Abstract
Despite the availability of PARP inhibitors for cancer therapy, a biomarker to clearly stratify patients for selection of this treatment remains lacking. Here we describe a radiotracer-based method that addresses this issue, using the novel compound [125I]KX1 as a PARP-1–selective radiotracer that can accurately measure PARP-1 expression in vitro and in vivo. The pharmacologic properties of the PARP radiotracer [125I]KX1 was characterized in multiple cell lines where single-agent sensitivity was correlated with [125I]KX1 binding to PARP-1. In vivo evaluation of [125I]KX1 verified in vitro results, validating PARP radiotracers to define PARP-1 enzyme expression as an in vivo biomarker. Notably, PARP-1 expression as quantified by [125I]KX1 correlated positively with the cytotoxic sensitivity of cell lines evaluated with PARP inhibitors. Overall, our results defined a novel technology with the potential to serve as a companion diagnostic to identify patients most likely to respond therapeutically to a PARP inhibitor.
Introduction
PARP-1 is the primary molecular therapeutic target for PARP inhibitor (PARPi) therapy; however, PARP-1 target enzyme expression is not part of the indication for patients to receive PARPi therapy. The cancer therapy field has focused primarily on downstream pathways that involve PARP-1 as a key mediator of DNA damage response in homologous recombination–deficient (HRD) cancers (1). There is a strong set of preclinical data to support this mechanistic approach to identifying cancers that will best respond to PARPi therapy (2–4). In support, a retrospective analysis of patients with ovarian cancer treated with olaparib showed patients who expressed HRD had better response rates than the group of patients that did not (5–7). This led to the first PARPi, olaparib, to receive FDA approval for the treatment of advanced ovarian cancer with BRCA mutations. Most recently, olaparib has been granted breakthrough designation by the FDA for the treatment of metastatic-resistant prostate cancer with BRCA or BRCA-like mutations (8). While genetic profiling of cancer subtypes is important, the phenotypic characteristics play an equally critical role in determining patient response to therapy. In the case of PARPis for cancer therapy, the target density defined by PARP-1 enzyme expression cannot be left out of consideration.
To date, there has never been a clinical trial with PARPi that evaluated the molecular subtyping of PARP-1 in cancer, despite PARP-1 being the primary molecular therapeutic target. It has been shown that cancers with mutations in critical DNA repair genes are synthetically lethal to PARPis (9, 10). What has not been shown is why 30% to 70% of patients that harbor mutations within DNA-repair genes do not respond to PARPi therapy (11). Nonresponders have been grouped together as a population with multiple resistant mechanisms, including loss of PARP-1 expression. Indeed, loss of PARP-1 significantly reduces PARPi toxicity to cultured cells and is relative to PARPi potency (12–14). The discovery of new genes related to synthetic lethality will aid in preselecting patients who could benefit from PARPi therapy; however, PARP-1 expression may be the singular most important determinant of patient response. It is therefore imperative to develop a quantitative and reliable means to measure PARP-1 expression in vivo.
Current modalities of measuring PARP-1 expression rely largely on immunohistochemical techniques that are difficult to reproduce and low yielding in biologic information relevant to the patient’s current clinical picture (15). In addition, biopsies are invasive and inherently restrict the ability to evaluate the entire properties of a tumor due to heterogeneity and sampling error. Furthermore, some tumor sites may be difficult or impossible to access, such as with metastatic disease. Finally, baseline biopsies are generally not repeated during therapy to allow for direct assessment of drug efficacy. Alternative approaches to assessing PARP include noninvasive PET and single-photon emission tomography (16–18). These reports consist of in vitro and in vivo proof-of-concept studies showing the basic capabilities of radiotracers to image PARP. To the best of our knowledge, there has not been a comprehensive study that (i) evaluates what PARP radiotracers are actually measuring (i.e., protein expression level or enzyme activity), and (ii) if PARP radiotracers can predict response to PARPi therapy.
We hypothesize that measuring PARP-1 enzyme expression can aid in predicting outcomes in patients who receive PARPi therapy. To test this hypothesis, we studied PARP-1 expression and activity, as well as their sensitivity to single-agent PARP inhibition in vitro. In this study, we define poly ADP-ribose (PAR), the biochemical product of PARP catalysis of NAD+ substrates, as a measure of PARP activity (19–21). In parallel, we investigated the utility of a novel radio-iodinated PARP-1 radiotracer, [125I]KX1, from a pharmacologic perspective in live biologic systems. This work provides a foundation for mechanistically understanding the utility of PARP-1 radiotracers in clinical translation.
