Abstract
Evaluating the factors responsible for differing species-specific sensitivities to declining seawater pH is central to understanding the mechanisms via which ocean acidification (OA) affects coral calcification. We report here the results of an experiment comparing the responses of the coral Acropora yongei and Pocillopora damicornis to differing pH levels (8.09, 7.81, and 7.63) over an 8-week period. Calcification of A. youngei was reduced by 35% at pH 7.63, while calcification of P. damicornis was unaffected. The pH in the calcifying fluid (pHcf) was determined using δ11B systematics, and for both species pHcf declined slightly with seawater pH, with the decrease being more pronounced in P. damicornis. The dissolved inorganic carbon concentration at the site of calcification (DICcf) was estimated using geochemical proxies (B/Ca and δ11B) and found to be double that of seawater DIC, and increased in both species as seawater pH decreased. As a consequence, the decline of the saturation state at the site of calcification (Ωcf) with OA was partially moderated by the DICcf increase. These results highlight that while pHcf, DICcf and Ωcf are important in the mineralization process, some corals are able to maintain their calcification rates despite shifts in their calcifying fluid carbonate chemistry.
Introduction
Ocean acidification (OA) results from a shift in seawater carbonate chemistry due to the uptake of anthropogenic CO2 by the oceans, and has been identified as a major threat for calcifying organisms1. Of particular concern are the effects of OA on scleractinian corals that form calcium carbonate reefs, a major ‘hotspot’ for marine biodiversity2. It has been extensively demonstrated, particularly in laboratory-based studies3, 4, that there is generally a negative effect of OA on coral calcification rates. However, recent studies indicate more nuanced responses to OA. For example, some species, such as massive Porites spp., are particularly tolerant to OA both in the laboratory5, 6 and in field studies7. Such species-specific responses have led to the hypothesis that ocean acidification could lead to a major shift in species composition between a small group of ecological winners and a larger group of ecological losers7.
It is therefore important to determine whether the susceptibility of corals to OA is determined by specific physiological traits, such as their morphology, rate of calcification6, 8, or their capacity to maintain high pH at the site of calcification when seawater pH declines9, 10. Maintaining elevated pH in the extracellular calcifying fluid, where precipitation of calcium carbonate occurs, is a critical step to initiate and sustain the mineralization process9. Direct measurements of pH in the calcifying fluid (pHcf) using pH microelectrodes, confocal microscopy, and isotopic methods using the boron isotope proxy have all shown that corals actively maintain pHcf well above seawater pH9, 11–13. It has also been shown under laboratory conditions that pHcf tends to partially follow changes in external pH, maintaining an offset between pHcf and external seawater pH even under acidified pH conditions9, 12, 13. Such capacity to maintain elevated pHcf could be one of the reasons explaining the resistance of some corals to modifications of the carbonate chemistry10. In addition to maintenance of pH at the site of calcification, physiological traits such as skeleton morphology or absolute rate of calcification have also been suggested as playing an important role in controlling the sensitivity of corals to OA6, 14. The rate of calcification in particular could be a key parameter, with fast calcifiers generally being more sensitive to OA than slow calcifiers, thus providing a possible explanation for the species-specific6 and intraspecific14 sensitivities to OA. While there are examples of slow calcifiers being affected by OA6, it is possible that the greater sensitivity of fast calcifiers can be explained by their greater requirement to export larger quantities of protons (H+) from their site of calcification. This requirement arises from the reaction: Ca2+ + HCO3 − → CaCO3 + H+ and the closely associated need to maintain a chemical micro-environment favoring mineralization through high pH (>8.6)12 and elevated CaCO3 saturation state (Ω)9, 15.
With increasing OA, the concentration of protons in seawater increases, which effectively steepens the proton concentration gradient between the site of calcification and the surrounding seawater. As a result, the energy required to transport protons across this larger gradient will also increase, potentially representing a significant additional metabolic cost for calcification as OA increases16. However it has also been questioned whether the maintenance of elevated pH and the export of protons against the proton concentration gradient is in fact an energetically expensive processes relative to the available energy resources9.
In addition to the maintenance of elevated levels of calcifying fluid pHcf, corals also have to maintain adequate supplies of dissolved inorganic carbon (DIC) and calcium at a sufficient level to elevate the aragonite saturation state in the calcifying fluid (Ωcf) to facilitate calcification. While several methods now exist to measure pHcf, which all provide similar results, measurements of the DIC concentration in the calcifying fluid (DICcf) has proved much more challenging. For example, DICcf derived from micro-sensors measurements17 of carbonate-ion concentration was interpreted as being similar to seawater DIC levels. However, these results were highly spatially dependent and could not be determined in conjunction with pHcf measured at the same location. In contrast, geochemical proxies18, 19 and modelling of aragonite saturation at the site of calcification9, 20 both indicate that DICcf is approximately double than DIC in seawater. As pHcf and DICcf have a direct effect on the chemical conditions (Ωcf) in the calcifying fluid, measuring these two parameters in parallel is a critical step when assessing the tolerance of coral calcification to OA.
The present study was designed to test two main hypotheses regarding the response of corals to OA. First, we tested whether a rapidly calcifying coral6 was more sensitive to OA than a slow calcifying coral from the same location. Second, we tested whether a coral species that has been identified as being particularly tolerant to OA (P. damicornis)21 was able to maintain constant carbonate chemistry conditions in its calcifying fluid independently of the surrounding seawater conditions. To test these hypotheses, we designed an experiment in which one coral species potentially sensitive to ocean acidification (Acropora yongei) and one expected to be resistant (Pocillopora damicornis) were incubated over an 8-week period under three different levels of seawater pH (~8.1, 7.8, and 7.6). We measured calcification rates from 36 individuals for the two species grown under these different pH conditions and determined both the pHcf and DICcf (calculated from estimates of [CO3 2−]cf and pHcf) using both isotopic (δ11B) and geochemical (B/Ca) proxies (see following).
Materials and Methods
Coral collection
The experiment was performed from October to December 2015 at the Indian Ocean Marine Research Centre’s new mesocosm facility at Watermans Bay, Western Australia, Australia. Corals used during the experiments consisted of 36 × 5-cm branches of Acropora yongei and 36 × 5-cm branches of Pocillopora damicornis that were collected 15 days prior to the beginning of the experiment from Salmon Bay, Rottnest Island, Western Australia, at ~1–2-m depth. Rottnest Island is located ~10 km offshore from Watermans Bay. Therefore, the translocated specimens were subjected to initially very similar conditions. After 2 days of recovery in continuously renewed seawater, the branches were glued to plastic bases (4 × 4 cm) with Z-Spar (A788 epoxy) to form nubbins. Nubbins were placed in the experimental tanks and allowed to recover from translocation and to acclimate to the laboratory conditions over a further 12 days.
