Abstract
Cystatin C, cathepsin S, and IL-1 are three important biomarkers of atherosclerosis. Previous studies emphasized the relationship between individual biomarkers in coronary artery disease (CAD) patients and severity of atherosclerostic lesions of the coronary arteries, while combined cystatin C, cathepsin S, and IL-1 have not been reported for clinical classification of CAD. We aimed to establish a link between cystatin C, cathepsin S, IL-1 and CAD in this cohort study. Totally 112 subjects were enrolled and divided into the stable angina pectoris group, the unstable angina pectoris group and the acute myocardial infarction (AMI) groups, and 50 healthy adults served as controls. The levels of the three biomarkers were detected by ELISA. The results showed that serum level of cystatin C (mg/L) was higher in CAD patients compared with those in the healthy controls (AMIvs. unstable angina pectoris vs. stable angina pectoris vs. controls: 1.27±0.18 vs. 1.09±0.19 vs. 0.91±0.05 vs. 0.78±0.07, all P<0.01). Cathepsin S (ng/mL) was also significantly different among the groups (AMI vs. unstable angina pectoris vs. stable angina pectoris vs. controls: 67.30±8.36 vs. 56.90±7.16 vs. 49.8±2.72 vs. 67.30±8.36, all P<0.01). IL-1 (pg/mL) was significantly different among the groups as well (AMIvs. unstable angina pectoris vs. stable angina pectoris vs. controls: 2.96±0.57 vs. 2.46±0.24 vs. 2.28±0.09 vs. 2.02±0.13, all P<0.01). Spearman's correlation test revealed positive correlation between cystatin C, cathepsin S, IL-1 and Gensini score (r=0.451, 0.491, 0.397, respectively). It is suggested that simultaneous detection of cystatin C, cathepsin S, and IL-1 in serum may be useful in clinical classification and assessment of severity of CAD.
Keywords: cystatin C, cathepsin S, IL-1, coronary artery disease
Introduction
Coronary artery disease (CAD) is a prevalent disease that poses a serious threat to public health. Early detection and treatment significantly increase the chance of survival for CAD patients. Although vascular intervention is the most direct and accurate method to diagnose and treat CAD and other atherosclerotic diseases, its clinical application is still limitated to early atherosclerosis, and it is hampered by its invasiveness, high cost, inconvenient continuous monitoring, and frequent follow-up visits. Identification of at-risk patients by biological markers in the peripheral blood could suggest preventative strategies to decrease cardiovascular risk.
Atherosclerosis is the pathological basis of CAD, and evidence has shown that inflammatory response takes part in the whole process of plaque formation, rupture and thrombogenesis[1–3]. Vascular remodeling and extracellular matrix (ECM) degradation also play important roles in the process[4–5], and vascular remodeling requires that the ECM be degraded by specific cathepsin cysteine protease[6]. Stimulated by inflammatory mediators, vascular smooth muscle cells secrete cathepsin cysteine proteases S (cathepsin S), whose elastolytic activity[7] accelerates destruction of arterial elastin. As a member of cysteine protease inhibitor super-families, cystatin C shows the highest inhibitory action on cathepsins S[8].
Although substantial literatures have reported the relationship between the three biomarkers and CAD, they emphasized the relationship of individual biomarkers in CAD patients and the severity of atherosclerotic lesions of the coronary arteries. The use of three biomarkers in clinical classification of CAD has not been adequately assessed. Meanwhile, whether serum levels of three biomarkers correlated with Gensini score has not been investigated. In this study, we simultaneously measured the serum levels of cystatin C, cathepsin S, and interleukin-1β (IL-1 b)in CAD subjectse to investigate whether these non-invasive biomarkers were useful in clinical classification and assessment of the severity of CAD.
