Abstract
Bisphenol-A (BPA) has been considered as an endocrine disrupting chemical (EDC) because it can exert estrogenic properties. For bisphenol-S (BPS) and bisphenol-F (BPF) that are BPA analogs and substitutes, their risk to estrogen-dependent cancer has been reported rarely compared with the numerous cases of BPA. In this study, we examined whether BPA, BPS, and BPF can lead to the proliferation, migration, and epithelial mesenchymal transition (EMT) of MCF-7 clonal variant (MCF-7 CV) breast cancer cells expressing estrogen receptors (ERs). In a cell viability assay, BPA, BPS, and BPF significantly increased proliferation of MCF-7 CV cells compared to control (DMSO) as did 17β-estradiol (E2). In Western blotting assay, BPA, BPS, and BPF enhanced the protein expression of cell cycle progression genes such as cyclin D1 and E1. In addition, MCF-7 CV cells lost cell to cell contacts and acquired fibroblast-like morphology by the treatment of BPA, BPS, or BPF for 24 hours. In cell migration assay, BPA, BPS, and BPF accelerated the migration capability of MCF-7 CV cells as did E2. In relation with the EMT process, BPA, BPS, and BPF increased the protein expression ofN-cadherin, while they decreased the protein expression of E-cadherin. When BPA, BPS, and BPF were co-treated with ICI 182,780, an ER antagonist, proliferation effects were reversed, the expression of cyclin D1 and cyclin E1 was downregulated, and the altered cell migration and expression ofN-cadherin and E-cadherin by BPA, BPS, and BPF were restored to the control level. Thus, these results imply that BPS and BPF also have the risk of breast cancer progression as much as BPA in the induction of proliferation and migration of MCF-7 CV cells by regulating the protein expression of cell cycle-related genes and EMT markersvia the ER-dependent pathway.
Keywords: human breast cancer cells, endocrine disrupting chemicals, bisphenol-A, bisphenol-S, bisphenol-F, epithelial-mesenchymal transition, migration
Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous chemical compounds which can interfere with hormone balance and the normal functions of endocrine system[1–2]. Generally, EDCs bind to hormone receptors such as the androgen receptor (AR) and estrogen receptor (ER) and thereby interrupt the functions of endogenous steroid hormones[3]. They can threaten health by generating reproductive and developmental disorders and cancer development and progression[4]. In spite of many widely known hazardous effects of EDCs, eliminating them completely in everyday life is almost impossible because they are applied to various industrial products frequently used such as plastics, pesticides, food packages, cosmetics, and detergents[5].
As one of the chemicals produced in high volumes globally, bisphenol-A [BPA, 2,2-bis(4-hydroxydiphenyl)propane] is an industrially important chemical which is abundantly and widely used as a basic raw material for the production of polycarbonate plastics, epoxy resins, and lacquer coatings[6]. Current researches indicate that over 8 billion pounds of BPA are supplied every year and approximately 100 tons may be released into the environment annually[7]. BPA is currently found in various plastic goods including electronic equipment, automobiles, children's toys, and water bottles. Also, it is contained in the internal coatings of beverage and food cans and line water pipes[8]. Meanwhile, BPA has long been a health concern associated with serious and chronic conditions due to its potency as a crucial EDC having an estrogenic effect in the body and its widespread exposure[7,9–10]. Specifically, BPA was reported to have the potential to increase cancer risk[11] and to have cell proliferation effects in estrogen-dependent breast and ovarian cancer cells as in case of endogenous estrogen, 17β-estradiol (E2), which has been reported to be closely linked to the pathogenesis of some cancers occurring in the reproductive organs[9,12–13]. In animal experiments, BPA forced breast and prostate cells to be predisposed to cancer[14–15].
These negative health effects of BPA seem to be caused by other bisphenol analogs such as bisphenol S [BPS, bis(4-hydroxyphenyl)sulfone] and bisphenol F [BPF, 4,4'-dihydroxydiphenyl-methane] which are used as BPA substitutes because they share a similar structure and versatility to BPA. BPS has been used as an electroplating solvent, a constituent of phenolic resin, and wash fastening agent in cleaning products[16]. It is also used as a developer in currency as well as in paper products marketed as "BPA free paper"[17]. BPF is used to produce epoxy resins and coatings, especially for systems needing increased thickness and durability, such as tank and pipe linings, industrial floors, and structural adhesives[16]. BPF epoxy resins are also used for several consumer products such as food packaging, adhesives, and plastics[16]. Currently, BPS and BPF have been also detected in many everyday products such as personal care products (e.g. body wash, hair care products, makeup, lotions, and toothpastes)[18]. Although BPS and BPF were anticipated to reduce the concern of using the original bisphenol compound, BPA, or to be at least far less toxic than BPA, a systemic review displayed that these substitutes are also EDCs being as hormonally active as BPA[16].
To gain better understandings of cancer-related toxicity of BPS and BPF in comparison with BPA, which has been rarely discussed, we investigated whether they could affect cancer progression associated with proliferation, epithelial mesenchymal transition (EMT), and migration of estrogen-responsive breast cancer cells. Commonly, cancer cells have the potential to proliferate with abnormal cell growth rate and to invade or spread to other parts of the body[19]. In connection with cell growth, the cell cycle that is the series of events that take place in a cell leading to its division into two daughter cells is a vital process for development and organ renewal and is elaborately regulated by cell cycle checkpoint components. However, cancer cells abnormally proliferate by active cell division due to a dysregulation of the cell cycle components[20]. In addition, the EMT process is an important cellular mechanism by which epithelial cells lose their cell polarity and cell-cell adhesion, instead gaining an migratory and invasive mesenchymal phenotype[21–22]. This process governs many developmental processes and organ fibrosis and is also considered as critical for malignant transformation and the initiation of cancer metastasis[23–25]. Therefore, the EMT process has been considered as a hallmark of cancer stemness and aggressiveness[26].
