MB using the Drosophila AD model. At the same dose, while static MB exhibited mild recovery in the locomotion defect, photoexcited MB almost fully rescued the AD phenotype in in vivo experiments performed with the Drosophila AD model; the loss in synaptic bouton, locomotion defect, and vacuolization in the brain were significantly reduced with the MB treatment under red LED light, indicating that photoexcited MB successfully prevented in vivo toxicity resulting from β-amyloidosis. We further verified that MB under illumination is also able to dissociate pre-existing aggregates and to suppress resulting cytotoxicity, while MB under dark conditions did not affect the aggregation state. Based on these results, we suggest that shining light on MB can be a breakthrough to enhance its efficacy beyond the conventional limit. While the recent report on clinical trials performed with MB was not satisfactory, this study hints at a new opportunity of inhibiting β-amyloidosis based on the photosensitizing property of MB, a therapeutic chemical that has been used for more than a century.
Methods
Preparation of Aβ42 Peptides
Recombinant β-amyloid (1–42) was purchased from rPeptide Co. Monomeric Aβ42 solution was prepared by dissolving the peptide in hexafluoro-2-propanol (HFIP) followed by sonication for 30 min and keeping it overnight at room temperature. The solution was aliquoted into 1.5 ml protein LoBind tubes (Eppendorf) and vacuum-dried for 2~3 h. The tubes were then stored at −20 °C for further use.
Light-induced inhibition of Aβ42 self-assembly
Aβ42 aliquot was dissolved in a 30 μL buffer comprised of CH3CN (144 μM), Na2CO3 (144 μM) and NaOH (8.5 mM) and was briefly sonicated for 1 min. The solution was diluted with 270 μL of phosphate buffer (8.5 mM) containing NaCl (8.5 mM), Na2CO3 (14 μM), NaOH (0.85 mM), and 6.0% acetonitrile (final pH 8.0) to yield a final concentration of 40 μM Aβ42 monomer. For the in vitro experiments, the solution was incubated in the absence or presence of methylene blue (MB) at 30 °C for 24 h under dark or light conditions. The power densities of the light sources (LEDs) were measured with ILT1400-A photometer (International Light Tech.). MB and all other chemicals were purchase from Sigma Aldrich.
Disassembly of pre-formed fibrils
The pre-formed Aβ42 aggregates were obtained by the incubation of the Aβ42 monomers (40 μM) for 48 h at 30 °C. Then, 10 μM MB was added to the solution containing aggregates, and further incubated for an additional 6 h under dark or light conditions at 30 °C.
Circular dichrosim (CD) analysis
After the incubation of 40 μM Aβ42 under various conditions at 30 °C for 24 h, far-UV (190–260 nm) CD spectra were measured using a JASCO J-810 (Jasco) spectropolarimeter at 20 °C.
Thioflavin T (ThT) assay
The 20 μl of incubated Aβ42 samples were mixed into 480 μl of ThT solution (20 μM) in the phosphate buffer. The fluorescence of ThT was measured at 440 nm (ex) and 485 nm (em) using RF-5301PC spectrofluorophotometer (Shimadzu Inc.). For the real-time monitor of Aβ42 aggregation, monomeric Aβ42 (5 μM) in the absence or presence of 2 μM MB in a glass vial was incubated at 4 °C for 30 min under dark or light conditions, prior to the measurement. Then, 90 μl of samples were moved to a 96-well plate, and 10 μl of ThT solution (final concentration, 20 μM) was added to each well. The fluorescence of ThT was monitored every 10 min using a 405 nm excitation filter and a 486 nm emission filter of the Victor 3 microplate reader (PerkinElmer Inc.). The temperature of the well plate was maintained at 30 °C during the measurement. Each experimental point was an average of the fluorescence signal of at least four wells containing the same solution.
Atomic Force Microscopy (AFM)
For the AFM measurement, 5 μl of Aβ42 sample solutions were deposited onto a cleaved mica substrate for 10 min and were rinsed several times with DI water to remove remaining salts and unbound peptides. After they were fully dried, AFM images were acquired in a tapping mode with an NCHR silicon cantilever (Nanosensors Inc.) using a Multimode AFM instrument equipped with a Nanoscope III controller and “E”-type scanner (Digital Instruments Inc.).
Native gel electrophoresis and silver staining
The Aβ42 solutions were transferred to a loading buffer containing 50 mM Tris HCl, pH 6.8, 1% SDS, 1% β-mercaptoethanol, 10% (v/v) glycerol, and 0.01% bromophenol blue. The samples were loaded onto 10% Gradi-Gel™ II gradient gel (Elpis Biotech) and peptide distribution was visualized by silver staining. Protein electrophoresis kit were purchased from Bio-rad.
