Abstract
Amazon comprises a vast variety of ecosystems, including savannah-like Canga barrens that evolved on iron-lateritic rock plateaus of the Carajás Mountain range. Individual Cangas are enclosed by the rain forest, indicating insular isolation that enables speciation and plant community differentiation. To establish a framework for the research on natural history and conservation management of endemic Canga species, seven chloroplast DNA loci and an ITS2 nuclear DNA locus were used to study natural molecular variation of the red flowered Ipomoea cavalcantei and the lilac flowered I. marabaensis. Partitioning of the nuclear and chloroplast gene alleles strongly suggested that the species share the most recent common ancestor, pointing a new independent event of the red flower origin in the genus. Chloroplast gene allele analysis showed strong genetic differentiation between Canga populations, implying a limited role of seed dispersal in exchange of individuals between Cangas. Closed haplotype network topology indicated a requirement for the paternal inheritance in generation of cytoplasmic genetic variation. Tenfold higher nucleotide diversity in the nuclear ITS2 sequences distinguished I. cavalcantei from I. marabaensis, implying a different pace of evolutionary changes. Thus, Canga ecosystems offer powerful venues for the study of speciation, multitrait adaptation and the origins of genetic variation.
Introduction
Plants present remarkable opportunities for studying adaptive radiation and speciation in action1. Interactions with pollinators and herbivores2, adaptation to soils and harsh environmental conditions3 are among the major ecological factors that drive adaptive radiation in plants. Ecogeographic factors such as mountain range uplifts and island formation set gene flow restrictive reproductive isolation barriers in plants and facilitate rapid speciation rates4. Speciation by hybridization is another very common process in plants4, 5.
The Carajás Mountain range is located in the south-eastern lowland Amazon. Part of the mountain range is protected within the Carajás National Forest (Fig. 1a,b). Rock weathering in Carajás Mountains and suitable climatic conditions, such as distinct wet and dry seasons, led to the localized formation of iron-laterite rocks at the surface of small plateaus elevated 650–800 m above sea level6. Plateaus host fire prone savannah-like ecosystems known as Canga that are isolated from each other by the rain forest7 (Supplementary Fig. S1). Canga soils are shallow (0–10 cm), unevenly distributed though rock fissures and landscape depressions; are edaphically restrictive and often contain potentially phytotoxic levels of metals8, 9. The rain fall precipitation varies through the dry-wet seasons between <60 mm to 1900 mm/month. Average monthly temperatures vary between 19 and 31 °C. Fires are common. Thus, low nutrients, heat, openness, drought prone and toxic metal rich conditions in combination with the insular isolation resulted in highly specialized Canga plant communities composed of more than 500 plant species9, 10.
Figure 1.

(a) Map of the Carajás National Forest. Forest boundary is indicated with the purple line. The patchy light green areas are deforestation areas used for agriculture and cattle pastures. Intense green color is a mountain rain forest. Paler green areas within the Forest are savannahs and granitic inselbergs. Established open-pit mines and the ongoing mine explorations are indicated by the red and yellow dots, respectively. (b) Location of the populations used in this study. (c) Distribution of I. cavalcantei (red flowers) and I. marabaensis (lilac flowers). The purple dot indicates the location where putative interspecies hybrids were found in 2016. (d) From left to right, flower of a representative I. cavalcantei individual, flower of a putative hybrid, and flower of I. marabaensis. Bar = 2 cm. (e) Leaves from I. cavalcantei (upper leaf, from Canga N4), I. marabaensis (lower leaf, from S11 Plateau). Individuals of I. marabaensis from populations N5, N6, N8 and Tarzan have narrow lanceolate leaves (middle leaf). Bar = 1 cm.
The iconic plant of Carajás, known as “flor de Carajás” - “flower of Carajás” in Portuguese, is a red flowered morning glory Ipomoea cavalcantei 11 from the family Convolvulaceae that comprises commonly known food crop sweet potatoes, which is Ipomoea batatas. I. cavalcantei is a narrow endemic that to the best of our knowledge is only found in Carajás National Forest at four northern Canga islands, measuring at most 20 km2 (Figs 1c and S2; Table S1). The lilac flowered I. marabaensis 12 is a related species that inhabits, but is not restricted to Cangas south and east from I. cavalcantei (Figs 1c and S2; Table S1). The leaf morphology distinguishes populations of I. marabaensis 12 (Fig. 1e). The flower traits differentiation between I. cavalcantei and I. marabaensis suggests adaptive radiation driven by pollinator preferences2. I. cavalcantei bright red tubular flowers with protruding reproductive organs are reminiscent of a hummingbird pollination syndrome, whereas large flowers of I. marabaensis with a broad tube display the features of a bee pollination syndrome13 (Fig. 1d). The assumed shift in pollinator preferences should favor the species co-occurrence in the same area, i.e. sympatry. Why I. cavalcantei and I. marabaensis, adapted to Canga environments, have very distinct areal is not understood. The herbarium of the Museum Emilio Goeldi (Belém, Pará, Brazil) holds specimens that were classified as interspecies I. cavalcantei × marabaensis hybrids14. There are no reports of the cytogenetic or molecular characterization of the putative natural hybrids (Fig. 1d), as well as their role in a gene flow and/or incipient speciation.
In the present study, seven chloroplast DNA loci and an ITS2 nuclear DNA locus were used to study natural molecular variation of I. cavalcantei and I. marabaensis in Cangas of the Carajás National Forest. Soil transplantation experiments with I. cavalcantei, I. marabaensis and four other Convolvulaceae species were carried out. The primary goals were to: (1) investigate the genetic structure and diversity of I. cavalcantei and I. marabaensis populations; (2) find molecular evidence for the hypotheses that I. cavalcantei and I. marabaensis are recently diverged, sister species12 that could hybridize in nature; (3) study the role of Canga soils in structuring plant communities and species distributions; (4) establish a framework for the future research on natural history and conservation management of Carajás morning glories.
Results
I. cavalcantei and I. marabaensis belong to a lineage within the clade Murucoides
To decipher the phylogenetic affinities of I. cavalcantei and I. marabaensis using molecular markers, partial coding sequences of seven genes encoded by the plastomes (cpDNA) were determined and compared to respective sequences computationally extracted from the assembled and sequenced plastomes of twenty six Ipomoea species15. Maximum parsimony, maximum likelihood and Bayesian likelihood analyses of the concatenated sequence alignments positioned I. cavalcantei and I. marabaensis within the clade of Murucoides (Fig. S3). In addition, alignments of psbA-trnH intergenic spacers showed that species from the Murucoides clade have a large deletion, i.e. approximately 150–190 bp as compared to the species from clades Pes-caprae and Quamoclit (Fig. S4).
I. cavalcantei and I. marabaensis share chloroplast DNA polymorphisms
cpDNA sequence analysis revealed molecular polymorphisms in rpoC1 and psbA-trnH intergenic (IGS) sequences (Table S2). The C/A single nucleotide polymorphism (SNP) in a coding region of rpoC1 was nonsynonymous, alternating the AAA codon for the positively charged lysine (K) and CAA codon for the hydrophilic noncharged glutamine (Q) amino acid residues. It is unlikely that plastidial RNA editing converts CAA codon to a translation termination codon UAA, thus we infer that rpoC1K and rpoC1Q subunit isoforms characterize the plastidial prokaryotic type RNA polymerase in I. cavalcantei and I. marabaensis. In the plant kingdom, rpoC1 polypeptides from many lineages are characterized by the K/Q variation at this site, e.g. among the top 35 hits in a blast-p search 19 species had K and 16 had Q. However, within the family Convolvulaceae, species such as Convolvulus arvensis, Turbina corymbosa, Operculina macrocarpa, Merremia quinquefolia, Evolvulus nuttallianus, Argyreia nervosa, Cuscuta reflexa, as well as twenty six Ipomoea species15 all had rpoC1K isoform, suggesting that rpoC1K is an ancestral state in morning glories, whereas the emergence of rpoC1Q in I. cavalcantei and I. marabaensis could be an example of convergent evolution in a 687 amino acid residues long rpoC1 polypeptide. Analysis of available 26 Ipomoea rpoC1 sequences showed additional SNP variation at 17 sites, of which two were nonsynonymous resulting in hydrophobic amino acid residue variation L/V and V/I.
The psbA-trnH IGS from I. cavalcantei and I. marabaensis individuals were identical in length, but differed in a sequence at three neighboring positions, i.e. AAA versus TTT.