In the present study, MCF-7 clonal variant (MCF-7 CV) breast cancer cell line was adopted as an estrogen-responsive cancer model because this cell line expresses both isoforms of ERα and ERβ and maintain high responsiveness to estrogen like its original cell line, MCF-7 breast cancer cell line[27]. In addition, the effects of ICI 182,780, an ER antagonist, co-treated with BPA, BPS, and BPF as well as E2 on cell proliferation, EMT, and migration of MCF-7 CV cells was also examined. Therefore, the present study aims to show that these bisphenol compounds may induce breast cancer progression by involving themselves in ER-dependent pathway, mimicking the mode of actions of endogenous estrogen. The outcomes obtained from this study may provide new insights for safer use of the original bisphenol compound as well as its widely-used substitutes based on their cancer-associated toxicity.
Materials and methods
Reagents and chemicals
E2, BPA, BPS, BPF, and fulvestrant (ICI 182,780) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). All chemicals were dissolved in 100% dimethyl sulfoxide (DMSO; Junsei Chemical Co., Tokyo, Japan).
Cell culture and media
MCF-7 CV cell line was originally provided by Dr. K. S. Korach (National Institute of Environmental Health Sciences, NIEHS, Research Triangle Park, NC, USA). Until a recent date, MCF-7 CV cell line has been known as BG-1 NIEHS ovarian cancer cell line, but Li Y et al. revealed that BG-1 NIEHS cell line is a clonal variant from the MCF-7 breast cancer cell line through short tandem repeat profile and gene expression comparisons[27]. MCF-7 CV cells were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone Laboratories Inc., Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories Inc.), 2% penicillin G and streptomycin (Cellgro; Mediatech, Inc., Manassas, VA, USA), and 1% HEPES (Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2-95% air. To prevent the effects of estrogenic components included in the DMEM and FBS, phenol red-free DMEM supplemented with 5% charcoal-dextran treated FBS (CD-FBS) was used to culture MCF-7 CV cells and to measure the estrogenicity of EDCs. The cells were detached with 0.05% Trypsin-EDTA (Life technologies, CA, USA).
Cell viability assay
To evaluate the effect of E2, BPA, BPS, and BPF on MCF-7 CV cell proliferation, a cell viability assay was conducted. MCF-7 CV cells were seeded at a density 3 × 103 cells per well in 96-well plates (SPL Life Science, Seoul, Republic of Korea) in a humidified atmosphere of 5% CO2 at 37°C. After the cells were incubated with phenol red-free DMEM with 5% CD-FBS medium for 48 hours, they were treated with various concentrations of E2, BPA, BPS, or BPF (E2: 10−9−10−7 mol/L, BPA: 10−7−10−5 mol/L, BPS: 10−7−10−5 mol/L, or BPF: 10−7−10−5 mol/L) in phenol red-free DMEM with 5% CD-FBS supplemented with 0.1% DMSO for 6 days. During this period, the media were changed to the same new media every second day. DMSO was used as a vehicle to carry the chemicals to the media. Cell viability was detected with the addition of 3-(4-5-dimethylthiazol-2-yl)-2.5-dyphenyltetrazolium bromide (MTT; Sigma-Aldrich) solution. MTT (10 mL of 5 mg/mL solution) was added to each well of 96-well plates, and the plates were incubated for 3 hours at 37°C in a humidified atmosphere of 5% CO2. Supernatants were removed, and 100 mL DMSO was added to each well to dissolve resultant formazan crystals. The optical density (OD) of each well was measured at 540 nm using an ELISA reader (Epoch, BioTek, VT, USA) and used to calculate the number of viable cells.
Protein extraction and Western blotting assay
MCF-7 CV cells were cultured to a density of 1.0×106 cells per 100-mm dish and then treated with DMSO, E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L), BPF (10−5 mol/L), and ICI 182,780 (10−8 mol/L) or combinations of ICI 182,780 (10−8 mol/L) and E2, BPA, BPS or BPF for 48 hours. After treatment, the proteins from MCF-7 CV cells were harvested with RIPA lysis buffer (50 mmol/L Tris-HCl, pH 8.0; 150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholic acid, and 0.1% SDS). Total protein concentrations were determined using bicinchoninic acid (BCA; Sigma-Aldrich Corp.) Total proteins (50 mg) were separated on 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories). The membrane was incubated with a mouse monoclonal antibody specific for cyclin D1 (1:2,000 dilution), cyclin E1 (1:2,000 dilution),E-cadherin (1:500 dilution), N-cadherin (1:2,000 dilution) or GAPDH (1:2,000 dilution, antibodies were all from Abcam, Hanam, Korea) overnight at 4°C. Primary antibody binding was detected with a horseradish peroxidase (HRP)-conjugated secondary antibody [goat anti-mouse lgG (H+L) or goat anti-mouse lgG (H+L) HRP conjugate (1:5,000 dilution, Bio-Rad Laboratories)]. Target proteins were detected with a West-Q Chemiluminescent Substrate Plus kit (GenDEPOT, Barker, TX, USA). Quantification of each protein was performed by scanning the densities of bands on a transfer membrane using Lumino graph II (ATTO, Japan).
Effects of E2, BPA, BPS or BPF on MCF-7 CV cells morphology
MCF-7 CV cells were seeded in 6-well and treated with E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) for 24 hours. Cellular morphology of MCF-7 CV cells was detected by Olympus IX-73 inverted microscope (Olympus, Japan) under magnification of 400 times before and after the treatment of the reagents.
Scratch-wound healing assay
MCF-7 CV cells were cultured more than 70% confluent growth (about 1.0×106 cells) in each well of 6-well plates at 37°C in a humidified atmosphere of 95% and 5% CO2 air. Monolayer of MCF-7 CV cells seeded in a well was scratched with a 1 mL micropipette tip in the same length and width. And the cells were treated with media containing 5% charcoal/dextran-treated FBS with DMSO (0.1%, control), E2 (10−9 mol/L, positive control), BPA (10−5 mol/L), BPS (10−5 mol/L), BPF (10−5 mol/L), ICI 182, 780 (10−8 mol/L), the combination of E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) and ICI 182,780 (10−8 mol/L), respectively, and incubated for 48 hours. The images of each treatment group were captured at ×40 magnification using a microscope (Olympus IX-73 Inverted Microscopy, Olympus, Japan). The percentage of wound healing area was calculated by Cell Sense Dimension software (Olympus, Japan).