Materials and Methods
Chemistry and radiochemistry
A detailed procedure for chemical and radiochemical synthesis is provided in Supplementary Information S1.
Cell culture
SKOV3, HCC1937, MDA-MB-231, UWB1.289, and UWB1.289-BRCA1 (isogenic pair to UWB1.289 with BRCA1 restored) cell lines were acquired from ATCC, whereas SNU-251 and isogenic MEF cell lines were acquired from the Basser Center for BRCA at the University of Pennsylvania. HCC1937, SKOV3, and SNU-251 cells were cultured in RPMI with 1% glutamax, 1% penicillin/streptomycin, and 10% FBS. Mouse embryonic fibroblast (MEF) cell lines were cultured in DMEM with 1% penicillin/streptomycin and 10% FBS. MDA-MB-231 cells were cultured in MEM with 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, and 10% FBS. UWB1.289 and UWB1.289-BRCA1 cells were cultured with 1:1 RPMI:MEGM with bullet kit and added G418 for selection in UWB1.289-BRCA1. All cells were cultured at 37°C with 5% CO2 and 15% O2. The gene profile of cell lines used in this study is described in the Supplementary Table S1. Cell line authentication was not performed for this study.
Competitive inhibition
SNU-251, SKOV3, HCC1937, or MDA-MB-231 cells were plated in 96-well format at 50,000 cells/well 24 hours before the assay. Plates were coincubated with 0.05 to 0.1 nmol/L [125I]KX1 and solutions of nonradioactive KX1, talazoparib, or olaparib at concentrations of 250 to 0.12 nmol/L. Reactions were allowed to incubate until equilibrium was reached at 1 hour and then solutions were removed, washed with 200 μL of PBS, and assayed for radioactivity on a Perkin Elmer Wizard gamma counter. Competitive inhibition curves were plotted using a nonlinear fit one-site Ki, Prism Version 6.0 GraphPad and competitive inhibition constants (Ki) were calculated.
Kinetic analysis
SNU-251, SKOV3, HCC1937, or MDA-MB-231 cells were plated in 96-well format at 50,000 cells/well 24 hours before the assay. Association and dissociation experiments were carried out using each cell line. On the day of the experiment solutions containing 3 varying concentrations of 0.625, 1.25, 2.5 nmol/L [125I]KX1 were prepared in RPMI and each solution represented a single concentration at which the rate of association was measured. At time points from 1 minute to 1 hour, each concentration of [125I]KX1 was separately added to the plate in quadruplicate. Dissociation experiments were performed by first incubating cells with a single concentration of [125I]KX1 (2.5 nmol/L), then at time points from 1 minute to 1 hour, 10 μmol/L olaparib was added to competitively force dissociation of [125I]KX1. Next, the plate was washed and radioactivity was assayed in each well as previously described in competitive inhibition experiments. Association and dissociation curves were graphed using a nonlinear fit association kinetics—two or more concentrations of hot ligand and one phase exponential decay (GraphPad Prism version 6.0).
PARP specificity
To evaluate the specificity of [125I]KX1, MEF WT, MEF PARP1 KO−/− and MEF PARP2 KO−/− cells were used. We chose this isogenic model for testing the specificity of [125I]KX1 due to the highly conserved structure of the PARP catalytic domain (CAD) across species including human and mouse (22). Within the PARP CAD is the NAD+-binding site where PARPis are believed to competitively inhibit NAD+ catalysis. MEF cell lines were harvested from double-knockout mice that were produced through genetic cross breading of PARP1/PARP2 heterozygously null mice. Cells were plated as described previously, and [125I]KX1 was added at varying concentrations (0.25–4 nmol/L) and was allowed to reach equilibrium for 1 hour. Plates were washed and radioactivity was measured as described in previous competitive inhibition experiments. Control wells were used for protein quantification by Lowry method. Data were plotted using a nonlinear one-site binding hyperbola, and dissociation constant (Kd) and maximum number of binding sites (Bmax) were generated using Prism Version 6.0.
Quantifying PARP-1 in vitro
Saturation experiments were carried out in SNU-251, SKOV3, HCC1937, MDA-MB-231, UWB1.289, and UWB1.289-BRCA1. These cell lines were chosen due to their respective BRCA1 mutations (SNU-251, UWB1.289, HCC1937) or wild-type (WT; SKOV3, UWB1.289-BRCA1, MDA-MB-231) control. Experiments were performed as described above in PARP specificity assays.
