Abstract
Background
Smart wearables such as the Fitbit wristband provide the opportunity to monitor patients more comprehensively, to track patients in a fashion that more closely follows the contours of their lives, and to derive a more complete dataset that enables precision medicine. However, the utility and efficacy of using wearable devices to monitor adolescent patients’ asthma outcomes have not been established.
Objective
The objective of this study was to explore the association between self‑reported sleep data, Fitbit sleep and physical activity data, and pediatric asthma impact (PAI).
Methods
We conducted an 8‑week pilot study with 22 adolescent asthma patients to collect: (1) weekly or biweekly patient‑reported data using the Patient-Reported Outcomes Measurement Information System (PROMIS) measures of PAI, sleep disturbance (SD), and sleep‑related impairment (SRI) and (2) real-time Fitbit (ie, Fitbit Charge HR) data on physical activity (F-AM) and sleep quality (F‑SQ). To explore the relationship among the self-reported and Fitbit measures, we computed weekly Pearson correlations among these variables of interest.
Results
We have shown that the Fitbit-derived sleep quality F-SQ measure has a moderate correlation with the PROMIS SD score (average r=−.31, P=.01) and a weak but significant correlation with the PROMIS PAI score (average r=−.18, P=.02). The Fitbit physical activity measure has a negligible correlation with PAI (average r=.04, P=.62).
Conclusions
Our findings support the potential of using wrist-worn devices to continuously monitor two important factors—physical activity and sleep—associated with patients’ asthma outcomes and to develop a personalized asthma management platform.
Keywords: mobile health, mHealth, asthma, Fitbit, physical activity, sleep, sleep quality
Introduction
Childhood morbidity due to asthma is particularly high among adolescent patients [1]. Adolescents have a higher prevalence of asthma compared with younger children [2]. They are at a higher risk for adverse health outcomes and are more likely to experience an exacerbation requiring hospitalization, intubation, or cardiopulmonary resuscitation [3]. The adolescent years are critically important for a person’s physical and psychological development, which in turn requires sufficient sleep [4] and physical activity; however, both sleep and physical activity can be significantly constrained by asthma [5,6]. Conversely, asthma symptoms can worsen because of sleep problems [7] and strenuous exercise [8]. Nevertheless, regular physical activity is an important component of pediatric asthma management [9]. Physical activity has also been associated with a decrease in the severity of the symptoms of asthma and an improvement in the quality of life among children with asthma [10-12]. Therefore, unveiling the relationship between asthma, sleep patterns [13], and physical activity can potentially aid health care providers when counseling adolescents on asthma self-management, improving quality of life, and facilitating the discovery of more effective interventions for asthma.
Prior studies have reported a relationship between asthma and sleep among adolescents [14,15]. Uncontrolled asthma is associated with impaired nighttime sleep, daytime sleepiness, and health-related quality of life. Compared with healthy children, children with asthma are more likely to report sleep problems due to greater increase in airway resistance during nighttime sleep, which in turn leads to exacerbated asthma symptoms such as coughing and wheezing [16]. However, there are substantial barriers to examining the relationship between asthma and sleep patterns. Such studies usually need to be carried out in a special environment (for capturing sleep patterns [17-20]) and over a relatively long time (to accumulate enough data), which incurs high costs. Specialized and expensive equipment is typically needed to collect sleep pattern data, and participants usually have to stay in an unfamiliar sleep study facility [19,20].
However, the advent of smart wearables (eg, wrist-worn mobile devices such as the Fitbit wristband) opens a new horizon for continuous, unobtrusive, and cost-effective data collection [21-24]. The concept is compelling: smart wearables provide the opportunity to monitor patients more comprehensively, to track patients in a fashion that more closely follows the contours of their lives, and to derive a more complete dataset that enables precision medicine [25]. Although multipurpose wrist‑worn devices present some limits with respect to accuracy, they are reasonable options for near-continuous data monitoring [26]. It has been reported that these devices have acceptable reliability and validity when used to measure physical activity and sleep in adults [27]. Specifically, for physical activity tracking, it has been reported [28-30] that these devices have acceptable accuracy at various speeds, attachment sites, and in both lab and real-world settings, particularly for step counting. More recently, it was shown that age and pathological gait changes might affect the accuracy of step counting devices. However, this does not apply to our specific study [31]. By wearing wrist-worn devices, long-term motion and biological data that patients generate in their living environment can be collected through device built-in sensors (eg, accelerometer, gyroscope, and heart rate sensors) in a user-transparent manner. Furthermore, there is a growing interest in using sensor-based observations through smartphones and wearables as a novel way to collect health information, accelerated as a part of the precision medicine initiative [25]. Technologies such as wearables will provide new opportunities for patient engagement and care delivery that will generate precision interventions, which will ultimately enhance clinical outcomes and reduce health care costs [32].
