Abstract
Leversen et al. (PLoS One 7(6):e38830, 2012) emphasise the importance of understanding the principles of life-long development. In their study of motor control, they found a common tendency towards improved motor performance from childhood to adulthood and a subsequent deterioration. The aim of our study was to examine this issue further by investigating fine motor behaviour (tracing a model line) in 196 participants (age range 12–95 years old) in two sensory conditions—proprioceptive + visual (PV) and proprioceptive only—in both hands and in two types of movement, frontal and transversal. Regression analyses of line length and task performance speed in relation to age were conducted for the different test conditions. The best performance was found in middle age, and a quadratic function provided the best fit for most of the test conditions. The corresponding inflection points (the age at which graphical analysis showed a change in performance as a peak of maturation before decline due to ageing) showed earlier ages in the proprioceptive condition. For most types of movement analysed, performance speed was slower under the PV condition. Paired correlation analysis showed that the symmetry of precision performance between hands became stronger with age. The results provide information on age-dependent differences in proprioception based on fine motor performance. They may be of use in the design of preventive strategies for preserving proprioceptive function by reducing the risk of falls and accidents or diseases such as Parkinson’s.
Keywords: Proprioception, Proprioceptive diagnostics, Fine motor behaviour, Age-related differences, Symmetry in motor lateralization
Introduction
Given that human life expectancy is increasing and the percentage of older individuals is also rising, especially in developed countries, the question of how to maintain health in the elderly is becoming ever more urgent. A key factor, needless to say, is cost: for instance, fall-related costs for people over 65 years of age alone are expected to exceed USD 32 billion by the year 2020 (Shaffer and Harrison 2007). In fact, the ageing process poses numerous challenges, and one of the most important is the design of preventive measures for maintaining individuals’ health and autonomy for as long as possible. An integrative approach comprising different research areas is clearly required. One of the areas involved is postural control and proprioception. In this field, studies have examined ways of preventing age-related sensorimotor deficits (Goble et al. 2009), and have highlighted the role of cognitive processes when older individuals have to reweight sensory inputs, a task that may heighten the risk of losing balance (Teasdale and Simoneaub 2001).
The famous riddle of the Sphinx “Which creature in the morning goes on four legs, at mid-day on two, and in the evening upon three, and the more legs it has, the weaker it be?” reflects a hypothesis about general evolution; and the development of proprioceptive and visuo-proprioceptive functions in relation to fine motor behaviour across the human lifespan follows the same law. Although this behaviour shows a complex polynomial pattern, with ‘ups’ and ‘downs’ and small cycles within a larger one, it can be regarded as consisting of two main stages. In the first stage, which corresponds to the period of growth and maturation, there is a gradual improvement in motor performance. In the second, performance begins to deteriorate with the passing of time, as part of the ageing process.
Neurological studies based on fMRI analysis, which reflect the results of motor control, have shown that brain maturation and ageing effects follow a complex pattern that is not always linear, and varies throughout the cortex (Sowell et al. 2003; Bartzokis et al. 2010). Specifically, Sowell et al. (2003) found that the left posterior temporal region gained grey matter density up to age 30, before entering a rapid decline; white matter density, on the other hand, increased between the ages of 19 and 40 years, after which it declined. Non-linear (quadratic) effects were observed in total white matter volume, which peaked at age 43 (Sowell et al. 2003).
Motor performance also approximately fits the quadratic function, as shown by Leversen et al. (2012) in a study of 338 participants (7–79 years, cross-sectional study). Performance improved from childhood (7–9) to young adulthood (19–25) and then deteriorated in old age (66–80). The authors stressed that our knowledge of general development throughout the lifespan is still insufficient and few motor domain studies have been conducted (Leversen et al. 2012). In psychology, motor control studies are underestimated and have been described by Rosenbaum (2005) as the “Cinderella” of psychological research.
Fine motor performance decreases with age, while the learning of gross motor skills with age appears to be more multidirectional (with some skills declining at earlier ages, while others improve or are at least maintained) (Voelcker-Rehage 2008). In addition, a progressive worsening in vision was usually observed after age 50 and in balance after age 65 (Sturnieksa et al. 2008). Proprioception also decreases with age and is closely related to loss of muscle and joint strength. Muscle strength has been found to remain stable until the fifth or sixth decade, but shows a 50 % decrease by age 80 (Cole et al. 1999; Sturnieksa et al. 2008). The winning performance of Senior Olympians (>50 years) declines approximately 3.4 % per year over 35 years of competition: slowly from age 50 to 75 years and then dramatically after age 75 years (Wright and Perricelli 2008). Furthermore, some researchers have shown decreased hand asymmetry (the performance difference between both hands) in motor tasks with ageing (Kalisch et al. 2006; Przybyla et al. 2011). Others have reported that the right hemisphere (left hand) worked faster on non-verbal and visual tasks, and found little change in this speed with age (Stern et al. 1980). Nonetheless, there are still very few studies on hand asymmetries and their development with age.
Engagement in daily physical activity (Ribeiro and Oliveira 2007), combined with a healthy diet, to avoid obesity and improve balance (Teasdale 2012), rich in antioxidants and essential fatty acids to reduce negative stress effects (Willis et al. 2009), could contribute to preserving proprioceptive function. It has also been shown that old people present limited improvements in cognitive performance after cognitive training (Martin et al. 2011). Thus, an alternative way to mitigate the loss of cognitive performance would be to preserve the proprioceptive function for as long as possible. This can be achieved by focusing on physical and psychological health (Liutsko 2013) in the form of exercises that improve or maintain proprioception (tai-chi, relaxing and stretching, yoga, Pilates, or simply dancing and singing and playing musical instruments); promoting healthy diet (apart from balance problems, certain substances can impair proprioception—alcohol, drugs or heavy metals and pesticides are risk factors for Parkinson’s disease) or in some way improve it, as in the case of medical drugs like l-dopa (Mongeon et al. 2009); promoting emotional intelligence in order to reduce the negative effects of stress that provoke oxidative processes and accelerate ageing (Guzman et al. 2010); and all other factors that help to maintain a healthy lifestyle. Thus, the maintenance or improvement of proprioception is a part of general health and can promote better cognitive status (since more attentional resources are available for cognitive tasks and attention does not have to be split in order to maintain balance) and quality of life.