Western blot analysis
For more details, see Supplementary Information S2 for Western blot analysis procedure. Protein expression was determined for PARP-1, PAR, and Histone H3 using the following commercial antibodies. Anti-PARP 1 (PARP 46D11 rabbit mAb, Cell Signaling Technology), anti-PAR (Trevigen) or anti-Histone H3 (Histone H3 antibody #9715, Cell Signaling Technology). Histone H3 was used as a loading control and it was assumed to have equal levels constitutively expressed in all cell lines evaluated. All antibodies were cross-reactive between human and mouse. PARP-1 antibody was verified in our laboratory to be specific for PARP-1 over PARP-2 or PARP-3. Bands were imaged on LiCOR imager and regions of interest (ROI) were drawn allowing for total fluorescent signal to be quantified. See Supplementary Information S2 for ROI generation.
PARPi growth inhibition assays
Cell lines were seeded at a density of 1,000 cells/well in a 96-well black wall–coated plate 24 hours before treatment. Cells were then treated with concentrations varying from 100 μmol/L to 0.02 pmol/L for 7 days. Cell viability was assayed using Cell-Titer Glo (Promega), a chemiluminescent assay that measures ATP. Dose–response curves were fitted using Prism version 6.0 and effective concentrations for 50% reduction in cell growth (EC50) were calculated.
Correlation of PARP-1 expression and PARPi sensitivity
The purpose of this analysis was to determine whether there was a linear relationship between PARPi sensitivity and PARP-1 expression. A linear-regression was used to correlate PARP-1 expression, measured by [125I]KX1, and sensitivity of cell lines to talazoparib or olaparib. PARP-1 and PAR expression measured by Western blot analysis was also correlated to [125I]KX1 to determine what PARP radiotracers measure whether PARP expression or activity.
Quantifying PARP-1 in vivo
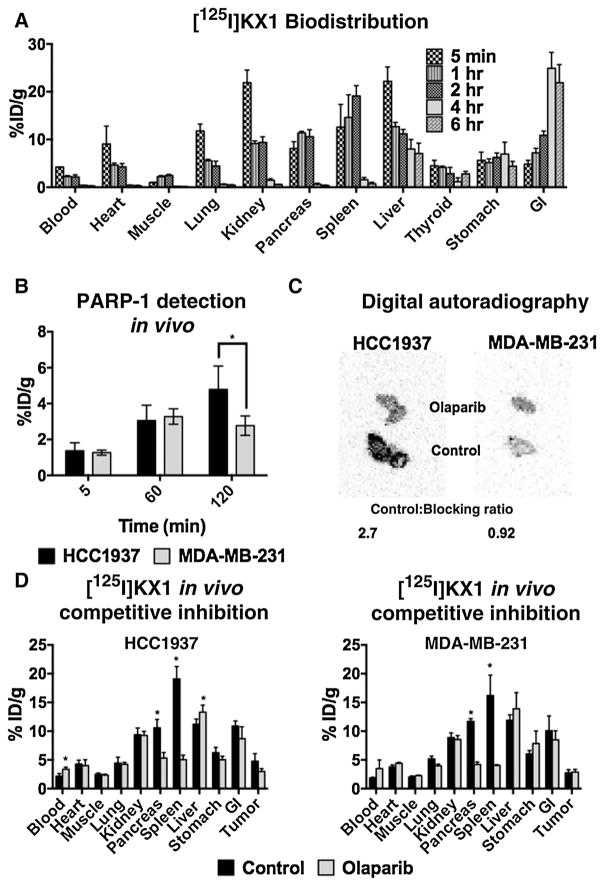
Biodistribution experiments using 135 Bq [125I]KX1 were carried out in 10-week-old NCr mice and organs/tissues were harvested at 5 minutes, 1-, 2-, 4-, and 6-hour time points (n = 4/time point). Radioactivity was measured as described previously. HCC1937 and MDA-MB-231 tumor-bearing models were also evaluated. Approximately 10 million cells of HCC1937 or MDA-MB-231 were subcutaneously injected into the left flank of 10-week-old Nu/Nu or SCID mice and tumors were allowed to grow for 2 weeks before experiments were carried out. Biodistribution of [125I]KX1 in tumor-bearing models were evaluated at time points of 5 minutes, 1 hour, and 2 hours (n = 4/time point), and in vivo competitive inhibition experiments were carried out at 2-hour time points (n = 4). For in vivo competitive inhibition, mice underwent intraperitoneal injections with 1.5 mg of olaparib in 15% PEG, 1% DMSO saline suspension. A blocking dose of olaparib was administered 24 and 4 hours before experiments. Ex vivo digital autoradiography was performed by harvesting tumors and freezing them at −80° C until cryosectioning occurred. Twenty-five micron sections were prepared using a Leica CM1950 Cryostat and were mounted on slides. Next, sections were exposed for 48 hours using a phosphor film, then were read on a GE Typhoon FLA Digital Imaging System.