Treatments and regulation of pCO2
Seawater pCO2 was manipulated in twelve 20 L header tanks in which seawater was continuously renewed at ~0.3 L min−1. Seawater was pumped from 12-m depth, 150-m offshore, in Waterman Bay and filtered over series of sand filters (corresponding to a mesh size of 20 μm) before delivery to the header tanks. Each header tank supplied via gravity three incubation containers (for a total of 36 incubation containers) in which individual organisms for each species were maintained, along with two individual coralline algae used in a separate experiment22. Light was provided by 150 W LED (Malibu LED, Ledzeal) that followed a natural diel cycle. Light was gradually ramped-up in the morning commencing from 6:00 h until noon to reach a maximum of ~400 μmol quanta m−2 s−1 at noon, and then ramped down until total darkness at 18:30 h. Temperature was kept at 21 °C, which is the average seawater temperature in Salmon Bay during the austral spring23. The total alkalinity levels were monitored periodically and were found to be relatively constant (see following) due to continuous delivery of fresh seawater that prevented the tank water chemistry from being altered by the organisms’ metabolism.
The twelve header tanks were used to create three pH treatments in quadruplicate that corresponded to: (1) a present day pH (pH ~ 8.1; pCO2 ~ 400 μatm), (2) a pH value commonly predicted by the end of the current century under representative concentration pathway (RCP) 6.0 (pH ~ 7.8; pCO2 ~ 750 μatm)24, and (3) a pessimistic pH projection for the end of the century under RCP 8.5 (pH = 7.6; pCO2 ~ 1200 μatm). pH treatments were controlled in the header tanks using pH-controllers (AquaController, Neptune systems, USA). The set-point pH was determined by a feed-back mechanism that varied the rate of bubbling of pure CO2 in the header tanks. The typical precision on the set-point pH was 0.03 unit over a full 24 hour period. Treatment water was continuously delivered from each header tank to their respective ×3 incubation tanks at ~100 mL min−1. Submersible water pumps provided continuous turbulent water motion in each incubation tank.
Carbonate chemistry
Seawater pH on the total scale (pHT) and temperature were measured at 09:00 h every ~2 d in each incubation tank, using a pH meter calibrated every 2 d on the total scale using Tris/HCl buffers25. Total alkalinity (A T) was measured weekly in the header tanks and 12 randomly chosen incubation tanks using a spectrophotometric method. A T was calculated using a modified Gran function25, and titrations of certified reference materials (CRM) provided by A.G. Dickson (batch 151) yielded A T values within 2 µmol kg−1 of the certified value. A T, pHT, temperature, and salinity were used to calculate the carbonate chemistry parameters using the seacarb package26 running in R software (R Foundation for Statistical Computing) (Table 1).
Table 1.
Mean carbonate chemistry for each treatment during the 8-week experiment.
| Treatment | pHT | A T (μmol kg−1) | C T (μmol kg−1) | pCO2 (μatm) | Ωarag | T (°C) |
|---|---|---|---|---|---|---|
| Ambient | 8.09 ± 0.05 | 2358 ± 5 | 2061 ± 2 | 369 ± 4 | 3.28 ± 0.02 | 20.9 |
| High | 7.81 ± 0.05 | 2358 ± 5 | 2201 ± 1 | 779 ± 6 | 1.94 ± 0.01 | 20.9 |
| Very High | 7.63 ± 0.05 | 2357 ± 5 | 2270 ± 1 | 1217 ± 9 | 1.36 ± 0.01 | 21.0 |
The mean ± SE dissolved inorganic carbon (C T), partial pressure of CO2 (pCO2), and the saturation states of aragonite (Ωarag) were calculated from pHT, total alkalinity (A T), and temperature (T).
Calcification rates
Prior to the incubation, the skeletons of the organisms were stained by placing the organisms for 30–60 min in seawater enriched with the fluorescent dye calcein at 50 mg L−1 with a pH adjusted to ~8.1. Single specimens of P. damicornis and A. yongei were placed randomly in each of the 36 incubation tanks, and calcification was measured over the 8-week period using buoyant weighing27. The difference in buoyant weight between the beginning and end of incubation period was converted to dry weight using the density of aragonite (2.93 g cm−3) and used to calculate net rate of calcification. The calcification rate was normalized to surface area of the coral tissue (mg cm−2 d−1) determined using the relationship between skeleton weight and surface area previously established for this coral species from Rottnest Island22.
Determination of pHcf, [CO32−]cf and DICcf
Determination of the calcifying fluid pHcf, [CO3 2−]cf and DICcf was undertaken for all organisms after completion of the experiment using the boron isotopic proxy method13, 28 and the recently developed19, 29 B/Ca method.
In brief, the use of the δ11B pH proxy is based on the assumption that of the two boron species (borate and boric), only the borate ion is incorporated into the carbonate skeleton of calcifiers precipitating aragonite. This behaviour has been confirmed from the δ11B isotopic systematics observed in aragonitic calcifiers30 and in inorganic aragonite precipitation experiments, and also by measurements made using a pH sensitive dye12. Therefore, the δ11B composition of the coral’s skeleton provides direct constraints on the pH of its calcifying fluid (pHcf). Measurements of the skeleton geochemistry were done on the tip of the branches (first 1–2 mm) that corresponded to material deposited during the 8-week incubation as shown by the calcein staining. The selected apical-tip portion of the skeleton was then crushed in a mortar and pestle. The samples were rinsed and bleached to remove any residual organic material. Dissolution of the sample powders was undertaken in 0.5 N HNO3. Once dissolved, boron ions were extracted using cation ion exchange resin31 with δ11B determined on a multicollector inductively coupled plasma mass spectrometry (NU II). Measurements of the international carbonate standard JCP-1 yielded a mean value of 24.37 ± 0.17‰ (mean ± 2 SD, n = 8), which was similar to the 24.33 ± 0.11‰ (SE) reported previously32.
