Abstract
A major goal for the treatment of opioid use disorder is to reduce or eliminate the use of illicit opioids. Buprenorphine, a µ-opioid receptor partial agonist and kappa opioid receptor antagonist, is now being developed as a monthly, sustained-release formulation (RBP-6000). The objective of this study was to demonstrate that RBP-6000 blocks the subjective effects and reinforcing efficacy of the µ-opioid receptor agonist hydromorphone (intramuscularly administered) in subjects with moderate or severe opioid use disorder. Subjects were first inducted and dose stabilized on sublingual buprenorphine/naloxone (8–24 mg daily; dose expressed as the buprenorphine component), then received two subcutaneous injections of RBP-6000 (300 mg) on Day 1 and Day 29. Hydromorphone challenges (6 mg, 18 mg or placebo administered in randomized order) occurred on 3 consecutive days of each study week before and after receiving RBP-6000. Subjects reported their responses to each challenge on various 100-mm Visual Analogue Scales (VAS). Subjects also completed a choice task to assess the reinforcing efficacy of each hydromorphone dose relative to money. At baseline, mean “drug liking” VAS scores for hydromorphone 18 mg and 6 mg versus placebo were 61 mm (95% confidence interval, 52.3–68.9) and 45 mm (95% confidence interval, 37.2–53.6), respectively. After 300 mg RBP-6000 was administered, mean VAS score differences from placebo were less than 10 mm through week 12. The reinforcing efficacy of hydromorphone decreased in a parallel manner. This study demonstrated that RBP-6000 at a 300 mg dose provides durable and potent blockade of the subjective effects and reinforcing efficacy of hydromorphone in subjects with moderate or severe opioid use disorder.
Keywords: sustained-release buprenorphine, opioid use disorder, hydromorphone challenge, pharmacokinetics, reinforcing efficacy
Opioid use disorder is a neurobehavioral syndrome characterized by the repeated, compulsive seeking, and use of an opioid despite adverse social, psychological, and/or physical consequences (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition). It can relate to illicit opioids (eg, heroin), legally or illegally obtained heroin substitutes (eg, methadone), or prescribed opioid analgesics (eg, morphine, codeine). Opioid abuse is a persistent problem that creates a social, economic, and medical burden.1 Opioid abuse treatment admissions for individuals aged 12 or older have increased 1.4-fold from 2002 (331,000 admissions) to 2012 (455,319 admissions) in facilities reporting to individual state administrative data systems.2 Although heroin abuse accounted for 63% of all opiate admissions in 2012, the proportion of non-heroin, opioid-related admissions approximately doubled from 14% in 2002 to 37% in 2012.2 The increase in non-herion opioid abuse and dependence highlights a major concern: the use of prescription drugs, over-the-counter medicines, and other pharmaceuticals for nonmedicinal purposes.
Current treatments for opioid use disorder include opioid medication-assisted treatment to reduce or eliminate the illicit use of opioids. These therapies are beneficial for relieving cravings and blocking the euphoric effects of other µ-opioid receptor (µOR) agonists. Buprenorphine, used in medication-assisted treatment, is a partial agonist at the µOR and has κ-opioid antagonist properties.3 The partial agonist activity of buprenorphine3 paired with its slow dissociation from the µOR has been associated with limited signs and symptoms of physical dependence and a mild opioid withdrawal syndrome.4
RBP-6000 is a new sustained-release (28 days) formulation of buprenorphine under development for the treatment of opioid use disorder. RBP-6000, using the ATRIGEL delivery system, is designed to be administered in a once a month subcutaneous (SC) injection. After SC injection, the ATRIGEL delivery system solidifies on contact with bodily fluids and subsequently delivers buprenorphine over an extended period of time. This formulation offers potential advantages over existing buprenorphine pharmacotherapies, including improved patient compliance, as well as reduced diversion, abuse, and unintended exposure (eg, pediatric).
During the clinical development of RBP-6000, 3 studies were conducted to assess its pharmacokinetics (PK), safety and tolerability: a phase 1, first-in-man study evaluating 20 mg of RBP-6000, a single ascending dose (SAD) study evaluating 50, 100, and 200 mg of RBP-6000, and a phase 2 multiple dose study evaluating 50, 100, 200, and 300 mg of RBP-6000 (NLM Identifier: NCT01738503).5 To support the dosing regimen for the current study, a population PK/pharmacodynamic (PD) model was developed to predict µOR occupancy (µORO) using a single-center, open-label, sequential-cohort, SAD study.6 Based on this model, a buprenorphine plasma concentration of 2 to 3 ng/mL was predicted to achieve sufficient µORO—approximately 70%7—to suppress opioid withdrawal signs and symptoms and to block the response to a µOR agonist.6 As a result of the SAD, multiple dose, and PK/PD modeling studies, a 300 mg dose of RBP-6000 was selected for use in the current study. The objective of this study was to evaluate the ability of RBP-6000 (300mg) to block the subjective drug liking effects and the reinforcing efficacy of the µOR agonist hydromorphone. The safety and tolerability of the RBP-6000 depot formulation were also evaluated.
MATERIALS AND METHODS
This was a single-center phase 2 multiple-dose study that involved adults with moderate or severe opioid use disorder (NLM Identifier: NCT02044094).8 The study was conducted at Vince & Associates Clinical Research, Overland Park, KS, in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, Food and Drug Administration regulations governing clinical study conduct, and the Declaration of Helsinki (1996) and was approved by the MidLands Institutional Review Board (Overland Park, KS).