Subjects and methods
Subjects
We enrolled consecutive 112 patients (91 men and 21 women) who had typical symptoms of chest pain or myocardial ischemia on electrocardiogram (ECG), or who had at least one vessel stenosis ≥ 50% on CAG. These patients visited the Department of Cardiology at the Affiliated Hospital of Xuzhou Medical College between January 2014 and January 2015. Meanwhile, 50 healthy volunteers (39 men and 11 women) all underwent physical examination, and each health check-up was given an overall health valuation by cardiologists. The white blood cell count and erythrocyte sedimentation rate of the study subjects were in the normal range.
The healthy controls were assigned to group I. Patients who had typical symptom of chest pain with triggers and transient ST segment depression ST ≥ 1 mm were assigned to the stable angina group (group II). Patients who had typical symptom of chest pain at rest and sustainable ST segment depression ST ≥ 1 mm (segment elevation: limb leads ≥ 1 mm, chest leads ≥ 2 mm) were assigned to the unstable angina pectoris group (group III). Patients who had sustainable symptom of chest pain with pathological Q wave were assigned to the acute myocardial infarction group (group IV). Subjects with suspected CAD underwent coronary angiography. We excluded patients with any history of cardiac valve disease (heart murmur on auscultation, or valve regurgitation on color Doppler ), cardiomyopathy, congestive heart disease, thyroid disease, renal insufficiency (creatine clearance rate<20 mL/minute), cerebrovascular disease, acute or chronic infectious disease, autoimmune disease, cancer or erythrocyte sedimentation rate (ESR>20 mm/hour). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula.
The study protocol was approved by the local institutional board at the authors' affiliated institution and informed consent was obtained from all study subjects.
Blood and biochemistry tests
White blood cell (WBC) count and ESR were determined by blood routine examination. Furthermore, after overnight fasting, peripheral blood samples of patients were respectively taken from an antecubital vein in the morning before CAG for measurement of total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN) and serum creatinine (Scr).
ELISA
Venous blood samples were taken from subjects after an overnight fast, and from group II patients immediately after they came to the emergency room. Blood samples were centrifuged at 3000 r/minute, and serum samples were collected and stored at −80°C. We measured concentrations of cystatin C by using the Human Cystatin C ELISA kit as instructed by the manufacturer (Elabscience Biotechnology Co., Ltd, Wuhan, Hubei, China). Concentrations of cathepsin S were measured using the Human Cathepsin S ELISA kit (Elabscience). Levels of IL-1 bwere detected by the Human IL-1 bELISA kit (Elabscience).
Coronary angiography and Gensini score
A technologist performed coronary angiography through right femoral catheterization on patients who had symptoms of exertional dyspnea, chest pain and changes of ST segment and T wave on ECG. Two blinded experienced interventional cardiologists interpreted degrees of coronary artery stenosis. A modified Gensini scoring system[9] was used to assess the degree of vascular stenosis according to the results of CAG.
Data analysis
Patients were grouped according to the results of coronary angiography. Continuous variables with a normal distribution at baseline were expressed as mean (SD), and variables with a non-normal distribution were expressed as median (25th-75th percentile). Independent-sample T test was used for analyzing baseline characteristics of the study participants. One-way ANOVA was used for testing differences of three biomarkers in the three groups of patients. We used Spearman rank correlation to investigate the relationship of cystatin C, cathepsin S, IL-1β and Gensini score. Bivariate correlations were indicated by Spearman coefficient. A bilateralP<0.05 was considered significant for all tests. Statistical analysis was performed with SPSS 15.0.
Results
Baseline characteristics of the study population
We included 112 patients with CAD and 50 healthy controls in this study. Among the 162 participants, significant differences were found in age, LDC-C, diastolic blood pressure (DBP), systolic blood pressure (SBP), WBC, ESR, history of drinking, smoking and diabetes (P<0.05). However, there were no significant differences in gender, HDL-C, TC, TG, Scr, and BUN between CAD patients and healthy subjects (P>0.05). Baseline characteristics of the participants are summarized in Table 1.
Tab.1.