Calculations of pHcf based on δ11B were made using the calculations (equation 1) of Trotter et al.28:
| 1 |
where: δ11Bsw represents the δ11B in seawater (δ11Bsw = 39.61‰)33 and α(B3-B4) = 1.027234. The dissociation constant of boric acid pK B has a well-established value at given temperatures and salinities35. Because of a measurement error on δ11B of 0.17‰, uncertainty on pHcf estimates was 0.01 units.
A recently developed method19, based on inorganic experiments, was utilized to calculate the [CO3 2−]cf and combined with the pHcf, the DIC at the site of calcification (DICcf)29. For the determination of [CO3 2−]cf, calculations were based on experiments19 that measured the ratio of boron to calcium in aragonite precipitated inorganically under a wide range of carbonate chemistries. These showed that the substitution of boron into aragonite is closely linked to the carbonate ion concentration19. Thus, in combination with δ11B derived pHcf, the B/Ca ratio provides a quantitative means to determine the [CO3 2−] and hence the [DIC] of the calcifying fluid (i.e. DICcf). B/Ca ratios were determined on the same aliquot of the solution used for pHcf estimates, and DICcf was calculated from estimates of carbonate ion concentrations using the following equation (2):
| 2 |
where K D = K D.0 exp(−k KD[H+]T) with K D,0 = 2.97 ± 0.17 × 10−3 (±95% CI), = 0.0202 ± 0.04229. The concentration of DICcf was then calculated from estimates of pHcf and [CO3 2−]cf 29.
Uncertainties on the estimates of DICcf and Ωcf were calculated by using Monte-Carlo simulations that randomly used values of the determined parameters (δ11B and B/Ca) between the mean ± SD (δ11B SD = 0.17‰ and B/Ca SD = 18) over 1000 iterations.
A “bio-inorganic model” of calcification based on abiotic rate kinetics of CaCO3 precipitation (IpHRAC model)9 was calculated for the range of Ωcf estimates calculated in this study and the experimental temperature. This model is based on an empirical exponential rate dependence law for carbonate precipitation (G): G = k a (Ω cf − 1)n, where k a = −0.0177T2 + 1.47 T + 14.9, n = 0.0628 T + 0.0985, and T is the temperature.
Statistical analysis
The assumptions of normality and equality of variance were evaluated through graphical analyses of residuals using the R software. Effects of each header tank on the response of calcification, pHcf and DICcf to the CO2 treatments were analyzed using a two-way ANOVA, with the physiological measurement (e.g., calcification, pHcf, and DICcf) as the dependent variable, CO2 treatments as fixed effect, and header tank as a random factor. When no significant effects of the header tank were detected and p > 0.25, header tank was dropped from the analysis36, and individual organisms were treated as statistical replicates. All statistical analyses were done with R.
Results
Carbonate chemistry was successfully manipulated in all the treatments, with seawater pHT maintained at 8.09 ± 0.05, 7.81 ± 0.05, and 7.63 ± 0.05 (mean ± SE) when grouped by pH treatments, which corresponded to pCO2 of 369 ± 4, 779 ± 6, and 1217 ± 9 μatm, respectively (Table 1). Hereafter these treatments will be referred to as pH 8.1, 7.8 and 7.6 for convenience. Because seawater was continuously renewed in the tanks, total alkalinity was maintained at ~2358 ± 5 μmol kg−1 in all treatments. At the end of the experiment all corals were alive in all treatments and no bleaching as a function of the pH was observed.
For Acropora youngei, calcification was the highest in the pH 8.1 treatment (1.97 ± 0.13 mgCaCO3 cm−2 d−1) and the lowest (1.34 ± 0.09 mgCaCO3 cm−2 d−1) in the pH 7.6 treatment (Fig. 1A, Table 2). This indicates a significant effect of seawater pH on calcification (p < 0.001) with calcification rates decreasing as a function of decreasing pH. There was no significant effect of seawater pH on calcification for P. damicornis (p = 0.674) (Fig. 1B, Table 2).
Figure 1.
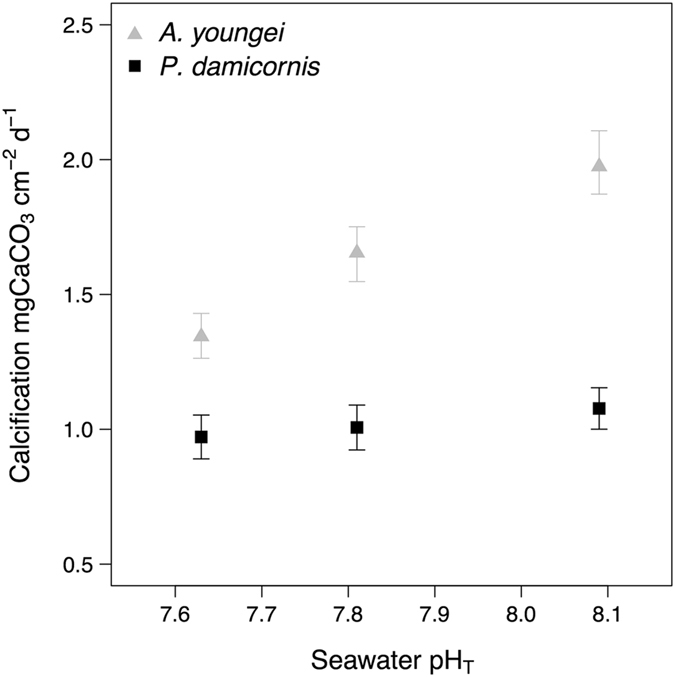
Effects of pCO2 on the surface area-normalized net calcification of the corals Acropora youngei (grey triangles) and Pocillopora damicornis (black dots). Calcification was measured on corals that were incubated during 8 weeks under pH = 8.09, 7.81, and 7.63. Values displayed are mean ± SE (n = 12).
Table 2.
Mean calcification rates, pH in the calcifying fluid (pHcf), Dissolved Inorganic Carbon in the calcifying fluid (DICcf), and aragonite saturation state in the calcifying fluid (Ωcf) determined on the coral A. youngei and P. damicornis incubated during 8 weeks under pH = 8.09, 7.81, and 7.63.
| Species | Treatment | Calcification (mgCaCO3 cm−2 d−1) | pHcf | DICcf (μmol kg−1) | Ωcf |
|---|---|---|---|---|---|
| A. youngei | pH 8.1 | 1.97 ± 0.13 | 8.51 ± 0.01 | 3866 ± 47 | 14.0 ± 0.1 |
| pH 7.8 | 1.65 ± 0.10 | 8.46 ± 0.02 | 4044 ± 87 | 13.3 ± 0.2 | |
| pH 7.6 | 1.34 ± 0.09 | 8.44 ± 0.02 | 4127 ± 82 | 13.1 ± 0.2 | |
| P. damicornis | pH 8.1 | 1.07 ± 0.08 | 8.48 ± 0.02 | 3493 ± 64 | 12.0 ± 0.3 |
| pH 7.8 | 1.00 ± 0.08 | 8.40 ± 0.02 | 3697 ± 74 | 10.9 ± 0.2 | |
| pH 7.6 | 0.97 ± 0.08 | 8.35 ± 0.02 | 3834 ± 70 | 10.2 ± 0.2 |
Values are mean ± SE (n = 12).