Subjects
All study participants provided written informed consent before any study procedures. Eligible subjects were men and nonpregnant women aged 18 to 55 years with moderate or severe opioid use disorder according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria, who were not seeking treatment. Other inclusion criteria included normal or no clinically significant electrocardiogram and laboratory (including hematology chemistry and urinalysis) findings at screening; current or past experience of parenteral abuse of opioids, and signs and symptoms of opioid withdrawal before starting the study dosing (as evidenced by a Clinical Opiate Withdrawal Scale score >12). Exclusion criteria included a “Drug-Liking” Visual Analogue Scale (VAS) score less than 40 mm for 18 mg hydromorphone and/or less than a 20-mm difference in score between 18 mg hydromorphone and placebo during screening; any current diagnosis requiring chronic opioid treatment; history of risk factors for Torsades de Pointes; diagnosis of moderate or severe substance use disorder for substances other than opioids, caffeine, or nicotine; uncontrolled medical or psychiatric illness; and abuse or use of buprenorphine within 14 days before informed consent. Full inclusion and exclusion criteria are detailed in Supplementary eTable 1, Supplemental Digital Content 1, http://links.lww.com/JCP/A343.
Procedures
Each subject received RBP-6000 as a single 300-mg SC injection on day 1 and day 29 of the study period. The study schedule was designed to include a minimum of 28 days separation between each RBP-6000 injection. Subjects received injections on alternate sides of the abdomen below the waist but above the hip bone.
Non-opioid rescue medications (eg, clonidine, hydroxyzine, loperamide, ibuprofen, methocarbamol, and acetaminophen) were permitted to help alleviate signs and symptoms of opioid withdrawal and for prophylaxis of opioid withdrawal throughout the trial as determined by the investigator. Methocarbamol, hydroxyzine, and clonidine were not given within 12 hours of the randomized hydromorphone challenges. Additionally, no rescue medications were given for the 5 hours after hydromorphone administration or during the subjective effects assessments for the hydromorphone challenge. Subjects were requested to abstain from using non-study opioids for the duration of the study.
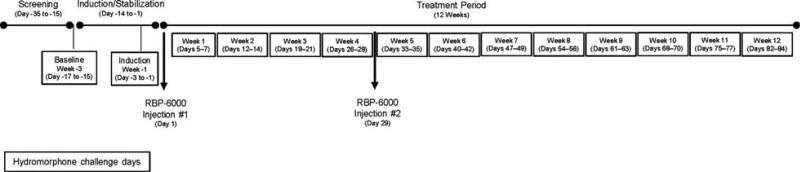
The study consisted of 3 periods: baseline hydromorphone challenge (week –3), sublingual buprenorphine/naloxone induction and stabilization (week –1), and treatment (weeks 1–12, with RBP-6000 administered on days 1 and 29). The study timeline is presented in Figure 1.
FIGURE 1.
Study design.
Baseline
Eligible subjects were admitted to the clinical unit for 3 consecutive days (admission the night before the first challenge day) on day –18 to undergo baseline blinded challenges with hydromorphone (10 mg/mL, Hospira Inc., Lake Forest, IL). On each day, subjects received one intramuscular injection of placebo (0 mg; 0.45% sodium chloride [half normal saline]) or 6 or 18 mg of hydromorphone (constant 1.8 mL volume) in 1 of 6 randomized sequences. “Drug liking” VAS and the drug versus money choice task were conducted on each of the 3 days preceding induction/stabilization on sublingual buprenorphine/naloxone.
Induction/Stabilization
Inducted subjects received 8 to 24 mg per day of sublingual buprenorphine/naloxone (SUBOXONE Sublingual Film, distributed by Indivior Inc., Richmond, VA) until a stable dose was established. Subsequent doses of buprenorphine/naloxone were administered at approximately the same time (±1 hour) each day, typically at approximately 5:00 pm. Once stabilized, subjects received hydromorphone challenges and 6 VAS assessments and the drug versus money choice task were conducted, as detailed above, on each of the 3 days preceding administration of RBP-6000 (days –3 to –1 during week –1).
Treatment
Subjects who continued to meet the inclusion and dosing criteria (Supplementary eTable 2, Supplemental Digital Content 1, http://links.lww.com/JCP/A343) stopped receiving sublingual buprenorphine/naloxone the day before the treatment period started (week 1 through week 12). On day 1, subjects were administered a single SC injection of RBP-6000 (300 mg) into the abdominal area. A second injection of RBP-6000 was administered on day 29. Hydromorphone challenges (administered in randomized study drug sequences similar to the induction period) and 6 VAS assessments and the drug versus money choice task were conducted on 3 consecutive residential days each week for a total of 12 weeks (4 weeks after the first injection of RBP-6000, and 8 weeks after the second injection of RBP-6000). During each 3-day hydromorphone challenge, the clinical staff and subjects remained blinded to the sequence. The final study visit was 9 weeks after the second injection of RBP-6000 or after early study termination.
Pharmacodynamic Assessments
VAS of Subjective Effects
The VAS of subjective drug effects was assessed during 3-day residential periods at baseline and once a week after each RBP-6000 injection. Subjects responded to each challenge on a 100-mm VAS anchored by “none” or “not at all” and “extreme” or “extremely” (modified from Bickel et al9). The scale measured reports of drug liking, as well as reports of “good drug effect,” “bad drug effect,” “any drug effect,” “high,” and “sedation.” Measurements were completed 30 minutes before and 15, 30, 45, 60, 75, 90, 120, 150, 180, 210, 240, 270, and 300 minutes after each hydromorphone challenge. Mean values from all postinjection time points were used.
Drug Versus Money Choice Task
At least 5 hours after the baseline and treatment period, randomized hydromorphone challenges subjects completed a 12-trial drug/money choice task.10–12 On each trial, the subject could choose to earn 1 of the 12 total hydromorphone (or placebo) unit doses (ie 0mg, 0.5mg or 1.5mg per trial) they had received that morning or US $2. To earn each choice, subjects had to click either the “drug” or “money” box displayed on the computer screen. The number of mouse clicks required to receive each reward (drug or money) increased exponentially across trials (5, 40, 70, 120, 180, 260, 395, 555, 775, 1110, 1558, 2160 mouse clicks), according to a progressive ratio schedule of reinforcement. The response requirement for both drug and money increased (independently from one another) until responding ceased, all 12 ratios were completed or the participant chose to work for the alternative option. The “breakpoint” was defined as the highest number of mouse clicks completed to receive the hydromorphone unit dose.
