Abstract
Background
CT-P10 is a biosimilar candidate of innovator rituximab (RTX) that demonstrated a comparable clinical profile to RTX in a phase I randomized controlled trial (RCT) in rheumatoid arthritis (RA) (ClinicalTrials.gov identifier: NCT01534884).
Objective
This open-label extension (OLE) study (NCT01873443) compared the efficacy and safety of CT-P10 in patients with RA who received CT-P10 from the outset (i.e., from the start of the RCT and also in the OLE; ‘maintenance group’) with those who received RTX during the RCT and switched to CT-P10 during the OLE (‘switch group’).
Methods
Patients who completed the RCT were recruited. Based on the Disease Activity Score using 28 joints (DAS28) and predefined safety criteria, patients could receive up to two courses of CT-P10 during the OLE. Efficacy [DAS28 and European League Against Rheumatism (EULAR) response], safety and immunogenicity were assessed.
Results
Eighty-seven patients were enrolled; 58 and 29 had previously received CT-P10 or RTX, respectively, in the RCT. Of these, 38 (65.5%) and 20 (69.0%) were treated with CT-P10 in the OLE and therefore comprised the maintenance and switch groups, respectively. The mean change in DAS28-erythrocyte sedimentation rate (ESR) from baseline (week 0 of RCT) at week 24 of the first OLE treatment course in the maintenance and switch groups was −2.7 and −2.4, respectively. The proportion of patients with good/moderate EULAR responses was also comparable between groups. Antidrug antibodies were detected in 13.2 and 15.0% of patients in the maintenance and switch groups, respectively, at week 24 of the first OLE course. CT-P10 treatment was well-tolerated when administered for up to 2 years or after switching from RTX.
Conclusion
In this study population, comparable efficacy and safety profiles were observed in patients who switched from RTX to CT-P10 and those maintained on CT-P10 throughout treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s40259-017-0233-6) contains supplementary material, which is available to authorized users.
Key Points
| Similar disease activity responses were observed between patients with rheumatoid arthritis who continued treatment with CT-P10, a rituximab biosimilar, and those who switched from innovator rituximab to CT-P10. |
| Switching from innovator rituximab to CT-P10 was not associated with any safety issues. |
| Long-term treatment with CT-P10 was efficacious and well-tolerated. |
Introduction
Rituximab is a chimeric monoclonal antibody that targets the CD20 protein primarily found on B lymphocytes. Used in the treatment of almost all B cell-related malignancies and autoimmune diseases, rituximab works by depleting CD20+ B cells in the peripheral blood and bone marrow [1, 2]. Randomized controlled trials (RCTs) have shown that ‘innovator rituximab’ (hereafter abbreviated as RTX) is an effective treatment for active rheumatoid arthritis (RA) when used in combination with methotrexate [3]. This combination is recommended for RA patients with an inadequate response or intolerance to anti-tumor necrosis factor (TNF) agents [4–6]. The development of biologic drugs such as rituximab has expanded the therapeutic options for RA and markedly improved patient outcomes [7].
As patents for a number of biologics have reached or approach expiration, interest in the development of biosimilar drugs has increased. The term ‘biosimilar’ refers to a follow-on drug with proven physiochemical, biological, immunochemical, and clinical similarity to its ‘innovator’ biologic [8]. Before regulatory approval of a biosimilar is considered, a rigorous comparability testing program must be undertaken to prove similarity with its innovator drug [9, 10]. Even after approval for clinical use from regulatory bodies, some concerns about widespread use of biosimilars still exist. A particularly common question is whether patients currently treated with an innovator biologic can be switched to a less expensive biosimilar without affecting treatment efficacy or safety.
CT-P10 is a biosimilar of RTX and shares an identical primary structure. CT-P10 and RTX are also highly similar with regard to their higher-order structures, post-translational modifications, and biological activities [11]. A phase I RCT in RA patients (ClinicalTrial.gov identifier: NCT01534884) demonstrated the pharmacokinetic equivalence of CT-P10 and RTX over 24 weeks of treatment [11]. The RCT also showed that these drugs had comparable efficacy and safety when patients were followed for up to 72 weeks [12]. CT-P10 is the first rituximab biosimilar to receive market authorization from the European Medicines Agency (EMA) and is approved for use in all indications held by RTX [13].
