Abstract
Synthesis of nanocomposites from antimicrobial biopolymers such as chitosan (CS) and lysozyme (LZ) is an important and promising area in bionanotechnology. Chitosan–lysozyme (CS–LZ) nanoparticles (NPs) were prepared by the nanoprecipitation method, using commercial chitosan of 153 kDa. TEM and dynamic light scattering (DLS) analysis were carried out to evaluate the morphology, size, dispersion, and Z potential. Association efficiency of lysozyme was determined using Coomassie blue assay. The antifungal activity of NPs against Aspergillus parasiticus was evaluated through cell viability (XTT), germination and morphometry of spores, and reducing sugars production; the effects on membrane integrity and cell wall were also analyzed. NPs’ size were found in the range of 13.4 and 11.8 nm for CS–LZ and CS NPs, respectively, and high Z potential value was observed in both NPs. Also, high association of lysozyme was presented in the CS matrix. With respect to the biological responses, CS–LZ NPs reduced the viability of A. parasiticus and a strong inhibitory effect on the germination of spores (100% of inhibition) was observed at 24 h in in vitro assays. CS–LZ and CS NPs affected the membrane integrity and the cell wall of spores of fungi with respect to control, which is consistent with the low amount of reducing sugars detected. CS–LZ NPs prepared by nanoprecipitation promise to be a viable and safe alternative for use in biological systems, with a possible low or null impact to humans and biota. However, the potential benefits and the environmental and health implications of NPs need to be globally discussed due to its possible negative effects.
Keywords: Chitosan–lysozyme nanocomposites; Nanoparticles; Cell viability; Morphometry; Aspergillus parasiticus; Membrane integrity; Cell wall; β-1,3-Glucanase
Introduction
Nanostructured systems constitute an increasingly important nanometric scale type of products, whose use has been significant in the last decade. Recent progress in nanotechnology provides improvements in processing, designs, and manufacturing systems (Camacho Elizondo et al. 2011), and nanocomposites are an emergent class of nanostructured hybrid materials composed of a natural polymer and an inorganic solid and, on minimum, one of its components possesses one nanometric scale dimension. These materials exhibit improved structural and functional properties for different applications, such as high performance and low weight (Patel et al. 2015). The nanostructured materials (i.e., nanoparticles, nanotubes) possess very particular characteristics, such as the ability to cross cell membranes due to their size, besides showing a large area/surface ratio and therefore a large superficial extension and high reactivity or functionality (Rhim et al. 2013). Due to these intrinsic properties, nanoparticles may be toxic, which can be associated with cellular damages caused by their bioaccumulation as a result of exposure, besides changes in biological activities including ROS generation (Manke et al. 2013). However, taking into account the environmental hazards, research has been focused on green nanotechnology, where biopolymers such as polysaccharides play diverse roles.
At the present time, the use of biodegradable polymers in nanocomposite technology has received special attention. These materials, called “bionanocomposites”, combine an interdisciplinary research based on materials science, nanotechnology, and biological science, which can lead to the development of bionanocomposites with drug delivery systems (Wang et al. 2016; Zhang et al. 2015) or environment-friendly applications (Ojijo and Ray 2013). Formulation of functional biopolymers, such as chitosan, into nanoparticles significantly increases its antimicrobial effect; therefore, it is feasible that these particles have the potential of becoming a promising, safe, and natural alternative for the control of microorganisms (Cota-Arriola et al. 2013a; El Guilli et al. 2016; Yien et al. 2012).
The production of nanocomposites, made from antimicrobial natural polymers with bioactive compounds such as chitosan and lysozyme, is an interesting topic in the development of functional bio-based materials for biotechnological applications. Several studies have focused on the application of bionanocomposites in the human medicine area, as they have been associated with properties such as antimicrobial, anti-inflammatory, antioxidant, anticancer, and antidiabetic activity, cholesterol lowering effect, and mats for pork preservation (Cota-Arriola et al. 2013b; Huang et al. 2012; Lin et al. 2007; Durango et al. 2006; Park et al. 2004; Hirano et al. 1990).
The antimicrobial effect of chitosan-based nanomaterials has been previously reported. Chitosan nanoparticles (NPs) exhibit a better inhibitory activity against fungi like Candida albicans and Fusarium solani compared to chitosan in solution, and the inhibitory effect was influenced by size and Z potential of NPs (Yien et al. 2012). In a previous work, a better in vitro antifungal effect of chitosan NPs against A. parasiticus spores was observed (Cota-Arriola et al. 2013a). Similarly, layer-by-layer structured nanocomposites of lysozyme/chitosan/organic rectorite enhanced the degree of inhibition against Escherichia coli and Staphylococcus aureus, extending the shelf life of pork for about 3 days (Huang et al. 2012). The antimicrobial activity of chitosan can be increased by enzymatic changes of the native chemical conformation of the biopolymer by using lysozyme, a non-specific enzyme that can be effortlessly obtained at low cost production. The enzyme catalyzes the hydrolysis of β-(1,4) glycosidic linkages between N-acetyl-d-glucosamine and N-acetylmuramic acid in polysaccharides from bacteria and chitosan (Zimoch-Korzycka et al. 2015), hence cutting down the molecular weight until fragments of very low molecular weight of chitooligomers are produced (Ren et al. 2005).
Chitooligomers display better antimicrobial activity compared to chitosan. For example, a high inhibition of Listeria monocytogenes growth was observed with chitooligomers of insoluble fractions obtained from enzymatic hydrolysis with lysozyme, compared to water-soluble fractions or chitosan (Zimoch-Korzycka et al. 2015). In addition to the chitooligomers production, the incorporation of lysozyme into chitosan-based formulations has proved to be an option to obtain coatings with antibacterial activity. Active coatings of chitosan–lysozyme effectively inhibited the growth of E. coli O157:H7 and S. aureus, which demonstrates the potential of using this material for antimicrobial packaging (Lian et al. 2012).
Also, coating solutions of lysozyme–chitosan increased the shelf life of chicken eggs during storage and delayed the loss of interior quality (Yuceer and Caner 2014). Chitosan nanomaterials loaded with lysozyme are biodegradable and biocompatible, with a potential for using it as wound dressings. In these materials, the amount of lysozyme loaded in the nanomaterial matrix decreases when the initial concentration of lysozyme increases (Charernsriwilaiwat et al. 2012).
Regarding the antimicrobial effect, chitosan NPs loaded with lysozyme preserve the antibacterial activity of the loaded enzyme against S. epidermidis up to 5 days of incubation and a slow release over 3 weeks in vitro was observed (Piras et al. 2014). Despite the enhanced antimicrobial potential that can result from the combination of chitosan and lysozyme in the form of nanoparticles, there is not enough information related to the effect of these materials on the spore viability and growth of phytopathogenic fungi of agronomic importance.
The objectives of this research work were: (a) to synthesize nanoparticles from chitosan–lysozyme solutions and to study its physicochemical properties, (b) to evaluate the antifungal effect on the viability, germination and morphometry of A. parasiticus spores and (c) to determine the effect on the membrane integrity and β-1,3-glucanase production quantified as reducing sugar production by fungi.
Materials and methods
Materials
Commercial chitosan flakes with medium molecular weight (153 kDa, 78% deacetylation degree, Aldrich cat. 448,877) were used. Commercial lysozyme from chicken egg white (lyophilized powder, protein ≥90%, ≥40, 000 units/mg protein, cat. L6876) was purchased from Sigma-Aldrich Co.
Strains and microorganisms cultures
Single spore cultures of Aspergillus parasiticus (ATCC 16,992) were prepared and maintained in potato dextrose agar media (PDA, Bioxon, USA) at 4 °C until their use (Xu et al. 2013).