Data analysis
All experiments were conducted at least three times, and all data were analyzed with Graph-pad Prism software (San Diego, CA, USA). Data were expressed as the mean±SD and analyzed with one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test and Student'st-test. P-values<0.05 were considered to be statistically significant.
Results
Cell growth effect by E2, BPA, BPS, or BPF in the absence or presence of ICI 182,780
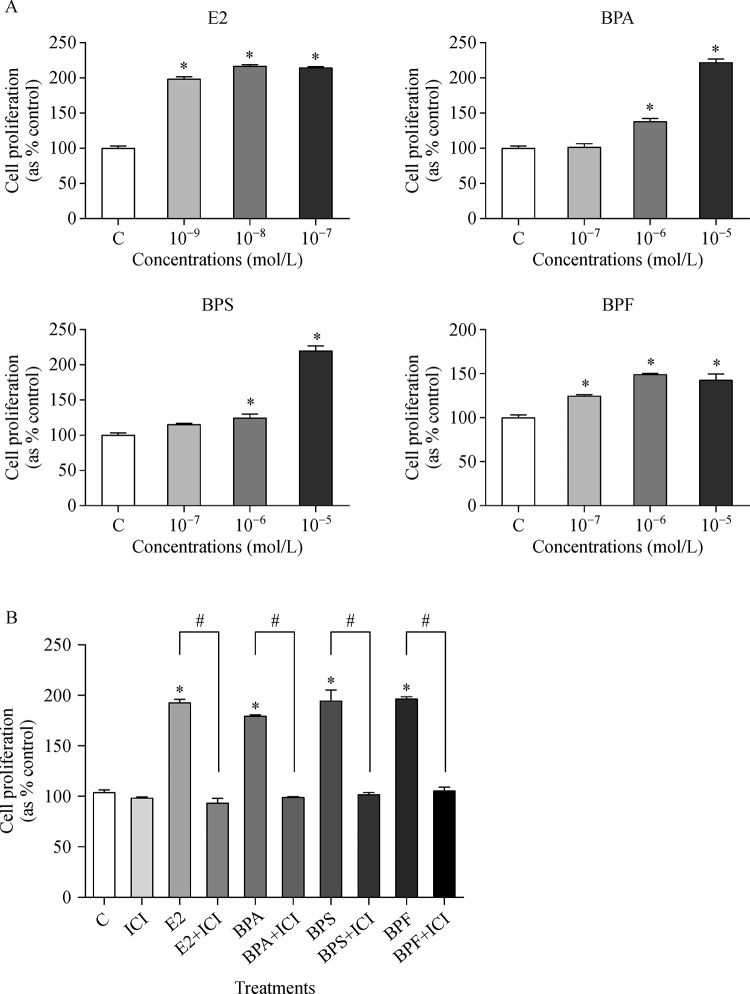
To examine the effect of E2, BPA, BPS, and BPF on cell proliferation of MCF-7 CV cells, a cell viability assay was conducted by using MTT. As indicated inFig. 1, E2 (10−9 mol/L to 10−7 mol/L), BPA (10−6 mol/L to 10−5 mol/L), BPS (10−6 mol/L to 10−5 mol/L), and BPF (10−7 mol/L to 10−5 mol/L) increased the cell viability compared to control (Fig. 1A). Even though higher concentrations of BPA, BPS, and BPF were needed to induce substantial cell proliferation compared to E2, these chemicals also stimulated cell proliferation from the concentration of 10−7 mol/L or 10−6 mol/L in a dose dependent manner. However, E2, BPA, BPS, and BPF did not lead to proliferation of MCF-7 CV cells when the ER receptors were blocked by ICI 182,780, an ER antagonist (Fig. 1B). This implies that BPA, BPS, and BPF can induce the proliferation of MCF-7 CV cells through ER-dependent pathway. From the result of cell viability, the concentration of BPA, BPS, and BPF for other experiments of the present study was identically chosen as 10−5 mol/L.
Fig.1.
Viability of MCF-7 CV cells following treatment with E2, BPA, BPS or BPF and co-treatment of ICI 182,780.
Altered protein expressions of cyclin D1& E1 by E2, BPA, BPS, or BPF in the absence or presence of ICI 182,780
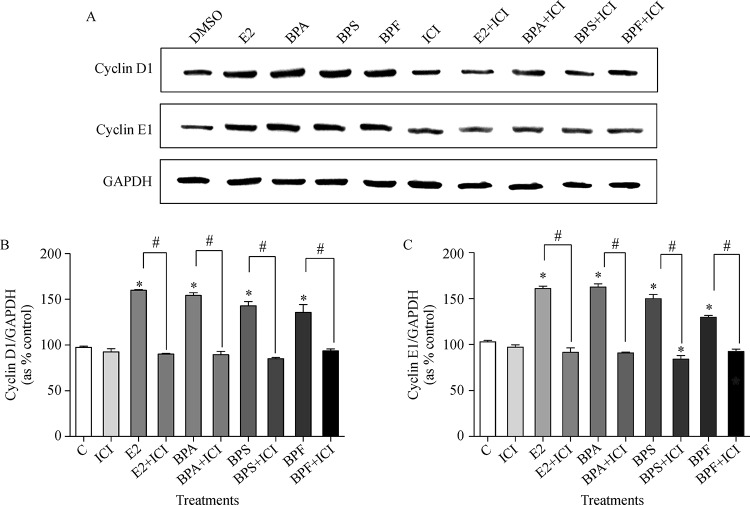
Western blot assay was conducted to investigate the influences of E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) alone or in combination with ICI 182,780 (10−8 mol/L) on the protein expression of cell cycle control genes such as cyclin D1 and cyclin E1. Cyclin D1 and E1 are proteins that regulate the proliferation of cells through the cell cycle progression by stimulating cyclin-dependent kinase (Cdk) enzymes[28]. E2, BPA, BPS, and BPF increased protein expressions of cyclin D1 (Fig. 2Aand2B) and cyclin E1 (Fig. 2Aand2C). In contrast, the expression of cyclin D1 and cyclin E1 were reduced by co-treatment of ICI 182,780 with E2, BPA, BPS, or BPF compared to the treatment of E2, BPA, BPS, or BPF alone as seen inFig. 2, indicating that BPA, BPS, and BPF can increase the protein expression of cell cycle progression genes, cyclin D1 and E1, through ER-dependent pathway.