Here we report the results of a multicenter, single-arm, open-label extension (OLE) study that enrolled patients with RA who had completed the phase I RCT described earlier. In this OLE study, all patients were assessed for eligibility for retreatment with CT-P10 and followed for up to 56 additional weeks. The key objective of the study was to compare the efficacy and safety of CT-P10 in patients with RA who received CT-P10 from the outset (i.e., from the start of the RCT and also in the OLE) with those who received RTX during the RCT and switched to CT-P10 during the OLE. In addition, the longer-term efficacy and safety of CT-P10 was investigated in RA patients in the maintenance group who were treated with the biosimilar for up to 104 weeks.
Methods
Patients
As previously described [11], the preceding phase I RCT recruited patients with active RA aged 18–75 years who had been diagnosed according to the revised 1987 American College of Rheumatology classification for at least 6 months and had shown an inadequate response or intolerance to anti-TNF therapy. Additional inclusion criteria for this OLE study included disease improvement during the last course of treatment in the RCT [according to Disease Activity Score using 28 joint counts (DAS28) combined with erythrocyte sedimentation rate (DAS28-ESR) or serum C-reactive protein (DAS28-CRP)], and completion of all scheduled visits in that study with no major protocol violations. Exclusion criteria included any medical problem that meant continued participation could be detrimental to the patient’s health according to the investigator’s opinion.
Study Design and Treatment
This was a multicenter, single-arm, phase I OLE study (ClinicalTrials.gov identifier: NCT01873443). In the preceding phase I RCT, patients with active RA were randomized 2:1 to receive CT-P10 (CELLTRION, Inc., Incheon, Republic of Korea) or RTX (Roche, Welwyn Garden City, UK) alongside continued methotrexate therapy. Patients received one or two courses of treatment during the RCT, with each course comprising two intravenous infusions of 1000 mg of CT-P10 or RTX separated by a 2-week interval [11, 12].
Patients were screened for the OLE study within 8 weeks of their last visit following the first or second course of treatment in the RCT (up to week 48 or 72 of the RCT, respectively). The OLE study had a maximum duration of 56 weeks and the maximum combined duration of both studies was 104 weeks. Eligible patients entered the monitoring period of the OLE study in which their eligibility for CT-P10 infusion was assessed every 8 weeks ± 14 days [Electronic Supplementary Material (ESM) Online Resource 1]. Patients were eligible to receive a course of CT-P10 treatment during the OLE if (1) they had responded to the previous course of treatment but disease activity had then worsened during the monitoring period (DAS28-ESR or DAS28-CRP response worsened by ≥20% vs. the best response during weeks 16–24 of the previous treatment course); and (2) their B cell or immunoglobulin (Ig)M levels were equal to or higher than the lower limit of normal or ≥50% of the baseline level (week 0 of the RCT). Patients received up to two courses of treatment during the OLE, each consisting of two intravenous infusions of CT-P10 (1000 mg) 2 weeks apart. As in the preceding RCT, methotrexate (10–25 mg/week orally or parenterally) and folic acid (≥5 mg/week orally) were co-administered throughout the study and patients received methylprednisolone, an antipyretic, and an antihistamine 30–60 min before each infusion. CT-P10 was not administered after week 80. All patients had an end-of-study visit between weeks 96 and 104 unless they withdrew from the study earlier. In the OLE, patients who received CT-P10 during the RCT and OLE were termed the ‘maintenance’ group, while those who received RTX during the preceding RCT and then CT-P10 during the OLE were termed the ‘switch’ group.
All patients provided written informed consent to participate in the OLE study which was performed according to the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice (ICH) guidelines. Study protocols and consent forms were approved by the relevant independent ethics committees.
Study Assessments and Endpoints
Efficacy was assessed before the first treatment and at 8, 16, and 24 weeks after the first infusion in the OLE. Efficacy endpoints included the mean change in disease activity measured by DAS28-ESR and DAS28-CRP compared with baseline (week 0 of the preceding RCT) [14] and the proportion of patients by response status, defined according to European League Against Rheumatism (EULAR) response criteria based on DAS28-ESR and DAS28-CRP [15]. Adverse events, including those of special interest such as infusion-related reactions, malignancies and infections, were monitored and recorded. Blood samples were analyzed for the presence of antidrug antibodies (ADAs) using an electrochemiluminescent immunoassay method (MSD, Rockville, MD, USA), and neutralizing antibodies (NAbs) using a complement-dependent cytotoxicity assay, as described previously [11]. ADAs and NAbs were analyzed at week 24 of the OLE study.