Preparation of the chitosan–lysozyme nanoparticles
Nanoparticles (NPs) were prepared according to the nanoprecipitation technique (Luque-Alcaraz et al. 2012; Bilati et al. 2005). A chitosan–lysozyme (CS–LZ) solution, 1:1 (w/w) proportion, was used as diffuse phase and absolute acetone was used as disperse phase. The diffuse phase was prepared at a concentration of 0.5 mg/mL (w/v) by dissolving 0.05 g of chitosan flakes in 100 mL of 1% (v/v) acetic acid solution. Then, the lysozyme was added at a concentration of 0.5 mg/mL (w/v) by dissolving 0.05 g of enzyme in the chitosan solution previously prepared. The CS–LZ solution was maintained under magnetic stirring at 500 rpm for 5 min and aliquots of 2.5 mL were directly dropped into a glass beaker containing 40 mL of absolute acetone and Tween 80 (0.05% v/v). The flux was set at 0.87 mL/min using a peristaltic pump (BIO-RAD, USA). Chitosan (CS) nanoparticles, prepared from a solution containing 0.5 mg/mL (w/v) (diffuse phase), were used as a control. The newly formed NPs were used for the characterization and subsequent trials.
Physicochemical characterization of nanoparticles
Morphology
The morphology of the CS–LZ nanoparticles was observed in a transmission electron microscope (TEM) (JEM 2010F JEOL, USA). A drop of sample was allowed to dry at room temperature in a carbon-coated film on a 400-mesh copper grid (FCF400-Cu) in a vacuum chamber for 18 h. Subsequently, observations were made in the field of 200 nm. The acceleration voltage used for the TEM observations was 200 kV.
Size, dispersion, and Z potential
Size, dispersion, and Z potential of NPs were determined by dynamic light scattering (DLS) analysis using a Mobiuz equipment (Wyatt Technologies, USA), at a controlled temperature. The system uses a single laser Mobiuz, longitudinal mode of 45 mW, operated at 532 nm and a scattering angle of θ = 163.5 °C. The analysis of the experimental data was carried out using the Dynamics 7.3.1.15 software (Wyatt Technology Corporation, USA).
Association efficiency of lysozyme
The amount of lysozyme attached to the nanoparticles was determined measuring the difference of weight between that of total lysozyme added to the diffuse phase and that of lysozyme contained in the supernatant (non-attached). Samples were centrifuged at 8500 rpm for 20 min and the association efficiency of nanoparticles was determined. A 100 μL aliquot of supernatant was combined with 900 μL of G-250 Coomassie blue stain solution, and this mixture was incubated during 5 min to allow protein staining. Absorbance of this mixture was measured in triplicate at 595 nm using a spectrophotometer (ELISA, Termolabsystem, China) (Zhang et al. 2013). A standard curve (R 2 = 0.9561) was prepared using a lysozyme solution (at concentrations of 50–300 μg/mL) in 1% (v/v) acetic acid solution. The association efficiency of lysozyme to chitosan in the nanoparticles was defined as the percentage of the associated lysozyme and was calculated using Eq. (1) (Luque-Alcaraz et al. 2012):
| 1 |
Biological characterization of nanoparticles
Effect on the viability of Aspergillus parasiticus
The colorimetric assay using tetrazolium salt XTT [2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide] for the quantification of the number of viable spores was carried out. Menadione was used as an electron-coupling agent (Meletiadis et al. 2001). A spore suspension containing 4 × 106 spores/mL was used as an inoculum (Luque-Alcaraz et al. 2016; Meletiadis et al. 2001).
The assays were carried out using 96-well microplates. A 100 μL aliquots of the spore suspension was added to each well and incubated for 4 h at 27 ± 2 °C. Then, 100 μL of nanoparticles (CS, CS–LZ) or lysozyme solution at different concentrations were added, to achieve final concentrations (at the well) of 300, 250, 200, 150, 100, and 50 μg/mL. Microplates were incubated for another 4 h at the same temperature. Finally, 57.06 μL of the XTT–menadione solution was added to each well and samples were again incubated for 3 h. The absorbance was measured in triplicate at 450 nm in a spectrophotometer (ELISA, Termolabsystem, China). Sterile Czapek liquid medium was used as control. From the obtained experimental data, the concentration of nanoparticles that inhibited 50% of the spore viability (IC50) was determined by Probit analysis using the NCSS statistical program (NCSS Inc., USA). The estimated IC50 values were used for subsequent antifungal assays.
Effect on the germination of Aspergillus parasiticus spores
A 700 μL aliquot of Czapek liquid medium was inoculated into the spore suspension mentioned above, to obtain a final concentration of 2 × 106 spores/mL and was deposited on flat-bottom wells of a microtiter plate (Costar, Corning, USA), previously conditioned with a sterile glass coverslip in each well. Then, 300 μL of either CS–LZ NPs or CS NPs suspension was added and homogenized. Plates were incubated (Nova-Tech, USA) for 24 h at 27 ± 2 °C. Finally, coverslips with the adhered cells were removed and 200 spores per coverslip (germinated and non-germinated) were randomly counted using an optical microscope (Olympus CX-311, Japan). The percentage of inhibition with respect to the control was calculated (Plascencia-Jatomea et al. 2003).
Morphometric analysis of spores
The effect of nanoparticles on the average diameter of the fungi spores was determined by image analysis using Image-Pro Plus version 6.3 software (Media Cybernetics Inc., USA). Images from the spore germination assay were captured using an optical microscope (Olympus CX31, Japan) connected to an Infinity 1 camera (Media Cybernetics, USA). At least 60 measurements per treatment were made using the 40× objective (Luque-Alcaraz et al. 2016; Cota-Arriola et al. 2016; Cota-Arriola et al. 2013a).
β-1,3-Glucanases enzymatic assay
β-1,3-Glucanase extracts were prepared in flasks containing 20 mL of Czapek broth, which were separately inoculated with 1 × 105 spores/mL of A. parasiticus and incubated for 96 h at 28 °C. The cultures were centrifuged, sonicated, and the supernatants were obtained following the procedure reported (Buitimea-Cantúa et al. 2013).
The effect of either CS nanoparticles or CS–LZ BNCs on the β-1,3-glucanase activity of A. parasiticus was determined by the production of reducing sugars, using 12 μL of the supernatant, 125 μL of laminarin 2.5% as substrate (Sigma-Aldrich, USA), 10 μL of bovine serum albumin (BSA, Sigma-Aldrich), and 362 μL of 50 mM sodium acetate buffer, pH 5.2. The mixture was incubated for 25 min at 37 °C and reducing sugars were estimated at 610 nm following the Somogyi–Nelson method using a standard curve of glucose (Buitimea-Cantúa et al. 2013).
Effect on Aspergillus parasiticus membrane and cell wall integrity
An inverted fluorescence microscope (Leica DMi8, USA), equipped with a 50 W Hg lamp and a filter set (DAPI excitation 350/50 and emission 460/50, FITC excitation 480/40 and emission 527/30, RHOD excitation 546/10 and emission 585/40), was used. The digital images were acquired with a DFC 450C camera (Leica Microsystem, USA) and a fluorescence overlay software (LAS AF version 3.1.0, Leica Microsystem, USA) for image acquisition and management. Cultures were prepared under the same conditions used in the spore germination probes and the glass coverslips with the fungi grown during 24 h were stained with the fluorescent biomarkers.
Damage or permeability of fungal membrane after exposure to NPs was observed using propidium iodide PI (Sigma, USA), a membrane-impermeable dye. The fungi was stained with a 3 µM PI solution and incubated for 3–6 h at 28 °C. Cells were visualized using a fluorescence microscope (Chavan and Tupe 2014; Riccardi and Nicoletti 2006). The effect of CS and CS–LZ nanoparticles on cellulose and chitin contained in fungi cell wall and septa was analyzed using the fluorescent stain calcofluor-white (Fluostain I, Sigma, USA). Fungi were stained with two drops of a solution containing 10 μg/mL of fluorochrome, incubated for 30 min at room temperature, and observed under the fluorescence microscope (Medina-López et al. 2016).