Fig.2.
Altered levels of cyclin D1 and cyclin E1 protein expression following treatment with E2, BPA, BPS or BPF and co-treatment of ICI 182,780.
Effects of E2, BPA, BPS or BPF on MCF-7 CV cell morphology
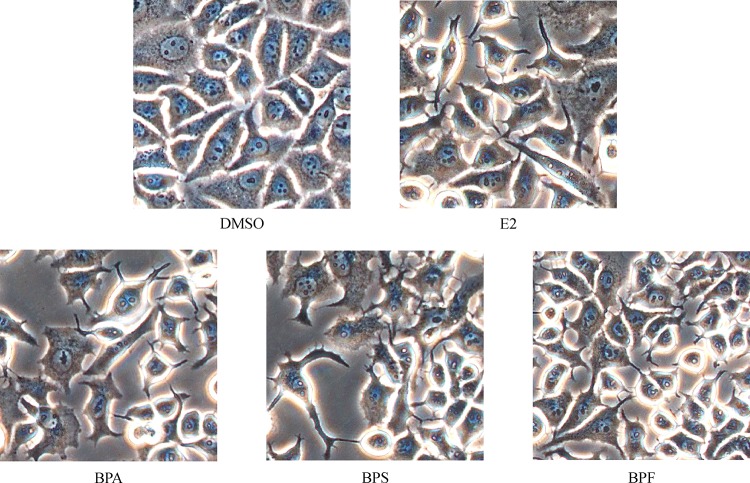
MCF-7 CV cells were seeded in 6-well and treated with E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) for 24 hours. After the treatment, it was microscopically found that MCF-7 CV cells acquired a spindle-shaped or a fibroblast-like morphology and lost cell to cell contacts whereas under the control condition, most MCF-7 CV cells maintained a cobble stone-like appearance typical of epithelial cell morphology as shown inFig. 3.
Fig.3.
Effects of E2, BPA, BPS or BPF on MCF-7 CV cell morphology.
Altered protein expressions of E- & N-cadherin by E2, BPA, BPS, or BPF in the absence or presence of ICI 182,780
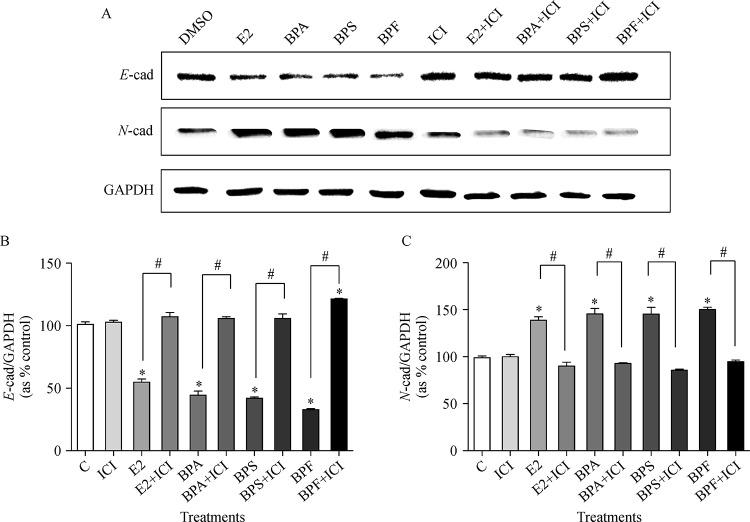
To clarify the effects of E2, BPA, BPS, and BPF on protein expressions of EMT-related genes, Western blot assay was conducted. The expression of E-cadherin, a crucial epithelial marker, was significantly decreased in the treatment with E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) compared to control as seen in Fig. 4Aand4B . On the other hand, when co-treated with ICI 182,780 (10−8 mol/L), the expression of E-cadherin was increased to the control level (Fig. 4B). In addition, the expression of N-cadherin, a mesenchymal marker, was increased in the treatment with E2, BPA, BPS or BPF compared to control as illustrated inFig. 4AandC. On the contrary, the expression of N-cadherin was reduced by treatment with the co-treatment of ICI 182,780 compared to the treatment of E2, BPA, BPS, or BPF alone (Fig. 4C). These results indicate that BPA, BPS, and BPF induced the EMT process in MCF-7 CV cells via ER-dependent pathway.
Fig.4.
Altered protein expression of E-cadherin and N-cadherin genes following treatment with E2, BPA, BPS or BPF and co-treatment of ICI 182,780.
