Abstract
An approximate 140-fold purification of the A1, adenosine receptor of bovine cerebral cortex has been obtained via affinity chromatography. The affinity column consists of Affi-Gel 10 coupled through an amide linkage to XAC, a high-affinity A1, adenosine receptor antagonist. As assessed by [3H]XAC binding, bovine brain membranes solubilized with the detergent CHAPS had a specific binding activity of 1.1 pmol/mg protein. Interaction of solubilized A1, adenosine receptors with the XAC-Affi-Gel was biospecific and 30% of the receptor activity was bound by the gel. Demonstration of [3H]XAC binding in the material eluted from the column with R-PIA required insertion of receptor into phospholipid vesicles. The specific activity of the affinity column purified receptor was 146 ± 22 pmol/mg protein with typically 5–15% of the bound receptor recovered. The purified receptor displayed high-affinity antagonist binding and bound agonists with the potency order expected of the bovine brain A1, adenosine receptor: R-PIA > S-PIA > NECA. In purified preparations, the photoaffinity probe [125I]PAPAXAC-SANPAH specifically labelled a protein of molecular mass 38 000 which has previously been shown to be the A1, adenosine receptor binding subunit.
Keywords: Adenosine receptor, Affinity chromatography
1. INTRODUCTION
Adenosine produces a variety of effects in the cardiovascular, central nervous, pulmonary and immunologic systems [1,2]. Many of the effects of adenosine occur as the result of its interaction with a specific cell surface receptor termed the A1 adenosine receptor (A1AR). The A1AR binding subunit in bovine brain and rat brain and fat is a glycoprotein of Mr, 38 000 [3,4]. Molecular properties of the A1AR, including its ligand binding activity and interaction with the associated G protein, have begun to be studied in solubilized preparations [5–8]. Complete determination of the receptor’s structure as well as a more complete characterization of its function at a molecular level requires its purification. This report describes the synthesis and use of a biospecific affinity column to obtain substantial purification of the AlAR from solubilized bovine cerebral cortex membranes. The affinity column consists of Affi-Gel 10 coupled through an amide linkage to XAC, a selective and high-affinity A1 adenosine receptor antagonist [9].
2. MATERIALS AND METHODS
2.1. Synthesis of XAC-Affi-Gel
Affi-Gel 10 (10 ml) was washed with 250 ml of DMSO over 15 min. The slurry was added to 20 ml of a 75% DMSO/25% ethanol solution containing 5 mg of XAC and incubated at 22°C for 15 h. The gel was washed extensively with DMSO to remove uncoupled XAC and the wash solution was gradually increased to 100% H2O. Finally, the gel was washed with 200 mM Tris-acetate, pH 8.0, for 24 h at 5°C to block any remaining free ester groups.
2.2. Membrane solubilization
Bovine cerebral cortex membranes prepared as previously described [7] were washed with 50 mM Tris, 5 mM EDTA, pH 8.26, at 5°C and then treated with adenosine deaminase (10 U/ml) for 30 min at 37°C. Membranes were pelleted by centrifugation and resuspended at a detergent/protein ratio of ~ 2.5/l in 1% CHAPS, 20 mM Tris, 5 mM EDTA, and 100 mM NaCI, pH 7.4, at 5°C. The mixture was dounce homogenized with 10 strokes and stirred on ice for 30 min followed by centrifugation at 105 000 × g for 40 min. The supernatant was taken as the soluble fraction and diluted with an equal volume of 50 mM Tris, 1 mM EDTA, and 125 mM NaCl, pH 7.4, at 5°C containing 20 µM Gpp(NH)p.
2.3. Affinity chromatography
Prior to a chromatography cycle, the XAC-Affi-Gel was equilibrated with 0.33% CHAPS, 50 mM Tris, 1 mM EDTA, and 125 mM NaCl, pH 7.4, at 5°C (Buffer A). Typically, 100–200 ml of solubilized membranes (100–200 pmol of receptor) were incubated with approximately 4 ml of XAC-Affi-Gel either batchwise with slow rotation or by recycling at a flow rate of 30 ml/h. For either method, incubation was conducted at 5°C for 10 h. The soluble preparation was then removed and the gel washed batchwise with 15 ml of Buffer A for 5 min. This wash buffer was removed and the gel then washed with Buffer A at a flow rate of 30 ml/h for 45 min. All washing was performed at 5°C. The gel was then brought to room temperature for elution. The elution buffer consisted of 0.05% L-α-phosphatidyl-choline suspended via sonication in 30 mM Hepes, 5 mM MgCl2, and 100 mM NaCl diluted with an equal volume of 0.66% CHAPS, 50 mM Tris, 10 mM MgCl2, and 250 mM NaCl. R-PIA was added to give a final concentration of 2 µM and the buffer was made pH 6.8 at 22°C. The elution buffer was applied to the gel at 10 ml/h and the eluate collected over the first 10 min was discarded. Eluted material was collected on ice over the next 65 min and immediately desalted at 5°C on Sephadex G50 columns preequilibrated with 30 mM Hepes, 5 mM MgCl2, and 100 mM NaCl, pH 7.4.