In A. youngei, δ11B was significantly affected by the pH treatment (p = 0.004) and decreased linearly with decreasing seawater pH following the equation δ11B = 2.37 pHSW + 4.41 (Fig. 2A; Raw data available in the Supplementary Table 1). pHcf, calculated from boron isotopes, decreased with seawater pH in A. youngei from 8.51 ± 0.01 in the ambient seawater pH (8.1) to 8.45 ± 0.02 in the pH 7.6 treatment (Fig. 2B, Table 2). Nevertheless there were small but significant effects of seawater pH on pHcf (p = 0.004), and posthoc analyses showed that pHcf differed between seawater pH 8.1 and pH 7.8 (TukeyHSD posthoc analyses, p = 0.044) and between seawater pH 8.1 and pH 7.6 (TukeyHSD posthoc analyses, p = 0.004). The linear decrease of pHcf with seawater pH for A. youngei followed the equation pHcf = 0.157 pHSW + 7.24. For P. damicornis, seawater pH also significantly affected δ11B (p < 0.001) that decreased linearly with decreasing seawater pH (δ11B = 4.37 pHSW −12.11; Fig. 2A; Raw data available in the supplementary Table 2). As a result, pHcf of P. damicornis also decreased linearly with seawater pH (pHcf = 0.30 pHSW + 6.08; Fig. 2B, Table 2). Treatments significantly affected pHcf (p < 0.001), with posthoc analyzes showing that pHcf differed between all treatments (p ≤ 0.05 for all comparisons). However, there was no relationship between calcification rates and pHcf for both corals (Supplementary Figure 1).
Figure 2.
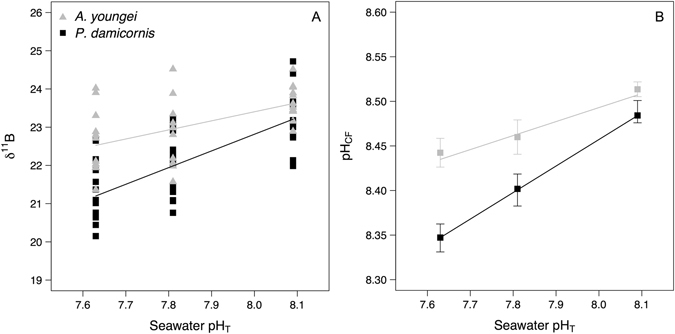
δ11B and estimates of pH in the calcifying fluid (pHcf) determined on the corals Acropora youngei (grey triangles) and Pocillopora damicornis (black dots) incubated during 8 weeks under pH = 8.09, 7.81, and 7.63. For both corals, the relationships between δ11B and seawater pH were best fit with linear models (δ11B = 2.37 pHSW + 4.41, p < 0.001, and δ11B = 4.37 pHSW − 12.11, p < 0.001, for A. youngei and P. damicornis, respectively). Therefore, the relationships between pHcf and seawater pH also were best fit with linear models (pHcf = 0.157 pHSW + 7.24, p < 0.001, and pHcf = 0.30 pHSW + 6.08, p < 0.001, for A. youngei and P. damicornis, respectively). Values displayed for δ11B are individual replicates and are mean ± SE (n = 12) for pHcf. Uncertainty on pHcf estimates was 0.01 pH unit.
The B/Ca ratio varied for A. pulchra from 618 ± 6 to 591 ± 10 μmol mol−1 at pH 8.1 and pH 7.6, respectively, but there was no significant pH treatment effect (p = 0.101) (Fig. 3A). For P. damicornis, there was a significant pH effect on the ratio B/Ca (p = 0.036) that decreased from 690 ± 10 μmol mol−1 at pH 8.1 to 649 ± 11 μmol mol−1 at pH 7.6 (Fig. 3A). Posthoc analysis showed that the B/Ca ratio differed between pH 8.1 and pH 7.6 (TukeyHSD; p = 0.027). For both corals, the B/Ca ratios increased linearly as a function of δ11B (and then pHcf) (B/Ca = 31.6 δ11B–124, p < 0.001, R2 = 0.78, and B/Ca = 25.8 δ11B + 98.5, p < 0.001, R2 = 0.57, for A. youngei and P. damicornis, respectively; Supplementary Figure 2).
Figure 3.
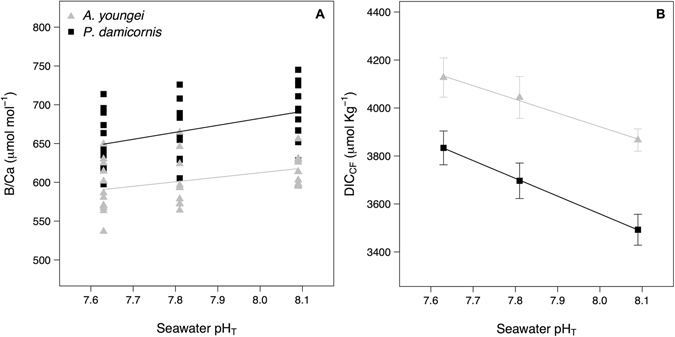
Measured B/Ca ratios (A) and DIC in the calcifying fluid (DICcf) estimates (B) for the corals Acropora youngei (grey triangles) and Pocillopora damicornis (black dots). Measurements and estimates were made on corals incubated at pH = 8.09, 7.81, and 7.63 during 8 weeks. Values for the B/Ca ratios are individual measurements and displayed DICcf are mean ± SE (n = 12). Uncertainty on DICcf estimates was 110 μmol. Kg−1.