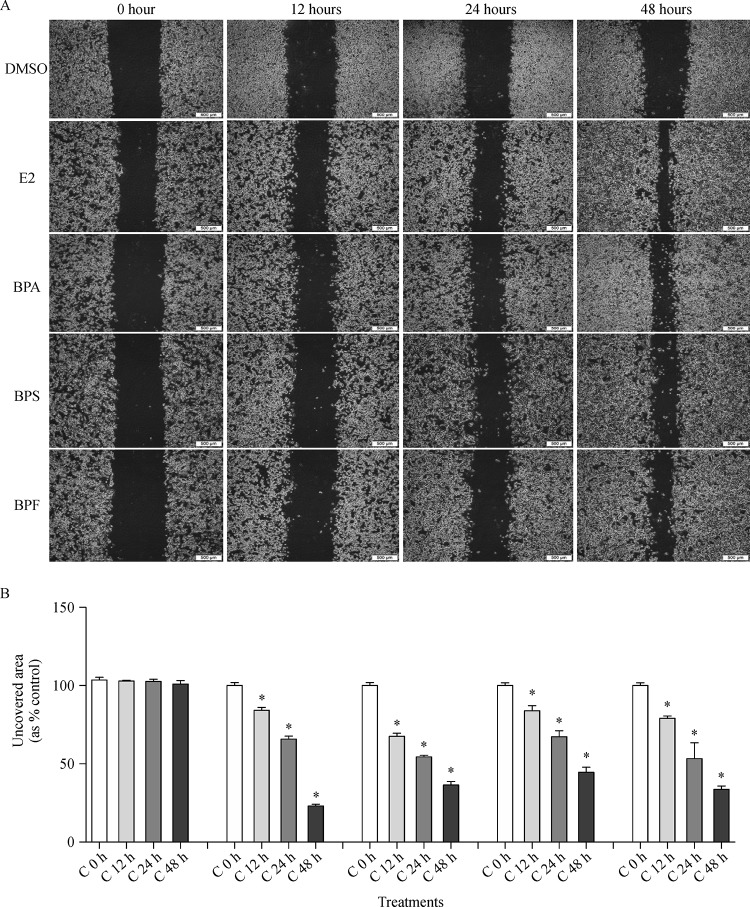
Effect of E2, BPA, BPS, and BPF on migration of MCF-7 CV cells
The altered migration activity of MCF-7 CV cells by the treatment of E2, BPA, BPS, and BPF was identified by cell migration assay. Cells were treated with 0.1% DMSO, E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L) after scratching with a 1 ml micropipette tip, and then the images showing the closure of wounded area were captured at 0, 12, 24 and 48 hours. By the treatment of E2, BPA, BPS, and BPF, unrecovered wound areas were remarkably reduced in a time-dependent manner compared to those of control as seen inFig. 5. Unrecovered wound areas were significantly decreased to approximately 23.03%, 36.48%, 44.61%, and 33.69% by the treatment of E2, BPA, BPS, and BPF for 48 h, respectively (Fig. 5B), implying that BPA, BPS, and BPF stimulated the migration of MCF-7 CV cells as did E2.
Fig.5.
Effects of E2, BPA, BPS or BPF on MCF-7 CV cell migration.
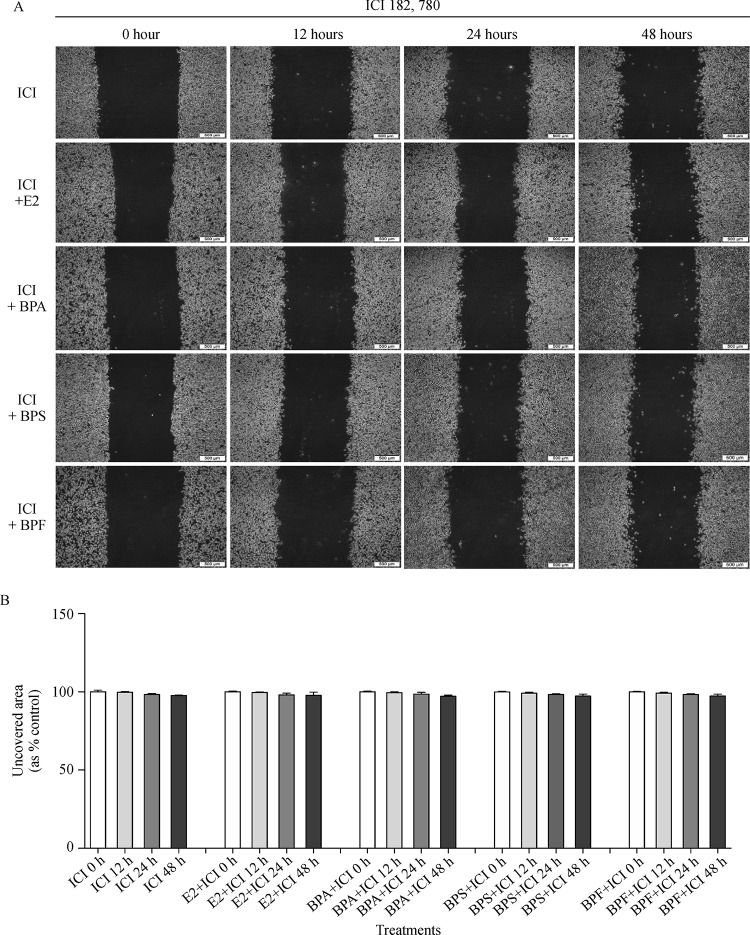
Inhibitory effect of ICI 182,780, an ER antagonist, on E2, BPA, BPS or BPF-induced migratory capability
To elucidate the involvement of bisphenol compounds in ER signaling to induce the cell migration, a migration assay was performed for MCF-7 CV cells treated with the combination of ICI 182,780 with E2 (10−9 mol/L), BPA (10−5 mol/L), BPS (10−5 mol/L) or BPF (10−5 mol/L). Percentages of unrecovered areas in the treatment of DMSO+ ICI, E2+ ICI, BPA+ ICI, BPS+ ICI and BPF+ ICI at 48 hours were on average 97.82%, 97.16%, 98.12%, and 97.29, respectively, as shown inFig. 6AandB. Therefore, unrecovered wounded areas of all treatment groups were not changed and maintained the similar level to that of a control (DMSO+ ICI) (Fig. 6B). According to these results, BPA, BPS, and BPF may induce the migration in MCF-7 CV cells via an ER-dependent pathway.
Fig.6.
Effect of ICI 182,780 co-treated with E2, BPA, BPS or BPF on the migratory capability of MCF-7 CV cells.