We assumed that the gene order in Ipomoea spp. plastomes is conserved, accordingly the polymorphisms at rpoC1 and psbA-trnH IGS were phased into haplotypes that identify at least four plastome types in I. cavalcantei and I. marabaensis (Fig. 2a; Table S3). The plastome type network was closed (Fig. 2b), i.e. all possible assortment of polymorphisms into four plastome haplotypes have been found in wild populations of I. cavalcantei and I. marabaensis. Observed haplotypes could have originated among ancestors of the species either from three mutational changes, i.e. mutation origin scenario, or two mutations and one recombination event, i.e. recombination origin scenario (Fig. 2c).
Figure 2.

(a) Distribution of the four plastome types of Carajás morning glories. (b) Parsimony network of plastome haplotypes. Bars across the network edges indicate the numbers of mutational changes. (c) The mutation and recombination scenarios of plastome diversity origin among ancestors of the species. Plastome types/haplotypes are represented by polymorphic sites identified in rpoC1 (A/C SNP) and psbA-trnH (AAA/TTT SNP stretch) sequences. Dash is a several thousand base pairs long gap between the genes. Plastome AT (A-TTT) was arbitrary chosen as ancestral.
The CA plastome was only identified among I. marabaensis individuals in this study, whereas three other plastome types occurred in both species. We cannot exclude that the observed absence of CA plastome in I. cavalcantei could be due to its very low frequencies in the species and was not captured by the population sampling in this study. The rpoC1 Q and rpoC1 K were predominant alleles in I. marabaensis (66 rpoC1 Q/17 rpoC1 K) and I. cavalcantei (12 rpoC1 Q/91 rpoC1 K), respectively. The overall interspecies haplotype diversity h and nucleotide diversity π calculated over 883 bp of rpoC1 and psbA-trnH sequences were higher in I. marabaensis (h = 0.6560; π = 0.001997), than in I. cavalcantei (h = 0.2581; π = 0.000429) (see also the results of the basic population statistic calculations for individual Cangas in Table S4).
Calculation of pairwise F ST values indicated significant genetic differentiation among populations (Table 1). Surprisingly, the I. marabaensis samples collected at S11 Plateau Canga were more similar to I. cavalcantei populations (F ST range 0.011–0.115) than to the conspecific I. marabaensis populations from the Northern Cangas N6, N7 and N8 or South-Eastern Canga Tarzan (F ST range 0.632–0.807) (Table 1), most probably due to the abundance of the plastome type AT (Fig. 2a).
Table 1.
Canga areas, geographic distances and genetic differentiation of I. cavalcantei and I. marabaensis populations. The values along diagonal (in bold) are areas of Cangas (km2). Values above diagonal are the shortest distances between Canga islands (km). The pairwise genetic differentiation measured by the population descriptive statistics is shown below the diagonal. The F ST values from the plastome (pt) gene alleles, emphasized by italics font, and internal transcribed rDNA spacer alleles (ITS2) are combined in the same cells. Asterisk are the p values: *<0.05; **<0.01; ***<0.001.
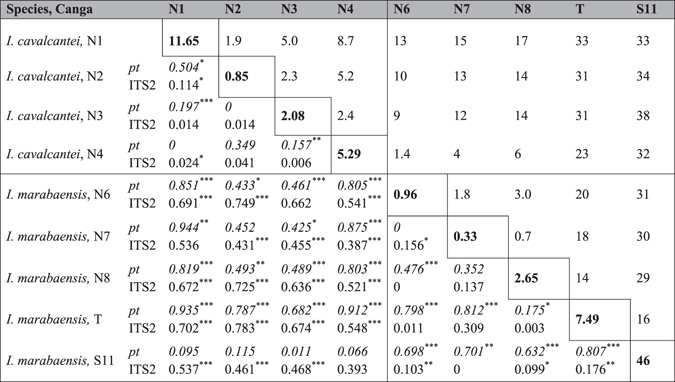
AMOVA analysis within I. cavalcantei suggested that 13.92% of variation can be explained by the genetic differentiations between Canga populations (Table 2). AMOVA analysis of eleven Canga group permutations indicated that most of the variation between populations could be due to the differences between the Canga N1 & N4 group versus Canga N2 & N3 group (Table 2). This population differentiation scenario was also supported by the analysis using software package STRUCTURE16 (Fig. S5). The differentiation of Canga N3 (n = 21) could be due to the three individuals with AA plastome, that was not found in N1 (n = 33) or N4 (n = 44) (Table S3). The AMOVA results do not appear to correlate with geographic distances, because only 1.9 km separate N1 from N2 and 2.4 km N3 from N4, whereas the closest distance between N1 and N4 is approximately 8.7 km (Table 1).
Table 2.
AMOVA results, partitioning molecular variation of plastomes.
| Species | Variance component | df | Variance % | Fixation index | P |
|---|---|---|---|---|---|
| I. cavalcantei & I. marabaensis a | Among species | 1 | 44.74 | ΦCT = 0.447 | <0.05 |
| Among populations within species | 7 | 28.71 | ΦSC = 0.519 | <0.001 | |
| Within populations | 174 | 26.55 | ΦST = 0.734 | <0.001 | |
| I. cavalcantei b | Among populations | 3 | 13.92 | ΦST = 0.139 | <0.01 |
| Within populations | 99 | 86.08 | |||
| I. cavalcantei c | Among population groups | 1 | 24.05 | ΦCT = 0.241 | 0.341 |
| Among populations within groups | 2 | 0 | ΦSC = 0 | 0.654 | |
| Within populations | 99 | 75.95 | ΦST = 0.229 | <0.01 | |
| I. marabaensis d | Among populations | 4 | 64.01 | ΦST = 0.64 | <0.001 |
| Within populations | 75 | 35.99 | |||
| I. marabaensis e | Among population groups | 2 | 62.47 | ΦCT = 0.625 | 0.086 |
| Among populations within groups | 2 | 5.65 | ΦSC = 0.151 | <0.05 | |
| Within populations | 75 | 31.87 | ΦST = 0.681 | <0.001 |
aCanga groups (N1, N2, N3, N4), (N6, N7, N8, T, S11).
bOne group of Canga populations (N1, N2, N3, N4).
cCanga populations groups (N1, N4), (N2, N3).
dOne group of Canga populations (N6, N7, N8, T, S11).
eCanga population groups (N6, N7), (N8, T), (S11).
AMOVA (Table 2) and STRUCTURE (Fig. S5) analyses of I. marabaensis populations suggested the plausible structure N6 & N7 vs N8 & Tarzan vs S11 Plateau. The northern populations N6, N7 and perhaps N5 (the latter was only represented by 2 individuals and not included in AMOVA analyses) have predominant plastome CT, the south-eastern group of Cangas N8, Tarzan and a single individual on a granitic inselberg Sossego mainly have plastome type CA. The plastome type AT was predominant (11 plants out of 13 analyzed) in S11 Plateau Canga (Table S3).
Diversification of transcribed internal ribosomal RNA gene spacer
Next, we characterized the ribosomal RNA gene (rDNA) internal spacer ITS2 that is a standard type of the nuclear genome loci used in plant phylogenetic analysis and population genetics studies17, 18. In all 217 analyzed sequences we did not detect insertions-deletions, thus ITS2 regions had the same length of 223 base pairs. Six positions that are clustered within 67 bp window closer to the 28S rRNA coding region showed SNP variation (Fig. 3). There was a species-specificity in distribution of polymorphic sites. Four sites varied in I. cavalcantei and two in I. marabaensis (Fig. 3a; Tables S5 and S6). Base 215 represented a polymorphic site that was shared between the species. This polymorphism was the only one that affected the length of the base-pairing in a helix IV (Fig. 3b). Analysis of more than fifty Ipomoea ITS2 sequence-structures at ITS2 Database revealed numerous insertion/deletion (indel) polymorphisms, as well as SNP’s distributed all over the Ipomoea ITS2. The ITS2 sequences of I. polpha, I. arborescens, I. carnea and I. conzattii, that could belong to Murucoides clade, were more similar to I. cavalcantei and I. marabaensis ITS2 and showed only two 1 base pair indels. Eighteen polymorphic SNP sites differentiated those species from I. cavalcantei and I. marabaensis in the upstream ITS2 region that comprise Helixes I and II, suggesting certain constrains on I. cavalcantei/I. marabaensis ITS2 polymorphisms distribution, which could result from co-evolvement of the rRNA precursor sequences and the cognate RNA processing machinery19.
Figure 3.