The calculated DICcf based on the combined δ11B and B/Ca systematics for A. pulchra ranged between 3866 ± 46 and 4127 ± 81 μmol kg−1 in the pH 8.1 and pH 7.6 treatments, respectively, while for P. damicornis DICcf ranged from 3493 ± 64 at pH 8.1 to 3833 ± 70 μmol kg−1 at pH 7.6 (Fig. 3B, Table 2). For both corals there was a significant effect of the seawater pH on DICcf (p = 0.041 and 0.005, for A. pulchra and P. damiconis, respectively).
Estimations of the aragonite saturation state in the calcifying fluid (Ωcf) from pHcf and DICcf showed that it was more elevated in A. pulchra than in P. damicornis (Fig. 4, Table 2). For both species there was a significant treatment effect on Ωcf (p = 0.001).
Figure 4.
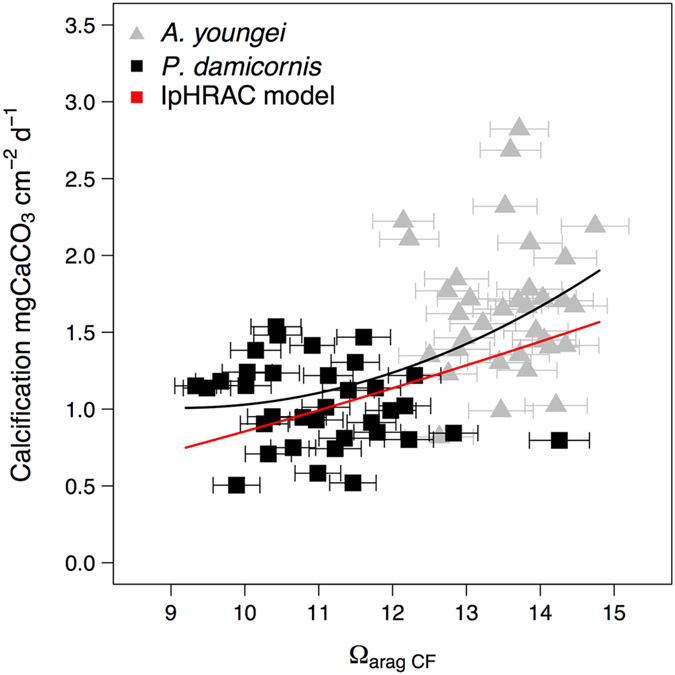
Calcification rates of Acropora youngei (grey triangles) and Pocillopora damicornis (black dots) as a function of estimates of the aragonite saturation state in the calcifying fluid (Ωarag cf) made using estimates of pHcf and DICcf. The horizontal error-bars represent the uncertainties on Ωarag cf estimates. The mean relationship between calcification and ΩaragCF for both species was best fitted by the polynomial relationship y = 0.025X 2 + 0.63x + 3.03 (black line). The red lines corresponded to a “bio-inorganic model” of calcification based on the known abiotic rate kinetics of CaCO3 precipitation as a function the aragonite saturation state Ωcf (IpHRAC)9. This model is based on an empirical exponential rate dependence law for carbonate precipitation (G): G = k a (Ω cf − 1)n; with k a = −0.0177T2 + 1.47 T + 14.9 and n = 0.0628 T + 0.0985.
Discussion
Understanding the role played by seawater carbonate chemistry on the chemistry of the calcifying fluid, and quantifying the physiological control of corals on their calcifying environment is of critical importance in assessing the future of coral in a high pCO2 world. Our study showed contrasting responses of calcification, pHcf, and DICcf to seawater pH in two subtropical corals from Western Australia. Calcification of the fast growing species A. youngei was more affected by OA than calcification of the slower growing P. damicronis, but pHcf was more affected by seawater pH in P. damicornis. DICcf increased in both species with decreasing seawater pH, with a larger increase in P. damicornis. These results were contrary to our original hypothesis that both calcification and pHcf would be more affected by OA in A. youngei than in P. damicornis. These findings were somewhat unexpected and demonstrate the complex link between calcification and carbonate chemistry within the calcifying fluid.
Calcification
The pH treatments had a very strong species-specific effect with rates of calcification declining by 35% between pH 8.1 and pH 7.6 in A. youngei, while they did not change significantly in P. damicornis. These results are consistent with previous findings; i.e. that the calcification of P. damicornis is generally insensitive to OA across several different locations21 (but see also ref. 37). The tolerance of P. damicornis from Rottnest Island to OA, confirms that these inferences extends even to those living in subtropical environments. The P. damicornis specimens calcified here at rates that were similar or higher to those in past studies at 27 °C under higher light21 and to those recorded in situ 23. This illustrates the capacity of this species to maintain optimal growth rates under various pCO2, temperature and light regimes.
The genus Acropora is generally highly sensitive to acidification38, 39 (but see also refs 40, 41): this sensitivity to OA can now be extended to A. youngei. Importantly rates of calcification (~1–2 mgCaCO3 cm−2 d−1), and the species-specific responses to OA of these corals growing in a sub-tropical region were highly comparable to those previously reported for the same species/genus in warmer more oligotrophic waters6. Together, the responses of both species examined demonstrate that sub-tropical coral have similar sensitivities to OA to their tropical counterparts.
It has been suggested that for Mediterranean corals8 and tropical corals and calcifying algae6, 14 that fast calcifiers might be more sensitive to a decline in seawater pH than slow calcifiers. As previously noted this maybe due to faster rates of calcification leading to the requirement for higher rates of export of protons from the calcifying fluid, a process that may become more difficult under OA6, 16, 42, 43, and whose energetic requirements are still questioned9. In contrast, slow calcification produces fewer protons and requires the import of less carbon to the site of calcification6. While the present study is only based on two corals species, it tends to support this hypothesis, since the rapidly growing coral such as A. youngei are more susceptible to OA. However, the export of protons from the calcifying fluid is not the only parameter controlling calcification. This is revealed by the capacity of P. damicornis to maintain constant calcification despite the relative sensitivity of its pHcf to decreasing seawater pH.
pHcf at the site of calcification
Determining the pHcf at the site of calcification is a powerful tool, providing insights into the likely mechanisms controlling the response of corals to OA. Past studies have shown that corals upregulate their pHcf well above seawater pH, and that a decrease of seawater pH leads to a decrease in pHcf 9, 12, 13. The present results are in agreement with these previously mentioned studies as both A. youngei and P. damicornis up-regulated their pHcf well above seawater (0.43 and 0.40 pH unit in ambient conditions for A. youngei and P. damicornis, respectively), and pHcf decreased significantly with decreasing seawater pH. The greater decrease in pHcf (~0.3 units per seawater pH unit) with decreasing seawater pH in P. damicornis demonstrates that the magnitude of the decrease in pHcf and rates of calcification are not directly correlated (Supplementary Figure 1). In contrast to Porites cylindrical - a coral able to maintain both calcification and pHcf at ambient values when exposed to pH 0.25 units below ambient10 – the coral P. damicornis was able to maintain calcification at ambient values across a range of treatments despite decreasing pHcf.