Although asthma is a serious health problem among adolescents, the utility and efficacy of using wearable devices to monitor adolescent patients’ asthma outcomes have not been established. In this pilot study, we aimed to explore the association among patient‑reported sleep data, Fitbit sleep and physical activity data, and pediatric asthma outcomes. We collected patient‑reported outcomes (PROs) on pediatric asthma impact (PAI), sleep disturbance (SD), and sleep‑related impairment (SRI) [33,34], as well as Fitbit-derived sleep and physical activity measures from 22 adolescents with asthma. The goals of the study were to explore the association between (1) asthma outcomes and Fitbit-derived sleep and physical activity measures and (2) patient‑reported sleep measures and Fitbit-derived sleep measures.
Methods
Study Design
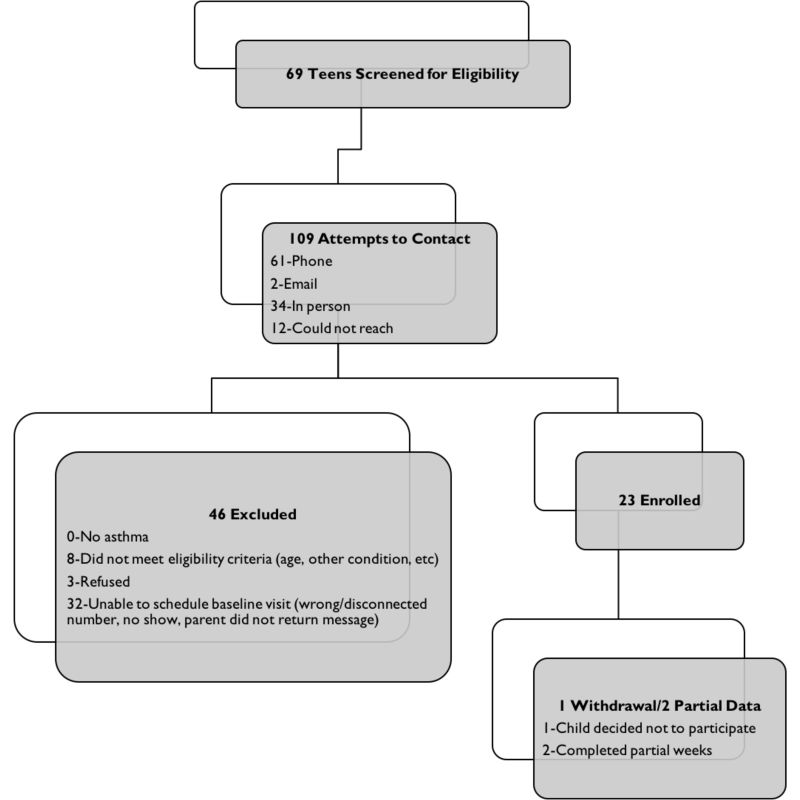
We conducted an 8-week pilot study (trial registration number NCT02556567) and recruited a convenience sample of adolescent asthma patients according to their medical chart from the Arkansas Children’s Hospital subspecialty asthma clinics. Patients were screened for eligibility after their outpatient visits, where the physician discussed the study briefly with the patient and the caregiver, and a research nurse carried out the rest of the recruitment activities. Inclusion criteria required the participants to: (1) be in the age group of 14 to 17 years with physician-diagnosed persistent asthma and (2) to have access to a smartphone or a computer that was compatible with the Fitbit app. Informed consent and assent were obtained from all participants and their caregivers, respectively, during the baseline visit. Figure 1 shows the cumulative screening and enrollment summary for the study. Of the 23 enrolled participants, 1 participant withdrew from the study at the beginning of the study, and 2 did not complete all 8 weeks of the study procedures. Our data analysis excluded the participant who withdrew, resulting in 22 adolescents in our data analysis. There were 17 participants with complete data (8 weeks). We also included participants with partial data where 2 participants had 7 weeks complete, one with 6 weeks, one with 4 weeks, and one with 3 weeks.
Figure 1.