Very few studies have explored the relationship between proprioceptive motor control and emotional state and cognitive performance. In an earlier experimental study by our group, negative sign correlations were found between certain indicators of fine motor imprecision in a proprioceptive condition and visual memory performance (Liutsko et al. 2012a). Moreover, imprecision was negatively related to academic performance and emotional equilibrium (Liutsko et al. 2012b). Ingram and colleagues reported a significant reduction in motor task performance in a patient with proprioceptive problems who showed a 60 % decrease (compared to a 10 % decrease in the control group) when the motor task was switched from single to dual (while subjects counted backwards). This experiment demonstrated the importance and value of cognition as a compensatory function for proprioceptive deficit in motor performance tasks (Ingram et al. 2000). The proprioception state may be even more challenging in older people and cause poor balance (Lin and Woollacott 2005) and irregularity of gait, especially with cognitive load (Shellenbach et al. 2010). Research using fMRI has also identified an age-related shift from automatic to more cognitively controlled movements as subjects get older (Heuninckx et al. 2005). In addition, elderly subjects were found to rely more on visual control when they learnt and performed a precision locomotor task (van Hedel and Dietz 2004).
Most studies compare proprioceptive state or acuity in relation to fine motor precision or speed in different age groups. Goble’s (2010) results showed U-shaped proprioceptive acuity in joint position matching tests (30-degree targets; individuals had to replicate a reference joint angle in the absence of vision) for the average absolute errors, with the highest errors in children (8–10 years), followed by the elderly group (70+ years), adolescents (16–18 years) and middle-aged (35–50 years), and the highest precision in the young adult group (20–30 years). Hurley et al. (1998) reported significantly lower proprioceptive acuity and functional performance in an elderly population (mean age 72 years) than in young (mean age 23 years) and middle-aged subjects (mean age 56 years). Moreover, the elderly group performed functional tasks significantly more slowly than the rest of the groups (Hurley et al. 1998), and more jerky movements were reported in senior adults (81.2 ± 1.8 years) than in young adults (25.2 ± 2.5 years) (Yan 2000).
In the light of these findings, we performed a cross-sectional study to examine whether age has a similar influence on proprioceptive and visuo-proprioceptive functions in relation to fine motor performance (tracing over a model line). Line length performance and task speed were measured in frontal and transversal directions (touch screen position was vertical for frontal movements and horizontal for transversal ones), in both hands and under two sensory conditions—proprioceptive only (P) and proprioceptive–visual (PV). Our main hypothesis was that age-related differences would fit a quadratic function. If this was the case, we sought to identify the ages that corresponded to changes in fine motor behaviour under both sensory conditions (P and PV), as inflection points of parabolas. The aim of our study was to broaden our understanding of the peculiarities of the developmental and ageing processing by performing fine motor precision tasks in both hands and in different movement types and sensory conditions.
Method
Participants
Participants had normal or corrected-to-normal vision (N = 196, age = 33 ± 21 years, range 12–95, male 75 %) and described themselves as healthy; none were receiving medication and none had neurological problems. Handedness was checked by means of the Lateral Preference Inventory (LPI; Coren 1993), which revealed right-hand dominance in 95 % of subjects. Individuals who had been forced to change their hand dominance at school were excluded. All subjects took part voluntarily, were informed about the aims of the research and gave consent prior to inclusion in the study. All tests were administered in accordance with ethical guidelines on human research.
Instruments
We controlled for conditions that might affect test performance, such as temperature or noise, and for the consumption of any substances that might influence fine motor activity. We developed a validated (Muiños 2008) computerized test (Tous and Viadé 2002; Tous et al. 2007) on the basis of myokinetic psychodiagnosis (MKP), a manual version of which was originally proposed by Mira (1958). The test equipment comprised a tactile screen (LGE, resolution 1,280 × 1,024, optimal frequency 60 Hz) and a sensory stylus (for hand drawings), both of which were connected to a laptop computer (Pentium IV) on which specially designed software was installed for data coding and analysis. A piece of cardboard (or an opaque screen) was also available for use in the P part of the test, to hide the active arm and prevent the subject from receiving movement feedback. All subjects sat at a table on an adjustable stool to perform the task. They were all given prior instructions to ensure that they performed the task correctly (Tous-Ral et al. 2012a, b).
Instructions
The following instruction was given to the study participants: ‘You have to trace over the model line from the starting point to the end of it; then trace backwards (return) to the starting point without interruption. Repeat these movements to reproduce the model line as accurately as possible. At first you will be able to see the model line, but after some trials a piece of cardboard will be placed between you and the screen, so that you cannot see your active hand position or the movement feedback and model line. You have to continue to draw the lines as before without stopping. During each section of the test, do not lift your stylus until the end of the task’. The starting point was the same for all participants, as once the subject had set the stylus at the correct coordinates, the line changed colour from red to green. The researcher only started to record the data once the line was green.
Procedure
Fine motor behaviour, assessed by the precision of line lengths (tracing over 40 mm model lines) and task speed1, was measured in frontal and transversal directions for both hands and under two test conditions: proprioceptive information only (P) and PV. Correct posture (body in the upright position looking straight ahead without leaning to the left or right during the performance of movements, and with the feet together on the floor) was ensured in all subjects, and stool and table heights were adjusted individually to allow free elbow movement. This meant that subjects were seated comfortably without having to bend their back or extend their arms in an unnatural way. The hand/arm used for the task was only in contact with the stylus with which the drawing was being made, and the wrist was kept rigid. The hand that was not being used in the task rested on the ipsilateral leg. Subjects held the stylus in the middle using their thumb, ring and index fingers, as when painting. The software recorded data on line length (LL) and performance speed for each subject under both test conditions (P and PV), and the line lengths were transformed from pixels into millimetres.