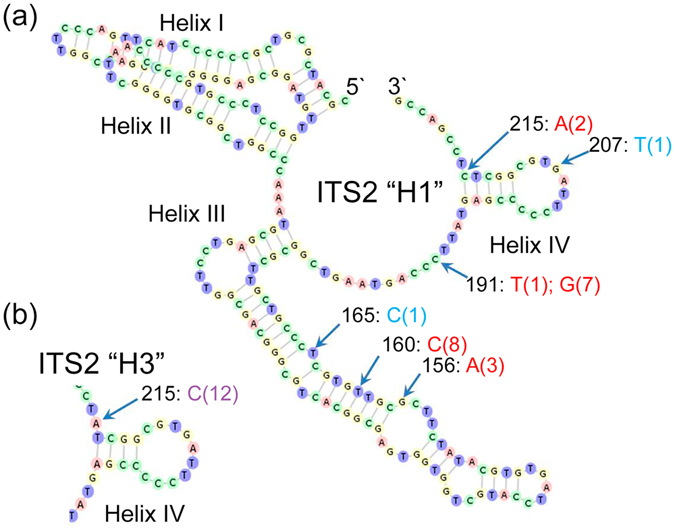
Distribution of SNP’s over the ITS2 secondary structure. (a) ITS2 haplotype “H1” and (b) the Helix IV of the folded ITS2 haplotype “H3”. Arrows point bases at which natural variation was observed. Numbers in black are the bases numbering from the 5′end. The letters next to arrows are the alternative bases, i.e. SNP; bracketed numbers are instances among fourteen recorded haplotypes. SNP are colored in red, when a polymorphism is only found in I. cavalcantei; in blue for I. marabaensis; in purple, when shared between the species.
We identified fourteen ITS2 haplotypes, of which eleven were found only in I. cavalcantei, two only in I. marabaensis. One haplotype H1 was shared between the species (Fig. 4a; Tables S5 and S6). Quantification of this variation showed six to ten-fold interspecific differences in haplotype and nucleotide diversities, I. cavalcantei (h = 0.6172; π = 0.004092); I. marabaensis (h = 0.1186; π = 0.000543), a situation opposite to the plastome diversity estimates (see the results of the basic population statistic calculations for individual Cangas in Supplementary Table S7). The ITS2 haplotype H1 was predominant in I. marabaensis. It could be either ancestral, or indicate a gene flow between the species20, 21.
Figure 4.
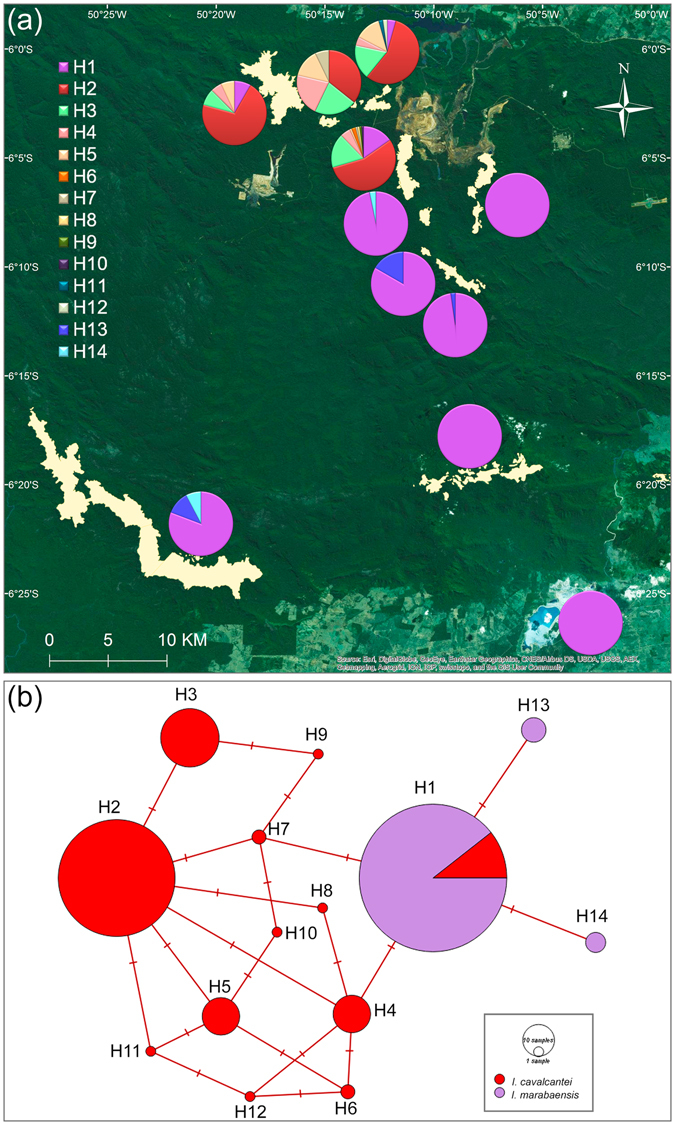
Nuclear genome ITS2 (a) allele distribution and (b) haplotypes network.
Maximum parsimony network modelling showed that all identified ITS2 haplotypes could derive from each other by single base pair substitutions (Fig. 4b). Interestingly, several I. cavalcantei haplotypes formed closed sub-networks, indicating gene recombination/conversion followed by rDNA repeat homogenization as a mechanism of their origin within heterozygous individuals20, 22. Indeed, the average observed heterozygosity in I. cavalcantei was five times higher than in I. marabaensis (Table S7).
The F ST pairwise differences between I. cavalcantei populations showed little genetic differentiation (Table 1). Moderate genetic differentiation characterized some of I. marabaensis populations. In this species, S11 Plateau Canga was well differentiated from N6, N8 and T, which fits its separation by the distance. However, a small (0.33 km2) 30 km distant Canga N7 was not differentiated from S11 (F ST = 0), but differentiated from N6 (F ST = 0.156) and N8 (F ST = 0.137) that are separated from N7 by 1.8 and 0.7 km, respectively.
AMOVA indicated that most of genetic variation in I. cavalcantei and I. marabaensis originates from ITS2 genetic differences within the populations (Table 3). AMOVA population analyses also showed the higher inbreeding levels in I. marabaensis (F IS = 0.78–1), as compared to I. cavalcantei (F IS = 0.31–0.59). ITS2 molecular data clustering using STRUCTURE revealed a clear interspecies differentiation, but, consistently with AMOVA analysis, indicated the absence of intraspecific genetic differentiation (Fig. S6).
Table 3.
AMOVA results, partitioning molecular variation in ITS2.
| Species | Variance component | Variance % | Fixation index | P |
|---|---|---|---|---|
| I. cavalcantei & I. marabaensis a | Among species | 58.86 | ΦCT = 0.588 | <0.01 |
| Among populations within species | 1.05 | ΦSC = 0.026 | <0.01 | |
| Within populations | 40.07 | ΦST = 0.599 | <0.001 | |
| I. cavalcantei b | Among populations | 2.39 | ΦST = 0.0233 | <0.05 |
| Within populations | 97.67 | |||
| I. cavalcantei c | Among population groups | 0.22 | ΦCT = 0.002 | 0.319 |
| Among populations within groups | 2.2 | ΦSC = 0.022 | 0.061 | |
| Within populations | 97.6 | ΦST = 0.024 | <0.05 | |
| I. marabaensis d | Among populations | 7.67 | ΦST = 0.0767 | <0.001 |
| Within populations | 92.33 | |||
| I. marabaensis e | Among population groups | 3.91 | ΦCT = 0.039 | 0.365 |
| Among populations within groups | 4.5 | ΦSC = 0.045 | <0.05 | |
| Within populations | 91.58 | ΦST = 0.084 | <0.05 |
aCanga groups (N1, N2, N3, N4), (N6, N7, N8, T, S11).
bOne group of Canga populations (N1, N2, N3, N4).
cCanga populations groups (N1, N4), (N2, N3).
dOne group of Canga populations (N6, N7, N8, T, S11).
eCanga population groups (N6, N7), (N8, T), (S11).
Molecular analysis of the putative hybrids
Flow cytometry analysis showed that I. cavalcantei and I. marabaensis have similar genome sizes (Table S8), presenting no evidence that differentiation in ploidy or genome size could establish barriers to gene flow between the species. The putative I. cavalcantei × marabaensis hybrids had a genome size like parental species, indicating that those phenotypically distinct individuals (Fig. 1d) are not autopolyploids or allopolyploids.
A single available I. marabaensis plant from a sympatric area in Canga N4 had plastome type CT and ITS2 haplotype H1, which is consistent with the allele frequencies in the closest Canga N6 I. marabaensis population. Three putative interspecies hybrid plants from the same area all had plastome type AT, suggesting I. cavalcantei as a mother, if we assume maternally-biased cytoplasmic inheritance in the species, although a reversion from maternal inheritance mode to paternal has been observed in interspecies crosses of Passiflora 23. All putative hybrids had ITS2 haplotype H1, which is shared between I. cavalcantei and I. marabaensis. Thus, available data did not conclusively demonstrate a hybrid nature of analyzed individuals.