Cumulative screening and enrollment summary.
During the baseline visit, all participants had their height and weight measured and completed a baseline survey to collect demographic data (eg, gender, race, and ethnicity), socioeconomic status (eg, household income), insurance information, baseline level of asthma control according to the national guidelines, medication history, and baseline PROs (ie, PAI, SD, and SRI [33]). The caregivers of the participants were also present during the baseline visit and helped answer some of the questions (eg, income). The research nurse also showed the participants how to navigate through the Fitbit user interface during the baseline visit. During the study, all participants were given a Fitbit Charge HR wristband (Figure 2) and instructed to wear the wristband at all times (24 hours every day) during the 8-week study period, except for when the device was being charged. Participants’ biological data were collected in real time and passively without user interactions with the Fitbit devices and then uploaded to the Fitbit/Fitabase servers [35]. In addition, participants were asked to complete Web-based surveys to collect the PROs. The asthma impact survey was administered weekly and the two sleep‑related surveys were administered biweekly to avoid survey fatigue. As an incentive for participation, participants received US $10 for completion of weekly surveys and synchronization of Fitbit data. Study procedures were approved by the institutional review board of the University of Arkansas for Medical Sciences.
Figure 2.
Fitbit Charge HR wristband.
Measures
Fitbit Measures
The Fitbit Charge HR wristband can collect a wide range of physical and biological data, including physical activity (eg, calories burned, steps, distance, and heart rate) and sleep data (eg, time in bed and awakenings count). We used two summary measures derived from Fitbit data, Active Minutes (F-AM) and Sleep Quality (F-SQ) score. F-AM was defined as daily minutes of moderate and vigorous activity, and F-SQ score was defined as the ratio of minutes asleep to minutes in bed.
Patient-Reported Outcomes
PROs were collected using the Patient-Reported Outcomes Measurement Information System (PROMIS) instruments [33,36], which included pediatric asthma impact (PAI; short form 8a), sleep disturbance (SD; short form 6a), and sleep-related impairment (SRI; short form 8a). These instruments were previously validated among adolescents [37,38].
The PAI short form 8a contains 8 questions asking whether the patients had asthma-specific symptoms such as cough, wheeze, shortness of breath, and avoidance of triggers in the past 7 days. The responses are on an ordinal scale going from Never (0 point) to Almost Always (4 points). A higher PAI score means worse asthma outcomes. Prior studies have reported good Cronbach alpha (>.8) for the PAI short form 8a [39].
The SD short form 6a contains 6 questions that evaluate self‑reported perceptions of sleep quality, sleep depth, and restoration associated with sleep in the past 7 days. For example, one question asks “I had difficulty falling asleep...,” and the responses include the following: Not at all (5 points), A little bit, Somewhat, Quite a bit, and Very much (1 point). A higher SD score means less sleep disturbance. The Cronbach alpha for the SD short form 6a has been reported to be good (>.8; [40-43]).
The SRI short form 8a contains 8 questions that evaluate self‑reported perceptions of alertness, sleepiness, and tiredness during usual waking hours and the perceived functional impairments during wakefulness associated with sleep problems or impaired alertness in the past 7 days. For example, one question asks “I had a hard time getting things done because I was sleepy…,” and the responses include the following: Not at all (5 points), A little bit, Somewhat, Quite a bit, and Very much (1 point). A higher SRI score means less sleep-related impairment. Prior studies have also reported good Cronbach alpha (>.8) for the SRI short form 8a [41-43].
All PROMIS measures are reported on a T-score scale (mean= 50, SD=10). This allows us to easily compare various PROMIS scores across scales, even if the original scores do not have identical variances or are measured on different scales [33]. For each instrument, we computed the raw scores by adding up scores from each question and converted the raw scores to T-scores for data analysis. All instruments are presented in Multimedia Appendices 1-.
Statistical Analysis
We first calculated descriptive statistics to summarize the participants’ characteristics. We then computed weekly Spearman rank-order correlations among the variables of interest, which included pediatric asthma outcome PAI, Fitbit-derived measures F‑SQ and F‑AM, and self-reported sleep measures SD and SRI. We used the following guidelines for interpreting correlation coefficients: <.3=poor, .3 to .7=moderate, and >.7=excellent. All analyses were conducted with Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc).