Pharmacokinetics Assessments
Blood samples for pharmacokinetic testing were collected immediately before each hydromorphone challenge; before and 1.5 hours after sublingual buprenorphine/naloxone; and before and 24 hours after the RBP-6000 injection. Plasma concentrations of buprenorphine and norbuprenorphine were quantified using a validated liquid chromatography coupled to tandem mass spectrometry method (methods unpublished). Human plasma, containing buprenorphine, norbuprenorphine, and the internal standards, buprenorphine-D4 and norbuprenorphine-D3, was extracted with an organic solvent mixture of cyclohexane and ethyl acetate after the addition of sodium hydroxide solution (liquid-liquid extraction). After extraction, the extract was evaporated, reconstituted, and an aliquot was injected on a Sciex API 5000 liquid chromatography coupled to tandem mass spectrometry system. The chromatographic separation was performed by a reverse phase C18 column and acidified water and methanol as the mobile phase under gradient conditions. The peak area of the m/z 468 → 414 buprenorphine product ion was measured against the peak area of the m/z 472 → 414 buprenorphine-D4 internal standard product ion. The peak area of the m/z 414 → 83 norbuprenorphine product ion was measured against the peak area of the m/z 417 → 83 norbuprenorphine-D3 internal standard product ion. Quantitation was performed using a separate weighted (1/×2) linear least-squares regression analysis generated from fortified plasma calibration standards prepared immediately before each run. The method was validated for specificity, linearity, lower limit of quantitation, precision, accuracy, recovery, and stability for a range of 0.050 to 25.0 ng/mL of buprenorphine and 0.040 to 20.0 ng/mL for norbuprenorphine based on the analysis of 0.500 mL of plasma. The overall precision for buprenorphine and norbuprenorphine was greater than 10.1%; the overall accuracy was within ± 9.6%. The recovery of buprenorphine, norbuprenorphine, and the internal standards were greater than 74%. The established short-term and long-term stabilities covered the maximum sample storage time.
Safety and Tolerability
Metabolic parameters and adverse events were assessed throughout the treatment period. Additional safety and tolerability parameters included local injection site reactions (using a grading scale), and subject-reported injection site pain using VAS scores.
Statistical Analyses
The intent-to-treat (ITT) population was used for assessing the ability of RBP-6000 to block the effects of hydromorphone and included all subjects who received at least 1 dose of RBP-6000 and had all doses for the hydromorphone challenges at least once after administration of RBP-6000. Safety analyses were performed using the safety population and included all subjects who received at least 1 dose of RBP-6000, SUBOXONE Film, or hydromorphone (starting with the baseline hydromorphone challenge).
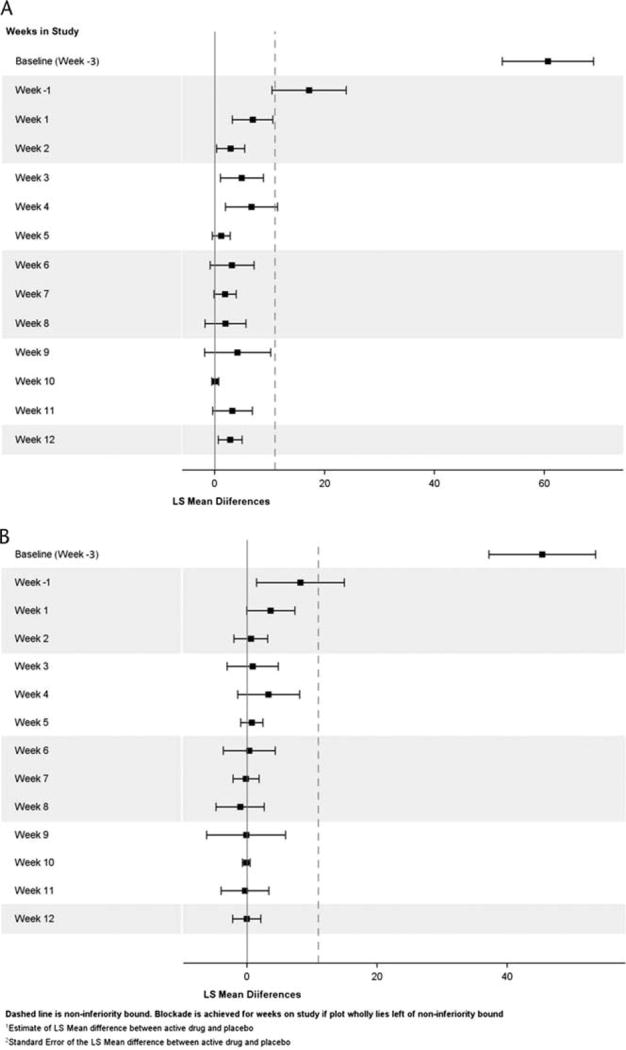
Drug liking VAS scores (primary endpoint) were analyzed using a mixed-effects model with period (ie, day), hydromorphone sequence, and hydromorphone dose as fixed effects and subject nested within hydromorphone sequence as a random effect. The difference in mean outcome between hydromorphone doses was compared using SAS® estimate statements. Opioid blockade of drug liking effects was achieved if the upper bound of the 95% confidence interval (CI) was less than or equal to the noninferiority margin (11 mm). As defined a priori, complete hydromorphone blockade of drug liking effects was achieved for RBP-6000 if opioid blockade occurred for both hydromorphone doses (6 mg and 18 mg) during each week of testing for the 4 weeks after the first dose of RBP-6000. Each of the above tests was performed at a 2-sided α = 0.05 level off significance. Because an intersection union test was used, there was no adjustment for multiple testing, and the overall test was a size-α test.13
Hydromorphone breakpoint values (secondary endpoint) for the drug-money choice task for all subjects were analyzed by week using a repeated measures mixed-effects model with period, hydromorphone sequence, and hydromorphone dose as fixed effects and subject nested within hydromorphone sequence as a random effect. Difference in mean outcome between hydromorphone doses was compared using SAS® estimate statements. Opioid blockade was achieved if the 95% CI for the reinforcing effects transformed breakpoint value enclosed zero. If the normality assumption was violated, the analyses were carried out on the log (base 10) transformed hydromorphone breakpoint value, as previously described.11
Sample size was calculated using a noninferiority hypothesis in a Williams’ square design, according to Chow et al.14 For a given type I error bound α and power 1-β, the number of replicates needed per sequence is:
where δ is the noninferiority margin (11 mm),15 is the variance of the individual differences between responses to dose 1 (or dose 2) and placebo, ε is the true mean of the differences in outcomes to dose 1 (or dose 2) and placebo, and m is the number of sequences (6 in this case).