Statistical Analyses
Descriptive statistics were used to analyze efficacy and safety data in the maintenance and switch groups. Efficacy parameters were also compared between groups using cumulative logistic regression and t tests. The efficacy analysis population consisted of all patients who received at least one dose of CT-P10 and provided data for at least one efficacy endpoint in the OLE study. The safety population included all patients who received at least one dose of study treatment in the OLE.
Results
Patients
A total of 87 patients who had completed up to 72 weeks of the preceding phase I RCT entered this single-arm OLE study (Fig. 1). Of these, 58 and 29 had been in the CT-P10 and RTX groups of the phase I RCT, respectively. Disease activity at week 24 of the RCT in these patients was comparable with patients who did not enter the OLE. For example, the mean [standard deviation (SD)] change from baseline in DAS28-ESR at week 24 of the RCT for patients in the CT-P10 and RTX groups who did not enter the OLE was −2.4 (1.2) and −2.3 (1.8), respectively. In patients who later entered the maintenance and switch groups of the OLE, these values were −2.0 (1.2) and −2.1 (1.3), respectively.
Fig. 1.
Patient flow (RCT and OLE). aPatients who completed the RCT received one or two courses of treatment. b45 patients completed the RCT but did not enter the OLE; reasons for this were absence of new signed informed consent form (n = 17), delay in timelines for ministry or institutional review board review/approval of the OLE protocol (n = 8), screening failure (n = 11), other (n = 9). cStable disease = DAS28-ESR or DAS28-CRP response had not worsened by ≥20% versus the best response during weeks 16–24 of the previous treatment course. dPatients enrolled in the OLE could receive up to two courses of treatment. However, all patients who completed the OLE received one course with the exception of one patient (in the maintenance group) who received two courses in the OLE. CRP C-reactive protein, DAS28 Disease Activity Score using 28 joints, ESR erythrocyte sedimentation rate, OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab
Thirty-eight (65.5%) and 20 (69.0%) patients from the CT-P10 and RTX groups of the RCT, respectively, met predefined disease activity and safety criteria and received CT-P10 in the current OLE study (Fig. 1). These 38 and 20 patients were therefore included in the maintenance and switch groups, respectively. Patient demographics and baseline disease characteristics were similar between these two groups (Table 1). For patients who received their second course of treatment in the OLE (i.e., received the first course in the phase I RCT), the mean (SD) time to retreatment was similar between the maintenance and switch groups [15.40 (2.60) and 15.30 (2.25) months, respectively]. Similarly, for patients who received their third course of treatment in the OLE (i.e., received the first and second courses in the phase I RCT), the time interval between the first and second course and the second and third course were also comparable between groups (Table 2). Only one patient (maintenance group) received a second course of CT-P10 treatment in the OLE study, having previously received two courses of CT-P10 in the RCT. For this patient, the change in DAS28-ESR from baseline (week 0 of the RCT) at week 24 after the first and second courses in the OLE was −2.49 and −4.00, respectively. Only data following the first course of treatment are reported in the rest of this article.
Table 1.
Demographics and baseline characteristics of patients who received study treatment (CT-P10) during the open-label extension study (safety population)
| Demographics and characteristics | Maintenance groupa (n = 38) | Switch groupb (n = 20) |
|---|---|---|
| Age (years) | 50.9 ± 11.4 | 49.6 ± 10.6 |
| Female [n (%)] | 35 (92.1) | 18 (90.0) |
| Caucasian [n (%)] | 23 (60.5) | 11 (55.0) |
| Height (cm) | 161.3 ± 6.6 | 162.7 ± 10.3 |
| Weight (kg) | 72.7 ± 16.3 | 73.2 ± 16.4 |
| Body mass index (kg/m2) | 28.0 ± 6.5 | 27.6 ± 5.3 |
| RA duration (years) | 11.6 ± 8.8 | 9.0 ± 5.2 |
| MTX dose (mg/week) | 15.5 ± 5.0 | 14.5 ± 3.6 |
| Anti-CCP positive [n (%)] | 33 (86.8) | 16 (80.0) |
| RF positive [n (%)] | 34 (89.5) | 16 (80.0) |
| C-reactive protein (mg/dL) | 1.4 ± 1.5 | 1.9 ± 3.3 |
| Erythrocyte sedimentation rate (mm/h) | 48.7 ± 23.6 | 39.6 ± 15.9 |
| Swollen joint count (66 joints assessed) | 16.8 ± 8.7 | 14.0 ± 7.5 |
| Tender joint count (68 joints assessed) | 29.1 ± 15.9 | 22.9 ± 10.1 |
| Number of treatment courses administered in RCT [n (%)] | ||
| 1 | 13 (34) | 9 (45) |
| 2 | 25 (65) | 11 (55) |
Values are mean ± standard deviation, unless otherwise stated
Anti-CCP anti-cyclic citrullinated peptide, MTX methotrexate, OLE open-label extension, RA rheumatoid arthritis, RCT randomized controlled trial, RF rheumatoid factor, RTX innovator rituximab
aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study
bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study
Table 2.