Data analysis
The line lengths drawn over the model line on the touch screen were measured under both sensory conditions (P and PV) for the two movement types (F—frontal and T—transversal) and for both hands (D—dominant and ND—non-dominant). For additional analysis (MANOVA), data were split into four age groups: (1) 12–17 (N = 41, age 14.1 ± 1.3); (2) 18–29 (N = 63, age 21.9 ± 3.3); (3) 30–64 (N = 67, age 38.8 ± 8.0), and (4) 65–95 (N = 25, age 80.3 ± 6.7). We also measured the time spent on the whole task and on each test condition (PV and P), thereby enabling us to calculate the average speed for the complete movement (forward/back or up/down) in both directions and with both hands. Linear and curvilinear regression analyses (second- and third-degree polynomials) were performed using SPSS v18 with the precision and task speed data to estimate the model that best fit the data, as well as to determine the inflection points for all observable variables so as to identify the approximate age corresponding to optimal motor precision under both test conditions and for both hands. Paired correlation coefficients (r) and differences (t) were also calculated to examine non-dominant and dominant hand performance under the PV- and P-test conditions and for both frontal and transversal movements in the four age groups.
Results
In general, sensorimotor development across the lifespan shows a complex behaviour that is similar to polynomial dependence on age, with its corresponding cycles and peaks. Although there is considerable variation at individual level, it is nonetheless possible to identify common population trends (means). Figure 1 shows a graphical analysis of the variation in means for the P and PV conditions, in relation to the subjects’ age. It can be seen that the P and PV feedback functions are well matched after 20 years of age, and begin to diverge more clearly after 48–50 years. Moreover, the P condition shows greater imprecision in fine motor performance after 48–50 years, which may also contribute to a greater change in overall performance under the PV-test condition (the amplitude of PV plots was also higher after this age, as can be seen in Fig. 1 for transversal movements). If we drew an adjusted smoothing line through the data, we would see that imprecision in transversal movements tends to increase with age. The means of frontal movements were generally more varied (presenting a sharper behaviour with many peaks) than the results for transversal performance, especially under the P-test condition. However, after the age of approximately 50 years, the plot showed a similar trend towards greater imprecision.
Fig. 1.
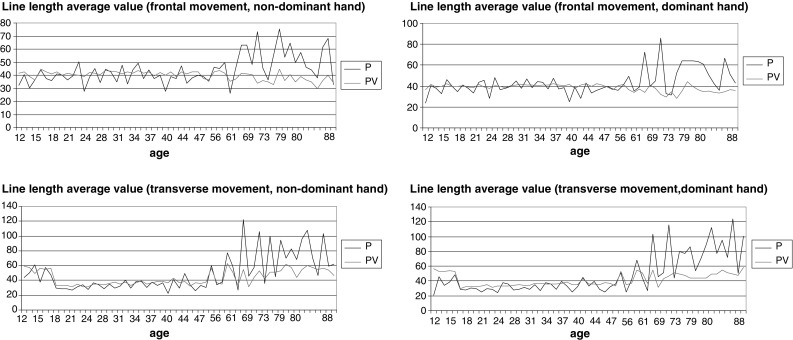
Line length average values (y axis, mm) per age (x axis, years) for frontal movements (above), transverse movements (below), non-dominant hand (on the left) and dominant hand (on the right) under the P (dark line)- and PV (light line)-test conditions
The linear regression analysis, together with the curvilinear estimations, showed that a quadratic function provided the best fit for most of the observed variables, while a cubic function was better (the explained variance was 17 % greater) for variables obtained in the transversal movement (in the PV-test conditions and for both hands). Although the cubic function fitted the data better, the quadratic function was preferred as a general or common model of the effect of age. For the cubic approximation, the graphical analysis showed a decline towards the end of life, due to the low number of subjects aged over 90.
In a quadratic model, the precision of fine motor performance was poorest at the initial measurement point (age 12). After this, it improved with age until it reached an optimum point at which the movement was made with the highest precision (middle-aged). It then started to decline again as subjects entered an advanced age. With the exception of line length in the frontal direction under the PV condition (for both hands), in other test condition regressions, the age2 parameter had positive coefficients (Table 1). This indicates a positive bias for line length at the youngest and oldest ages for the majority of test conditions. In any case, all peaks (minimum and maximum) of parabolas corresponded to the values for the greatest precision and best performance speed. Therefore, these inflection points, assessed by an average population mean for the given sample (196 subjects), provide an estimate of the point on the x axis (age) that reflects the best fine motor performance (line lengths, in mm, y axis). Quadratic equations related to changes in fine motor precision for subjects of different ages (12–95 years) were derived separately for each test condition. The best regressions, together with the highest R/R 2 adj values (Table 1), were obtained for transversal movements, which, therefore, provide a better prediction of the inflection points.
Table 1.