A bound was chosen on the 2-sided type I error of 5% (2-sided α = 0.05 and zα/2 = 1.96) and a power of 80% (β = 0.2 and zβ = 0.84). It was estimated a minimum of 24 subjects (with 4 subjects assigned to each sequence of a Williams’ 6×3 design) who completed all challenges for weeks 1 to 4 were required for the analysis.
RESULTS
Of the 342 subjects who provided informed consent, 39 subjects were randomized. The remaining 303 subjects were ineligible based on the exclusion criteria. One subject was lost to follow-up, leaving 38 subjects in the ITT population. Eight additional subjects withdrew from the study due to physician decision or self-withdrawal. Thirty subjects completed the study in the ITT (78.9%) and safety (76.9%) groups. The mean age was 34.8 years (range, 20 to 55 years) and the highest proportion of subjects were men (89.7%) and white (65.8%). The mean body mass index was 25.35 kg/m2 (range, 20.7–31.5 kg/m2).
Pharmacodynamics
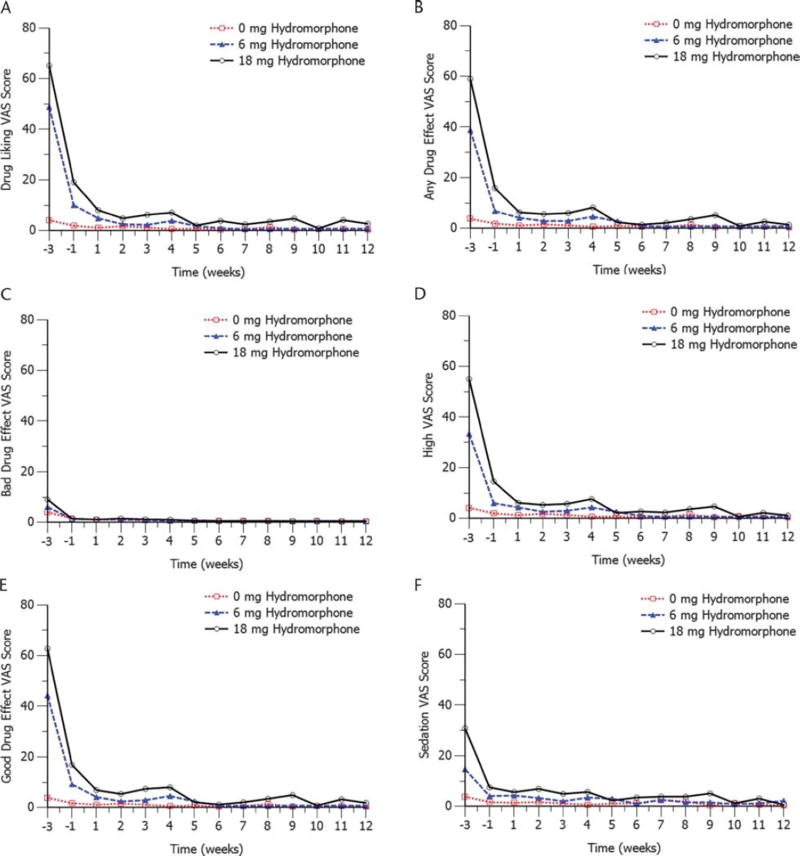
Drug liking for both hydromorphone doses was reduced, as measured by VAS scores, after the first injection of RBP-6000 (day 1) and remained low throughout the treatment period. Results for the drug liking VAS scores, the primary endpoint measure, are presented in Table 1 and Figure 3A. At baseline, the least squares (LS) mean difference from placebo for drug liking VAS scores was 45 mm (95% CI, 37.2–53.6) for 6 mg of hydromorphone and 61 mm (95% CI, 52.3–68.9) for 18 mg of hydromorphone. After stabilization on sublingual buprenorphine/naloxone, the LS mean difference from placebo for drug liking scores decreased to 8 mm (95% CI, 1.5–14.9) for 6 mg of hydromorphone and 17 mm (95% CI, 10.4–23.9) for 18 mg of hydromorphone (Fig. 2). The VAS scores generally decreased until the end of the study, where the LS mean difference from placebo for drug liking scores was −0.03 mm (95% CI, −2.19 to 2.12) for 6 mg of hydromorphone and 2.78 (95% CI, 0.61–4.96) for 18 mg of hydromorphone. Because the upper bound of the 95% CI for drug liking VAS scores was 11 mm or less (the noninferiority margin) for both hydromorphone doses from week 1 to week 4, these results suggest clinically non inferior drug liking effects of hydromorphone compared with placebo. As presented in Figure 3B to Figure 3F, a trend similar to drug liking was observed for the other 5 VAS assessments (ie, “high,” “any drug effect,” “good drug effect,” “bad drug effect,” and “sedation”).
TABLE 1.