2.4. Radioligand binding assays
Binding assays in soluble preparations were performed as previously described [7]. Saturation assays of affinity chromatography-purified receptor consisted of 150 µl of phospholipid reconstituted material, 50 µl of [3H] XAC (0.2–2.0 nM) and 50 µl of H2O or 10 µM R-PIA to define non-specific binding. Gpp(NH)p was present in all assay tubes at a concentration of 10 µM. In competition binding experiments, the assay consisted of 150 µl of receptor preparation, 50 µl of 0.5 nM [3H]XAC and 50 µl of competitor. All incubations were for 2 h at 22°C and terminated by washing with three 3 ml aliquots of 0.01% CHAPS in 50 mM Tris, 10 mM MgCl2, and 1 mM EDTA with rapid vacuum filtration over polyethylenimine-treated glass fiber filters. Saturation and competition curves were analyzed using a nonlinear least-squares curve fitting technique with statistical analysis as previously described [10].
2.5. Photoaffinity labelling
[125I]PAPAXAC-SANPAH was synthesized as described [11]. Labelling was performed by incubating 1 ml of reconstituted affinity column eluate with 0.8 nM [125I]PAPAXAC-SANPAH for 45 min at 25°C. The mixture was diluted to 3 ml with 30 mM Hepes, 5 mM MgCl2, and 100 mM NaCl, pH 7.4, and exposed to UV light for 3 min from a model UVCG-25 mineral lamp. The mixture was then centrifuged at 105 000 × g for 1 h. The pellet was solubilized in SDS buffer and subjected to polyacrylamide gel electrophoresis and auto-radiography as previously described [11].
2.6. Protein assays
All protein concentrations were determined by the method of Bradford [12] with the exception of the affinity column-purified material which was analyzed by either the aminoschwarz method of Schaffner and Weismann [13] or the Bradford assay.
3. RESULTS
Solubilization conditions were optimized to ensure the maximal efficiency of solubilization and stability of the A1AR. We tested a variety of conditions to enhance [3H]XAC binding and found that elimination of MgCl2 and the addition of NaCl and Gpp(NH)p enhanced solubilization and stabilization of the receptor. As determined with [3H]XAC binding, maximum binding capacity of the solubilized membranes (without Gpp(NH)p and with MgCl2) was ~ 1 pmol/mg protein, however, this value declined by approximately 50% after 16 h storage at 5°C. It was then determined that the exclusion of MgCl2 and the addition of Gpp(NH)p to the postsolubilization diluting buffer, not only increases 13H]XAC binding, but stabilizes the solubilized receptor so that the Bmax value declines by less than 10% after storage at 5°C for 16 h. Using these conditions, approximately 50–60% of the membrane receptor is solubilized.
Maximum binding of the A1AR to the XAC-Affi-Gel occurs after approximately 10 h of incubation at 5°C. At this time, approximately 30% of the applied A1ARs adsorb to the XAC-Affi-Gel while less than 5% of the total protein is retained on the column. Both the length of time and the volume of the wash buffer were found to be important. Less washing decreased fold purification, while more prolonged washing resulted in decreased yield.