Statistical analysis
Statistical analysis (one-way analysis of variance) on a completely randomized design was performed using the JMP software (version 10.0.0, SAS Institute Inc.). Means for groups in homogeneous subsets were carried out using the Tukey multiple comparisons test (Tukey’s post hoc test) at a 95% confidence interval. All data were presented as the mean value with their indicated standard error (mean ± SE). The significance level was set at 0.05. Probit analysis was performed using the NCSS software (version 2001, NCSS Statistical Software, USA).
Results and discussion
Physicochemical characterization of nanoparticles
Morphology
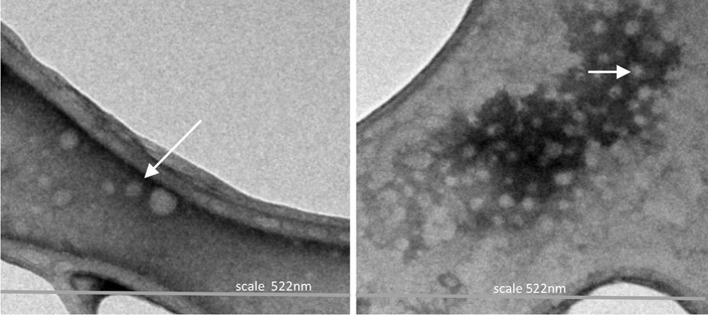
The CS–LZ nanoparticles obtained by nanoprecipitation showed a defined spherical and regular structure, with average diameter between 15 and 30 nm (Fig. 1). Some authors who have used the same nanoprecipitation method (Luque-Alcaraz et al. 2012) and ionotropic gelation (Piras et al. 2014) have reported obtaining chitosan nanoparticles with size about 280–500 and 140–159 nm, respectively, which may vary depending on chitosan molecular weight, solvent employed, and the bioactive compound associated with the nanomaterial matrix. The CS–LZ nanoparticles diameter obtained in this study were smaller than those reported by Luque-Alcaraz et al. (2012) and Piras et al. (2014); however, the shape was similar.
Fig. 1.
TEM images of chitosan–lysozyme (CS–LZ) nanoparticles. Scale of image: 522 nm. Asterisk samples of NPs were fixed on a carbon-coated film on a 400 mesh copper grid (FCF400-Cu)
Size, dispersion, and Z potential
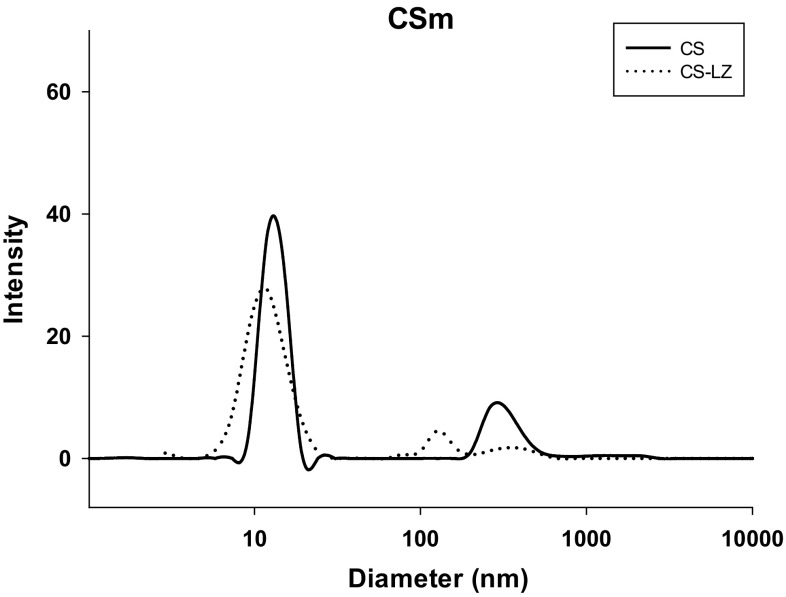
Figure 2 shows the size distribution determined by dynamic light scattering (DLS) analysis of the CS and CS–LZ NPs prepared with chitosan of 153 kDa. The average size was 13.4 nm and 11.8 nm for the CS and CS–LZ NPs, respectively, and a bimodal distribution was observed (Table 2). Regardless of the lysozyme loaded, the CS–LZ NPs showed a slight size reduction compared to CS nanoparticles; however, based on the statistics results, both NPs did not showed significant differences. The lower distribution of the average sizes might be related to a shorter length of the CS chains, favoring the formation of three-dimensional networks. A population of particles with larger diameter was observed at significantly lower intensity.
Fig. 2.
Size distribution of chitosan (CS) and chitosan–lysozyme (CS–LZ) nanoparticles, obtained by dynamic light scattering (DLS) analysis
Table 2.
Spore germination, spore diameter, and reducing sugar quantification by Aspergillus parasiticus inoculated in culture media with added chitosan nanoparticles and chitosan–lysozyme nanoparticles, at 24 h, at 27 °C
| Treatment | Spore germination (%) | Spore diameter (μm) | Reducing sugars production (μm/mL) |
|---|---|---|---|
| Control | 87.6 ± 2.5 | 4.69 ± 0.074a | 501.11 ± 7.25c |
| Chitosan–lysozyme nanoparticles | 0 | 4.76 ± 0.070a | 173.06 ± 13.71b |
| Chitosan nanoparticles | 0 | 4.90 ± 0.074ª | 124.54 ± 6.25a |
Data, followed by their standard errors, are means of at least three experiments. Treatment means were separated using the Tukey test (P > 0.05). Different letters in superscript indicate significant differences (P < 0.05)
The size of the NPs obtained by the nanoprecipitation method was significantly smaller compared to that of lysozyme-loaded CS nanoparticles prepared by ionotropic gelation with TPP (Piras et al. 2014). Besides the elaboration method, other factors, such as CS concentration, CS–protein weight ratio, CS deacetylation degree, and pH of solution, could have an influence on the particle size (Deng Q-y et al. 2006; Yien et al. 2012). Moreover, it has been suggested that when the molecular weight of chitosan is low, there is a greater flexibility of the biopolymer chains and a quicker formation of bridges between bacterial cells and polymeric chains, leading to a strong in vitro bactericidal effect against S. aureus, Bacillus subtilis, B. cereus, E. coli, Pseudomonas aeruginosa, Salmonella typhimurium, Vibrio cholerae, Shigella dysenteriae, Prevotella melaninogenica, and Bacteroides fragilis (Benhabiles et al. 2012). Also, the growth and spores germination of fungi such as Botrytis cinerea and Mucor piriformis were inhibited by chitooligomers (Rahman et al. 2015).
The Z potential of all NPs was positive (Table 1), which suggests that nanoparticles prepared by nanoprecipitation may have greater stability in suspension as a result of repulsive forces, avoiding crowds and precipitation of material. The surface charge could be attributed to the presence of the chitosan amino groups (–NH2) on the particle surface, associated with the polycationic character of the biopolymer (pH 4.5). The authors suggest that portions of the positively charged lysozyme (pH 6.6) can be adsorbed onto the CS–LZ nanoparticle surface (Piras et al. 2014).
Table 1.