Differential plant growth on Canga soils
I. cavalcantei and I. marabaensis ten-week-old plantlets produced 0.27 g (±0.059 s.d.) and 0.24 g (±0.06 s.d.) of the dry leaf biomass, respectively (Fig. S7), when grown on horticultural soil that had high levels of available nutrients (Table S9). The leaf biomass produced on three nutrient-rationed Canga soils, which had lower contents of major nutrients, such as P, S, K, Ca+2, Mg+2 (Table S9), was 20–30% (I. cavalcantei) and 17–22% (I. marabaensis) of the biomass produced on horticultural soil (Figs 5a,b and S7), indicating an absence of specific species dependence on Canga soils and potential suitability of abandoned agricultural land for ex situ conservation.
Figure 5.

The growth of (a) I. cavalcantei, (b) I. marabaensis, and (c) I. asarifolia on soils collected from Canga N4 (N4 site#1, N4 site#2), S11 Plateau Canga (S11), and horticultural soil (Control). Pictures taken ten weeks after germination. Operculina hamiltonii (d), Merremia aegyptia (e) and Ipomoea grandifolia (f) grown on horticultural soil (C) or Canga soil (N4). Seedlings grown on Canga soil (N4) were retarded, shed leaves that developed necrotic lesions (lower close up images).
Operculina hamiltonii is common in edaphically restrictive, very dry Carajás granitic inselberg ecosystem, which is similar to Canga (Fig. S1). However, seedlings of O. hamiltonii grown on Canga soils showed little shoot growth, whereas both cotyledon leaves and young shoots developed extensive necrotic lesions. After the death of the primary shoot, the development of the new side shoots was as well aborted soon after emergence (Fig. 5d). Similar degree of growth inhibition, shoot tissue necrosis and leaf abscission characterized seedlings of Merremia aegyptia (Fig. 5e) and Ipomoea grandifolia (Fig. 5f). Unexpectedly, Ipomoea asarifolia seedlings grown on Canga soils produced leaf biomass that was 19–25% of leaf biomass produced by the species on horticultural soil (Fig. 5c and S7). The tested here Ipomoea asarifolia accession originated from a population found on rocky barrens in Atlantic coastal areas very remote from Cangas (>600 km). The species occurrence within Carajás National Forest ecosystems has not been reported, indicating species preadaptation to Canga soils.
Discussion
We show that I. cavalcantei and I. marabaensis are closely related species from a lineage within the morning glory clade Murucoides. Studies of the Ipomoea L. showed that the Murucoides clade includes species with vastly different morphologies and biogeographic distributions15, 24, 25. Australian endemic I. polpha is a ground trailing vine, that develops tuberous roots harvested by the indigenous people for food. I. murucoides and I. pauciflora are deciduous trees that grow in arid regions of Central America. The neotropical I. carnea and I. cuneifolia are erect shrubs. Thus, it appears that the genetic makeup of the Murucoides ancestors enabled high adaptability of the derived modern species to very different environments, in particular, arid and edaphically restrictive semi-deserts, savannahs and rocky mountainous ridges.
Sequence analysis in this study suggested a Central American endemic I. populina as one of the closest relatives to I. cavalcantei and I. marabaensis. Similar to I. cavalcantei, I. populina has obovate leaves and grows as a deciduous climbing woody stemmed liana. Austin noted similarities between I. cavalcantei, I. argentea and a red flowered I. longistaminea O’Donnel from the Brazilian Cerrado and Caatinga ecosystems11. In addition to I. longistaminea, there are several other woody Caatinga Ipomoea species that develop edible tuberous roots for which they are known as mountain potatoes, “batata da serra” in Portuguese26. Clearly, the higher phylogenetic resolution of I. cavalcantei and I. marabaensis lineage is required to reconstruct the speciation history and to detect possible gene-flow mediated sharing of adaptive gene alleles, which could have facilitated colonization of Cangas by the morning glories.
Our results show overlaps in gene allele distributions between I. cavalcantei and I. marabaensis, which indicates that the species are sisters12, sharing the most recent common ancestor. Thus, I. cavalcantei could represent a new example of independent origin of red flowers in morning glories. It is generally accepted that the blue/purple flower color due to cyanidin accumulation is ancestral in Ipomoea L.27, implying that flowers of I. marabaensis are closer to the ancestral type. The reverse transitions from red to blue flowers are unlikely27, 28. Parsimony reconstructions indicated that red-flowered lineages have arisen four times independently in the Ipomoea tribe Astripomoeinae, i.e. in the lineages leading to I. quamoclit/I. hederifolia, I. udeana, I. conzattii and I. horsfalliae 27. The I. conzattii and I. horsfalliae are likely to belong to Murucoides clade and have evolved the shared red flower phenotype through different genetic mechanisms27. Therefore, it is possible that red flowers in the Murucoides clade independently originated at least three times. Plants employ different strategies to color flowers in red27, 29. Further biochemical and genetic studies of I. cavalcantei will advance our understanding of mechanisms that underpin convergent evolution of the red flower trait in plants.
Analysis of plastomes revealed significant genetic differentiation of I. cavalcantei and I. marabaensis populations, indicating the combined effects of genetic drift and population bottlenecks as mechanisms of population evolvement in Cangas. In contrast, little spatial structure was displayed by the distribution of the nuclear ITS2 polymorphisms. Thus, natural variation of plastome and ITS2 sequences suggested alternative demographic histories of Ipomoea populations in Carajás Cangas, which could reflect differences in gene allele dispersal by the seed or/and pollen18, 30. Such contrasting genetic differentiations indicate maternally-biased plastome inheritance that limits plastome gene flow to the seed dispersal30. Although empiric studies suggest that the mode of plastome inheritance cannot always be extrapolated within a genus31, strict maternal inheritance in Japanese morning glory Ipomoea nil was inferred from the genetic analysis of a cytoplasmic mutation32. However, I. nil pollen sperm cells have plastids33, indicating a potential for biparental inheritance like in ca. 20% of angiosperm species33, 34. Furthermore, the rates of paternal plastome transmission, the so-called paternal leakage, increased among the offspring of interpopulation crosses in Helianthus verticillatus 31 and Campanulastrum americanum 35. The reversal from maternal to paternal plastome inheritance was found in interspecies hybrids of Passiflora 23. Thus, the potential plasticity of the plastome inheritance modes in I. cavalcantei and I. marabaensis species cannot be disregarded and should be studied further both in laboratory and natural settings.
We show that plastome haplotype network of I. cavalcantei and I. marabaensis has closed topology. We consider two scenarios to explain this property (Fig. 2c). The mutation scenario requires a repetitive origin for either rpoC1 or psbA-trnH polymorphism in the species ancestry, which implies a strong selective pressure at a given polymorphic site. Only about twenty polymorphisms in rbcL amino acid residue sequences are responsible for the most cases of positive selection in CO2-fixing Ribulose-1,5-bisphospate carboxylase/oxygenase (Rubisco)36. Positive selection at Rubisco correlates with adaptation to altitude variation37 or aquatic environments38. The rpoC1 or psbA-trnH polymorphisms could affect plastid gene expression. The recombination scenario is consistent with a neutral evolution mode, but requires: (i) heteroplasmy due to biparental inheritance, or paternal leakage; and (ii) encounter of the different parentage chloroplast DNA molecules within the same cellular compartment by the plastid fusion, which is thought to be a rare event in a plant life cycle34. Common axiomatic assumptions in plant plastome evolutionary studies are uniparental inheritance, powerful functional constraints, and absent sexual recombination39. However, plastome recombination was detected in artificial interspecies somatic cell hybrids34 and proposed as the source of the markedly different phylogenies; unusual patterns of plastome polymorphism and linkage disequilibrium in Silene 40, Picea 41, Pinus 42 and Cycas 43. Thus, closed topologies of the plastid haplotype networks could be a signature of plastome sexual recombination in a history of plant taxa.
The closed subnetworks in a nuclear genome ITS2 locus that characterize I. cavalcantei can be explained by recombination/gene conversions between the parental ITS2 copies. The unexpected finding was a greater diversity in species-specific ITS2 alleles of I. cavalcantei (eleven species-specific alleles), as compared to I. marabaensis (two species-specific alleles). It could be that the species differ in the death/birth rates of repetitive rDNA operons that comprise ITS2 sequences44. Importantly, speciation events are likely to be accompanied by the bursts of repeat amplifications, which is reflected in the abundance of the species-specific repeat families that discriminate closely related plant species. The toxicity of metal ions often includes genotoxicity45. Thus, metal rich soils could influence the spontaneous rates of mutations and recombination46. We provide an evidence for the toxicity of Canga soils. Comparative analysis of spontaneous mutation rates in I. marabaensis versus I. cavalcantei, and assessment of Canga soil effects on genome stability will advance our understanding of repetitive sequence dynamics in Carajás morning glories.