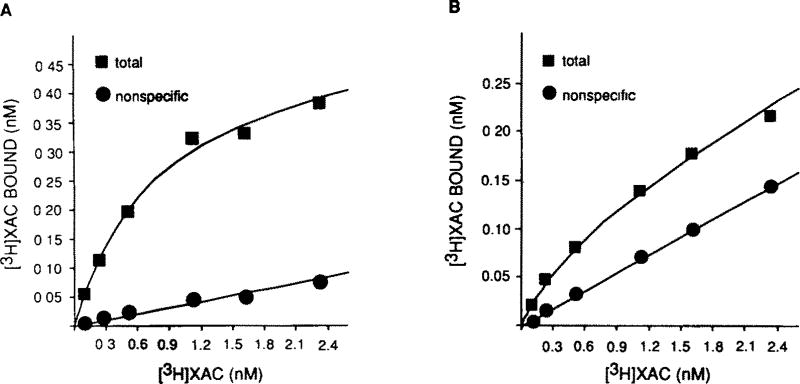
The results of a typical purification procedure with XAC-Affi-Gel are presented in fig. 1. In 5 experiments, an average specific activity of 146 ± 22 pmol/mg protein was obtained for XAC-Affi-Gel-purified receptor. This represents an approximate 140-fold purification of the A1AR from the starting solubilized material (Bmax = 1.10 ± 0.09 pmol/mg). For both the purified and solubilized receptor, [3H]XAC binding is saturable and of high affinity. KD values are 0.31 ± 0.02 nM and 0.53 ± 0.05 nM for solubilized membranes and purified receptor, respectively. In solubilized membranes, at [3H]XAC concentrations slightly above the KD, non-specific binding represents approximately 10% of total binding. This value is 25–30% in the reconstituted material with the increase most likely attributable to the presence of phospholipids. The Bmax value calculated for the purified product is likely an underestimation since the efficiency of insertion of proteins into phospholipid vesicles is likely less than 100%. Inclusion of phospholipids in the elution buffer and subsequent insertion of receptors into vesicles is required for A1AR activity as assessed by [3H]XAC binding. Most of the A1AR activity was eluted between 10 and 65 min and hence these aliquots were pooled for all subsequent assays. The interaction of the receptor with XAC-Affi-Gel is biospecific as no [3H]XAC binding activity can be recovered if R-PIA is omitted from the phospholipid elution buffer or if the solubilized preparation is applied to Affigel 10 or Sepharose 6B without XAC coupled to it. A1AR activity comparable to that presented in fig. 1 can be obtained if theophylline (5 mM), an adenosine receptor antagonist, is used as a counter-ligand during elution (data not shown). Typically, 5–15% of the receptor activity bound to the affinity column is recovered in the eluate. The yield of affinity-purified receptor as calculated from starting solubilized membranes is therefore approximately 3%.
Fig.1.
[3H]XAC saturation curves in (A) solubilized bovine brain membranes and (B) XAC-Affi-Gel-purified preparation. Membrane solubilization, affinity chromatography and binding assays were performed as described in section 2. Non-specific binding was defined with 10 µM R-PIA or 5 mM theophylline. Gpp(NH)p (10 µM) was present in all assay tubes. These plots are representative of 5 similar experiments.
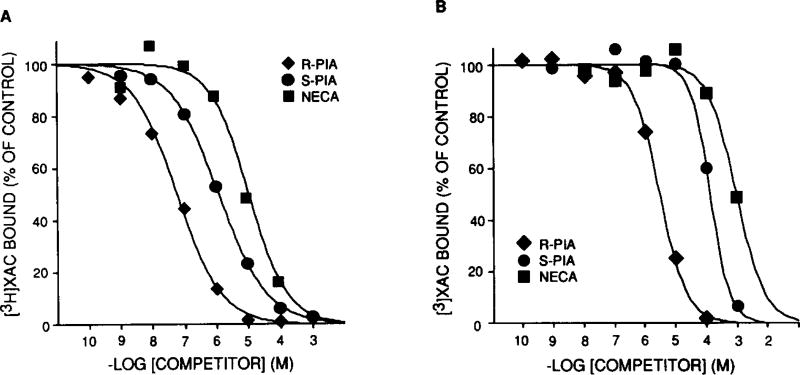
To demonstrate that the purified receptor possesses all the characteristics expected of the A1AR, we fully characterized the affinity-purified preparation. First, antagonist saturation curves demonstrate high affinity, saturable binding with no change in KD compared to solubilized A1AR. Second, in purified preparations, agonists compete for [3H]XAC binding in the order of R-PIA > S-PIA > NECA which is as expected for the bovine cerebral cortex A1AR [4]. The results of a representative competition binding experiment in solubilized membranes and purified preparation are shown in fig. 2. For the experiment shown, the IC50 values of R-PIA, S-PIA and NECA are increased by 52-, 90- and 124-fold, respectively, in purified as compared to starting material. This decrease in affinity is agonist specific and has been observed in other receptor systems following purification [14]. The A1AR antagonist, XCC, displayed a similar affinity in solubilized and partially purified material (data not shown).