Size and Z potential of chitosan and chitosan–lysozyme nanoparticles
| Chitosan (153 kDa) | Size (nm) | Z potential (mV) |
|---|---|---|
| CS | 13.4 ± 6.3; 295.8 | +34.9 ± 4.0 |
| CS–LZ | 11.8 ± 5.1; 127.1 | +33.5 ± 2.0 |
Data, followed by their standard errors, are means of ten experiments
Association efficiency of lysozyme
The percentage of association between the lysozyme and chitosan in the CS–LZ nanoparticles matrix was indirectly determined by quantification of the amount of non-linked lysozyme (free lysozyme). Nanoparticles showed a high (P < 0.05) association value (77.6 ± 6.5%) of lysozyme on the chitosan matrix. These results confirm the enzyme entrapment in the NPs matrix, which could be reversible at neutral pH at 25 °C and release the lysozyme to the media. The association value obtained in this study was significantly higher than those reported by Piras et al. (2014) and Deng et al. (2006) for the CS–LZ matrixes (8.3–35.0% of association of lysozyme) obtained by ionotropic gelation (Deng et al. 2006). It has been reported that ionic interactions between the enzyme and the chitosan as support provide less leaching and a high entrapment of the compound due to its hydrophilic nature (Datta et al. 2013).
The elaboration method of NPs is one factor that can affect the association capacity. It is feasible that the chemical cross-linking between chitosan and tripolyphosphate (TPP, used in ionic gelation techniques) leads to a major rigidity of nanoparticles and therefore to a major stability of the polymeric matrix. However, if the matrix is chemically more stable, a weaker association of lysozyme in the CS–LZ–TPP complex can occur; therefore, these nanoparticles produced by the nanoprecipitation method could associate higher quantity of lysozyme to the matrix, which does not involve the use of cross-linking agents. However, particles with strong gelation and hydrogen bonds can be obtained when TPP and chitosan with high % DD are used, due to the high amount of chitosan amino groups linked to tripolyphosphate groups (Xu and Du 2003).
The authors suggest that the coacervation of chitosan (of high Mw) particles increases the rigidity and package of chains due to inter and intramolecular interactions, resulting in a minor permeability of the particle membrane surface, which can also affect the release rate of the encapsulated compound. Besides, chitosan molecules with extended lineal chain conformations are more flexible, entangle easier and form more intermolecular hydrogen bonding, hydrophobic interaction and electrostatic interaction, which leads to an increase in the surface tension; moreover, for the high molecular weight chitosans, the increase of surface tension is more prominent than that of low molecular weight chitosans (Qun and Ajun 2006).
Considering the above, it is possible that CS chains induced the formation of more compact molecular conformations and/or three-dimensional networks due to a compacted molecular arrangement in the diffuse phase, reducing the surface tension and allowing greater association with the lysozyme.
Biological characterization of nanoparticles
Effect on the viability of Aspergillus parasiticus spores
The antimicrobial effect of chitosan, biologically active chitooligomers and chitosan nanoparticles has been demonstrated, mainly in bacterial species. Chitooligomers have been effective against Gram-positive bacteria, such as Listeria monocytogenes (Zimoch-Korzycka et al. 2015), S. aureus, B. subtilis, and B. cereus; Gram-negative bacteria like E. coli, P. aeruginosa, S. typhimurium and V. cholera (Benhabiles et al. 2012); and filamentous fungi such as B. cinerea and M. piriformis (Rahman et al. 2015). In fungi, the plasma membrane has been reported as the main chitosan target and the cell wall composition plays an important role in the sensitivity of filamentous fungi such as Neurospora crassa and Pochonia chlamydosporia (Aranda-Martinez et al. 2016).
Regarding the antifungal activity of chitosan nanomaterials, a better inhibitory activity against Candida albicans and Fusarium solani was reported for CS NPs prepared by ionic gelation from different concentrations of low and high molecular weight compared to the solution form. Aspergillus niger was found to be resistant to chitosan NPs except for those prepared from higher concentrations of high molecular weight chitosan, and the inhibitory effect was influenced by particle size and Z potential of CS NPs (Yien et al. 2012).
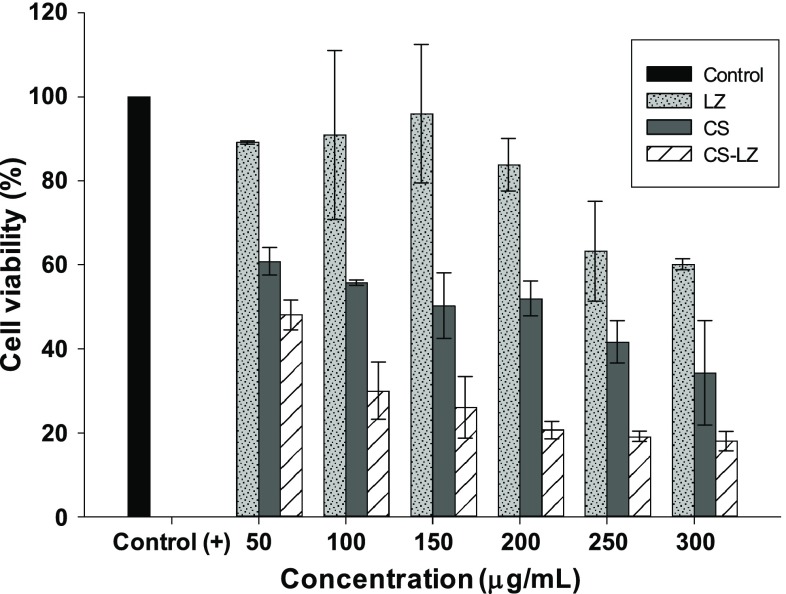
In this work, the XTT method was used to evaluate the antifungal effect of the chitosan–lysozyme NPs on the spore viability of A. parasiticus. The results showed that lysozyme had a moderate effect on the viability of fungal spores and around 60% of viable spores were detected at the highest concentration tested (250 and 300 µg/mL). A higher spore viability (>80%) was observed when the concentration of lysozyme decreased (Fig. 3).
Fig. 3.
Effect of lysozyme (LZ), chitosan nanoparticles (CS) and chitosan–lysozyme nanoparticles (CS–LZ) on the spores viability of Aspergillus parasiticus
Since Aspergillus species are widely distributed in nature and are able to grow in a variety of substrates or environments such as humans, animals, soil, plants, decaying organic matter, and stored food such as grains (Plascencia-Jatomea et al. 2014), the results suggests that A. parasiticus exhibit a low sensitivity to lysozyme that could be associated with its ability to assimilate low protein concentrations as carbon and nitrogen sources.
Some lysozymes can kill bacteria by stimulating autolysin activity upon interaction with the cell surface and extensive hydrolysis of peptidoglycan by lysozyme results in cell lysis and death in a hypo-osmotic environment (Salazar and Asenjo 2007). In fungi, lysozyme isolated from mungbean seeds (Phaseolus mungo) showed strong activity toward Fusarium oxysporum, and some activity toward F. solani, Sclerotium rolfsii, Pythium aphanidermatum, and B. cinerea; however, it had hardly any antifungal effect on Mycosphaerella arachidicola (Wang et al. 2005). Also, the inhibitory activity of lysozyme (from hen egg white) against 18 phytopathogens was lower than those by low molecular weight CS and CS oligosaccharides. This strongly suggests that the depolymerized products of CS are effective for growth inhibition. Only a medium inhibition was reported against Valsa mali and F. oxysporum F. sp. lycopersici (Hirano and Nagao 1989).
When Aspergillus parasiticus was grown in media with added CS NPs, a spore viability of 38–60% was observed, which was proportionally reduced when the concentration of CS NPs in the culture media increased (Fig. 3). This demonstrates the inherent antimicrobial potential of chitosan, in solution and/or chitosan particles, which has been previously tested (Cota-Arriola et al. 2013a; Luque-Alcaraz et al. 2016; Yien et al. 2012). For example, A. niger was found to be resistant to chitosan NPs except for those prepared from concentrations of 2 and 3 mg/mL of high molecular weight chitosan (310 kDa Mw, 85% DD); the inhibitory activity was measured by broth microdilution procedure and could only be detected for solutions from 310 and 70 kDa chitosans (Yien et al. 2012). In agreement with the authors, fungi that have chitosan in the cell are more resistant to exogenous chitosan, which could therefore explain the high resistance of Aspergillus species such as A. fumigatus and A. niger as it contains about 10% of chitin in its cell wall (Yien et al. 2012; Klis et al. 2007; Bernard and Latgé 2001).