Time to retreatment in the phase I randomized controlled trial and open-label extension study (safety population)
| Number of treatment courses received | Time to retreatment (months) | Maintenance groupa (n = 38) | Switch groupb (n = 20) |
|---|---|---|---|
| 2 (1st course in RCT and 2nd course in OLE) | Between 1st and 2nd course | 15.40 ± 2.60 | 15.30 ± 2.25 |
| 3 (1st and 2nd course in RCT and 3rd course in OLE) | Between 1st and 2nd course | 8.75 ± 1.41 | 8.92 ± 1.27 |
| Between 2nd and 3rd course | 8.14 ± 1.20 | 7.99 ± 1.15 |
Values are mean ± standard deviation
OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab
aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study
bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study
Twenty (34.5%) patients in the maintenance group and nine (31.0%) patients in the switch group did not receive CT-P10 in the OLE study (Fig. 1). Among these 29 patients, 27 were not eligible for retreatment due to a stable disease state. Two patients in the RTX group during the RCT did not receive CT-P10 at the investigator’s discretion because their B cell count had not adequately recovered.
Efficacy
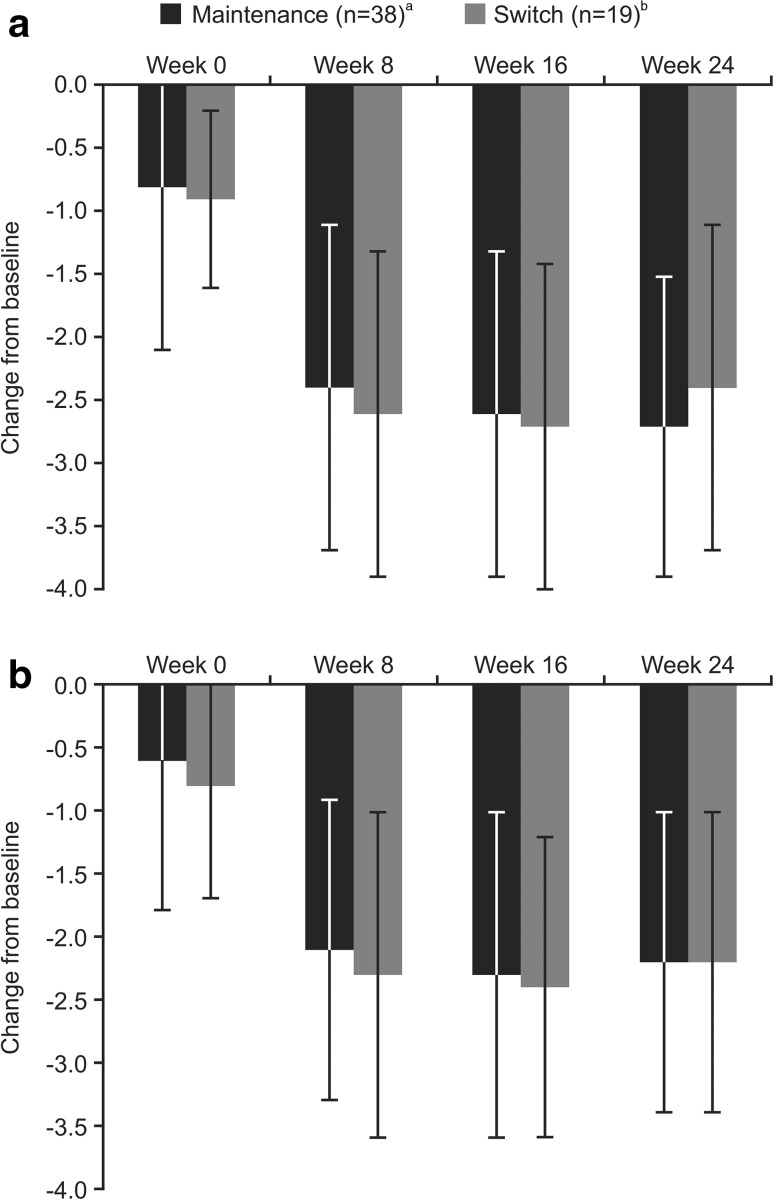
All efficacy endpoints were comparable between the maintenance and switch groups and no statistically significant differences were observed (P ≥ 0.05). Improvements in DAS28, as assessed by the mean change from baseline (week 0 of the preceding RCT), did not differ between the maintenance and switch groups at each time-point (Fig. 2a, b). The mean change from baseline in DAS28-ESR for the maintenance and switch groups was −2.4 and −2.6 at week 8, −2.6 and −2.7 at week 16, and −2.7 and −2.4 at week 24 of the OLE, respectively. For DAS28-CRP, the mean change from baseline was −2.1 and −2.3 at week 8, −2.3 and −2.4 at week 16, and −2.2 and −2.2 at week 24 of the OLE, respectively.