Blockade Effects of 18 mg and 6 mg Hydromorphone Challenge (ITT Population)
| Buprenorphine Concentration (ng/mL), (SD) |
Estimated µORO (%), (SD) |
Drug Liking VAS Scores (mm)—Change From Placebo, LS Mean (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Phase | Week | 18 mg | 6 mg | 18 mg | 6 mg | 18 mg vs Placebo | 6 mg vs Placebo |
| Baseline | −3 | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 60.61 (52.32–68.90) | 45.36 (37.16–53.56) |
| Sublingual | −1 | 1.24 (0.82) | 1.32 (0.87) | 53.63 (14.53) | 54.64 (15.38) | 17.17 (10.43–23.90) | 8.20 (1.47–14.94) |
| RBP-6000 | 1 | 2.04 (0.77) | 2.16 (0.88) | 67.09 (6.59) | 67.88 (6.81) | 6.93 (3.24–10.61) | 3.66 (−0.03 to 7.34) |
| 2 | 1.97 (0.76) | 1.87 (0.61) | 66.49 (6.67) | 66.06 (5.69) | 2.90 (0.33–5.47) | 0.59 (−1.98 to 3.15) | |
| 3 | 1.92 (0.61) | 1.88 (0.66) | 66.44 (5.93) | 66.00 (6.01) | 4.93 (1.02–8.84) | 0.86 (−3.05 to 4.78) | |
| 4 | 1.81 (0.63) | 1.78 (0.60) | 65.35 (5.85) | 65.11 (5.53) | 6.68 (1.94–11.42) | 3.32 (−1.43 to 8.06) | |
| 5 | 3.67 (1.04) | 3.65 (1.12) | 76.42 (3.86) | 76.29 (3.94) | 1.21 (−0.43 to 2.85) | 0.74 (−0.94 to 2.42) | |
| 6 | 3.52 (1.18) | 3.53 (1.10) | 75.69 (4.37) | 75.70 (4.69) | 3.16 (−0.83, 7.15) | 0.35 (−3.62 to 4.32) | |
| 7 | 3.47 (1.14) | 3.50 (1.11) | 75.43 (4.60) | 75.64 (4.37) | 1.88 (−0.11 to 3.87) | −0.15 (−2.16 to 1.86) | |
| 8 | 3.50 (1.30) | 3.37 (1.16) | 75.18 (5.30) | 74.79 (5.50) | 1.93 (−1.79 to 5.66) | −1.05 (−4.77 to 2.68) | |
| 9 | 3.21 (1.20) | 3.12 (1.03) | 74.04 (5.69) | 73.97 (5.13) | 4.17 (−1.84 to 10.17) | −0.12 (−6.20 to 5.96) | |
| 10 | 3.19 (1.14) | 3.04 (1.15) | 74.18 (5.36) | 73.08 (6.58) | 0.13 (−0.50 to 0.76) | −0.09 (−0.69 to 0.51) | |
| 11 | 3.08 (1.12) | 2.99 (1.08) | 73.51 (5.90) | 73.05 (6.10) | 3.24 (−0.35 to 6.83) | −0.32 (−3.97 to 3.34) | |
| 12 | 2.62 (0.95) | 2.65 (1.04) | 71.30 (5.86) | 71.31 (5.93) | 2.78 (0.61–4.96) | −0.03 (−2.19 to 2.12) | |
FIGURE 3.
Mean scores for the 6 VAS assessments. A, Mean drug liking VAS scores by hydromorphone challenge dose. B, Mean “any drug effect” VAS scores by hydromorphone challenge dose. C, Mean “bad drug effect” VAS scores by hydromorphone challenge dose. D, Mean “drug high” VAS scores by hydromorphone challenge dose. E, Mean “good drug effect” VAS scores by hydromorphone challenge dose. F, Mean “sedation” VAS scores by hydromorphone challenge dose.
FIGURE 2.
Plot of mean difference and 95% confidence interval for drug liking VAS score. A, Comparison of 18-mg hydromorphone versus placebo. B, Comparison of 6-mg hydromorphone versus placebo.
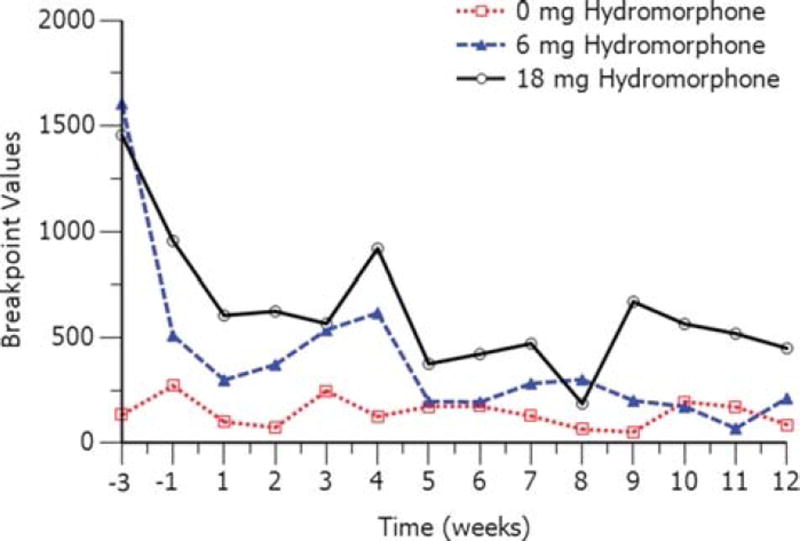
Breakpoint values for both doses of hydromorphone decreased after injections of RBP-6000. Because the raw breakpoint values were not normally distributed, log-transformed (based 10) hydromorphone breakpoint values were used.9 The log-transformed LS mean difference from placebo in breakpoint values is presented in Table 2, and mean breakpoint values per week are illustrated in Figure 4 for 6 and 18 mg of hydromorphone. The difference from placebo in log-transformed breakpoint decreased from 2.1 at baseline (week −3) to 1.9 (95% CI, 1.1–2.8) for 6 mg hydromorphone and 1.3 (95% CI, 0.5–2.2) for 18 mg hydromorphone during stabilization (week −1). During the treatment period (weeks 1 to 12), breakpoint values decreased after each injection of RBP-6000, and by the end of the treatment period, the difference from placebo in log-transformed breakpoint values was 0.6 (95% CI, −0.573 to 1.8) for 6 mg of hydromorphone and 1.6 (95% CI, 0.50–2.7) for 18 mg of hydromorphone.