Discussion
Coupled with the fact that endogenous estrogens are closely linked to the pathogenesis of cancers occurring in estrogen-responsive organs[29–31], there is a greater probability that natural and environmental estrogens may have carcinogenic activities in the relevant organs[32]. According to recent studies, EDCs having an estrogenic effect have been related to the development and progression of cancers of estrogen-responsive organs such as breast, ovarian, and uterine[33–39]. For instance, BPA, an original bisphenol compound, has been identified to definitely act as an EDC with some estrogenic properties by binding to the ERs and likely to induce or predispose to pre-neoplastic lesions of the mammary and prostate gland[40]. Additionally, it has been reported that prenatal exposure to environmentally relevant doses of BPA may increase markers of breast cancer risk in humans[40]. It was also found that low BPA dose (10−9 mol/L) stimulated the cell proliferation of human breast carcinoma cells and that the combined effect of low BPA dose with physiologic E2 concentration led to the reduced rate of apoptosis[41].
For BPS and BPF, which are BPA analogs and frequently used substitutes for BPA, a systemic review displayed that their hormonal activities are in the same order of magnitude and of similar action to BPAin vitro and in vivo, and they also have endocrine disrupting effects[16]. However, there has been no reported the risk of BPS and BPF to estrogen-dependent cancer compared with the numerous cases of BPA. For this reason, we proved the cancer progression potential as a crucial endocrine disrupting activity of BPA as well as these BPA analogs (BPS and BPF) targeting estrogen-responsive breast cancer cell line, MCF-7 CV, comparing with the effects of E2, a positive control.
In cell viability assay, BPA, BPS, and BPF significantly induced the proliferation of MCF-7 CV cells compared to control (DMSO) as did E2. In Western blot assay, BPA, BPS, and BPF enhanced protein expressions of cyclin D1 and cyclin E1. The cell cycle control genes, D-type cyclins and cyclin E, are involved with cell cycle progression through the G1 phase by forming complexes with Cdks including cdk4, cdk6, or cdk2[42–43]. Actually, cyclin D1 is frequently overexpressed in ductal carcinoma, and its expression appears to be closely linked with carcinogenesis[44]. Also, high-level cyclin E expression represents a tumor-associated abnormality and is considered a potential prognostic marker of breast cancer[45–46]. On the other hand, cell proliferation effect and upregulation of cyclin D1 and E1 by BPA, BPS, and BPF as well as E2 were restored to the control level by the co-treatment of ICI 182,780, an ER antagonist. From these results, it can be suggested that BPA, BPS, and BPF promoted the growth of MCF-7 CV breast cancer cells by upregulating the cell cycle progression genes through ER-dependent pathway.
Following the cancer cell proliferation effect, the metastatic potential of BPA, BPS, and BPF was identified by their involvement in the EMT process and cell migration. BPA, BPS, and BPF changed cell morphology of MCF-7 CV cells from cobblestone appearance of epithelial cells to a spindle-shaped or fibroblast-like mesenchymal cell morphology as did E2, indicating that they have a potential to induce the EMT process in MCF-7 CV cells. The activation of EMT was validated by the altered protein expression of EMT markers such as E-cadherin and N-cadherin by BPA, BPS, and BPF. However, co-treatment of ICI 172,780 reversed the altered protein expression by these bisphenol compounds and E2 to the control level. E-cadherin that is a transmembrane protein responsible for adherens junction and highly expressed in epithelial cells is a typical epithelial marker[47]. The EMT event is induced by E-cadherin loss, and E-cadherin expression was found to be reduced or absent in poorly differentiated carcinomas of diverse cancers[48]. On the contrary, N-cadherin is a mesenchymal marker associated with an increased invasive potential in cancer metastasis[49]. A recent study demonstrated that overexpression of N-cadherin in breast carcinoma correlates with invasiveness as a result of N-cadherin-mediated interactions between cancer and stromal cells[50].
In a migration assay, unrecovered areas were declined by the treatment of BPA, BPS or BPF within the periods (12, 24, and 48 h) in which the influence of cell proliferation was nearly excluded, indicating that BPA, BPS, and BPF effectively induced cell migration as did E2. Therefore, it seems that MCF-7 CV cells acquiring mesenchymal phenotypes from the EMT process induced by the treatment of the bisphenol compounds simultaneously achieved the migration ability. As with other results, the increased cell migration ability of MCF-7 CV cells by these bisphenol compounds and E2 were reduced to the control level by the co-treatment of ICI 182,780.
In our previous study, it was already demonstrated that BPA have the ability to increase ovarian cancer cell migration and metastatic potentialvia regulating EMT markers such as E-cadherin, snail, slug, and vimentin[51]. Furthermore, the present study identified that BPS and BPF have the similar hormonal activities to BPA in the induction of the EMT process and migration as well as proliferation of MCF-7 CV breast cancer cells.