Fig.2.
Agonist competition for [3H]XAC binding in (A) solubilized bovine brain membranes and (B) XAC-Affi-Gel-purified preparation. Membrane solubilization, affinity chromatography and binding assays were performed as described in section 2. The final concentration of [3H]XAC was ~ 0.5 nM in both assays. These plots are representative of 3 similar experiments.
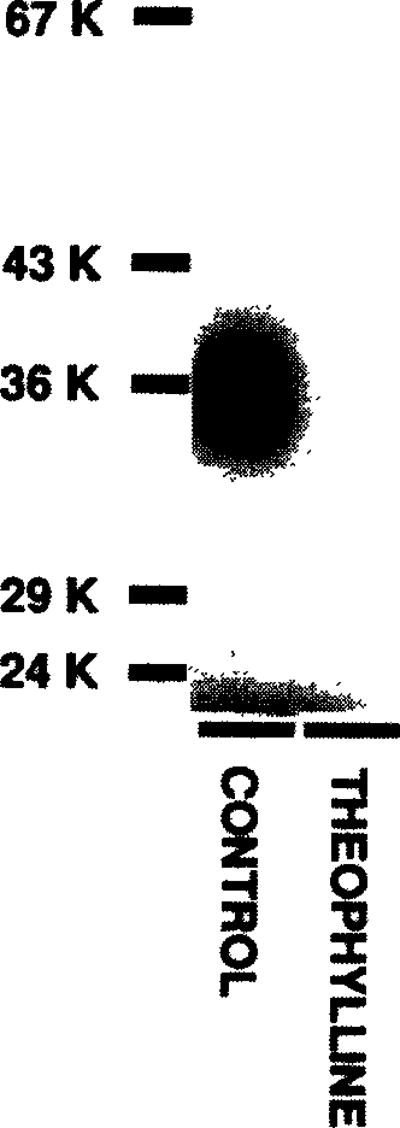
Finally, to ensure that we had purified the same A1AR binding subunit as that present in membranes, we photoaffinity-labelled the purified A1AR with [125I]-PAPAXAC-SANPAH (an A1AR selective photoaffinity probe) [4, 11]. As shown in fig. 3, the same 38 000 Da protein is specifically labelled in the affinity-purified preparation as that previously demonstrated [4,11].
Fig.3.
Photoaffinity labelling of XAC-Affi-Gel-purified A1 adenosine receptor with [125I]PAPAXAC-SANPAH. Chromatography and photoaffinity labelling were performed as described in section 2. Labelling was performed in the absence (control) or presence of 5 mM theophylline. Following labelling, the sample was solubilized in SDS and electrophoresed on a 12% acrylamide gel. The positions of iodinated molecular mass standards are shown to the left.
4. DISCUSSION
In this report we describe the synthesis and use of XAC-Affi-Gel to obtain an approximate 140-fold purification of the A1AR of bovine cerebral cortex. Through the exclusion of MgCl2 during the solubilization and subsequent treatment of the preparation with Gpp(NH)p, we have solubilized the A1AR in a state which binds antagonists with high affinity and is stable over the time required for completion of the affinity chromatography procedure. This procedure was required in that, in the presence of MgCl2 and the absence of guanine nucleotides, the solubilized receptor was rather labile as assessed by radioligand binding. A similar finding has been reported by others [15].
The interaction of the A1AR with the XAC-Affi-Gel is biospecific as the receptor does not bind to the support matrix itself and the presence of R-PIA as a counterligand is required to obtain receptor activity in the gel eluate. A1AR binding activity is evident in the XAC-Affi-Gel product only under the elution conditions described. Phospholipids must be present in the elution buffer and the eluate must be desalted to permit the removal of CHAPS and insertion of the receptor into phospholipid vesicles. A similar requirement for phospholipid reconstitution procedure was reported for the purification of the D2-dopamine receptor via affinity chromatography [16]. It is not known if the phospholipids are necessary for the removal of the receptor from the gel, the stabilization of the receptor once it is dissociated from the gel, or if they act to allow the receptor to assume the conformation necessary to demonstrate [3H]XAC binding.
The affinity-purified receptor possesses the characteristics expected of the A1AR. The binding of antagonists displays similar properties in both solubilized membranes and the affinity column eluate. Furthermore, adenosine analogs bind to the partially purified preparation in the order expected for bovine brain A1AR [4]. Finally, as it does in membranes, the photo-affinity probe [125I]PAPAXAC-SANPAH specifically labels a protein of Mr 38 000 in the affinity column eluate. This protein is the A1AR binding subunit [4,11].