Sedimentation assay
The sedimentation assay was performed according to the previous study54. Briefly, Aβ42 monomers (40 μM) were centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was collected and the optical density (OD) at 214 nm was measured using V/650 spectrophotometer (Jasco Inc.). The supernatant was then moved to the glass vials and MB (2 μM) was introduced to the vials. The samples were incubated under dark or light conditions for 24 h at 30 °C. Then the samples were ultracentrifuged at 100,000 g for 10 min at 4 °C. The OD214 of collected supernatant was measured. The aggregation was derived from the difference between the OD214 before and after the incubation as described by Yoshiike et al.
DNPH assay
The DNPH assay was performed according to Dalle-Donne et al 48. Aβ42 solutions (40 μM) with or without 10 μM MB were incubated under dark or light conditions for 24 h at 30 °C. The samples were contained in the glass vials and illuminated with red LED (λmax = 630 nm, 3 mW/cm2) The samples were then precipitated with a trichloroacetic acid (TCA, 20% final concentration) solution for 10 min in an ice bath and were then collected by centrifuge. Then, 2 M HCl containing 10 mM DNPH (2 M HCl only for reagent blanks) was added and incubated for 1 h under room temperature. After the precipitation with 20% TCA and centrifugation, the remaining pellets were washed three times with 1 ml ethanol-ehtyl acetate (1:1, v/v) solution. The samples were then dissolved in 6 M guanidine hydrochloride solution (in 20 mM potassium phosphate, pH 2.3 adjusted with TCA) and were incubated at 37 °C for 15 min. The absorbance spectrums of the samples were measured using V/650 spectrophotometer (Jasco Inc.).
Mass spectrometry
Aβ42 monomer (40 μM) was treated with MB (1 μM) and irradiated for 0, 0.5, 1, 2, and 3 h at 4 °C. MALDI-TOF spectra was recorded with Bruker autoflex III (Bruker Daltonics) using sinapinic acid as a matrix. 1.5 μl of samples mixed with matrix (1:1) was spotted on the plate.
Fly strains
The UAS-Aβ42 was provided by Dr. K Iijima-Ando55, the Mhc-GAL4 driver was provided by Dr. T Littleton; and the elav-GAL4 driver was provided by Bloomington Stock Center. For the pharmacological approach, either MB or PBS was added to fly food at 1, 10, 100, 1000, and 10000 nM concentration. All flies were reared at 25 °C.
Brain vacuole analysis
For analysis of brain vacuolization, 30-days-aged fly heads were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) and were processed for paraffin sections as described32. Embedded paraffin was cut into 4 um-thick coronal sections. These sections were stained with hematoxylin and eosin (Vector laboratories). For quantification of vacuole phenotypes in the fly head section, we measured the area of the vacuoles in the cell body region using ImageJ. Five to ten hemispheres were analyzed for each genotype. For the pharmacological approach of the brain vacuole analysis, either MB or PBS was added to fly food at 100 nM concentration.
Immunohistochemistry
Third instar larvae were dissected in PBS, fixed in 4% formaldehyde (Ted Pella) in PBS for about 15 minutes and washed 3x in 0.1% Triton X-100 in PBS. FITC-conjugated anti-HRP (Jackson ImmunoResearch Laboratories) were used at 1:100 and were incubated for about 1.5 hours at room temperature. Laval samples were mounted in SlowFade Antifade kit (Invitrogen). Confocal images were collected from OLYMPUS FLUOVIEW FV-1000 confocal microscopes SP2 equipped with 40x UPlans FL N inverted oil lens. OLYMPUS Fluoview software was used to capture, process, and analyze the images. Analysis of the NMJ was performed essentially as described34.
Crawling assay
Wandering 3rd instar larvae were briefly washed with PBS to remove residual fly food. Larvae were dried for a short time on clean filter paper and were placed on a 2% agar-grape juice coated petri dish. Each genotype was allowed to crawl freely for 90 sec. To quantify the crawling distance, we drew lines to track crawled larvae and measured the distance using Image J software. Approximately 10–20 animals were tested for each genotype.
Electronic supplementary material
Acknowledgements
This study was supported by grants from the National Research Foundation via the Creative Research Initiative Center (NRF-2015R1A3A2066191) and National Research Council of Science and Technology (CRC-15-04-KIST), Republic of Korea.