Results
Competitive inhibition
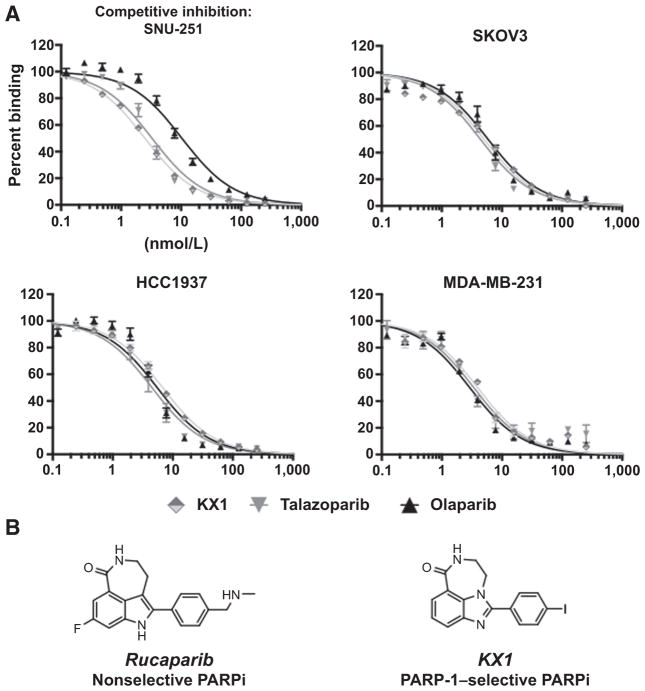
KX1, talazoparib, and olaparib showed small differences in calculated Ki among the different cell lines. See Fig. 1A and Supplementary Table S2 for competitive inhibition curves and Ki values. Chemical structures for KX1 and analogue PARPi rucaparib are illustrated in Fig. 1B.
Figure 1.
A, competitive inhibition curves of [125I]KX1 with various PARPis in multiple cell lines. B, the structural similarities of KX1 and analogue PARPi rucaparib are illustrated.
Kinetic analysis
The dissociation rate of [125I]KX1 measured in SNU-251 cells was much slower than dissociation rates measured in the three other cell lines. This may be reflective of pharmacologic differences of PARPis in the presence of DNA damage, similar to how NAD+metabolism by PARP-1 increases when PARP-1 is bound to DNA (23). The BRCA1-mutant cell line SNU-251 has higher levels of γH2AX at baseline. See Supplementary Fig. S1 and Supplementary Table S3. The association rates were similar and the calculated Kd was within an order of magnitude between cell lines. This observation highlights the fact that there are subtle differences in how KX1 interacts with PARP-1 in different cell lines.
PARP specificity
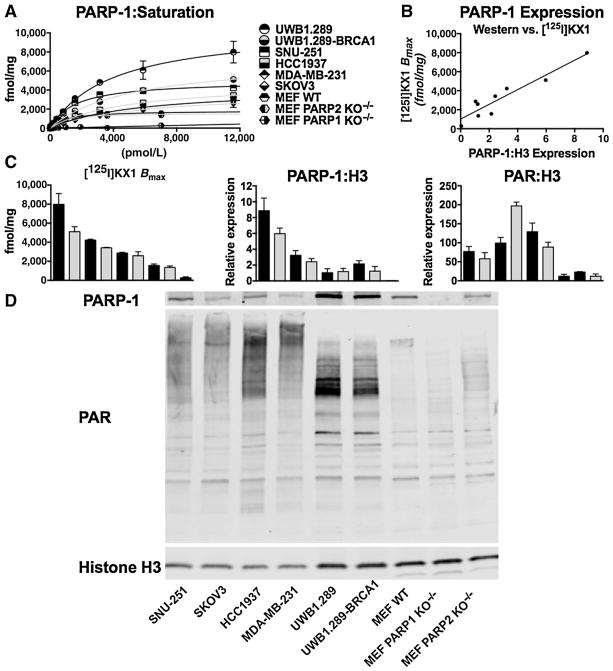
[125I]KX1 was tested in MEF WT and PARP1/PARP2 KO−/− cells to evaluate PARP isozyme specificity. We found that [125I]KX1 had no specific binding in MEF PARP1 KO−/− cells. This can be attributed to the compounds selective nature for PARP-1. These results were surprising as rucaparib, a structural congener of KX1 (Fig. 1A), has been reported as a nonselective PARPi (24). Also, these experiments may reflect the relative expression of PARP-1 versus PARP-2, with PARP-1 being more highly expressed (see Fig. 2A). [125I]KX1 exhibited no change in Kd in the absence of PARP-2, providing evidence for PARP-1 selectivity at the molecular level.