The Canga soils tested in the laboratory conditions were found to be restrictive for the seedling establishment of Convolvulaceae family species Merremia aegyptia, Ipomoea grandifolia and Operculina hamiltonii, the latter two species occur in the Carajás National Forest. The result is consistent with a proposal that soil properties are the main drivers of vegetation composition and structure in Canga47. The symptoms of tissue necrosis in O. hamiltonii, M. aegyptia and I. grandifolia seedlings resembled calcium deficiency, which could be exacerbated by the aluminum toxicity48. In line with the foliar damage symptoms, composition analysis of the tested Canga soil samples showed lower Ca+2 contents as compared to horticultural soil (0.1–1.4 cmol/dm3 vs 6.9 cmol/dm3). However, soil fertility depends on complex and often poorly understood interactions between the biological, chemical and physical properties of soil3, 49. Further experiments are required to decipher causality between Canga soil properties and arrest O. hamiltonii, M. aegyptia and I. grandifolia seedling growth. It also remains to be seen whether soil differentiation in Canga islands drives largely allopatric distribution of I. cavalcantei and I. marabaensis.
We show that I. asarifolia pregerminated seeds developed on Canga soils into the plantlets that produced leaf biomass quantities that were comparable to those of I. cavalcantei and I. marabaensis plantlets, indicating that Canga ecosystems of the Carajás National Forest potentially could be occupied by the invasive species. In the Serra do Rola Moça State Park (Minas Gerais State, Brazil), 60% of grassy fields in Canga biomes has been invaded by the African grass Melinis minutiflora, which is thought to constitute a severe threat to the biodiversity50. Many species of Ipomoea are noxious weeds51 of which I. asarifolia is an emerging problem in Amazon and Unites States. We searched for and identified Ipomoea asarifolia road-side populations at ca. 5 km distances from the Carajás National Forest boundaries. Thus, the Canga conservation management must include continuous monitoring for and eradication of the invasive species in mining areas and along interconnected road system that infringed historical Canga island isolation by the forest.
Molecular analyses of population sampling in this work showed that some polymorphisms could be restricted to specific locations. For example, I. cavalcantei ITS2 haplotypes H6, H9, H8 and H10 were only observed among Canga N4 individuals; whereas plastome type AA was only found in Canga N3 individuals. Large areas of vegetation cover of Canga N5, Canga N4 and the southern part of the Canga S11 Plateau were12 and are lost due to mining that sustains the global economic growth. I. cavalcantei is thought to be endemic to Carajás Canga ecosystems10. It is difficult to predict whether the loss of hidden molecular diversity in Canga N4 will be detrimental to the species long-term survival in Cangas N1, N2 and N3 that are currently protected. Here, we show that species can be maintained in ex situ collection on standard horticultural soils. One outcome of our work is an ex situ collection of several hundred living I. cavalcantei and I. marabaensis individuals in our laboratories. Noteworthy, we also established clonal propagation of unique genotypes. Thus, reintroduction18 of the lost in a wild unique genotypes and/or rare gene alleles into the less disturbed Canga islands can become an element of the species conservation management.
Methods
Study organisms
Species of the several genera of the family Convolvulaceae populate Carajás National Forest14, including the genus of morning glories Ipomoea L. that is the largest within family Convolvulaceae with estimated 650 species15. Ipomoea cavalcantei 11 and Ipomoea marabaensis 12 were the major focus of this work. The surveyed populations and species sampling are listed in a Table S1. Taxonomical treatment of the American morning glories positioned I. cavalcantei and I. marabaensis within an ill-defined, unnamed series containing sixty one species from the section Eriospermum, subgenus Eriospermum52. Species are similar in vegetative morphological traits (Fig. S2), indicating that they could share the ancestry. However, I. cavalcantei and I. marabaensis are likely to be differentiated by the pollination syndromes (Figs 1d and S2). Such species pairs could provide useful models for studies of speciation and adaptive gene pleiotropy13, 51. I. cavalcantei and I. marabaensis are adapted to similar habitats that characterize Canga savannahs (Fig. S1), however I. marabaensis range is broader and those species were also found on granitic inselbergs (Fig. S1), for example. In Carajás National Forest, species ranges are altered by the mining industry (Fig. 1a). The molecular diversity information necessary for conservation planning and management is not available.
It is thought that I. cavalcantei and I. marabaensis could hybridize in a sympatry area in Canga N414. In 2016, we found four I. marabaensis individuals among the abundance of I. cavalcantei in Canga N4. No I. cavalcantei were found in Canga N6 that is only 1.4 km away from N4. In the sympatric area of Canga N4, our survey identified three putative hybrid individuals. Open anthers of putative hybrid plants had abundant pollen like I. cavalcantei, indicating proper meiosis and pollen development. We could collect a few viable seeds from three hybrids. It remains to be seen whether the progeny is from self-fertilization or cross-pollination by I. cavalcantei. However, fifty four controlled reciprocal pollinations between I. cavalcantei and I. marabaensis individuals that grew in a wild failed, indicating certain genetic or environmental constrains to interspecies hybridization.
The anthropogenic perturbation of Canga savannahs provides opportunities for invasive species, among which many Convolvulaceae species are known noxious weeds51. The effects of Canga soils on survival of three potentially invasive Convolvulaceae species: Ipomoea asarifolia, Ipomoea grandifolia and Merremia aegyptia (Table S10), has been tested. Because I. marabaensis can survive in the extreme environments of both Canga savannahs and granitic inselbergs, it was relevant to understand the invasive potential of Convolvulaceae species the distribution of which at present is limited to Carajas granitic inselbergs. Thus, the Operculina hamiltonii (Fig. S1; Table S10) was studied as well.
Geographic maps of the locations
The geographic maps were generated with ArcGIS version 10.2 (www.esri.com) based on satellite imagery source (http://goto.arcgisonline.com/maps/World_Imagery): Esri, DigitalGlobe, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community. The map images were processed in Adobe Photoshop CS6 to indicate agricultural and mining activities (Fig. 1a); species ranges (Fig. 1b and c); and gene allele distributions (Figs 2 and 4).
Plant material, DNA extraction, PCR amplification and DNA sequencing
DNA was extracted from the leaf tissues that were collected and stored in a NaCl-saturated 2% CTAB solution53. The DNA extractions were performed following the automated QIAcube HT (QIAGEN) protocol, using the QIAamp 96 DNA kit (QIAGEN), with some modifications. For each sample, a piece of approximately 30 mg of leaf tissue was cut, placed in a racked 1.2 mL polypropylene tube, frozen overnight at −80 °C (~18 h) and then macerated with two 3 mm carbide tungsten beads in the TissueLyser II (QIAGEN) for 1 min at 30 Hz. The powdered tissue was lysed in 600 µL of 2% CTAB lysis buffer [2% CTAB, 0.1 mM Tris-HCl (pH 8.0), 20 mM EDTA (pH 8.0), 1.4 M NaCl]. The tubes were gently mixed and incubated for 40 min at 60 °C in a water bath, followed by a 1 min spin at 4000 rpm, and then 300 µL of the supernatant were collected and transferred to a 96 square deep well plate, in which the automated extraction was carried out.
The PCR conditions for each of the analyzed regions (partial coding sequences of the rpoB, rpoC1, rbcL and matK and the intergenic spacers atpF-atpH, psbK-psbI and psbA-trnH) were set according to Shaw et al.54, with some modifications: 1.5 µL of the extracted DNA, 1.25 µL of the 10 × reaction buffer [100 mM Tris-HCl (pH 8.3) and 500 mM KCl], 1.2 µL of 25 mM MgCl2, 1.0 µL of the dNTP mix (2 mM each), 0.25 µL of each the forward and reverse primers (10 pmol) and 0.5 U of Taq polymerase, in a 12 µL reaction volume. The ITS2 amplification reactions were additionally supplemented with 1 µL of DMSO. Cycling conditions were the same for all amplicons, consisting of an initial denaturation at 94 °C for 3 min, followed by 30 cycles of 1 min denaturation at 94 °C, 1 min annealing at 54 °C (except for the matK reactions, in which the annealing temperature was 46 °C) and 1 min extension at 72 °C, and a final extension at 72 °C for 7 min. After the purification of the PCR products, 2 µL of the amplified DNAs were used in the bi-directional cycle sequencing reactions with the BigDye Terminator v3.1 kit (Applied Biosystems) using the manufacturer’s instructions. Amplicons were sequenced in a ABI 3730 Genetic Analyzer (Applied Biosystems). Primer sequences can be found in Supplementary Table S11.