Author Contributions
B.L. and C.P. conceived the research. K.Y. and C.P. supervised the research. B.L. and Y.S. designed and performed experiments, and analyzed data. Y.S. and K.Y. conducted in vivo experiments with Drosophila AD models. Y.C. conducted in vitro cellular experiment and peptide preparation. B.L., Y.S., K.Y., and C.P. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Byung Il Lee and Yoon Seok Suh contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07581-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahu A, Choi WI, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34:6239–6248. doi: 10.1016/j.biomaterials.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 3.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and Molecular Actions of Methylene Blue in the Nervous System. Med Res Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wainwright M, Crossley KB. Methylene Blue - a therapeutic dye for all seasons? J Chemotherapy. 2002;14:431–443. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 5.Kupfer A, Aeschlimann C, Wermuth B, Cerny T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994;343:763–764. doi: 10.1016/S0140-6736(94)91839-2. [DOI] [PubMed] [Google Scholar]
- 6.Sontag EM, et al. Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models. J Neurosci. 2012;32:11109–11119. doi: 10.1523/JNEUROSCI.0895-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochemical pharmacology. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Wischik CM, Bentham P, Wischik DJ, Seng KM. O3-04-07: Tau aggregation inhibitor (TAI) therapy with rember™ arrests disease progression in mild and moderate Alzheimer’s disease over 50 weeks. Alzheimer’s & Dementia. 2008;4:T167. doi: 10.1016/j.jalz.2008.05.438. [DOI] [Google Scholar]
- 9.Paban V, et al. Therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in a transgenic mouse model. Neuropharmacology. 2014;76(Pt A):68–79. doi: 10.1016/j.neuropharm.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Medina DX, Caccamo A, Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthier S, et al. Phase 3 Trial of the Tau Aggregation Inhibitor Leuco-Methylthioninium-Bis (Hydromethanesulfonate) (LMTM) in Mild to Moderate Alzheimer’s Disease. Alzheimer’s & Dementia. 2016;12:P351–P352. doi: 10.1016/j.jalz.2016.06.650. [DOI] [Google Scholar]
- 12.Alzheimer’s A. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 14.Hamley IW. The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chemical reviews. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 15.Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 16.Nie Q, Du XG, Geng MY. Small molecule inhibitors of amyloid beta peptide aggregation as a potential therapeutic strategy for Alzheimer’s disease. Acta Pharmacol Sin. 2011;32:545–551. doi: 10.1038/aps.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BI, et al. Photoexcited Porphyrins as a Strong Suppressor of beta-Amyloid Aggregation and Synaptic Toxicity. Angewandte Chemie. 2015;54:11472–11476. doi: 10.1002/anie.201504310. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Lee BI, Park CB. Photo-induced inhibition of Alzheimer’s beta-amyloid aggregation in vitro by rose bengal. Biomaterials. 2015;38:43–49. doi: 10.1016/j.biomaterials.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi A, et al. Attenuation of the aggregation and neurotoxicity of amyloid-beta peptides by catalytic photooxygenation. Angewandte Chemie. 2014;53:1382–1385. doi: 10.1002/anie.201308001. [DOI] [PubMed] [Google Scholar]
- 20.Tardivo JP, et al. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn Ther. 2005;2:175–191. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- 21.Wainwright M, McLean A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dyes and Pigments. 2017;136:590–600. doi: 10.1016/j.dyepig.2016.09.015. [DOI] [Google Scholar]
- 22.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–61. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed MH, Keyes TE, Byrne JA. The photocatalytic inactivation effect of Ag-TiO2 on beta-amyloid peptide (1-42) J Photoch Photobio A. 2013;254:1–11. doi: 10.1016/j.jphotochem.2012.12.019. [DOI] [Google Scholar]
- 25.Deisseroth K. Optogenetics. Nature methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dugue GP, Akemann W, Knopfel T. A comprehensive concept of optogenetics. Prog Brain Res. 2012;196:1–28. doi: 10.1016/B978-0-444-59426-6.00001-X. [DOI] [PubMed] [Google Scholar]