Figure 2.
A, saturation curves for [125I]KX1 in all cell lines evaluated are represented. B, [125I]KX1 Bmax (fmol/mg) values correlated with PARP-1 expression (r2 = 0.88), validating [125I]KX1 as a biomarker for PARP-1 expression. C, the comparison of PARP-1 expression versus activity as defined by [125I]KX1, PARP-1:H3, or PAR:H3, highlighting that [125I]KX1 binding corresponds with PARP-1 expression but not PAR expression. Cell lines listed in A from top to bottom represent bars in bar graphs in C from left to right. D, a representative Western blot analysis of PARP-1, PAR, and Histone H3 is shown.
Quantifying PARP-1 in vitro
[125I]KX1 saturation experiments were performed in multiple cancer cell lines and revealed differences in PARP-1 expression. These levels correspond to pharmacologically active PARP-1 NAD+–binding sites (see Fig. 2A). The UWB1.289 and UWB1.289-BRCA1 cell lines had the highest levels of PARP-1 defined by Bmax followed by the SNU-251 cell line. Values for Bmax are presented in Supplementary Table S4. These data establish that PARP-1 can be quantified in vitro using a highly sensitive live cell–based assay.
Western blot analysis
PARP-1 expression correlated with the [125I]KX1 saturation experiments. Of note, PAR expression did not correlate with PARP expression and the HCC1937 cell line showed the highest level of PAR. See Fig. 2C for relative PARP-1 and PAR protein expression normalized to Histone H3 expression and Fig. 2D for representative Western blots.
PARPi growth inhibition assays
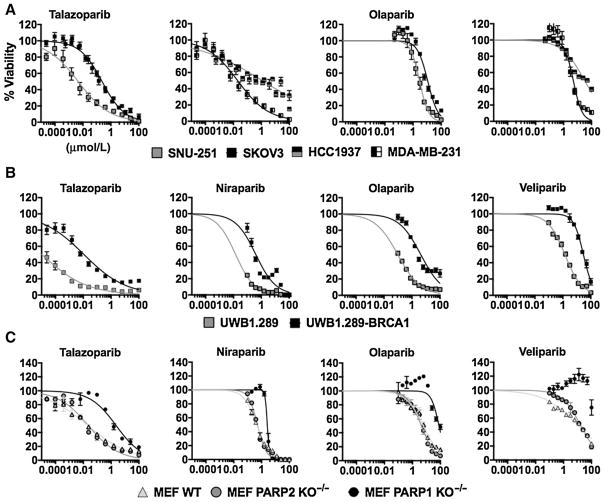
The UWB1.289 cell line was the most sensitive cell line evaluated to single-agent talazoparib with an EC50 value of 10 pmol/L. See Fig. 3A and B for PARPi dose-response curves in cell lines SNU-251, SKOV3, HCC1937, MDA-MB-231, UWB1.289, and UWB1.289-BRCA1. See Supplementary Table S5 for a list of cell line/drug-specific EC50 values. Figure 3 represents dose–response curves for single-agent PARPis. Using the MEF WT, PARP1 KO−/−, and PARP2 KO−/−, we demonstrated the importance of PARP-1 protein expression for the mechanism of action of talazoparib, olaparib, niraparib, and veliparib (see Fig. 3C). In the absence of PARP-1, a large rightward shift in EC50 values for all PARPis was observed except for niraparib, which only had a 5-fold decrease in potency. Niraparib is believed to be equally potent for PARP-1 and PARP-2, and this property is in agreement with our growth inhibition data in MEF cell lines (22). There was no difference in PARPi sensitivity between MEF WT and PARP2 KO−/− cell lines. The HCC1937 and SKOV3 cell lines were the most resistant to talazoparib and olaparib.
Figure 3.
A, in vitro growth inhibition curves for SNU-251, SKOV3, HCC1937, and MDA-MB-231 cells treated with either talazoparib or olaparib. Notice the leftward shift in ovarian cancer BRCA1-mutated cell line SNU-251 compared with SKOV3. B, growth inhibition curves for ovarian cancer and BRCA1-mutated/restored isogenic pair UWB1.289 and UWB1.289-BRCA1. Restoration of BRCA1 caused a significant decrease in sensitivity. C, growth inhibition assays carried out in MEF cell lines. No difference was observed in the absence of PARP2; however, drastic reductions in sensitivity were noticed in the MEF PARP1 KO−/− cell line.