Results
The participants’ characteristics are summarized in Table 1. The average age of our participants was 15.5 years (SD 1.1). There were 12 boys (55%) and 10 girls (45%). The race distributions of the participants were 12 whites (55%), 7 African Americans (32%), and 3 other races (13%). The majority (64%; 14 participants) of the participants were covered by state insurance, 7 (32%) were covered by private insurance, and 1 (4%) was uninsured. The annual household income was below $60,000 for most of the participants.
Table 1.
Participants’ characteristics.
| Characteristic | n (%) or mean (SD) (N=22) |
||
| Age in years, mean (SD) | 15.5 (1.1) | ||
| Sex, n (%) | |||
| Female | 10 (45) | ||
| Male | 12 (55) | ||
| Race, n (%) | |||
| White | 12 (55) | ||
| Black | 7 (32) | ||
| Other | 3 (13) | ||
| Insurance, n (%) | |||
| Private | 7 (32) | ||
| State | 14 (64) | ||
| Uninsured | 1 (4) | ||
| Household income in US dollars, n (%) | |||
| <15,000 | 2 (9) | ||
| 15,000-60,000 | 10 (45) | ||
| >60,000 | 5 (23) | ||
| Missing | 5 (23) | ||
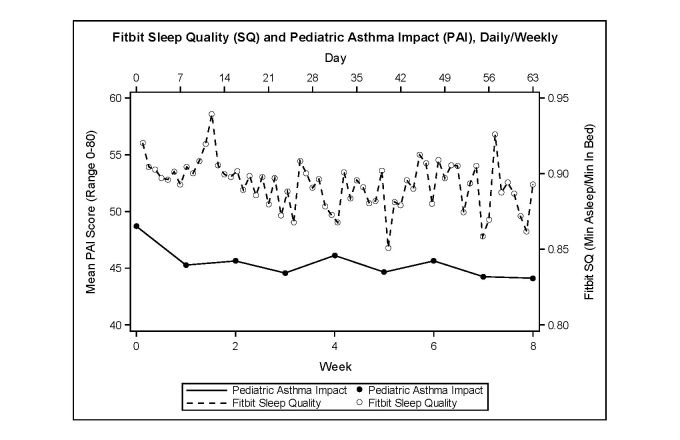
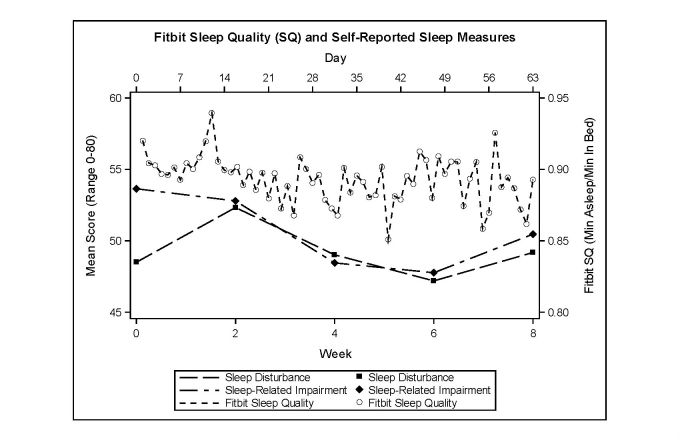
The variables of interest are summarized in Table 2 and to visually examine how the variables of interest changed across the study period, we plotted the Fitbit SQ score with the PAI score (Figure 3) and with the self‑reported SD and SRI scores (Figure 4) across the 8 weeks of our study. As seen in Figure 3, the average weekly PAI score varied between 44.12 and 48.70, with an average of 45.44. The average daily Fitbit SQ score varied between 0.85 and 0.94, with an average of 0.89. As seen in Figure 4, the biweekly self-reported SD score varied between 47.19 and 52.33, with an average of 49.24. The biweekly self-reported SRI score varied between 47.76 and 53.64, with an average of 50.62.
Table 2.
Patient-reported outcomes and Fitbit measure statistics.
| Measure | Median | Mean | Standard deviation |
Skewness | Kurtosis |
| PAIa | 46.50 | 44.99 | 10.45 | 0.15 | −1.12 |
| SDb | 49.00 | 49.49 | 9.27 | 0.03 | −0.36 |
| SRIc | 50.30 | 49.93 | 11.27 | 0.07 | −0.97 |
| F-AMd | 0.00 | 10.59 | 21.59 | 4.08 | 24.21 |
| F-SQe | 0.93 | 0.90 | 0.12 | −2.69 | 6.37 |
aPAI: pediatric asthma impact.
bSD: sleep disturbance.
cSRI: sleep-related impairment.
dF-AM: Fitbit-Active Minutes.
eF-SQ: Fitbit-Sleep Quality.