Sequence datasets, alignments, secondary structure, phylogenetic and genetic variation analyses
It has been proposed that I. cavalcantei and I. marabaensis could be sister species12. It is expected that sister species could share gene alleles due to the incomplete sorting of alleles present in the ancestor21, 55. Hence, we initiated characterization of the natural molecular variation in I. cavalcantei and I. marabaensis. For that purpose, plastome (cpDNA) sequences of seven genes from several individuals were analyzed56. The plastome sequences at rpoB, rbcL, matK, psbK-psbI and atpF-atpH loci were identical (Table S2). Polymorphisms characterized rpoC1 and intergenic psbA-trnH sequences, hence the frequencies of those polymorphisms were analyzed in a larger number of individuals (Table S2). The genetic differentiation of I. cavalcantei and I. marabaensis was also characterized by sequence analysis of the nuclear ribosomal RNA gene (rDNA) internal spacer ITS217, 18, 20. The ITS2 analyses and discussions assumed that all analyzed ITS2 sequences are derived from the homogenized rDNA operon repeats positioned at a single locus that also functions as a nucleolar organizer. It is well possible that this assumption is an oversimplification of the actual rDNA genes organization in I. cavalcantei and I. marabaensis nuclear genomes57. To support taxa identification, the ITS2 and cpDNA sequences Ipomoea asarifolia, Ipomoea grandifolia, Merremia aegyptia and Operculina hamiltonii were also analyzed (Table S10).
DNA sequences were edited and trimmed using the software package Geneious version 1058. Five datasets were generated. In one dataset, only partial protein-coding sequences of the plastome genes rpoC, psbK, rbcL, matK and rpoB were concatenated. This dataset did not require significant additional editing of gaps with an exception of an in-frame 30 base pairs deletion in I. murucoides matK. After removal of matK indel variation, all sequences had equal length 2767 bp. In the second set the, atpF-trnH sequences were added, i.e. 3086 bp long for I. cavalcantei/marabaensis. Other concatenates were different in length due to very high indel variation of the atpF-trnH IGS (Fig. S4). Finally, the full set had added sequences of psbA-trnH IGS sequences (3532 bp for I. cavalcantei/marabaensis). The derived subsets were prepared, in which gaps due to indels were deleted manually. Four plastome types that characterize I. cavalcantei and I. marabaensis were included in the analyses. The gene orthologs from Ipomoea spp. and an outgroup species Merremia quinquefolia were extracted from published complete plastome sequences15. Sequences were aligned using the software MUSCLE59. The maximum likelihood bootstrap analyses sampling 1000 pseudoreplicates was performed using the software RAxML version 8.2.760 and the software PHYML61. Bayesian analyses were performed using MrBayes version 3.2.662, 63. A maximum parsimony bootstrap analysis was performed in the program PAUP* v40b1064.
Ipomoea nuclear rDNA amplicon sequences were annotated65 and ITS2 regions were extracted using the software available at ITS2 Database website. ITS2 were analyzed for direct folding66. Sequences with a direct fold were saved as possible templates. For the ITS2 sequences that did not fold directly, the best modeling template was searched amongst a pool of structures comprising Ipomoea ITS2 sequences reported in ITS2 Database and I. cavalcantei and I. marabaensis ITS2 that folded directly. The ITS2 haplotype H1 that is shared between I. cavalcantei and I. marabaensis was found to be the best template. Structured sequence alignments were analyzed in the alignment and editing tool 4SALE67, 68, which was also used to generate the images of the ITS2 secondary structures. Haplotype assignments were done manually. The ITS2 sequences that had no ambiguities indicated homozygous plants. ITS2 sequences from some individuals showed ambiguities. To infer the SNP phasing into haplotypes, we verified whether ambiguous SNP positions could be explained by hybridization between homozygous parents (Tables S5 and S6).
A minimum spanning network tree depicting the relationships of the haplotypes was produced using TCS computer program69, 70. Calculations of the standard diversity metrics, F-statistics and Analysis of Molecular Variance (AMOVA) were carried out using an integrated software package for population genetics data analysis Arlequin71. Genetic differentiation F ST values and AMOVA were done for a single internal transcribed spacer rDNA (ITS2) locus differentiated by 14 alleles and for two plastome loci represented by two alleles each. The nucleotide diversity (π), Tajima’s D and Fu’s F S were calculated using DNA sequences of nuclear genome locus ITS2 (223 bp); plastome loci rpoC1 (501 bp) and psbA-trnH (382 bp). In AMOVA analysis, the variation was first estimated in a single group that comprised four populations of I. cavalcantei, or five populations of I. marabaensis. Next, eleven group permutations were analyzed. The intraspecific population group assemblies that gave variation distribution similar to that of a single species group were considered to be biologically significant. We also assessed the genetic relationships by clustering of molecular data with the software package STRUCTURE version 2.3.416. The datasets were analyzed in four models with or without admixture; with or without consideration of the sampling locations as prior information to assist the clustering16. Twenty replicate runs were conducted for every value of K between 1 and the number of locations plus 3, with a burn-in of 10.000 Markov Chain Monte Carlo (MCMC) steps followed by 10.000 or 50 000 iterations. To determine the optimal number of clusters (K), ΔK values were calculated72.
Genomic size analysis
To determine whether I. cavalcantei, I. marabaensis and putative interspecies hybrids have similar genome sizes, and thus are likely to have similar ploidy, we used flow cytometry51. The nuclei suspensions were prepared essentially as described73 with a modification in the composition of the lysis buffer, which included the non-ionic detergent Triton X-100 and the phenolics absorbent poly-vinyl-pyrrolidone (PVP-40), 1% final concentrations each. The nuclei were stained with propidium iodide (PI) at a final concentration 50 µg/ml. Nuclei were obtained from fresh leaf tissues of plants grown in the laboratory. The procedure steps of leaf maceration, lysate clearing by filtration through 30 µm nylon mesh, and storage of nuclei suspensions were done on ice. For the comparative analyses, equal amounts (100 mg) of leaf tissues from two individuals were processed in 1 ml of extraction buffer. Nuclei suspensions were analyzed on a BD FACS Aria II flow cytometer, using a 488 nm laser. For each specimen, 1000 nuclei were acquired and three runs were made per sample. PI fluorescence mean was collected under 585/42 bandpass filter. The nuclei of Petroselinum crispum served as a reference standard (2 C = 4.50 pg)74.
Plant growth and soil test experiments
To understand the role of soils both in a natural species distribution and in susceptibility of Canga ecosystems to invasive plants, we analyzed Convolvulaceae species seedling establishment on top soils. Twenty to twenty five kilograms of a mixture of the organic matter and degraded lateritic rocks to the depth of ca. 0–20 cm, referred to as “soil”, were collected at sites near growing I. cavalcantei (two sites at Canga N4) or I. marabaensis (one site at S11 Plateau Canga). The control soil commonly used for horticultural applications was purchased from a local supplier Yamanaka Comércio Ltda. (Belém, Pará, Brazil). This soil is successfully used as a gardening potting soil for growing common ornamentals, such as hibiscus, bougainvillea, several species of palms etc. This quality of the control soil was satisfactory for our purpose to determine whether I. cavalcantei and I. marabaensis could be easily maintained in ex situ collections.
The major goal of the experimental design was to reduce the effect of all the environmental variables, except the composition of the plant growth substrate. To eliminate the effect of the soil biotic components, soil samples were autoclaved. Equal amounts of soil by weight were distributed into plastic pots, measuring 6 × 6 × 9,5 cm. One pot was placed on a balance and water was added until the first drop of water leaked from the bottom of the pot. The same amount of water minus 5 g was then added to all pots of a given soil sample. Thus, the water content was close to the water holding potential for each soil type. The water losses due to evaporation and plant evapotranspiration were replenished every day by bringing the weight of each individual pot to its original value. In this watering regime, there was no leaching of elements from the growth substrates, because water was never leaking out of the pots. Bi-distilled deionized water (resistance 16 MOhms) was used in experiments. To facilitate synchronous germination, seeds were scarified with a sanding paper and pre-germinated on wet paper disks in Petri plates. Seeds with just emerged roots were planted on soil. Two seeds per pot were planted in triplicate. Plants were cultured at 25 °C/22 °C with 12/12 hours light/dark photoperiod in a walk-in plant growth room. The selection and identification of the control Convolvulaceae species is explained in a Supplementary Table S9. Accessions of I. cavalcantei from Cangas N1, N3, N4; and I. marabaensis from Cangas N8, Tarzan, Plateau S11 were tested in these experiments. To assess plant growth, leaves was collected, dried at 65 °C and weighted (Fig. S7). The dried leaf sample comprised leaves from two plants grown on a soil in a single pot. Nine samples per species were measured for soils from N4 site#1, N4 site #2 and Control (i.e. 9 pots with a given soil sample, 18 individuals per species). Due to limited amount of soil from S11 Plateau Canga, six dried leaf samples per species were measured.