The effect of chitosan nanoparticles on the fungi viability may be related to its toxicity. Chitosan NPs (200 nm) prepared by ionic cross-linking with sodium tripolyphosphate caused malformations (bent spine, pericardial edema, and an opaque yolk) in zebrafish (Danio rerio) embryos. Also, a decreased hatching rate and increased mortality (concentration dependent) was observed, besides a high expression of reactive oxygen species and an overexpression of heat shock protein 70 (Hu et al. 2011).
In this work, the antifungal effect was higher in A. parasiticus grown in media with added CS–LZ nanoparticles, and a significant (P < 0.05) reduction on the spore viability was observed with respect to the control. The maximal value of viable spores was 48% at the lowest NPs concentration tested (50 µg/mL), which was reduced to a value of 18% at a higher concentration (300 µg/mL) (Fig. 3). At all concentrations tested, the antifungal activity of CS–LZ particles was significantly higher (P < 0.05) compared with treatments alone, the chitosan NPs and lysozyme. This suggests that lysozyme immobilization in chitosan is feasible to reduce the spore viability of toxigenic fungi such as A. parasiticus, since a more effective antifungal activity can be resulted, which could be related to a possible synergistic effect when chitosan and lysozyme are combined. Due to the positive Z potential, interactions between positive charges on the CS–LZ nanoparticles surface with negative charges of the cell wall of fungus can occur. Besides the hydrolytic capacity of lysozyme (Salazar and Asenjo 2007), other explanation may be related to the chitooligomers production by lysozyme (Zimoch-Korzycka et al. 2015) and the consequent formation of oligomers–lysozyme complexes, with enhanced antimicrobial activity.
Based on inhibition zone diameters using the disc diffusion method, integration of lysozyme into CS nanoparticles (488 and 613 nm, Z potential of +21 and +14 mV, prepared by ionotropic gelation with TPP at pH of 4 and 5, respectively) enhanced the antibacterial activity against Escherichia coli and Bacillus subtilis. For E. coli, breakage of the basement membrane, leakage of some cytoplasm, degradation of cells from a round column to an irregular shape and complete rupture were observed (Wu et al. 2017). Using the same ionotropic gelation method, Piras et al. (2014) prepared chitosan (Mw 50–190 kDa, DD 75–85%) NPs loaded with lysozyme, which showed a full in vitro cytocompatibility toward murine fibroblasts and effectively preserved the antibacterial activity of the enzyme against S. epidermidis (in about 2 log reduction of the number of viable bacteria) compared to chitosan NPs.
Although the activity of CS–LZ nanoparticles against bacteria species has been previously tested, no studies regarding its effect on the viability of toxigenic filamentous fungi were reported before, thus hampering the comparison of the obtained results with others. In a previous work, chitosan–pepper tree essential oil bionanocomposites (754 ± 7.5 nm and Z potential of + 9.1 ± 1.74 mV) prepared by the nanoprecipitation technique reduced the viability of fungal spores of A. parasiticus, having about 50% of viable spores in the treatment with higher concentrations (200 µg/mL) of chitosan/pepper tree bionanocomposites with respect to the control (Luque-Alcaraz et al. 2016). As occurred for CS–LZ nanoparticles, the antifungal effect of CS–pepper tree oil particles decreased when the bionanocomposite concentration was reduced, which was attributed to electrostatic interactions of van der Waals type and hydrogen bonds between the components present in the fungus cell membrane and the bionanocomposites.
From the experimental data of the spore viability of Aspergillus parasiticus assay, CI50 values of 155.2 ± 19.6 and 41.7 ± 6.4 µg/mL were estimated for CS nanoparticles and CS–LZ nanoparticles, respectively, which confirmed the strong inhibitory effect of the CS–LZ nanoparticles on the in vitro viability of fungi. The CI50 estimated doses were used for subsequent biological assays to assess the antimicrobial activity.
Effect on the spore germination of Aspergillus parasiticus
The CS and CS–LZ nanoparticles strongly affected the spore germination of fungi with respect to the control and an inhibition of 100% was observed at 24 h (Table 2). This can be attributed to the electrostatic interactions between chitosan amino groups with fungi cell wall components, besides the high surface charge (Z potential) and the small particle size. The inhibitory effect of CS–LZ nanoparticles against A. parasiticus was higher compared to previous results for CS–TPP particles (20–80 µm) prepared by ionotropic gelation, where an inhibition of spore germination of 77% was observed for A. parasiticus; a lower inhibitory effect was observed when fungus was exposed to smaller size particles, which was attributed to the agglomeration of particles that provides a surface where the spore may adhere to germinate (Cota-Arriola et al. 2013a). Also, the incorporation of ferulic acid into micro- and nanoparticles of chitosan potentiated the inhibitory effect on the spore germination, which was attributed to the increase of the Z potential of the particles, besides the calcium chelating potential and electrostatic interactions caused by chitosan ( Cota-Arriola et al. 2013a).
It has been reported that Cu–chitosan nanoparticles are more effective at 0.1% concentration and show 89.5, 63.0, and 60.1% growth inhibition of Macrophomina phaseolina, Alternaria alternata, and Rhizoctonia solani, respectively, in in vitro model. The maximum inhibition rates of 87.4 and 87.1% of A. alternate germination were observed for Cu–CS and CS NPs, respectively, which could be due to the high superficial charge density that provides a major binding affinity for negatively charged compounds in the fungal membrane (Saharan et al. 2015). At 0.12% concentration, Cu–CS NPs (374.3 ± 8.2 nm, Z potential of + 22.6 mV) caused 70.5 and 73.5% inhibition of mycelia growth and 61.5 and 83.0% inhibition of spore germination in A. solani and F. oxysporum, respectively (Saharan et al. 2015). According to the authors, the Z potential of Cu–CS NPs is crucial to assess the antifungal activity and nanoparticle stability.
Morphometric analysis of spores
Changes in morphometric parameters indicated the morphological changes undergone by the fungus in the presence of adverse factors in their adaptation and development (Cota-Arriola et al. 2013a). For fungi, plasma membrane has been reported as the main chitosan target (Aranda-Martinez et al. 2016) and morphological anomalies such as swelling, echinulate spores, delay in breaking dormancy, polarization, and germ tube emergence have been observed in spores of A. niger grown in media with added chitosan, which was explained by interferences caused by chitosan on the nutrients or minerals (calcium) (Cota-Arriola et al. 2013a; Plascencia-Jatomea et al. 2003).
The exposition of Aspergillus parasiticus to CS–LZ nanoparticles resulted in a slight increment (P > 0.05) of the average spore diameter with respect to control (Table 2). Based on the above, the swelling caused by the CS–LZ nanoparticles may be related to electrostatic interactions that eventually could lead to an osmotic or oxidative stress. Changes in chemical structure and physiological characteristics of nanoparticles lead to changes in biological properties and the ROS generation is the most commonly reported NPs-associated toxicities, where cellular responses (NP–cell interaction, mitochondrial respiration, and immune cell activation) are responsible of damages mediated by ROS (Manke et al. 2013).
The effect of various nanomaterials is different. Some nanoparticles induce higher cytotoxicity and oxidative impairment, whereas others cause higher DNA damage. Surface properties and chemical composition of particles may have a key role in the ROS generation. However, the genotoxicity of nanoparticles at lower exposure doses may be primarily due to particle shape (Yang et al. 2009).
The swelling of A. parasiticus spores can be also attributed to the low particle size (<14 nm), which allows them to penetrate more easily through the fungal cell wall and membrane, being able to interact with the cellular material within the spore, thereby causing and unbalance or stopping metabolic functions. This is supported by the strong inhibitory effect observed in the germination of spores of fungi (Table 2), where no germinated spores were observed at 24 h.