The δ11B values measured here for A. youngei were within the same range as that previously reported for this genus44–46 (Supplementary Figure 3). However, the limited decrease of pHcf (δ11B) with decreasing seawater pH in A. youngei (0.16 pHcf unit per seawater pH unit) is unusual for species from this genus, for which a greater sensitivity of pHcf to seawater pH has previously been reported (e.g., 0.51 pHcf unit per seawater pH unit9). Different analytical methods (MC-ICPMS vs NTIMS) could explain a portion of the differences between the present study and previous measurements done on Acropora 44. However, the large differences found at low seawater pH cannot be only explained by analytical discrepancies. Furthermore, the decrease in pHcf may not always be linear in Acropora. For example, the coral Acropora digitifera had a pHcf that decreased rapidly between pH 8.2 and 7.8 before leveling out at pH 7.446. The difference between the non-liner relationship found previously46, and the linear relationship found in the present study, was principally caused by higher values of δ11B in this previous study46 at control seawater pH (Supplementary Figure 3). At lower seawater pH, the δ11B values and the direction of the curves of the present study and that of Tanaka et al.46 were in agreement (Supplementary Figure 3). This demonstrates a very similar response of pHcf to seawater pH in these two Acropora species.
The contradictory strong decrease in calcification and limited decrease in pHcf for A. youngei can potentially be explained by two non-exclusive hypotheses. First, as daytime calcification is about ~3 fold higher than dark calcification47, the isotopic composition of the skeleton mostly represents conditions in the calcifying fluid during the day. In the case of A. youngei, we can hypothesize that this coral was able to maintain elevated calcification and pHcf during the day, while dark calcification could have been suppressed or negative (i.e., night dissolution48) in the high pCO2 treatments. As a result, calcification could be strongly affected by pH because of decreased nighttime calcification. Under this scenario, pHcf would remain unaffected, as it represents what is occurring in the light. The second potential explanation is that pHcf is not the only driver of calcification in corals that varies due to external seawater carbonate chemistry. For example, in the low pH seawater, the synthesis of organic matrix49, 50 could have been reduced because of less energy available for this process. This would limit the ability of A. youngei to precipitate calcium carbonate, regardless of chemically favorable conditions.
Furthermore, pHcf is not the only parameter governing the chemistry in the calcifying fluid. Other parameters such as calcium and DIC concentrations can affect both the saturation state and the rates of calcium carbonate precipitation. Finally, a reduction in calcification while pHcf remained almost constant could demonstrate that this coral requires elevated pHcf to precipitate calcium carbonate, and is not able to elevate its rate of proton export under OA; i.e., the gradient in external to internal proton concentration increases, regardless of internal pHcf. As a result, a reduction in calcification due to reduced proton production export, could have been the only option available for A. youngei to maintain elevated pHcf and precipitation of calcium carbonate, though at a lower rate.
DICcf at the site of calcification
Here we provide one of the first estimations of DICcf using geochemical proxies for corals grown under a range of controlled pH conditions. In both corals, DICcf (between ~3500 and 4100 μmol kg−1) was well above seawater DIC (between ~2060 and 2270 μmol kg−1). This is similar to that estimated for Porites spp., using δ11B and B/Ca ratio18, and previous studies that have estimated DICcf to be about double the seawater DIC9, 12. Recently, McCulloch et al.29 also showed using δ11B and B/Ca ratio that DICcf in massive Porites corals varies seasonally well above seawater DIC and can reach values as high as 3.2 times the seawater DIC in summer. The agreement between these past studies and the present results lends support for the validity of our approach. Only a recent study has reported much lower estimates of DICcf using carbonate micro-probe17. The discrepancy between geochemical and micro-probe approaches are still unresolved, and could be due to the spatio-temporal differences between the two methods. Micro-probe methods provide spot measurements of pH and DIC at different times and location of what is inferred to be the chemical conditions in the calcifying fluid, while geochemical proxies indicate the average chemistry in the calcifying fluid responsible for skeletal formation averaged over weeks of carbonate precipitation.
DICcf increased as a function of decreasing seawater pH for both A. youngei and P. damicornis. As a result, the two species exhibited strong linear relationships between DICcf and pHcf, where decreasing pHcf resulted in higher DICcf (Supplementary Figure 4). This is similar to what has been found at the colony level on massive Porites spp. where seasonal increases in DICcf were associated with decreases in pHcf. Increasing the import of DIC to the calcifying fluid when pH decreases might be one strategy for corals to alleviate some of the negative effects of decreasing pH. Thereby maintaining constant DIC/proton ratios in the calcifying fluid to maintain precipitation rates. Interestingly, the increase in DICcf was larger in P. damicornis, which could explain part of the capacity of this species to maintain calcification despite decreasing pHcf.
Increasing DICcf with OA could be the result of several processes. Firstly, with ocean acidification, more DIC is available in seawater - DIC increased by ~200 μmol kg−1 between pH 8.1 and pH 7.6 - which could favor the uptake of DIC if this process is substrate limited. Secondly, a reduction in calcification could lead to less DIC being consumed, therefore favoring its accumulation in the calcifying fluid if the import of DIC remain constant. This hypothesis is mostly valid for A. youngei for which calcification decreased. Thirdly, corals might actively increase the rate at which they import DIC in the calcifying fluid under OA by actively increasing the activity of their Cl-/bicarbonate transporter51 to increase DIC concentration in the calcifying cell or/and the calcifying fluid52. Finally, the increase in DICcf (and therefore total alkalinity in the calcifying fluid) with decreasing seawater pH could be the cause of the decrease in pHcf, because maintaining elevated pHcf in a calcifying fluid with higher buffering capacity (higher TAcf) is chemically more challenging.
Ωcf at the site of calcification
Estimates of the aragonite saturation state in the calcifying fluid showed that Ωcf was well above Ωarag in seawater, for both corals. When pooled by treatments, Ωcf appeared to be more affected by pH in P. damicornis than A. youngei because of the stronger decrease in pHcf for P. damicornis (Supplementary Figure 5).