Characteristics of patients and controls
| Variable | Healthy controls (n=50) | SAP (n=42) | UAP (n=40) | AMI (n=30) | P |
|---|---|---|---|---|---|
| Age | 55.1±11.8 | 54.2±7.8 | 56.3±8.6 | 58.2±9.5 | 0.03 |
| Male (%) | 78.0 | 76.1 | 69.1 | 83.3 | 0.21 |
| LDL-C (mmol/L) | 3.0±0.8 | 3.4±0.7 | 3.9±0.6 | 3.3±0.3 | 0.04 |
| HDL-C (mmol/L) | 1.2±0.2 | 1.2±0.1 | 1.6±0.3 | 1.1±0.2 | 0.45 |
| TC (mmol/L) | 4.2±0.8 | 4.6±0.7 | 4.3±0.2 | 4.5±0.4 | 0.32 |
| TG (mmol/L) | 1.1±0.5 | 1.8±0.4 | 2.0±0.2 | 2.3±0.1 | 0.16 |
| Scr (µmol/L) | 77.3±6.2 | 78.1±7.4 | 75.6±8.1 | 73.1±10.2 | 0.30 |
| BUN (mmol/L) | 6.6±3.4 | 6.8±3.5 | 6.7±3.0 | 6.9±2.7 | 0.26 |
| Diastolic BP (mm Hg) | 76.2±9.4 | 79.1±5.6 | 78.0±10.2 | 85.3±12.4 | 0.01 |
| Systolic BP (mm Hg) | 119.5±10.6 | 121.0±7.6 | 129.4±11.2 | 134.6±7.9 | 0.02 |
| Diabetes (%) | 12.0 | 15.0 | 15.6 | 18.1 | 0.00 |
| Drinking (%) | 72.0 | 71.1 | 81.2 | 76.0 | 0.01 |
| Smoking (%) | 56.0 | 75.4 | 65.4 | 80.2 | 0.00 |
| WBC (×10 9/L) | 4.6±0.2 | 4.4±0.3 | 10.4±1.5 | 11.2±0.8 | 0.01 |
| SR (mm/L) | 15.1±2.8 | 14.9±2.5 | 18.1±3.2 | 20.5±5.6 | 0.03 |
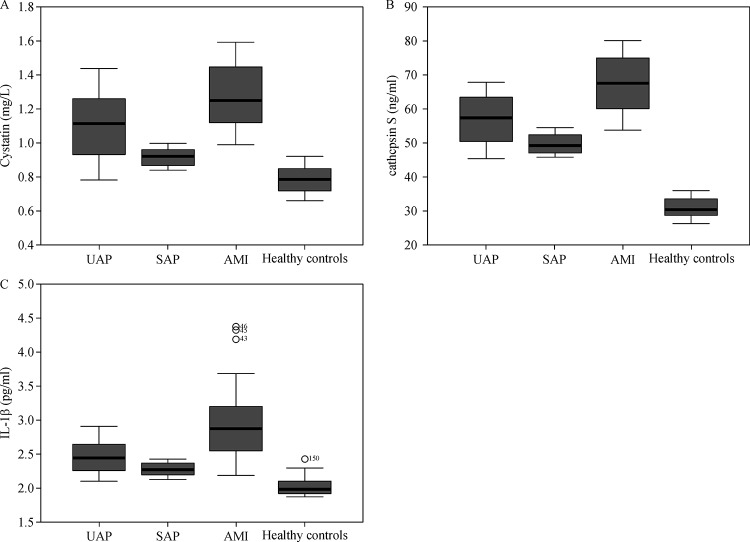
Patients with acute myocardial infarction exhibit significant elevations in cystatin C, cathepsin S and IL-1β
Group I had the lowest mean levels of cystatin C (0.78±0.07 mg/mL) and group IV had the highest levels (1.27±0.18 mg/mL) (Table 2). In addition, group I had the lowest mean levels of cathepsin S (30.88±2.94 ng/mL) and group IV had the highest levels (67.30±8.36 ng/mL). The mean levels of IL-1 bwere also the lowest in healthy controls (2.02±0.13 pg/mL) and the highest in group IV (2.96±0.57 pg/mL). One-way ANOVA test showed that the F value of cystatin C levels, cathepsin S levels, IL-1 blevels in different groups was 92.70, 320.87, and 68.77, respectively. Furthermore, cathepsin S showed the most obvious variance between healthy controls and patients with CAD (Fig. 1A–C). There were significant differences in the mean levels of these biomarkers between the groups (P<0.05, Table 2).