The extractable nutrient composition and properties of the soil samples were analyzed at the Center of Agricultural and Environmental Technologies (Centro de Tecnologia Agrícola e Ambiental (CAMPO), http://www.campo.com.br/cta/), following standardized procedures developed by the Brazilian Agricultural Research Corporation (Empresa Brasileira de Pesquisa Agropecuária, EMBRAPA)75. Organic matter (O.M.) and organic carbon content measurements were done according to Leite et al.76; and extractable phosphorus content on anion exchange resin according to Camargo et al.77. The measured parameters and respective values are summarized in a Table S9.
Electronic supplementary material
BABIYCHUK_SUPPLEMENTAL INFORMATION_SREP-16-51438B
Acknowledgements
The authors would like to thank Tasso Guimarães, Pedro Viana, Ana Maria Giulietti-Harley and Liziane Vilela Vasconcelos for stimulating discussions; Manoel João Pereira Lopes and Cinthia Helena Miléo de Miranda Bandeira for technical support and plant care. The work was funded by VALE S.A. The Brazilian National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) supported M.C.D. (process number 380290/2016-2), G.L.N. (process number 301659/2015-0), G.O. (process number 309312/2012-4). Biological material collection has been carried out in accordance with the authorization #48272-3 by SISBIO (http://www.icmbio.gov.br/sisbio/); Chico Mendes Institute of Biodiversity Conservation (ICMBio); Brazilian Ministry of Environment (MMA).
Author Contributions
E.B., S.K. and A.C. designed research; L.T., D.F.S., A.C., E.B. and S.K. collected samples; E.B., S.K., S.V., M.C.D., N.C.-F., G.L.N., J.F.S., V.L.I.F., G.O. performed research; E.B. and S.K. analyzed data; E.B. and S.K. wrote the main manuscript; E.B., J.F.S. and S.K. produced the figures, all authors revised the manuscript; E.B. finalized the manuscript.
Competing Interests
VALE S.A. supported the research at Instituto Tecnológico Vale (ITV). L.T., D.F.S. and A.C. are VALE S.A. employees. VALE S.A. did not influence the study design, data analysis or the interpretation of the results.
Footnotes
Elena Babiychuk and Sergei Kushnir contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07398-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–4. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Des Marais DL, Rausher MD. Parallel evolution at multiple levels in the origin of hummingbird pollinated flowers in Ipomoea. Evolution. 2010;64:2044–54. doi: 10.1111/j.1558-5646.2010.00972.x. [DOI] [PubMed] [Google Scholar]
- 3.Brady KU, Kruckeberg AR, Bradshaw HD., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Ann. Rev. Ecol. Evol. & Systematics. 2005;36:243–266. doi: 10.1146/annurev.ecolsys.35.021103.105730. [DOI] [Google Scholar]
- 4.Stebbins, G. L. Variation and Evolution in Plants (Columbia Univ. Press; New York: 1950).
- 5.Soltis PS, Soltis DE. The role of hybridization in plant speciation. Ann. Rev. Plant Biol. 2009;60:561–88. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer, C. E. et al. The physical environment of rupestrian grasslands (Campos Rupestres) in Brazil: geological, geomorphological and pedological characteristics, and interplays. (In Ecology and Conservation of Mountaintop Grasslands in Brazil pp. 15–53). Springer International Publishing. ISBN 978-3-319-29808-5 (2016).
- 7.Fernandes, G. W. ed. Ecology and Conservation of Mountaintop Grasslands in Brazil. Springer International Publishing. ISBN 978-3-319-29808-5 (2016).
- 8.Oliveira RS, et al. Mineral nutrition of campos rupestres plant species on contrasting nutrient‐impoverished soil types. New Phytologist. 2015;205:1183–1194. doi: 10.1111/nph.13175. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer, C. E., Cândido, H. G., Corrêa, G. R., Nunes, J. A. & Arruda, D. M. Soils Associated with Rupestrian Grasslands. In Ecology and Conservation of Mountaintop Grasslands in Brazil, pp. 55–69, Springer International Publishing. ISBN 978-3-319-29808-5 (2016).
- 10.Viana PL, et al. Flora das cangas da Serra dos Carajás, Pará, Brasil: história, área de estudos e metodologia. Rodriguésia. 2016;67:1107–1125. doi: 10.1590/2175-7860201667501. [DOI] [Google Scholar]
- 11.Austin DF. Novidades nas Convolvulaceae da flora Amazonica. Acta Amazonica. 1981;11:291–295. doi: 10.1590/1809-43921981112291. [DOI] [Google Scholar]
- 12.Austin DF, Secco RDS. Ipomoea marabaensis, nova Convolvulaceae da Serra dos Carajás (PA) Boletim Museu Paraense Emilio Goeldi, sér. Bot. 1988;4:187–194. [Google Scholar]
- 13.Wessinger CA, Hileman LC. Accessibility, constraint, and repetition in adaptive floral evolution. Dev. Biol. 2016;419:175–183. doi: 10.1016/j.ydbio.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simão-Bianchini R, Vasconcelos LV, Pastore M. Flora das cangas da Serra dos Carajás, Pará, Brasil: Convolvulaceae. Rodriguésia. 2016;67:1301–1318. doi: 10.1590/2175-7860201667530. [DOI] [Google Scholar]
- 15.Eserman LA, Tiley GP, Jarret RL, Leebens-Mack JH, Miller RE. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot. 2014;101:92–103. doi: 10.3732/ajb.1300207. [DOI] [PubMed] [Google Scholar]
- 16.Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resources. 2009;9:1322–32. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.China Plant BOL Group et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc. Natl. Acad. Sci. USA108, 19641–19646 (2011). [DOI] [PMC free article] [PubMed]
- 18.Cennamo P, et al. Genetic structure of Ipomoea imperati (Convolvulaceae) in the Mediterranean region and implications for its conservation. Phytotaxa. 2013;141:40–54. doi: 10.11646/phytotaxa.141.1.3. [DOI] [Google Scholar]
- 19.Coleman AW. Nuclear rRNA transcript processing versus internal transcribed spacer secondary structure. Trends in Genetics. 2015;31:157–163. doi: 10.1016/j.tig.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q, et al. Genetic homogenization of the nuclear ITS loci across two morphologically distinct gentians in their overlapping distributions in the Qinghai-Tibet Plateau. Sci. Reports. 2016;6:34244. doi: 10.1038/srep34244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper EA, Whittall JB, Hodges SA, Nordborg M. Genetic variation at nuclear loci fails to distinguish two morphologically distinct species of Aquilegia. PLoS ONE. 2010;5:e8655. doi: 10.1371/journal.pone.0008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elder JF, Turner BJ. Concerted evolution of repetitive DNA sequences in eukaryotes. Q. Rev. Biol. 1995;70:297–320. doi: 10.1086/419073. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AK, Escobar LK, Gilbert LE, Jansen RK. Paternal, maternal, and biparental inheritance of the chloroplast genome in Passiflora (Passifloraceae): implications for phylogenetic studies. Am. J. Bot. 2007;94:42–46. doi: 10.3732/ajb.94.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Manos PS, Miller RE, Wilkin P. Phylogenetic analysis of Ipomoea, Argyreia, Stictocardia, and Turbina suggests a generalized model of morphological evolution in morning glories. Systematic Botany. 2001;26:585–602. [Google Scholar]
- 25.McDonald JA, Hansen DR, McDill JR, Simpson BB. A phylogenetic assessment of breeding systems and floral morphology of North American Ipomoea (Convolvulaceae) J. Bot. Res. Inst. Texas. 2011;5:159–177. [Google Scholar]
- 26.Vasconcelos LV, Simão-Bianchini R, França F. Two new species of Ipomoea (Convolvulaceae) from the Chapada Diamantina of Bahia, Brazil. Brittonia. 2016;68:142–147. doi: 10.1007/s12228-016-9411-y. [DOI] [Google Scholar]
- 27.Streisfeld MA, Rausher MD. Genetic changes contributing to the parallel evolution of red floral pigmentation among Ipomoea species. New Phytologist. 2009;183:751–763. doi: 10.1111/j.1469-8137.2009.02929.x. [DOI] [PubMed] [Google Scholar]
- 28.Wessinger CA, Rausher MD. Predictability and irreversibility of genetic changes associated with flower color evolution in Penstemon barbatus. Evolution. 2014;68:1058–70. doi: 10.1111/evo.12340. [DOI] [PubMed] [Google Scholar]
- 29.Ng J, Smith SD. How to make a red flower: the combinatorial effect of pigments. AoB Plants. 2016;8:plw013. doi: 10.1093/aobpla/plw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCauley DE. Contrasting the distribution of chloroplast DNA and allozyme polymorphism among local populations of Silene alba: implications for studies of gene flow in plants. Proc. Nat. Acad. Sci. USA. 1994;91:8127–8131. doi: 10.1073/pnas.91.17.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis JR, Bentley KE, McCauley DE. Detection of rare paternal chloroplast inheritance in controlled crosses of the endangered sunflower Helianthus verticillatus. Heredity. 2008;100:574–580. doi: 10.1038/hdy.2008.11. [DOI] [PubMed] [Google Scholar]