Descriptive statistics for the four age groups regarding the precision of fine motor performance with post-hoc MANOVA analysis; quadratic regression (R/Radj 2) values for line length in different test conditions, with the estimated inflection points for ages derived from the equations
| MT | Transverse | Frontal | ||||||
|---|---|---|---|---|---|---|---|---|
| Hand | ND | D | ND | D | ||||
| TC | P | PV | P | PV | P | PV | P | PV |
| Age group (N) | Descriptive statistics M ± SD (mm) and MANOVA post-hoc analysis (with Bonferroni correction) | |||||||
| (1) 12–17 (41) | 50.79*(2,3) ± 20.26 | 55.70*(2,3) ± 5.22 | 42.73*(2) ± 14.33 | 52.81*(2,3) ± 4.41 | 39.08 ± 8.83 | 41.61 ± 7.39 | 39.65 ± 12.36 | 41.13*(2) ± 2.78 |
| (2) 18–29 (63) | 30.31*(1) ± 7.00 | 33.25*(1,3) ± 3.92 | 29.31*(1) ± 8.67 | 33.01*(1,3) ± 2.59 | 40.34 ± 12.25 | 41.11 ± 3.12 | 39.13 ± 9.92 | 39.57*(1) ± 2.85 |
| (3) 30–64 (67) | 36.07*(1) ± 10.46 | 38.21*(1,2) ± 5.94 | 34.17 ± 10.11 | 37.35*(1,2) ± 5.76 | 40.05 ± 9.43 | 41.40 ± 3.15 | 39.96 ± 7.66 | 40.54 ± 2.42 |
| (4) 65–95 (25) | 86.27*(1−3) ± 54.60 | 51.65*(1−3) ± 6.93 | 86.24*(1−3) ± 41.51 | 46.93*(1−3) ± 11.19 | 55.92*(1−3) ± 27.63 | 35.97*(1−3) ± 3.80 | 54.55*(1−3) ± 27.35 | 34.40*(1−3) ± 3.74 |
| Regression analysis for all data | ||||||||
| R/R 2 adj | 0.59/0.34 | 0.59/0.34 | 0.70/0.48 | 0.49/0.24 | 0.34/0.11 | 0.39/0.14 | 0.33/0.10 | 0.55/0.30 |
| B (age) t |
−1.59 −4.37*** |
−1.16 −8.73*** |
−1.36 −4.83*** |
−0.95 −7.28*** |
−0.18 −0.83 |
0.09 1.27 |
−0.23 −1.10 |
0.05 1.14 |
| B (age2) t |
0.023 6.26*** |
0.013 9.59*** |
0.022 7.48*** |
0.01 7.78*** |
0.004 1.90 |
−0.002 −2.49* |
0.005 2.12* |
−0.001 −3.10** |
| ANOVA (F) | 51.07*** | 50.14*** | 92.25*** | 31.20*** | 12.85*** | 17.00*** | 12.12*** | 42.29*** |
| Inflection points (age) | 35*** | 45*** | 31*** | 48*** | 23 | 23 | 23 | 26 |
Post-hoc differences are presented for at least a statistical level of *p < 0.05 and with the corresponding age groups indicated in the brackets (for the 4th age group with all other three age groups and for the first three age groups, only the differences between them)
MT movement type, TC test condition, P proprioceptive feedback only, PV proprioceptive+visual feedback, ND and D non-dominant and dominant hands
Significance level for regression analysis: * p < 0.05, ** p < 0.01, *** p < 0.001; F (193, 2)
The inflection points were calculated by equalling the first derivations of the function f(x) = B 2* age2 + B*age + C to zero and resolving the equation for each test condition (the corresponding coefficients B and B 2 were taken from the regression analysis). This process revealed that for transversal movements the inflection points for the proprioceptive function (P condition) were at ages 35/31 for ND/D hands, respectively, whereas for the visuo-proprioceptive function (PV condition) they were at 45/48. The regressions showed a poorer fit for frontal movements, although the results were almost the same for all test conditions: a critical age of 26 for the right hand under the PV condition, and a critical age of 23 for the other cases (Table 1).
The analysis of task performance speed (Table 2) for the age groups also revealed a quadratic function, with higher values at the two ends, and faster, less variable performance in the 30–64 age group. As in the line length evaluations, we conducted a regression analysis for performance speed (the average time spent on the task under each test condition), in order to find the inflection points (Table 2). They ranged from 33 to 40 years old for different hands and sensory test conditions. The most significant and best-fitting models corresponded to the non-dominant hand in the transversal and frontal directions (40 and 37 years old, respectively) under the PV condition, and to the dominant hand in the frontal direction under the same PV condition (36 years old).
Table 2.
Descriptive statistics for the four age groups regarding the precision of fine motor performance with post hoc of MANOVA analysis; and quadratic regression parameters for performance speed with respect to age and the test conditions, with inflection points estimated from the equations
| MT | Transverse | Frontal | ||||||
|---|---|---|---|---|---|---|---|---|
| Hand | ND | D | ND | D | ||||
| TC | P | PV | P | PV | P | PV | P | PV |
| Age group (N) | Descriptive statistics of average time spent for complete movement M ± SD (ms) and MANOVA post-hoc analysis (with Bonferroni correction) | |||||||
| (1) 12–17 (41) | 6,518 ± 5,042 | 7,613 ± 4,234 | 6,269 ± 3,010 | 9,361 ± 4,604 | 5,353 ± 2,613 | 7,280 ± 4,620 | 5,778 ± 3,392 | 7,117 ± 4,072 |
| (2) 18–29 (56) | 6,277 ± 3,326 | 7,024 ± 3,141 | 6,528 ± 3,490 | 8,621 ± 4,502 | 5,741 ± 3,178 | 6,574 ± 3,373 | 5,643 ± 3,178 | 6,543 ± 3,349 |
| (3) 30–64 (12) | 4,641 ± 1,170 | 5,414 ± 1,149 | 4,464 ± 1,094 | 5,784 ± 1,625 | 4,553 ± 2,057 | 5,565 ± 3,406 | 4,282 ± 1,704 | 5,003 ± 2,307 |
| (4) 65–95 (25) | 9299*(1−3) ± 6745 | 10,478*(2,3) ± 5215 | 11,142*(1−3) ± 6942 | 13,644*(1−3) ± 7683 | 7816*(1−3) ± 4588 | 10,888*(1,3) ± 5353 | 8584*(1−3) ± 5600 | 12,470*(1−3) ± 7049 |
| Regression analysis for all data | ||||||||
| R/R 2 adj | 0.27/.06 | 0.36/0.12 | 0.41/0.16 | 0.35/0.11 | 0.30/0.07 | 0.39/0.14 | 0.34/0.10 | 0.49/0.22 |
| B (age) t |
−152.26 −1.43 |
−205.26 −2.31* |
−138.90 −1.44 |