Tab.2.
Levels of the three biomarkers in CAD patients
| Bio-marker | Controls
(n=50) |
SAP
(n=42) |
UAP
(n=40) |
AMI
(n=30) |
F | P |
|---|---|---|---|---|---|---|
| Cystatin C
(mg/L) |
0.78±0.07 | 0.91±0.05 | 1.09±0.19 | 1.27±0.18 | 92.70 | 0.000 |
| Cathepsin S
(ng/mL) |
30.88±2.94 | 49.80±2.72 | 56.90±7.16 | 67.30±8.36 | 320.87 | 0.000 |
| IL-1β
(pg/mL) |
2.02±0.13 | 2.28±0.09 | 2.46±0.24 | 2.96±0.57 | 68.77 | 0.000 |
Fig.1.
Comparision of mean cystatin-C (A), IL-1β(B), and cathepsin S(C) levels among healthy contrlos (n=50), stable angina pectoris patients (SAP, n=42), unstable angina (The uppermost line presents 75 percentiles of variable, the line in the middle presents 50 percentiles of variable, and the bottom line presents 25 percentiles of variable) pectoris patients (UAP,n=40) and acute myocardial infarction patients (AMI, n=30).
Cystatin C, cathepsin S, and IL-1β levels correlate with Gensini score
Bivariate correlations were performed to investigate relationships of cystatin C, cathepsin S, IL-1β and Gensini score. Positive correlations were found among cystatin C, cathepsin S, IL-1 band Gensini score. Coefficient of rank correlations was 0.451, 0.491, and 0.397, respectively, andP value was 0.004, 0.000, and 0.000, respectively. Significant difference was found between the levels of these biomarkers and Gensini score.
Discussion
Existing literature mostly focuses on association of cathepsin S and CAD, cystatin C and CAD, as well as inflammatory cytokines and CAD. However, the prognostic value of combined cathepsin S, cystatin C and IL-1β on differentiating clinical classification of CAD has not been reported. Levels of cystatin C change in nephropathy patients, cathepsin S changes in patients with vascular disease, and levels of IL-1 bincrease in inflammatory diseases. In this study, we aimed to assess the prognostic value of combined cathepsin S, cystatin C and IL-1 bon differentiating clinical classification of CAD. The results of this study suggested that IL-1β, cathepsin S, and cystatin C were all significantly different among the groups and the levels of the three biomarkers correlated with severity of CAD.
Jernberg et al. considered cystatin C as a novel predictor of suspected or confirmed non-ST-elevation acute coronary syndrome[10]. Deoet al. studied association of cystatin C with ischemia in patients with coronary heart disease[11]. Koc et al. investigated clinical utility of serum cystatin C in predicting CAD[12]. Ichimoto et al. studied the prognostic significance of cystatin C in patients with ST-elevation myocardial infarction[13]. Their studies all showed a close relationship between cystatin C and CAD. In our study, we observed that levels of cystatin C in CAD patients were much higher than those in healthy controls, and high levels of cystatin C had a close association with the severity of CAD. Therefore, cystatin C may be a promising and clinically useful marker that provides complementary information to the established risk determinants in patients with CAD. Our results are in accordance with some studies[10,12–14] that also showed a rising trend of more severe CAD and worse clinical outcomes with higher cystatin C levels, and are contrary to those by Sukhovaet al.[15] and Noto et al.[16], Koc et al.[17], Sekizuca et al.[18], and Wang et al.[19] who suggested that elevated cystatin C was significantly associated with the presence and severity of CAD in patients with normal renal function.