β-1,3-Glucanase enzymatic assay
Cellulases are high molecular colloidal proteinaceous biocatalyst and the extracellular cellulolytic enzyme system consists of three major enzyme components: endoglucanase, exoglucanases or cellobiohydrolases, and β-glucosidase or cellobiases. β-Glucanases are required for hyphal morphogenesis and are believed to play cell wall remodeling roles during growth and morphogenesis in filamentous fungi. β-1,3-Glucanase is produced by many bacteria, plants, and fungi such as Trichoderma and Aspergillus, and are related to the hyphal transformation in Candida albicans (Xu et al. 2013).
In this work, a significantly (P < 0.05) low production of reducing sugars was detected in the culture media with added CS and CS–LZ NPs with respect to control, obtaining an inhibition of 75.2% for A. parasiticus grown in media with added CS–LZ NPs (Table 2). These results confirm the strong inhibitory effect on the first stage of fungal growth. Differences on the reducing sugar production could be related to affectations on the β-1,3 glucan production and therefore the β-1,3-glucanase activity, the main component of the fungi cell wall and enzyme involved in the synthesis of β-1,3-glucan, respectively.
At the nanoscale level, supported by limited experimental evidence, it was reported that materials have effects such as oxidative stress and protein denaturation/degradation, whose possible pathophysiology outcomes are phase II enzyme induction via transcriptional activation and loss of enzyme activity, respectively, among others (Nel et al. 2006). The exposition of E. coli to silver NPs resulted in the leakage of reducing sugars and proteins and induced the respiratory chain dehydrogenases into inactive state. Many pits and gaps were observed and the membrane was fragmentary, suggesting that Ag NPs affect the permeability of the bacterial membranes (Li et al. 2010).
Also, a significantly improved antibacterial activity against S. aureus has been reported for water-soluble quaternized carboxymethyl chitosan–Ag NPs (Huang et al. 2016) and Ag NPs coated with chitosan (with different sizes and shapes) presented antibacterial activity against clinical stocks of Streptococcus mutans, which was associated with smaller NPs (Martínez-Robles et al. 2016). For chitosan, the regulatory functions in a simple fungal–plant interaction can involve the activation of specific genes in plants and the complete inhibition of all RNA synthesis in some fungal organisms and thus suppress gene activity (Hadwiger et al. 1986). If chitosan can affect the RNA synthesis in fungi, it is feasible to predict that enzymatic function may be also compromised.
However, to date no information is available about the effect of bio-based polymeric nanoparticles on the enzyme production in bacteria or filamentous fungi species. So, this is the first report that describes the effect of chitosan and chitosan–lysozyme NPs on the viability, germination, and reducing sugars production (associated with the production of enzymes involved with the cell wall synthesis and cell differentiation) in toxigenic fungi such as A. parasiticus.
Taking into account that the interaction between enzymes and NPs is governed by the properties of NPs, such as structure, size, charge, surface chemistry and shape (Wu et al. 2009), it is possible that CS–LZ nanoparticles may damage the structure of fungi cell wall and depress the activity of membranous enzymes like glucanases, reducing the glucan synthesis and therefore causing the interruption of the spore germination. Other cellular components or important enzymes for metabolism of the fungus can also be affected.
Effect on the membrane integrity and cell wall of Aspergillus parasiticus
Silver metallic NPs could enhance the membrane leakage of reducing sugars from cytoplasm in E. coli. Also, empty membrane vesicles, disorganized and dispersed membrane components, were observed (Li et al. 2010). In contrast, from live/dead assay, the ratio obtained from the microbial suspensions of nitrifying bacteria and E. coli, PHL628-gfp treated with Ag NPs (1 mg/L Ag) showed no difference compared to controls, suggesting that there is no evidence of cell membrane leakage caused by Ag NPs, Ag+ ions or AgCl colloids (Choi et al. 2008).
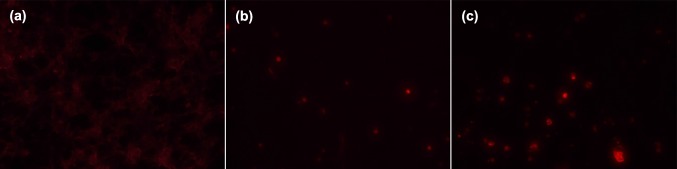
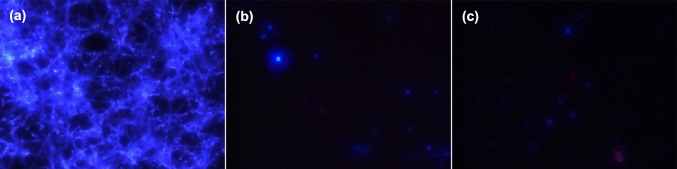
In this study, effects caused by CS and CS–LZ nanoparticles on the membrane of A. parasiticus were observed using propidium iodide (PI) dye, which enter into the cell when the membrane integrity is compromised. A high amount of non-stained germinated spores and hyphae were observed in the control media (Fig. 4a); however, the fungi cells treated with both CS and CS–LZ nanoparticles were stained with PI, which shows the damage to the cell membrane caused by NPs (Fig. 4b, c). Only non-germinated spores were observed in media with added NPs, which is consistent with the results found in the spore germination assay.
Fig. 4.
Mycelium and spores of Aspergillus parasiticus grown in culture media with added chitosan and chitosan–lysozyme nanoparticles stained with propidium iodide. a Branched mycelium of fungi in the control media; b Non-germinated spores treated with chitosan nanoparticles; c Non-germinated spores treated with chitosan–lysozyme nanoparticles, showing membrane integrity damages
The effect of CS and CS–LZ nanoparticles on the fungi cell wall polymeric components was determined by staining with calcofluor-white (CW) dye, which binds chitin and glucan allowing display the cell wall. When spores and hyphae grown in control media were observed, a high amount of stained mycelium was noted (Fig. 5a) that could be related with the normal growth of A. parasiticus. In contrast, only a few stained spores grown in media with CS and CS–LZ NPs were observed (Fig. 5b, c), suggesting a low content or deposition of glucan and chitin in the fungi cell wall. This is consistent with the low amount of reducing sugars (Table 2) detected in media with added NPs, suggesting a low activity or a low production of glucanase enzymes.
Fig. 5.
Mycelium and spores of Aspergillus parasiticus grown in culture media with added chitosan and chitosan–lysozyme nanoparticles stained with calcofluor-white. a Branched mycelium of fungi in control media; b Non-germinated spores treated with chitosan nanoparticles, and c Non-germinated spores treated with chitosan–lysozyme nanoparticles, showing low glucan/chitin content (b and c)
The efg1 and cek1 pathways control the filamentation process in C. albicans. This process is induced by β-1,3 glucanase, which may be an adaptive and protective response to enzymes that damage the cell wall glucan (Xu et al. 2013). Based on the above, it is possible that the CS and the CS–LZ nanoparticles affected the integrity of the cellular membrane of A. parasiticus by disturbing the metabolic pathways related to the glucans synthesis.
With regard to the action mechanisms of nanoparticles, the model for Ag metallic NPs may be described in two stages: (1) Ag NPs make a break through the permeability of outer membrane firstly, resulting in the leakage of cellular materials. (2) Ag NPs enters the inner membrane and inactivate respiratory chain dehydrogenases, thus inhibiting respiration and growth of cells. Simultaneously, Ag NPs may affect proteins and phosphate lipids and induce collapse of membrane, resulting in cell decomposition and death (Li et al. 2010). Considering the obtained results, it is possible to deduce that the CS and CS–LZ nanoparticles affect the viability and growth of A. parasiticus in two ways: (a) by causing membrane destabilization through intra- and intermolecular chemical interactions between functional groups of CS and lysozyme with components of the cell wall, and (b) by reducing the viability and preventing the germination of spores, possibly due to damages in the synthesis of the cell wall-associated components or enzymes (Xu et al. 2013; Hadwiger et al. 1986), as well as due to the small size of NPs (Wu et al. 2009).