When calcification rates of the two corals species determined in this study were pooled together and plotted against Ωcf, calcification followed a polynomial relationship with Ωcf (Fig. 4) that increased with Ωcf. A “bio-inorganic model” of calcification based on the known abiotic rate kinetics of CaCO3 precipitation as a function of saturation state (IpHRAC)9 was also fitted using estimates of Ωcf (Fig. 4, red curve). The IpHRAC model (Fig. 4) was in agreement with the data measured here. Thus, although coral calcification is a biologically mediated process that is species-specific, a simple model (IpHRAC) was able to explain most of the mean link between calcification rates and Ωcf.
Nevertheless, the IpHRAC model did not capture the entire complexity of the responses, as calcification tended to drop rapidly with Ωcf was elevated (for Ωcf > 12) but leveled out at lower Ωcf (<12). This finding tends to demonstrate that the coral with low Ωcf were less affected by changes in seawater pH. Potentially, this result could indicate two different and non-exclusive means of maintaining calcification in corals. For corals calcifying at low Ωcf, the precipitation of CaCO3 could be dependent on an active biological catalyzation such as the one provided by proteins from the organic matrix, which have been shown to favor precipitation of calcium carbonate at low saturation state50. Such corals would therefore be less sensitive to changes in their calcifying fluid chemistry. In contrast, calcification in corals with elevated Ωcf could be less dependent on the activity of catalyzers and be governed by the chemical conditions in the calcifying fluid (i.e., precipitation of CaCO3 occurs because Ωcf is elevated). As a result, calcification in such corals would be more sensitive to changes in Ωcf. However it is important to bear in mind that this experiment was performed on only two corals species and more experimental data where DICcf and pHcf are determined at the same time on corals maintained in identical experimental conditions and exposed to various pH levels would be necessary to confirm its broad significance. Furthermore this experiment was performed under constant conditions of temperature, light, and pH, which did not capture the complexity of natural variations that affect coral physiology and coral calcifying fluid composition in situ 29.
Conclusion
The current study shows that pHcf is not the only driver of the response to OA in sub-tropical corals. Indeed, while calcification behaved as expected, with a stronger decline in A. youngei than in P. damicornis under OA, the decline in pHcf under OA was more important in P. damicornis. DICcf, estimated using a novel proxy, increased in both corals with pCO2, but this increase was not sufficient to compensate for declining pHcf as shown by the decrease in Ωcf in both corals. There was a non-linear relationship between Ωcf and calcification suggesting that the corals with lower rates of calcification and lower Ωcf will be less affected by changes in pCO2. Maintaining elevated Ωcf allow corals to catalyze the rapid precipitation of calcium carbonate, however, the present results indicate that maintaining such high rates of precipitation will become more challenging in the future. As a result, OA could reduce the variance of growth between corals, which could potentially have ecological repercussion on coral competition53. Further investigations into the potential role of the relationship Ωcf – calcification rates and sensitivity to OA are necessary to estimate the effects of OA on the specific composition of future coral reefs.
Electronic supplementary material
Acknowledgements
We thank C. Ross, V. Schoepf, A.-M. Nisumaa-Comeau, K. Rankenburg and J. Trotter for field or laboratory assistance. This study was supported by funding provided by an ARC Laureate Fellowship (LF120100049) awarded to Professor M. McCulloch, the ARC Centre of Excellence for Coral Reef Studies, and an ARC Discovery Early Career Researcher Award (DE160100668) awarded to S. Comeau.
Author Contributions
S.C., C.C., and M.M. conceived and designed research. S.C. and C.C. performed the experiments. S.C. analyzed the data. S.C., C.C., and M.M. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08003-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC Climate Change 2014: The Physical Science Basis (eds Field, C. B. et al.) (Cambridge Univ. Press, 2014).
- 2.Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999;29:215–233. doi: 10.1016/S0921-8009(99)00009-9. [DOI] [Google Scholar]
- 3.Chan NCS, Connolly SR. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 2013;19:282–290. doi: 10.1111/gcb.12011. [DOI] [PubMed] [Google Scholar]
- 4.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 5.Edmunds PJ, Brown D, Moriarty V. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob. Change Biol. 2012;18:2173–2183. doi: 10.1111/j.1365-2486.2012.02695.x. [DOI] [Google Scholar]
- 6.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 2014;59:1081–1091. doi: 10.4319/lo.2014.59.3.1081. [DOI] [Google Scholar]
- 7.Fabricius KE, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim. Change. 2011;1:165–169. doi: 10.1038/nclimate1122. [DOI] [Google Scholar]
- 8.Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso J-P. Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences. 2010;7:289–300. doi: 10.5194/bg-7-289-2010. [DOI] [Google Scholar]
- 9.McCulloch M, Falter J, Trotter J, Montagna P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Clim. Change. 2012;2:623–627. doi: 10.1038/nclimate1473. [DOI] [Google Scholar]
- 10.Georgiou L, et al. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl. Acad. Sci. USA. 2015;112:13219–13224. doi: 10.1073/pnas.1505586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Horani FA, Al-Moghrabi SM, Beer DDe. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003;142:419–426. doi: 10.1007/s00227-002-0981-8. [DOI] [Google Scholar]
- 12.Venn AA, et al. Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA. 2013;110:1634–1639. doi: 10.1073/pnas.1216153110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holcomb M, et al. Coral calcifying fluid pH dictates response to ocean acidification. Scientific Reports. 2014;4:5207. doi: 10.1038/srep05207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw EC, Carpenter RC, Lantz CA, Edmunds PJ. Intraspecific variability in the response to ocean warming and acidification in the scleractinian coral Acropora pulchra. Mar. Biol. 2016;163:210. doi: 10.1007/s00227-016-2986-8. [DOI] [Google Scholar]
- 15.Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem. Geophys. Geosystems. 2009;10:Q07005. doi: 10.1029/2009GC002411. [DOI] [Google Scholar]
- 16.Ries JB. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 2011;403:54–64. doi: 10.1016/j.jembe.2011.04.006. [DOI] [Google Scholar]
- 17.Cai W-J, et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nature Comm. 2016;7:11144. doi: 10.1038/ncomms11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison N, et al. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nature Comm. 2014;5:5741. doi: 10.1038/ncomms6741. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb M, DeCarlo TM, Gaetani GA, McCulloch M. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 2016;437:67–76. doi: 10.1016/j.chemgeo.2016.05.007. [DOI] [Google Scholar]
- 20.Venn A, Tambutté E, Holcomb M, Allemand D, Tambutté S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE. 2011;6:e20013. doi: 10.1371/journal.pone.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comeau S, et al. Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc. R. Soc. B. 2014;281:20141339. doi: 10.1098/rspb.2014.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornwall, C. E., Comeau, S., & McCulloch, M. T. Coralline algae elevate pH at the site of calcification under ocean acidification. Glob. Change Biol., doi:10.1111/gcb.13673 (2017). [DOI] [PubMed]
- 23.Ross CL, Falter JL, Schoepf V, McCulloch MT. Perennial growth of hermatypic corals at Rottnest Island, Western Australia (32°S) PeerJ. 2015;3:e781. doi: 10.7717/peerj.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss RH, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463:747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- 25.Gattuso, J.-P., Epitalon, J.-M., Lavigne H., & Orr J. Seacarb: seawater carbonate chemistry. R package version 3.1.1. http://CRAN.R-project.org/package5seacarb (Date of access: 01/10/2016).