Fig. 2.
Mean (standard deviation) change in a DAS28-ESR and b DAS28-CRP from baseline in patients who received CT-P10 during the OLE study (efficacy population). Baseline refers to week 0 of the preceding phase I RCT. aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study. bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study. One patient was excluded from the efficacy population due to major protocol deviations [non-compliance with the eligibility criteria (received >2 biologic agents prior to start of study) plus received prohibited medication after study commencement]. CRP C-reactive protein, DAS28 Disease Activity Score using 28 joints, ESR erythrocyte sedimentation rate, OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab
The proportion of patients in the maintenance and switch groups achieving a good or moderate EULAR-ESR response was also comparable at each timepoint (78.9 and 84.2%, respectively, at weeks 8, 16, and 24; Fig. 3). EULAR-CRP response data were also similar between the maintenance and switch groups (81.6 and 78.9%, respectively, at both weeks 8 and 16; 73.7 and 84.2% at week 24; Fig. 3). The clinical response in patients who switched from RTX to CT-P10 was similar before and after switching.
Fig. 3.
Proportion of patients achieving a good/moderate EULAR response in patients who received CT-P10 during the OLE study (efficacy population). aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study. bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study. One patient was excluded from the efficacy population due to major protocol deviations [non-compliance with the eligibility criteria (received >2 biologic agents prior to start of study) plus received prohibited medication after study commencement]. CRP C-reactive protein, ESR erythrocyte sedimentation rate, EULAR European League Against Rheumatism, OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab
Safety and Immunogenicity
One or more adverse events were reported in 23.7 and 20.0% of patients in the maintenance and switch groups, respectively (Table 3). A full list of reported adverse events is provided in ESM Online Resource 2. One patient from both the maintenance and switch groups experienced a serious adverse event. Both were cases of spinal osteoarthritis that were considered unrelated to study treatment. Infections occurred in three (7.9%) and two (10.0%) patients in the maintenance and switch groups, respectively. One patient in each group experienced an infusion-related reaction. There were no malignancies or lymphomas and no adverse events led to permanent discontinuation from study treatment. No deaths occurred during the study.
Table 3.
Summary of adverse events in patients who received study treatment (CT-P10) during the open-label extension study (safety population)
| Adverse events | Number of patients with ≤1 event (%) | |
|---|---|---|
| Maintenance groupa (n = 38) | Switch groupb (n = 20) | |
| Any adverse event | 9 (23.7) | 4 (20.0) |
| Any serious adverse event | 1 (2.6) | 1 (5.0) |
| Spinal osteoarthritis | 1 (2.6) | 1 (5.0) |
| Infusion-related reaction | 1 (2.6) | 1 (5.0) |
| Infection | 3 (7.9) | 2 (10.0) |
| Gastroenteritis | 1 (2.6) | 0 |
| Respiratory tract infection | 1 (2.6) | 0 |
| Upper respiratory tract infection | 2 (5.3) | 1 (5.0) |
| Urinary tract infection | 2 (5.3) | 1 (5.0) |
| Malignancy or lymphoma | 0 | 0 |
| Study drug discontinuation due to adverse event | 0 | 0 |
OLE open-label extension, RCT randomized controlled trial, RTX innovator rituximab
aPatients treated with CT-P10 during the preceding phase I RCT and also during the OLE study
bPatients treated with RTX during the preceding phase I RCT and with CT-P10 during the OLE study
At week 24 of the first OLE treatment course, ADAs were detected in five (13.2%) and three (15.0%) patients in the maintenance and switch groups, respectively; however, ADAs had pre-existed in all these patients since the RCT and none of the patients had seroconverted after switching from RTX to CT-P10. NAbs were detected in one patient (maintenance group) at week 24.