Figure 3.
Fitbit sleep quality and pediatric asthma impact.
Figure 4.
Fitbit sleep quality and self-reported sleep measures.
We summarized the correlations among the variables of interest by week in Table 3. Our results showed that across the study period, higher PAI score was weakly associated with a lower SQ score. The weekly F‑SQ –PAI correlation ranged from −.48 to .19, with the average being −.18. On the other hand, the Fitbit physical activity measure (F‑AM) did not have a significant correlation with PAI, where the weekly F‑AM –PAI correlation ranged from a weak negative association to moderate positive association (−.26 to .39), suggesting a potentially complex relationship between these two variables. There was a moderate to strong positive association between PAI and the two sleep measures, SD and SRI, with the average weekly correlation being .71 and .64 for PAI –SD and PAI –SRI, respectively. Finally, we observed that as F-SQ increased, there was a moderate decrease in the PRO sleep disturbance (average weekly correlation=−.31).
Table 3.
Spearman rank-order correlations among the variables of interests across the study period.
| Variable Pairs | Week (P value) | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Average | |
| PAIa–F-SQb | .04 (.88) | −.48 (.02) | −.57 (.01) | −.07 (.78) | −.07 (.77) | .19 (.41) | −.17 (.51) | −.21 (.44) | −.18 (.02) |
| PAI–F-AMc | −.09 (.70) | −.01 (.97) | .09 (.70) | .04 (.86) | −.26 (.28) | −.02 (.94) | .39 (.09) | .10 (.69) | .04 (.62) |
| PAI–SDd | .75 (<.01) | .62 (<.01) | .71 (<.01) | .75 (<.01) | .71 (<.01) | ||||
| PAI–SRIe | .53 (.01) | .74 (<.01) | .67 (<.01) | .59 (<.01) | .64 (<.01) | ||||
| F-SQ–SD | −.63 (<.01) | −.09 (.72) | −.16 (.49) | −.37 (.17) | −.31 (.01) | ||||
| F-SQ–SRI | −.32 (.15) | −.09 (.70) | .08 (.73) | −.05 (.86) | −.08 (.50) | ||||
aPAI: pediatric asthma impact.
bF-SQ: Fitbit-Sleep Quality.
cF-AM: Fitbit-Active Minutes.
dSD: sleep disturbance.
eSRI: sleep-related impairment.
Discussion
Principal Findings
In this pilot study, we have shown that the Fitbit-derived sleep quality measure F‑SQ has a weak but significant inverse association with the PROMIS PAI score. In addition, F‑SQ has a moderate inverse correlation with the PROMIS SD measure. Overall, our study suggests that there is a potential association between Fitbit measures or other wrist-worn activity tracking devices and asthma outcomes. Further studies, with more power, would be able to determine whether Fitbit measures could predict the symptoms of pediatric asthma.
Fitbit Sleep Quality and PROMIS Sleep Measures
The Fitbit-derived F‑SQ score has a moderate negative correlation with the SD measure but no correlation with the SRI measure. One potential reason is that the F‑SQ score is the ratio of minutes asleep over minutes spent in bed, which is an indicator of sleep quality. Therefore, it is conceptually more similar to the SD measure, which evaluates perceived difficulties with getting to sleep or staying asleep. On the other hand, the SRI measure is not a measure of sleep quality. SRI is concerned with functional impairments, such as alertness, sleepiness, and tiredness, associated with sleep problems during usual waking hours.
Fitbit Physical Activity and Asthma Impact
The Fitbit physical activity measure was not associated with the asthma impact despite what the existing literature suggests (ie, physical activity is associated with asthma outcomes [10,12]). There are several potential reasons that we did not find such association, which include the following: (1) our pilot study was not sufficiently powered and (2) the relationship between physical activity and asthma is more complex. For example, to capture exercise-induced bronchoconstriction, also called exercise-induced asthma, asthmatic events will likely need to be captured in real time in addition to weekly patient-reported asthma impact. Furthermore, the effects of physical activity on patients’ general asthma outcomes are long term and may not be observed in an 8-week study. In short, more studies and data are needed to further explore the relationship between Fitbit data and patient‑reported measures.