−230.58 −1.91 |
−88.16 −1.17 |
−193.20 −2.00* |
−156.61 −1.89 |
−247.75 −2.44* |
| B (age2) t |
2.00 1.04 |
2.59 2.84** |
2.14 2.17* |
3.04 2.47* |
1.28 1.66 |
2,60 2.63* |
2.05 1.33** |
3.41 3.29** |
| ANOVA (F) | 5.00** | 9.76*** | 13.42*** | 9.27 | 6.30** | 11.61*** | 8.76*** | 20.02*** |
| Inflection points (age) | 38 | 40* | 33 | 38 | 34 | 37* | 38 | 36** |
Post-hoc differences are presented for at least statistical level of *p < 0.05 and with the corresponding age groups indicated in the brackets (for the 4th group age with all other three age groups and for the first three age groups, only the differences between them)
Test condition: P proprioceptive only, PV proprioceptive+visual, ND and D non-dominant and dominant hands
Significance level: * p < 0.05, ** p < 0.01, *** p < 0.001; F(131, 2)
In the MANOVA analysis with Bonferroni correction for data split into four age subgroups, the oldest group (65–95) presented statistically significant differences with regard to the other three age subgroups in both precision and speed in all test conditions (Table 2). However, differences in precision were also found in the 12–17 and 18–29 age groups in frontal movement, D hand, and PV test (p = 0.038). In transversal movement type and ND hand but on the P test, the 12–17 age group’s precision presented statistically significant differences with regard to all three groups, being poorer than in the two middle-aged groups but better than in the oldest one. However, the 12–17 age group performed significantly worse on the ND hand and the PV test than the other ages. As regards transversal movement, in the PV test, all four groups presented statistically significant difference.
Hand symmetry in performance
The lowest correlations for coherent performance (symmetry of motor lateralization) of the dominant and non-dominant hands (Table 3) corresponded to the 12–17 age group, in which significant moderate correlations were obtained only under the P test condition. However, correlations for the performance of both hands under the P-test condition increased with age for both transversal and frontal movements, and ranged from 0.37 to 0.87 (Table 3). In addition, significant correlations were found for hand coherence under the PV-test condition in the age groups 18–29 (r = 0.31 and r = 0.54) for frontal movements and 30–64 (r = 0.45 and r = 0.84) for transversal movements. However, in the old group (65–95 years) the correlation was only significant (r = 0.46) for the frontal movement. The greatest symmetry between hand performance was found in the oldest group (65–95), except for the transversal movement under the PV-test condition, for which the maximum correlation (r = 0.84) was observed in the late middle-aged group (30–64 years), after which it declined (Table 3).
Table 3.
Paired correlations for precision and speed
| Age: | 12–17 | 18–29 | 30–64 | 65–95 | |
|---|---|---|---|---|---|
| Paired correlations (r) for precision between ND and D hands | |||||
| Movement type | Test condition | r | r | r | r |
| Frontal | P | 0.57*** | 0.76*** | 0.68*** | 0.87*** |
| PV | 0.10 | 0.31* | 0.45*** | 0.46* | |
| Transverse | P | 0.37** | 0.49*** | 0.68*** | 0.69*** |
| PV | 0.08 | 0.54*** | 0.84*** | 0.24 | |
| Paired correlations (r) for speed of ND and D hands | |||||
|---|---|---|---|---|---|
| Movement type | Test condition | r | r | r | r |
| Frontal | P | 0.94*** | 0.90*** | 0.97*** | 0.91*** |
| PV | 0.90*** | 0.95*** | 0.95*** | 0.94*** | |
| Transverse | P | 0.89*** | 0.78*** | 0.61*** | 0.82*** |
| PV | 0.51*** | 0.86*** | 0.72** | 0.85*** | |
| Age: | 12–17 | 18–29 | 30–64 | 65–95 | |
|---|---|---|---|---|---|
| Paired correlations (r) for precision between P and PV condition | |||||
| Movement type | Hand | r | r | r | r |
| Frontal | ND | 0.08 | 0.23 | 0.40*** | 0.28 |
| D | 0.53*** | 0.46*** | 0.38** | 0.21 | |
| Transverse | ND | 0.13 | 0.38** | 0.68*** | 0.23 |
| D | 0.11 | 0.37*** | 0.69*** | −0.44 | |
| Paired correlations (r) for speed between P and PV condition | |||||
|---|---|---|---|---|---|
| Movement type | Hand | r | r | r | r |
| Frontal | ND | 0.88*** | 0.85*** | 0.97*** | 0.86*** |
| D | 0.83*** | 0.89*** | 0.95*** | 0.77*** | |
| Transverse | ND | 0.68*** | 0.73*** | 0.90*** | 0.80*** |
| D | 0.80*** | 0.86*** | 0.81** | 0.75*** | |
ND non-dominant and D dominant (hand), P proprioceptive only and PV proprioceptive–visual (sensory condition)
Level of significance: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001
Paired t-test differences in speed between the PV and P conditions showed that performance was significantly slower in the PV condition, except for the non-dominant hand in transversal movement in the youngest (12–17) and oldest (65–95) age groups, in which the difference did not reach statistical significance. In fact, both movement types were associated with high and significant correlations between PV and P line length performance, with the highest peaks corresponding to the 30–64 age group (for both hands in frontal movements and for the non-dominant hand in transversal movements) and the 18–29 age group (for the dominant hand in the transversal movement). High correlations suggest that although proprioception plays a crucial role in the PV-test condition, the size of this influence depends on age (Table 3).