TABLE 2.
Reinforcing Effects of 18 mg and 6 mg Hydromorphone Challenge (ITT Population)
| Hydromorphone (18 mg or 6 mg) Versus Placebo Challenge Ratio, LS Mean (95% CI)* |
Reinforcing effects (breakpoint)—Change From Placebo, LS Mean of Log Transformation (95% CI) |
||||
|---|---|---|---|---|---|
| Phase | Week | 18 mg vs Placebo | 6 mg vs Placebo | 18 mg vs Placebo | 6 mg vs Placebo |
| Baseline | −3 | 7.85 (4.39–14.01) | 8.50 (4.71–15.18) | 2.06 (1.48–2.64) | 2.14 (1.55–2.72) |
| SL induction/stabilization | −1 | 6.89 (3.00–15.80) | 3.78 (1.57–9.21) | 1.93 (1.10–2.76) | 1.33 (0.45–2.22) |
| RBP-6000 | 1 | 2.56 (1.42–4.62) | 1.84 (1.00–3.42) | 0.94 (0.35–1.53) | 0.61 (−0.003 to 1.23) |
| 2 | 4.31 (1.92–9.68) | 2.10 (0.89, 5.00) | 1.46 (0.65–2.27) | 0.74 (−0.12 to 1.61) | |
| 3 | 2.48 (1.34–4.57) | 2.59 (1.36–4.85) | 0.91 (0.29–1.52) | 0.95 (0.31–1.58) | |
| 4 | 6.62 (3.42–12.81) | 4.01 (2.03–7.92) | 1.89 (1.23–2.55) | 1.39 (0.71–2.07) | |
| 5 | 1.45 (0.58–3.67) | 0.91 (0.34–2.46) | 0.37 (−0.55 to 1.30) | −0.09 (−1.09 to 0.90) | |
| 6 | 2.66 (0.93–7.54) | 1.13 (0.39–3.22) | 0.98 (−0.07 to 2.02) | 0.12 (−0.94 to 1.17) | |
| 7 | 2.39 (1.11–5.21) | 2.05 (0.99–4.22) | 0.87 (0.10–1.65) | 0.72 (−0.01 to 1.44) | |
| 8 | 2.34 (0.90–6.11) | 1.68 (0.68–4.10) | 0.85 (−0.11 to 1.81) | 0.52 (−0.38 to 1.41) | |
| 9 | 3.00 (0.73–12.18) | 1.09 (0.24–4.90) | 1.10 (−0.31 to 2.50) | 0.09 (−1.41 to 1.59) | |
| 10 | 1.57 (0.61–4.10) | 1.03 (0.39–2.75) | 0.45 (−0.50 to 1.41) | 0.03 (−0.94 to 1.01) | |
| 11 | 2.12 (0.52–8.58) | 0.80 (0.18–3.49) | 0.75 (−0.66 to 2.15) | −0.22 (−1.69 to 1.25) | |
| 12 | 4.90 (1.65–14.44) | 1.80 (0.57–5.81) | 1.59 (0.50–2.67) | 0.59 (−0.57 to 1.76) | |
Values were presented after back exponential conversion from natural log-transformed data.
SL indicates sublingual.
FIGURE 4.
Mean breakpoint values for the drug versus money choice task.
Pharmacokinetics
Mean weekly buprenorphine and norbuprenorphine concentrations increased 24 hours after each RBP-6000 injection and slowly decreased thereafter (Supplementary eFigure 1A, Supplemental Digital Content 1, http://links.lww.com/JCP/A343). Mean buprenorphine concentrations were 1.3 ng/mL (±0.8 ng/mL) immediately after the first injection and increased to 5.0 ng/mL (±1.6 ng/mL) 24 hours later. Compared with the first RBP-6000 injection, the concentration of buprenorphine was slightly higher after the second injection of RBP-6000 (1.8 ± 0.7 ng/mL) and 24 hours later (6.6 ± 2.1 ng/mL). Mean weekly concentrations of norbuprenorphine, however, decreased slightly 24 hours after the second RBP-6000 injection compared with the first RBP-6000 injection (1.5 ± 1.0 ng/mL vs 3.8 ± 2.2 ng/mL) (Supplementary eFigure 1B, Supplemental Digital Content 1, http://links.lww.com/JCP/A343).
Safety and Tolerability
RBP-6000 was generally well tolerated. At least 1 treatment-emergent adverse event (TEAE) was reported by all 39 subjects in the safety population. A list of reported TEAEs is presented in Table 3. The proportion of subjects experiencing treatment-related TEAEs was 64.1%. The most common related TEAEs (occurring in ≥10% of subjects) in this study were sedation (10.3%), nausea (12.8%), constipation (30.8%), and injection site reactions (79.5%). The majority of the injection site reactions (which included tenderness, pain, induration, and erythema) were determined to be mild in severity; however, 3 subjects (7.7%) were determined to have severe injection site tenderness. The mean pain VAS score was 52 mm at 15 and 30 seconds after the first injection, although these scores varied widely by subject (from 2 to 95 mm). By 5 minutes after injection, however, pain VAS scores rapidly decreased to about 10 mm (data not shown). These results were also similar for the second injection of RBP-6000 (data not shown). There were no withdrawals due to TEAEs reported during this study.
TABLE 3.