Discussion
This multicenter, single-arm OLE study investigated the efficacy and safety of CT-P10 over 56 weeks in RA patients previously treated for up to 72 weeks with either CT-P10 (maintenance group) or RTX (switch group) [11, 12]. At week 24 of the preceding RCT, comparable clinical responses were observed between patients who were subsequently enrolled in the OLE and patients that were not, implying that non-responders were not selectively excluded from the OLE. During the OLE study, the efficacy of treatment—as measured by DAS28 scores and EULAR responses—was highly similar between the maintenance and switch groups. Adverse event profiles were also comparable between the groups and were consistent with the known safety profile of RTX. Overall, switching from RTX to CT-P10 did not appear to adversely affect the efficacy or safety of treatment when patients with RA were followed for up to 56 weeks after switching.
Data from the maintenance arm of this study demonstrated the longer-term efficacy of treatment with CT-P10 following repeated courses of treatment. During the first course of treatment in the RCT preceding this study, mean changes from baseline in DAS28-ESR and DAS28-CRP at week 24 in the CT-P10 arm were −2.1 and −1.9, respectively [11]. In this OLE, these values in the maintenance group were −2.7 and −2.2, respectively. Previous reports have shown that repeated treatment with RTX provided similarly sustained efficacy in patients with RA [4, 16, 17]. In a post hoc analysis of the REFLEX (Randomized Evaluation of Long-term Efficacy of Rituximab in RA) trial and its OLE study, the mean change from baseline in DAS28-ESR at week 24 of the third course of RTX treatment (−2.99) was comparable with that in the maintenance group of the current study (−2.7) [17]. The proportion of patients in the maintenance group with a good/moderate EULAR-ESR response at 24 weeks after two to three courses of CT-P10 treatment was 78.9%. This compares with 88.8% observed at week 24 after three courses of RTX in REFLEX [17]. The good/moderate EULAR-ESR response rate observed in the current study was an improvement on that observed after one course in the CT-P10 group of the preceding RCT (73.0%) [11], and comparable with week 24 after the first treatment course of RTX in REFLEX (77.2%) [17]. However, there are limitations to comparing these studies as results from REFLEX were from the observational post hoc analysis of the original 24-week RCT and many patients were withdrawn from the study following the first course [17].
CT-P10 was well-tolerated during this study and showed a safety profile at least as favorable as that observed following multiple courses of RTX [16, 18]. Importantly, there were no noticeable differences in the proportion of patients experiencing adverse events between the maintenance and switch groups. Of note, infusion-related reactions occurred in one patient in each treatment group, suggesting that switching from RTX to its biosimilar did not increase the likelihood of these events. In the maintenance group, cumulative exposure to CT-P10 did not increase the proportion of patients experiencing adverse events. On the contrary, during the first course of treatment in the preceding phase I RCT, the proportion of CT-P10-treated patients experiencing at least one adverse event (including events unrelated to treatment), infection, or infusion-related reaction was 51.0, 23.5, and 16.7%, respectively; this compares with 23.7, 7.9, and 2.6% in the OLE maintenance group [11]. A reduction in the proportion of patients experiencing adverse events has also been observed with repeated RTX treatment [4, 17].
The proportion of patients with ADAs or NAbs at week 24 was similar between maintenance and switch groups in the current study [ADAs: 5 (13.2%) and 3 (15.0%); NAbs: 1 (2.6%) and 0, respectively]. The eight patients who were ADA-positive at week 24 all had pre-existing ADAs before treatment in the OLE. The presence of ADAs in these patients did not appear to impact treatment efficacy or safety; all except one patient (maintenance group) demonstrated a good or moderate EULAR response at week 24 of the first treatment course of the OLE and none experienced infusion-related reactions or infections.