Regarding the symmetry of motor lateralization, the only group in which the performance speed did not differ significantly between ND and D hands in all test variables was the 30–64-year-old group. The only age group in which no significant differences were found for N and ND precision performances was the 65–95-year-old group. However, the difference between P test and PV test precision was significant for all observed variables (as per results of paired t test).
This is an exploratory study whose main aim was to report the details of fine motor behaviour in terms of age-dependent differences. For this reason the descriptive statistics are given in detail. The regression analysis revealed the best fit of the quadratic function and the inflection points showing the times of best performance. Moreover, the symmetry/asymmetry age-dependent features were described for different test conditions.
Discussion
Our results are consistent with the hypothesis of a shift to symmetrical hand performances with ageing. There were no statistically significant differences between dominant and non-dominant hands in the eldest group (65–95), which presented the highest correlations between the performances of both hands. Significant differences between D and ND hands were found mainly for the PV conditions: in the 30–64 age group the precision was higher in the ND hand in transversal movement, whereas in the rest of the cases, the opposite was true: the D hand was more precise for ages 30–64 and 18–29 in frontal movement. In the 12–17 age group (transversal movement), the D hand was found to be more precise in both the P- and PV-test conditions. The most significant differences between P and PV performance in the eldest group (65–95 years) may reflect the higher dissociation between performances in both sensory-type outputs. This can also be observed graphically, as the gap between the two lines (mean P and PV performances) increases with age.
The dominant hand only performed significantly faster in the 12–17 age group (transversal movement type, P-test condition). At 18–29 years, the situation was reversed in favour of the non-dominant hand (statistical significance was found only in transversal movement, P test). These findings (excluding the 12–17 age group) are consistent with the results of Stern et al. (1980), who showed that the right hemisphere (left hand) works faster. From 30 to 64 years, no significant difference was found in task performance speed, and at ages 65–95, the ND hand was faster than the D hand for most movement types (in transversal movement for both sensory conditions, P and PV, and in frontal movement in the P-test condition).
The hypothesis of the quadratic distribution of fine motor precision (line length performance) was confirmed in this sample and helped to calculate the approximate ages (for different test conditions) as the points of the highest fine motor performance. Therefore, the results can contribute to a better understanding of the developmental (maturation) and ageing process. For some movement types the ages were exactly the same or very similar (in frontal movements, for example: 23-23-23-26). Nevertheless, the fit to the regression line was not as good for the frontal movement (percentage of explained variance from 10 to 30 %) as for the transversal one (with higher percentages of explained variance, from 24 to 48 %). Inflection points corresponded to middle age, which was consistent with the results of the neurological brain maturity and motor precision studies described in the “Introduction” section.
This study has limitations that should be borne in mind. First, the health status of participants was self-reported and not checked by any other means (however, participants were not diagnosed with any neurological or motor impairment disease or any other illness; they were also asked about their medication intake). Moreover, the test itself is a kind of measure of neurological status, since participants started at the same point (the data were started to be recorded once the correct point had been reached, as stated in the instructions). Therefore, any severe problems or other qualitative changes in performance were observed in the proprioceptive part of the test (for example, loss of linearity). In our previous study with Parkinson’s patients (early stage of disease, medication on/state off), line length was only significantly greater in the dominant hand of male Parkinson group members for the frontal movement in the PV condition (Gironell et al. 2012). However, for the frontal movement in this study, the oldest groups drew shorter lines in the PV condition (35.97 ± 3.80 mm for the ND hand and 34.40 ± 3.74 mm for the D hand) and longer lines in the P condition than the model line length (40 mm) and the rest of the groups’ performances.
Second, since this was a cross-sectional study, the results highlighted the age-dependent differences in proprioception. Longitudinal studies are required to assess age-related changes; however, we can analyse differences in the performance of both hands due to hemispherical proliferation and differences between the two sensory conditions (P vs. PV). Another limitation is that we had fewer subjects in the older age groups. This is a question of time, since the test is applied individually and depends on the general willingness of volunteers (especially if they are healthy and still working). Nevertheless, this gap may be covered by future research. As life expectancy and retirement age vary in different countries, the information obtained by current research is more reliable for Spain and countries with indicators similar to those described above.
One important finding of this exploratory analysis was that in the more difficult sensory test condition (without visual guidance), age-related declines seem to set in earlier, and age-related differences become more pronounced. The ages identified as inflection points should be paid special attention and considered as periods of change, in order to prevent the risk of possible associated pathological states or crises. The ages found for the P condition can be related to the appearance of diseases like Parkinson’s. The inflection point for the PV condition (transversal movement) occurred at the ages of 45/48, which is consistent with the age at which vision starts to decline (50 years) (Sturnieksa et al. 2008). Moreover, these ages can be taken into account in age group comparisons (especially young vs. elder), in order to break down and interpret the results more accurately. The age-dependent polynomial can be split into two, which simplifies it into two linear regressions. The first one (up to the inflection point) may be related to developmental maturity and skill acquisition processes, and the second may indicate changes that can be attributed mainly to the natural ageing process and/or age-cohort differences.
If proprioception is crucial for automatic locomotor behaviour and spatial orientation, then its deterioration must be compensated by other senses, principally, by vision and cognition. In other words, more attention needs to be paid to controlling the action, which can indirectly affect cognitive performance. This may explain why cognitive exercises were found to have little or no effect on maintaining cognitive performance with ageing, and why people with professional training that involves cognitive functioning (such as pilots and architects) exhibited similar age-related trends to those who had no such training (Salthouse 2006). Moreover, sensory–sensorimotor variables were found to be statistically good predictors of age-based differences in general intelligence in the old population (Lindenberger and Baltes 1997). Another example of the effect of proprioception on cognition (distribution of attention), especially in dual-task performance, is reflected in studies by Ingram and colleagues (Ingram et al. 2000). It has also been reported that older adults need to invest more cognitive resources in sensorimotor performance, and so these resources are no longer available (or, at least, less available than in the case of a younger group for the execution of the secondary cognitive task) (Lövdén et al. 2005) and their balance (trunk angle variability) is affected (Shellenbach et al. 2010).