Total TEAEs That Occurred in >10% of Subjects and TEAEs Related* to the Study Drug (Safety Population) by MedDRA Preferred Term
| Total TEAEs n = 39 (%) |
Related* TEAEs n = 39 (%) |
|
|---|---|---|
| Subjects with ≥1 TEAE | 39 (100) | 25 (64.1) |
| Drug withdrawal syndrome | 31 (79.5) | |
| Headache | 25 (64.1) | 3 (7.7) |
| Sedation | 12 (30.8) | 4 (10.3) |
| Dizziness | 4 (10.3) | 1 (2.6) |
| Constipation | 22 (56.4) | 12 (30.8) |
| Nausea | 13 (33.3) | 5 (12.8) |
| Vomiting | 11 (28.2) | 1 (2.6) |
| Diarrhoea | 8 (20.5) | |
| Anxiety | 19 (48.7) | |
| Abnormal dreams | 5 (12.8) | 2 (5.1) |
| Musculoskeletal pain | 12 (30.8) | 3 (7.7) |
| Upper respiratory tract infection | 4 (10.3) | |
| Weight decreased | 4 (10.3) | 3 (7.7) |
As determined by the investigator. Related: the cause of the AE is related to the investigational product and cannot be reasonably explained by other factors (eg, the subject’s clinical state, concomitant therapy, and/ or other interventions); not related: data are available to identify a clear alternative cause for the AE other than the investigational product.
DISCUSSION
Hydromorphone has been used in various studies as a challenge drug to investigate the ability of buprenorphine to block its agonist effects.3,9,16–18 The 18 mg hydromorphone dose selected for this study was based, in part, on a previous study that used an 18 mg cumulative dose,9 which is equivalent to approximately 135 mg of morphine.19 This dose, therefore, has substantial clinical relevance to concerns about possibly overriding the opioid blockade effect by using large opioid agonist doses.19 Furthermore, Greenwald et al7,10, 11 previously found that repeated administration of 24 mg bolus doses of hydromorphone in addition to daily buprenorphine doses to be safe. The 6 mg hydromorphone parenteral dose is approximately equivalent in analgesic potency to 45 mg of parenterally administered morphine, a dose in the range commonly used in opioid abuse liability assessment studies.20
The current study included both subject-reported (VAS scores) and reinforcing effects outcomes (breakpoint values) because other studies have found these 2 outcomes do not necessarily correlate,21 suggesting subjective effects alone may not adequately indicate a drug’s abuse liability under varying conditions. Drug liking VAS scores from both hydromorphone challenges were significantly reduced during treatment with 300 mg RBP-6000, and the noninferiority analysis did not indicate any clinically relevant changes in drug liking of either hydromorphone challenge doses during the RBP-6000 treatment period, suggesting blockade of the drug liking effects of hydromorphone. This blockade was maintained during the full intended dosing interval of 28 days.
The drug versus money choice task, which evaluated the reinforcing efficacy of hydromorphone, used a standard amount of money as a choice alternative to hydromorphone. This paradigm has been adopted in various clinical studies of drug reinforcement.10–12,22–24 and is intended to model drug abuse liability. The breakpoint values generally decreased following RBP-6000 treatment during the hydromorphone challenges. The statistical analysis indicated clinically relevant differences from placebo in the log-transformed breakpoint values during week 3 and week 4 after the 6 mg hydromorphone challenge and during weeks 1 to 4 and weeks 7 and 12 after the 18 mg hydromorphone challenge.
Results of the current study using RBP-6000 are consistent with earlier studies which demonstrated that sublingual buprenorphine blocks the subjective effects and reduces the reinforcing effects of a hydromorphone challenge.3,9,16,18,25 Although the hydromorphone challenge doses were compared with placebo in a blinded manner and there was no placebo control for RBP-6000, self-controlled responses were compared to baseline, when subjects received no buprenorphine. Two studies have also evaluated the effects of sustained-release buprenorphine, using a polymer microcapsule depot formulation at a dose of 58 mg.17,26 These studies included an open-label trial (n = 5)22 and a randomized placebo-controlled trial (n = 15).17 Both studies found that sustained-release buprenorphine provided effective relief from opioid withdrawal and produced opioid blockade in opioid challenge sessions, an effect that persisted for 6 weeks.17,26
An overall correlation between buprenorphine concentration with drug liking VAS scores after the 18 mg hydromorphone challenge (blue line) and µORO (red line) is presented in Supplementary eFigure 2, Supplemental Digital Content 1, (http://links.lww.com/JCP/A343). The µORO was predicted using the observed buprenorphine concentrations and the previously published model from Nasser et al.6 After the first injection of SC RBP-6000 (Fig. 3A), the mean buprenorphine plasma concentration was less than 2 ng/mL coupled with greater than 70% µORO, which corresponded to drug liking VAS that was noninferior to placebo. During week 4 (before the second injection of RBP-6000), a slight decrease in mean buprenorphine plasma concentration (from 1.9 to 1.8 ng/mL) correlated with a 65% µORO, which corresponded to a slight increase in VAS scores. The same pattern was observed for breakpoint values (drug seeking) on the drug versus money choice task. After the second RBP-6000 injection, an average buprenorphine plasma concentration greater than 3 ng/mL was achieved that correlated with greater than 70% µORO, which corresponded to drug liking VAS scores that approached zero. Together, these results indicate that monthly injections of RBP-6000 produce clinically relevant levels of µORO leading to a subsequent blockade of opioid subjective effects and reinforcing efficacy. In addition, the monthly depot formulation of RBP-6000 may reduce and perhaps cease illicit opioid use while improving patient adherence and reducing diversion, abuse, and unintended exposure to buprenorphine.
In conclusion, the drug liking VAS scores measured after challenge with 6 and 18 mg of hydromorphone were noninferior to placebo after both RBP-6000 injections. The results of the present study indicate that monthly injections of RBP-6000 produce clinically relevant plasma levels of buprenorphine (and predicted µORO) which translate into an almost complete blockade of the subjective effects of hydromorphone and a significant reduction in the reinforcing effects of hydropmorphone. RBP-6000 was also safe and well tolerated. RBP-6000 is currently being assessed for efficacy and safety in a phase 3 clinical trial.