Correlation of PARP-1 expression and PARPi sensitivity
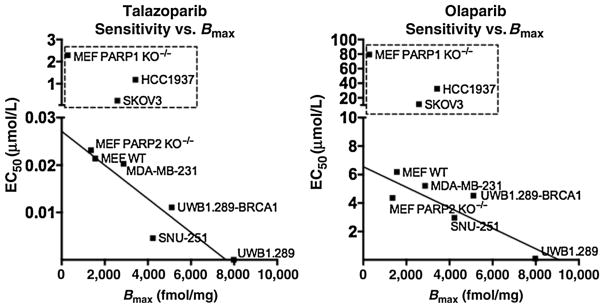
We found that there was a linear correlation between PARP-1 protein expression measured by Western blot analysis and PARP-1 binding measured by [125I]KX1 (Fig. 2B and C). Also, PARPi-sensitive cell lines shared a linear correlation between PARPi sensitivity and PARP-1 expression (Fig. 4). Cell lines that required more than 10 times the Kd for cytotoxic efficacy were excluded from the analysis due to the likelihood that the observed toxicity was due to nonspecific effects of the PARPi and were pharmacologically not related to PARP-1. We chose 10 times the Kd as a threshold because it represents the theoretical concentration needed to occupy 90.9% of PARP-1 enzymes present.
Figure 4.
Linear-regression plots correlating sensitivity to talazoparib (r2 = 0.84, P = 0.001) or olaparib (r2 = 0.77, P = 0.02) with PARP-1 expression. Resistant cell lines were not included in analysis and are outlined. Cell lines were excluded because of concentrations required for cytotoxic efficacy exceeded concentrations needed to occupy 90.9% of PARP-1 enzymes and therefore can be attributed to nonspecific effects.
Quantifying PARP-1 in vivo
Biodistribution studies are presented in Fig. 5A. Significant differences in PARP-1 expression were observed in tumor-bearing models (Fig. 5B) and corresponded with in vitro data on PARP-1 expression presented in Fig. 2C. In vivo competitive inhibition resulted in significant decreases in [125I]KX1 in the pancreas and spleen; however, no significant differences were observed in tumor (P = 0.0743; Fig. 5C or D). We postulate the observed lack of significant pharmacologic blocking of olaparib is due to differences in pharmacokinetics between olaparib and [125I]KX1, resulting in lower tumor uptake of the competing olaparib. Olaparib has very quick clearance from tissues due to p-glycoprotein efflux mechanisms and fast renal clearance (25, 26). Autoradiography of tumors from HCC1937 and MDA-MB-231 xenograft models confirmed the small differences observed in vivo (Fig. 5C).
Figure 5.
A, total organ distribution of [125I] KX1 over the course of 6 hours (n = 4/time point). B, PARP-1 expression measured in HCC1937 and MDA-MB-231 tumors at time points up to 2 hours (n = 4/time point). C, ex vivo autoradiography of HCC1937 and MDA-MB-231 tumors at 2 hours. Olaparib-treated mice show a reduction in signal in HCC1937 but not in MDA-MB-231. D, in vivo competitive inhibition of [125I] KX1 using olaparib.*, signi of [125I] KX1 using olaparib cant change was measured in the organ.
Discussion
In our study, the loss of PARP-1 significantly increased all PARPi EC50 values in MEF PARP1 KO−/− compared with WT or PARP2 KO−/− showing that PARP-1 expression is essential for drug efficacy. Loss of PARP-2 had little to no effect and might be due to relative protein expression between PARP-1 and PARP-2 in MEF cell lines. In vitro PARPi sensitivity has been previously associated with PARP-1 expression in multiple neuroblastoma, and astrocytoma/glioma cells (27, 28). PARPi pharmacology has also been controversial regarding specific allosteric trapping mechanisms possessed by some PARPis but not others (14, 29, 30). Regardless of the differences in potency, all clinical PARPis require PARP-1 expression for cytotoxic effects.