- 26.Dickson, A. G., Sabine, C. L. & Christian, J. R. (Eds) Guide to best practices for CO2measurements, PICES Special Publication 3 (2007).
- 27.Spencer-Davies P. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989;101:389–395. doi: 10.1007/BF00428135. [DOI] [Google Scholar]
- 28.Trotter J, et al. Quantifying the pH “vital effect” in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 2011;303:163–173. doi: 10.1016/j.epsl.2011.01.030. [DOI] [Google Scholar]
- 29.McCulloch MT, D’Olivo Cordero JP, Falter J, Holcomb M, Trotter JA. Coral calcification in a changing World and the interactive dynamics of pH and DIC up-regulation. Nature Comm. 2017;8:15686. doi: 10.1038/ncomms15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCulloch MT, et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta. 2012;87:21–34. doi: 10.1016/j.gca.2012.03.027. [DOI] [Google Scholar]
- 31.McCulloch MT, Holcomb M, Rankenburg K, Trotter JA. Rapid, high-precision measurements of boron isotopic compositions in marine carbonates. Rapid Comm. Mass Spectro. 2014;28:2704–2712. doi: 10.1002/rcm.7065. [DOI] [PubMed] [Google Scholar]
- 32.Foster GL, et al. Interlaboratory comparison of boron isotope analyses of boric acid, seawater and marine CaCO3 by MC-ICPMS and NTIMS. Chem. Geol. 2013;358:1–14. doi: 10.1016/j.chemgeo.2013.08.027. [DOI] [Google Scholar]
- 33.Foster GL, Pogge von Strandmann PaE, Rae JWB. Boron and magnesium isotopic composition of seawater. Geochem. Geophys. Geosystems. 2010;11:Q08015. doi: 10.1029/2010GC003201. [DOI] [Google Scholar]
- 34.Klochko K, Kaufman AJ, Yao W, Byrne RH, Tossell JA. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 2006;248:276–285. doi: 10.1016/j.epsl.2006.05.034. [DOI] [Google Scholar]
- 35.Dickson AG. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1990;37:755–766. doi: 10.1016/0198-0149(90)90004-F. [DOI] [Google Scholar]
- 36.Quinn, G. P. & Keough, M. J. Experimental design and data analysis for biologists, Cambridge Univ. Press (2002).
- 37.Bahr KD, Jokiel PL, Rodgers KS. Relative sensitivity of five Hawaiian coral species to high temperature under high-pCO2 conditions. Coral Reefs. 2016;35:729–738. doi: 10.1007/s00338-016-1405-4. [DOI] [Google Scholar]
- 38.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA. 2008;105(45):17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoepf V, et al. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE. 2013;8:e75049. doi: 10.1371/journal.pone.0075049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi A, Kurihara H. Ocean acidification does not affect the physiology of the tropical coral Acropora digitifera. Coral Reefs. 2012;32:305–314. doi: 10.1007/s00338-012-0979-8. [DOI] [Google Scholar]
- 41.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Mar. Ecol. Progr. Ser. 2014;501:99–111. doi: 10.3354/meps10690. [DOI] [Google Scholar]
- 42.Jokiel PL. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull. Mar. Sci. 2011;87:639–657. doi: 10.5343/bms.2010.1107. [DOI] [Google Scholar]
- 43.Comeau, S. & Cornwall, C. E. Contrasting effects of ocean acidification on coral reef “animal forests” versus seaweed “kelp forests” In Marine Animal Forests (ed. Rossi, S.) Springer International Publishing, Switzerland, doi:10.1007/978-3-319-17001-5_29-1 (2016).
- 44.Hönisch B, et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim Cosmochim Acta. 2004;68:3675–3685. doi: 10.1016/j.gca.2004.03.002. [DOI] [Google Scholar]
- 45.Reynaud S, Ferrier-Pages C, Boisson F, Allemand D, Fairbanks R. Effect of light and temperature on calcification and strontium uptake in the scleractinian coral Acropora verweyi. Mar Ecol-Prog Ser. 2004;279:105–112. doi: 10.3354/meps279105. [DOI] [Google Scholar]
- 46.Tanaka K, et al. Response of Acropora digitifera to ocean acidification: constraints from δ11B, Sr, Mg, and Ba compositions of aragonitic skeletons cultured under variable seawater pH. Coral Reefs. 2015;34:1139–1149. doi: 10.1007/s00338-015-1319-6. [DOI] [Google Scholar]
- 47.Gattuso J-P, Allemand D, Frankignoulle M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. American Zoologist. 1999;39:160–183. doi: 10.1093/icb/39.1.160. [DOI] [Google Scholar]
- 48.Schneider K, Erez J. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 2006;51:1284–1293. doi: 10.4319/lo.2006.51.3.1284. [DOI] [Google Scholar]
- 49.Mass T, et al. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 2013;23:1126–1131. doi: 10.1016/j.cub.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Muscatine L, Tambutte E, Allemand D. Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs. 1997;16:205–213. doi: 10.1007/s003380050075. [DOI] [Google Scholar]
- 51.Zoccola D, et al. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Scientific Reports. 2015;5:09983. doi: 10.1038/srep09983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comeau S, et al. Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. B. 2017;284:20161669. doi: 10.1098/rspb.2016.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evensen NR, Edmunds PJ. Interactive effects of ocean acidification and neighboring corals on the growth of Pocillopora. Mar. Biol. 2016;163:1–11. doi: 10.1007/s00227-016-2921-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