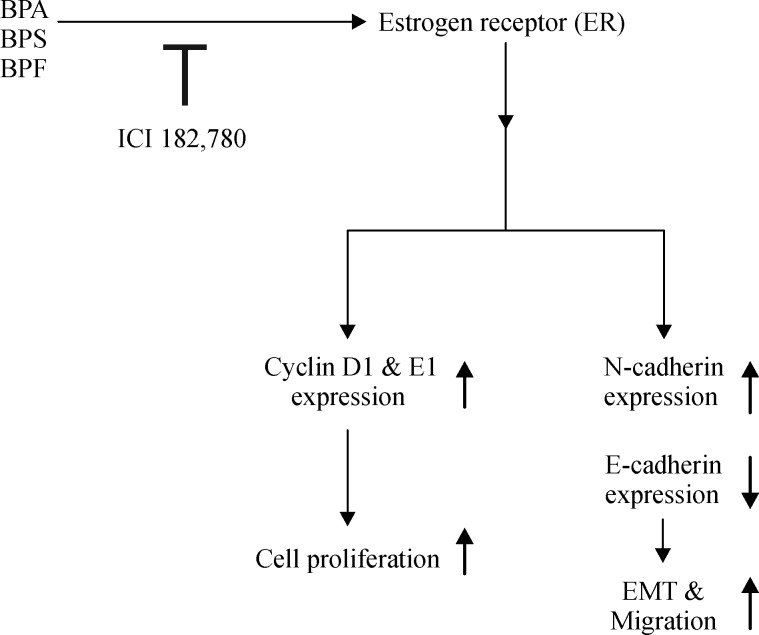
Collectively as shown in Fig. 7, BPS and BPF were displayed to act as xenoestrogens by promoting the proliferation of MCF-7 CV cells via ER-dependent signaling pathway similar to BPA. These bisphenol compounds all induced cell cycle progression by upregulating the expression of cyclin D1 and cyclin E1. They were also found to increase the metastatic potential of MCF-7 CV cells by inducing the EMT process and cell migration through regulating EMT-related markers such asE-cadherin and N-cadherin via ER-dependent pathway. Therefore, this study may enlighten that BPS and BPF also have the risk of breast cancer progression as much as BPA does and that their use as BPA substitutes should be cautious especially in cancer patients. Further studies are needed to shed light on thein vivo effect of these bisphenols on breast cancer progression as well as to elucidate their cancer-related endocrine disrupting risk using other various cancer models.
Fig.7.
Involvement of BPA, BPS or BPF in the proliferation, EMT, and migration of MCF-7 CV cells via ER-dependent pathway.
Acknowledgment
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ011355-2015), Rural Development Administration, Republic of Korea. In addition, this work was supported by Priority Research Centers Program through NRF funded by the Ministry of Education, Science and Technology (2015R1A6A1A04020885).
Contributor Information
Ji-Youn Kim, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
Ho-Gyu Choi, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
Hae-Miru Lee, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
Geum-A Lee, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
Kyung-A Hwang, Email: hka9400@naver.com, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
Kyung-Chul Choi, Email: kchoi@cbu.ac.kr, Laboratory of Biochemistry and Immunology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644 Republic of Korea..
References
- 1. Kim CW, Go RE, Choi KC. Treatment of BG-1 ovarian cancer cells expressing estrogen receptors with lambda-cyhalothrin and cypermethrin caused a partial estrogenicity via an estrogen receptor-dependent pathway[J]. Toxicol Res, 2015, 31(4): 331–337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diamanti-Kandarakis E, Palioura E, Kandarakis SA, et al. The impact of endocrine disruptors on endocrine targets[J]. Horm Metab Res, 2010, 42(8): 543–552 . [DOI] [PubMed] [Google Scholar]
- 3. Choi KC, Jeung EB. The biomarker and endocrine disruptors in mammals[J]. J Reprod Dev, 2003, 49(5): 337–345 . [DOI] [PubMed] [Google Scholar]
- 4. Kopras E, Potluri V, Bermudez ML, et al. Actions of endocrine-disrupting chemicals on stem/progenitor cells during development and disease[J]. Endocr Relat Cancer, 2014, 21(2): T1–T12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jung EM, Choi KC, Yu FH, et al. Effects of 17beta-estradiol and xenoestrogens on mouse embryonic stem cells[J]. Toxicol In Vitro, 2010, 24(6): 1538–1545 . [DOI] [PubMed] [Google Scholar]
- 6. Chen MY, Ike M, Fujita M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols[J]. Environ Toxicol, 2002, 17(1): 80–86 . [DOI] [PubMed] [Google Scholar]
- 7. Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A[J]. Cien Saude Colet, 2012, 17(2): 407–434 . [DOI] [PubMed] [Google Scholar]
- 8. Staples CA, Dorn PB, Klecka GM, et al. A review of the environmental fate, effects, and exposures of bisphenol A[J]. Chemosphere, 1998, 36(10): 2149–2173 . [DOI] [PubMed] [Google Scholar]
- 9. Lee HR, Hwang KA, Park MA, et al. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway[J]. Int J Mol Med, 2012, 29(5): 883–890 . [DOI] [PubMed] [Google Scholar]
- 10. Lee HS, Park EJ, Oh JH, et al. Bisphenol A exerts estrogenic effects by modulating CDK1/2 and p38 MAP kinase activity[J]. Biosci Biotechnol Biochem, 2014, 78(8): 1371–1375 . [DOI] [PubMed] [Google Scholar]
- 11. Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens[J]. Nat Rev Endocrinol, 2010, 6(7): 363–370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park MA, Choi KC. Effects of 4-nonylphenol and bisphenol A on stimulation of cell growth via disruption of the transforming growth factor-β signaling pathway in ovarian cancer models[J]. Chem Res Toxicol, 2014, 27(1): 119–128 . [DOI] [PubMed] [Google Scholar]
- 13. Russo J, Hasan Lareef M, Balogh G, et al. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells[J]. J Steroid Biochem Mol Biol, 2003, 87(1): 1–25 . [DOI] [PubMed] [Google Scholar]
- 14. Murray TJ, Maffini MV, Ucci AA, et al. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure[J]. Reprod Toxicol, 2007, 23(3): 383–390 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho SM, Tang WY, Belmonte de Frausto J, et al. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4[J]. Cancer Res, 2006, 66(11): 5624–5632 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol a substitutes[J]. Environ Health Perspect, 2015, 123(7): 643–650 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao C, Liu F, Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues[J]. Environ Sci Technol, 2012, 46(12): 6515–6522 . [DOI] [PubMed] [Google Scholar]
- 18. Liao C, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States[J]. Arch Environ Contam Toxicol, 2014, 67(1): 50–59 . [DOI] [PubMed] [Google Scholar]
- 19. Hanahan D, Weinberg RA. The hallmarks of cancer[J]. Cell, 2000, 100(1): 57–70 . [DOI] [PubMed] [Google Scholar]
- 20. Champeris Tsaniras S, Kanellakis N, Symeonidou IE, et al. Licensing of DNA replication, cancer, pluripotency and differentiation: an interlinked world[J]? Semin Cell Dev Biol, 2014, 30(174–180. [DOI] [PubMed] [Google Scholar]