In this study, PARPi sensitivity was directly related to the level of PARP-1 protein expression. BRCA1 restoration in the UWB1.289 cell line caused significant shifts in EC50 values; however, the cell line remained relatively sensitive compared with the SKOV3, an ovarian cancer cell line with functional BRCA1 and low PARP-1 expression. In comparison with the SNU-251 BRCA1-mutant cell line, the UWB1.289-BRCA1 restored had similar PARPi sensitivities and PARP-1 expression although they differed in BRCA1 mutational status. Conversely, the HCC1937 BRCA1-mutant cell line had PARP-1 expression but was the most resistant cancer cell line evaluated. Interestingly, HCC1937 had the highest expression of PAR, which could be a marker of PARPi resistance. The path forward in understanding the importance of this work is to clinically evaluate the negative and positive predictive value of PARP-1 expression for PARPi therapy.
PARP radiotracers were first developed to image cell death pathways such as parthanatos or necroptosis (17). Currently, there have been multiple reports of PARP radiotracers and fluorescent probes (18, 31–35). This study was designed to identify what PARP radiotracers measure and we have observed that KX1 correlates to PARP-1 expression in vitro and in vivo. In addition, we also observed that KX1 is PARP-1 specific as tested in the genetically engineered MEF cell lines. Understanding these two fundamental properties of PARP radiotracers will help guide clinicians in early investigational studies.
Currently, there are two Investigational New Drug Applications open at Washington University (St. Louis, MO) and The University of Pennsylvania (Philadelphia, PA) for clinical PET imaging of PARP-1 with a fluorine-18 analogue (FluorThanatrace) of KX1. With this technology available for clinical research, PARP-1 can now serve as an additional biomarker in precision medicine for the clinical management of cancer.
Conclusion
[125I]KX1 is capable of specifically quantifying PARP-1 expression in vitro and in vivo and serves as a foundation for the development of clinical protocols that encompass the utilization of PARP-1 PET imaging agents. This technology can provide accurate and reproducible quantitative methods for measuring the expression of PARP-1, which is especially important in the prospective selection of appropriate patients for PARPi therapy.
Supplementary Material
Acknowledgments
The authors thank S. Franco (Johns Hopkins Medical School) for generously providing PARP1−/− and PARP2−/− MEFs.
Grant Support
This study was supported by NIH grants CA138835 and CA17494 (R.A. Greenberg), DOE Training Grant DE-SC0012476, the Basser Center for BRCA, and the K12 Abramson Cancer Paul Calabresi Career Development program.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: M. Makvandi, B.P. Lieberman, R.-C. Anderson, E.S. McDonald, D.A. Pryma, R.A. Greenberg, R.H. Mach Development of methodology: M. Makvandi, K. Xu, B.P. Lieberman, R.-C. Anderson, C. Zeng, E.S. McDonald, D.A. Pryma
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M. Makvandi, S.S. Effron, D.A. Pryma
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M. Makvandi, S.S. Effron, H.D. Winters, D.A. Pryma, R.H. Mach
Writing, review, and/or revision of the manuscript: M. Makvandi, B.P. Lieberman, R.-C. Anderson, S.S. Effron, E.S. McDonald, D.A. Pryma, R.A. Greenberg, R.H. Mach
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K. Xu, C. Zeng, D.A. Pryma
Study supervision: D.A. Pryma, R.A. Greenberg, R.H. Mach
References
- 1.Feng FY, de Bono JS, Rubin MA, Knudsen KE. Chromatin to clinic: the molecular rationale for PARP1 inhibitor function. Mol Cell. 2015;58:925–34. doi: 10.1016/j.molcel.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 3.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 4.Gottipati P, Vischioni B, Schultz N, Solomons J, Bryant HE, Djureinovic T, et al. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Res. 2010;70:5389–98. doi: 10.1158/0008-5472.CAN-09-4716. [DOI] [PubMed] [Google Scholar]
- 5.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 7.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 8.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18:5806–15. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16:475. doi: 10.1186/s13058-014-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med. 2015;13:188. doi: 10.1186/s12916-015-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettitt SJ, Rehman FL, Bajrami I, Brough R, Wallberg F, Kozarewa I, et al. A genetic screen using the PiggyBac transposon in haploid cells identifies Parp1 as a mediator of olaparib toxicity. PLoS One. 2013;8:e61520. doi: 10.1371/journal.pone.0061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, Digiammarino EL, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13:1465–77. doi: 10.1158/1541-7786.MCR-15-0191-T. [DOI] [PubMed] [Google Scholar]