Thus, proprioception deterioration during ageing can also decrease cognitive task performance, due to the additional cognitive efforts required to maintain balance and gait. However, performance in the proprioceptive condition was more variable than in the task adjusted by vision, and this dispersion in performance also follows the quadratic function: it is greater in young and old people, who show higher variability in performance. Proprioception is important in maturation and ageing processes, and can influence the speed of individual progress in age-related development. It has also been found to be related to brain plasticity, which reflects the unity of body–mind states. For example, music positively affects proprioception and creates new nerve connections in the brain (Wan and Schlaug 2010). It is used in the recovery of neurological patients after strokes (Schauer and Mauritz 2003). Furthermore, music education has been found to be a precursor of higher intellectual preparation and performance in children (Glozman and Pavlov 2007). Moreover, Baltes and Lindenberger (1997) stress the importance of addressing cognitive ageing from the perspective of common factors for sensory and intellectual domains. Goble (2010) underlined a crucial role for proprioceptive feedback in the reorganization and subsequent recovery of the nervous system, and van Hedel and Dietz (2004) described the optimization of remaining proprioceptive inputs in the elderly. Finally, positive effects on balance (dynamic postural control) were reported in elderly individuals who regularly practiced low-energy proprioceptive physical activities such as soft gymnastics or yoga (Gauchard et al. 1999).
To sum up the main findings, this exploratory study shows the age-dependent differences in fine motor performance and contributes detailed information by reporting how performance of line length changes depending on age in different test conditions (movement type, hand or sensory input). Age-related differences in proprioception based on fine motor behaviour have not been widely studied to date. Regression analysis revealed the complex relationship of age-dependent differences to fine motor performance and helped to identify the inflection points of quadratic function. MANOVA analysis showed whether the age-related differences in performance were significant across four age groups and different test conditions. Finally, the correspondence between paired correlations and differences highlighted the symmetry/asymmetry between both hands and sensory test conditions.
These results could be the basis for further investigation to explore some of the questions that have arisen—for example, why is line length in old age underperformed in the PV test and overperformed in the P test? The identification of ages of change (as a peak of maturation and start of decline due to ageing processes) may shed light on other related developmental stages and on the mid-life crisis period.
Acknowledgments
We are grateful to all the participants, to the University of Barcelona for supporting this study, and to the UB Language Service for revising the English.
Conflict of interest
None.
Footnotes
The principal aim of the study was precision (i.e. to trace the line as accurately as possible). Participants were not instructed to perform the task quickly.
Contributor Information
Liudmila Liutsko, Phone: +34-93-312-51-08, FAX: +34-93-402-13-62, Email: lliutsko@ub.edu.
Josep Maria Tous-Ral, Email: jmtous@ub.edu.
References
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12–21. doi: 10.1037/0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall K, Finn JP, Villablanca P, Thomson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neuorobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL, Harner JG. Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci. 1999;19(8):3238–3247. doi: 10.1523/JNEUROSCI.19-08-03238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S. The lateral preference inventory for measurement of handedness, footedness, eyedness and earedness. Norms for young adults. Bull Psychonom Soc. 1993;31(1):1–3. doi: 10.3758/BF03334122. [DOI] [Google Scholar]
- Gauchard GC, Jeandel C, Tessier A, Perrin PP. Beneficial effect of proprioceptive physical activities on balance control in elderly human subjects. Neurosci Lett. 1999;273:81–84. doi: 10.1016/S0304-3940(99)00615-1. [DOI] [PubMed] [Google Scholar]
- Gironell A, Luitsko L, Muiños R, Tous JM. Differences based on fine motor behaviour in Parkinson’s patients compared to an age matched control group in proprioceptive and visuo-proprioceptive test conditions. Anuario de Psicología. 2012;42(2):183–197. [Google Scholar]
- Glozman JM, Pavlov AE. Bлияниe зaнятий мyзыкoй нa paзвитиe пpocтpaнcтвeнныx и кинeтичecкиx фyнкций y дeтeй млaдшeгo шкoльнoгo вoзpacтa [Effects of musical practice on development of spatial and kinetic functions in children of primary school age, In Russian] Пcиxoлoгичecкaя нayкa и oбpaзoвaниe [Psychological science and education] 2007;3:35–46. [Google Scholar]
- Goble D. Proprioceptive acuity assessment via joint position matching: from basic science to general practice. Phys Ther. 2010;90:1176–1184. doi: 10.2522/ptj.20090399. [DOI] [PubMed] [Google Scholar]
- Goble D, Coxona JP, Wenderotha N, Impea AV, Swinnena SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Behav Rev. 2009;33(3):271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergetic neurons is attenuated by DJ-1 (Letter) Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoch N, Debaere F, Peters R, Swinnena S. Neural basis of aging: the penetration cognition into action control. J Neurosci. 2005;25(29):6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MV, Rees J, Newham J. Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects. Age Ageing. 1998;27:55–62. doi: 10.1093/ageing/27.1.55. [DOI] [PubMed] [Google Scholar]