Supplementary Material
Acknowledgments
The authors wish to express their profound gratitude and appreciation to Dr Rolley (Ed) Johnson for his mentorship and vision for the treatment of opioid use disorder; Nicole Buzin for her medical writing assistance; and Worldwide Clinical Trials Early Phase Services for their bioanalytical analysis.
AUTHOR DISCLOSURE INFORMATION
This work was sponsored and supported by Indivior Inc. NIH grant R01 DA015462, Joe Young, Sr./Helene Lycaki Funds (State of Michigan), and the Detroit Wayne Mental Health Authority supported the effort of M.K. Greenwald.
At the time this manuscript was submitted for publication, A.F. Nasser, P.J. Fudala, P. Twumasi-Ankrah, Y. Liu, J.P. Jones III and C. Heidbreder were full-time employees of Indivior Inc. M.K. Greenwald was a full-time employee of Wayne State University, and was a paid consultant for Indivior Inc. B. Vince was an investigator of this study, and was a full time employee of Vince & Associates Clinical Research, Inc.
Footnotes
Supplemental digital contents are available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
References
- 1.Boutwell AE, Nijhawan A, Zaller N, et al. Arrested on heroin: a national opportunity. J Opioid Manag. 2007;3:328–332. doi: 10.5055/jom.2007.0021. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services. Treatment Episode Data Set (TEDS) 2002–2012. [Accessed February 1, 2015];National Admissions to Substance Abuse Treatment Services. 2012 Available at: http://www.samhsa.gov/data/sites/default/files/TEDS2012N_Web.pdf.
- 3.Walsh SL, Preston KL, Bigelow GE, et al. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- 4.Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharm Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 5.Inc Reckitt Benckiser Pharmaceuticals. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [[cited 2015 Jun 30]]. Multiple dose pharmacokinetics depot buprenorphine in opioid-dependent subjects. Available at: http://clinicaltrials.gov/show/NCT01738503:NCT01738503. [Google Scholar]
- 6.Nasser AF, Heidbreder C, Gomeni R, et al. A population pharmacokinetics and pharmacodynamic modelling approach to support the clinical development of RBP-6000, a new, subcutaneously injectable, long-acting, sustained-release formulation of buprenorphine, for the treatment of opioid dependence. Clin Pharmacokinet. 2014;53:813–824. doi: 10.1007/s40262-014-0155-0. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald MK, Johanson CE, Bueller J, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61:101–110. doi: 10.1016/j.biopsych.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Inc Reckitt Benckiser Pharmaceuticals. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [[cited 2015 Jun 30]]. Multiple dose study of blockade opioid effects of injections of buprenorphine in participants with opioid disorder. Available at: http://clinicaltrials.gov/show/NCT02044094:NCT02044094. [Google Scholar]
- 9.Bickel WK, Stitzer ML, Bigelow GE, et al. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. [PubMed] [Google Scholar]
- 10.Greenwald MK, Hursh SR. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend. 2006;85:35–48. doi: 10.1016/j.drugalcdep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald MK, Steinmiller CL. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Depend. 2009;104:84–93. doi: 10.1016/j.drugalcdep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald MK, Lundahl LH, Steinmiller CL. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology (Berl) 2013;225:811–824. doi: 10.1007/s00213-012-2868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger RL, Hsu JC. Bioequivalence trials, intersection-union tests and equivalence confidence sets. Statist Sci. 1996;11:283–319. [Google Scholar]
- 14.Chow SC, Wang H, Shao J. Sample Size Calculations in Clinical Research. 2. New York: CRC Press; 2007. [Google Scholar]
- 15.Chen L. Science of Abuse Liability Assessment. Rockville, MD: 2011. Analysis of DATA From Human Abuse Potential Studies [abstract] [Google Scholar]
- 16.Correia CJ, Walsh SL, Bigelow GE, et al. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology (Berl) 2006;189:297–306. doi: 10.1007/s00213-006-0571-4. [DOI] [PubMed] [Google Scholar]
- 17.Sigmon SC, Wong CJ, Chausmer AL, et al. Evaluation of an injection depot formulation of buprenorphine: placebo comparison. Addiction. 2004;99:1439–1449. doi: 10.1111/j.1360-0443.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosen MI, Wallace EA, McMahon TJ, et al. Buprenorphine: duration of blockade of effects of intramuscular hydromorphone. Drug Alcohol Depend. 1994;35:141–149. doi: 10.1016/0376-8716(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 19.Brunton LL, Blumenthal DK, Murri N, et al. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12. New York: McGraw-Hill; 2011. [Google Scholar]
- 20.Jasinski DR, Henningfield JE. Human abuse liability assessment by measurement of subjective and physiological effects. NIDA Res Monogr. 1989;92:73–100. [PubMed] [Google Scholar]
- 21.Comer SD, Sullivan MA, Whittington RA, et al. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwald MK. Effects of experimental unemployment, employment and punishment analogs on opioid seeking and consumption in heroin-dependent volunteers. Drug Alcohol Depend. 2010;111:64–73. doi: 10.1016/j.drugalcdep.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwald MK, Schuh KJ, Hopper JA, et al. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology (Berl) 2002;160:344–352. doi: 10.1007/s00213-001-0975-0. [DOI] [PubMed] [Google Scholar]
- 25.Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/naloxone and buprenorphine. Psychopharmacology (Berl) 2002;159:161–166. doi: 10.1007/s002130100920. [DOI] [PubMed] [Google Scholar]
- 26.Sobel BF, Sigmon SC, Walsh SL, et al. Open-label trial of an injection depot formulation of buprenorphine in opioid detoxification. Drug Alcohol Depend. 2004;73:11–22. doi: 10.1016/j.drugalcdep.2003.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.