The fold purification of the A1AR we report is appreciable, however, the yield remains less than 10%. A loss in the yield may in part be attributable to the desalting procedure which follows elution as well as the less than 100% efficiency of insertion of receptors into phospholipid vesicles. It is possible that a population of receptors are eluted from the gel but are in a conformation which is no longer recognized by [3H]XAC. Receptor degradation during the procedure could also diminish the recovery of receptors. However, the use of several protease inhibitors or the stabilizing agent, glycerol, during the solubilization and chromatography steps provided no improvements in the yield of receptors.
The theoretical specific activity of the A1AR, based on a molecular mass of 38 000 Da for the binding subunit [3,4] is 26 nmol/mg. Therefore, a further 150–200-fold purification of the affinity column-purified receptor we have obtained is required for a homogeneous preparation. Techniques such as wheat germ agglutinin chromatography and high-performance steric exclusion liquid chromatography may permit for the complete purification of the A1AR thus permitting the determination of its structure and further study of the molecular mechanisms involved in its signal transduction activity.
Acknowledgments
G.L.S. is an Established Investigator of the American Heart Association and is supported in part by Grant RO1HL-35134 from the National Heart, Lung and Blood Institute and a grant-in-aid from the American Heart Association and 3M Riker.
Abbreviations
- A1AR
A1 adenosine receptor
- G protein
guanine nucleotide-binding protein
- XAC
xanthine amine congener
- DMSO
dimethyl sulfoxide
- CHAPS
3-[(3-cholamidopropyI)dimethylam-monio]-1-propanesulfonate
- Gpp(NH)p
guanyl-5-yl-(β,γ-imido)di-phosphate
- R-PIA
N6-R-phenyl-2-propyladenosine
- S-PIA
N6-S-1-phenyl-2-propyladenosine
- NECA
N-ethyl adenosine-5′-uronic acid
- XCC
xanthine carboxylic acid congener
- PAPAXAC
8-[4-[[[[[2-(4-aminophenylacetylamino)ethyl]carbonyl]methyl]oxy]phenyl]-l,3-di-propylxanthine
- SANPAH
N-succinimidyl 6-(4′-azido-2′-nitro-phenylamine)hexanoate
References
- 1.Ramkumar V, Pierson G, Stiles GL. In: Progress in Drug Research. Jucker E, editor. Vol. 32. Birkhauer, Basel; 1988. pp. 195–247. [DOI] [PubMed] [Google Scholar]
- 2.Williams M. Annu. Rev. Pharmacol. Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- 3.Stiles GL. J. Biol. Chem. 1986;261:10839–10843. [PubMed] [Google Scholar]
- 4.Stiles GL, Jacobson KA. Mol. Pharmacol. 1987;32:184–188. [PMC free article] [PubMed] [Google Scholar]
- 5.Gavish M, Goodman RR, Snyder SH. Science. 1983;215:1633–1635. doi: 10.1126/science.6280275. [DOI] [PubMed] [Google Scholar]
- 6.Stiles GL. J. Biol. Chem. 1985;260:6728–6732. [PubMed] [Google Scholar]
- 7.Stiles GL. J. Neurochem. 1988;51:1592–1598. doi: 10.1111/j.1471-4159.1988.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 8.Klotz KN, Lohse MJ, Schwabe U. J. Neurochem. 1986;46:1528–1534. doi: 10.1111/j.1471-4159.1986.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson KA, Ukena D, Kirk KL, Daly JW. Proc. Natl. Acad. Sci. USA. 1986;83:4087–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLean A, Hancock AA, Lefkowitz RJ. Mol. Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- 11.Barrington WW, Jacobson KA, Stiles GL. J. Biol. Chem. 1989;264:13157–13164. [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Schaffner W, Weissman C. Anal. Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 14.Gingrich MA, Amlaiky N, Senogles SE, Chang WK, McQuade RD, Berger JG, Caron MG. Biochemistry. 1988;27:3907–3912. doi: 10.1021/bi00411a003. [DOI] [PubMed] [Google Scholar]
- 15.Helmke SM, Cooper DMF. Biochem. J. 1989;257:413–418. doi: 10.1042/bj2570413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senogles SE, Amlaiky N, Falardeau P, Caron MG. J. Biol. Chem. 1988;263:18996–19002. [PubMed] [Google Scholar]