Conclusions
This study demonstrated that with the use of nanoprecipitation technique, it is feasible to obtain chitosan–lysozyme NPs, with a small size and a strong antifungal activity against A. parasiticus. In in vitro assays, the CS–LZ NPs proved to be more effective than CS NPs to reduce the viability and to inhibit the germination of spores. Structural affectations could be associated with the β-1,3-glucanase production, since a diminution in the reducing sugar production was detected. The CS–LZ nanoparticle systems promise to be a viable alternative for agricultural purposes; however, the potential benefits and applications must be balanced with environment and health implications, due to the potential adverse effects on microbial activity.
Acknowledgements
The study was funded by the Mexican Council for Science and Technology (CONACyT) through the project No. 219786 (CB-2013-01) and for the scholarship to Cynthia Nazareth Hernández-Téllez for postgraduate studies. The authors acknowledge the University of Sonora for the assistantship grant to Francisco Julián Rodríguez-Córdova for undergraduate studies.
Abbreviations
- CS–LZ
Chitosan–lysozyme
- NPs
Nanoparticles
- CS
Chitosan
- LZ
Lysozyme
- TEM
Transmission electron microscopy
- DLS
Dynamic light scattering
- XTT
Tetrazolium salt
- Mw
Molecular weight
- ROS
Reactive oxygen species
- kDa
Kilo Daltons
- PDA
Potato dextrose agar
- µg/mL
Microgram per milliliter
- BSA
Bovine serum albumin
- PI
Propidium ioidide
- CW
Calcofluor-white
- CS
Chitosan of 153 kDa
Author contribution
Maribel Plascencia-Jatomea conceived and designed the experiments. Cynthia Nazareth Hernández-Téllez, Francisco Julián Rodríguez-Córdova and Aaron Martínez-Higuera performed the experiments. Ema Carina Rosas Burgos, Mario Onofre Cortez-Rocha, Armando Burgos-Hernández, Wilfrido Torres-Arreola and Jaime Lizardi-Mendoza analyzed the data and contributed with reagents, materials and analysis tools. Cynthia Nazareth Hernández-Téllez and Maribel Plascencia-Jatomea wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Cynthia Nazareth Hernández-Téllez, Email: naza08qb@gmail.com.
Francisco Julián Rodríguez-Córdova, Email: julianrc15@gmail.com.
Ema Carina Rosas-Burgos, Email: ecrosas@guayacan.uson.mx.
Mario Onofre Cortez-Rocha, Email: mcortez@guayacan.uson.mx.
Armando Burgos-Hernández, Email: armando.burgos@unison.mx.
Jaime Lizardi-Mendoza, Email: jalim@ciad.mx.
Wilfrido Torres-Arreola, Email: wilfrido.torres@unison.mx.
Aarón Martínez-Higuera, Email: zar_aron@hotmail.com.
Maribel Plascencia-Jatomea, Phone: +52 662 25922-07, Email: mplascencia@guayacan.uson.mx.
References
- Aranda-Martinez A, Lopez-Moya F, Lopez-Llorca LV. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan. J Basic Microbiol. 2016;56:1059–1070. doi: 10.1002/jobm.201500775. [DOI] [PubMed] [Google Scholar]
- Benhabiles M, Salah R, Lounici H, Drouiche N, Goosen M, Mameri N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocol. 2012;29:48–56. doi: 10.1016/j.foodhyd.2012.02.013. [DOI] [Google Scholar]
- Bernard M, Latgé J-P. Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol. 2001;39:9–17. doi: 10.1080/mmy.39.1.9.17. [DOI] [PubMed] [Google Scholar]
- Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Buitimea-Cantúa GV, Rosas-Burgos EC, Cinco-Moroyoqui FJ, Burgos-Hernández A, Plascencia-Jatomea M, Cortez-Rocha MO, Gálvez-Ruiz JC. In vitro effect of antifungal fractions from the plants baccharis glutinosa and jacquinia macrocarpa on chitin and β-1, 3-glucan hydrolysis of maize phytopathogenic fungi and on the fungal β-1, 3-glucanase and chitinase activities. J Food Saf. 2013;33:526–535. doi: 10.1111/jfs.12085. [DOI] [Google Scholar]
- Camacho Elizondo M, Vega Baudrit J, Campos Gallo A. Uso de nanomateriales en polímeros para la obtención de bioempaques en aplicaciones alimentarias. Revista de la Sociedad Química del Perú. 2011;77:292–306. [Google Scholar]
- Charernsriwilaiwat N, Opanasopit P, Rojanarata T, Ngawhirunpat T. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm. 2012;427:379–384. doi: 10.1016/j.ijpharm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Chavan PS, Tupe SG. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control. 2014;46:115–120. doi: 10.1016/j.foodcont.2014.05.007. [DOI] [Google Scholar]
- Choi O, Deng KK, Kim N-J, Ross L, Surampalli RY, Hu Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Cota-Arriola O, Cortez-Rocha MO, Ezquerra-Brauer JM, Lizardi-Mendoza J, Burgos-Hernández A, Robles-Sánchez RM, Plascencia-Jatomea M. Ultrastructural, morphological, and antifungal properties of micro and nanoparticles of chitosan crosslinked with sodium tripolyphosphate. J Polym Environ. 2013;21:971–980. doi: 10.1007/s10924-013-0583-1. [DOI] [Google Scholar]
- Cota-Arriola O, Onofre Cortez-Rocha M, Burgos-Hernández A, Marina Ezquerra-Brauer J, Plascencia-Jatomea M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: development of new strategies for microbial control in agriculture. J Sci Food Agric. 2013;93:1525–1536. doi: 10.1002/jsfa.6060. [DOI] [PubMed] [Google Scholar]
- Cota-Arriola O et al (2016) Preparation of chitosan matrices with ferulic acid: physicochemical characterization and relationship on the growth of Aspergillus parasiticus. CyTA-J Food 15(1):65–74. doi:10.1080/19476337.2016.1213317
- Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials 3. Biotech. 2013;3:1–9. doi: 10.1007/s13205-012-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Q-Y Deng, C-R Zhou, B-H Luo. Preparation and characterization of chitosan nanoparticles containing lysozyme. Pharm Biol. 2006;44:336–342. doi: 10.1080/13880200600746246. [DOI] [Google Scholar]
- Durango A, Soares N, Benevides S, Teixeira J, Carvalho M, Wobeto C, Andrade N. Development and evaluation of an edible antimicrobial film based on yam starch and chitosan. Packag Technology Sci. 2006;19:55–59. doi: 10.1002/pts.713. [DOI] [Google Scholar]
- El Guilli M, Hamza A, Clément C, Ibriz M, Ait Barka E. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture. 2016;6:12. doi: 10.3390/agriculture6020012. [DOI] [Google Scholar]
- Hadwiger L, Kendra D, Fristensky B, Wagoner W (1986) Chitosan both activates genes in plants and inhibits RNA synthesis in fungi. In: Muzzarelli R, Jeuniaux C, Gooday GW (eds) Chitin in nature and technology. Springer, Boston, MA, pp 209–214
- Hirano S, Nagao N. Effects of chitosan, pectic acid, lysozyme, and chitinase on the growth of several phytopathogens. Agric Biol Chem. 1989;53:3065–3066. [Google Scholar]
- Hirano S, Yamaguchi R, Fukui N, Iwata M. A chitosan oxalate gel: its conversion to an N-acetylchitosan gel via a chitosan gel. Carbohyd Res. 1990;201:145–149. doi: 10.1016/0008-6215(90)84231-I. [DOI] [Google Scholar]
- Hu Y-L, Qi W, Han F, Shao J-Z, Gao J-Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int J Nanomed. 2011;6:3351–3359. doi: 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xu H, Xue Y, Huang R, Deng H, Pan S. Layer-by-layer immobilization of lysozyme–chitosan–organic rectorite composites on electrospun nanofibrous mats for pork preservation. Food Res Int. 2012;48:784–791. doi: 10.1016/j.foodres.2012.06.026. [DOI] [Google Scholar]