Limitations of the current study include the fact that it was not powered to formally assess the efficacy or safety equivalence of switching from RTX to CT-P10 versus maintained treatment with CT-P10. Also, due to the study’s relatively small size, it was not possible to include an additional control group of patients who were maintained on RTX. An OLE study of an ongoing phase III trial of CT-P10 in RA (ClinicalTrials.gov identifier: NCT02149121) will compare efficacy and safety between patients maintained on RTX and those patients switching from RTX to CT-P10.
In this study, no detrimental effects on efficacy or safety were observed following the switch from RTX to CT-P10. This finding concurs with other biosimilar switching studies involving relatively ‘simpler’ and lower molecular-weight biosimilars such as the epoetins [19–21]. In addition, the efficacy, safety, and immunogenicity of treatment were not affected when patients with RA or ankylosing spondylitis previously treated with the anti-TNF agent infliximab were switched to its biosimilar CT-P13 in two OLE studies [22, 23]. The absence of adverse switching effects in most studies reported to date is likely a result of manufacturers following the strict guidelines imposed during biosimilar development by the EMA, the US Food and Drug Administration and other regulatory authorities [9, 10].
Conclusions
This study demonstrated that switching from RTX to CT-P10 had no notable impact on the efficacy or safety of treatment in this population of patients with RA. Comparable improvements in RA disease activity and response rates were observed in patients who continued CT-P10 treatment in this OLE study and also in those who had switched from RTX to CT-P10. In addition, CT-P10 was shown to be efficacious and well-tolerated in patients with active RA treated repeatedly with this biosimilar for up to 2 years.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the patients and study personnel who made this trial possible, and the study investigators—Brazil: M. Scheinberg, S. Radominski; Republic of Korea: M.J. Lim, E.Y. Lee, J.Y. Choe; Mexico: M. Salazar-Paramo, J.A. Simon Campos, F. Medina-Rodriguez, F. Cons Molina; Poland: P. Hrycaj, J. Brzezicki, M. Piotrowski, Z. Ruzga, S. Jeka, P. Wiland; Russia: M. Stanislav, L. Myasoutova, P. Shesternya, T. Raskina, I. Vinogradova; Spain: J.S. Rey, F.J. Navarro Blasco; Ukraine: V. Kovalenko. Medical writing support for this manuscript was provided by Alice Wareham at Aspire Scientific Limited (Bollington, UK) and was funded by CELLTRION, Inc. (Incheon, Republic of Korea).
Author contributions
DHY, SJL, and SYL were involved in conception and design of the study, acquisition of data and/or analysis, and interpretation of data; drafting of the manuscript or revising it critically for important intellectual content; and final approval of the version to be published. SHK was involved in acquisition of data and technical support of the bioanalytical method development and sample analysis; revising the manuscript critically for important intellectual content; and final approval of the version to be published. WP, C-HS, and SCS were involved in the conception and design of the study; drafting of the manuscript or revising it critically for important intellectual content; and final approval of the version to be published. SJ, FFCM, PH, PW, EYL, FGM-R, PS, SR, MS, VK, LM, MJL, and J-YC were involved in the acquisition of data; drafting of the manuscript or revising it critically for important intellectual content; and final approval of the version to be published. The project management, clinical and medical monitoring, pharmacovigilance, data management, analysis of pharmacokinetic data, biostatistical analysis, and medical writing were performed under contract with PPD, Inc. in collaboration with CELLTRION, Inc. WP is the guarantor for the overall content.
Compliance with Ethical Standards
Funding
The study was sponsored by CELLTRION, Inc. (Incheon, Republic of Korea). The funding body contributed to the design of the study, the collection, analysis, and interpretation of data, and the review of drafts and final version of the manuscript.
Conflict of interest
WP received consulting fees during the conduct of the study and grants outside the submitted work from CELLTRION. PH and SR received grants form CELLTRION for conducting the clinical trial. MJL received grants from CELLTRION during the conduct of the study. SJL is an employee of, and holds stock options in, CELLTRION. SYL and SHK are employees of CELLTRION. DHY is a scientific consultant and on the speaker’s bureau of CELLTRION and has received research grants not related to this clinical study. C-HS, SCS, FFCM, SJ, FGM-R, PW, EYL, PS, VK, LM, MS, and J-YC declare no conflicts of interest.