- Ingram HA, Donkelaar CJ, Vercher J-L, Gaithier GM, Miall RC. The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res. 2000;132:114–126. doi: 10.1007/s002219900322. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, Dinse HR. Age-Related attenuation of dominant hand superiority. PLoS One. 2006;1(1):e90. doi: 10.1371/journal.pone.0000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leversen JSR, Haga M, Sigmundsson H. From children to adults: motor performances across the life-span. PLoS One. 2012;7(6):e38830. doi: 10.1371/journal.pone.0038830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-I, Woollacott M. Association between sensorimotor function and functional and reactive balance control in the elderly. Age Ageing. 2005;34:358–363. doi: 10.1093/ageing/afi089. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12(3):410–432. doi: 10.1037/0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Liutsko L (2013, in press) Age and sex differences in proprioception based on fine motor precision. PhD dissertation (directed by Tous, J.M.), University of Barcelona, Barcelona
- Liutsko L, Tous JM, Muiños R. The effects of proprioception on memory: a study of proprioceptive errors and results from the Rey-Osterrieth Complex Figure in a healthy population. Acta Neuropsychologica. 2012;10(4):489–497. [Google Scholar]
- Liutsko L, Muiños R, Tous J (2012) Relación entre inteligencia emocional basada en la información propioceptiva y rendimiento académico en alumnos de secundaria. [Relationship between emotional intelligence based on the proprioceptive information and academic performance in pupils of the secondary school]. In: Libro de abstracts [Book of the abstracts], I Congreso Nacional de Inteligencia Emocional [The I National Congress of Emotional Intelligence], 8-10 of November, Barcelona
- Lövdén M, Schellenbach M, Grossman-Hutter B, Krüger A, Lindenberger U. Environmental topography and postural control demands shape aging-associated decrements in spatial navigation performance. Psychol Aging. 2005;20:683–694. doi: 10.1037/0882-7974.20.4.683. [DOI] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people with mild cognitive impairment . Cochrane database Syst Rev. 2011;1:CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- Mira E. Myokinetic psychodiagnosis. (M. K. P.) New York: Logos; 1958. [Google Scholar]
- Mongeon D, Blanchet P, Messier J. Impact of Parkinson’s disease and dopaminergic medication on proprioceptive processing. Neuroscience. 2009;158(2):426–440. doi: 10.1016/j.neuroscience.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Muiños R (2008) El psicodiagnóstico miokinético: desarrollo, descripción y análisis factorial confirmatorio [thesis in Spanish, abstract in English], UB, Barcelona
- Przybyla A, Haaland K, Bagesteiro LB, Sainburg R. Motor asymmetry reduction in older adults. Neurosci Lett. 2011;489(2):99–104. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro F, Oliveira J. Aging effects on joint proprioception: the role of physical activity in proprioception preservation. Eur Rev Aging Phys Act. 2007;4:71–76. doi: 10.1007/s11556-007-0026-x. [DOI] [Google Scholar]
- Rosenbaum DA. The Cinderella of psychology. The neglect of motor control in the science of mental life and behavior. Am Psychol. 2005;60(4):308–317. doi: 10.1037/0003-066X.60.4.308. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and mental health aging: evaluating the validity of the “use it or lose it” hypothesis. Perspect Psychol Sci. 2006;1(1):68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Schauer M, Mauritz K-H. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin Rehabil. 2003;17(7):713–722. doi: 10.1191/0269215503cr668oa. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AI. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Shellenbach M, Lövdén M, Verrel J, Krüger A, Linderberger U. Sensoriomotor-cognitive couplings in the context of assistive spatial navigation for older adults. GeroPsych. 2010;23:69–77. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical changes across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stern J, Oster P, Newport K (1980) Reaction time measures, hemispheric specialization, and age. In: Poon L (ed) Aging in the 1980s: Psychological issues. Washington, DC: American Psychological Association, pp 309–326
- Sturnieksa DL, St Georgea R, Lord SR. Balance disorders in the elderly. Clin Neurophysiol. 2008;38(6):467–478. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Teasdale, N. (2012). Movement and balance control limitations related to obesity. In: Slomka K, Juras G (eds) International Scientific Conference Motor Control 2012—From Theories to Clinical Applications, 27–29 September, 2012, Wisla, Poland, p 19
- Teasdale N, Simoneaub M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture. 2001;14(3):203–210. doi: 10.1016/S0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Tous Ral JM, Muiños R, Tous O, Tous Rovirosa JM. Diagnóstico propioceptivo del temperamento y el carácter. Barcelona: Universidad de Barcelona; 2012. [Google Scholar]
- Tous JM, Viadé A. Advances in MKP-R. Psicologia em Revista. 2002;8(12):95–110. [Google Scholar]
- Tous JM, Viadé A, Muiños R. Validez estructural de los lineogramas del psicodiagnóstico miokinético, revisado y digitalizado (PMK-RD) Psicothema. 2007;19(2):350–356. [PubMed] [Google Scholar]
- Tous-Ral JM, Muiños R, Liutsko L, Forero CG. Effects of sensory information, movement direction and hand use on fine motor precision. Percept Mot Skills. 2012;115(1):261–272. doi: 10.2466/25.22.24.PMS.115.4.261-272. [DOI] [PubMed] [Google Scholar]
- van Hedel HJA, Dietz V. The influence of age on learning a locomotor task. Clin Neurophysiol. 2004;115(9):2134–2143. doi: 10.1016/j.clinph.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C. Motor-skill learning in older adults—a review of studies on age-related differences. Eur Rev Aging Phys Activity. 2008;5(1):5–16. doi: 10.1007/s11556-008-0030-9. [DOI] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16(5):566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis LM, Shukitt-Hale B, Joseph JA. Modulation of cognition and behaviour in aged animals: role for antioxidant- and essential fatty acid-rich plant foods. Am J Clin Nutr. 2009;89(5):1602S–1606S. doi: 10.3945/ajcn.2009.26736J. [DOI] [PubMed] [Google Scholar]
- Wright VJ, Perricelli B. Age-related rates of decline in performance among elite senior athletes. Am J Sports Med. 2008;36(3):443–450. doi: 10.1177/0363546507309673. [DOI] [PubMed] [Google Scholar]
- Yan JH. Effects of aging on linear and curvilinear aiming arm movements. Exp Aging Res. 2000;26:393–407. doi: 10.1080/036107300750015778. [DOI] [PubMed] [Google Scholar]