The success of the pilot study demonstrates the utility of passively collected sensory data for monitoring factors related to asthma, and that ultimately, these data could be potentially incorporated into a disease management tool. In our previous study [44], we pilot-tested a smartphone-—based mobile asthma action plans (mAAP) app and showed its acceptance among adolescents. Incorporating models for detecting asthma triggers into the mAAP app will allow us to build a mobile health (mHealth) platform that can give us a more complete picture of patient’s disease states and then provide them with real-time personalized asthma management strategies. Nevertheless, to achieve this goal, we shall further explore sources that would have data on other asthma triggers beyond physical activity and sleep. For example, allergens and irritants in the air are common asthma triggers that can be measured with particle sensors.
Limitations
Due to the pilot nature of this project, our study has distinct limitations. First, with only 22 participants, the study was not adequately powered for complex statistical analyses. Therefore, influences from covariates, such as gender and race, on the outcome-predictor relationships were not considered. Second, the study participants were recruited as a convenience sample rather than as a random sample. It is therefore unknown whether the results from this study generalize well to other populations. Third, the PAI survey has a question about trouble sleeping at night that could explain the correlations between the PAI measure and our sleep measures. Last, the accuracy of using wearable devices for long-term monitoring of physical activity and sleep is suboptimal, and device dependent [26]. Future studies are warranted to further explore the use of different wearable devices to replicate our findings using Fitbit.
Conclusions
Wrist-worn activity tracking devices such as Fitbit are associated with pediatric asthma PROs, and with further research, have the potential to closely monitor patients’ biological data and to help manage chronic diseases where sleep quality and physical activity are factors. Our long-term goal is to develop an mHealth platform with a wrist-worn device that can collect sensory data related to asthma triggers and create models that predict patients’ asthma outcomes with these factors, thereby providing personalized asthma management strategies to patients in real time.
Acknowledgments
The work was supported by NIH grant UL1TR000039, and in part by NIH grant UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- F-AM
Fitbit-Active Minutes
- F-SQ
Fitbit-Sleep Quality
- mAAP
mobile asthma action plans
- mHealth
mobile health
- PAI
pediatric asthma impact
- PRO
patient-reported outcome
- PROMIS
Patient-Reported Outcomes Measurement Information System
- SAS
Statistical Analysis Software
- SD
sleep disturbance
- SRI
sleep-related impairment
Pediatric Asthma Impact short form 8a.
Sleep Disturbance short form 6a.
Sleep Related Impairment short form 8a.
Footnotes
Authors' Contributions: JB, YG, MX, and TP designed the study protocol. YG and AP performed the statistical analysis. MX and IW helped with the study and data management. RB and TP carried out the subject recruitment. JB, YG, DZ, and TP wrote the manuscript. All authors have provided feedback and edited the manuscript.
Conflicts of Interest: None declared.
References
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ, Centers for Disease Control and Prevention (CDC) National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007 Oct 19;56(8):1–54. https://www.cdc.gov/mmwr/preview/mmwrhtml/ss5608a1.htm. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009 Mar;123(Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Calmes D, Leake BD, Carlisle DM. Adverse asthma outcomes among children hospitalized with asthma in California. Pediatrics. 1998 May;101(5):845–50. doi: 10.1542/peds.101.5.845. [DOI] [PubMed] [Google Scholar]