- 21. Son H, Moon A. Epithelial-mesenchymal transition and cell invasion[J]. Toxicol Res, 2010, 26(4): 245–252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta N, Duda DG. Role of stromal cell-derived factor 1α pathway in bone metastatic prostate cancer[J]. J Biomed Res, 2016, 30(3): 181–185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease[J]. Cell, 2009, 139(5): 871–890 . [DOI] [PubMed] [Google Scholar]
- 24. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis[J]. Science, 2011, 331(6024): 1559–1564 . [DOI] [PubMed] [Google Scholar]
- 25. Oh K, Moon HG, Lee DS, et al. Tissue transglutaminase-interleukin-6 axis facilitates peritoneal tumor spreading and metastasis of human ovarian cancer cells[J]. Lab Anim Res, 2015, 31(4): 188–197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chunhacha P, Sriuranpong V, Chanvorachote P. Epithelial-mesenchymal transition mediates anoikis resistance and enhances invasion in pleural effusion-derived human lung cancer cells[J]. Oncol Lett, 2013, 5(3): 1043–1047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Arao Y, Hall JM, et al. Research Resource: STR DNA profile and gene expression comparisons of human BG-1 cells and a BG-1/MCF-7 clonal variant[J]. Molecular endocrinology (Baltimore, Md, 2014, 28(12): 2072–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sherr CJ. Cancer cell cycles[J]. Science, 1996, 274(5293): 1672–1677 . [DOI] [PubMed] [Google Scholar]
- 29. Santen RJ, Boyd NF, Chlebowski RT, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model[J]. Endocr Relat Cancer, 2007, 14(2): 169–187 . [DOI] [PubMed] [Google Scholar]
- 30. Giacalone PL, Daurés JP, Ouafik L, et al. Steroids and adrenomedullin growth patterns in human ovarian cancer cells: estrogenic-regulation assay[J]. Gynecol Oncol, 2003, 91(3): 651–656 . [DOI] [PubMed] [Google Scholar]
- 31. Chung SH, Franceschi S, Lambert PF. Estrogen and ERalpha: culprits in cervical cancer[J]? Trends Endocrinol Metab, 2010, 21(8): 504–511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelekanou V, Leclercq G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis[J]. Int J Dev Biol, 2011, 55(7-9): 869–878 . [DOI] [PubMed] [Google Scholar]
- 33. Conzen SD. Minireview: nuclear receptors and breast cancer[J]. Mol Endocrinol, 2008, 22(10): 2215–2228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derouiche S, Warnier M, Mariot P, et al. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling[J]. Springerplus, 2013, 2(1): 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression[J]. J Clin Oncol, 2010, 28(26): 4038–4044 . [DOI] [PubMed] [Google Scholar]
- 36. Hsieh TH, Tsai CF, Hsu CY, et al. n-Butyl benzyl phthalate promotes breast cancer progression by inducing expression of lymphoid enhancer factor 1[J]. PLoS One, 2012, 7(8): e42750 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis[J]. Int J Breast Cancer, 2012, 2012: 654698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu L, Shi J, Cheng S, et al. Estrogen promotes prostate cancer cell migration via paracrine release of ENO1 from stromal cells[J]. Mol Endocrinol, 2012, 26(9): 1521–1530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng S, Huang J, Zhou K, et al. 17β-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein ezrin[J]. PLoS One, 2011, 6(7): e22439 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keri RA, Ho SM, Hunt PA, et al. An evaluation of evidence for the carcinogenic activity of bisphenol A[J]. Reprod Toxicol, 2007, 24(2): 240–252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mlynarcikova A, Macho L, Fickova M. Bisphenol A alone or in combination with estradiol modulates cell cycle- and apoptosis-related proteins and genes in MCF7 cells[J]. Endocr Regul, 2013, 47(4): 189–199 . [DOI] [PubMed] [Google Scholar]
- 42. Bates S, Bonetta L, MacAllan D, et al. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1[J]. Oncogene, 1994, 9(1): 71–79 . [PubMed] [Google Scholar]
- 43. Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner[J]. Mol Cell Biol, 1994, 14(3): 2077–2086 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barnes DM, Gillett CE. Cyclin D1 in breast cancer[J]. Breast Cancer Res Treat, 1998, 52(1–3): 1–15 . [DOI] [PubMed] [Google Scholar]
- 45. Keyomarsi K, O’Leary N, Molnar G, et al. Cyclin E, a potential prognostic marker for breast cancer[J]. Cancer Res, 1994, 54(2): 380–385 . [PubMed] [Google Scholar]
- 46. Nielsen NH, Arnerlöv C, Emdin SO, et al. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status[J]. Br J Cancer, 1996, 74(6): 874–880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiao D, He J. Epithelial mesenchymal transition and lung cancer[J]. J Thorac Dis, 2010, 2(3): 154–159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graff JR, Herman JG, Lapidus RG, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas[J]. Cancer Res, 1995, 55(22): 5195–5199 . [PubMed] [Google Scholar]
- 49. Hazan RB, Phillips GR, Qiao RF, et al. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis[J]. J Cell Biol, 2000, 148(4): 779–790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakajima S, Doi R, Toyoda E, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma[J]. Clin Cancer Res, 2004, 10(12 Pt 1): 4125–4133 . [DOI] [PubMed] [Google Scholar]
- 51. Kim YS, Hwang KA, Hyun SH, et al. Bisphenol A and nonylphenol have the potential to stimulate the migration of ovarian cancer cells by inducing epithelial-mesenchymal transition via an estrogen receptor dependent pathway[J]. Chem Res Toxicol, 2015, 28(4): 662–671 . [DOI] [PubMed] [Google Scholar]