Furthermore, serum cathepsin S is suggested as a potential biomarker. Protein expression studies showed that normal human arteries sparsely expressed cathepsin S[20]. Our study suggest that cathepsin S between healthy controls and patients with CAD had the largest gap among the three biomarkers, and its level increased with the aggravation of CAD, which is the same as the findings by Suzanneet al.[20–21]. It might be speculated that elevated plasma concentrations of cystatin C in patients with manifest cardiovascular disease and a future secondary event represent in part a compensatory mechanism for the increased activity of elastolytic proteases in an attempt to restore the physiologic balance between proteases and their main inhibitor[22]. Imbalance in cystatin C and cathepsin S in serum may explain our results that levels of cystatin C and cathepsin S increase in CAD .
Inflammatory response exists in the whole process of atherosclerosis[23]. IL-1β is a typical pro-inflammatory cytokine, which recruits inflammatory cells to the lesion site leading to injury of vascular tissues[24]. Stimulated by IL-1β, vascular smooth muscle cells secreted large numbers of cathepsin S and K. Permeating into cytoplasm and tissue space, cathepsin S and K promoted over-expression of cysteine protease in arterial elastin injured part. The present study showed that elevated IL-1β is associated with the presence and severity of CAD, and the result is the same with the previous studies[25–28]. During injury of blood vessels, production of inflammatory cytokines increase, which in turn stimulate the production of elastolytic cysteine proteases such as cathepsin S. This could explain increased levels of IL-1 band cathepsin S increase in CAD.
In our study, Gensini score that presents the severity of coronary artery stenosis is different in three clinical classifications of CAD. In an analysis of bivariate correlation, Gensini score shows positive correlations to cystatin C (r=0.451), cathepsin S (r=0.491), and IL-1β (r=0.397). Clear correlations suggest that serum levels of the three biomarkers could reflect the severity of coronary artery stenosis indirectly. Higher levels of mutiple biomarkers in patients with more severe CAD suggest that their clinical usefulness as potential biomarkers for identification of high risk CAD patients.
In summary, simultaneous detection of cystatin C, cathepsin S and IL-1β may be useful for differentiating clinical classification and diagnosing the severity of CAD. However, these data are limited, and we need greater samples to delineate the relationship between the biomarkers and CAD.
Acknowledgment
This research was founded by science and technology planning project of Xuzhou City (No. KC14SH088). We would like to thank all participants for their technical assistance and advice regarding statistical analysis.
References
- 1. Murtagh BM, Anderson HV. Inflammation and atherosclerosis in acute coronary syndromes [J] . J Invasive Cardiol, 2004, 16(7): 377–384. [PubMed] [Google Scholar]
- 2. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease[J]. N Engl J Med, 2005, 352(16): 1685–1695. [DOI] [PubMed] [Google Scholar]
- 3. Libby P. Inflammation in atherosclerosis[J]. Nature, 2002, 420(6917): 868–874. [DOI] [PubMed] [Google Scholar]
- 4. Siasos G, Tousoulis D, Kioufis S, et al. Inflammatory mechanisms in atherosclerosis: the impact of matrix metalloproteinases[J]. Curr Top Med Chem, 2012, 12(10): 1132–1148. [DOI] [PubMed] [Google Scholar]
- 5. Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans[J]. Cardiovasc Res, 2008, 79(1): 14–23. [DOI] [PubMed] [Google Scholar]
- 6. Iglseder B, Cip P, Malaimare L, et al. The metabolic syndrome is a stronger risk factor for early carotid atherosclerosis in women than in men[J]. Stroke, 2005, 36(6): 1212–1217. [DOI] [PubMed] [Google Scholar]
- 7. Obermajer N, Jevnikar Z, Doljak B, et al. Role of cysteine cathepsins in matrix degradation and cell signalling[J]. Connect Tissue Res, 2008, 49(3): 193–196. [DOI] [PubMed] [Google Scholar]