- 27.Rossi MA, et al. A wirelessly controlled implantable LED system for deep brain optogenetic stimulation. Front Integr Neurosci. 2015;9:8. doi: 10.3389/fnint.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomakin A, Chung DS, Benedek GB, Kirschner DA, Teplow DB. On the nucleation and growth of amyloid beta-protein fibrils: detection of nuclei and quantitation of rate constants. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1125–1129. doi: 10.1073/pnas.93.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Necula M, et al. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry. 2007;46:8850–8860. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- 30.Bitan G, Lomakin A, Teplow DB. Amyloid beta-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. The Journal of biological chemistry. 2001;276:35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 31.Prussing K, Voigt A, Schulz JB. Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol Neurodegener. 2013;8:35. doi: 10.1186/1750-1326-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iijima K, et al. Abeta42 mutants with different aggregation profiles induce distinct pathologies in Drosophila. PloS one. 2008;3:e1703. doi: 10.1371/journal.pone.0001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annual review of pathology. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Wang JW, Yu W, Lu B. Phospho-dependent ubiquitination and degradation of PAR-1 regulates synaptic morphology and tau-mediated Abeta toxicity in Drosophila. Nature communications. 2012;3:1312. doi: 10.1038/ncomms2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mhatre SD, et al. Synaptic abnormalities in a Drosophila model of Alzheimer’s disease. Disease models & mechanisms. 2014;7:373–385. doi: 10.1242/dmm.012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HY, et al. EPPS rescues hippocampus-dependent cognitive deficits in APP/PS1 mice by disaggregation of amyloid-beta oligomers and plaques. Nature communications. 2015;6:8997. doi: 10.1038/ncomms9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, et al. Tanshinones Inhibit Amyloid Aggregation by Amyloid-beta Peptide, Disaggregate Amyloid Fibrils, and Protect Cultured Cells. ACS chemical neuroscience. 2013;4:1004–1015. doi: 10.1021/cn400051e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood A, et al. Disassembly of preformed amyloid beta fibrils by small organofluorine molecules. Bioorg Med Chem Lett. 2011;21:2044–2047. doi: 10.1016/j.bmcl.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busche MA, et al. Decreased amyloid-[beta] and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci. 2015;18:1725–1727. doi: 10.1038/nn.4163. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, et al. Inhibition of beta-amyloid peptide aggregation by multifunctional carbazole-based fluorophores. Angewandte Chemie. 2012;51:1804–1810. doi: 10.1002/anie.201104150. [DOI] [PubMed] [Google Scholar]
- 41.Cooper, A. & Royal Society of Chemistry (Great Britain). Biophysical chemistry. 2nd edn, (RSC Pub., 2011).
- 42.Sundd M, Robertson AD. Illuminating proteins with Aladan’s lamp. Nat Struct Biol. 2002;9:500–501. doi: 10.1038/nsb0702-500. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed M, et al. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nature structural & molecular biology. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mourtas S, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-beta 1-42 peptide. Biomaterials. 2011;32:1635–1645. doi: 10.1016/j.biomaterials.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Junqueira MV, et al. Functional Polymeric Systems as Delivery Vehicles for Methylene Blue in Photodynamic Therapy. Langmuir: the ACS journal of surfaces and colloids. 2016;32:19–27. doi: 10.1021/acs.langmuir.5b02039. [DOI] [PubMed] [Google Scholar]
- 46.He X, Wu X, Wang K, Shi B, Hai L. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials. 2009;30:5601–5609. doi: 10.1016/j.biomaterials.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Bulina ME, et al. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat Protoc. 2006;1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- 48.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 49.Mesquita CS, et al. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Analytical biochemistry. 2014;458:69–71. doi: 10.1016/j.ab.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Q, Zhu X, Sayre LM. Chemical nature of stochastic generation of protein-based carbonyls: metal-catalyzed oxidation versus modification by products of lipid oxidation. Chem Res Toxicol. 2007;20:129–139. doi: 10.1021/tx600270f. [DOI] [PubMed] [Google Scholar]
- 51.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334:1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou L, et al. Solution NMR studies of the A beta(1-40) and A beta(1-42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. Journal of the American Chemical Society. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 53.Pattison DI, Rahmanto AS, Davies MJ. Photo-oxidation of proteins. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2012;11:38–53. doi: 10.1039/C1PP05164D. [DOI] [PubMed] [Google Scholar]
- 54.Yoshiike Y, et al. New insights on how metals disrupt amyloid beta-aggregation and their effects on amyloid-beta cytotoxicity. The Journal of biological chemistry. 2001;276:32293–32299. doi: 10.1074/jbc.M010706200. [DOI] [PubMed] [Google Scholar]
- 55.Iijima K, Gatt A, Iijima-Ando K. Tau Ser262 phosphorylation is critical for A beta 42-induced tau toxicity in a transgenic Drosophila model of Alzheimer’s disease. Hum Mol Genet. 2010;19:2947–2957. doi: 10.1093/hmg/ddq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