- Huang S, Wang J, Zhang Y, Yu Z, Qi C. Quaternized carboxymethyl chitosan-based silver nanoparticles hybrid: microwave-assisted synthesis. Charact Antibact Act Nanomat. 2016;6:118. doi: 10.3390/nano6060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F, Ram A, De Groot P (2007) A molecular and genomic view of the fungal cell wall. In: Howard RJ, Gow NAR (eds) Biology of the fungal cell. Springer, Heidelberg, pp 97–120
- Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, You-Sheng O-Y, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- Lian Z-X, Ma Z-S, Wei J, Liu H. Preparation and characterization of immobilized lysozyme and evaluation of its application in edible coatings. Process Biochem. 2012;47:201–208. doi: 10.1016/j.procbio.2011.10.031. [DOI] [Google Scholar]
- Lin C-W, Chen L-J, Lee P-L, Lee C-I, Lin J-C, Chiu J-J. The inhibition of TNF-α-induced E-selectin expression in endothelial cells via the JNK/NF-κB pathways by highly N-acetylated chitooligosaccharides. Biomaterials. 2007;28:1355–1366. doi: 10.1016/j.biomaterials.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Luque-Alcaraz AG, et al. Characterization and antiproliferative activity of nobiletin-loaded chitosan nanoparticles. J Nanomater. 2012;2012:100. doi: 10.1155/2012/265161. [DOI] [Google Scholar]
- Luque-Alcaraz AG, et al. Enhanced antifungal effect of chitosan/pepper tree (Schinus molle) essential oil bionanocomposites on the viability of Aspergillus parasiticus spores. J Nanomater. 2016;2016:38. doi: 10.1155/2016/6060137. [DOI] [Google Scholar]
- Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013:15. doi:10.1155/2013/942916 [DOI] [PMC free article] [PubMed]
- Martínez-Robles ÁM, et al. Antimicrobial properties of biofunctionalized silver nanoparticles on clinical isolates of streptococcus mutans and its serotypes. Nanomaterials. 2016;6:136. doi: 10.3390/nano6070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-López CF, Plascencia-Jatomea M, Cinco-Moroyoqui FJ, Yépiz-Gómez MS, Cortez-Rocha MO, Rosas-Burgos EC. Potentiation of antifungal effect of a mixture of two antifungal fractions obtained from Baccharis glutinosa and Jacquinia macrocarpa plants. J Environ Sci Health. 2016;51:760–768. doi: 10.1080/03601234.2016.1198641. [DOI] [PubMed] [Google Scholar]
- Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE, Network E. Colorimetric assay for antifungal susceptibility testing of aspergillus species. J Clin Microbiol. 2001;39:3402–3408. doi: 10.1128/JCM.39.9.3402-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Ojijo V, Ray SS. Processing strategies in bionanocomposites. Prog Polym Sci. 2013;38:1543–1589. doi: 10.1016/j.progpolymsci.2013.05.011. [DOI] [Google Scholar]
- Park P-J, Je J-Y, Byun H-G, Moon S-H, Kim S-K. Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J Microbiol Biotechnol. 2004;14:317–323. [Google Scholar]
- Patel S, Jammalamadaka U, Sun L, Tappa K, Mills DK. Sustained release of antibacterial agents from doped halloysite nanotubes. Bioengineering. 2015;3:1. doi: 10.3390/bioengineering3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras AM, Maisetta G, Sandreschi S, Esin S, Gazzarri M, Batoni G, Chiellini F. Preparation, physical–chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int J Biol Macromol. 2014;67:124–131. doi: 10.1016/j.ijbiomac.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Plascencia-Jatomea M, Viniegra G, Olayo R, Castillo-Ortega MM, Shirai K. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromol Biosci. 2003;3:582–586. doi: 10.1002/mabi.200350024. [DOI] [Google Scholar]
- Plascencia-Jatomea S, Gómez Y, Vales-Haro J (2014) Aspergillus spp. (Black Mold). In: Bautista-Baños S (ed) Postharvest decay: control strategies. Elsevier Inc., Academic Press, New York, pp 267–286
- Qun G, Ajun W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohyd Polym. 2006;64:29–36. doi: 10.1016/j.carbpol.2005.10.026. [DOI] [Google Scholar]
- Rahman MH, Hjeljord LG, Aam BB, Sørlie M, Tronsmo A. Antifungal effect of chito-oligosaccharides with different degrees of polymerization . Eur J Plant Pathol. 2015;141:147–158. doi: 10.1007/s10658-014-0533-3. [DOI] [Google Scholar]
- Ren D, Yi H, Wang W, Ma X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohyd Res. 2005;340:2403–2410. doi: 10.1016/j.carres.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Rhim J-W, Park H-M, Ha C-S. Bio-nanocomposites for food packaging applications. Prog Polym Sci. 2013;38:1629–1652. doi: 10.1016/j.progpolymsci.2013.05.008. [DOI] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Saharan V, et al. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol. 2015;75:346–353. doi: 10.1016/j.ijbiomac.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Salazar O, Asenjo JA. Enzymatic lysis of microbial cells. Biotechnol lett. 2007;29:985–994. doi: 10.1007/s10529-007-9345-2. [DOI] [PubMed] [Google Scholar]
- Wang S, Ng TB, Chen T, Lin D, Wu J, Rao P, Ye X. First report of a novel plant lysozyme with both antifungal and antibacterial activities. Biochem Biophys Res Commun. 2005;327:820–827. doi: 10.1016/j.bbrc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li P, Truong-Dinh Tran T, Zhang J, Kong L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer . Nanomaterials. 2016;6:26. doi: 10.3390/nano6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhang B, Yan B. Regulation of enzyme activity through interactions with nanoparticles . Int J Mol Sci. 2009;10:4198–4209. doi: 10.3390/ijms10104198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wu C, Fu S, Wang L, Yuan C, Chen S, Hu Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohyd Polym. 2017;155:192–200. doi: 10.1016/j.carbpol.2016.08.076. [DOI] [PubMed] [Google Scholar]
- Xu Y, Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 2003;250:215–226. doi: 10.1016/S0378-5173(02)00548-3. [DOI] [PubMed] [Google Scholar]
- Xu H, Nobile CJ, Dongari-Bagtzoglou A. Glucanase induces filamentation of the fungal pathogen Candida albicans. PloS One. 2013;8:e63736. doi: 10.1371/journal.pone.0063736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- Yien L, Zin NM, Sarwar A, Katas H (2012) Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater 2012:9. doi:10.1155/2012/632698 [DOI] [PMC free article] [PubMed]
- Yuceer M, Caner C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J Sci Food Agric. 2014;94:153–162. doi: 10.1002/jsfa.6322. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao J, Wen Y, Zhu C, Yang J, Yao F. Carboxymethyl chitosan-poly (amidoamine) dendrimer core–shell nanoparticles for intracellular lysozyme delivery. Carbohyd Polym. 2013;98:1326–1334. doi: 10.1016/j.carbpol.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu T, Xiao Y, Yu D, Zhang N. Hyaluronic acid-chitosan nanoparticles to deliver Gd-DTPA for MR cancer imaging . Nanomaterials. 2015;5:1379–1396. doi: 10.3390/nano5031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimoch-Korzycka A, Gardrat C, Castellan A, Coma V, Jarmoluk A. The use of lysozyme to prepare biologically active chitooligomers. Polímeros. 2015;25:35–41. doi: 10.1590/0104-1428.1630. [DOI] [Google Scholar]