- 32.Miyake K, Imai Y. Maternal transmission of mutated plastids in the Japanese morning glory. Bot. Gazette. 1935;96:571–574. doi: 10.1086/334503. [DOI] [Google Scholar]
- 33.Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 1988;75:1443–1458. doi: 10.2307/2444695. [DOI] [Google Scholar]
- 34.Greiner S, Sobanski J, Bock R. Why are most organelle genomes transmitted maternally? Bioessays. 2015;37:80–94. doi: 10.1002/bies.201400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnard‐Kubow KB, McCoy MA, Galloway LF. Biparental chloroplast inheritance leads to rescue from cytonuclear incompatibility. New Phytol. 2017;213:1466–1476. doi: 10.1111/nph.14222. [DOI] [PubMed] [Google Scholar]
- 36.Kapralov MV, Filatov DA. Widespread positive selection in the photosynthetic Rubisco enzyme. BMC Evol. Biol. 2007;7:73. doi: 10.1186/1471-2148-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, et al. Plastome organization and evolution of chloroplast genes in Cardamine species adapted to contrasting habitats. BMC Genomics. 2015;16:306. doi: 10.1186/s12864-015-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iida S, et al. Molecular adaptation of rbcL in the heterophyllous aquatic plant Potamogeton. PLoS One. 2009;4:e4633. doi: 10.1371/journal.pone.0004633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfe AD, Randle CP. Recombination, heteroplasmy, haplotype polymorphism, and paralogy in plastid genes: implications for plant molecular systematics. Syst. Bot. 2004;29:1011–1020. doi: 10.1600/0363644042451008. [DOI] [Google Scholar]
- 40.Erixon P, Oxelman B. Reticulate or tree-like chloroplast DNA evolution in Sileneae (Caryophyllaceae)? Mol. Phylogenet. Evol. 2008;48:313–325. doi: 10.1016/j.ympev.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan, A. R., Schiffthaler, B., Thompson, S. L., Street, N. R. & Wang, X. R. Interspecific Plastome Recombination Reflects Ancient Reticulate Evolution in Picea (Pinaceae). Mol. Biol. Evol., doi:10.1093/molbev/msx111 (2017). [DOI] [PMC free article] [PubMed]
- 42.Marshall HD, Newton C, Ritland K. Sequence-repeat polymorphisms exhibit the signature of recombination in lodgepole pine chloroplast DNA. Mol. Biol. Evol. 2001;18:2136–2138. doi: 10.1093/oxfordjournals.molbev.a003757. [DOI] [PubMed] [Google Scholar]
- 43.Huang S, Chiang YC, Schaal BA, Chou CH, Chiang TY. Organelle DNA phylogeography of Cycas taitungensis, a relict species in Taiwan. Mol. Ecol. 2001;10:2669–2681. doi: 10.1046/j.0962-1083.2001.01395.x. [DOI] [PubMed] [Google Scholar]
- 44.Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Ann. Rev. Genet. 2005;39:121–152. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gichner T, Patková Z, Száková J, Demnerová K. Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotoxicol. Environ. Safety. 2006;65:420–426. doi: 10.1016/j.ecoenv.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Kovalchuk O, Titov V, Hohn B, Kovalchuk I. A sensitive transgenic plant system to detect toxic inorganic compounds in the environment. Nature Biotech. 2001;19:568–572. doi: 10.1038/89327. [DOI] [PubMed] [Google Scholar]
- 47.Nunes JA, et al. Soil-vegetation relationships on a banded ironstone ‘island’, Carajás Plateau, Brazilian Eastern Amazonia. Anais da Academia Brasileira de Ciências. 2015;87:2097–2110. doi: 10.1590/0001-376520152014-0106. [DOI] [PubMed] [Google Scholar]
- 48.Rout G, Samantaray S, Das P. Aluminium toxicity in plants: a review. Agronomie. 2001;21:3–21. doi: 10.1051/agro:2001105. [DOI] [Google Scholar]
- 49.Parker SS. Buried treasure: soil biodiversity and conservation. Biodiversity & Conservation. 2010;19:3743–3756. doi: 10.1007/s10531-010-9924-8. [DOI] [Google Scholar]
- 50.Ribeiro PC, et al. Invasion of the Brazilian campo rupestre by the exotic grass Melinis minutiflora is driven by the high soil N availability and changes in the N cycle. Sci. Total Environ. 2017;577:202–211. doi: 10.1016/j.scitotenv.2016.10.162. [DOI] [PubMed] [Google Scholar]
- 51.Duncan TM, Rausher MD. Morphological and genetic differentiation and reproductive isolation among closely related taxa in the Ipomoea series Batatas. Am. J. Bot. 2013;100:2183–2193. doi: 10.3732/ajb.1200467. [DOI] [PubMed] [Google Scholar]
- 52.Austin DF, Huáman Z. A Synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon. 1996;45:3–38. doi: 10.2307/1222581. [DOI] [Google Scholar]
- 53.Rogstad SH. Saturated NaCl-CTAB solution as a means of field preservation of leaves for DNA analyses. Taxon. 1992;41:701–708. doi: 10.2307/1222395. [DOI] [Google Scholar]
- 54.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am. J. Botany. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- 55.Funk DJ, Omland KE. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Ann. Rev. Ecol., Evol. & Systematics. 2003;34:397–423. doi: 10.1146/annurev.ecolsys.34.011802.132421. [DOI] [Google Scholar]
- 56.Fazekas AJ, et al. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE. 2008;3:e2802. doi: 10.1371/journal.pone.0002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrasekhara C, Mohannath G, Blevins T, Pontvianne F, Pikaard CS. Chromosome-specific NOR inactivation explains selective rRNA gene silencing and dosage control in Arabidopsis. Genes & Dev. 2016;30:177–190. doi: 10.1101/gad.273755.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kearse M, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–90. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 61.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 62.Ronquist F, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–42. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huelsenbeck JP, Ronquist F. MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 64.Swofford, D. L. (Paup*: Phylogenetic Analysis Using Parsimony (and other methods) 4.0. Sinauer, Sunderland, Massachusetts, USA, 2001).
- 65.Keller A, et al. 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene. 2009;430:50–57. doi: 10.1016/j.gene.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Koetschan C, et al. ITS2 database IV: Interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Mol. Phylogenet. & Evol. 2012;63:585–588. doi: 10.1016/j.ympev.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Seibel P, Müller T, Dandekar T, Schultz J, Wolf. M. 4 SALE - A tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics. 2006;7:498–510. doi: 10.1186/1471-2105-7-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seibel P, Müller T, Dandekar T, Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4 SALE. BMC Res. Notes. 2008;1:91–10. doi: 10.1186/1756-0500-1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 70.Leigh JW, Bryant D. Popart: full‐feature software for haplotype network construction. Meth. Ecol.& Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 71.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 72.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 2005;14:2611–20. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 73.Loureiro J, Rodriguez E, Doležel J, Santos C. Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann. Bot. 2007;100:875–888. doi: 10.1093/aob/mcm152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obermayer R, Leitch IJ, Hanson L, Bennett MD. Nuclear DNA C‐values in 30 species double the familial representation in pteridophytes. Ann. Bot. 2002;90:209–217. doi: 10.1093/aob/mcf167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.EMBRAPA Manual de métodos de análise do solo. EMBRAPA, Rio de Janeiro, 2nd edition, 1–230 (2011).
- 76.Leite C, Bernardes RS, Oliveira SAD. Walkley-Black method for organic matter determination in soils contaminated by leachate. Revista Brasileira de Engenharia Agrícola e Ambiental. 2004;8:111–115. doi: 10.1590/S1415-43662004000100016. [DOI] [Google Scholar]
- 77.Camargo OA, Moniz AC, Jorge JA, Valadares JMAS. Métodos de análise química, mineralógica e física de solos do IAC. Campinas, Instituto Agronômico de Campinas. Boletim Técnico. 2009;106:1–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BABIYCHUK_SUPPLEMENTAL INFORMATION_SREP-16-51438B