- 4.Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation's children. Pediatrics. 2007 Feb;119(Suppl 1):S29–37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- 5.Walker TJ, Reznik M. In-school asthma management and physical activity: children's perspectives. J Asthma. 2014 Oct;51(8):808–13. doi: 10.3109/02770903.2014.920875. http://europepmc.org/abstract/MED/24796650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer LJ, Ullrich M, Szefler SJ. Sleep duration, sleep hygiene, and insomnia in adolescents with asthma. J Allergy Clin Immunol Pract. 2014 Oct;2(5):562–9. doi: 10.1016/j.jaip.2014.02.005. http://europepmc.org/abstract/MED/25213049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, Chung KF, Erzurum S, Israel E, Strollo PJ, Wenzel SE. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012 Dec;16(4):1129–37. doi: 10.1007/s11325-011-0616-8. http://europepmc.org/abstract/MED/22102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanrooij VH, Willeboordse M, Dompeling E, van de Kant KD. Exercise training in children with asthma: a systematic review. Br J Sports Med. 2014 Jul;48(13):1024–31. doi: 10.1136/bjsports-2012-091347. [DOI] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute NIH. 2007. [2017-07-20]. National asthma education and prevention program expert panel report 3: guidelines for the diagnosis and management of asthma https://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf . [PubMed]
- 10.Welsh L, Kemp JG, Roberts RG. Effects of physical conditioning on children and adolescents with asthma. Sports Med. 2005;35(2):127–41. doi: 10.2165/00007256-200535020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Basaran S, Guler-Uysal F, Ergen N, Seydaoglu G, Bingol-Karakoç G, Ufuk AD. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J Rehabil Med. 2006 Mar;38(2):130–5. doi: 10.1080/16501970500476142. https://www.medicaljournals.se/jrm/content/abstract/10.1080/16501970500476142. [DOI] [PubMed] [Google Scholar]
- 12.Welsh L, Roberts RG, Kemp JG. Fitness and physical activity in children with asthma. Sports Med. 2004;34(13):861–70. doi: 10.2165/00007256-200434130-00001. [DOI] [PubMed] [Google Scholar]
- 13.Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. New York: Cambridge University Press; 2010. Sep 9, [Google Scholar]
- 14.Chugh IM, Khanna P, Shah A. Nocturnal symptoms and sleep disturbances in clinically stable asthmatic children. Asian Pac J Allergy Immunol. 2006;24(2-3):135–42. http://thailand.digitaljournals.org/index.php/apjai/article/viewFile/28047/27256. [PubMed] [Google Scholar]
- 15.Li Z, Huang I, Thompson L, Tuli S, Huang S, DeWalt D, Revicki D, Shenkman E. The relationships between asthma control, daytime sleepiness, and quality of life among children with asthma: a path analysis. Sleep Med. 2013 Jul;14(7):641–7. doi: 10.1016/j.sleep.2013.04.002. http://europepmc.org/abstract/MED/23684939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg H, Cohen RI. Nocturnal asthma. Curr Opin Pulm Med. 2012 Jan;18(1):57–62. doi: 10.1097/MCP.0b013e32834d098e. [DOI] [PubMed] [Google Scholar]
- 17.Mouthon A, Huber R. Methods in pediatric sleep research and sleep medicine. Neuropediatrics. 2015 Jun;46(3):159–70. doi: 10.1055/s-0035-1550232. [DOI] [PubMed] [Google Scholar]
- 18.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008 Sep;9(Suppl 1):S10–7. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- 19.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015 Jan 13;313(2):156–64. doi: 10.1001/jama.2014.17822. http://europepmc.org/abstract/MED/25585327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng Y, Chiang L, Lue K, Chang S, Wang L, Lee S, Ting H, Lee S. Poor sleep quality measured by polysomnography in non-obese asthmatic children with or without moderate to severe obstructive sleep apnea. Sleep Med. 2014 Sep;15(9):1062–7. doi: 10.1016/j.sleep.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004 Aug 01;27(5):923–33. doi: 10.1093/sleep/27.5.923. http://europepmc.org/abstract/MED/15453551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantelopoulos A, Bourbakis N. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans Syst Man Cybern C. 2010;40(1):1–12. doi: 10.1109/TSMCC.2009.2032660. [DOI] [Google Scholar]
- 23.Kay M, Choe E, Shepherd J, Greenstein B, Watson N, Consolvo S, Kientz JA. Lullaby: a capture & access system for understanding the sleep environment. Proceedings of the ACM Conference on Ubiquitous Computing; September 05-08, 2012; Pittsburgh, PA. 2012. Sep, pp. 226–34. [DOI] [Google Scholar]
- 24.Adam NJ, Spierer DK, Gu J, Bronner S. Comparison of steps and energy expenditure assessment in adults of Fitbit tracker and ultra to the actical and indirect calorimetry. J Med Eng Technol. 2013 Oct;37(7):456–62. doi: 10.3109/03091902.2013.831135. [DOI] [PubMed] [Google Scholar]
- 25.Precision Medicine Initiative (PMI) Working Group NIH. 2015. Sep 17, [2017-07-19]. The precision medicine initiative cohort program – building a research foundation for 21st century medicine https://www.nih.gov/sites/default/files/research-training/initiatives/pmi/pmi-working-group-report-20150917-2.pdf .