- 8. Hall A, Ekiel I, Mason RW, et al. Structural basis for different inhibitory specificities of human cystatins C and D[J]. Biochemistry, 1998, 37(12): 4071–4079. [DOI] [PubMed] [Google Scholar]
- 9. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease[J]. Am J Cardiol, 1983, 51(3): 606-606. [DOI] [PubMed] [Google Scholar]
- 10. Jernberg T, Lindahl B, James S, et al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome[J]. Circulation, 2004, 110(16): 2342–2348. [DOI] [PubMed] [Google Scholar]
- 11. Deo R, Shlipak MG, Ix JH, et al. Association of cystatin C with ischemia in patients with coronary heart disease[J]. Clin Cardiol, 2009, 32(11): E18–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koc M, Batur MK, Karaarslan O, et al. Clinical utility of serum cystatin C in predicting coronary artery disease[J]. Cardiol J, 2010, 17(4): 374–380. [PubMed] [Google Scholar]
- 13. Ichimoto E, Jo K, Kobayashi Y, et al. Prognostic significance of cystatin C in patients with ST-elevation myocardial infarction[J]. Circ J, 2009, 73(9): 1669–1673. [DOI] [PubMed] [Google Scholar]
- 14. Keller T, Messow CM, Lubos E, et al. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study[J]. Eur Heart J, 2009, 30(3): 314–320. [DOI] [PubMed] [Google Scholar]
- 15. Sukhova GK, Shi GP, Simon DI, et al. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells[J]. J Clin Invest, 1998, 102(3): 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noto D, Cefalu’ AB, Barbagallo CM, et al. Cystatin C levels are decreased in acute myocardial infarction: effect of cystatin C G73A gene polymorphism on plasma levels[J]. Int J Cardiol, 2005, 101(2): 213–217. [DOI] [PubMed] [Google Scholar]
- 17. Koc M, Batur MK, Karaarslan O, et al. Clinical utility of serum cystatin C in predicting coronary artery disease[J]. Cardiol J, 2010, 17(4): 374–380. [PubMed] [Google Scholar]
- 18. Sekizuka H, Akashi YJ, Kawasaki K, et al. Cystatin C: a better marker to detect coronary artery sclerosis[J]. J Cardiol, 2009, 54(3): 359–367. [DOI] [PubMed] [Google Scholar]
- 19. Wang Gan-nan, Sun Kai, Hu De-liang, Wu Hong-hao, Wang Xiao-zhi, Zhang Jin-song . Serum cystatin C levels are associated with coronary artery disease and its severity[J]. Clinical Biochemistry 2014 Jul 30. pii: S0009-9120(14)00530-X 10.1016/j.clinbiochem.2014.07.013. [DOI]
- 20. Lutgens SP, Cleutjens KB, Daemen MJ, et al. Cathepsin cysteine proteases in cardiovascular disease[J]. FASEB J, 2007, 21(12): 3029–3041. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Ma L, Yang J, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes[J]. Atherosclerosis, 2006, 186(2): 411–419. [DOI] [PubMed] [Google Scholar]
- 22. Koenig W, Twardella D, Brenner H, et al. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate[J]. Clin Chem, 2005, 51(2): 321–327. [DOI] [PubMed] [Google Scholar]
- 23. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease[J]. N Engl J Med, 2005, 352(16): 1685–1695. [DOI] [PubMed] [Google Scholar]
- 24. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family[J]. Annu Rev Immunol, 2009, 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 25. Cignarella A. Targeting interleukin-1ß hampers atherosclerosis progression- is there great promise [J]. [Journal homepage: www.elsevier.com/locate /atherosclerosis]. Atherosclerosis, 2011, 217(1): 64–66. [DOI] [PubMed] [Google Scholar]
- 26. Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice[J]. Arterioscler Thromb Vasc Biol, 2003, 23(4): 656–660 . [DOI] [PubMed] [Google Scholar]
- 27. Isoda K, Sawada S, Ishigami N, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice[J]. Arterioscler Thromb Vasc Biol, 2004, 24(6): 1068–1073. [DOI] [PubMed] [Google Scholar]
- 28. Olofsson PS, Sheikine Y, Jatta K, et al. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta:interleukin-1 receptor antagonist balance in atherosclerosis[J]. Circ J, 2009, 73(8): 1531–1536. [DOI] [PubMed] [Google Scholar]