- 15.Ozretic L, Rhiem K, Huss S, Wappenschmidt B, Markiefka B, Sinn P, et al. High nuclear poly(adenosine diphosphate-ribose) polymerase expression is predictive for BRCA1- and BRCA2-deficient breast cancer. J Clin Oncol. 2011;29:4586–8. doi: 10.1200/JCO.2011.38.1988. [DOI] [PubMed] [Google Scholar]
- 16.Salinas B, Irwin CP, Kossatz S, Bolaender A, Chiosis G, Pillarsetty N, et al. Radioiodinated PARP1 tracers for glioblastoma imaging. EJNMMI Res. 2015;5:123. doi: 10.1186/s13550-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu Z, Chu W, Zhang J, Dence CS, Welch MJ, Mach RH. Synthesis and in vivo evaluation of [11C]PJ34, a potential radiotracer for imaging the role of PARP-1 in necrosis. Nucl Med Biol. 2005;32:437–43. doi: 10.1016/j.nucmedbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Chu W, Xu J, Jones LA, Peng X, Li S, et al. Synthesis, [(1)(8)F] radiolabeling, and evaluation of poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors for in vivo imaging of PARP-1 using positron emission tomography. Bioorg Med Chem. 2014;22:1700–7. doi: 10.1016/j.bmc.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–82. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- 21.Barkauskaite E, Jankevicius G, Ahel I. Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-ribosylation. Mol Cell. 2015;58:935–46. doi: 10.1016/j.molcel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Steffen JD, Brody JR, Armen RS, Pascal JM. Structural implications for selective targeting of PARPs. Front Oncol. 2013;3:301. doi: 10.3389/fonc.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336:728–32. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlberg E, Karlberg T, Kouznetsova E, Markova N, Macchiarulo A, Thorsell AG, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol. 2012;30:283–8. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor D, Martin P, Busschots S, Thery J, O’Leary JJ, Hennessy BT, et al. PARP inhibitors as P-glyoprotein substrates. J Pharm Sci. 2014;103:1913–20. doi: 10.1002/jps.23952. [DOI] [PubMed] [Google Scholar]
- 26.Henneman L, van Miltenburg MH, Michalak EM, Braumuller TM, Jaspers JE, Drenth AP, et al. Selective resistance to the PARP inhibitor olaparib in a mouse model for BRCA1-deficient metaplastic breast cancer. Proc Natl Acad Sci U S A. 2015;112:8409–14. doi: 10.1073/pnas.1500223112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chornenkyy Y, Agnihotri S, Yu M, Buczkowicz P, Rakopoulos P, Golbourn B, et al. Poly-ADP-ribose-polymerase as a therapeutic target in pediatric diffuse intrinsic pontine glioma and pediatric high grade astrocytoma. Mol Cancer Ther. 2015;14:2560–8. doi: 10.1158/1535-7163.MCT-15-0282. [DOI] [PubMed] [Google Scholar]
- 28.Newman EA, Lu F, Bashllari D, Wang L, Opipari AW, Castle VP. Alternative NHEJ pathway components are therapeutic targets in high-risk neuroblastoma. Mol Cancer Res. 2015;13:470–82. doi: 10.1158/1541-7786.MCR-14-0337. [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Aoyagi-Scharber M, Wang B. Trapping poly(ADP-ribose) polymerase. J Pharmacol Exp Ther. 2015;353:446–57. doi: 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
- 30.Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13:433–43. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin CP, Portorreal Y, Brand C, Zhang Y, Desai P, Salinas B, et al. PARPi-FL–a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia. 2014;16:432–40. doi: 10.1016/j.neo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney B, Carlucci G, Salinas B, Di Gialleonardo V, Kossatz S, Vansteene A, et al. Non-invasive PET Imaging of PARP1 expression in glioblastoma models. Mol Imaging Biol. 2016;18:38–92. doi: 10.1007/s11307-015-0904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer JP, Houghton JL, Kozlowski P, Abdel-Atti D, Reiner T, Pillarsetty NV, et al. (18)F-Based pretargeted PET imaging based on bioorthogonal diels-alder click Chemistry. Bioconjug Chem. 2016;27:298–301. doi: 10.1021/acs.bioconjchem.5b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlucci G, Carney B, Brand C, Kossatz S, Irwin CP, Carlin SD, et al. Dual-modality optical/PET imaging of PARP1 in glioblastoma. Mol Imaging Biol. 2015;17:848–55. doi: 10.1007/s11307-015-0858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zmuda F, Malviya G, Blair A, Boyd M, Chalmers AJ, Sutherland A, et al. Synthesis and evaluation of a radioiodinated tracer with specificity for poly(ADP-ribose) polymerase-1 (PARP-1) in vivo. J Med Chem. 2015;58:8683–93. doi: 10.1021/acs.jmedchem.5b01324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.