- 26.Rosenberger ME, Buman MP, Haskell WL, McConnell MV, Carstensen LL. Twenty-four hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc. 2016 Mar;48(3):457–65. doi: 10.1249/MSS.0000000000000778. http://europepmc.org/abstract/MED/26484953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015 Dec 18;12:159. doi: 10.1186/s12966-015-0314-1. https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortune E, Lugade V, Morrow M, Kaufman K. Validity of using tri-axial accelerometers to measure human movement - Part II: step counts at a wide range of gait velocities. Med Eng Phys. 2014 Jun;36(6):659–69. doi: 10.1016/j.medengphy.2014.02.006. http://europepmc.org/abstract/MED/24656871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tudor-Locke C, Barreira TV, Schuna JM. Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc. 2015 Apr;47(4):839–42. doi: 10.1249/MSS.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 30.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015 Feb 10;313(6):625–6. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 31.Klassen T, Simpson L, Lim S, Louie D, Parappilly B, Sakakibara B, Zbogar D, Eng J. “Stepping Up” activity poststroke: ankle-positioned accelerometer can accurately record steps during slow walking. Phys Ther. 2016 Mar;96(3):355–60. doi: 10.2522/ptj.20140611. http://europepmc.org/abstract/MED/26251478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milani RV, Franklin NC. The role of technology in healthy living medicine. Prog Cardiovasc Dis. 2017;59(5):487–91. doi: 10.1016/j.pcad.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Assessment Center Assessment Center. 2016. [2017-07-19]. Instruments available for use in assessment center https://www.assessmentcenter.net/documents/InstrumentLibrary.pdf .
- 34.Fayers P, Machin D. Quality of life: the assessment, analysis, and interpretation of patient-reported outcomes. Chichester: J Wiley; 2007. [Google Scholar]
- 35.Fitbase Fitbase. 2016. [2017-07-19]. Fitabase - research device data and analytics https://www.fitabase.com/
- 36.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries J, Gershon R, Hahn E, Lai J, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, PROMIS Cooperative Group The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010 Nov;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. http://europepmc.org/abstract/MED/20685078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeatts K, Stucky B, Thissen D, Irwin D, Varni J, DeWitt E, Lai J, DeWalt D. Construction of the pediatric asthma impact scale (PAIS) for the patient-reported outcomes measurement information system (PROMIS) J Asthma. 2010 Apr;47(3):295–302. doi: 10.3109/02770900903426997. http://europepmc.org/abstract/MED/20394514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanish A, Lin-Dyken D, Han J. PROMIS sleep disturbance and sleep-related impairment in adolescents: examining psychometrics using self-report and actigraphy. Nurs Res. 2017;66(3):246–51. doi: 10.1097/NNR.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandhi PK, Kenzik KM, Thompson LA, DeWalt DA, Revicki DA, Shenkman EA, Huang I. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir Res. 2013 Feb 23;14:26. doi: 10.1186/1465-9921-14-26. https://respiratory-research.biomedcentral.com/articles/10.1186/1465-9921-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen R, King-Kallimanis B, Sexton E, Reeve B, Moinpour C, Potosky A, Lobo T, Teresi J. Measurement properties of PROMIS sleep disturbance short forms in a large, ethnically diverse cancer cohort internet. Psychol Test Assess Model. 2016;58(2):353–70. http://www.psychologie-aktuell.com/fileadmin/download/ptam/2-2016_20160627/06_Jensen.pdf. [Google Scholar]
- 41.Bartlett SJ, Orbai A, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, Bingham CO. Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PLoS One. 2015;10(9):e0138543. doi: 10.1371/journal.pone.0138543. http://dx.plos.org/10.1371/journal.pone.0138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogelberg DJ, Vitiello MV, Hoffman JM, Bamer AM, Amtmann D. Comparison of self-report sleep measures for individuals with multiple sclerosis and spinal cord injury. Arch Phys Med Rehabil. 2015 Mar;96(3):478–83. doi: 10.1016/j.apmr.2014.10.001. http://europepmc.org/abstract/MED/25450136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas KA, Spieker S. Sleep, depression, and fatigue in late postpartum. MCN Am J Matern Child Nurs. 2016 Apr;41(2):104–9. doi: 10.1097/NMC.0000000000000213. http://europepmc.org/abstract/MED/26909724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry TT, Marshall A, Berlinski A, Rettiganti M, Brown RH, Randle SM, Luo C, Bian J. Smartphone-based vs paper-based asthma action plans for adolescents. Ann Allergy Asthma Immunol. 2017 Mar;118(3):298–303. doi: 10.1016/j.anai.2016.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pediatric Asthma Impact short form 8a.
Sleep Disturbance short form 6a.
Sleep Related Impairment short form 8a.