Ethical approval
This study was performed according to the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. Study protocols and consent forms were approved by the relevant independent ethics committees. The following ethical bodies approved the study in the centers involved—Brazil: Comitê de Ética em Pesquisa da Associação de Assistência à Criança Deficiente; Comitê de Ética em Pesquisa do Hospital de Clínicas da Universidade Federal do Paraná; Republic of Korea: IRB of IN-HA University Hospital; SNUMC/SNUH IRB; IRB of Daegu Catholic University Medical Center; Mexico: Comité de Ética ICLE SC; Comités de Ética e Investigación en Salud Centro de Especialidades Médicas del Sureste SA de CV; Comité de Ética e Investigación para Estudios en Humanos del “Hospital Médica Sur” S.A.B de C.V.; Comité Bioetico para la Investigación Clínica S.C; Poland: The Bioethics Committee at the Regional Medical Council of Wielkopolska Medical Chamber; Russia: Council on Ethics, Annex to the Order of the Ministry of Healthcare of the Russian Federation; Spain: Comité Ético de Investigación Clínica de Andalucía; Ukraine: Commission on Ethics Questions of State Institution National Scientific Center.
Informed consent
All patients provided written informed consent to participate in the OLE study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40259-017-0233-6) contains supplementary material, which is available to authorized users.
References
- 1.Weiner GJ. Rituximab: mechanism of action. Sem Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JCW, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Olivo MA, Amezaga Urruela M, McGahan L, et al. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev. 2015;1:CD007356. [DOI] [PMC free article] [PubMed]
- 4.Tak PP, Rigby W, Rubbert-Roth A, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis. 2012;71:351–357. doi: 10.1136/annrheumdis-2011-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–260. doi: 10.1136/pgmj.2006.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Expert Committee on Biological Standardization: Geneva, 19 to 23 October 2009. Guidelines on evaluation of similar biotherapeutic products (SBPs). 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed 14 Feb 2017.
- 9.Committee for Medicinal Products for Human Use (CHMP). European Medicines Agency. Guideline on Similar Biological Medicine Products. 23 October 2014. CHMP/437/04 Rev 1. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 14 Feb 2017.
- 10.US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Guidance for Industry. 2015. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291134.pdf. Accessed 14 Feb 2017.
- 11.Yoo DH, Suh C, Shim SC, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–570. doi: 10.1136/annrheumdis-2016-209540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo DH, Suh C, Shim SC, et al. Efficacy and safety of rituximab biosimilar candidate (CT-P10) and innovator rituximab in patients with rheumatoid arthritis: results from phase I randomized controlled trial over 72 weeks [abstract] Arthritis Rheumatol. 2015;67(Suppl 10):2449–2452. [Google Scholar]
- 13.Generics and Biosimilars Initiative (GaBI). EMA approval for rituximab biosimilar Truxima. 2017. http://www.gabionline.net/Biosimilars/News/EMA-approval-for-rituximab-biosimilar-Truxima. Accessed 14 Feb 2017.
- 14.Prevoo MLL, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 15.van Gestel AM, Prevoo ML, van’t Hof MA, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39:34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- 16.Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56:3896–3908. doi: 10.1002/art.23059. [DOI] [PubMed] [Google Scholar]
- 17.Keystone EC, Cohen SB, Emery P, et al. Multiple courses of rituximab produce sustained clinical and radiographic efficacy and safety in patients with rheumatoid arthritis and an inadequate response to 1 or more tumor necrosis factor inhibitors: 5-year data from the REFLEX study. J Rheumatol. 2012;39:2238–2246. doi: 10.3899/jrheum.120573. [DOI] [PubMed] [Google Scholar]
- 18.Van Vollenhoven RF, Emery P, Bingham CO, et al. Long-term safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010;37:558–567. doi: 10.3899/jrheum.090856. [DOI] [PubMed] [Google Scholar]
- 19.Davis-Ajami ML, Wu J, Downton K, et al. Epoetin zeta in the management of anemia associated with chronic kidney disease, differential pharmacology and clinical utility. Biologics. 2014;8:155–167. doi: 10.2147/BTT.S27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flodmark CE, Lilja K, Woehling H, et al. Switching from originator to biosimilar human growth hormone using dialogue teamwork: single-center experience from Sweden. Biol Ther. 2013;3:35–43. doi: 10.1007/s13554-013-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wizemann V, Rutkowski B, Baldamus C, et al. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008;24:625–637. doi: 10.1185/030079908X273264. [DOI] [PubMed] [Google Scholar]
- 22.Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2016;76:346–354. doi: 10.1136/annrheumdis-2015-208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2016;76:355–363. doi: 10.1136/annrheumdis-2015-208786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.